SUMMARY
Ticks transmit a diverse array of microbes to vertebrate hosts, including human pathogens, which has led to a human-centric focus in this vector system. Far less is known about pathogens of ticks themselves. Here, we discover that a toxin in blacklegged ticks (Ixodes scapularis) horizontally acquired from bacteria—called domesticated amidase effector 2 (dae2)—has evolved to kill mammalian skin microbes with remarkable efficiency. Secreted into the saliva and gut of ticks, Dae2 limits skin-associated staphylococci in ticks while feeding. In contrast, Dae2 has no intrinsic ability to kill Borrelia burgdorferi, the tick-borne Lyme disease bacterial pathogen. These findings suggest ticks resist their own pathogens while tolerating symbionts. Thus, just as tick symbionts can be pathogenic to humans, mammalian commensals can be harmful to ticks. Our study underscores how virulence is context-dependent and bolsters the idea that “pathogen” is a status and not an identity.
In Brief
Hayes et al. demonstrate that bacteria on the skin of humans can be pathogenic to ticks, but blacklegged ticks have horizontally acquired a bacterial toxin—Dae2—that efficiently kills mammalian skin microbes. Dae2 is secreted into the tick digestive system and kills off skin-associated staphylococci during feeding, but not Borrelia burgdorferi, the bacterial cause of Lyme disease in humans.
Graphical Abstract
INTRODUCTION
Bacteria have shaped the evolution of animal hosts through a vast continuum of interactions, ranging from pathogenic to beneficial (McFall-Ngai et al., 2013). In the case of vector-borne disease systems, a symbiotic vector-microbe partnership can lead to disastrous consequences for other animals with which they collide (Shaw et al., 2018). For example, blood-feeding ticks are vectors that transmit a more diverse array of microbes to vertebrate hosts than any other arthropod known, resulting in a multitude of diseases for both humans and livestock (Goodman et al., 2005; Sonenshine, 2005). The microbiome of the tick vector Ixodes scapularis is dominated by a few human pathogens, such as Rickettsia spp. and Borrelia burgdorferi, the causative agent of Lyme disease (Stewart and Bloom, 2020). The persistence of tick-borne pathogens results from stable tick colonization by these symbionts, which likely have reduced pathogenic potential against their tick vectors (Casadevall, 2017). Except for these human pathogens, the tick microbiota composition is highly variable and context-dependent (Clay and Fuqua, 2010; Couper et al., 2019; Narasimhan and Fikrig, 2015; Stewart and Bloom, 2020), which is suggestive of many transient interactions that could become infectious for ticks. However, we know markedly little about antagonistic interactions between bacteria and tick vectors themselves, because ticks have largely been studied through the lens of human disease.
Blood-feeding (hematophagy) is a hazardous lifestyle that arose independently in at least six different arthropod lineages ~50–150 million years ago (mya), coincident with the emergence of many immune and host-modulating genes predicted to support this behavior (Mans, 2011; Mans and Neitz, 2004; Ribeiro et al., 1985). The prevalence of antibacterial defense mechanisms encoded by innate immune systems of ticks suggests that effective control of the microbes they encounter is important for successful parasitic interactions with a bloodmeal host (Gulia-Nuss et al., 2016; Smith and Pal, 2014). Hard ticks, such as the blacklegged tick vector of Lyme disease, Ixodes scapularis, have especially prolonged continuous bloodmeals that can last over a week (Sonenshine, 2005), during which ticks must embed within the skin of their hosts and maintain an intimate, perilous attachment. Field microbiome studies of hard ticks suggest that they encounter a diverse assemblage of environmental and host-associated bacteria, including species commonly found on the skin of mammalian hosts (Carpi et al., 2011; Clay and Fuqua, 2010; Greay et al., 2018; Narasimhan and Fikrig, 2015; Stewart and Bloom, 2020). A subset of these could be pathogenic to ticks (Lormendez et al., 2019; Szczepańska et al., 2018; Zhioua et al., 1999), particularly those residing at the tick-host skin interface, which could gain entry into ticks as they imbibe blood. Identification of bona fide tick pathogens would offer a critical point of comparison for understanding how tick innate immune systems control microbial interactions in support of hematophagy and may also inform future para-transgenic strategies, which have experienced recent success against mosquito vectors (Gao et al., 2020; Shaw and Catteruccia, 2019; Wilke and Marrelli, 2015).
The function and selectivity of invertebrate innate immune systems can evolve in response to specific microbial threats (Schulenburg et al., 2007; Tetreau et al., 2019). We previously found that I. scapularis and other ticks acquired a potent antibacterial enzyme approximately 40 mya by horizontal gene transfer of an interbacterial competition toxin gene from bacteria (Chou et al., 2015). These domesticated amidase effector 2 (dae2) genes were co-opted through acquisition of bacterial type VI secretion (T6S) amidase effector 2 (tae2) genes, which encode lytic cell wall-degrading toxins that bacteria use to break down cell wall peptidoglycan (PG) of neighboring competitor microbes. The evolutionary timing and extraordinary interkingdom journey of dae2 reinforce the notion that the antibacterial defense it provides is important for the hematophagous parasitism of I. scapularis ticks. However, the microbes driving dae2 domestication are not known. Acquisition of B. burgdorferi by ticks during feeding activates expression of dae2 from I. scapularis (dae2Is) (Smith et al., 2016), although induction is mediated through interferon signaling from the bloodmeal host and not directly by the bacterium (Smith et al., 2016). Our prior work revealed that dae2Is modestly limits B. burgdorferi levels in I. scapularis (Chou et al., 2015). Paradoxically, B. burgdorferi is a stably associated tick microbe that is not cleared by I. scapularis immunity and does not appear to negatively impact tick fitness (Couper et al., 2020; Eisen, 2020). These observations suggest that Dae2Is may promote tick tolerance of B. burgdorferi by preventing its excessive proliferation in the vector through direct killing or immunomodulation, similar to what has been observed for host-commensal interactions in other systems (Hooper et al., 2012; Nyholm and Graf, 2012). Alternatively, Dae2 may primarily function to resist and protect against less stably associated microbes that are more akin to true pathogens of ticks.
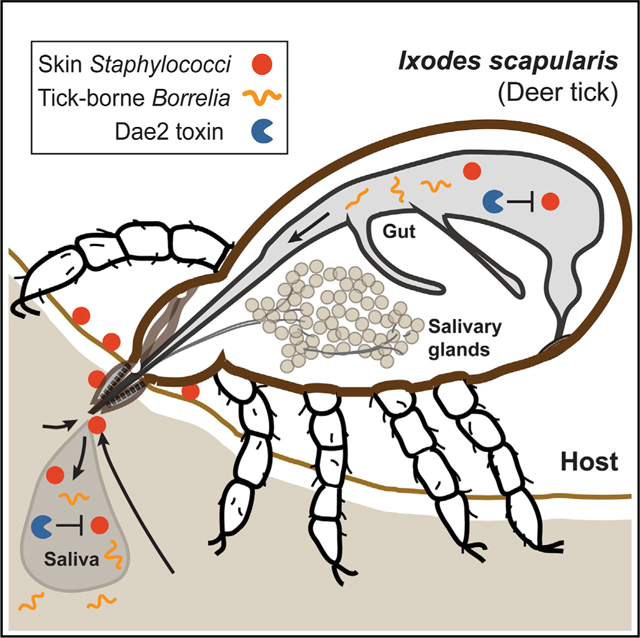
In this study, we consider the possibility that the Dae2 immune factor protects against the natural pathogens of ticks themselves by examining the specificity and biological function of this toxin in the tick disease vector I. scapularis. Given that incorporation of domesticated genes into the biology of recipient organisms can be accompanied by evolutionary and functional divergence(Wiedenbeck and Cohan, 2011), we approached this problem by investigating differences between bacterial Tae2 and tick Dae2 toxins. We report that I. scapularis-encoded Dae2Is has structurally and biochemically diverged from its ancestral bacterial counterparts, which has led to broadened antimicrobial specificity. Notably, Dae2Is is a particularly effective bactericidal agent against Gram-positive bacteria that are enriched in the skin microbiota of mammals. Through its action in the tick gut and saliva during feeding, Dae2Is directly antagonizes skin commensals of bloodmeal host, which are detrimental to both tick feeding success and viability.
RESULTS
Tae2 and Dae2 Enzymes Are Structurally Divergent
The bacterial Tae enzymes are delivered by specialized T6SS injection machinery into the outer membrane-encased cell wall compartment (periplasm) of recipient Gram-negative bacteria (Russell et al., 2011, 2012), resulting in osmotic stress and cell lysis. In contrast, the eukaryotic dae genes are not associated with any specialized secretion apparatus. Although Dae enzymes have retained the ability to degrade PG, the eukaryotic representatives are not intrinsically capable of traversing the protective outer membrane of Gram-negative bacteria (Chou et al., 2015). Thus, we investigated whether Dae effectors may act against other microbial groups by first conducting a sequence-based comparison of dae2 genes of ticks and mites with bacterial tae2 homologs to identify potential differences that could contribute to altered specificity. This showed several discrete regions within the primary sequence that varied between these groupings (Figures S1A and S1B), suggestive of divergent structural signatures. Although we were unable to find definitive evidence of positive selection due to a low overall number of representatives (Chou et al., 2015), these variances were unique to eukaryotic representatives and may encode important functional differences between ancestral and domesticated enzymes.
