Abstract
BACKGROUND & AIMS:
Fecal microbiota transplantation (FMT) is used commonly for treatment of Clostridioides difficile infections (CDIs), although prospective safety data are limited and real-world FMT practice and outcomes are not well described. The FMT National Registry was designed to assess FMT methods and both safety and effectiveness outcomes from North American FMT providers.
METHODS:
Patients undergoing FMT in clinical practices across North America were eligible. Participating investigators enter de-identified data into an online platform, including FMT protocol, baseline patient characteristics, CDI cure and recurrence, and short and long-term safety outcomes.
RESULTS:
Of the first 259 participants enrolled at 20 sites, 222 had completed short-term follow-up at 1 month and 123 had follow-up to 6 months; 171 (66%) were female. All FMTs were done for CDI and 249 (96%) used an unknown donor (eg, stool bank). One-month cure occurred in 200 patients (90%); of these, 197 (98%) received only 1 FMT. Among 112 patients with initial cure who were followed to 6 months, 4 (4%) had CDI recurrence. Severe symptoms reported within 1-month of FMT included diarrhea (n = 5 [2%]) and abdominal pain (n = 4 [2%]); 3 patients (1%) had hospitalizations possibly related to FMT. At 6 months, new diagnoses of irritable bowel syndrome were made in 2 patients (1%) and inflammatory bowel disease in 2 patients (1%).
CONCLUSIONS:
This prospective real-world study demonstrated high effectiveness of FMT for CDI with a good safety profile. Assessment of new conditions at long-term follow-up is planned as this registry grows and will be important for determining the full safety profile of FMT.
Keywords: Risk, Microbiome, Bacteriotherapy
Recurrent Clostridioides difficile infection (CDI) is common and increasing within the United States.1 It is associated with significant morbidity and mortality2 and frequent failure of standard medical treatments.3 Fecal microbiota transplantation (FMT) has proven to be a highly efficacious therapeutic modality to prevent recurrent CDI4 and increasing data support its use in severe or refractory cases.5 FMT is permissible for CDI not responsive to standard therapy under a policy of enforcement discretion of the US Food and Drug Administration. This policy, the widespread availability of the therapeutic substrate, and the ease of administration have facilitated the use of FMT for treatment of CDIs. However, early adoption and expansion of FMT in clinical practice has allowed FMT to bypass the standard investigatory pathway in which large randomized controlled trials (RCTs) contribute important short- and long-term safety data before a product comes to market.
Although the value of FMT in treating recurrent CDIs is clear, the potential long-term consequences are not known. The gut is estimated to contain 1000 bacterial species containing 100-fold more genes than the human genome. Viruses, bacteriophages, archaea, and fungi contribute to this microbial community,6 which functions as an “organ” with an immense impact on human health and disease, including host metabolism, physiology, nutrition, and immune function.7 Recent evidence demonstrates long-term engraftment of donor microbes into the recipients of FMT.8 A priori knowledge is not available regarding the impact of transferring these complex communities from one individual to another, although animal models and human studies indicate that manipulation of gut microbiota can affect host susceptibility to diseases such as obesity9 and inflammatory bowel disease (IBD).10-12 As the practice of FMT and related therapeutic methods rapidly expand, it is crucial to determine the effectiveness and best practices of FMT techniques, and to assess short-term and long-term safety. Furthermore, understanding FMT effectiveness and safety in real-world clinical settings is important because many recurrent CDI patients are not eligible for clinical trials due to common comorbidities, such as IBD and immunocompromised status,13 and because interest in FMT for other indications is increasing.
To address these needs, the American Gastroenterological Association (AGA) Institute, in partnership with other professional organizations, has developed an FMT National Registry to collect clinical and patient-reported outcomes. This registry, funded by a grant from the National Institute of Allergy and Infectious Diseases, primarily aims to assess the short-term and long-term safety of FMT and other gutrelated microbiota products. Secondary objectives include characterizing the effectiveness of FMT and other gutrelated microbiota products, gathering information on FMT practice in North America, and promoting scientific investigation in FMT and the gut microbiome. Here we report on the first 259 participants enrolled in the FMT National Registry.
Methods
Study Design
The FMT National Registry is an ongoing, prospective, observational, multicenter registry of North American participants who receive FMT for any indication and began enrolling patients in December 2017. The registry is administered by the AGA with grant support from the National Institute of Allergy and Infectious Diseases (award R24AI118629). Partner organizations include the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; the Infectious Diseases Society of America; and the Crohn’s & Colitis Foundation.
This observational registry has no study-mandated protocol for FMT or follow-up visits. Rather, participants are treated at the discretion of their providers. Diagnosis of CDI and need for FMT or subsequent anti-CDI therapies were determined by individual registry sites. The registry was approved by Western Institutional Review Board, Inc, and all subjects provide written informed consent. De-identified data were entered by investigators at participating sites into electronic case report forms on a data capture system maintained by Viedoc (ACI Clinical, Bala Cynwyd, PA). AGA oversees data collection remotely under the supervision of a steering committee comprising experts in FMT, gut microbiome, clinical research, epidemiology, and informatics. Participant safety is reviewed at least yearly by an Observational Study Monitoring Board (Supplementary Material).
