Abstract
Prognostic assessment of patients with liver cirrhosis allocated for implantation of a transjugular intrahepatic portosystemic shunt (TIPS) is a challenging task in clinical practice. The aim of our study was to assess the prognostic value of the CLIF‐C AD (Acute Decompensation) score in patients with TIPS implantation. Transplant‐free survival (TFS) and 3‐month mortality were reviewed in 880 patients who received de novo TIPS implantation for the treatment of cirrhotic portal hypertension. The prognostic value of the CLIF‐C AD score was compared with the Model for End‐Stage Liver Disease (MELD) score, Child‐Pugh score, and albumin‐bilirubin (ALBI) score using Harrell’s C concordance index. The median TFS after TIPS implantation was 40.0 (34.6‐45.4) months. The CLIF‐C AD score (c = 0.635 [0.609‐0.661]) was superior in the prediction of TFS in comparison to MELD score (c = 0.597 [0.570‐0.623], P = 0.006), Child‐Pugh score (c = 0.579 [0.552‐0.606], P < 0.001), and ALBI score (c = 0.573 [0.545‐0.600], P < 0.001). However, the CLIF‐C AD score did not perform significantly better than the MELD‐Na score (c = 0.626 [0.599‐0.653], P = 0.442). There were no profound differences in the scores’ ranking with respect to indication for TIPS implantation, stent type, or underlying liver disease. Subgroup analyses revealed that a CLIF‐C AD score >45 was a predictor of 3‐month mortality in the supposed low‐risk group of patients with a MELD score ≤12 (14.7% vs. 5.1%, P < 0.001). Conclusion: The CLIF‐C AD score is suitable for prognostic assessment of patients with cirrhotic portal hypertension receiving TIPS implantation. In the prediction of TFS, the CLIF‐C AD score is superior to MELD score, Child‐Pugh score, and ALBI score but not the MELD‐Na score.
In the present study we investigated the value of the CLIF‐C AD score for prognostic assessment of patients with liver cirrhosis allocated for implantation of a transjugular intrahepatic portosystemic shunt (TIPS) for the treatment of portal hypertension. Indeed, the CLIF‐C AD score performed significantly better in the prediction of transplant‐free survival following TIPS implantation in comparison to the MELD score (P = 0.008), the Child Pugh score (P < 0.001) and the ALBI score (P < 0.001). However, the CLIF‐C AD score performed only marginally better than the MELD‐Na score (P = 0.442).

Abbreviations
- ACLF
acute‐on‐chronic liver failure
- ALBI
albumin‐bilirubin
- MELD
Model for End‐Stage Liver Disease
- PSG
portosystemic pressure gradient
- TFS
transplant‐free survival
- TIPS
transjugular intrahepatic portosystemic shunt
Implantation of a transjugular intrahepatic portosystemic shunt (TIPS) is a safe and effective treatment for complications of portal hypertension in patients with liver cirrhosis.( 1 ) However, patients that require TIPS implantation due to decompensated liver cirrhosis are a population with impaired prognosis per se. Reported in‐hospital mortality following TIPS implantation ranges between 2.3% and 16.3%, and 3‐month mortality between 7.9% and 27.0%.( 2 , 3 , 4 , 5 , 6 ) For optimum patient care after TIPS implantation, it is crucial to identify those patients who are at high risk for a poor outcome. Originally developed for this purpose, the MELD score has prevailed as primary prognostic tool for patients with TIPS implantation.( 7 , 8 ) In 2015 the European Association for the Study of the Liver (EASL)–CLIF Consortium published the CLIF‐C AD (Acute Decompensation) score that was designed to predict 3‐month mortality in patients with decompensated liver cirrhosis—excluding patients with acute‐on‐chronic liver failure (ACLF).( 9 ) So far, the CLIF‐C AD score has not been validated in the TIPS setting. Hence, the aim of our study was to assess the CLIF‐C AD score as a prognostic tool in patients with implantation of TIPS in comparison to the MELD score, the Child‐Pugh score, and the albumin‐bilirubin (ALBI) score.
Patients and Methods
Patient Selection
A total of 1,235 patients who received de novo TIPS implantation at the University Medical Centers Freiburg (n = 489) and Bonn (n = 746) between January 2004 and September 2016 were screened. Thirty patients with Budd‐Chiari‐syndrome, 16 patients with noncirrhotic portal vein thrombosis, and 46 patients with hepatocellular carcinoma were excluded. Furthermore, 55 patients with missing or incomplete clinical were excluded. The remaining 1,088 patients were screened for fulfilling the criteria for ACLF according to the definition of the EASL‐CLIF consortium.( 10 ) A total of 208 patients (19.1%) with ACLF before TIPS implantation were excluded. Eventually, 880 patients were enrolled in the study, as summarized in Fig. 1.
Fig. 1.
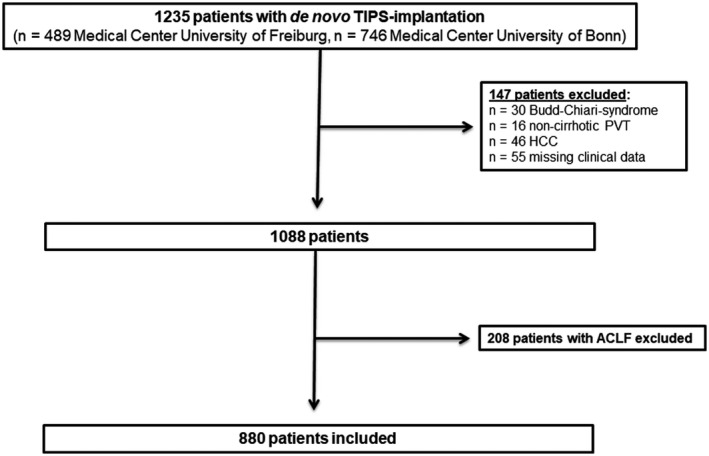
Flow chart summarizing patient inclusion. A total of 1,235 patients who received de novo TIPS implantation were screened. A total of 147 patients with noncirrhotic portal hypertension, hepatocellular carcinoma, or missing clinical data and 208 patients with acute‐on‐chronic liver failure according to the definition of the EASL‐CLIF consortium were excluded.( 10 ) Eventually, 880 patients were enrolled in the study. Abbreviations: ACLF, acute‐on‐chronic liver failure; HCC, hepatocellular carcinoma; PVT, portal vein thrombosis.