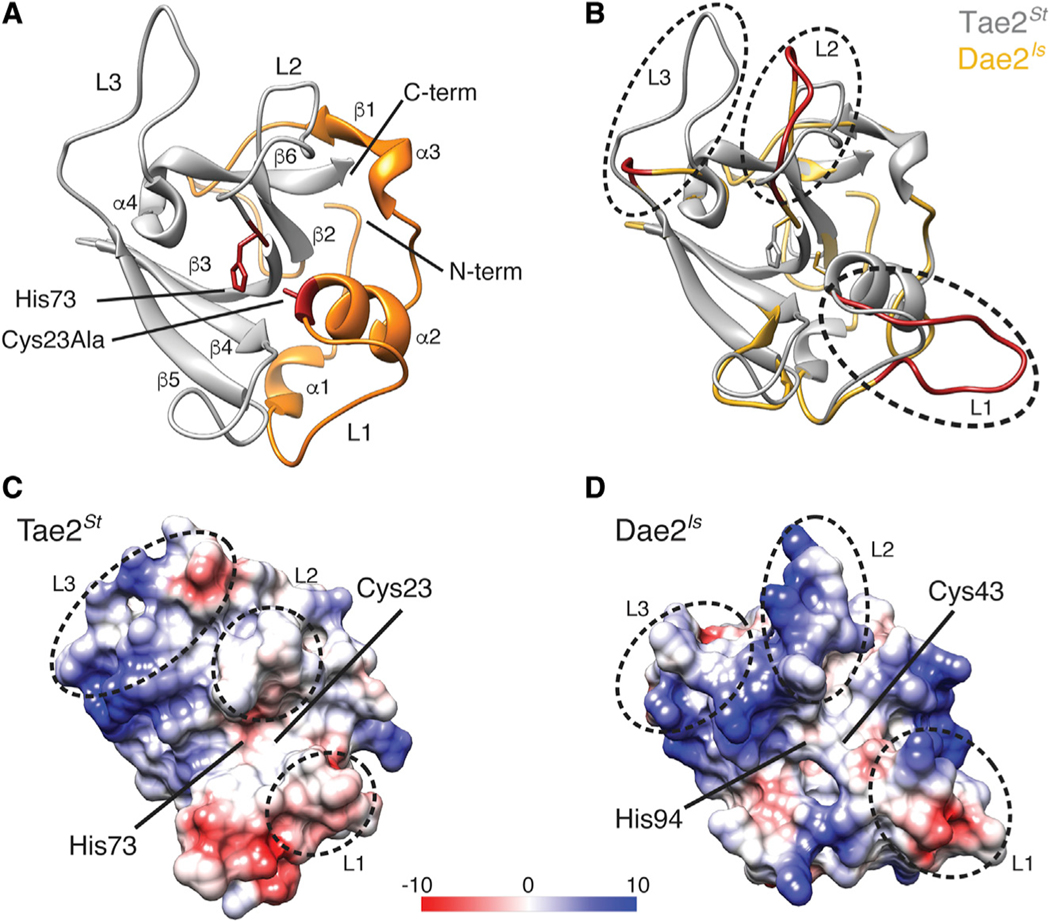
We next pursued a structural comparison of Tae2 and Dae2 enzymes. After crystallization trials with several Tae2/Dae2 family members, we were able to determine the high-resolution X-ray crystal structure of Tae2 from Salmonella enterica Typhi (Tae2St) at a resolution of 2.05 Å (Figure 1A; Table S1, PDB: 6WIN). Tae2St adopts an amidase fold conserved across papain-like proteases and most similar to endolysin LysK from staphylococcal phage K (Figure S2) (Sanz-Gaitero et al., 2014). From this structure, we generated threaded homology models of remaining family members at a confidence interval of >90% that allowed high probability fold prediction (Kelley et al., 2015). Alignment of the Tae2St structure and a Dae2Is homology model (112 aligned residues; 32% identity) pointed to several substantial structural divergences between eukaryotic and prokaryotic subgroups that correlated with our sequence-based analyses, with the three major regions of sequence divergence mapping to the loops (L1–L3) that abut the catalytic channel (Figure 1B). This comparison also revealed substantial differences in the topology and electrostatic properties of a deep hydrophobic substrate-binding groove that extends from the active site (Figures 1C and 1D). The catalytic residues themselves are more accessible in Dae2Is than in Tae2St, with a predicted pore behind the catalytic cysteine of Dae2Is (Figure 1D). Importantly, the modeled differences in loop structures, the substrate-binding groove and the catalytic channel led us to hypothesize that the substrate specificity of Dae2 could either be distinct from or expanded compared to the bacterial enzymes.
Figure 1. Tae2 and Dae2 Enzymes Have Divergent Structural Features.
(A) Ribbon diagram Tae2 from Salmonella enterica Typhi (Tae2St). Canonical papain-like secondary structures are labeled (α1–4, β1–6, Loop1–3 [L1–3]), and subdomains are colored (gray, orange). Catalytic residues are denoted (labeled His 73 and Cy-s23Ala, red).
(B) Structural alignment of Tae2St (gray) and a Tae2-based homology model of Dae2Is (golden). Loops 1, 2, and 3 are colored red in Dae2Is.
(C and D) Electrostatic charges in surfaces proximal to the catalytic residues for Tae2St and Dae2Is (scale is in kcal mol−1e−1). The loops are boxed in sequence alignment in Figure S1B.
Tae2/Dae2 Catalytic Channels Are Predicted to Accommodate Distinct Substrate Positions
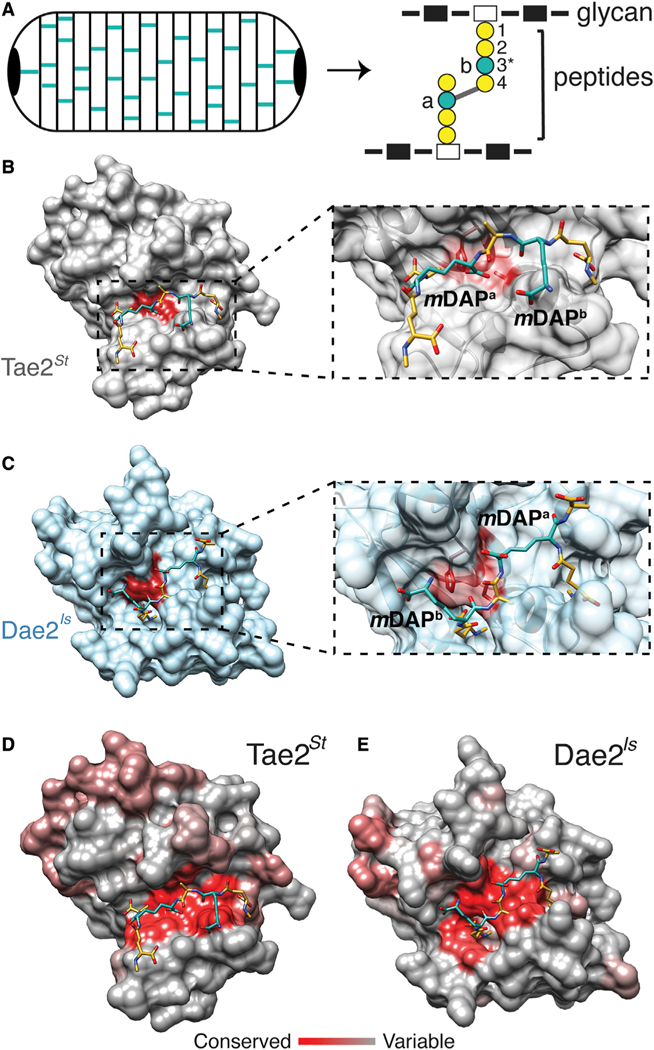
Divergent Dae2 specificity could have important biological implications for tick immunity. Given that cell wall composition varies considerably between bacterial taxa (Turner et al., 2014), enzyme selectivity for PG types directly dictates which microbes can be inhibited. Of note, Gram-negative bacteria incorporate meso-diaminopimelic acid (mDAP) at the third position in the peptide stem (Figure 2A) (Turner et al., 2014), whereas Gram-positive Bacilli have an amidated mDAP, and other Gram-positive species have a lysine (Turner et al., 2014). We previously found both Tae2 and Dae2 can hydrolyze Gram-negative PG (Chou et al., 2015), and a Tae toxin from Pseudomonas aeruginosa is highly selective for mDAP-containing Gram-negative PG (Chou et al., 2012). We used an in silico approach to further probe specificity by computationally docking a fragment of crosslinked mDAP PG onto Tae2St and Dae2Is (Figures 2B and 2C). Docked PG adopted highly dissimilar poses between the two structures. We calculated an overall narrower cleft on Tae2St with 852.9 Å2 active site surface area versus 1,086.0 Å2 for Dae2Is, which also has an additional deep binding pocket. Upon binding, the cross-linked PG interacted with Dae2Is across a larger area of the catalytic site (624.9 Å2) compared to Tae2St (450.2 Å2) (Figure S3, red). Our modeling and metadynamics simulations predicted a flipped PG orientation mediated through different residue interactions at the enzyme-substrate binding interface (Figure S3) as well as tighter Dae2-PG binding. Finally, surface conservation binding interface. Surface conservation profiles of Tae2 and Dae2 subgroups revealed distinct properties that are conserved within the bacterial and eukaryotic enzyme families (Figures 2D and 2E). We hypothesize that altered binding and positioning of the PG substrate could lead to an enzyme specificity profile for Dae2 that is distinct from their bacterial antecedents, which may be important for their domesticated function in ticks. The widened catalytic channel of Dae2 leads us to predict that the eukaryotic enzymes can act on a broader panel of PG structural groups relative to Tae2 toxins, widening the range of targeted bacteria.
Figure 2. Modeling of Tae2/Dae2 Interactions with PG Substrate.
(A) Cartoon schematic of bacterial peptidoglycan (PG) sacculus (left) and PG chemical structure (right) with glycans (rectangles), peptides (circles), and cross-link position (gray line) shown. Residue positions in PG peptide stem are labeled (1–4) with key variable third position (3*) colored in teal.
(B) Surface representation of Tae2St ligand docking using a stem peptide substrate. Both mDAP residues in the stem peptide are colored in teal.
(C) Close-up of the Tae2St active site and docked substrate. The scissile amide bond is between mDAPa and the adjacent D-ala (position 4), proximal to the active site Cys23 residue.
(D) Surface representation of Dae2Is ligand docking, using the same substrate as for Tae2St in (C).