Participating sites were primarily self-identified through an online survey created by AGA to gauge interest in the registry. Other sites were referred by members of the steering committee.
Subjects
Inclusion criteria were ability to provide informed consent, receipt of FMT or another gut microbial therapeutic product within 90 days after providing consent, and access to Internet and/or telephone. The only exclusion criterion was incarceration.
Data Collection
Data were collected regarding FMT methods (eg, screening of donor and recipient, stool preparation, and FMT delivery method), baseline characteristics of donors and recipients of FMT (eg, demographic information, details on indication for FMT, body mass index, medical history, and medications) (Supplementary Table 1). Severity of CDI was classified per published guidelines.14 Predefined safety (short-term symptoms, new medical conditions, other adverse events) and effectiveness (eg, cure and recurrence for CDI and IBD status) outcomes were provided on the electronic case report form (Supplementary Table 2), which also allows free-text entry of additional outcomes.
Data were collected by the participating sites at baseline and at the following points after the participant’s FMT procedure: 1 month, 6 months, 1 year, and 2 years. The registry protocol allowed for these follow-up data to be collected either at clinic visits or by telephone interview, as per local preferences at each site. This publication reported data at baseline, 1 month, and 6 months. The data for this publication were extracted from the database on September 2, 2019.
Outcomes for Analysis
The primary outcome of effectiveness was cure of CDI assessed at 1 month (window 20–60 days) after FMT. Cure was defined as resolution of diarrhea without need for additional anti-CDI therapy. We also assessed cure of CDI at 6 months (window 120–240 days) after FMT.
Safety outcomes included patient symptoms, infections, hospitalizations, deaths, and changes in current medical conditions or development of new medical conditions. One-month adverse events included those reported up to 60 days after FMT, and 6-month adverse events included those reported between 61 and 240 days after FMT.
Results are reported descriptively without hypothesis testing. For categorical variables, proportions are presented. For continuous variables, mean, median, SD, and range are provided.
Results
Demographic Characteristics
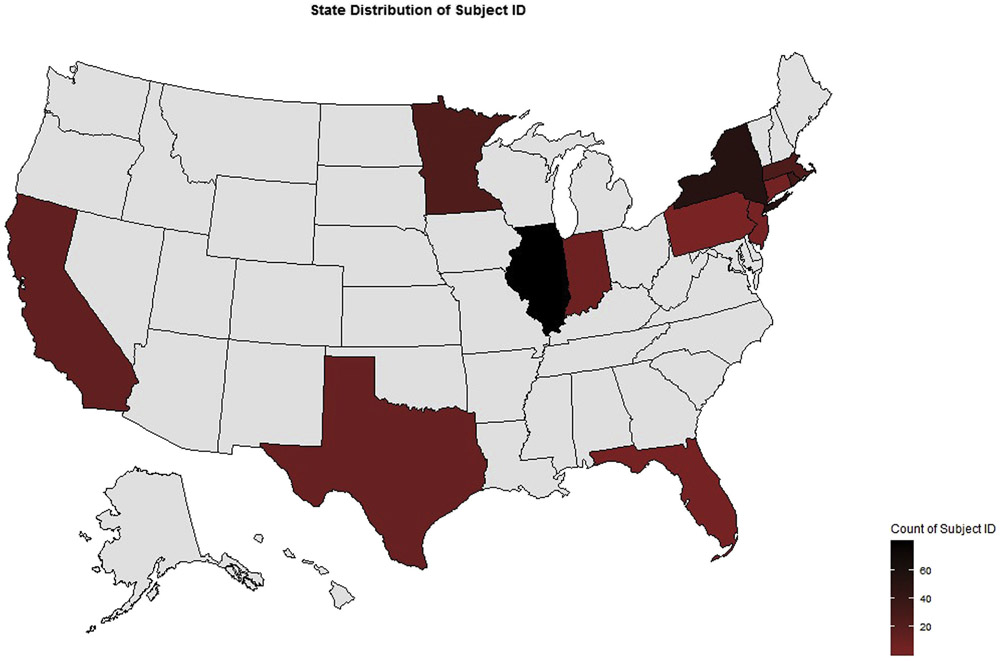
Between December 5, 2017 and September 2, 2019, 259 participants with post-FMT follow-up were enrolled at 20 sites (Figure 1).
Figure 1.
Distribution of registry sites by total number of subjects enrolled.
Table 1 lists baseline characteristics of the 259 participants. Treated participants ranged in age from 1 to 98 years (median, 63 years). Twenty participants (8%) were younger than 18 years. Recipients were more commonly female than male (n = 171 [66%] and n = 88 [34%], respectively) and 238 (92%) were White. Of note, baseline comorbidities included irritable bowel syndrome in 30 participants (12%), ulcerative colitis in 24 participants (9%), and Crohn’s disease in 19 participants (7%). Regional distribution in the United States was primarily in the Northeast (45%) and Midwest (43%).
Table 1.
