Abstract
Identifying patients at higher risk for poor outcomes from nonalcoholic fatty liver disease (NAFLD) remains challenging. Metabolomics, the comprehensive measurement of small molecules in biological samples, has the potential to reveal novel noninvasive biomarkers. The aim of this study was to determine if serum metabolite profiles in patients with NAFLD associate with future liver‐related events. We performed a retrospective single‐center cohort study of 187 participants with biopsy‐proven NAFLD. Metabolomic analysis was performed on serum using ultrahigh performance liquid chromatography/tandem mass spectrometry and gas chromatography/mass spectrometry. We identified liver‐related events (variceal bleeding, ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, hepatocellular carcinoma, hepatopulmonary or hepatorenal syndrome) by manual chart review between index biopsy (2007‐2013) and April 1, 2018. Generalized linear models and Cox proportional hazards models were used to test the association of metabolites with liver‐related events and time to first liver‐related event, controlling for covariates and fibrosis stage. Over a mean ± SD follow‐up of 6.9 ± 3.2 years, 11 participants experienced 22 liver‐related events. Generalized linear models revealed 53 metabolites significantly associated with liver‐related events (P < 0.05). In Cox proportional hazards modeling, 69 metabolites were significantly associated with time to future liver‐related events (P < 0.05), seven of which met the false discovery rate threshold of 0.10: vitamin E metabolites gamma‐carboxyethyl‐hydroxychroman (gamma‐CEHC) and gamma‐CEHC glucuronide; primary bile acid metabolite taurochenodeoxycholate; serotonin metabolite 5‐hydroxyindoleacetate; and lipid metabolites (i) 2‐hydroxyglutarate, (ii) 3beta,17beta‐diol disulfate 1, and (iii) eicosenoyl sphingomyelin. Conclusion: Metabolites of a primary bile acid, vitamin E, and serotonin were associated with future liver‐related events. Our results suggest metabolite pathways may be useful for predicting which patients with NAFLD are at higher risk for hepatic decompensation.

Abbreviations
- 5‐HIAA
5‐hydroxyindoleacetic acid
- BMI
body mass index
- DHEA
dehydroepiandrosterone
- FDR
false discovery rate
- FIB‐4
fibrosis‐4
- gamma‐CEHC
gamma‐carboxyethyl‐hydroxychroman
- GC
gas chromatography
- MS/MS
tandem mass spectrometry
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TCDCA
taurochenodeoxycholate
It is estimated that one quarter of the world’s population has nonalcoholic fatty liver disease (NAFLD), making it a major source of liver‐related morbidity and mortality and a leading indication for liver transplantation.( 1 , 2 ) The prevalence of NAFLD is projected to increase, paralleling the global rise in obesity, and will result in increased liver‐related morbidity and mortality.( 3 ) Among adults with NAFLD in the United States, complications of cirrhosis are the leading cause of death.( 4 )
At present, staging NAFLD, which encompasses nonalcoholic fatty liver (NAFL; simple steatosis) and nonalcoholic steatohepatitis (NASH; the more severe form of NAFLD), is challenging. The gold standard for quantifying inflammation and fibrosis remains a liver biopsy, an invasive test that comes with risks of bleeding and infection. About one quarter of patients with NASH will progress to cirrhosis, and among those with cirrhosis, about 5% per year develop clinical decompensation. Fibrosis stage is a strong predictor of overall clinical outcomes, liver‐related decompensation, and cardiovascular events.( 5 , 6 ) However, beyond the fibrosis stage, it remains unknown which specific patients with NAFLD are at high risk for clinical progression and subsequent decompensation. As such, improving noninvasive biomarkers to diagnose, stage, or risk stratify NAFLD is essential. Biomarkers would potentially identify patients needing intensive monitoring or screening, identify key dysregulated pathways associated with specific outcomes of disease, and promote personalized treatment given the possibility of U.S. Food and Drug Administration‐approved drugs for NAFLD in the near future.