(E) Close-up of the Dae2Is active site and docked substrate. The scissile bond is similarly situated between mDAPa and the adjacent D-ala, proximal to the active site Cys43 residue. The substrate directionality being mDAPb – D-ala – mDAPa is the inverse of that for Tae2St, mDAPa – D-ala – mDAPb.
(F) Consurf server analyses of Tae2St and Dae2Is with highly conserved residues shown in red and least conserved in gray. The docked PG substrate is shown.
See also Figures S3 and S4.
Biochemical and Antibacterial Expansion of Dae2Is Enables Killing of Skin Commensals
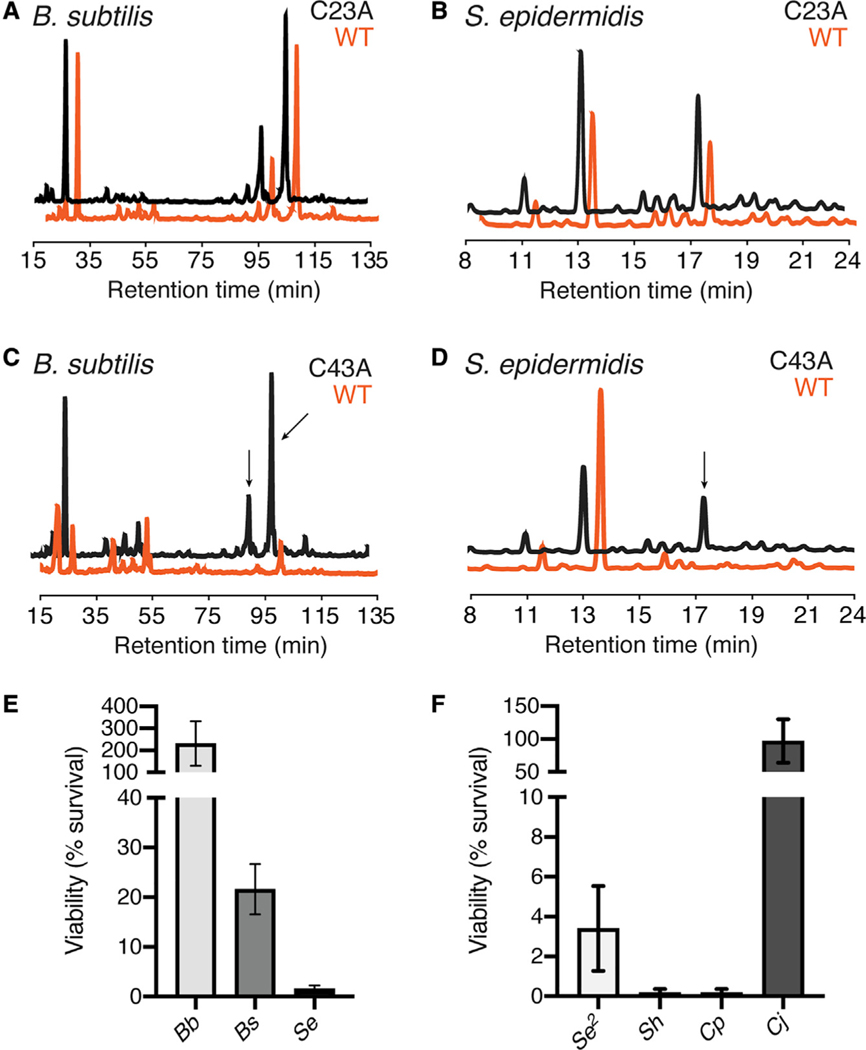
We experimentally tested our specificity model by comprehensively assessing the in vitro substrate specificity of Dae2Is and Tae2St enzymes. We previously observed that Dae2Is and Tae2St can degrade PG from the Gram-negative bacterium E. coli (Chou et al., 2015; Russell et al., 2012). We further tested purified recombinant proteins against a set of PG sacculi prepared from two bacterial species that we and others have found associated with field I. scapularis ticks: an environmental bacterium (Bacillus subtilis, Gram-positive) and a mammalian skin-associated bacterium (Staphylococcus epidermidis, Gram-positive) (Narasimhan and Fikrig, 2015; Ross et al., 2018). Analysis of PG degradation by high performance liquid chromatography (HPLC) showed that the bacterial Tae2St did degrade Gram-negative PG (Figure S4A), but not any Gram-positive cell walls assayed (Figures 3A and 3B), consistent with the role of Tae2 toxins in T6S interbacterial competition between Gram-negative bacteria (S4A). On the other hand, Dae2Is hydrolyzed all PG sacculi types tested (Figures 3C and 3D), including those from lysine-containing Gram-positive cell walls, which has not been previously reported for Tae enzymes. Expansion of Dae2Is activity to Gram-positive S. epidermidis PG was especially remarkable in light of how chemically divergent its cell wall composition is from Gram-negative PG (Figures S4B–S4D). Our biochemical observations open up the exciting possibility that Dae2Is has evolved to lyse a wider range of microbes, which may include skin-associated microbes found at the tick-host feeding interface.
Figure 3. Biochemical Analyses of Tae2/Dae2 Cell Wall and Antibacterial Specificities.
(A and B) HPLC chromatograms of PG treated with wild-type (WT) (orange) or catalytically inactive (C23A, black) Tae2St for cell wall sacculi prepared from (A) B. subtilis and (B) S. epidermidis.
(C and D) HPLC analyses for WT (orange) and catalytically inactive (C43A, black) Dae2Is against (C) B. subtilis and (D) S. epidermidis are also shown. Arrows indicate PG substrate peaks that change in response to enzyme treatment.
(E) Dae2Is lysis assays against Gram-negative Borrelia burgdorferi strain S9 (Bb), Gram-positive Bacillus subtilis 168 (Bs), and Gram-positive Staphylococcus epidermidis BCM060 (Se).
(F) Dae2Is lysis assays against Gram-positive skin associated bacteria S. epidermidis strain SK135 (Se2), S. hominis strain SK119 (Sh), Corynebacterium propinquum DSM44285 (Cp), and C. jeikeium DSM7171 (Cj). Results are reported as percent survival with Dae2Is WT normalized against catalytically inactive enzyme (C43A).
See also Figure S4.
To examine whether our Dae2Is in vitro specificity profile reflects broadened antibacterial activity, we next assayed Dae2Is-dependent effects on bacterial cell viability. We carried out bacterial killing assays at a physiologically relevant concentration of 2 μM Dae2Is, which was determined through quantification of levels in dissected I. scapularis salivary glands (Figure S5). As previously reported, E. coli is intrinsically resistant to Dae2Is, likely because Dae2Is cannot penetrate its outer membrane to access the PG (Chou et al., 2015). This likely also explains the inability of Dae2Is to directly kill the tick-borne spirochete, B. burgdorferi (Figure 3E), despite its outer membrane being highly distinct from that of E. coli (Jutras et al., 2016, 2019). However, given that Dae2Is can degrade purified B. burgdorferi PG fragments (Chou et al., 2015), we cannot rule out the possibility that Dae2Is acts in synergy with an additional host factor or enzymatically processes B. burgdorferi PG to modulate canonical innate immune signaling pathways encoded by I. scapularis (Gulia-Nuss et al., 2016; Shaw et al., 2018). In stark contrast, Dae2Is was highly efficient at directly killing S. epidermidis, a mammalian skin commensal, and moderately efficient at killing B. subtilis, a common environmental bacterium (Figure 3E). These dramatic differences lead us to hypothesize that skin microbes, which are frequently encountered by feeding ticks, are targeted by Dae2Is.
We further tested Dae2Is against three additional skin commensal species: another S. epidermidis strain, S. hominis, and Corynebacterium propiquum, and we observed immediate lysis of all (Figure 3F). Although related to C. propiquum and associated with human skin and mucus membranes, we did not observe Dae2Is-dependent killing of C. jeikeium (Figure 3F) here or Streptococcus pneumoniae previously (Chou et al., 2015), suggesting that Dae2Is specificity is broad but not indiscriminate. It is possible that this more invasive strain of Corynebacterium has evolved mechanisms of resistance to cell wall targeting enzymes (Bernard, 2012). Together, our analyses led to two major conclusions: expanded Dae2 specificity allows the eukaryotic enzymes to target a broader and distinct set of microbes compared to prokaryotic Tae2 enzymes, and Dae2 kills common mammalian skin commensals with incredible potency.
Dae2Is Is Delivered to Host Bite Site via Tick Saliva during Feeding
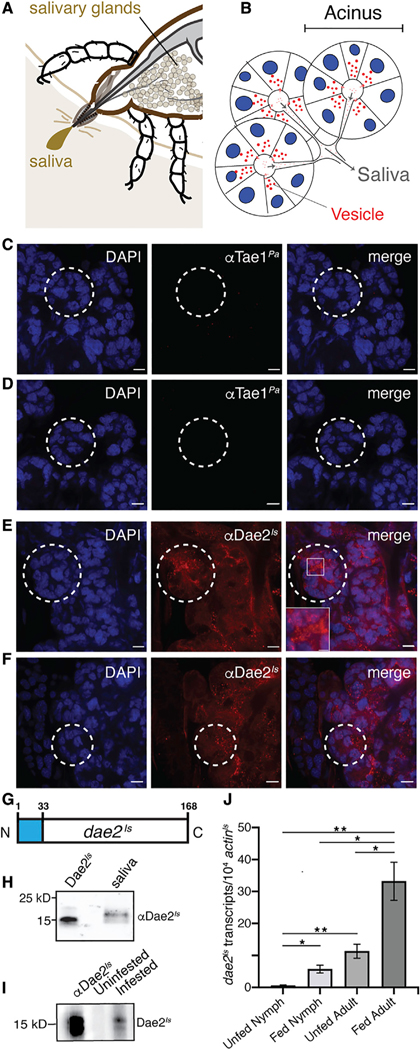
Hard ticks stay attached to the host skin dermis for days, sometimes over a week. This sustained interaction likely leads to intimate exchanges between feeding ticks and microbial commensals of host skin. Our biochemical observations potentiate the model that Dae2Is has the enzymatic capacity to kill bacterial groups commonly found at this critical tick-host bloodmeal interface. It is well documented that ticks and other hematophagous arthropods deliver a variety of secreted molecules, such as antimicrobial and host-modulating effectors, to host bite sites via saliva to ensure successful bloodmeal completion (Figures 4A and 4B) (Kim et al., 2016; Ribeiro et al., 1985). We previously observed that Dae2Is is highly enriched in I. scapularis salivary glands, suggesting the enzyme could be delivered to the bite site via saliva (Chou et al., 2015).
