Abstract
Percutaneous thermal ablation is a validated treatment option for small hepatocellular carcinoma (HCC). Steatotic HCC can be reliably detected by magnetic resonance imaging. To determine the clinical relevance of this radiological variant, we included 235 patients (cirrhosis in 92.3%, classified Child‐Pugh A in 97%) from a prospective database on percutaneous thermal ablation for <3 cm HCC. Among these patients, 52 (22.1%) had at least one steatotic HCC nodule. Nonalcoholic steatohepatitis was more frequent in patients with than without steatotic HCC (P = 0.057), whereas body mass index, diabetes mellitus, liver steatosis, and liver fat content did not differ between groups. Liver disease was less advanced in patients with than without steatotic HCC: lower total bilirubin (2.1 µmol/L; P = 0.035), higher albumin (+0.8 g/L; P = 0.035), and lower Model for End‐Stage Liver Disease score (‐0.8; P = 0.014). Tumor phenotype was less aggressive in patients with steatotic HCC: lower alpha‐fetoprotein (AFP) concentration (P = 0.019), less frequent AFP > 100 ng/mL (P = 0.045), and multifocality (P = 0.015). During the follow‐up (median: 28.3 months), overall mortality (3.8% vs. 23.5%; P = 0.001) and HCC‐specific mortality (0.0% vs. 14.2%; P = 0.002) rates were lower in patients with steatotic HCC. Early (<2 years) recurrence was also less frequent (32.7% vs. 49.2%; P = 0.041). The mean time to intrahepatic distant recurrence (16.4 vs. 9 months, P = 0.006) and the median time to recurrence and recurrence‐free survival (32.4 vs. 18.6 months, P = 0.024 and 30.4 vs. 16.4 months, P = 0.018) were longer in patients with steatotic versus nonsteatotic HCC. The 3‐year overall survival was 94.4% and 70.9% in steatotic and nonsteatotic HCC (P = 0.008). In multivariate analysis, steatotic HCC (hazard ratio = 0.12; P = 0.039) and AFP (HR=1.002; P < 0.001) independently predicted overall survival. Conclusion: Small steatotic HCC detected by magnetic resonance imaging is associated with a less aggressive tumor phenotype. In patients with such radiological variant, percutaneous thermal ablation results in improved outcome.
Small steatotic HCC is reliably detected by MRI and is associated with slightly less advanced liver disease and a less aggressive tumor phenotype. PTA of such radiological HCC variant results in low rate of early recurrence and HCC‐related deaths, longer time‐to‐recurrence, longer recurrence‐free and overall survivals.
Abbreviations
- AFP
alpha‐fetoprotein
- ALBI
albumin‐bilirubin
- ASA
American Society of Anesthesiologists
- BMI
body mass index
- CI
confidence interval
- CT
computed tomography
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- IDR
intrahepatic distant recurrence
- IQR
interquartile range
- LTP
local tumor progression
- MELD
Model for End‐Stage Liver Disease
- MRI
magnetic resonance imaging
- NASH
nonalcoholic steatohepatitis
- NLR
neutrophil‐to‐lymphocyte ratio
- OS
overall survival
- PLR
platelet‐to‐lymphocyte ratio
- PTA
percutaneous thermal ablation
- RFS
recurrence‐free survival
- SH‐HCC
steatohepatitic HCC
- SII
systemic immune‐inflammation index
- TTR
time to recurrence
Liver cancer is the sixth most common cancer and the third leading cause of death from cancer worldwide.( 1 ) Hepatocellular carcinoma (HCC) represents 80%‐90% of all liver cancer types. For early‐stage HCC, percutaneous thermal ablation (PTA) is a validated treatment option, together with surgical resection and liver transplantation.( 2 ) Specifically, PTA has become the first‐line curative treatment of small (i.e., <3 cm) HCC due to its excellent tolerance, particularly in patients with portal hypertension or comorbidity.( 3 ) However, the rate of intrahepatic distant recurrence is high (i.e., 60%‐80%) after PTA of HCC,( 3 , 4 , 5 ) like after surgical resection.( 6 , 7 ) As HCC is a very heterogeneous cancer, both at the molecular and histological level, considerable efforts have been made to identify HCC subclasses based on histological features, molecular subgroups, genetic alterations, or oncogenic pathways, to guide tumor management.( 8 , 9 , 10 )
Among the different HCC histological variants, steatohepatitic HCC (SH‐HCC) was described by Salamao et al. in 2010( 11 ) and is defined by ballooning change, Mallory‐Denk bodies, and inflammatory infiltrate, in addition to intratumoral fat. A limited number of reports investigated the outcome of patients with SH‐HCC( 12 , 13 , 14 , 15 ) and exclusively after surgical resection. Only one showed a trend in favor of longer disease‐free survival,( 14 ) although SH‐HCC belongs to the non‐proliferative HCC subgroup.( 9 ) These HCC subclasses are determined by analysis of biopsy specimens. However, histological samples are rarely available in clinical practice because in patients with cirrhosis, HCC is primarily detected and diagnosed using noninvasive imaging criteria, according to the European Association for the Study of the Liver (EASL)/American Association for the Study of Liver Disease recommendations.( 2 )
Interestingly, SH‐HCC represents a subset of steatotic HCCs.( 12 ) The diagnosis of steatotic HCC, contrary to that of SH‐HCC, does not require any biopsy specimen and can be achieved noninvasively using chemical‐shift imaging, which is part of routine magnetic resonance imaging (MRI) protocols.( 16 , 17 ) We previously reported that steatotic HCC (among 32 covariates related to patient, liver disease, tumor, and technical factors) was surprisingly associated with improved overall survival (OS) after PTA.( 18 ) Comparative analyses between patients with small steatotic versus nonsteatotic HCC were yet to be conducted to provide much‐needed data to (1) elucidate the mechanisms by which the outcome of patients with small steatotic HCC might be improved and (2) determine whether the detection of such radiological variant might be helpful in treatment decision making.
Therefore, the objective of this study was to explore the baseline characteristics of patients with small steatotic HCC detected by MRI, their outcome, and tumor recurrence after PTA compared with patients with nonsteatotic HCC.
Materials and Methods
This retrospective analysis was performed using data from a prospective database of patients who underwent PTA for HCC at our institution. This study was approved by our institutional review board (NCT03428321 [www.clinicaltrials.gov]), and written informed consent for the procedure and the prospective anonymized data collection was obtained from all patients.
Inclusion criteria were HCC diagnosed by histopathology or by imaging criteria, no extrahepatic metastasis or macrovascular invasion, tumor size ≤30 mm, World Health Organization performance status 0 or 1, prothrombin time ratio >50%, and platelet count higher than 50 G/L.