Metabolomics, the identification of small molecules or metabolites, holds promise in NAFLD biomarker development. Metabolites point to pathways implicated in NAFLD pathogenesis, potentially identifying promising drug targets. Lipid metabolism has been of interest given that the hallmark feature of NAFLD is abnormal fat accumulation in the liver. Studies have found that triglyceride levels( 7 , 8 ) and polyunsaturated fatty acids( 9 ) are able to distinguish NAFL from NASH. Phospholipid metabolism appears to be altered in mouse models of NASH.( 10 ) Bile acids are increasingly recognized as signaling molecules with pleiotropic effects on liver physiology, and bile acid metabolites may be future NAFLD biomarkers. In one study, participants with NAFLD had higher serum levels of glycocholate, taurocholate, and glycochenodeoxycholate compared to healthy controls.( 11 )
Amino acid metabolism has also been found to differ across the NAFLD spectrum; this has been measured both in serum( 12 ) and in urine.( 13 ) Vitamin and nutrient metabolism has been of interest based on the clinical observation that low vitamin D levels are associated with NAFLD.( 14 ) Levels of beta‐carotene and retinol were associated with histologic severity in one study,( 15 ) while another identified an association between serum homocysteine (a choline metabolite) and histologic severity in NAFLD.( 16 )
A recent large cross‐sectional study from a prospective cohort of 156 participants with biopsy‐proven NAFLD identified a 10‐metabolite signature that outperformed the fibrosis‐4 (FIB‐4) score and NAFLD fibrosis score in predicting advanced fibrosis in two separate validation cohorts. The signature included eight lipids (six of which were cholesterol‐derived steroid hormones), one amino acid (taurine), and one carbohydrate (fucose). In total, 32 metabolites were significantly associated with advanced fibrosis.( 17 )
Although the majority of proposed NAFLD biomarkers have been studied using cross‐sectional histology (including studies of progression evaluating individuals with different stages of NAFLD cross‐sectionally), few efforts have been made to study NAFLD progression in a longitudinal manner. One study from China found that serum sex hormone‐binding globulin levels were independently associated with NAFLD development and regression.( 18 ) However, detailed metabolomic profiling of patients with NAFLD who experience clinical progression has not been performed.
Metabolomic studies in NAFLD to date have been limited by small sample sizes, and many relied on clinical or imaging diagnosis of NAFL or NASH. Here, we attempt to address these limitations by presenting a longitudinal follow‐up of a cohort of 187 individuals with biopsy‐proven NAFLD. We hypothesized that metabolite profiles assessed at the time of index liver biopsy differ in those who suffer future liver‐related events compared to those who do not. The specific aim of the present study was to determine if serum metabolite profiles in patients with NAFLD associate with future liver‐related events.
Participants and Methods
Design and Procedures
We performed a retrospective single‐center cohort study of individuals with biopsy‐proven NAFLD. The Duke University Health System NAFLD Biorepository is approved by the Duke University Institutional Review Board and contains clinical information and frozen liver and serum specimens from study participants who underwent standard of care diagnostic liver biopsies between 2007 and 2013. Further details regarding the biorepository and its enrollment criteria have been published.( 19 ) For this study, participants specifically consented for genomic and metabolomic analysis of their specimens through the NAFLD biorepository. Patient and sample selection are detailed in Supporting Fig. S1. All authors had access to the study data and approved the final manuscript.
NAFLD was defined as (1) presence of >5% hepatic steatosis on liver biopsy; (2) absence of histologic and serologic evidence for other forms of chronic liver disease; and (3) little or no alcohol consumption (<20 g/day for women and <30 g/day for men). Demographic and clinical data were obtained at the time of liver biopsy. Serum samples were collected from participants at the time of liver biopsy following a 12‐hour fast. The study cohort was selected for inclusion into the metabolomic cohort based on severity of hepatic fibrosis at the time of biopsy.
The study cohort included different stages of fibrosis in roughly equal numbers (mild, Metavir fibrosis stage 0‐1; intermediate, stage 2; advanced, stage 3‐4( 20 )) and included only those participants who had high‐quality serum and liver biopsy collected at the same time. Fibrosis groups were matched for sex, age, and body mass index (BMI). Liver biopsy specimens were independently reviewed and scored by one pathologist (C.G.) using NASH Clinical Research Network scoring criteria.( 21 )
Sample Handling and Analysis
All samples were stored at −80°C until processed on the metabolomics platform at Metabolon, Inc. (Durham, NC). Patient serum samples underwent metabolomic analysis with ultrahigh performance liquid chromatography/tandem mass spectrometry (UHPLC/MS/MS) optimized for acidic species, UHPLC/MS/MS for basic species, and gas chromatography/MS (GC/MS). Metabolites were then identified using automated comparison of the ion features in the experimental samples to a reference library of chemical standard entries that included retention time, molecular weight (m/z), preferred adducts, and in‐source fragments as well as their associated MS/MS spectra. This library allowed for rapid identification of metabolites in the experimental samples with high confidence. A detailed explanation of the metabolite analysis procedure has been published.( 22 ) Quality control details are found in the Supporting Methods.
Lipidomics Panel
Lipids were extracted using chloroform:methanol in the presence of authentic internal standards. Lipids were trans‐esterified in 1% sulfuric acid in methanol in a sealed vial under a nitrogen atmosphere at 100°C for 45 minutes. The resulting fatty acid methyl esters were extracted from the mixture with hexane containing 0.05% butylated hydroxytoluene and prepared for GC by sealing the hexane extracts under nitrogen. Fatty acid methyl esters were separated and quantified by capillary GC (Agilent Technologies 6890 Series GC) equipped with a 30‐m DB 88 capillary column (Agilent Technologies) and a flame ionization detector.