Figure 4. Dae2Is Is Found in Tick Saliva and Upregulated during Feeding.
(A) Schematic of the tick at the bite-site interface showing the salivary glands. The salivary glands produce the saliva that is injected into the host.
(B) Cartoon representation of salivary gland acini. Nuclei (blue), vesicles (red), and saliva flow routes (gray arrows) are shown.
(C–F) Confocal images of dissected I. scapularis salivary glands from adult females. Whole mounts were stained with DAPI (left panels, blue). Control mounts (C and D) were immunostained with rabbit antibody raised against a the bacterial Tae1Pa toxin (αTae1Pa). Experimental mounts (E and F) were immunostained with rabbit αDae2Is. Merged images are shown (right). Scale bars, 15 μm. Selected acini are marked (dashed white lines). A zoomed-in region is shown (box).
(G) Diagram of the dae2Is gene structure showing the predicted N-terminal signal sequence from nucleotide position 1–33 (blue).
(H) Western blot of collected saliva from partially fed I. scapularis adult females demonstrating presence of Dae2Is in saliva. First lane has recombinant Dae2Is as a positive control for rabbit αDae2Is reactivity.
(I) Western blot showing reactivity of mouse serum to Dae2Is. Recombinant Dae2Is protein was probed with mouse serum before (uninfested) or after I. scapularis feeding (infested). Rabbit αDae2Is was used as positive control for reactivity (first lane).
(J) dae2Is expression (normalized against actinIs transcripts) before (unfed) and after feeding (fed) on mice for nymph and adult ticks. *p < 0.05, **p < 0.01.
See also Figure S5.
We tested this possibility by tracking its precise subcellular localization and expression profile during feeding. We imaged Dae2Is distribution in I. scapularis salivary glands by confocal microscopy using an affinity-purified rabbit antibody raised against recombinant Dae2Is (αDae2Is) with specificity validated by western blot analysis (Figure S6A). We found that Dae2Is localizes to vesicle-like puncta (Figures 4C–4F, red). Vesicle-mediated delivery of effector molecules to hosts has been observed in ticks and other hematophagous arthropods (Chávez et al., 2019; Zhou et al., 2020). The dae2Is N terminus encodes a predicted signal peptide (Figure 4G) (Chou et al., 2015); thus, we posited that Dae2Is can be secreted out of tick salivary cells into saliva, enabling access to microbes near the bite site. To test this, we used a glass capillary to harvest saliva produced by live, partially fed I. scapularis ticks. Immunoblot analysis of the extracted sample showed that Dae2Is is indeed secreted into the outgoing saliva (Figure 4H). We observed a slight increase in protein size relative to recombinant protein, suggestive of posttranslational modifications in the tick.
We also tested whether Dae2Is from saliva may physically enter the bloodmeal host by examining immunoreactivity of blood sera collected from mice that had been fed on by I. scapularis ticks (infested). Mouse host sera was immunoreactive to Dae2Is after a single round of tick infestation, whereas sera from mice that had never been exposed to ticks was nonreactive (Figure 4I). These results indicate that the tick injects Dae2Is via saliva into the bite site where it is recognized as an antigen by the murine immune system. We also quantified dae2Is transcripts throughout two feeding cycles and found dramatic upregulation of dae2Is expression in both nymph and adult ticks following bloodmeal consumption (Figure 4J). The localization and timing of Dae2Is in tick saliva strongly suggests that this effector has antibacterial functions at the bite site during feeding.
Dae2Is-Dependent Inhibition of Host Microbes Promotes Tick Feeding during a Bloodmeal
To ask whether Dae2Is activity in ticks contributes to resistance against host commensals during feeding, we next tested whether genetic disruption of dae2Is in I. scapularis affects the levels of the host skin-associated bacteria present in nymphal ticks. We knocked down dae2Is expression through microinjection-based RNA interference (RNAi) prior to feeding I. scapularis nymphs on mice (Figure 5A). We also blocked pre-existing Dae2Is protein in tick midguts or saliva by immunizing mouse bloodmeal hosts with αDae2Is 24 h before tick feeding (Figure 5A). Antibody-dependent neutralization of Dae2Is activity was confirmed through in vitro lysis assays (Figure S6B). We found that αDae2Is remained stable and detectable in mouse sera up to at least 2 weeks post-immunization (Figure 5B), allowing us to inhibit Dae2Is activity throughout the entirety of a tick bloodmeal.
Figure 5. Dae2Is-Dependent Effects on I. scapularis Ticks during a Bloodmeal.
(A) Schematic of in vivo dae2Is knockdown and immunization experiment. Mice were injected with sterile saline control (gray) or 2.5 mg rabbit αDae2Is antibody and I. scapularis nymphs were microinjected with small interfering RNAs (siRNAs) targeting dae2Is (red) or scrambled controls (gray). After 24 h of recovery, dae2Is and control ticks were placed on αDae2Is antibody immunized mice or control mice, respectively. Ticks were forcibly detached at 3 days post-host attachment and assessed by qPCR, scutal index, and weight.
(B) Western blot analysis against recombinant Dae2Is using serum collected from control (left) and αDae2Is-immunized (right) mice at 18 days following antibody injection. A secondary αRabbit antibody was used to specifically detect rabbit αDae2Is antibodies the mice were immunized with. αDae2Is was used as a control (far left).
(C) Staphylococcus load was detected in DNA isolated from pooled dae2Is knockdown (red) or control ticks (black) by qPCR with primers designed to target all species from the genus Staphylococcus. Results are presented as the average log2 Staphylococcus per tick.
(D) Diagram of how scutal index (SI) changes during feeding. Scutal index is the ratio of the length of the entire tick body to the width of the scutum (Meiners et al., 2006).
(E and F) Scutal index (E) and tick weight (F) of individual ticks are shown. *p < 0.05 based on Student’s t test. Blinded measurements were made from tick images using ImageJ. Weight was measured with an analytical balance with an error of ±0.1 mg.
We retrieved ticks during the fast-feeding phase of their bloodmeals (day 3) and measured levels of host-associated bacteria present in ticks by qPCR, using genus-specific primers that reflect the diversity of Staphylococcus commensal strains colonizing mouse skin (Table S2). Ticks where Dae2Is activity was significantly reduced or absent supported higher levels of Staphylococcus bacteria than the control ticks (Figure 5C). To test impacton tick health, we compared feeding metrics of dae2Is and control ticks at day 3 by first quantifying tick body expansion across two axes. The ratio of these measurements, known as the scutal index (SI), reflects how long ticks have been feeding on a bloodmeal host (Figure 5D) (Meiners et al., 2006). Control ticks had significantly higher SIs than dae2Is knockdown ticks (Figure 5E). We also weighed individual ticks and found that dae2Is ticks were lighter than controls (Figure 5F), suggesting increased host bacteria interfered with feeding. Impaired feeding may be due to slowed attachment, reduced blood intake, or some combination of these, ultimately resulting in decreased feeding or more prolonged, risky interactions with a bloodmeal host. Our combined in vitro and in vivo analyses show that Dae2Is limits levels of Gram-positive host skin commensals, such as S. epidermidis, suggesting resistance against these damaging infections is important for tick fitness.
Dae2Is in the Tick Gut Limits Damaging Infection by S. epidermidis
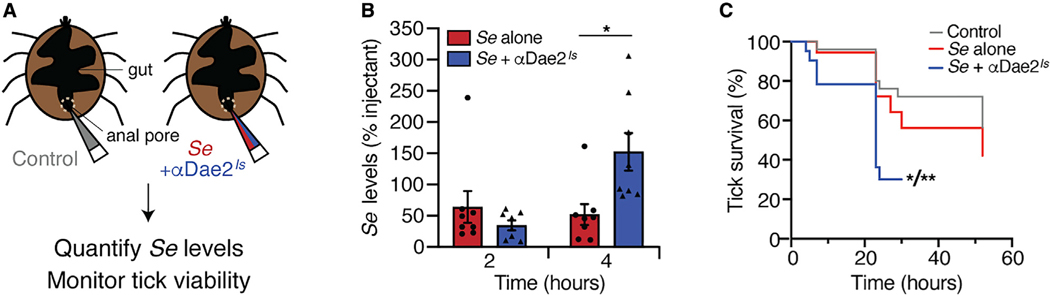
In addition to Dae2Is activity in the saliva, we reason that because the enzyme also localizes to the tick gut, it may play a second, additional role within this compartment in response to microbial entry. This would enable ticks to control skin-associated microbes through multiple barriers of defense. To investigate this possibility, we assessed whether physiological levels of Dae2Is can inhibit S. epidermidis levels in vivo through artificial challenge assays using live nymphal I. scapularis ticks (Figure 6A). Midguts of individual nymphs were infected with 102 log-phase S. epidermidis cells through needle-based microinjections into their anal pores. To deplete Dae2Is activity within the tick midgut for our challenge assays, we co-injected ticks with S. epidermidis and our neutralizing αDae2Is. At 2- and 4-h post-injection, we compared levels of S. epidermidis from ticks with and without antibody-based biochemical inhibition of Dae2Is in their guts. Inhibition of Dae2Is led to higher levels of S. epidermidis, suggesting Dae2Is has the capacity to directly antagonize S. epidermidis in vivo (Figure 6B) by killing or inhibiting cell growth. Strikingly, we also observed a notable concomitant increase in the death of the ticks themselves in the S. epidermidis+αDae2Is group (Figure 6C) compared to S. epidermidis alone and control groups. These effects represent a clear fitness cost for ticks harboring high levels of S. epidermidis and are highly suggestive of an antagonistic, pathogenic relationship.