Demographics Characteristics of Participants
| Characteristic | n (%) |
|---|---|
| Age | |
| <18 y | 20 (8) |
| 18–55 y | 77 (29) |
| >55 y | 162 (63) |
| Sex | |
| Female | 171 (66) |
| Male | 88 (34) |
| Race | |
| White | 238 (92) |
| Black/African American | 5 (2) |
| Asian | 3 (1) |
| Other | 4 (2) |
| Unknown | 9 (3) |
| Ethnicity | |
| Not Hispanic/Latino | 246 (95) |
| Hispanic/Latino | 11 (4) |
| Not reported | 2 (1) |
| Region | |
| US Northeast | 117 (45) |
| US Midwest | 111 (43) |
| US South | 18 (7) |
| US West | 13 (5) |
| Comorbidities at baseline (≥5% prevalence)a | |
| Hypertension | 82 (32) |
| Hyperlipidemia | 70 (27) |
| Anxiety | 48 (19) |
| IBD | 45 (17) |
| Ulcerative colitis | 24 (9) |
| Crohn’s disease | 19 (7) |
| Indeterminate colitis | 2 (1) |
| Depression | 40 (15) |
| Cancer | 35 (14) |
| Hypothyroidism | 32 (12) |
| Cardiovascular disease | 31 (12) |
| Irritable bowel syndrome | 30 (12) |
| Type 2 diabetes | 28 (11) |
| Asthma or allergic/atopic conditions | 15 (6) |
Note some participants had more than 1 comorbidity.
Indications
All participants were treated with FMT for a diagnosis of CDI. Most were diagnosed with CDI based on symptoms and stool testing, including polymerase chain reaction (n = 163 [63%]), enzyme immunoassay (n = 54 [21%]), or both (n = 20 [8%]) (Table 2).
Table 2.
Fecal Microbiota Transplantation Indication and Related Characteristics
| Characteristics | n (%) |
|---|---|
| FMT indication | |
| CDI | 259 (100) |
| Method of CDI diagnosis | |
| Symptoms and PCR | 163 (63) |
| Symptoms and EIA | 54 (21) |
| Symptoms, EIA and PCR | 20 (8) |
| Symptoms only | 12 (5) |
| PCR only | 8 (3) |
| EIA only | 1 (<1) |
| Not reported | 1 (<1) |
| CDI severity | |
| Mild | 92 (36) |
| Moderate | 115 (44) |
| Severe | 48 (19) |
| Severe-complicated | 2 (1) |
| Not reported | 2 (1) |
| CDI duration | |
| <1 mo | 14 (5) |
| 1–6 mo | 149 (58) |
| 7–12 mo | 61 (24) |
| 13–24 mo | 17 (7) |
| >24 mo | 16 (6) |
| Not reported | 2 (1) |
| No. of prior CDI episodes | |
| 1 | 15 (6) |
| 2 | 34 (13) |
| 3 | 110 (42) |
| 4 | 56 (22) |
| 5 or more | 42 (16) |
| Not reported | 2 (1) |
| Prior treatments for CDI | |
| Vancomycin | 236 (91) |
| Vancomycin taper/pulse | 141 (54) |
| Metronidazole | 104 (40) |
| Fidaxomicin | 73 (28) |
| Probiotics | 44 (17) |
| Other treatments | 14 (5) |
| No prior treatments | 5 (2) |
EIA, enzyme immunoassay; PCR, polymerase chain reaction.
Of the 259 participants with reported baseline CDI severity, most were moderate (n = 115 [44%]) or mild (n = 92 [36%]) (Table 2). Duration of CDI diagnosis pre-FMT varied from less than 1 week to 9 years (median, 20 weeks). Mean number of CDI episodes reported before FMT was 3.5 (range, 1–15). Among the 15 participants with 1 earlier CDI episode, 4 were severe, 7 were moderate, and 4 were mild; all were presumed to have failed standard anti-CDI therapy. Almost all participants (n = 236 [91%]) had an earlier course of vancomycin and 141 (55%) had also received a vancomycin taper or pulse regimen. Median duration of vancomycin treatment was 14 days (range, 1–203 days) and 146 of these participants (62%) had 2 or more courses of treatment. Among other earlier CDI treatments, 103 (40%) had received metronidazole and 73 (28%) had been treated with fidaxomicin.
Fecal Microbiota Transplantation Methodology
Almost all participants (n = 249 [96%]) received fecal microbiota transplant from an unknown donor, primarily stool banks (OpenBiome, n = 167 [67%]; other stool banks (n = 73 [24%]) (Table 3). Sites using an alternate source of stool described site-specific donor identification and screening techniques. Median volume administered was 250 mL (range, 21–400 mL). The primary method of FMT delivery was colonoscopy (n = 221 [85%]) followed by upper endoscopy (n = 15 [6%]) (Table 3). In all cases the site investigators planned a single-dose protocol of FMT for CDI therapy.
Table 3.