Clinical and Outcomes Data
Demographics, including age, sex, BMI, smoking status, medical comorbidities, and medications, were collected as part of the clinical database at the time of liver biopsy. Laboratory values, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet count were collected within 6 months of the liver biopsy and used to estimate the amount of liver fibrosis by the FIB‐4 score using the following formula: (age × AST) / (platelet count × square root[ALT]).( 23 ) The follow‐up period for each participant was defined as the date of the index liver biopsy until April 1, 2018. The primary outcome was the occurrence of liver‐related events. Liver‐related events were collected through manual systematic chart review and were defined as any one or more of the following: ascites (on imaging or requiring paracentesis), spontaneous bacterial peritonitis (ascitic fluid neutrophil count >250 cells/mm3), variceal bleeding (diagnosed endoscopically), hepatocellular carcinoma (HCC; by cross‐sectional imaging or tissue diagnosis), hepatic encephalopathy (clinician diagnosed or requiring lactulose or rifaximin therapy), and hepatopulmonary syndrome (diagnosed by presence of a shunt on echocardiography and arterial blood gas revealing arterial to alveolar oxygen gradient of 15 mm Hg or greater). We defined liver‐related death as any death that occurred after a previous liver‐related event and in which complications of liver disease were the primary cause of death. Participants with liver‐related events before the index biopsy or who had previously undergone liver transplantation before the index biopsy were excluded.
Statistical analysis
Chi‐square tests were used to compare baseline characteristics between participants with and without liver‐related events. We performed generalized linear models to evaluate for liver‐related events by metabolite while controlling for covariates of age, sex, diabetes, hypertension, BMI, and fibrosis stage. Statistical significance was defined as P < 0.05. We then estimated Cox proportional hazards models( 24 ) for time to liver‐related events, controlling for multiple comparisons and age, sex, BMI, diabetes, hypertension, and fibrosis stage. Statistical significance in the Cox proportional hazards models was defined as P < 0.05, and we presented those metabolites with a false discovery rate (FDR) <0.10. Generalized linear models were also calculated to evaluate the association between FIB‐4 score and liver‐related events, controlling for age, sex, diabetes, hypertension, and BMI. All analyses were done using MATLAB (MathWorks, Inc).
Results
Study Participant Characteristics
A total of 196 participants had metabolomic analysis performed. Nine participants were excluded from this analysis after further histologic and chart review revealed they had undergone liver transplantation before NAFLD diagnosis or steatosis resulted from an alternative etiology (i.e., alcohol not disclosed on initial questionnaire, autoimmune hepatitis, drug‐induced liver injury). As such, 187 participants were included in the final cohort and analyses. Baseline characteristics of the entire cohort and the cohort stratified according to liver‐related events are shown in Table 1. Overall, the mean ± SD age at liver biopsy was 50.2 ± 10.5 years, 53% of the cohort were women, and 89% of participants were Caucasian. Participants with and without liver‐related events were similar except for fibrosis stage; as expected, more participants who experienced liver‐related events had advanced (stage 3‐4) fibrosis (73% vs. 23% without liver‐related events).
Table 1.
Baseline Characteristics of the Cohort
| Characteristic | Overall Cohort (n = 187), n (%) | Patients Without Liver‐Related Events (n = 176) (%) | Patients With Liver‐Related Events (n = 11) (%) |
|---|---|---|---|
| Age at biopsy, years (mean ± SD) | 50.2 ± 10.5 | 50.0 ± 10.7 | 53.6 ± 6.2 |
| Race | |||
| Caucasian | 166 (89) | 89 | 91 |
| African American | 12 (6) | 7 | 0 |
| Female sex | 99 (53) | 53 | 55 |
| BMI (mean ± SD) | 35.3 ± 7.2 | 35.3 ± 7.2 | 34.2 ± 6.1 |
| Hypertension | 121 (65) | 65 | 64 |
| Diabetes mellitus | 67 (36) | 35 | 55 |
| Hyperlipidemia | 73 (39) | 39 | 45 |
| Current smoker | 25 (13) | 14 | 9 |
| Fibrosis stage* | |||
| 0 | 12 (6) | 7 | 0 |
| 1 | 36 (19) | 20 | 0 |
| 2 | 90 (48) | 49 | 27 |
| 3 | 41 (22) | 19 | 64 |
| 4 | 8 (4) | 4 | 9 |
Statistically significant for comparison between those with events and those without.
Liver‐Related Outcomes
Over a mean ± SD follow‐up of 6.9 ± 3.2 years, 11 participants experienced 22 liver‐related events, shown in Table 2. The time from baseline liver biopsy to first liver‐related event ranged from 7 months to 9.2 years. A total of 12 participants died during follow‐up; six of these deaths were due to liver‐related causes. Of the 6 participants who experienced liver‐related death, 3 had prior hepatic encephalopathy, 2 had ascites, and 1 had both hepatic encephalopathy and ascites. Of the remaining 6 participants who died, 2 had advanced malignancy, 1 had fatal intracerebral hemorrhage, and 3 lacked documentation of cause of death.