Figure 6. Dae2Is-Dependent Effects on Tick-S. epidermidis Interactions in the Gut.
(A) Schematic of experimental setup. I. scapularis nymphs were microinjected into the anal pore with αDae2Is antibody (gray, left), live S. epidermidis alone (red, right), or live S. epidermidis and αDae2Is together (blue, right). Ticks were then monitored for levels of S. epidermidis and tick survival.
(B) Colony-forming unit (CFU) levels of S. epidermidis alone (red) or together with αDae2Is antibody to neutralize the lytic activity of Dae2Is (blue) at 2 and 4 h post injection. Results are shown as percentage of CFU at time 0 (injectant). *p < 0.05 based on paired t test.
(C) Tick survival after microinjection of αDae2Is control (gray), S. epidermidis alone (red), or S. epidermidis together with αDae2Is antibody to neutralize the lytic activity of Dae2Is (blue). *p < 0.05 compared to S. epidermidis alone and **p < 0.01 compared to antibody alone based on log-rank (Mantel-Cox) test.
See Figure S6.
In conclusion, these data suggest that Dae2Is mediates tick resistance to host skin commensals, which are their natural pathogens encountered during feeding. Bloodmeal host skin commensals are highly adapted microbial partners of mammalian skin, an environment that is very distinct from the tick and its gut. Dae2Is-dependent immune function for this ectoparasite is most likely mediated through inhibition of bacteria both within the tick gut and outside at the vital bloodmeal host feeding site. Thus, this work identifies a major microbial threat to ticks and identifies an immune mechanism ticks have acquired to cope with it.
DISCUSSION
The phylogenetic history of dae2 as a horizontally transferred gene from bacteria granted us the unique opportunity to investigate how this immune effector has structurally, biochemically, and functionally diverged from its ancestral form to support the unique biology of ticks (Chou et al., 2015). Invertebrates rely solely on innate immunity to distinguish between virulent and benign bacteria by employing pattern recognition receptors (PRRs) that recognize conserved microbe-associate molecular patterns (MAMPs), phagocytic cells, and a variety of humoral downstream effectors, including antimicrobial peptides (AMPs) and lytic enzymes (Humphreys and Reinherz, 1994; Little et al., 2005; Schulenburg et al., 2007). These defense mechanisms can vary in their location, timing, and sensitivity to microbial signatures, contributing to the overall specificity of invertebrate-microbe interactions (Bosch et al., 2019; McFall-Ngai et al., 2013). We discovered that Dae2Is has evolved the capacity to selectively kill skin-associated bacteria of bloodmeal hosts, and these microbes pose a threat to tick health. The timing and location of Dae2Is action in the saliva of ticks further contributes to its specific role in protecting against host microbes during feeding, suggesting this function is of biological importance to ticks.
The role of dae2 in tick innate immunity also supports the prediction that expansion of antimicrobial strategies was a key facilitator for the unique lifestyle of hematophagous arthropods. The timing of dae2 acquisition by ticks and mites soon after the emergence of hematophagy within this arthropod clade implicates Dae2 as an enabler of blood-feeding. This may be particularly important for hard ticks, which imbibe blood continuously for several days at a time and interface with a wide range of vertebrate hosts and associated microbes (Sonenshine, 1991). The possibility that Dae2Is could act in both the gut and saliva of ticks would suggest that immune function both in- and outside of the organism could be of great biological importance to ectoparasites. Further, skin bacteria such as staphylococci are able to propagate in blood from humans (Nguyen et al., 2017); thus, antimicrobial activity in saliva could prevent bloodmeal contamination by these bacteria, further protecting the tick from infection and preventing a cascade of antagonistic host responses within the dermis. Finally, the connection between tick immunity and skin commensals raises the interesting possibility that vertebrate skin microbiota, which has conserved features such as enrichment of Gram-positive bacteria for mammals and Gram-negative for reptiles (Ross et al., 2019), may have evolved to protect against prevalent ectoparasites. Comparative analyses of antimicrobial activities from other tick species with a strong preference for reptilian blood sources, such as I. pacificus of the far western United States (Eisen et al., 2004), may shed light on this potential link. Together, our findings highlight the inseparability of animals from their microbiota: animals confront not only each other but also their associated microbes. For blood-feeding parasites, such as ticks, successful navigation of such complex, interkingdom clashes is critical for survival.
More broadly, whether a bacterium is beneficial or pathogenic is highly host-dependent (Ehrlich et al., 2008), and this is reflected in the differential specificity of animal immune systems. Our study of an innate immune factor from the blacklegged tick I. scapularis underscores the context-specific nature of relationships between animals and bacteria (Ehrlich et al., 2008). Ticks harbor microbes, such as B. burgdorferi, which are tolerated by their tick vector but are pathogenic to humans upon transmission (Shaw et al., 2018). Here, we have shown that the mirror opposite is also true: ticks are vulnerable to infection by bacterial commensals found on the skin of vertebrates they feed on. These observations underscore how microbial virulence is not a fixed transitive property but rather a fluid status-dependent on host or growth context, challenging the classical disease-driven view in support of a more contextual one known as “ecological Koch’s postulates” (Falkow, 2004; Vonaesch et al., 2018). Indeed, contextual pathogenesis lies at the crux of how interactions between vectors, their hosts, and associated microbes lead to disease (Shaw et al., 2018). Asking mechanistic questions with this tenet in mind will surely yield new insights into the beautifully complex world of vector biology.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Seemay Chou (seemay.chou@ucsf.edu).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact with a complete Material Transfer Agreement.
Data and Code Availability
The crystal structure generated during this study is available under PDB accession number 6WIN.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
Animal experiments were conducted in accordance with the approval of the Institutional Animal Care and Use Committee (IACUC) at UCSF, which maintains full AAALAC accreditation. 4–6 week old female C3H/HeJ mice, acquired from Jackson Laboratories, were used for tick-feeding studies. Daily health checks were performed by UCSF Laboratory animal resource center (LARC) or by trained Chou lab personnel.
Microbe Strains
Escherichia coli (XL1Blue, BL21 (DE3) and Rosetta2 (DE3)), Bacillus subtilis 168, Staphylococcus epidermidis (BCM060 and SK135), Staphylococcus hominis (SK119), Corynebacterium propinquum (DSM44285), and Corynebacterium jeikeium (DSM7171), Borrelia burgdorferi S9
METHOD DETAILS
Cloning, expression, and purification of Tae2 and Dae2 enzymes
Full-length Tae2 from Salmonella typhi (Tae2St) in the pET29b+ expression vector was used for protein expression (Gene locus: T2586; UniProt ID: Q8Z969) (Russell et al., 2012). Briefly, the gene was cloned into the NdeI/XhoI restriction sites, creating an in-frame C-terminal fusion with a His5 epitope tag. To create the catalytically dead mutant (C23A), we used a quick-change protocol employing Tae2St-C23A-QC-Forward and reverse primers (Table S2) followed by DpnI digestion before transforming into chemically competent cells (Macrolab). Luria Broth (LB) was used for routine cloning with DH5alpha or XL1Blues and proteins were expressed and purified from BL21 or Rosetta2 (DE3) E. coli strains grown in Terrific Broth (TB).
Protein expression was induced at an optical density at 600 nm (OD600) of 0.6 by the addition of 1.0 mM IPTG. Tae2 was induced for 3 hr at 37°C and cells were lysed by sonication. Cell lysate was incubated with metal-chelating affinity resin, followed by resin washes, and final protein elution with the same buffer (except using 400 mM imidazole). The protein was further purified via size exclusion chromatography (GE Healthcare). For Dae2Is, E. coli was grown to an OD600 of 0.6, followed by cell resuspension in 20 mM HEPES pH 7.5, 0.1 M NaCl, 25 mM imidazole. Cells were then induced with 1.0 mM IPTG overnight at 16°C. The protein was further purified via a metal-chelating affinity column and size-exclusion chromatography (GE healthcare) as described above.
To prepare selenomethionine (SeMet; Sigma-Aldrich) protein derivatives for determining crystal structure, proteins were purified as above but with the following differences. E. coli cells were grown in M9 minimal media to an OD600 of 0.6 before 60 mg SeMet, 100 mg each of threonine, lysine hydrochloride, and phenylalanine, and 50 mg each of leucine, isoleucine, and valine, were added as solids (Van Duyne et al., 1993). The culture was incubated for 15 min before inducing with 1.0 mM IPTG.
Protein structure determination and analysis
Tae2 C23A crystals were generated by hanging drop vapor diffusion at 25°C from a 1:1 mixture of 4.32 mg/ml protein in 20 mM HEPES pH 7.5, 0.1 M NaCl, 0.02% Na azide with 0.1 M MES pH 6.6, 1 M sodium citrate, and 4% formamide for 24 hr. Crystals were directly used for diffraction data collection at the Lawrence Berkeley National Laboratory Advanced Light Source Beamline 8.3.1. Phases were obtained experimentally with data from a SeMet-substituted crystal with the PHENIX software suite (Liebschner et al., 2019). Coot (Emsley, 2017) and maximum likelihood refinement with PHENIX (Liebschner et al., 2019) were used for iterative building and refinement. Coordinates and structural factors were deposited in the Protein Data Bank (PDB ID: 6WIN). The closest structural homolog (4CSH) identified through the DALI server (Holm and Rosenström, 2010) was visualized with Chimera (Pettersen et al., 2004). Structural models of Tae2 family homologs, as well as Dae2 family homologs, were built with the PHYRE One-to-One threading algorithm using the default homology modeling parameters, including a local alignment and weighted secondary structure scoring (weight of 0.1) (Kelley et al., 2015). Structures were aligned using Chimera (Pettersen et al., 2004).