Summary of Fecal Microbiota Transplantation Methodology
| Variable | n (%) |
|---|---|
| Stool donor type | |
| Unknown donor | 249 (96) |
| OpenBiome | 167 (67) |
| Other stool bank | 73 (29) |
| Source not reported | 9 (4) |
| Known donor | 8 (3) |
| Not reported | 2 (1) |
| Primary method of FMT delivery | |
| Colonoscopy | 221 (85) |
| Upper endoscopy | 15 (6) |
| Oral capsule | 8 (3) |
| Sigmoidoscopy | 2 (1) |
| Naso-intestinal tube | 2 (1) |
| Colonoscopy and upper endoscopy (same procedure date) | 2 (1) |
| Other | 3 (1) |
| Not reported | 6 (2) |
Outcomes
Effectiveness.
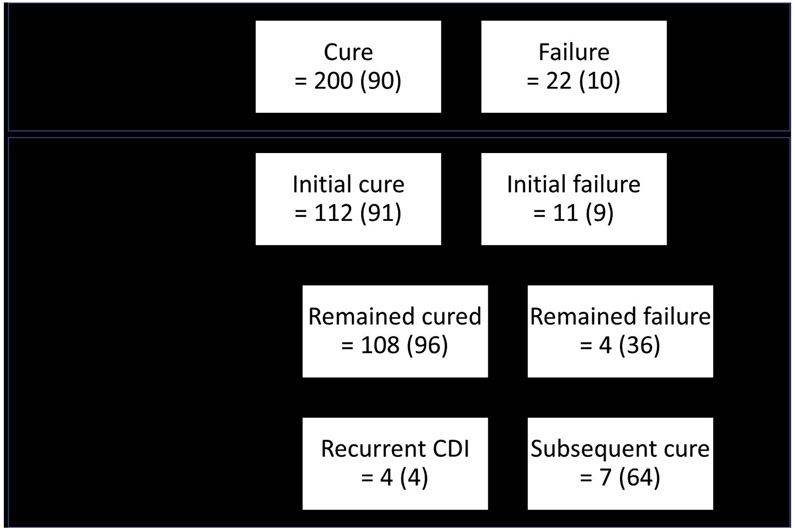
Of the 259 participants, 222 returned within the 1-month follow-up window. Of these, 200 (90%; 95% confidence interval [CI], 85%–93%) had CDI cure, with 197 (98%) requiring only 1 FMT to achieve cure (Figure 2). Participants not included in this effectiveness outcome calculation included those whose first visit occurred before day 20 (n = 9), after day 60 (n = 28), or who were missing first visit data (n = 6). Of the participants whose first visit occurred before 20 days, all were reported as cured and 6 participants remained cured at subsequent follow-up points; 3 participants did not have follow-up beyond this first visit. Post-hoc analysis of the 256 participants who had a follow-up visit recorded at least 20 days after FMT (first day of the 1-month visit window) was performed, and 224 (88%; 95% CI, 83%–91%) were reported as cured. Analysis of an intent-to-treat population of all participants entered, including those lost to follow-up, still revealed a cure rate of 224 of 259 (86%; 95% CI, 82%–90%).
Figure 2.
FMT effectiveness.
Of the 259 participants, 123 had both 1-month and 6-month follow-up within the prespecified windows. Of the 112 participants cured at 1-month and with follow-up at the 6-month point, 4 participants (4%) had developed recurrent CDI at a median of 8 weeks (range, 8–14 weeks) post FMT (Figure 2). Of the 11 participants failing initial FMT who were followed to 6 months, 7 (64%) were reported as cured at this later point. Treatments administered to these 7 participants included metronidazole and/or vancomycin (n = 6 [86%]) or repeat FMT (n = 1 [14%]). Participants not included in the 6-month effectiveness outcome calculation included those who reported cure at the first visit but withdrew (n = 4) or whose visit occurred after the data pull cutoff (n = 80). Post-hoc analysis of 145 participants with data reported within the 6-month visit window, regardless of whether they had 1-month follow-up visit, was performed and 128 (88%; 95% CI, 82%–93%) were reported as cure.
Short-term (1-month) adverse events.
The following 3 procedure-related complications occurred: 1 colonoscopic perforation and 2 episodes of gastrointestinal bleeding. The perforation occurred at a biopsy site in an 82-year-old woman with a history of microscopic colitis; she underwent a colectomy with full recovery. One episode of mild and self-limited rectal bleeding was reported at telephone follow-up after colonoscopic FMT. One participant experienced post-polypectomy bleeding, which required hospitalization.
Post-FMT symptoms were common, with 106 participants (45%) reporting at least 1 symptom. The most common symptoms were non-CDI diarrhea (n = 69), abdominal pain (n = 39), bloating (n = 34), and constipation (n = 24). A total of 212 symptoms were reported, of which 13 (6%) were severe (Table 4).
Table 4.