Exclusion criteria were follow‐up <3 months, Child‐Pugh class ≥B8, HCC nodule adjacent to the hepatic hilum, history of biliary‐digestive anastomosis or endoscopic sphincterotomy, combined treatment with embolization or chemoembolization, and no baseline MRI.
Treatment was validated at a multidisciplinary meeting that included interventional radiologists, liver surgeons, oncologists, hepatologists, and radiation oncologists. PTA was considered as first‐line treatment for patients with Barcelona Clinic Liver Cancer (BCLC) 0‐A HCC.( 2 )
All patients underwent contrast‐enhanced dynamic MRI including chemical‐shift gradient‐echo imaging, within 1 month before PTA.
Patient and Tumor Data
The following patient data were collected: age, sex, American Society of Anesthesiologists physical status score (assessed by the anesthesiologist), body mass index (BMI), diabetes mellitus, liver disease (cirrhosis was defined as typical hepatic dysmorphia on imaging, or by noninvasive evaluation of fibrosis, or by histological analysis of liver biopsy; patients were considered noncirrhotic based on liver biopsy or noninvasive evaluation of fibrosis; the others were considered undetermined), steatosis (defined as signal intensity loss on opposed‐phase gradient‐echo sequences at baseline MRI), liver fat content,( 19 ) and Child‐Pugh, Model for End‐Stage Liver Disease (MELD), albumin‐bilirubin (ALBI), and AFP scores.
Steatotic HCC was defined by two radiologists (5 and 15 years of experience in liver imaging, respectively) when signal intensity loss was noted on opposed‐phase compared with in‐phase gradient‐echo images for at least one HCC nodule. HCC location also was recorded( 18 ): dome, subcapsular, adjacent to large vessels, or adjacent to at‐risk organs. The following inflammation‐based scores were calculated: platelet‐to‐lymphocyte ratio (PLR), neutrophil‐to‐lymphocyte ratio (NLR), lymphocyte‐to‐monocyte ratio (LMR), and systemic immune‐inflammation index (SII).( 20 )
Percutaneous Thermal Ablation
All procedures were done under general anesthesia with endotracheal intubation in a multimodality interventional suite. PTA was performed by four interventional radiologists (5‐15 years of expertise in liver PTA) using a radiofrequency or a microwave device, depending on the operator’s choice. Ultrasonography was the first‐line guidance modality, but computed tomography (CT) guidance was used after tumor tagging whenever necessary.( 18 ) Contrast‐enhanced CT (portal phase) was performed immediately after the procedure to evaluate the ablation zone (i.e., the area of low attenuation) and to detect postprocedural complications. In the case of incomplete ablation or insufficient margins, the ablation needle(s) was re‐inserted during the same procedure to achieve complete ablation.
Follow‐up and Outcome
Complications were recorded and classified according to the Society of Interventional Radiology (SIR) guidelines.( 21 ) Technical success was defined as complete ablation of the target tumor(s)( 21 ) on the immediate post‐PTA CT images.
Patients were monitored by MRI (including dynamic acquisitions) at week 4‐6 after PTA, and then every 3 months, and by chest CT scan every 6 months.
Complete ablation observed on the first follow‐up MRI was considered as the primary treatment success. Secondary treatment success was treatment success observed only after a second PTA performed within 8 weeks after the first one.
Local tumor progression (LTP) was defined as any growing or enhanced tumor focus within or at the edge (direct contact) of the ablation zone, after complete ablation documented by at least one MRI.( 22 ) Intrahepatic distant recurrence (IDR) was defined as any new HCC nodule in the liver, defined according to EASL criteria.( 22 )
Statistical Analysis
Normally distributed continuous variables were described using means ± SD, and nonnormally distributed continuous variables using medians and interquartile range (IQR). Categorical variables were compared with the Fischer’s exact test, and continuous variables with the two‐sided t‐test or Kruskal‐Wallis test, as appropriate.
Time to recurrence (TTR) was defined as the interval between PTA and death, recurrence, or last follow‐up.
Recurrence‐free survival (RFS) was defined as the time from PTA until the first recurrence, death, or last follow‐up. OS was defined as the interval between PTA and death (any cause) or last follow‐up. For TTR and survival analyses, patients who underwent liver transplantation were censored at transplantation date.
The median (and 95% confidence interval [CI]) follow‐up was calculated using the reverse Kaplan‐Meier method. Survival curves were estimated using the Kaplan‐Meier method and compared with the log‐rank test. Univariate and multivariate Cox proportional‐hazards models of all potential baseline predictors were built to compute the hazard ratios (HRs) with their 95% CI. A robust variance estimator was used systematically. Log linearity was checked using fractional polynomials.
All analyses were performed with the Stata software version 16.1 (Stata Corporation, College Station, TX). A P value < 0.05 was considered significant.
Results
Study Population
Between January 2015 and November 2019, 279 consecutive patients underwent PTA for HCC at our institution. Forty‐four patients were excluded due to (1) absence of baseline liver MRI (n = 27), (2) combined treatment with embolization (n = 9), (3) metastatic progression discovered at PTA day (n = 1), (4) HCC > 30 mm (n = 4), and (5) follow‐up <2 months (n = 3). Finally, 235 patients (median age of 65 years [IQR: 58‐72 years]; 79.2% [186 of 235] men) who underwent PTA of 419 small HCC nodules were included in this study. Cirrhosis was detected in 92.3% (217 of 235) of patients, primarily of alcoholic origin (55.7%). Nevertheless, liver function was well‐preserved, because 97% of patients with cirrhosis were classified as Child‐Pugh A (A5 = 83% and A6 = 14%) with a median MELD score of 8.8 (95% CI: 6‐13) and ALBI grade 1 or 2 in 65.9% and 34.1% of patients, respectively. All patients had an AFP score ≤2. Liver biopsy was obtained in 19.2% patients (Fig. 1).
FIG. 1.
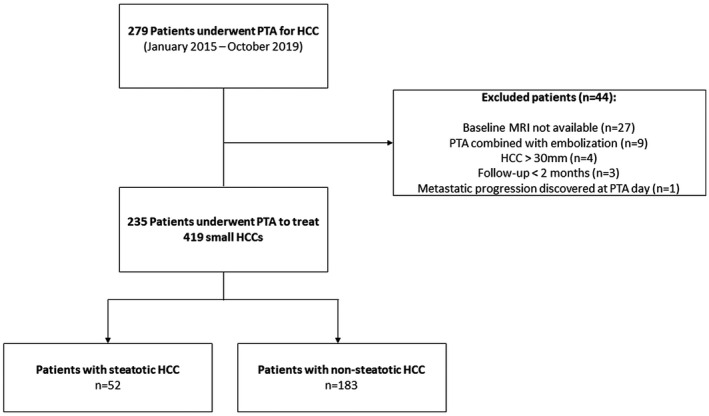
Study flowchart.