All patients were diagnosed with liver cirrhosis and clinically significant portal hypertension. Diagnosis of liver cirrhosis and portal hypertension was based on imaging and endoscopic studies, clinical findings, and laboratory tests. In addition, the portosystemic pressure gradient (PSG) was measured in all patients during the TIPS intervention before stent implantation, to confirm the presence of portal hypertension (≥10 mmHg). Indication for TIPS implantation was based on the recommendations of the Baveno consensus conference and the German guidelines for the treatment of ascites and variceal bleeding.( 11 , 12 , 13 ) Patients received TIPS implantation in standard clinical care.
TIPS Procedure
TIPS implantation was performed as described previously.( 1 ) Summarizing, a puncture needle was advanced into a hepatic vein using a transjugular approach. After successful puncture of the portal vein under ultrasound guidance, spleno‐portography was performed to confirm correct positioning of the catheter and to detect varices. In case of suitable varices, angiographic embolization was performed. Subsequently, the parenchymal tract was dilated and the stent graft was placed. Portal vein and inferior vena cava pressure were measured before and after placement of the stent graft to determine the PSG. Different types of noncovered stents and covered stents were used, as the choice of stent type was not defined in the study protocol but was made by the respective interventionalist.
Ethics Approval
Written informed consent for TIPS implantation and for data collection was obtained from all patients. The multicenter study was approved by the local ethics committee (No. EK 355/20) and is in accordance with the Declaration of Helsinki.
Statistical Analyses
The study was a retrospective observational analysis. Patients’ demographic data, interventional data, and laboratory parameters were reviewed in the medical records. Liver function was assessed using the CLIF‐C AD score, the MELD score, the Child‐Pugh score and the ALBI score. Parameters used to calculate the scores were assessed within 48 hours before TIPS implantation. The CLIF‐C AD score was calculated according to the formula presented on the EASL‐CLIF consortium website.( 14 ) Patients were analyzed from the day of TIPS implantation until death (n = 671; 76.3%), liver transplantation (n = 47; 5.3%), or last contact. The primary endpoint was transplant‐free survival (TFS); 3‐month mortality after TIPS implantation was defined as a secondary endpoint. The cutoff point for survival data was January 15, 2019.
Continuous variables were expressed as mean with SD; categorial variables were expressed as frequencies and percentages. Differences in continuous variables were determined using Wilcoxon Mann‐Whitney and Kruskal–Wallis tests, as there was no Gaussian distribution of the data. The χ2 tests or Fisher’s exact tests were used for categorial variables. P values < 0.05 were considered significant. TFS was calculated according to the Kaplan‐Meier method with death and liver transplantation being recorded as event. Differences in survival were assessed using log‐rank tests.
For survival analyses, patients were stratified according to MELD score, applying a cutoff of ≤12, as this value has been used previously to define patients with relatively preserved liver function.( 15 ) Patients were stratified according to CLIF‐C AD score, applying cutoff values of ≤45 and ≥60, as these defined low‐risk and high‐risk patients in the CLIF‐C AD score’s original publication.( 9 ) Discriminatory performance of the CLIF‐C AD score in comparison to the Child‐Pugh and MELD score was assessed using Harrell’s C concordance index (c‐index). Statistical comparison of the c‐indices for TFS was performed using STATA’s Somers’ D package. To analyze the percentage improvement of the CLIF‐C AD score in comparison to the other scores, the prediction error rate was determined. Prediction error rate was assessed as percentage reduction in discordance rate of the CLIF C‐AD versus the other scores, respectively (100 * [c‐indexCLIF‐C AD − c‐indexscore]/[1 − c‐indexscore]). Statistical analyses were performed with SPSS (Version 25.0; IBM, New York, NY), GraphPad Prism (Version 8; GraphPad Software, San Diego, CA), and STATA (Version 15.0; StataCorp, College Station, TX). The study was conducted in accordance with the STROBE guidelines.( 16 )
Results
Patient Characteristics
Table 1 summarizes patient characteristics. Indication for TIPS implantation was treatment of refractory ascites including hepatic hydrothorax in 458 patients (52.0%) and secondary prophylaxis of variceal bleeding in 422 patients (48.0%). The mean MELD score of the patients included was 11.1 ± 3.6; most of the 614 patients (69.8%) presented with a MELD score ≤12. A total of 183 patients (20.8%) were classified as Child‐Pugh A, 559 patients (63.5%) as Child‐Pugh B, and 138 patients (15.7%) as Child‐Pugh C. A total of 93 patients (10.6%) were in ALBI stage 1, 531 patients (60.3%) in ALBI stage 2, and 256 patients (29.1%) in ALBI stage 3. The mean CLIF‐C AD score in the patient collective was 48.4 ± 7.6, with most of the 522 patients (59.3%) presenting with a CLIF‐C AD score between 46 and 59, 294 patients (33.4%) with a CLIF‐C AD score of ≤ 45, and 64 patients (7.3%) with a CLIF‐C AD score of ≥ 60.
Table 1.