Table 2.
Liver‐Related Events*
| Liver‐Related Events | Number of Patients |
|---|---|
| Hepatic encephalopathy | 8 |
| Liver‐related death | 6 |
| Ascites | 5 |
| Spontaneous bacterial peritonitis | 1 |
| Hepatocellular carcinoma | 1 |
| Variceal bleeding | 1 |
Clinical variables independently associated with time to liver‐related events: age, diabetes, fibrosis stage.
Metabolites Detected
Metabolomics data from the cohort yielded 1,151 metabolites (760 identified and 361 unnamed). These metabolites derived from eight superpathways: amino acids (n = 157), peptides (n = 55), carbohydrates (n = 28), energy related (n = 7), lipids (n = 307), nucleotides (n = 34), cofactors/vitamins (n = 34), and xenobiotics (n = 67). The unnamed metabolites were excluded. We also excluded metabolites for which >50% of values were missing, which was presumably due to levels below the limit of detection. For the remaining 683 metabolites, missing values were imputed to half of the observed minimum value for each metabolite,( 25 ) and batch effects were corrected by removing mean day‐wise effects from each analyte independently after log‐transformation.
Association of Metabolites With Liver‐Related Events
In the generalized linear models, we identified 53 metabolites significantly associated with liver‐related events (P < 0.05) after controlling for age, sex, BMI, hypertension, diabetes, and fibrosis stage. FDR values for the top 10 metabolites ranged from 0.17 to 0.21. The most commonly represented superpathways were lipids (26 metabolites) and amino acids (11 metabolites). The superpathways and pathways represented are shown in Fig. 1. A full list of significant metabolites is provided in Supporting Table S1. The top 10 metabolites (based on FDR) are shown in Table 3. Of note, two primary bile acid metabolites (taurochenodeoxycholate [TCDCA] and taurocholate), two vitamin E metabolites (gamma‐carboxyethyl‐hydroxychroman [CEHC] and gamma‐CEHC glucuronide), and one serotonin metabolite (5‐hydroxyindoleacetate) were among the top 10 altered metabolites associated with liver‐related outcomes. Ten participants were taking vitamin E and 32 participants were taking serotonergic medications at the time of liver biopsy. Serotonergic medications but not vitamin E were associated with liver‐related events in a univariate model. Controlling for serotonergic medications did not cause 5‐hydroxyindoleacetate to lose statistical significance at the cutoff P value of 0.05.
FIG. 1.
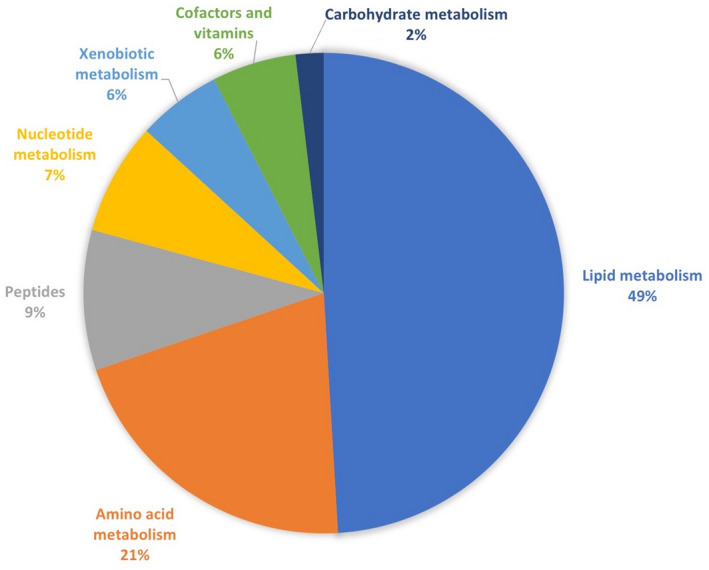
Superpathways and pathways represented by the 53 metabolites that were significantly associated with liver‐related events (P < 0.05) in generalized linear models after controlling for age, sex, diabetes, BMI, hypertension, and fibrosis stage.
Table 3.