Adverse Events Reported Up to 1 Month After Fecal Microbiota Transplantation
| Adverse event | n (%) |
|---|---|
| Common symptoms of any severity with >2% prevalence | |
| Diarrhea | 69 (30) |
| Abdominal pain | 39 (17) |
| Bloating | 34 (15) |
| Constipation | 24 (10) |
| Nausea and/or vomiting | 15 (6) |
| Severe symptoms | |
| Diarrhea | 5 (2) |
| Abdominal pain | 4 (2) |
| Bloating | 1 (<1) |
| Constipation | 1 (<1) |
| Other | 2 (1) |
| New infections | |
| None | 219 (95) |
| Unrelated to FMT | 9 (4) |
| Possibly related to FMT | 2 (1) |
| Bacteroides fragilis | 1 (<1) |
| Enteropathogenic Escherichia coli | 1 (<1) |
| Not reported | 1 (<1) |
| Hospitalizations | |
| None | 204 (88) |
| Unrelated to FMT | 24 (10) |
| Possibly related to FMT | 3 (1) |
| Perforation | 1 (<1) |
| Ulcerative colitis flare | 1 (<1) |
| Diarrhea, abdominal pain, fever | 1 (<1) |
Infections were reported in 11 participants (5%), including 2 (1%) that were believed by the site investigator to be possibly related to the procedure: Bacteroides fragilis bacteremia in 1 participant with severe diarrhea pre- and post-FMT and enteropathogenic Escherichia coli on a multiplex polymerase chain reaction stool panel in a participant who reported soft stools after FMT. Other infections in 9 participants were believed to be unrelated and included urinary tract infection (UTI) (n = 4), pneumonia (n = 3), E coli bacteremia (n = 1), and tooth infection (n = 1) (Table 4).
Hospitalizations were reported in 27 participants (12%) within 1 month after FMT. The most common reason for hospitalization was CDI recurrence (n = 6). Three hospitalizations for the following symptoms were reported as possibly related to the FMT procedure: continued diarrhea, abdominal pain, dehydration, and fever (n = 1); ulcerative colitis flare (n = 1); and perforation (n = 1). No participant deaths were reported at 1-month follow-up. Participants who missed the visit (n = 6) or whose visit fell after 61 days post FMT (n = 22) were not included in the short-term safety analysis.
Six-month adverse events.
Among the 156 participants with data collected at 6-month follow-up, 6 (4%) had 1 or more new infections diagnosed between 1 and 6 months, including pneumonia; Campylobacter infection; cellulitis and infected arteriovenous fistula with methicillin-resistant Staphylococcus aureus bacteremia; pneumonia with streptococcus pharyngitis; UTI; and pneumonia, UTI, and acute kidney injury with or without aspiration pneumonia (Table 5). All infections resolved.
Table 5.
Adverse Events Reported Between 1 and 6 Months After Fecal Microbiota Transplantation
| Adverse event | n (%) |
|---|---|
| New diagnoses | |
| No | 125 (80) |
| Yesa | 21 (13) |
| Irritable bowel syndrome, diarrhea | 2 (1) |
| Ulcerative colitis | 2 (1) |
| Otherb | 22 (14) |
| Not reported | 10 (6) |
| Serious infections | |
| No | 140 (90) |
| Yesa | 6 (4) |
| Pneumonia | 1 (<1) |
| UTI | 1 (<1) |
| Campylobacter infection | 1 (<1) |
| Cellulitis | 1 (<1) |
| Infected AV fistula/MRSA bacteremia | 1 (<1) |
| Streptococcus pharyngitis | 1 (<1) |
| Pneumonia/acute kidney injury with or without aspiration pneumonia/UTI | 1 (<1) |
| Not reported | 10 (6) |
| Hospitalizations | |
| No | 126 (81) |
| Yesa | 30 (19) |
| Infection other than CDI | 8 (6) |
| CDI recurrence | 3 (2) |
| Otherc | 30 (20) |
| Deaths | |
| Unrelated to FMT | 4 (3) |
| Related to FMT | 0 (0) |
AV, arteriovenous; MRSA, methicillin-resistant Staphylococcus aureus.
Note some participants had more than 1 diagnosis or reason for hospitalization.
All other conditions were n = 1: abdominal aortic aneurysm, Barrett’s esophagus, belching, cardiovascular disease, chronic obstructive pulmonary disease, colon cancer, dermatitis, fistula between Indiana pouch and bowel, gastroesophageal reflux disease, hair loss, jaw cancer, left heart failure, left inguinal hernia, neurologic disorder, osteopenia, osteoporosis, overflow diarrhea, pelvis floor dysfunction, pregnancy, prostate cancer, snoring, and UTI.
Other reasons for hospitalization included acute episode of croup, acute right anterior cerebral artery stroke, breast implant removal, breathing, cirrhosis, colitis, constipation, Crohn’s disease flare, dehydration (2 cases), diverticulitis, abnormal liver function tests, fainting, hysterectomy, intestinal obstruction, lung transplant symptoms, overflow diarrhea, peritoneal dialysis catheter placement, potential ulcerative colitis flare, primary sclerosing cholangitis flare (worsening of left hepatic biliary duct inflammation), robotic prostatectomy, seizure, severe sepsis/septic shock, surgery for recurrent colon cancer, ulcerative colitis flare, ulcerative colitis laparoscopic ileal pouch anal anastomosis with ileostomy, urinary retention, UTI, vomiting, and weakness.
Hospitalizations were reported in 30 participants (19%) between 1 and 6 months after FMT (Table 5). The most common reason was an infection other than CDI, as follows: UTI (n = 2); pneumonia (n = 1); methicillin-resistant Staphylococcus aureus (n = 1); septicemia (n = 1); and 1 participant with cellulitis, infected arteriovenous fistula, and methicillin-resistant Staphylococcus aureus bacteremia. Three participants were hospitalized for CDI recurrence.