Ligand model generation
Initial protein preparation commenced from the crystal structure of Tae2St C23A. The mutation of the catalytic residue Cys23 to Ala for crystalline stability was reverted back to wild-type Cys. This ‘WT’ crystal structure was used as the input for building a homology model of Dae2Is. For initial structural studies using Molecular Operating Environment (MOE) 2018 (Chemical Computing Group), protonation states were assigned with MOE2018. Later structural studies were performed using Maestro (Schrodinger) and these systems were prepared by the Protein Preparation Wizard. Maestro’s Binding Surface Area Analysis tool was used to compare the available binding surface between Tae2St and Dae2Is in the context of the docked conformations (see below).
Computational analysis of docking poses
Given the conformational complexity of a cross-linked peptidoglycan (PG), and the resultant sampling problem that occurs when trying to dock this highly flexible compound to a shallow binding surface, we used crystal structures of a single PG, MLD (PDB IDs: 2CB3, 2F2L, 4QRB, 4QR7, 4QTF, 6I9O) to form guesses at the cross-linked PG conformational space. Conformational space for these PGs was sampled using the Conformational Search algorithm within MOE, and all moderate-to-low-energy (50 kcal/mol cut-off) conformations were retained for docking. Initial rigid docking of these linked fragments to Tae2St and Dae2Is was performed with MOE2018, using the AMBER10:EHT forcefield and default options for scoring and returning poses. Based on these results, we elected to truncate the PG to the cross-linked tripeptide dimer as a means of simplifying the search space. We present results wherein the cross-linked peptidoglycan has been truncated to a simplified representation of the true substrate; two tripeptide stems consisting of D-glutamic acid (D-Glu)—meso-diaminopimelic acid (mDAP)—D-Alanine (D-Ala), with an amide bond (cross-link) between D-Ala in one stem and mDAP in the other stem.
Peptidoglycan purification, analysis, and enzyme assays
PG was purified according to established protocols and all necessary details are provided (Glauner, 1988; Stankeviciute et al., 2019). Bacterial strains were grown to an OD600 of 0.6, harvested by centrifugation and boiled in SDS (4% final concentration) for 4 hours with stirring. After washing in purified water to remove SDS, the PG was treated with Pronase E for 2 h at 60°C (0.1 mg/ml final concentration in 10 mM Tris-HCl pH7.2 and 0.06% NaCl; pre-activated for 2 h at 60°C). Pronase E was heat inactivated at 100°C for 10 min and washed with sterile filtered water (5 × 20 min at 21k × g). PG from Gram-positive bacteria was purified as described above and treated with 48% HF at 4°C for 48 h to remove teichoic acids, followed by washes with sterile filtered water before Tae2St and Dae2Is enzyme degradation (0.1–1 μM, 4h at 37°C in 10 mM Tris-HCl pH 7.2 and 0.06% NaCl). Enzymes were heat inactivated at 100°C for 10 min. Mutanolysin (Sigma M9901, final concentration 20 μg/ml) or cellosyl (kindly provided by Hoechst, Frankfurt Germany) was directly added to the purified PG and incubated overnight at 37°C. Following adjustment of pH to 8.0 with borate buffer (0.5M, pH 9.0), PG fragments were reduced with sodium borohydride for 1 hour at room temperature and then acidified with concentrated o-phosphoric acid to pH 3.0–4.0. Examination by HPLC was performed using a standard protocol (Glauner, 1988; Stankeviciute et al., 2019), which included a multi-step method at a flow rate of 0.5 ml/min, 55°C with the Hypersil ODS C18 HPLC column (Thermo Scientific, Cat.# 30103–254630). In summary, samples were separated by the following: 100% buffer A, ramp to 100% buffer B over 20 min, maintain 100% buffer B for 2 min, ramp to 100% buffer A over 2 min, maintain 100% buffer A over 30 min.
Tick feeding
I. scapularis nymphs and adults were purchased from the Tick Lab at Oklahoma State University (OSU) or provided by BEI Resources, a division of the Center for Disease Control. Ticks were maintained in glass jars with a relative humidity of 95% (saturated solution of potassium nitrate) in a sealed incubator at 22°C with a light cycle of 16h/8h (light/dark). Animal experiments were conducted in accordance with the approval of the Institutional Animal Care and Use Committee (IACUC) at UCSF. Ticks were fed on young female C3H/HeJ mice acquired from Jackson Laboratories. Mice were anesthetized with ketamine/xylazine before placing ≤ 30 nymphs or ≤ 6 adult female ticks. Ticks were either pulled off of isoflurance anesthetized mice at various times during feeding (1–7 days) or allowed to feed to repletion and collected from mouse cages.
Tick saliva collection
Partially fed (5–7 days) female ticks were taped to glass slides. Saliva production was stimulated by pipetting 2–5μL of 5% pilocarpine hydrochloride (Tokyo Chemical Industries) in methanol onto the scutum. Saliva was collected in capillary tubes fitted onto the hypostome (mouthparts) of the ticks during a period of 24 hours in a humid chamber. Saliva was stored at −20°C before SDS-PAGE analysis.
Mouse serum collection
One to two weeks following tick feeding, blood was collected from mice. Whole blood was centrifuged at 7k × g for 8 minutes at 4°C. Serum (top layer) was transferred to new tubes and stored at −20° until use.
SDS-PAGE and Western Blotting
Proteins were separated by electrophoresis and transferred to a nitrocellulose membrane with a BioRad Trans-Blot Turbo system. Membranes were blocked in 5% nonfat milk in TBST (Tris-buffered Saline 0.1% Tween20). Rabbit αDae2Is (Chou et al., 2015) was used at 1:2000 in TBST followed by Goat αRabbit-HRP (Advansta R-05072–500) at 1:5000 in TBST. Chemiluminescence was detected using Biorad Clarity Western ECL substrates and imaged on an Azure c400 instrument. For tick saliva western blot, recombinant Dae2Is was used as the positive control. For mouse serum reactivity to Dae2Is, Dae2Is was loaded into a single well 15% acrylamide gel, transferred to a membrane and blocked as described above. Reactivity with Rabbit αDae2Is antibody was used as a positive control. Mouse serum was diluted 1:200 in TBST followed by Goat αMouse-HRP (Advansta R-05071–500) at 1:5000.
Microscopy
Salivary glands dissected from adult female I. scapularis were fixed in formalin for 3 days at room temperature. After washing 3 times in PBST (PBS with 0.3% Triton X-100) for 15 minutes at 37°C, salivary glands were incubated in blocking buffer (PBST with 10% fetal bovine serum, Sigma) for 30 minutes at 37°C (Chou et al., 2015). Primary antibodies, αDae2Is and control αTae1Pa (Chou et al., 2015) were diluted at 1:500 in blocking buffer and incubated overnight at 4°C. After washing 3 times in PBST, donkey αRabbit Alexa Fluor 647 (Invitrogen) diluted 1:1000 in the blocking buffer was added for 1 hour at room temperature in the dark. Samples were washed 3 times in PBST and stained with DAPI (Invitrogen, 50ng/ml in PBST) for 30 minutes at room temperature in the dark. After washing 3 times in PBST salivary glands were mounted on microscope slides with 10 μl ProLong Diamond Antifade Mounting media (Invitro gen). Fluorescence imaging was performed on a Nikon spinning disk confocal microscope using a 60x/1.49 objective.
Tick RNA isolation and qRT-PCR
RNA and DNA from ticks was isolated using Trizol reagent (Fisher Scientific) according to the manufacturer’s protocol. I. scapularis dae2 and actin transcripts were quantified with RT-PCR using the SsoAdvanced Universal SYBR Green Supermix (Biorad) (Chou et al., 2015). Analyses of dae2 gene expression include three technical replicates of three biological replicates. Transcript or DNA copy numbers were calculated using a standard curve generated from pure, quantified target PCR products. Primers spanning an intron-containing region were used to allow examination of samples for genomic contamination through gel electrophoretic analysis of qPCR product size. Any samples containing genomic contamination were excluded from further analysis. Primers specific to the staphylococcus genus were used to quantify bacterial load in tick DNA samples using the Applied Biosystems PowerUp SYBR Green Master Mix. All primers are listed in Table S2.
Bacterial Growth
Borrelia burgdorferi strain S9 (Rego et al., 2011) was provided by Dr. Patricia Rosa (NIAID, NIH, RML) and cultured in BSK II media at 35°C, 2.5% CO2. Staphylococcus epidermidis (BCM060 and SK135), S. hominis (SK119), Corynebacterium propinquum (DSM44285), and C. jeikeium (DSM7171) isolates were provided by Dr. Tiffany Scharschmidt (UCSF). Staphylococcus species were grown in Tryptic Soy Broth at 37°C with shaking. Corynebacterium were cultured in Blood Heart Infusion (BHI) supplemented with 10% Tween 80. Bacillus subtilis (168) was provided by Dr. Carol Gross and cultured in LB at 37°C with shaking.
Lysis Assays
Log-phase bacteria were incubated with Dae2Is or Tae2St (wt or catalytic mutant) at 0.5–5μM in low salt buffer (20mM HEPES pH 7.0, 100mM NaCl) for 4 hours. The reaction was plated on appropriate solid media (see above) and incubated to determine CFU. Results are presented as percent survival (CFU surviving in wt normalized to its respective catalytic mutant).
Tick Injection/Viability Assays
Log-phase bacteria were diluted in PBS to a concentration of 107 CFU/mL and injected into I. scapularis nymphs using an Eppendorf FemtoJet 4x microinjector. At varying times post injection, ticks were crushed in PBS and plated on appropriate solid media to determine CFU. Injections of PBS alone was used as a monitor of tick fitness and survival of the injection trauma. To assess tick viability, ticks were monitored every hour after injection for movement and reaction to CO2 (human breath). Ticks that had curled their front legs, did not respond to CO2, and were not mobile even after picking up with forceps (no reflex straightening of legs) were considered dead.