Patient Characteristics
| Total of Patients | 880 |
| Age (years) | 58.1 ± 12.3 |
| Etiology of liver disease | |
| Viral liver disease | |
| HCV | 95 (10.8%) |
| HBV | 35 (4.0%) |
| Nonviral liver disease | |
| Alcoholic | 554 (63.0%) |
| NASH | 8 (0.9%) |
| Others | 188 (21.4%) |
| Indication for TIPS implantation | |
| Ascites | 458 (52.0%) |
| Varices | 422 (48.0%) |
| History of HE before TIPS | |
| No HE | 768 (87.3%) |
| Grade I | 84 (9.5%) |
| Grade II | 28 (3.2%) |
| CLIF‐C AD score | 48.4 ± 7.6 |
| ≤ 45 | 294 (33.4%) |
| 46‐59 | 522 (59.3%) |
| ≥ 60 | 64 (7.3%) |
| MELD score | 11.1 ± 3.6 |
| ≤ 12 | 614 (69.8%) |
| > 12 | 266 (30.2%) |
| Child‐Pugh score | 7.9 ± 1.7 |
| A | 183 (20.8%) |
| B | 559 (63.5%) |
| C* | 138 (15.7%) |
| MELD‐Na score | 14.3 ± 3.8 |
| ALBI score | −1.7 ± 1.8 |
| 1 | 93 (10.6%) |
| 2 | 531 (60.3%) |
| 3 | 256 (29.1%) |
| Hepatic hemodynamical data | |
| PSG before TIPS (mmHg) | 20.7 ± 5.7 |
| PSG after TIPS (mmHg) | 9.0 ± 4.3 |
| Stent type | |
| Noncovered | 268 (30.5%) |
| Covered | 612 (69.5%) |
| Stent size | |
| Diameter (mm) | 10.1 ± 1.5 |
| Length (mm) | 60.0 ± 10.9 |
| TIPS revision † | 206 (23.4%) |
| Dilatation/overstenting | 199 (22.6%) |
| Reduction | 7 (0.8%) |
| Laboratory | |
| WBC (103/µL) | 6.7 ± 3.6 |
| INR | 1.2 ± 0.2 |
| Creatinine (mg/dL) | 1.1 ± 0.3 |
| Bilirubin (mg/dL) | 1.6 ± 1.2 |
| Albumin (g/L) | 30 ± 8 |
| Sodium (mmol/L) | 136 ± 5 |
| Mortality | |
| TFS (months) | 40.0 [34.6 ‐ 45.4] |
| 3‐month mortality | 129 (14.7%) |
Decision for TIPS implantation was made on an individual basis.
Patients receiving TIPS revision in the course of the observation period.
Abbreviations: CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HE, hepatic encephalopathy; INR, international normalized ratio; NASH, nonalcoholic steatohepatitis; PSG, portosystemic gradient; WBC, white blood count.
TFS and 3‐Month Mortality After TIPS Implantation According to MELD, Child‐Pugh, ALBI, and CLIF‐C AD Score
Median TFS after TIPS implantation was 41.0 (35.3‐46.7) months. TFS was significantly related to the MELD score. Patients with a MELD score >12 displayed significantly reduced TFS of 21.0 (14.4‐27.6) months compared with 48.0 (40.8‐55.2) months in patients with a MELD score of ≤12 (P < 0.001; Fig. 2A). TFS was also linked to the Child‐Pugh score, as it was 63.0 (45.9‐80.1) versus 37.0 (30.5‐43.5) versus 27.0 (19.0‐35.0) months in patients with Child‐Pugh stadiums A, B and C, respectively (P < 0.001; Fig. 2B). However, discrimination between Child‐Pugh stadiums B and C was not statistically significant (P = 0.068). TFS declined with ALBI grade from 65.0 (48.3‐81.7) months in grade 1 to 41.0 (35.4‐46.6) months in grade 2 to 29.0 (20.1‐37.9) months in grade 3 (P < 0.001; Fig. 2C). Similar to the Child‐Pugh score, the ALBI score did not offer significant discrimination between stadiums 2 and 3 (P = 0.075). The CLIF‐C AD score also offered substantial differentiation with regard to survival: TFS declined with increasing CLIF‐C AD score from 67.0 (58.3‐75.7) to 30.0 (23.0‐37.0) to 11 (0‐22.2) months in patients with a CLIF‐C AD score of ≤45, 46‐59, and ≥60, respectively (P < 0.001; Fig. 2D).
Fig. 2.
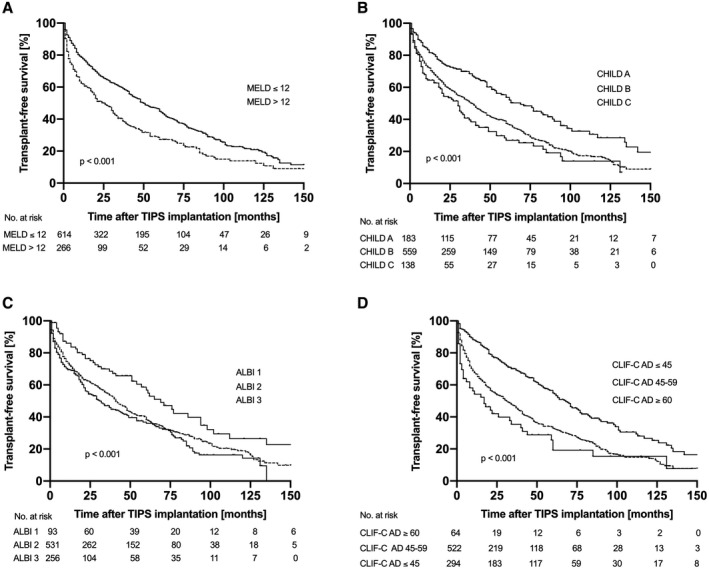
Transplant‐free survival according to MELD, Child‐Pugh, ALBI, and CLIF‐C AD score. MELD, Child‐Pugh, ALBI, and CLIF‐C AD scores were all able to stratify patients according to TFS following TIPS implantation. Patients with a MELD score > 12 showed significantly reduced TFS of 21.0 (14.4‐27.6) months compared with 48.0 (40.8‐55.2) months in patients with a MELD score of ≤ 12 (P < 0.001). TFS was 63.0 (45.9‐80.1) versus 37.0 (30.5‐43.5) versus 27.0 (19.0‐35.0) months in patients with Child‐Pugh stadiums A, B and C, respectively (P < 0.001). TFS was 65.0 (48.3‐81.7) months in ALBI stadium 1, 41.0 (35.4‐46.6) months in ALBI stadium 2, and 29.0 (20.1‐37.9) months in ALBI stadium 3 (P < 0.001). TFS declined with increasing CLIF‐C AD score from 67.0 (58.3‐75.7) to 30.0 (23.0‐37.0) to 11 (0‐22.2) months in patients with a CLIF‐C AD score of ≤ 45, 46‐59, and ≥ 60, respectively (P < 0.001).