Top 10 Metabolites from Generalized Linear Models
| Category | Subcategory | Metabolite | P Value | FDR |
|---|---|---|---|---|
| Amino acids | Tryptophan metabolism | 5‐hydroxyindoleacetate | 0.003 | 0.22 |
| Cofactors and vitamins | Tocopherol metabolism | gamma‐CEHC glucuronide | 0.0007 | 0.17 |
| gamma‐CEHC | 0.002 | 0.21 | ||
| Lipids | Primary bile acid metabolism | taurochenodeoxycholate | 0.0009 | 0.17 |
| taurocholate | 0.003 | 0.21 | ||
| Sterol | beta‐sitosterol | 0.003 | 0.21 | |
| sphingolipid metabolism | eicosenoyl sphingomyelin | 0.001 | 0.17 | |
| Peptides | Polypeptide | HWESASXX | 0.001 | 0.17 |
| Dipeptide | glycylproline | 0.003 | 0.21 | |
| Xenobiotics | Benzoate metabolism | benzoate | 0.002 | 0.21 |
Abbreviation: HWESASXX, inflammation associated complement component 3 peptide.
Next, we performed Cox proportional hazards modeling to identify metabolites associated with time to future liver‐related events. After controlling for age, sex, BMI, diabetes, hypertension, serotonergic medications, and fibrosis stage, 69 metabolites were significantly associated with time to future liver‐related events with P < 0.05. Of these, seven metabolites met the FDR threshold of 0.10; these are shown in Table 4. Gamma‐CEHC, gamma‐CEHC glucuronide (both decreased), taurochenodeoxycholate (increased), and 5‐hydroxyindoleacetate (decreased) were all associated with time to liver‐related events. The other significant metabolites were 2‐hydroxyglutarate (increased), 4‐androsten‐3beta,17beta‐diol disulfate 1 (increased), and eicosenoyl sphingomyelin (decreased).
Table 4.
Cox Proportional Hazards Modeling of Time to Liver‐Related Events
| Category | Metabolite | P Value | FDR | Beta |
|---|---|---|---|---|
| Amino acid | 5‐hydroxyindoleacetate | 0.0007 | 0.06 | −2.83 |
| Cofactors and vitamins | gamma‐CEHC | 0.0007 | 0.07 | −2.07 |
| gamma‐CEHC glucuronide | 0.0001 | 0.07 | −0.90 | |
| Lipids | taurochenodeoxycholate | 0.0006 | 0.07 | 0.87 |
| 2‐hydroxyglutarate | 0.0004 | 0.08 | 1.89 | |
| 4‐androsten‐3beta,17beta‐diol disulfate 1 | 0.0006 | 0.09 | 0.79 | |
| eicosenoyl sphingomyelin | 0.0003 | 0.09 | −2.42s |
We calculated the FIB‐4 score for each patient to compare its performance predicting liver‐related events to the top metabolites. For participants who experienced liver‐related events, FIB‐4 scores ranged from 1.17 to 7.34 (mean, 3.27); for those who did not experience liver‐related events, the range was 0.39‐5.98 (mean, 1.57). FIB‐4 was significantly associated with liver‐related events (P < 0.001). Thus, the top metabolites performed comparably to a well‐validated scoring system despite the fact that metabolites were evaluated in models adjusted for fibrosis stage.
Discussion
Here, we report an association between serum metabolites and the development of future liver‐related morbidity and mortality. Using a well‐characterized cohort of participants with NAFLD and longitudinal follow‐up, we found that seven metabolites measured at the time of liver biopsy were significantly associated with time to future liver‐related events (P < 0.05 and FDR, <0.10), independent of fibrosis stage. Approximately one quarter of participants with liver‐related events had stage 2 fibrosis on index biopsy, and therefore a noninvasive biomarker could have prioritized these individuals for intensified surveillance or identified candidates for treatment before the occurrence of decompensation. Our study is unique in that prior studies of serum metabolites have examined NAFLD histology in a cross‐sectional manner. While such studies have yielded valuable insights into NAFLD pathophysiology as well as potential for noninvasive diagnosis of NASH or advanced fibrosis, we have attempted to identify serum biomarkers associated with future events associated with NAFLD in individual patients. This proof of concept study suggests that noninvasive markers associated with NAFLD may be important predictors identifying patients who experience future liver‐related morbidity and mortality. This brings up the possibility that modulation of such metabolites could affect disease progression. Additionally, altered metabolite panels could be used to risk stratify patients at the time of diagnosis.