Pre-immunization of mice against Dae2
24 hours before tick feeding, 0.5mg affinity purified Rabbit αDae2 custom generated against recombinant Dae2Is protein (Genscript) was diluted in sterile PBS and injected into the intraperitoneal cavity of female C3H/HeJ mice to neutralize against pre-made Dae2Is protein in tick saliva. Sterile PBS was injected into control mice.
RNAi knockdown of dae2 in feeding nymphs
dae2Is targeting and scrambled primers listed in Table S2 were used with the Ambion Silencer siRNA Construction Kit (AM1620) to generate three different siRNA constructs targeting dae2Is, or equivalent scramble controls. Constructs were pooled such that each siRNA had a final concentration of 600 ng/μl. Five microliters of the siRNA pool was loaded into a capillary tube and injected into the midgut of I. scapularis nymphs. Microinjected nymphs were allowed to recover overnight in a humid environment. Nymphs were fed on mice pre-immunized with αDae2 (see above) and forcibly removed from mice after 3 days. Ticks were weighed and imaged to determine scutal indices. RNA was isolated from pools of 3 ticks (ordered by increasing scutal index) and dae2Is KD efficiency was determined by qRT-PCR as described above.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical methods used are described in the legends for relevant figures. GraphPad Prism Software was used for statistical data analysis. In summary, for bacterial lysis assays presented in Figure 3, experimental assays were run at least 3 times on separate days (biological replicates) with 3 technical replicates per group of bacteria. Statistical significance of differences between groups were analyzed as described in the figure legend, accounting for sample size and type of comparison. For analysis of dae2 gene expression by qRT-PCR in Figure 4, each data point represents a pool of 3 ticks, and each bar represents averaged data from at least 4 data points (biological replicates) resulting from averages of technical triplicates for each qRT-PCR run. Statistical significance of differences between groups were analyzed as described in figure legend. Animal experiments detailed in Figure 5 were conducted twice (biological replicates) with 3–4 mice per group (internal biological replicates) as denoted in the figure legends. qPCR data generated from DNA isolated from at least 4 pools of ticks (3 individual ticks/pool), scutal indices (from individual ticks) and weights (from individual ticks) are shown for a representative experiment. Scutal indices and weights were measured independently by two experimentalists, both of whom were blinded to groups. Measurements for individual ticks are reported from sample set of n ≥ 12. Statistical significance of observed difference between experimental groups were analyzed using t tests described in the corresponding figure legend. Given the number of biological variables inherent to our animal experiments, we consulted with a biostatistician Dr. Karla Lindquist at UCSF to advise on whether experimental assumptions were appropriate for our statistical analyses. In brief, we conducted a pilot assay measuring CFU of B. burgdorferi cells from control and KD ticks to generate a sample set of data to examine data distribution across groups and mice. We assessed the log-transformed distribution of CFU values to determine whether these could be assumed to follow a normal distribution. We tested for batch effects for ticks that were fed on different mice or feeding experiments conducted on different dates to guide our selection of sample size. In addition, we also conducted analogous data distribution and batch effect analyses for each animal experiment reported in this article using GraphPad Prism software. For injection experiments presented in Figure 6, starting groups of ticks (n = 25) were injected. To determine CFU, 8 ticks/group/time point were plated across several dilutions and enumerated through counting. Accuracy of plating was assessed by comparing consistency of CFU back calculations for at least two dilutions per set. Separate groups of 25 ticks were visually inspected to monitor death by an experimentalist who was blinded to the experimental groups. The recommended log-rank (Mantel-Cox) test was used to determine statistical significance of differences between survival curves.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Rabbit αDae2Is antibody | Chou et al., 2015 | N/A |
| Rabbit αTae1Pa antibody | Chou et al., 2012 | N/A |
| Donkey αRabbit Alexa Fluor 647 | Invitrogen | Cat.# A31573 |
| Goat αRabbit-HRP | Advansta | Cat.# R-05072-500 |
| Bacterial and Virus Strains | ||
| Escherichia coli (XL1Blue, BL21 (DE3) and Rosetta2 (DE3)) | MacroLab | N/A |
| Bacillus subtilis 168 | Carol Gross lab (UCSF) | N/A |
| Staphylococcus epidermidis (BCM060 and SK135) | Tiffany Scharschmidt lab (UCSF) | N/A |
| Staphylococcus hominis (SK119) | Tiffany Scharschmidt lab (UCSF) | N/A |
| Borrelia burgdorferi S9 | Patricia Rosa lab (NIH) | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Recombinant protein Tae2St | This paper | UniProt: Q8Z969 |
| Recombinant protein Dae2Is | This paper | Locus: XP_002434728 |
| Critical Commercial Assays | ||
| Ambion Silencer siRNA Construction Kit | Ambion | Cat.# AM1620 |
| Deposited Data | ||
| Tae2St protein crystal structure | This paper | PDB: 6WIN (https://www.rcsb.org/structure/6win) |
| Experimental Models: Organisms/Strains | ||
| Mouse: C3H/HeJ | Jackson laboratories | Cat.# 000659 |
| Oligonucleotides | ||
| Table S2 | This paper | N/A |
| Recombinant DNA | ||
| Overexpression construct pET29b::Tae2St | Russell et al., 2012 | N/A |
| Overexpression construct pET29b::Dae2Is | Chou et al., 2015 | N/A |
| Software and Algorithms | ||
| Phenix | NIH General Medical Sciences | https://www.phenix-online.org |
| Molecular Operating Environment | Chemical Computing Group | https://www.chemcomp.com |
| Maestro | Schrodinger | https://www.schrodinger.com |
Highlights.
A domesticated antibacterial effector in ticks has evolved broadened specificity
The effector is in the tick gut and saliva, reaching host bite sites during feeding
Ticks use this effector to protect themselves against host skin commensals
Pathogenicity of host microbes to ticks illustrates context-dependency of virulence
ACKNOWLEDGMENTS
We are grateful to George Meigs and James Holton at the Advance Light Source (Lawrence Berkeley National Laboratory), the Stroud lab (UCSF), and Michael Thompson (Fraser lab, UCSF) for help with X-ray crystallography. We thank Karla Lindquist (UCSF) for her assistance with our statistical analyses of mouse experiments. We also thank Carol Gross, Tiffany Scharschmidt, and Joe Bondy-Denomy (all at UCSF) as well as Patricia Rosa (Rocky Mountain Laboratories, NIAID, NIH) for providing bacterial strains. We also appreciate Dyche Mullins and Samuel Lord (UCSF) for their assistance with microscopy. The Chou lab thanks Soraya Pedemonte and Ethel Enoex-Godonoo for lab and administrative help, respectively, without which the lab would not run as smoothly. We are also grateful to Carol Gross (UCSF), Wallace Marshall (UCSF), Tiffany Scharschmidt (UCSF), Jeremy Reiter (UCSF), Thea Mauro (UCSF), Sophie Dumont (UCSF), and the entire Chou lab for helpful discussions and review of the manuscript. This project was funded in part by grants from the NIH (R01AI132851 to S.C. and R01AI134696 and R01AI116523 to J.P.), Research Councils UK (EP/T002778/1 to W.V.), UCSF Program for Breakthrough Biomedical Research (to S.C.), and the Sandler Foundation (to S.C.). Additional support for S.C. came from the Chan Zuckerberg Biohub, the Johnson & Johnson WiSTEM2D Award, Pew Biomedical Research Foundation, and the Sangvhi-Agarwal Innovation Award. F.Y. was supported by the National Science Foundation (1650113) and a grant to UCSF from the Howard Hughes Medical Institute through the James H. Gilliam Fellowships for Advanced Study program.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.cell.2020.10.042.