Three‐month mortality in the patient collective investigated was 14.7%. It was also significantly linked to MELD score, as 3‐month mortality was 10.1% compared to 25.2% in patients with a MELD score of ≤12 and >12, respectively (P < 0.001). Three‐month mortality was also significantly related to the Child‐Pugh score, as it accounted for 7.1%, 16.1%, and 18.8% in patients with Child‐Pugh stadiums A, B, and C (P = 0.004), and to the ALBI score, as it was 4.3%, 14.1%, and 19.5% in ALBI grades 1, 2, and 3 (P = 0.002). Again, the difference between Child‐Pugh stadiums B and C and ALBI grades 2 and 3 was not statistically significant (P = 0.445 and P = 0.052). The CLIF‐C AD score also offered significant discrimination, as 3‐month mortality was 6.5% in patients with a score ≤45, 16.7% in patients with a score of 46‐59, and 35.9% in patients with a score ≥60 (P < 0.001; Supporting Fig. S1).
Prognostic Discrimination of CLIF‐C AD, MELD, Child‐Pugh, and ALBI Score
To assess the prognostic discrimination of the different scores, the patients’ Harrell c‐indices were calculated. The CLIF‐C AD score achieved the highest c‐index of 0.635 (0.609‐0.661) with regard to prediction of TFS. The MELD score achieved a c‐index of 0.597 (0.570‐0.623), followed by the Child‐Pugh score with a c‐index of 0.579 (0.552‐0.606) and the ALBI score with a c‐index of 0.573 (0.545‐0.600). The CLIF‐C AD score performed significantly better in comparison to MELD score (P = 0.006), Child‐Pugh score (P < 0.001), and ALBI score (P < 0.001). The percentage improvement of the prediction error rate obtained with the CLIF‐C AD score compared with MELD score, Child‐Pugh score, and ALBI score was 9%, 13% and 15%, respectively. However, incorporating the modified MELD‐Na score into the analysis revealed that the CLIF‐C AD score’s c‐index was only marginally higher compared with the MELD‐Na score with a c‐index of 0.626 (0.599‐0.653). Subsequently, the CLIF‐C AD score’s improvement of prediction toward the MELD‐Na score was not significant (P = 0.442).
With regard to 3‐month TFS, the CLIF‐C AD score (c = 0.688 [0.638‐0.738]) performed significantly better than Child‐Pugh score (c = 0.614 [0.564‐0.664]; P = 0.026). However, the CLIF‐C AD score’s performance was not significantly better in comparison to the MELD score (c = 0.656 [0.603‐0.710]; P = 0.259) and the ALBI score (c = 0.621 [0.570‐0.672]; P = 0.077). In fact, the MELD‐Na score even predicted 3‐month mortality marginally better than the CLIF‐C AD score (c = 0.698 [0.647‐0.747]). However, the difference was not significant (P = 0.668).
Incorporating further survival endpoints revealed that the CLIF‐C AD score’s performance in relation to the other scores investigated improved with time, as summarized in Table 2.
Table 2.
C‐Indices of Scores for Prediction of Different Survival Endpoints
| C‐Index [95% CI] P Value* | |||||
|---|---|---|---|---|---|
| CLIF‐C AD | MELD | Child‐Pugh | MELD‐Na | ALBI | |
| 1‐month TFS | 0.673 [0.607‐0.740] | 0.618 [0.544‐0.703] 0.175 | 0.600 [0.523‐0.677] 0.146 | 0.673 [0.601‐0.746] 0.999 | 0.621 [0.542‐0.699] 0.364 |
| 3‐month TFS | 0.688 [0.638‐0.738] | 0.656 [0.603‐0.710] 0.259 | 0.614 [0.564‐0.664] 0.026 | 0.698 [0.647‐0.747] 0.668 | 0.621 [0.570‐0.672] 0.077 |
| 6‐month TFS | 0.688 [0.646‐0.730] | 0.648 [0.603‐0.692] 0.090 | 0.599 [0.555‐0.642] 0.001 | 0.694 [0.652‐0.736] 0.747 | 0.598 [0.553‐0.643] 0.004 |
| 1‐year TFS | 0.672 [0.636‐0.708] | 0.631 [0.594‐0.669] 0.045 | 0.592 [0.555‐0.628] < 0.001 | 0.674 [0.638‐0.710] 0.850 | 0.585 [0.547‐0.623] 0.001 |
| 2‐year TFS | 0.671 [0.638‐0.704] | 0.625 [0.591‐0.660] 0.014 | 0.599 [0.565‐0.632] < 0.001 | 0.647 [0.616 −0.679] 0.654 | 0.585 [0.553‐0.618] 0.002 |
| TFS | 0.635 [0.609‐0.661] | 0.597 [0.570‐0.623] 0.006 | 0.579 [0.552‐0.606] < 0.001 | 0.626 [0.599‐0.653] 0.442 | 0.573 [0.545‐0.600] < 0.001 |
P value against CLIF‐C AD score.