Baseline Characteristics of Patients With Steatotic and Nonsteatotic HCC
Both radiologists independently classified (100% interobserver agreement) 52 patients (22.1%) as having at least one steatotic HCC (Fig. 2), and 183 (77.9%) as without. Among the 6 patients with multifocal steatotic HCC, 3 (50%) presented fatty change in all HCC nodules. Nonalcoholic steatohepatitis (NASH) was more frequent in patients with than without steatotic HCC (21.2% vs. 10.4%; P = 0.057). Except for BMI, which did not differ between patients with and without steatotic HCC, metabolic conditions tended to be more frequent in the group with steatotic HCC group (diabetes mellitus: 46.2% vs. 38.8%, and liver steatosis: 40.4% vs. 32.8%, respectively; not significant). Liver fat content was also higher in patients with steatotic HCC (6% vs. 4.6%), but the difference was not significant (Table 1).
FIG. 2.
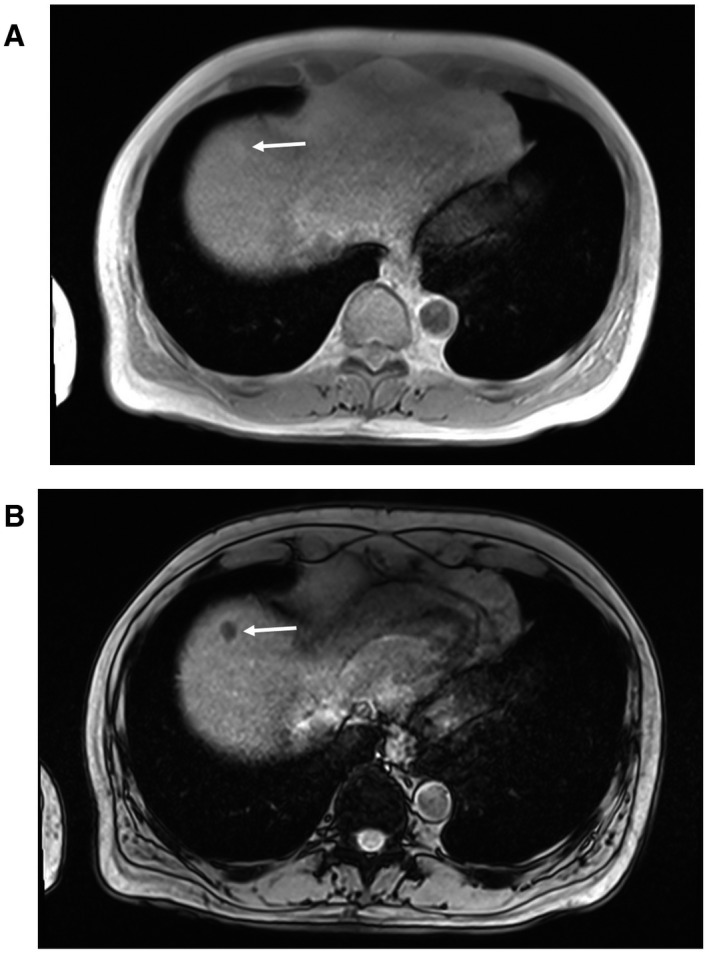
In‐phase (A) and opposed‐phase (B) chemical‐shift imaging showing signal intensity loss on the opposed‐phase in a typical steatotic HCC (white arrow).
Table 1.
Characteristics of Patients With Steatotic and Nonsteatotic Small HCC Nodules Treated by PTA
| Characteristic | Steatotic HCC | Nonsteatotic HCC | P Value |
|---|---|---|---|
| Patients | n = 52 | n = 183 | |
| Age, years (mean ± SD) | 65.3 ± 9.1 | 64.7 ± 9.9 | 0.69 |
| Sex (n [%]) | |||
| Male | 41 (78.9%) | 145 (79.2%) | 1 |
| Female | 11 (21.1%) | 38 (20.8%) | |
| ASA score (n [%]) | |||
| 1‐2 | 31 (59.6%) | 87 (47.5%) | 0.12 |
| 3‐4 | 21 (40.4%) | 96 (52.5%) | |
| Diabetes (n [%]) | |||
| No | 28 (53.8%) | 112 (61.2%) | 0.34 |
| Yes | 24 (46.2%) | 71 (38.8%) | |
| Metformin treatment (n [%]) | 10 (19.2%) | 32 (17.5%) | 0.77 |
| Statin treatment (n [%]) | 9 (17.3%) | 29 (15.8%) | 0.83 |
| BMI, kg/m2 (mean ± SD) | 27.8 ± 5.3 | 27.4 ± 5 | 0.56 |
| Prior treatment for HCC (n [%]) | |||
| Naive patient | 26 (50%) | 86 (47%) | 0.7 |
| Yes | 26 (50%) | 97 (53%) | |
| Liver disease | |||
| Cirrhosis (n [%]) | |||
| No | 3 (5.8%) | 15 (8.2%) | 0.56 |
| Yes | 49 (94.2%) | 168 (91.8%) | |
| Causes of liver disease (n [%]) | |||
| Alcohol (with or without viral hepatitis) | 28 (53.8%) | 103 (56.3%) | 0.24 |
| Viral hepatitis B or C | 10 (19.2%) | 51 (27.9%) | |
| Hemochromatosis | 3 (5.8%) | 8 (4.4%) | |
| NASH | 11 (21.2%) | 19 (10.4%) | |
| Unknown | 0 | 2 (0.9%) | |
| Steatosis (n [%]) | |||
| Absent | 31 (59.6%) | 121 (67.2%) | 0.31 |
| Present | 21 (40.4%) | 59 (32.8%) | |
| MRI quantification (%) | 6% ± 5.6 | 4.6% ± 4.3 | 0.12 |
| Child‐Pugh class | |||
| A5 | 46 (88.5%) | 149 (81.4%) | 0.3 |
| A6 | 4 (7.7%) | 29 (15.9%) | |
| B7 | 2 (3.8%) | 5 (2.7%) | |
| MELD score (mean ± SD) | 8.2 ± 1.9 | 9 ± 2.3 | 0.014 |
| Laboratory data (mean ± SD) | |||
| AFP (ng/mL) | 9.4 ± 13.5 | 44.7 ± 186.6 | 0.019 |
| AFP > 100 ng/mL (n [%]) | 0 | 13 (8.2%) | 0.044 |
| Total bilirubin (µmol/L) | 11.9 ± 5.6 | 14 ± 9.1 | 0.035 |
| Albumin (g/L) | 41.7 ± 5 | 39.9 ± 4.7 | 0.035 |
| Prothrombin activity (%) | 87.3 ± 11.1 | 84.4 ± 13.1 | 0.11 |
| AST (UI/mL) | 41 ± 38 | 38 ± 23 | 0.61 |
| ALT (UI/mL) | 34 ± 32 | 33 ± 31 | 0.77 |
| GGT (UI/mL) | 189 ± 204 | 155 ± 171 | 0.28 |
| Platelet count (103/mm3) | 139 ± 66 | 136 ± 74 | 0.76 |
| Neutrophils (103/mm3) | 3.65 ± 1.48 | 3.31 ± 1.32 | 0.15 |
| Lymphocytes (103/mm3) | 1.58 ± 0.77 | 1.62 ± 1.21 | 0.78 |
| Monocytes (103/mm3) | 0.51 ± 0.22 | 0.64 ± 0.22 | 0.34 |
| Creatinine (µmol/L) | 79.8 ± 24.9 | 83 ± 28.7 | 0.44 |
| ALBI score | |||
| 1 | 34 (69.4%) | 113 (64.9%) | 0.56 |
| 2 | 15 (30.6%) | 61 (35.1%) | |
| HCC | |||
| Tumor size (mean ± SD) | 16.3 ± 5 | 16.3 ± 5.5 | 0.98 |
| <20 mm | 44 (84.6%) | 149 (81.4%) | 0.6 |
| >20 mm | 8 (15.4%) | 34 (18.6%) | |
| No. of nodules per patient (n [%]) | |||
| 1 | 46 (88.5%) | 132 (72.1%) | 0.017 |
| >1 | 6 (11.5%) | 51 (27.9%) | |
| Dome location (n [%]) | 10 (19.2%) | 47 (25.7%) | 0.34 |