Two (1%) participants had a new diagnosis of irritable bowel syndrome and 2 (1%) had a new diagnosis of IBD (both ulcerative colitis) within 6 months after FMT (Table 5).
Four participants died, but no deaths were attributed to FMT; the causes reported by the site investigators were chronic obstructive pulmonary disease, ovarian cancer, septicemia, and worsening dementia.
Discussion
This is the largest prospective study to date of effectiveness and safety outcomes after FMT. CDI cure rates were excellent at approximately 90%, and were in line with those reported in RCTs of FMT15-18 and in a National Pediatric FMT Registry.19 Patients can expect to achieve high rates of success with FMT for refractory CDI in standard clinical practice. In addition, CDI cure could be achieved with only 1 FMT in virtually all cases. Finally, we found FMT response to be durable, with recurrence in the 6 months after successful FMT seen in only 4% of participants, occurring most often within 2 months. For those with unsuccessful FMT at 1 month, most could still achieve cure by 6 months using standard antibiotic therapy or after repeated FMT.
Risk of infection transmission from FMT has been of great concern, highlighted by a recent Food and Drug Administration report of 2 cases of extended spectrum β-lactamase producing E coli infections (1 was fatal) believed to have been transmitted by donor stool.20 Most donors in our cohort were from a stool bank that screens extensively for transmissible pathogens, including multidrug-resistant organisms. Short-term infections reported in registry participants, so far, have been few in number, occurring in 5% of those treated, with only 1% believed to be possibly related to the FMT. Infectious complications after FMT appear remarkably rare, as was reported in a recent systematic review that showed infections occurred in only 2.5% of more than 1000 patients treated.21 Even high-risk immunocompromised patients appear to have a low risk of contracting an infection related to FMT.22,23 However, infections after FMT might not be recognized or recorded in retrospective studies that are not designed specifically to assess safety outcomes, highlighting the importance of continued collection of data such as these in prospective studies with predefined safety and effectiveness outcomes.
Other short-term adverse events after FMT can be the result of the procedure used to administer the treatment. Fatal aspiration pneumonia was previously reported as a complication of FMT administered by nasoduodenal tube.24 Most sites in this registry administer FMT colonoscopically and 3 colonoscopy-related complications were reported. They were considered related to the procedure itself rather than the FMT. There may be advantages to administering FMT via lower endoscopy, including possibly increased efficacy25,26 and the ability to examine the colon for other underlying pathology, such as IBD. However, it is important to weigh the risk of procedural complications and associated sedation, as well as medical costs, against the benefits, especially in patients who are financially vulnerable, frail, or have significant cardiopulmonary comorbidities. The preferred method of administration can vary depending on the clinical situation and local expertise. The majority of FMTs in this country are being done by gastroenterologists using material from donor stool banks with the preferred method of administration being colonoscopic. As the registry expands, we plan to target additional sites that use other methods of delivery, as we hope to be able to compare the effectiveness and safety of FMT administered by different routes, such as capsule, enema, or naso-intestinal tubes.
The risk of additional CDI recurrences is known to increase with each subsequent CDI episode, with rates as high as 65% after a second recurrence.3,27 Although uncontrolled, our data are compelling in that the cycle of recurrent CDIs was broken in a large number of participants who had failed 2 or more previous courses of conventional antibiotic therapy. RCTs have restrictive inclusion and exclusion criteria and are not fully representative of an unselected real-world population of patients with CDIs. In fact, most patients with recurrent CDIs do not qualify for RCTs of microbiota-based therapeutics13 because of comorbidities, such as IBD or immunocompromised status, highlighting the value of registry studies such as this, that allow the collection of important effectiveness and safety data that are more reflective of the real-world population.
Weaknesses of this study are inherent to the design and included missing or incomplete data, multiple follow-up visits falling outside of prespecified windows, patients lost to follow-up, and potential for recall bias when patients were queried at follow-up. Donor screening protocols also varied across the participating sites, leading to possible differences in the potential for transmission of infectious agents, such as enteropathogenic E coli. In addition, because this was an observational study rather than a clinical trial, FMT and post-FMT practice were not mandated but rather were at the discretion of FMT providers at each site. Indications for FMT, diagnoses of CDI, and determinations of cure were made based on clinical grounds by site investigators. Transient episodes of diarrhea that resolved without further anti-CDI therapy were not considered failures of FMT and differing CDI testing methods (eg, polymerase chain reaction and toxin enzyme immunoassay) were used by investigators. In real-world settings, the diagnosis of recurrent CDI is commonly made based on compatible symptoms and anti-CDI therapy started without confirmatory testing. Registry staff queried site investigators to obtain missing or incomplete data or for clarification or additional information around serious adverse events. Although this report includes results from 20 different geographically diverse centers around the United States, we cannot conclude that they are wholly representative of FMT practice and results nationally. The registry sites tend to be larger-volume centers with an interest in FMT.
