Abstract
Adverse events in childhood and adolescence, such as social neglect or drug abuse, are known to lead to behavioral changes in young adulthood. This is particularly true for the subset of people who are intrinsically more vulnerable to stressful conditions. Yet the underlying mechanisms for such developmental trajectory from early life insult to aberrant adult behavior remains elusive. Adolescence is a period of dynamic physiological, psychological, and behavioral changes, encompassing a distinct neurodevelopmental stage called the ‘critical period’. During adolescence, the brain is uniquely susceptible to stress. Stress mediators may lead to disturbances to biological processes that can cause permanent alterations in the adult stage, even as severe as the onset of mental illness when paired with genetic risk and environmental factors. Understanding the molecular factors governing the critical period and how stress can disturb the maturation processes will allow for better treatment and prevention of late adolescent/young adult onset psychiatric disorders.
Keywords: Adolescence, Critical period, Developmental trajectory, Brain maturation, Adult behavior
1. Introduction
In the past, many scientists have addressed the question of how early life events may contribute to the shaping of adult behavior. These investigators include psychologists like Erikson and Piaget who stated that during the long-term trajectory there are several specific stages that contribute to the proper development towards adult behavior. For example, Erikson’s theory of psychosocial development suggests there are eight stages of psychosocial conflicts in ego growth from infancy to adulthood.1 Piaget’s four stages of cognitive development takes into consideration how biological maturation and environmental factors facilitate cognitive development throughout the lifespan.1
Consistent with such a conceptual framework of psychological development, recent clinical and epidemiological evidence has indicated that any early life disturbances, such as abuse or neglect, may lead to aberrant behaviors in adulthood, and potentially enhance the risk for a wide range of psychiatric conditions.2–6 For example, aberrant use of drugs, such as marijuana, in adolescence has been correlated to a heightened risk of psychosis.7
In rodent models, early life stress also reportedly impairs mood, cognition, memory, and learning in adulthood.3,5 Many of these studies have found that early stress elicits several molecular and structural changes in the brain, which accompany the behavioral deficits in adulthood.8 Thus, rodent models may be useful in studying mechanisms of how adverse events in early life may underlie adult behavior during the developmental trajectory.
Major psychiatric conditions, such as schizophrenia or severe cases of bipolar disorder, emerge in late adolescence and young adulthood, and most display a chronic deteriorating course after onset. Thus, there are two major mechanistic questions: the first being why these disorders often emerge after puberty, although the initial risk events may occur years earlier, sometimes even during prenatal stages; and the second being why the emerged deficits frequently become sustained throughout adulthood.
2. Dynamic changes in the adolescent brain
During adolescence, the brain, including the cerebral cortex, changes and matures drastically (Fig. 1). For example, in the first half of adolescence, massive elimination of synapses occurs, specifically among glutamatergic neurons.9 This event occurs not only in a cell autonomous mechanism, but is also influenced by non-neuronal cells such as microglia and astrocytes.10 In the cerebral cortex, dopaminergic projections from the midbrain region undergo alterations, accompanied by increases in dopamine levels until late adolescence.11,12 Response to γ-aminobutyric acid (GABA)-ergic neurons also critically changes during adolescence.13 An increase in myelination is prominent in late adolescence to young adulthood as well, which is associated with overall changes to molecular expression profiles.14,15
Fig. 1.