| Subcapsular location (n [%]) | 15 (28.9%) | 72 (39.3%) | 0.17 |
| Near large vessel (n [%]) | 16 (30.8%) | 37 (20.2%) | 0.11 |
| Near surrounding organ (n [%]) | 7 (10.6%) | 27 (7.7%) | 0.43 |
| PTA | |||
| PTA modality (n [%]) | |||
| Radiofrequency | 26 (50%) | 77 (42%) | 0.31 |
| Microwave | 26 (50%) | 106 (57.9%) | |
| Imaging guidance (n [%]) | |||
| Ultrasonography guidance | 33 (63.5%) | 93 (50.8%) | 0.11 |
| CT guidance | 19 (36.5%) | 90 (49.2%) |
Unless otherwise indicated, results are presented as numbers (percentages).
Significant P values are indicated in bold.
Abbreviations: ALT, alanine aminotransferase; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; GGT, gamma‐glutamyltransferase.
Liver disease was slightly less advanced in patients with than without steatotic HCC: Baseline total bilirubin concentration was lower (−2.1 µmol/L; P = 0.035); albumin concentration was higher (+0.8 g/L; P = 0.035); and MELD score was lower (0.8; P = 0.014) in patients with steatotic HCC. These data also significantly differed in a per‐tumor comparison (data not shown).
Tumor phenotype differed significantly between groups. AFP concentration was lower (P = 0.019), and AFP > 100 ng/mL (P = 0.045) and multifocality were less frequent (P = 0.015) in patients with steatotic HCC, whereas tumor size did not differ between groups. Similar results were obtained in a per‐tumor comparison (data not shown). Finally, PTA was more frequently performed under ultrasonography guidance (vs. CT, P = 0.01) and using radiofrequency (vs. microwave ablation, P = 0.02) in patients with steatotic HCCs.
None of the inflammation‐based scores (NLR, PLR, LMR, and SII) differed significantly in patients with and without steatotic HCCs (Table 2).
Table 2.
Inflammation‐Based Scores in Patients with Steatotic and Nonsteatotic HCC
| Scores | Steatotic HCC (n = 52 patients) | Nonsteatotic HCC (n = 183 patients) | P Value |
|---|---|---|---|
| NLR | 2.91 ± 1.85 | 2.53 ± 1.27 | 0.18 |
| PLR | 102 ± 50.2 | 99.3 ± 58.2 | 0.74 |
| LMR | 3.25 ± 1.53 | 3.18 ± 2.63 | 0.82 |
| SII | 364.7 ± 223.3 | 329.5 ± 251.9 | 0.34 |
Significant P values are indicated in bold.
Follow‐up and Outcome (Table 3)
Technical success was 100% in both groups. Primary treatment success was obtained in 100% of patients with steatotic and 98.9% of patients with nonsteatotic HCC (P = 0.9), whereas secondary treatment success was 100% in both groups.
Complications (SIR grade B/C) were observed after 3% and 3.7% of PTA in patients with and without steatotic HCC, respectively (P = 1). No PTA‐related death, needle track seeding, or liver abscess was recorded.
The median follow‐up of the whole cohort was 28.3 months (95% CI: 25.6‐33.4) and did not differ between patients with and without steatotic HCC (median: 27.5 and 29.8 months; P = 0.59). Twenty‐six (11.1%) patients underwent liver transplantation. In 12 (46.2%) patients, PTA was performed in a context of bridge to transplant. The number of transplanted patients did not differ between groups (P = 0.9).
During the follow‐up, 3.8% (2 of 52) and 23.5% (43 of 183) of patients with and without steatotic HCC died (P = 0.001). HCC‐specific mortality also was much lower in patients with steatotic HCC (0 vs. 14.2% [26 of 183], P = 0.002).
Overall, recurrence was less frequent in patients with than without steatotic HCC (42.3 vs. 54.6%), but the difference was not significant (P = 0.12). Conversely, early (i.e., <2 years) recurrences were significantly less frequent in patients with steatotic HCC (32.7% vs. 49.2%, P = 0.041).
LTP and IDR tended to be less frequent in patients with than without steatotic HCC (7.6% vs. 15.9%, P = 0.12; and 40.4% vs. 49.2%, P = 0.27). The mean time to IDR was significantly longer in patients with steatotic than nonsteatotic HCCs (16.4 ± 13.1 months vs. 9 ± 8.7 months, P = 0.006).