REFERENCES
- Bernard K. (2012). The genus corynebacterium and other medically relevant coryneform-like bacteria. J. Clin. Microbiol 50, 3152–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch TCG, Guillemin K, and McFall-Ngai M. (2019). Evolutionary “Experiments” in Symbiosis: The Study of Model Animals Provides Insights into the Mechanisms Underlying the Diversity of Host-Microbe Interactions. BioEssays 41, e1800256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpi G, Cagnacci F, Wittekindt NE, Zhao F, Qi J, Tomsho LP, Drautz DI, Rizzoli A, and Schuster SC (2011). Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PLoS ONE 6, e25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A. (2017). The Pathogenic Potential of a Microbe. MSphere 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez ASO, O’Neal AJ, Santambrogio L, Kotsyfakis M, and Pedra JHF (2019). Message in a vesicle - trans-kingdom intercommunication at the vector-host interface. J. Cell Sci 132, jcs224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S, Bui NK, Russell AB, Lexa KW, Gardiner TE, LeRoux M, Vollmer W, and Mougous JD (2012). Structure of a peptidoglycan amidase effector targeted to Gram-negative bacteria by the type VI secretion system. Cell Rep. 1, 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S, Daugherty MD, Peterson SB, Biboy J, Yang Y, Jutras BL, Fritz-Laylin LK, Ferrin MA, Harding BN, Jacobs-Wagner C, et al. (2015). Transferred interbacterial antagonism genes augment eukaryotic innate immune function. Nature 518, 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay K, and Fuqua C. (2010). The tick microbiome: diversity, distribution and influence of the internal microbial community for a blood-feeding disease vector. Critical Needs and Gaps in Understand Prevention, Amelioration, and Resolution of Lyme and Other Tick-Borne Diseases: the Short-Term and Long--Term Outcomes. Institute of Medicine Committee on Lyme Disease and Other Tick-Borne Diseases: The State of the Science: Washington DC. [Google Scholar]
- Couper LI, Kwan JY, Ma J, and Swei A. (2019). Drivers and patterns of microbial community assembly in a Lyme disease vector. Ecol. Evol 9, 7768–7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper LI, Yang Y, Yang XF, and Swei A. (2020). Comparative vector competence of North American Lyme disease vectors. Parasit. Vectors 13, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich GD, Hiller NL, and Hu FZ (2008). What makes pathogens pathogenic. Genome Biol. 9, 225–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L. (2020). Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: A review. Ticks Tick Borne Dis. 11, 101359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ, and Lane RS (2004). The roles of birds, lizards, and rodents as hosts for the western black-legged tick Ixodes pacificus. J. Vector Ecol 29, 295–308. [PubMed] [Google Scholar]
- Emsley P. (2017). ModelBuilding with Coot (University of Oxford; ). [Google Scholar]
- Falkow S. (2004). Molecular Koch’s postulates applied to bacterial pathogenicity–a personal recollection 15 years later. Nat. Rev. Microbiol 2, 67–72. [DOI] [PubMed] [Google Scholar]
- Glauner B. (1988). Separation and quantification of muropeptides with high-performance liquid chromatography. Anal. Biochem 172, 451–464. [DOI] [PubMed] [Google Scholar]
- Goodman JL, Dennis DT, and Sonenshine DE (2005). Tick-Borne Diseases of Humans. Emerg. Infect. Dis 11, 1808–1809. [Google Scholar]
- Greay TL, Gofton AW, Paparini A, Ryan UM, Oskam CL, and Irwin PJ (2018). Recent insights into the tick microbiome gained through next-generation sequencing. Parasit. Vectors 11, 12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia-Nuss M, Nuss AB, Meyer JM, Sonenshine DE, Roe RM, Water-house RM, Sattelle DB, de la Fuente J, Ribeiro JM, Megy K, et al. (2016). Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Commun 7, 10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, and Rosenström P. (2010). Dali server: conservation mapping in 3D. Nucleic Acids Res. 38 (Web Server issue), W545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys T, and Reinherz EL (1994). Invertebrate immune recognition, natural immunity and the evolution of positive selection. Immunol. Today 15, 316–320. [DOI] [PubMed] [Google Scholar]
- Jutras BL, Scott M, Parry B, Biboy J, Gray J, Vollmer W, and Jacobs-Wagner C. (2016). Lyme disease and relapsing fever Borrelia elongate through zones of peptidoglycan synthesis that mark division sites of daughter cells. Proc. Natl. Acad. Sci. USA 113, 9162–9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras BL, Lochhead RB, Kloos ZA, Biboy J, Strle K, Booth CJ, Govers SK, Gray J, Schumann P, Vollmer W, et al. (2019). Borrelia burgdorferi peptidoglycan is a persistent antigen in patients with Lyme arthritis. Proc. Natl. Acad. Sci. USA 116, 13498–13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LA, Mezulis S, Yates CM, Wass MN, and Sternberg MJE (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc 10, 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Tirloni L, Pinto AFM, Moresco J, Yates JR 3rd, da Silva Vaz I Jr., and Mulenga A. (2016). Ixodes scapularis Tick Saliva Proteins Sequentially Secreted Every 24 h during Blood Feeding. PLoS Negl. Trop. Dis 10, e0004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebschner D, Afonine PV, Baker ML, Bunkóczi G, Chen VB, Croll TI, Hintze B, Hung LW, Jain S, McCoy AJ, et al. (2019). Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol 75, 861–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TJ, Hultmark D, and Read AF (2005). Invertebrate immunity and the limits of mechanistic immunology. Nat. Immunol 6, 651–654. [DOI] [PubMed] [Google Scholar]
- Lormendez CC, Fernandez-Ruvalcaba M, Adames-Mancebo M, Hernandez-Velazquez VM, Zuñiga-Navarrete F, Flores-Ramirez G, Lina-Garcia L, and Peña-Chora G. (2019). Mass production of a S-layer protein of Bacillus thuringiensis and its toxicity to the cattle tick Rhipicephalus microplus. Sci. Rep 9, 17586–17589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans BJ (2011). Evolution of vertebrate hemostatic and inflammatory control mechanisms in blood-feeding arthropods. J. Innate Immun 3, 41–51. [DOI] [PubMed] [Google Scholar]
- Mans BJ, and Neitz AWH (2004). Adaptation of ticks to a blood-feeding environment: evolution from a functional perspective. Insect Biochem. Mol. Biol 34, 1–17. [DOI] [PubMed] [Google Scholar]
- McFall-Ngai M, Hadfield MG, Bosch TCG, Carey HV, Domazet-Lošo T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, et al. (2013). Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 110, 3229–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiners T, Hammer B, Göbel UB, and Kahl O. (2006). Determining the tick scutal index allows assessment of tick feeding duration and estimation of infection risk with Borrelia burgdorferi sensu lato in a person bitten by an Ixodes ricinus nymph. Int. J. Med. Microbiol 296 (Suppl 40 ), 103–107. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, and Fikrig E. (2015). Tick microbiome: the force within. Trends Parasitol. 31, 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Park MD, and Otto M. (2017). Host Response to Staphylococcus epidermidis Colonization and Infections. Front. Cell. Infect. Microbiol 7, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE (2004). UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- Rego ROM, Bestor A, and Rosa PA (2011). Defining the plasmid-borne restriction-modification systems of the Lyme disease spirochete Borrelia burgdorferi. J. Bacteriol 193, 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JM, Makoul GT, Levine J, Robinson DR, and Spielman A. (1985). Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J. Exp. Med 161, 332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross BD, Hayes B, Radey MC, Lee X, Josek T, Bjork J, Neitzel D, Paskewitz S, Chou S, and Mougous JD (2018). Ixodes scapularis does not harbor a stable midgut microbiome. ISME J. 12, 2596–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AA, Rodrigues Hoffmann A, and Neufeld JD (2019). The skin microbiome of vertebrates. Microbiome 7, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AB, Hood RD, Bui NK, LeRoux M, Vollmer W, and Mougous JD (2011). Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475, 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AB, Singh P, Brittnacher M, Bui NK, Hood RD, Carl MA, Agnello DM, Schwarz S, Goodlett DR, Vollmer W, and Mougous JD (2012). A widespread bacterial type VI secretion effector superfamily identified using a heuristic approach. Cell Host Microbe 11, 538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Gaitero M, Keary R, Garcia-Doval C, Coffey A, and van Raaij MJ (2014). Crystal structure of the lytic CHAP(K) domain of the endolysin LysK from Staphylococcus aureus bacteriophage K. Virol. J 11, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenburg H, Boehnisch C, and Michiels NK (2007). How do invertebrates generate a highly specific innate immune response? Mol. Immunol 44, 3338–3344. [DOI] [PubMed] [Google Scholar]
- Shaw DK, Tate AT, Schneider DS, Levashina EA, Kagan JC, Pal U, Fikrig E, and Pedra JHF (2018). Vector Immunity and Evolutionary Ecology: The Harmonious Dissonance. Trends Immunol. 39, 862–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AA, and Pal U. (2014). Immunity-related genes in Ixodes scapularis–perspectives from genome information. Front. Cell. Infect. Microbiol 4, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AA, Navasa N, Yang X, Wilder CN, Buyuktanir O, Marques A, Anguita J, and Pal U. (2016). Cross-Species Interferon Signaling Boosts Microbicidal Activity within the Tick Vector. Cell Host Microbe 20, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE (1991). Biology of TicksVolume 1 (Oxford University Press; ). [Google Scholar]
- Sonenshine DE (2005). The biology of tick vectors of human disease. In Tick-Borne Diseases of Humans, Goodman JL, Dennis DT, and Sonenshine DE, eds. (ASM Press; ), pp. 12–36. [Google Scholar]
- Stankeviciute G, Miguel AV, Radkov A, Chou S, Huang KC, and Klein EA (2019). Differential modes of crosslinking establish spatially distinct regions of peptidoglycan in Caulobacter crescentus. Mol. Microbiol 111, 995–1008. [DOI] [PubMed] [Google Scholar]
- Stewart PE, and Bloom ME (2020). Sharing the Ride: Ixodes scapularis Symbionts and Their Interactions. Front. Cell. Infect. Microbiol 10, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepańska A, Kiewra D, and Guz-Regner K. (2018). Sensitivity of Ixodes ricinus (L., 1758) and Dermacentor reticulatus (Fabr., 1794) ticks to Bacillus thuringiensis isolates: preliminary study. Parasitol. Res 117, 3897–3902. [DOI] [PubMed] [Google Scholar]
- Tetreau G, Dhinaut J, Gourbal B, and Moret Y. (2019). Trans-generational Immune Priming in Invertebrates: Current Knowledge and Future Prospects. Front. Immunol 10, 1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RD, Vollmer W, and Foster SJ (2014). Different walls for rods and balls: the diversity of peptidoglycan. Mol. Microbiol 91, 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, and Clardy J. (1993). Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J. Mol. Biol 229, 105–124. [DOI] [PubMed] [Google Scholar]
- Vonaesch P, Anderson M, and Sansonetti PJ (2018). Pathogens, microbiome and the host: emergence of the ecological Koch’s postulates. FEMS Microbiol. Rev 42, 273–292. [DOI] [PubMed] [Google Scholar]
- Wiedenbeck J, and Cohan FM (2011). Origins of bacterial diversity through horizontal genetic transfer and adaptation to new ecological niches. FEMS Microbiol. Rev 35, 957–976. [DOI] [PubMed] [Google Scholar]
- Zhioua E, Heyer K, Browning M, Ginsberg HS, and LeBrun RA (1999). Pathogenicity of Bacillus thuringiensis variety kurstaki to Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol 36, 900–902. [DOI] [PubMed] [Google Scholar]
- Zhou W, Tahir F, Wang JC-Y, Woodson M, Sherman MB, Karim S, Neelakanta G, and Sultana H. (2020). Discovery of Exosomes From Tick Saliva and Salivary Glands Reveals Therapeutic Roles for CXCL12 and IL-8 in Wound Healing at the Tick-Human Skin Interface. Front. Cell Dev. Biol 8, 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The crystal structure generated during this study is available under PDB accession number 6WIN.