Prognostic Effects of TIPS Indication, Stent Type, and Underlying Liver Disease
TFS was significantly reduced in patients with ascites as leading TIPS indication, compared to patients with varices as leading TIPS indication (48.0 [39.5‐56.5] months vs. 30.0 [21.0‐39.0] months; P < 0.001; Supporting Fig. S2). The prognostic values of the scores investigated were comparable in both subgroups, with the CLIF‐C AD score and the MELD‐Na score performing best (cCLIF‐C AD ascites = 0.611 [0.572‐0.650] vs. cMELD‐Na ascites = 0.610 [0.571‐0.649]; P = 0.957; cCLIF‐C AD varices = 0.638 [0.601‐0.676] vs. cMELD‐Na varices = 0.617 [0.579‐0.0.656]; P = 0.230). The type of implanted stent was also a relevant prognostic factor, as patients with a covered stent displayed a significantly longer TFS in comparison to patients with a noncovered stent (44.0 [36.6‐51.4] months vs. 31.0 [21.6‐40.4] months; P < 0.001; Supporting Fig. S3). The CLIF‐C AD score and the MELD‐Na score performed best in both patients with covered and noncovered stent. Notably, both scores’ prognostic values were higher among patients with a covered stent (cCLIF‐C AD covered = 0.652 [0.619‐0.685] vs. cMELD‐Na covered = 0.640 [0.606‐673]; P = 0.401; cCLIF‐C AD non‐covered = 0.598 [0.554‐0.642] vs. cMELD‐Na non‐covered = 0.597 [0.552‐0.642]; P = 0.978). There were no significant differences in TFS between patients with smaller (≤9 mm) and larger (≥10 mm) stent diameter (41.0 [33.6‐48.4] months vs. 39.0 [32.0‐40.0] months; P = 0.772) or patients with and without TIPS revision during the observational period (38.0 [32.2.‐43.8] months vs. 48.0 [32.3‐63.7] months; P = 0.861). With regard to underlying liver disease, there was no significant difference in TFS between patients with alcoholic and viral liver disease (38.0 [31.7‐44.3] months vs. 48.0 [30.7‐65.3] months; P = 0.386). While the scores investigated performed better in patients with viral liver disease in absolute measures, there were no profound differences in their relative performance between the two etiologies. Supporting Table S1 summarizes the prognostic scores’ c‐indices for patient subgroups.
Prognostic Effect of the CLIF‐C AD Score in Patients With Low MELD Score
Exploratory analyses of the data showed that 614 patients (69.8%) presented with a MELD score ≤12, suggesting low‐risk patients with comparatively good liver function. However, 3‐month mortality in this subgroup still accounted for 10.1%, illustrating poor prognosis despite a low MELD score. We assessed whether the CLIF‐C AD score could substratify this patient group. As only 14 (2.3%) of patients with a MELD score ≤12 had a CLIF‐C AD score ≥60, this cutoff proved unfeasible for a sensible substratification. However, 256 (41.7%) of the patients with a MELD score ≤12 had a CLIF‐C AD score ≤45. Applying this cutoff value allowed significant prognostic discrimination of the patients with low MELD scores, as those with a CLIF‐C AD score ≤45 displayed a significantly lower 3‐month mortality of 5.1% compared to 14.7% in those with a CLIF‐C AD score >45 (P < 0.001). The difference in 3‐month mortality between patients with a MELD score of ≤2 and a CLIF‐C AD score of >45 and patients with a MELD score >12 was also significant (14.7% vs. 25.2%; P < 0.001) (Fig. 3). In contrast, the CLIF‐C AD score was not able to substratify patients with a MELD score >12 (P = 0.224).
Fig. 3.
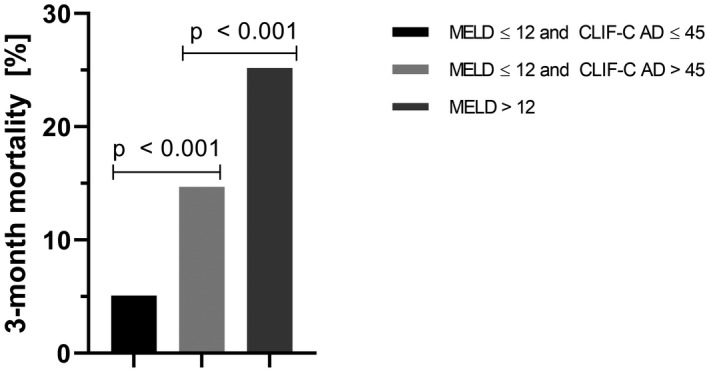
Stratification according to 3‐month mortality by the CLIF‐C AD score in the low‐MELD patient group. Among the supposed low‐risk patients with a low MELD score of ≤ 12, patients with a CLIF‐C AD score ≤ 45 displayed a significantly lower 3‐month mortality of 5.1% compared with 14.7% in those with a CLIF‐C AD score > 45 (P < 0.001). The difference in 3‐month mortality between patients with a MELD score of ≤ 12 and a CLIF‐C AD score of > 45 and patients with a MELD score > 12 was also significant (14.7% vs. 25.2%; P < 0.001).
The significant prognostic discrimination within the low‐MELD subgroup of patients applying a cutoff of a CLIF‐C AD score ≤45 was also consistent for long‐term survival (TFS 71.0 months [60.7‐81.3] vs. 34.0 months [26.2‐41.8]; P < 0.001).