The first distant recurrence was beyond the Milan criteria in 5.8% of patients with steatotic HCC and in 8.5% of patients without steatotic HCC (P = 0.77). In these patients, the median time to recurrence was 10.5 months (IQR: 8.1‐32.4) and 5.1 months (IQR: 3.5‐12.2), respectively. At first IDR, the AFP score was > 2 in 1.9% and 3.3% of patients with and without steatotic HCC (P = 1), respectively.
IDR was treated by a new curative approach in 72.7% and 56.7% of patients with and without steatotic HCC (P = 0.23). During the follow‐up, extrahepatic metastases were detected in 1 (1.9%) and 17 (9.3%) patients with and without steatotic HCC (P = 0.12) (Table 3).
Table 3.
Follow‐up and Outcome After PTA in 235 Patients With Steatotic (n = 52) and Nonsteatotic HCCs (n = 183) Small HCC Nodules
| Steatotic HCC | Non‐steatotic HCC | P Value | |
|---|---|---|---|
| Follow‐up (median, 95% CI) | 27.5 months (20.6‐34.8) | 29.1 months (25.2‐33.6) | 0.54 |
| Liver transplant (n [%]) | 6 (11.5%) | 20 (10.9%) | 0.9 |
| Death, all causes (n [%]) | 2 (3.8%) | 43 (23.5%) | 0.001 |
| HCC‐related death (n [%]) | 0 | 26 (14.2%) | 0.002 |
| Death from liver cause, except HCC (n [%]) | 2 (3.8%) | 8 (4.4%) | 0.62 |
| Overall recurrence (n [%]) | 22 (42.3%) | 100 (54.6%) | 0.16 |
| Early (<2 years) recurrence (n [%]) | 17 (32.7%) | 90 (49.2%) | 0.041 |
| First distant recurrence beyond Milan criteria (n [%]) | 3 (5.8%) | 17 (8.5%) | 0.58 |
| LTP, per tumor | |||
| Occurrence (n [%]) | 5 (7.6%) | 55 (15.5%) | 0.12 |
| Time to LTP (mean ± SD) | 18.9 ± 12 months | 12.1 ± 8.7 months | 0.18 |
| IDR, per patient | |||
| Occurrence (%) | 21 (40.4%) | 90 (49.2%) | 0.27 |
| Time to IDR (mean ± SD) | 16.4 ± 13.1 months | 9 ± 8.7 months | 0.006 |
| Largest HCC nodule (mean ± SD) | 18 ± 7 mm | 17 ± 12 mm | 0.81 |
| AFP score > 2 at first IDR (n [%]) | 1 (1.9%) | 6 (3.3%) | 1 |
| Curative treatment of IDR | 16 (72.7%) | 51 (56.7%) | 0.23 |
| Extrahepatic recurrence (n [%]) | 1 (1.9%) | 17 (9.3%) | 0.13 |
Unless otherwise indicated, data are presented per patient.
Significant P values are indicated in bold.
TTR, RFS, and OS
The 3‐year cumulative recurrence rate was 40.4% and 49.7% in patients with and without steatotic HCC. TTR was longer in patients with steatotic (median: 32.4 months; 95% CI: 17.1‐not reached) than without steatotic HCC (median: 18.6 months; 95% CI: 12‐23.6]) (HR = 0.6 [95% CI: 0.39‐0.94], P = 0.024), but in multivariate analysis, steatotic HCC was not independently associated with TTR.
The 3‐year RFS was 44.6% and 30.2% in patients with and without steatotic HCC. RFS was longer in patients with steatotic (median: 30.4 months [95% CI: 14.6‐not reached) than without steatotic HCC (median: 16.4 months [95% CI: 11.4‐21.1]) (HR = 0.6, P = 0.018), but in multivariate analysis, steatotic HCC was not independently associated with RFS.
The 3‐year OS was 94.4% (95% CI: 79.2%‐98.6%) and 70.9% (95% CI: 61.3%‐78.6%) in patients with and without steatotic HCC. OS was longer in patients with than without steatotic HCC (HR = 0.14 [95% CI: 0.03‐0.61], P = 0.008). In multivariate analysis, only steatotic HCC (HR = 0.12; P = 0.039) and AFP (HR = 1.002; P < 0.001) independently predicted OS (Fig. 3 and Table 4).
FIG. 3.
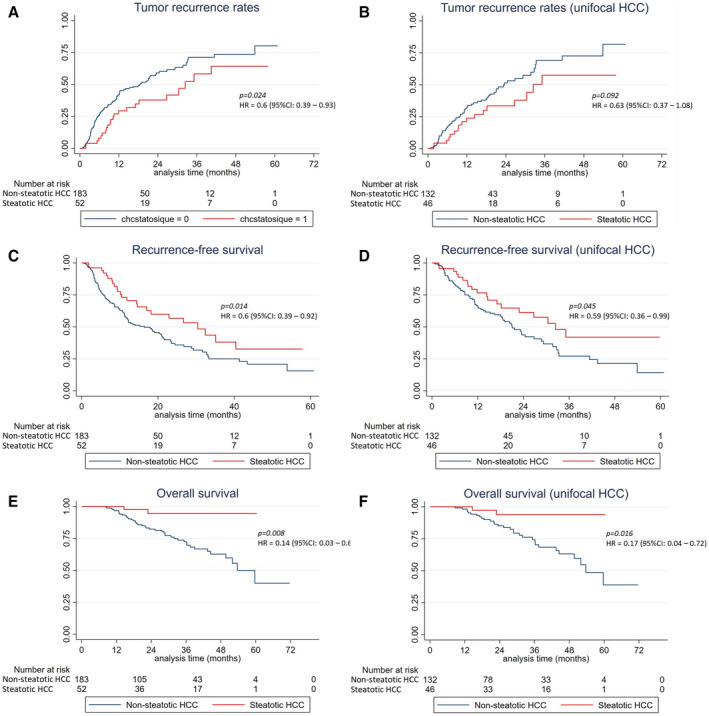
TTR (A), RFS (B), and OS (C) after PTA in patients with small steatotic and nonsteatotic HCCs.
Table 4.