We developed the FMT National Registry to provide a real-world view of clinical practice, patient outcomes, safety, and comparative effectiveness.28 Our registry is designed to provide long-term assessment for up to 10 years to answer the most pressing safety question regarding FMT, that is, whether FMT increases the risk of developing other medical conditions in the years after it is performed. This was a lesson the medical community learned from the inadvertent transmission of human immunodeficiency virus and hepatitis C virus via blood transfusion. A variety of conditions postulated to potentially be influenced by the gut microbiota (eg, diabetes, cancer, cardiovascular events, and autoimmune disease) are prespecified to be collected from physicians for up to 2 years and patients for up to 10 years in the registry. To assess whether such conditions are increased in patients receiving FMT, we plan to use a retrospective control group from an insurance claims database of patients with recurrent CDI treated with at least 3 courses of antibiotics rather than FMT.
We anticipate adding registry sites within the next 6 months, which will increase the number as well as geographic and demographic diversity of participants recruited. Obtaining information on current FMT methods in North America is an important first step in characterizing FMT practice, with the future goal of standardizing and optimizing FMT. In addition, several gut microbiota-derived products are in late phases of clinical development and will likely be approved for use in the near future. These products are hoped to reduce concerns regarding donor screening and infection transmission, but their real-world safety and effectiveness will be important to determine. We also anticipate the registry will evaluate patients treated with these new products once released.29-31
Real-world evidence is becoming increasingly important in health care and is a recent interest of the Food and Drug Administration in monitoring for adverse events and regulatory decision-making.32 FMT practitioners, together with the research community, have the responsibility to protect the safety of patients receiving FMT and the opportunity to gain tremendous new insights into the biology of the human gut microbiome.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
Fecal microbiota transplantation (FMT) is widely used for treatment of C difficile infection unresponsive to standard therapy, although a paucity of prospective and real-world data exists.
NEW FINDINGS
FMT led to a cure of C difficile infection in 90% of patients in a registry including 20 North American FMT practice sites.
LIMITATIONS
Follow-up beyond 6 months is not yet available.
IMPAC
The effectiveness and safety of FMT is similar in a real-world experience to that reported in research studies.
Acknowledgments
The members of the Steering Committee, Participating Site Investigators, and Observational Study Monitoring Board are listed in the Supplementary Material.
Funding
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R24AI118629. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used in this paper:
- AGA
American Gastroenterological Association
- CDI
Clostridioides difficile infection
- FMT
fecal microbiota transplantation
- IBD
inflammatory bowel disease
- RCT
randomized controlled trial
- UTI
urinary tract infection
Footnotes
Conflicts of interest
These authors disclose the following: Colleen R. Kelly: research support from Finch Therapeutics for a clinical trial; unpaid clinical advisor to OpenBiome. Ari M. Grinspan: lecture fees from Merck. Stacy A. Kahn: unpaid research collaboration with OpenBiome; pilot award (principal investigator) from Cures Within Reach; Mooney Fund Research Award (principal investigator). James D. Lewis: consulting for Merck and Pfizer, outside of submitted work. David T. Rubin: no disclosures relevant to the submitted work, but has received research support from Takeda and serves as a consultant for Abbvie, Abgenomics, Allergan, Inc, Arena Pharmaceuticals, Biomica, Bristol-Myers Squibb, Dizal Pharmaceuticals, Ferring Pharmaceuticals, Inc, Genentech/ Roche, Janssen Pharmaceuticals, Lilly, Mahana Therapeutics, Medtronic, Merck & Co., Inc, Napo Pharmaceuticals, Pfizer, Prometheus Laboratories, Shire, Takeda, and Target PharmaSolutions, Inc. Jessica R. Allegretti: consults for Finch Therapeutics, Servatus, and Artugen and is an unpaid advisor to Openbiome. Jessica R. Allegretti has research support from Merck. Sahil Khanna: research grants from Rebiotix, Inc (A Ferring Company), consulting fees from Shire Plc, Premier Inc, Facile therapeutics, ProbioTech Inc, outside of the submitted work. Carl V. Crawford: research support from Finch Therapeutics, Summit, Ferring, and Artugen for clinical trials. Speaker for Merck and Romark. Monika Fischer: on data and safety monitoring board for Rebiotix; unpaid clinical advisor to OpenBiome. Paul Feuerstadt: consulting fees for Merck and Co., Rebiotix (A Ferring Company), Roche Diagnostics and Premier Inc David Kerman: Consultant to Abbvie, Cleveland Clinic, Advisory Board Rebiotix. Jonathan Goldstein: research support from Rebiotix. Gary D. Wu: research support from Intercept Pharmaceuticals, Seres Therapeutics, and Takeda Pharmaceuticals; scientific consultant for the Hitachi Corporation; and a scientific advisory board member for Biocodex and Danone. The remaining authors disclose no conflicts.
Supplementary Material
To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.09.038.