Our analysis found that higher serum levels of TCDCA were associated with a shorter time to liver‐related events. Primary bile acids are potentially of mechanistic and therapeutic significance in NAFLD. TCDCA results from the conjugation of the bile acid chenodeoxycholate with taurine. Like other bile acids, TCDCA acts as a detergent by solubilizing fats in the intestine and is recycled in the terminal ileum. Bile acids have been linked to NAFLD through the farnesoid X receptor (FXR); FXR agonist obeticholic acid has demonstrated beneficial effects on histology in NASH.( 26 ) Norursodeoxycholic acid, a secondary bile acid, has also been found to have beneficial effects on histology.( 27 ) TCDCA specifically has been linked to progression in NAFLD in previous studies. One study found higher postprandial concentrations of taurine‐containing bile acids in participants with NAFLD compared to healthy controls.( 28 ) In animal models, TCDCA is retained in the liver to a greater degree compared to other bile acids. In one study, promoting excretion of TCDCA and other hydrophobic bile acids in mice prevented development of HCC.( 29 ) Bile acid derivatives have also been studied as therapeutics but with limited data to date.( 30 )
The salt form of the principal metabolite of serotonin 5‐hydroxyindoleacetic acid (5‐HIAA) is 5‐hydroxyindoleacetate. In our analysis, participants with biopsy‐proven NAFLD and lower levels of 5‐HIAA experienced increased risk and a shorter time interval for liver‐related events. Serotonin is produced in the liver by cholangiocytes and stellate cells( 31 ) and is also produced in the gut to be converted to 5‐HIAA in the liver. Through its effects on stellate‐cell proliferation, serotonin likely regulates fibrosis progression.( 32 ) Specific to NAFLD, one recent paper demonstrated that inhibiting gut synthesis of serotonin in a mouse model reduced hepatic steatosis induced by a high‐fat diet.( 33 ) In rat models, fetal and neonatal exposure to fluoxetine increased the incidence of NAFLD; changes in the inflammasome and hepatic lipogenesis were observed and were considered possible mechanisms.( 34 ) Interestingly in our analysis, controlling for serotonergic medications did not cause this metabolite to lose statistical significance, suggesting that exogenous serotonin is not the cause of this observed association between serotonin metabolites and liver‐related events. Given that serotonin modulation is already possible through existing therapies, better understanding of the relationship between serotonin and NAFLD could have direct applications to patient care.
Our finding of altered vitamin E metabolites in those participants with liver‐related outcomes of NAFLD is of particular interest. Vitamin E has been studied as a NAFLD therapy in adults without diabetes in the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial and was found to be superior to placebo for improvement in NAFLD histology.( 35 ) Vitamin E is thought to mitigate steatohepatitis through its antioxidant properties and may inhibit ferroptosis, a form of cell death associated with inflammation and ischemia.( 36 ) Unsurprisingly, therefore, we found that lower levels of two vitamin E metabolites, gamma‐CEHC and gamma‐CEHC glucuronide, were associated with increased hazard of liver‐related events. Exogenous vitamin E therapy was assessed as a possible confounder (data not presented), but it was not independently associated with liver‐related events. Further studies of vitamin E will hopefully clarify specific populations with expected benefit.
The remaining three metabolites that were significantly associated with time to liver‐related events have less robust mechanistic links to NAFLD progression. It is thought that 2‐hydroxyglutarate accumulates abnormally in malignant cells, but its links to oncogenesis are incompletely characterized.( 37 ) The metabolite 4‐androsten‐3beta,17beta‐diol disulfate is an intermediate in the pathway of testosterone synthesis from dehydroepiandrosterone (DHEA). In our analysis, higher levels were associated with increased hazard of liver‐related events. DHEA is thought to modulate the liver’s response to oxidative stress, and lower circulating levels have been associated with advanced histology in NAFLD.( 38 ) Of note, increased free testosterone levels in premenopausal women were associated with increased prevalence of NAFLD later in life in one study, independent of other NAFLD risk factors.( 39 ) The precise balance of sex hormones that facilitates or ameliorates NAFLD remains to be determined, but this area is under active investigation, rightfully so, as therapies already exist.
Our results overlap with published work. Of the 32 metabolites found to associate with advanced fibrosis by Caussy et al.,( 17 ) three were primary bile acids, including taurocholate. That cohort included 45.5% of participants who identified as non‐Hispanic white, indicating that at least some metabolites may remain significant in multiple ethnic or racial groups. Taurine itself was also included in the metabolite signature in that study. This and our results suggest that taurine metabolism is an area for future study. In addition, 4‐androsten‐3beta,17betadiol monosulfate 1 was associated with advanced fibrosis in that analysis, whereas we found association between the disulfate form and liver‐related events. DHEA and testosterone metabolism, therefore, could be areas relevant to NAFLD progression.
Serum metabolites have the potential to enhance noninvasive clinical prediction scores. Such scores are widely used for assessing risk of advanced fibrosis, but they have not been validated to predict liver‐related decompensation. In our analysis, the strength of association between top metabolites and future liver‐related events was comparable to the FIB‐4 score, despite the adjustment of our models for fibrosis stage. Serum metabolites could complement noninvasive scoring systems, particularly for participants with intermediate‐stage fibrosis, in determining risk for future liver‐related events.
In addition, outcomes beyond liver‐related clinical deterioration should be considered in future studies. NAFLD has been independently associated with cardiovascular disease, including coronary artery disease, left ventricular hypertrophy, and diastolic heart failure.( 40 , 41 ) Cardiovascular disease represents a major source of morbidity and mortality for patients with NAFLD, and whether serum metabolites are associated with cardiovascular outcomes will be explored in subsequent studies of this cohort. External validation of our findings would be worthwhile as well.