Univariate and Multivariate Cox Regression Models to Predict OS (per Patient Analysis)
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Patients | ||||
| Age | ||||
| Sex female vs. male | 0.70 (0.32‐1.54) | 0.38 | ||
| ASA (>2 vs. ≤2) | 2.13 (1.17‐3.9) | 0.014 | 1.92 (0.88‐4.17) | 0.1 |
| Diabetes | 1.36 (0.76‐2.42) | 0.3 | ||
| Metformin treatment | 0.99 (0.43‐2.3) | 0.98 | ||
| Statin treatment | 0.51 (0.18‐1.42) | 0.19 | ||
| Treatment‐naive patient | 0.59 (0.31‐1.09) | 0.09 | ||
| Local recurrence | 0.94 (0.49‐1.81) | 0.85 | ||
| IDR | 1.83 (0.96‐3.47) | 0.07 | ||
| Cirrhosis | 0.8 (0.33‐1.94) | 0.62 | ||
| Child‐Pugh (B vs. A) | 0.72 (0.08‐6.63) | 0.78 | ||
| Cause of liver disease (vs. alcohol) | ||||
| Viral hepatitis B or C | 0.91 (0.46‐1.8) | 0.79 | ||
| Hemochromatosis | 0.38 (0.05‐2.6) | 0.32 | ||
| Others (including NASH) | 0.56 (0.2‐1.52) | 0.25 | ||
| Steatosis | 0.76 (0.41‐1.4) | 0.38 | ||
| AFP ≥ 100 vs. <100 ng/mL | 4.71 (2.03‐10.9) | <0.001 | ||
| AFP (per unit) | 1.002 (1.001‐1.003) | <0.001 | 1.002 (1.001‐1.003) | <0.001 |
| MELD (>9 vs. ≤9) | 2.59 (1.44‐4.67) | 0.001 | 2.01 (0.95‐4.26) | 0.07 |
| Tumor size (per mm) | 1.01 (0.96‐1.06) | 0.73 | ||
| Tumor size <20 mm | 1.48 (0.65‐3.33) | 0.35 | ||
| Nb. of HCC (1 vs. >1) | 1.52 (0.78‐2.96) | 0.22 | ||
| Steatotic HCC | 0.14 (0.03‐0.61) | 0.008 | 0.12 (0.02‐0.9) | 0.039 |
| Dome tumor | 0.79 (0.39‐1.63) | 0.53 | ||
| Subcapsular | 1.58 (0.89‐2.81) | 0.12 | ||
| Near large vessel | 0.95 (0.49‐1.84) | 0.88 | ||
| Near surrounding organ | 0.53 (0.17‐1.63) | 0.27 | ||
| PTA modality: MWA vs. RF | 1.54 (0.84‐2.8) | 0.16 | ||
| US vs. CT guidance | 0.76 (0.42‐1.37) | 0.36 | ||
Otherwise indicated, numeric predictors are investigated per unit.
Significant P values are indicated in bold.
Abbreviations: MWA, microwave ablation; RF, radiofrequency; US, ultrasonography.
Discussion
This study compares the baseline characteristics of patients with small steatotic versus nonsteatotic HCCs, and their outcome after PTA. We confirm that steatotic HCC is an independent predictor of improved OS in patients treated by PTA for early‐stage tumors. In our Western population, liver disease was slightly less advanced in patients with steatotic HCC (mean difference between groups: +0.8 g/L [albumin], −2.1 µmol/L [bilirubin], −0.8 point [MELD score]). Although these differences were significant, it is very unlikely that the underlying liver disease explains the survival benefit. Indeed, PTA was performed in patients with BCLC 0‐A HCC and well‐compensated cirrhosis in nearly all cases (97% were Child‐Pugh A), and the number of deaths from liver cause (outside HCC) was similar in both groups (3.8% in steatotic vs. 4.4% in nonsteatotic HCC). On the other hand, despite a comparable follow‐up, no death from HCC was reported in the group with steatotic HCC, whereas it concerned 14.2% of patients with nonsteatotic HCC. Both TTR and RFS were also longer in patients with steatotic HCC (median of 32.4 vs. 18.6 months and 30.4 vs. 16.4 months, respectively).
Early (<2 years) tumor relapse is primarily related to metastatic spread.( 7 ) The lower rate of early recurrence (32.7% vs. 49.2%; P = 0.041) and the longer time to IDR (16.4 vs. 9 months; P = 0.006) in patients with steatotic HCC strongly pleads for slower metastatic escape in this group.
Limited data are available on steatotic HCC.( 12 , 23 ) In these studies, fatty change was detected by pathology analysis in approximately 20% of patients with HCC treated by surgical resection. We observed a similar rate (22.1%) in our series based on noninvasive MR diagnosis. The perfect interobserver agreement is not surprising, as intratumoral fat deposition can be detected by chemical‐shift MRI with 100% specificity,( 16 , 17 ) due to the difference in resonance frequency between water and fat protons. In a large series (516 patients), Chan et al.( 12 ) showed that steatotic HCC was associated with diabetes mellitus (36.7% vs. 22.1% in patients without steatotic HCC). Our European population with high prevalence of alcohol‐related cirrhosis and high rate of diabetes mellitus strongly differs from the Chinese patients with high hepatitis B virus infection rate of this previous study.( 12 ) In addition, our study only included small HCCs, whereas Chan et al. retrospectively collected data on patients undergoing curative surgery basically for larger tumors.
More data are available on SH‐HCC, which was described by Salamao et al. in 2010.( 11 ) SH‐HCC should be regarded as a subset (57.8% in Chan et al( 12 )) of steatotic HCC, in which—besides intratumoral fat—ballooning changes, Mallory‐Denk bodies, and intratumoral inflammatory infiltrate are present. In most reports, SH‐HCC has been associated with underlying fatty liver, steatohepatitis, diabetes mellitus, and generally with metabolic syndrome risk.( 11 , 12 , 13 , 14 , 24 ) The association between SH‐HCC and metabolic syndrome risk factors or liver steatosis( 11 , 12 , 13 , 14 , 24 , 25 , 26 ) led us to consider that in HCC, the presence of steatohepatitic features is due to a global liver response (seen in both benign and malignant tissue) to the metabolic syndrome.( 27 ) However, this hypothesis has been called into question, because HCC can also develop steatohepatitic morphology outside the setting of fatty liver disease or metabolic syndrome,( 28 ) possibly related to genetic alterations inherent to HCC itself. In agreement, recurrent loss of chromosome 9q12‐q31 has been reported in a subset of SH‐HCC, but not in NASH‐associated HCC.( 28 ) Although none of the metabolic factors (BMI, diabetes mellitus, metformin consumption, liver steatosis) investigated in our series was significantly associated with steatotic HCC, most of them were more frequent in this subgroup, including NASH (21.2% vs. 10.4%, P = 0.057). Inflammation is involved in NASH and SH‐HCC, and is recognized as a cancer hallmark. The host inflammatory response is associated with cancer progression and patient survival.( 29 ) The NLR, PLR, and SII inflammation‐based prognostic scores are predictors of early HCC recurrence after surgical resection.( 30 , 31 , 32 ) SII also independently predicts post‐recurrence survival.( 20 ) In our study, these inflammation‐based scores were comparable in patients with and without non‐steatotic HCC, suggesting that inflammation does not play a major role in the occurrence of steatotic HCC and in their improved outcome.