Discussion
TIPS implantation is the acknowledged interventional therapy for the treatment of portal hypertension in specific patients with liver cirrhosis, such as patients with refractory ascites or patients who suffered from variceal bleeding.( 1 ) The TIPS not only offers effective portal decompression, and therefore therapy of complications of portal hypertension, but can also lead to an improved survival.( 17 , 18 ) At the same time, apart from hepatic encephalopathy that occurs in up to one third of patients after TIPS implantation procedure‐related complications are rare.( 2 , 19 ) Despite the efficacy and safety of TIPS implantation, it is important to realize that patients with liver cirrhosis requiring treatment of refractory ascites or secondary prophylaxis of variceal bleeding by TIPS are a high‐risk patient cohort. These patients present with decompensated liver cirrhosis, which is associated with significantly impaired prognosis per se.( 20 ) In clinical practice, identifying those patients who are at high risk for a poor outcome is an important factor in making the decision for or against TIPS implantation. On the other hand, known high‐risk patients can be monitored more closely than in routine care after TIPS implantation. Identification of prognostic factors in TIPS patients is subject to intensive research. For example, the NEPTUN study (NCT03628807), an observational, real‐world TIPS cohort with structured follow‐up described sarcopenia and echocardiographic parameters as prognostic factors.( 21 , 22 ) In addition, several instrument‐based parameters of liver function and portal hypertension, which could serve to assess patients’ prognoses, have emerged in recent years, such as the liver maximum function capacity test (LiMax) or transient elastography.( 23 , 24 ) Furthermore, several scoring systems have been evaluated for prognostic assessment of TIPS patients. Since its publication in 2000, the MELD score, designed to predict 3‐month mortality in TIPS patients, is still likely to be considered the most valid tool to assess prognosis in patients undergoing TIPS implantation. Various studies demonstrated that the MELD score is superior to the widely used Child‐Pugh classification in this setting.( 7 , 8 ) The ALBI score has been discussed as a possible alternative model, but so far has not proven suitable as predictor for survival after TIPS implantation.( 25 , 26 ) Importantly, the MELD score has several limitations, too. Indeed, it was observed that the MELD score tends to overestimate mortality and, designed to predict short‐term mortality, its prognostic value significantly decreases in the long term.( 4 ) An alternative prognostic model is the modified MELD‐Na score, which was designed to improve the MELD score’s performance in patients awaiting liver cirrhosis.( 27 ) However, the MELD‐Na is not established as prognostic tool in patients undergoing TIPS implantation, as it has been evaluated with mixed results in this context.( 28 , 29 ) In 2015 the EASL‐CLIF Consortium published the CLIF‐C AD score for prognostic assessment of patients hospitalized for decompensated liver cirrhosis (without ACLF).( 9 ) We set out to investigate the prognostic value of the CLIF‐C AD score in patients undergoing TIPS implantation and to compare it with the MELD score, the Child‐Pugh score, and the ALBI score. Indeed, in our analysis of 880 patients the CLIF‐C AD score allowed significant stratification according to TFS following TIPS implantation. Thereby, the CLIF‐C AD score’s prognostic performance increased with time. Compared with the MELD score, the Child‐Pugh score and ALBI score demonstrated that the CLIF‐C AD score was superior in the prediction of long‐term survival among these scores, but performed only marginally better in comparison with the modified MELD‐Na score. With regard to the prediction of short‐term survival 3 months after TIPS implantation, the CLIF‐C AD was not superior to the MELD score and ALBI score, with the MELD‐Na score even performing minimally better. Subgroup analyses demonstrated impaired TFS in patients with a noncovered stent in comparison to patients with a covered stent, which is in accordance with previous studies.( 30 ) With respect to stent size, we observed no significant difference in TFS between patients with smaller (≤9 mm) and larger (≥10 mm) shunt diameter. This result is in contrast to a recent study in which a smaller nominal shunt diameter was associated with better survival after TIPS implantation.( 31 ) Importantly, our data do not allow us to differentiate whether the reported shunt diameter was nominal or due to underdilation, which may influence our survival analyses. With regard to the prognostic scores’ ranking, we observed no profound differences with respect to technical TIPS specifics, indication for TIPS implantation, or underlying liver disease.
To the best of our knowledge our study is the first to investigate the prognostic value of the CLIF‐C AD score in patients with TIPS implantation. In a recent study, Lv et al. found the CLIF‐C AD score to be a strong predictor for mortality in 608 patients with liver cirrhosis in Child‐Pugh stadium B and active variceal bleeding.( 32 ) Fifty‐four of these patients (8.9%) received a rescue TIPS; patients with early or elective TIPS were excluded from the analysis. Due the low number of TIPS patients and the narrowly defined patient collective investigated, the significance of the study’s findings in the context of TIPS implantation in general appears limited. Allegretti et al. compared the CLIF‐C ACLF score to the MELD score and Child‐Pugh score in 440 patients.( 5 ) The study reported the prognostic discrimination of the MELD score to be higher than that of the CLIF‐C ACLF score. However, the authors did not specify whether patients included in the study met the criteria of ACLF according to the EASL consortium.( 10 ) Because the CLIF‐C ACLF score was developed particularly for patients with ACLF, this may be a reason for the weaker performance of the score in comparison with the MELD score. In contrast, we applied the CLIF‐C AD score and excluded patients meeting the criteria for ACLF.
An interesting finding is that the CLIF‐C AD score was able to substratify patients according to 3‐month mortality in the supposed low‐risk group of patients with a MELD score ≤12. As 3‐month mortality increased from 5.1% to 13.7% to 25.2% between patients with a MELD score ≤2 and CLIF‐C AD score ≤45, with a MELD score ≤12 and CLIF‐C AD score >45, and with a MELD score >12 (P < 0.001), one may propose a classification of patients with TIPS into a low‐risk, intermediate‐risk, and high‐risk groups according to these criteria.