The strengths of this study are that the cohort was drawn from a well‐annotated repository and database in which all participants had biopsy‐proven NAFLD and other causes for liver disease had been excluded. In addition, thorough baseline clinical data, including medications, were available for the cohort. The limitations are the single‐center design and our racially homogeneous population, which was almost 90% Caucasian. Our ascertainment of liver‐related events using manual retrospective chart review leaves open the possibility that events were not captured in our system or were missed due to incomplete documentation. As serum metabolites were evaluated at the time of index liver biopsy, serial measurements to indicate metabolite trends were not available and could have potentially yielded additional insights. This would be a valuable area for future study. Limitations in the metabolite assay itself include the possible presence of unmeasured or unnamed metabolites that could be of mechanistic or prognostic significance. This study was not powered to assess metabolites in the subgroup of participants with stage 2 fibrosis, but this population warrants particular attention, given the potential additional predictive value of noninvasive biomarkers. Finally, the study design precludes assessment of causality between metabolite levels and liver‐related events.
In summary, we found that primary bile acid, serotonin, and vitamin E metabolites were associated with future liver‐related events in NAFLD, independent of fibrosis stage at the time of diagnosis. Our findings have potential diagnostic and therapeutic implications and should be validated in larger and additional cohorts.
Supporting information
Fig S1
Table S1
Supplementary Material
Acknowledgment
We gratefully acknowledge the contributions of Metabolon, Inc. (Durham, NC) to this manuscript.
Supported by Metabolon, Inc. (funding to Duke University), American Association for the Study of Liver Diseases Foundation Bridge Award (to C.A.M.), National Institutes of Health (funding 5T32DK007568‐29 to K.W.), and a Duke Endowment (to A.M.D.).
Potential conflict of interest: Dr. Abdelmalek advises and has received grants from Intercept, BMS, NGM, Madrigal, Novo‐Nordisk, Allergan, and Inventiva; she consults for Promethera and TaiwanJ and advises Hanmi and Allergan; she has received grants from Metabolon, GalMed, Viking, Celgene, DURECT, Poxel, Enyo, Enanta, Gilead, Novartis, Genfit, Target NASH, Progenity, and Boehringer‐Ingelheim. Dr. Guy consults for NGM, Madrigal, and CymaBay. Dr. Moylan is a consultant for GLG. The other authors have nothing to report.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274‐285. [DOI] [PubMed] [Google Scholar]
- 3. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paik JM, Henry L, De Avila L, Younossi E, Racila A, Younossi ZM. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun 2019;3:1459‐1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shili‐Masmoudi S, Wong GL, Hiriart JB, Liu K, Chermak F, Shu SS, et al. Liver stiffness measurement predicts long‐term survival and complications in non‐alcoholic fatty liver disease. Liver Int 2020;40:581‐589. [DOI] [PubMed] [Google Scholar]
- 6. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mayo R, Crespo J, Martinez‐Arranz I, Banales JM, Arias M, Minchole I, et al. Metabolomic‐based noninvasive serum test to diagnose nonalcoholic steatohepatitis: results from discovery and validation cohorts. Hepatol Commun 2018;2:807‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kashyap SR, Diab DL, Baker AR, Yerian L, Bajaj H, Gray‐McGuire C, et al. Triglyceride levels and not adipokine concentrations are closely related to severity of nonalcoholic fatty liver disease in an obesity surgery cohort. Obesity (Silver Spring) 2009;17:1696‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loomba R, Quehenberger O, Armando A, Dennis EA. Polyunsaturated fatty acid metabolites as novel lipidomic biomarkers for noninvasive diagnosis of nonalcoholic steatohepatitis. J Lipid Res 2015;56:185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 2012;56:118‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalhan SC, Guo L, Edmison J, Dasarathy S, McCullough AJ, Hanson RW, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011;60:404‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lake AD, Novak P, Shipkova P, Aranibar N, Robertson DG, Reily MD, et al. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids 2015;47:603‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dong S, Zhan ZY, Cao HY, Wu C, Bian YQ, Li JY, et al. Urinary metabolomics analysis identifies key biomarkers of different stages of nonalcoholic fatty liver disease. World J Gastroenterol 2017;23:2771‐2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, et al. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med 2011;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang L, Ding C, Zeng F, Zhu H. Low levels of serum beta‐carotene and beta‐carotene/retinol ratio are associated with histological severity in nonalcoholic fatty liver disease patients. Ann Nutr Metab 2019;74:156‐164. [DOI] [PubMed] [Google Scholar]
- 16. Lai Z, Chen J, Ding C, Wong K, Chen X, Pu L, et al. Association of Hepatic Global DNA Methylation and Serum One‐Carbon Metabolites With Histological Severity in Patients With NAFLD. Obesity (Silver Spring) 2020;28:197‐205. [DOI] [PubMed] [Google Scholar]