In surgical studies, steatotic HCCs are more frequently well‐differentiated,( 23 ) associated with lower serum AFP level, earlier stage, and lower frequency of major vessel and microvascular invasion.( 12 ) Similarly, in our series, tumor phenotype was less aggressive in patients with small steatotic HCC: Serum AFP concentration was significantly lower (no patient with AFP > 100 ng/mL in this group), and multifocality was less frequent. In studies on SH‐HCC outcome after resection, no difference was noted in terms of OS, development of metastatic disease or local recurrence,( 12 , 13 , 14 , 15 ) except in one that found improved disease‐free survival for typical SH‐HCC.( 14 ) Conversely, in our series of small tumors treated by PTA, steatotic HCC was associated with improved outcome. Studies on PTA for early‐stage HCC usually report LTP rates between 10% and 30%, and 3‐year cumulative recurrence, RFS, and OS rates of 51%‐57%, 29%‐40%, and 60%‐83%, respectively.( 3 , 5 , 33 , 34 , 35 , 36 , 37 ) In our study, patients with small steatotic HCC showed excellent oncological outcome (LTP rate = 7.6%, and 3‐year cumulative recurrence, RFS, and OS = 40.4%, 44.6%, and 94.4%, respectively). These results suggest that focus should be redirected toward steatotic HCC rather than SH‐HCC, especially because steatotic HCC can be noninvasively diagnosed by MRI in routine practice.
It remains quite uncommon to discover a predictive factor of improved outcome. Indeed, the commonly used predictors (macrotrabecular‐massive histological subtype,( 10 ) AFP,( 5 , 14 , 18 , 36 , 37 , 38 ) and multifocality( 18 , 34 , 38 )) are associated with poor prognosis. Outside liver transplantation, in which some of them are used to exclude poor candidates, they are of limited interest in clinical practice due to the absence of any validated neo‐/adjuvant treatment. On the other hand, the radiological detection of steatotic HCC as a predictor of good outcome could help in treatment decision making. In early‐stage steatotic HCC, PTA is certainly the right treatment option, given the improved outcome of patients, its low complication rate, and improved cost‐effectiveness compared with resection.( 39 ) In these patients with small steatotic HCC and well‐compensated cirrhosis, liver transplant might be unnecessary or could be kept as a salvage therapy. Indeed, at first recurrence, the risk of recurrence outside the Milan criteria was very low in steatotic HCC (5.8%), compared with the 12.6% risk commonly reported in <3 cm HCC.( 33 ) Additionally, at first recurrence, the AFP score was > 2 only in 1.9% of patients with steatotic HCC.
Several limitations to our study must be acknowledged. First, this is a retrospective, monocentric study, although data came from a prospectively maintained database with a strict imaging follow‐up policy. Second, the median follow‐up was relatively short (28.3 months). Finally, like in most HCC studies, the very low percentage of patients with liver biopsy did not allow exploring histological/molecular/genetic features of steatotic HCC. In this context, the SH‐HCC subtype is associated with overexpression of C‐reactive protein, and lack of satellite nodules and vascular invasion.( 9 , 40 ) These tumors belong to the G4 subclass with more favorable prognosis.( 9 ) Genetically, SH‐HCCs very rarely harbor activated Wnt/beta‐catenin pathway, and they lack CTNNB1, TP53, and TERT promoter mutations.( 9 , 40 ) It is certainly worth exploring molecular and genetic pathways in the wider subgroup of steatotic HCCs.
In conclusion, small steatotic HCC detected by MRI is associated with a less aggressive tumor phenotype. In patients with such radiological variant, PTA results in improved outcome and might be the treatment of choice.
Acknowledgment
The authors thank Elisabetta Andermarcher and Sandrine Guiu for revising the English.
Potential conflict of interest: Nothing to report.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. EASL Clinical Practice Guidelines . Management of hepatocellular carcinoma. J Hepatol 2018;69:182‐236. [DOI] [PubMed] [Google Scholar]
- 3. Nault JC, Sutter O, Nahon P, Ganne‐Carrie N, Seror O. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol 2018;68:783‐797. [DOI] [PubMed] [Google Scholar]
- 4. Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008;47:82‐89. [DOI] [PubMed] [Google Scholar]
- 5. N'Kontchou G, Mahamoudi A, Aout M, Ganne‐Carrié N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: long‐term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology (Baltimore, MD) 2009;50:1475‐1483. [DOI] [PubMed] [Google Scholar]
- 6. Liver. EAftSot . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182‐236. [DOI] [PubMed] [Google Scholar]
- 7. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200‐207. [DOI] [PubMed] [Google Scholar]
- 8. Calderaro J, Couchy G, Imbeaud S, Amaddeo G, Letouzé E, Blanc J‐F, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol 2017;67:727‐738. [DOI] [PubMed] [Google Scholar]
- 9. Calderaro J, Ziol M, Paradis V, Zucman‐Rossi J. Molecular and histological correlations in liver cancer. J Hepatol 2019;71:616‐630. [DOI] [PubMed] [Google Scholar]
- 10. Ziol M, Poté N, Amaddeo G, Laurent A, Nault J‐C, Oberti F, et al. Macrotrabecular‐massive hepatocellular carcinoma: a distinctive histological subtype with clinical relevance. Hepatology 2018;68:103‐112. [DOI] [PubMed] [Google Scholar]
- 11. Salomao M, Yu WM, Brown RS, Emond JC, Lefkowitch JH. Steatohepatitic hepatocellular carcinoma (SH‐HCC): a distinctive histological variant of HCC in hepatitis C virus‐related cirrhosis with associated NAFLD/NASH. Am J Surgical Pathol 2010;34:1630‐1636. [DOI] [PubMed] [Google Scholar]
- 12. Chan AWH, Yu S, Yu Y‐H, Tong JHM, Wang L, Tin EKY, et al. Steatotic hepatocellular carcinoma: a variant associated with metabolic factors and late tumour relapse. Histopathology 2016;69:971‐984. [DOI] [PubMed] [Google Scholar]
- 13. Salomao M, Remotti H, Vaughan R, Siegel AB, Lefkowitch JH, Moreira RK. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol 2012;43:737‐746. [DOI] [PubMed] [Google Scholar]