Our study has some limitations that need to be discussed. We performed a multicenter analysis including 880 patients. As prior studies evaluating prognostic scores in patients with TIPS implantation investigated smaller patient numbers, a quantum of 880 patients appears reasonably high to achieve dependable results.( 5 , 8 , 25 , 26 , 33 ) Notably, the cohort in which the CLIF‐C AD score was developed accounted for 1,016 patients (plus 225 patients for validation).( 9 ) Still, including a larger number of patients and more than two centers in the analysis may contribute to obtaining even more valid results. Another limitation that should also be mentioned is that we did not perform survival analyses in patients with similar stages of cirrhosis without TIPS implantation in comparison. Another aspect that needs to be addressed is that, in order to include a sufficiently high number of patients, data were obtained over a comparatively long observation period of almost 12 years. Along with this goes a certain heterogeneity regarding technical aspects of TIPS implantation. This is apparent in the use of noncovered versus covered stents, which were both implemented in our analysis. An issue to be discussed is the absolute prognostic value of the CLIF‐C AD score. Realizing that a c‐index of 1.0 would be a perfect result while a c‐index of 0.50 represents coin‐flip probability, it is important to keep in mind that the CLIF‐C AD score’s c‐index of 0.635 (0.609‐0.661) for TFS in our analysis equates to a relevant number of incorrect predictions when applying the score to assess patients’ prognoses. Notably, in the cohort of patients outside the TIPS setting, in which the CLIF‐C AD score was originally developed, the c‐index accounted for 0.67 regarding prediction of 12‐month mortality.( 9 ) This indicates that prediction of survival in patients with decompensated liver cirrhosis is a challenging task in general.
The present study demonstrates that the CLIF‐C AD score is suitable for the prognostic assessment of patients with cirrhotic portal hypertension allocated for TIPS implantation. In comparison with the MELD score, Child‐Pugh score and ALBI score, the CLIF‐C AD score offers the best prediction of long‐term TFS following TIPS implantation. However, it performs only marginally better in comparison to the modified MELD‐Na score. In the prediction of short‐term TFS, the CLIF‐C AD score is superior to the Child‐Pugh score, but offers no significant advantage over MELD score and ALBI score.
Supporting information
Fig S1
Fig S2
Fig S3
Supplementary Material
Table S1
Acknowledgment
L.S. and D.B. are supported by the Berta‐Ottenstein‐Programme, Faculty of Medicine, University of Freiburg. M.P. is supported by the BONFOR research program of the University of Bonn and the Ernst‐und‐Berta Grimmke Foundation. Open access funding enabled and organized by ProjektDEAL.
Supported by Deutsche Forschungsgemeinschaft (SFB TRR57 P18 and CRC1382), H2020 European Institute of Innovation and Technology (668031 and 825694), Fundaci¢n Cellex (PREDICT), and H2020 Society (731875).
Potential conflict of interest: Dr. Bettinger consults for Bayer Healthcare and Boston Scientific. He is on the speakers’ bureau for Falk Foundation. Dr. Meyer consults for and is on the speakers’ bureau for W.L. Gore.
References
- 1. Rössle M. TIPS: 25 years later. J Hepatol 2013;59:1081‐1093. [DOI] [PubMed] [Google Scholar]
- 2. Bettinger D, Schultheiss M, Boettler T, Muljono M, Thimme R, Rössle M. Procedural and shunt‐related complications and mortality of the transjugular intrahepatic portosystemic shunt (TIPSS). Aliment Pharmacol Ther 2016;44:1051‐1061. [DOI] [PubMed] [Google Scholar]
- 3. Lee EW, Kuei A, Saab S, Busuttil RW, Durazo F, Han SH, et al. Nationwide trends and predictors of inpatient mortality in 83884 transjugular intrahepatic portosystemic shunt. World J Gastroenterol 2016;22:5780‐5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salerno F, Merli M, Cazzaniga M, Valeriano V, Rossi P, Lovaria A, et al. MELD score is better than Child‐Pugh score in predicting 3‐month survival of patients undergoing transjugular intrahepatic portosystemic shunt. J Hepatol 2002;36:494‐500. [DOI] [PubMed] [Google Scholar]
- 5. Allegretti AS, Frenk NE, Li DK, Seethapathy H, Vela Parada XF, Long J, et al. Evaluation of model performance to predict survival after transjugular intrahepatic portosystemic shunt placement. PLoS One 2019;14:e0217442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarwar A, Zhou L, Novack V, Tapper EB, Curry M, Malik R, et al. Hospital volume and mortality after transjugular intrahepatic portosystemic shunt creation in the United States. Hepatology 2018;67:690‐699. [DOI] [PubMed] [Google Scholar]
- 7. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, Ter Borg PCJ. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864‐871. [DOI] [PubMed] [Google Scholar]
- 8. Angermayr B, Cejna M, Karnel F, Gschwantler M, Koenig F, Pidlich J, et al. Child‐Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut 2003;52:879‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland‐Fischer P, et al. The CLIF Consortium Acute Decompensation score (CLIF‐C ADs) for prognosis of hospitalised cirrhotic patients without acute‐on‐chronic liver failure. J Hepatol 2015;62:831‐840. [DOI] [PubMed] [Google Scholar]
- 10. Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, et al. Development and validation of a prognostic score to predict mortality in patients with acute‐on‐chronic liver failure. J Hepatol 2014;61:1038‐1047. [DOI] [PubMed] [Google Scholar]