- 17. Caussy C, Ajmera VH, Puri P, Hsu CL, Bassirian S, Mgdsyan M, et al. Serum metabolites detect the presence of advanced fibrosis in derivation and validation cohorts of patients with non‐alcoholic fatty liver disease. Gut 2019;68:1884‐1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Xie J, Pang J, Zhang H, Chen X, Lin J, et al. Serum SHBG is associated with the development and regression of nonalcoholic fatty liver disease: a prospective study. J Clin Endocrinol Metab 2020;105:dgz244. [DOI] [PubMed] [Google Scholar]
- 19. Moylan CA, Pang H, Dellinger A, Suzuki A, Garrett ME, Guy CD, et al. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology 2014;59:471‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. No authors listed . Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994;20:15‐20. [PubMed] [Google Scholar]
- 21. Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander‐Tetri BA; NASH Clinical Research Network (CRN) . Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011;53:810‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small‐molecule complement of biological systems. Anal Chem 2009;81:6656‐6667. [DOI] [PubMed] [Google Scholar]
- 23. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al.; APRICOT Clinical Investigators . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317‐1325. [DOI] [PubMed] [Google Scholar]
- 24. Cox DR. Regression models and life‐tables. J Roy Stat Soc: Ser B (Methodol) 1972;34:187‐220. [Google Scholar]
- 25. Wei R, Wang J, Su M, Jia E, Chen S, Chen T, et al. Missing value imputation approach for mass spectrometry‐based metabolomics data. Sci Rep 2018;8:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al.;NASH Clinical Research Network . Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 2015;385:956‐965. Erratum in: Lancet 2015;385:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Traussnigg S, Schattenberg JM, Demir M, Wiegand J, Geier A, Teuber G, et al.; Austrian/German NAFLD‐norUDCA study group . Norursodeoxycholic acid versus placebo in the treatment of non‐alcoholic fatty liver disease: a double‐blind, randomised, placebo‐controlled, phase 2 dose‐finding trial. Lancet Gastroenterol Hepatol 2019;4:781‐793. [DOI] [PubMed] [Google Scholar]
- 28. Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, Jia W, et al. Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig Dis Sci 2015;60:3318‐3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xie G, Wang X, Huang F, Zhao A, Chen W, Yan J, et al. Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis. Int J Cancer 2016;139:1764‐1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chavez‐Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 2017;152:1679‐1694.e3. [DOI] [PubMed] [Google Scholar]
- 31. Omenetti A, Yang L, Gainetdinov RR, Guy CD, Choi SS, Chen W, et al. Paracrine modulation of cholangiocyte serotonin synthesis orchestrates biliary remodeling in adults. Am J Physiol Gastrointest Liver Physiol 2011;300:G303‐G315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oben JA, Roskams T, Yang S, Lin H, Sinelli N, Torbenson M, et al. Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut 2004;53:438‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi W, Namkung J, Hwang I, Kim H, Lim A, Park HJ, et al. Serotonin signals through a gut‐liver axis to regulate hepatic steatosis. Nat Commun 2018;9:4824. Erratum in: Nat Commun 2019;PMID:30622275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Long NE, Hardy DB, Ma N, Holloway AC. Increased incidence of non‐alcoholic fatty liver disease in male rat offspring exposed to fluoxetine during fetal and neonatal life involves the NLRP3 inflammasome and augmented de novo hepatic lipogenesis. J Appl Toxicol 2017;37:1507‐1516. [DOI] [PubMed] [Google Scholar]
- 35. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al.; NASH CRN . Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hinman A, Holst CR, Latham JC, Bruegger JJ, Ulas G, McCusker KP, et al. Vitamin E hydroquinone is an endogenous regulator of ferroptosis via redox control of 15‐lipoxygenase. PLoS One 2018;13:e0201369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gelman SJ, Mahieu NG, Cho K, Llufrio EM, Wencewicz TA, Patti GJ. Evidence that 2‐hydroxyglutarate is not readily metabolized in colorectal carcinoma cells. Cancer Metab 2015;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Charlton M, Angulo P, Chalasani N, Merriman R, Viker K, Charatcharoenwitthaya P, et al. Low circulating levels of dehydroepiandrosterone in histologically advanced nonalcoholic fatty liver disease. Hepatology 2008;47:484‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sarkar M, Wellons M, Cedars MI, VanWagner L, Gunderson EP, Ajmera V, et al. Testosterone levels in pre‐menopausal women are associated with nonalcoholic fatty liver disease in midlife. Am J Gastroenterol 2017;112:755‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol 2012;10:646‐650. [DOI] [PubMed] [Google Scholar]
- 41. Sao R, Aronow WS. Association of non‐alcoholic fatty liver disease with cardiovascular disease and subclinical atherosclerosis. Arch Med Sci 2018;14:1233‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Supplementary Material