- 14. Shibahara J, Ando S, Sakamoto Y, Kokudo N, Fukayama M. Hepatocellular carcinoma with steatohepatitic features: a clinicopathological study of Japanese patients. Histopathology 2014;64:951‐962. [DOI] [PubMed] [Google Scholar]
- 15. Lee JS, Yoo JE, Kim H, Rhee H, Koh MJ, Nahm JH, et al. Tumor stroma with senescence‐associated secretory phenotype in steatohepatitic hepatocellular carcinoma. PLoS One 2017;12:e0171922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guiu B, Petit JM, Loffroy R, Ben Salem D, Aho S, Masson D, et al. Quantification of liver fat content: comparison of triple‐echo chemical shift gradient‐echo imaging and in vivo proton MR spectroscopy. Radiology 2009;250:95‐102. [DOI] [PubMed] [Google Scholar]
- 17. Min JH, Kim YK, Lim S, Jeong WK, Choi D, Lee WJ. Prediction of microvascular invasion of hepatocellular carcinomas with gadoxetic acid‐enhanced MR imaging: impact of intra‐tumoral fat detected on chemical‐shift images. Eur J Radiol 2015;84:1036‐1043. [DOI] [PubMed] [Google Scholar]
- 18. Hermida M, Cassinotto C, Piron L, Aho‐Glele S, Guillot C, Schembri V, et al. Multimodal percutaneous thermal ablation of small hepatocellular carcinoma: predictive factors of recurrence and survival in Western patients. Cancers (Basel) 2020;12:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guiu B, Petit J‐M, Loffroy R, Ben Salem D, Aho S, Masson D, et al. Quantification of liver fat content: comparison of triple‐echo chemical shift gradient‐echo imaging and in vivo proton MR spectroscopy. Radiology 2009;250:95‐102. [DOI] [PubMed] [Google Scholar]
- 20. Wang C, He W, Yuan Y, Zhang Y, Li K, Zou R, et al. Comparison of the prognostic value of inflammation‐based scores in early recurrent hepatocellular carcinoma after hepatectomy. Liver Int 2020;40:229‐239. [DOI] [PubMed] [Google Scholar]
- 21. Cardella JF, Kundu S, Miller DL, Millward SF, Sacks D, Society of Interventional Radiology . Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2009;20:S189‐S191. [DOI] [PubMed] [Google Scholar]
- 22. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image‐guided tumor ablation: standardization of terminology and reporting criteria—a 10‐year update. J Vasc Interv Radiol 2014;25:1691‐1705.e1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kutami R, Nakashima Y, Nakashima O, Shiota K, Kojiro M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J Hepatol 2000;33:282‐289. [DOI] [PubMed] [Google Scholar]
- 24. Jain D, Nayak NC, Kumaran V, Saigal S. Steatohepatitic hepatocellular carcinoma, a morphologic indicator of associated metabolic risk factors: a study from India. Arch Pathol Lab Med 2013;137:961‐966. [DOI] [PubMed] [Google Scholar]
- 25. Alexander J, Torbenson M, Wu TT, Yeh MM. Non‐alcoholic fatty liver disease contributes to hepatocarcinogenesis in non‐cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol 2013;28:848‐854. [DOI] [PubMed] [Google Scholar]
- 26. Taniai M, Hashimoto E, Tobari M, Kodama K, Tokushige K, Yamamoto M, et al. Clinicopathological investigation of steatohepatitic hepatocellular carcinoma: a multicenter study using immunohistochemical analysis of adenoma‐related markers. Hepatol Res 2018;48:947‐955. [DOI] [PubMed] [Google Scholar]
- 27. Olofson AM, Gonzalo DH, Chang M, Liu X. Steatohepatitic variant of hepatocellular carcinoma: a focused review. Gastroenterology Res 2018;11:391‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yeh MM, Liu Y, Torbenson M. Steatohepatitic variant of hepatocellular carcinoma in the absence of metabolic syndrome or background steatosis: a clinical, pathological, and genetic study. Hum Pathol 2015;46:1769‐1775. [DOI] [PubMed] [Google Scholar]
- 29. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493‐e503. [DOI] [PubMed] [Google Scholar]
- 30. Chua DW, Koh YX, Liew YX, Chan C‐Y, Lee S‐Y, Cheow P‐C, et al. Pre‐operative predictors of early recurrence/mortality including the role of inflammatory indices in patients undergoing partial hepatectomy for spontaneously ruptured hepatocellular carcinoma. J Surg Oncol 2018;118:1227‐1236. [DOI] [PubMed] [Google Scholar]
- 31. Hu B, Yang X‐R, Xu Y, Sun Y‐F, Sun C, Guo W, et al. Systemic immune‐inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212‐6222. [DOI] [PubMed] [Google Scholar]
- 32. Wang B‐L, Tian LU, Gao X‐H, Ma X‐L, Wu J, Zhang C‐Y, et al. Dynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection. Clin Chem Lab Med 2016;54:1963‐1969. [DOI] [PubMed] [Google Scholar]
- 33. Doyle A, Gorgen A, Muaddi H, Aravinthan AD, Issachar A, Mironov O, et al. Outcomes of radiofrequency ablation as first‐line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J Hepatol 2019;70:866‐873. [DOI] [PubMed] [Google Scholar]
- 34. Hocquelet A, Balageas P, Laurent C, Blanc J‐F, Frulio N, Salut C, et al. Radiofrequency ablation versus surgical resection for hepatocellular carcinoma within the Milan criteria: a study of 281 Western patients. Int J Hyperthermia 2015;31:749‐757. [DOI] [PubMed] [Google Scholar]
- 35. Kim Y‐S, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten‐year outcomes of percutaneous radiofrequency ablation as first‐line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol 2013;58:89‐97. [DOI] [PubMed] [Google Scholar]
- 36. Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, et al. Radiofrequency ablation of hepatocellular carcinoma as first‐line treatment: long‐term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014;270:900‐909. [DOI] [PubMed] [Google Scholar]
- 37. Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long‐term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma </=3 cm. Results of a multicenter Italian survey. J Hepatol 2013;59:89‐97. [DOI] [PubMed] [Google Scholar]
- 38. Hasegawa K, Kokudo N, Makuuchi M, Izumi N, Ichida T, Kudo M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol 2013;58:724‐729. [DOI] [PubMed] [Google Scholar]
- 39. Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, et al. Cost‐effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013;59:300‐307. [DOI] [PubMed] [Google Scholar]
- 40. Nault J‐C, Martin Y, Caruso S, Hirsch TZ, Bayard Q, Calderaro J, et al. Clinical impact of genomic diversity from early to advanced hepatocellular carcinoma. Hepatology 2020;71:164‐182. [DOI] [PubMed] [Google Scholar]