References
- 1.Ma GK, Brensinger CM, Wu Q, et al. Increasing incidence of multiply recurrent clostridium difficile infection in the United States: a cohort study. Ann Intern Med 2017; 167:152–158. [DOI] [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 2002; 97:1769–1775. [DOI] [PubMed] [Google Scholar]
- 4.Moayyedi P, Yuan Y, Baharith H, et al. Faecal microbiota transplantation for Clostridium difficile-associated diarrhoea: a systematic review of randomised controlled trials. Med J Aust 2017;207:166–172. [DOI] [PubMed] [Google Scholar]
- 5.Fischer M, Sipe B, Cheng YW, et al. Fecal microbiota transplant in severe and severe-complicated Clostridium difficile: a promising treatment approach. Gut Microbes 2017;8:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome medicine 2016;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Ther Adv Gastroenterol 2013;6:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smillie CS, Sauk J, Gevers D, et al. Strain tracking reveals the determinants of bacterial engraftment in the human gut following fecal microbiota transplantation. Cell Host Microbe 2018;23:229–240.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031. [DOI] [PubMed] [Google Scholar]
- 10.Borody TJ, Warren EF, Leis S, et al. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003;37:42–47. [DOI] [PubMed] [Google Scholar]
- 11.Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149:102–109.e6. [DOI] [PubMed] [Google Scholar]
- 12.Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017;389:1218–1228. [DOI] [PubMed] [Google Scholar]
- 13.Kelly CR, Fischer M, Grinspan A, et al. Patients eligible for trials of microbe-based therapeutics do not represent the population with recurrent Clostridioides difficile infection. Clin Gastroenterol Hepatol 2020;18:1099–1101. [DOI] [PubMed] [Google Scholar]
- 14.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013;108:478–498; quiz 499. [DOI] [PubMed] [Google Scholar]
- 15.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013;368:407–415. [DOI] [PubMed] [Google Scholar]
- 16.Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med 2016;165:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2017;318:1985–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther 2015;41:835–843. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson MR, Mitchell PD, Alexander E, et al. Efficacy of fecal microbiota transplantation for Clostridium difficile infection in children. Clin Gastroenterol Hepatol 2020; 18:612–619.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration. Information Pertaining to Additional Safety Protections Regarding Use of Fecal Microbiota for Transplantation—Screening and Testing of Stool Donors for Multi-Drug Resistant Organisms. Rockville, MD: US Food and Drug Administration, 2019. [Google Scholar]
- 21.Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One 2016;11(8):e0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 2014;109:1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shogbesan O, Poudel DR, Victor S, et al. A systematic review of the efficacy and safety of fecal microbiota transplant for Clostridium difficile infection in immunocompromised patients. Can J Gastroenterol Hepatol 2018;2018:1394379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baxter M, Ahmad T, Colville A, et al. Fatal aspiration pneumonia as a complication of fecal microbiota transplant. Clin Infect Dis 2015;61:136–137. [DOI] [PubMed] [Google Scholar]
- 25.Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther 2017;46:479–493. [DOI] [PubMed] [Google Scholar]
- 26.Cohen NA, Livovsky DM, Yaakobovitch S, et al. A retrospective comparison of fecal microbial transplantation methods for recurrent Clostridium difficile infection. Isr Med Assoc J 2016;18:594–599. [PubMed] [Google Scholar]
- 27.McFarland LV, Surawicz CM, Rubin M, et al. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol 1999; 20:43–50. [DOI] [PubMed] [Google Scholar]
- 28.Gliklich RE, Dreyer NA, Leavy MB, eds. Registries for Evaluating Patient Outcomes: A User’s Guide. 3rd ed. Rockville, MD: Agency for Healthcare Research and Quality, 2014. [PubMed] [Google Scholar]
- 29.Finch Therapeutics Announces positive topline results from randomized controlled trial of CP101, an oral microbiome drug, for the prevention of recurrent C. difficile infection. Finch Therapeutics. Published June 19, 2020. Available at: https://finchtherapeutics.com/blog/finch-therapeutics-announces-positive-topline-results-from-randomized-controlled-trial-of-cp101-an-oral-microbiome-drug-for-the-prevention-of-recurrent-cdiff. Accessed October 23, 2020. [Google Scholar]
- 30.Rebiotix and Ferring announce world’s first with positive preliminary pivotal phase 3 data for investigational microbiome-based therapy RBX2660. Business Wire. Available at: https://www.businesswire.com/news/home/20200506005454/en/Rebiotix-and-Ferring-Announce-World%E2%80%99s-First-With-Positive-Preliminary-Pivotal-Phase-3-Data-for-Investigational-Microbiome-based-Therapy-RBX2660. Published May 6, 2020. Accessed October 23, 2020. [Google Scholar]
- 31.Seres Therapeutics to host virtual SER-109 focused symposium on May 27, 2020, ahead of phase 3 ECO-SPOR III study read-out. BioSpace. Available at: https://www.biospace.com/article/releases/seres-therapeutics-to-host-virtual-ser-109-focused-symposium-on-may-27-2020-ahead-of-phase-3-ecospor-iii-study-read-out/. Published May 18. 2020. Accessed October 23, 2020. [Google Scholar]
- 32.Franklin JM, Glynn RJ, Martin D, et al. Evaluating the use of nonrandomized real-world data analyses for regulatory decision making. Clin Pharmacol Ther 2019; 105:867–877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.