- 11. Gerbes AL, Labenz J. Leitlinie Komplikationen der Leberzirrhose. Z. Gastroenterol 2019;57:571‐573. [DOI] [PubMed] [Google Scholar]
- 12. Lynen Jansen P, Götz M, Trebicka J. S2k‐Leitlinie Gastrointestinale Blutung. Z Gastroenterol 2017;55:937‐946. [DOI] [PubMed] [Google Scholar]
- 13. de Franchis R, Faculty BVI. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743‐752. [DOI] [PubMed] [Google Scholar]
- 14. European Foundation for the Study of Chronic Liver Failure . Score calculators. http://www.efclif.com/scientific‐activity/score‐calculators/clif‐c‐ad. Accessed May 29, 2020. [Google Scholar]
- 15. Luca A, Miraglia R, Maruzzelli L, Damico M, Tuzzolino F. Early liver failure after transjugular intrahepatic portosystemic shunt in patients with cirrhosis with model for end‐stage liver disease score of 12 or less: incidence, outcome, and prognostic factors. Radiology 2016;280:622‐629. [DOI] [PubMed] [Google Scholar]
- 16. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting Of Observational Studies In Epidemiology (STROBE): explanation and elaboration. PLoS Medicine 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trebicka J, Gu W, Ibáñez‐Samaniego L, Hernández‐Gea V, Pitarch C, Garcia E, et al. Rebleeding and mortality risk are increased by ACLF but reduced by pre‐emptive TIPS. J Hepatol 2020;73:1082‐1091. [DOI] [PubMed] [Google Scholar]
- 18. Bureau C, Thabut D, Oberti F, Dharancy S, Carbonell N, Bouvier A, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant‐free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017;152:157‐163. [DOI] [PubMed] [Google Scholar]
- 19. Schultheiss M, Bettinger D, Boettler T, Thimme R, Roessle M. Severe hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS): value of shunt reduction and occlusion. JSM Hepat 2017;2:1009. [Google Scholar]
- 20. D’Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217‐231. [DOI] [PubMed] [Google Scholar]
- 21. Praktiknjo M, Clees C, Pigliacelli A, Fischer S, Jansen C, Lehmann J, et al. Sarcopenia is associated with development of acute‐on‐chronic liver failure in decompensated liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. Clin Transl Gastroenterol 2019;10:e00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jansen C, Schröder A, Schueler R, Lehmann J, Praktiknjo M, Uschner FE, et al. Left ventricular longitudinal contractility predicts acute‐on‐chronic liver failure development and mortality after transjugular intrahepatic portosystemic shunt. Hepatol Commun 2019;3:340‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reichert MC, Schulz A, Massmann A, Buecker A, Glanemann M, Lammert F, et al. Predictive power of liver maximum function capacity test in transjugular intrahepatic portosystemic shunt patients: a pilot study. Dig Dis 2020;38:251‐258. [DOI] [PubMed] [Google Scholar]
- 24. Berzigotti A. Transient elastography and prognosis of cirrhosis. Hepatology 2012;55:1629‐1631. [DOI] [PubMed] [Google Scholar]
- 25. Khabbaz RC, Lokken RP, Chen YF, Lipnik AJ, Bui JT, Ray CE, et al. Albumin‐bilirubin and platelet–albumin–bilirubin grades do not predict survival after transjugular intrahepatic portosystemic shunt creation. Cardiovasc Intervent Radiol 2018;41:1029‐1034. [DOI] [PubMed] [Google Scholar]
- 26. Ronald J, Wang Q, Choi SS, Suhocki PV, Hall MD, Smith TP, et al. Albumin‐bilirubin grade versus MELD score for predicting survival after transjugular intrahepatic portosystemic shunt (TIPS) creation. Diagn Interv Imaging 2018;99:163‐168. [DOI] [PubMed] [Google Scholar]
- 27. Leise MD, Kim WR, Kremers WK, Larson JJ, Benson JT, Therneau TM. A revised model for end‐stage liver disease optimizes prediction of mortality among patients awaiting liver transplantation. Gastroenterology 2011;140:1952‐1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahmed R, Santhanam P, Rayyan Y. MELD‐Na as a prognostic indicator of 30‐ and 90‐day mortality in patients with end‐stage liver disease after creation of transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol 2015;27:1226‐1227. [DOI] [PubMed] [Google Scholar]
- 29. Young S, Rostambeigi N, Golzarian J, Lim N. MELD or Sodium MELD: a comparison of the ability of two scoring systems to predict outcomes after transjugular intrahepatic portosystemic shunt placement. Am J Roentgenol 2020;215:215‐222. [DOI] [PubMed] [Google Scholar]
- 30. Qi X, Tian Y, Zhang W, Yang Z, Guo X. Covered versus bare stents for transjugular intrahepatic portosystemic shunt: an updated meta‐analysis of randomized controlled trials. Therap Adv Gastroenterol 2017;10:32‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trebicka J, Bastgen D, Byrtus J, Praktiknjo M, Terstiegen S, Meyer C, et al. Smaller‐diameter covered transjugular intrahepatic portosystemic shunt stents are associated with increased survival. Clin Gastroenterol Hepatol 2019;17:2793‐2799.e1. [DOI] [PubMed] [Google Scholar]
- 32. Lv Y, Wang Z, Li K, Wang Q, Bai W, Yuan X, et al. Risk stratification based on CLIF consortium acute decompensation score in patients with child‐pugh b cirrhosis and acute variceal bleeding. Hepatology 2020;hep.31478. [DOI] [PubMed] [Google Scholar]
- 33. Gaba RC, Couture PM, Bui JT, Grace Knuttinen M, Walzer NM, Kallwitz ER, et al. Prognostic capability of different liver disease scoring systems for prediction of early mortality after transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol 2013;24:411‐420.e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Supplementary Material
Table S1