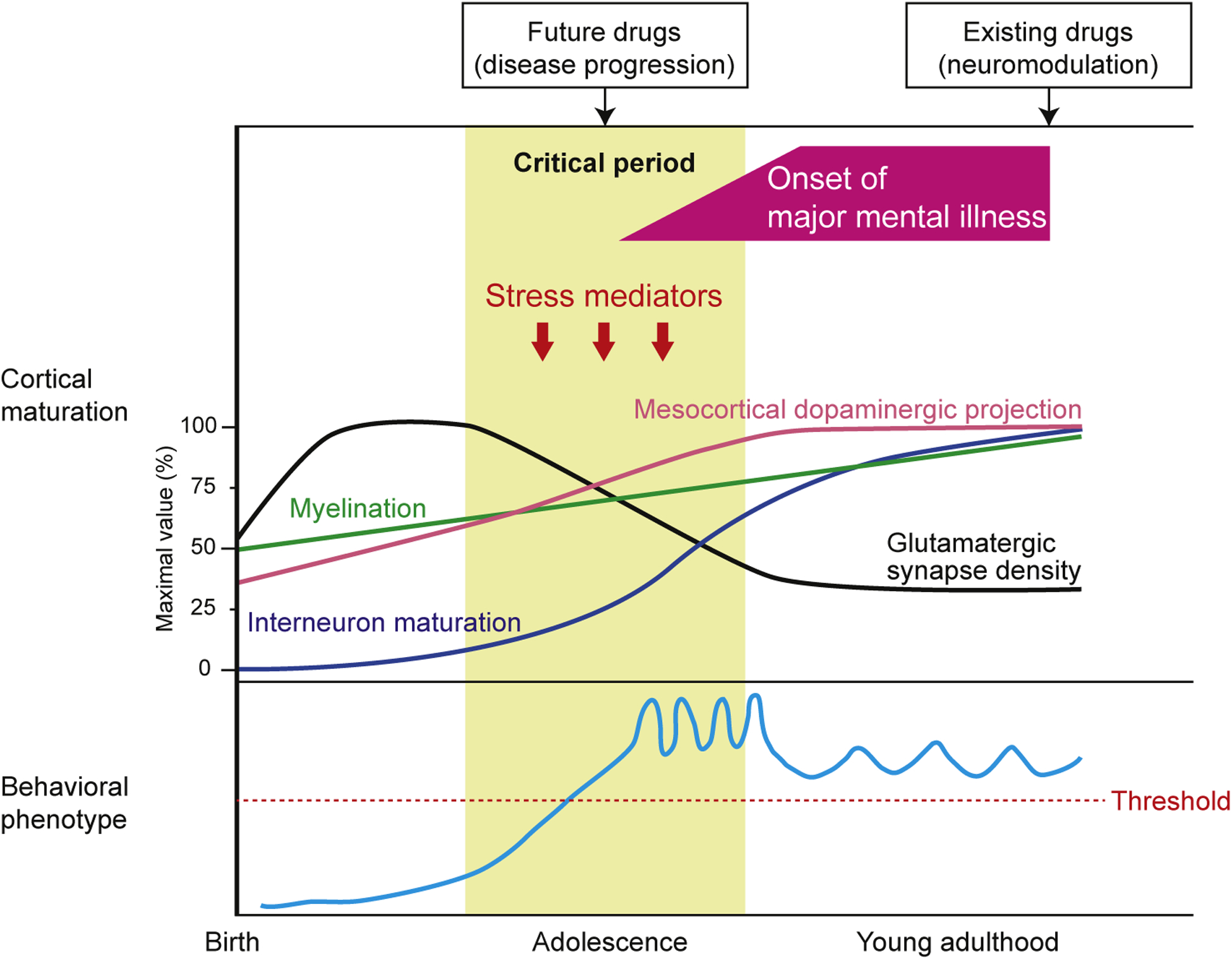
Cortical maturation and behavioral patterns in developmental trajectory of major mental illnesses. The upper panel shows the developmental trajectory of brain maturation. Brain maturation, including maturation of interneurons and dopaminergic projections, pruning of glutamatergic synapses, and increased myelination, occurs from birth to young adulthood, most dynamically during adolescence. Stress mediators regulated by genetic and environmental factors affect this maturation processes during this critical period. Aberrant brain maturation induced by stress mediators might be an essential mechanism underlying the disease. The lower panel shows the course of abnormal behavioral patterns related to major mental illnesses. Disturbance of brain maturation caused by stress mediators during the critical period may increase vulnerability to major mental illnesses, for which the devastating behavioral phenotype (over the threshold) will begin to emerge in late adolescence and early adulthood. Adapted from Jaaro-Peled and colleagues, with permission from Elsevier.30
At the physiological level, the dynamics of neuronal activity changes during adolescence as cortical regions are formed into functional networks. There is also increased synchrony at multiple brain wave frequencies as well as changes in the balance between excitatory and inhibitory synapses (E/I balance).16 Neuronal oscillations, particularly at gamma-band frequencies, may play an important role in cognitive and behavioral responses, such as perceptual grouping, attention-dependent stimulus selection, working memory, and consciousness.17
3. Critical periods
The classic concepts in neuropsychology that describe multiple developmental stages can be applied to the concepts of critical periods in neuroscience. The notion of the critical period originated from the observation by Wiesel and Hubel that monocular light deprivation during the neonatal stage resulted in blindness that could not be recovered by light exposure later in life, despite no prior intrinsic anatomical or biological abnormalities.18 Recent studies in molecular neuroscience have indicated that there are indeed critical developmental windows governed by several concrete molecular factors: molecules associated with GABA neurotransmission and some immune-related molecules have been underscored.19,20
Experimental validation of critical periods has encouraged investigators to extend this approach to understand how stress during the critical period may even create vulnerability and resilience to psychopathology, and affect behaviors in later stages. Adolescents show an attenuated fear memory and extinction, suggesting that adolescence is indeed a sensitive period to stress that differs from other developmental and adult stages.21 One study found that adolescents with anxiety disorders show a decreased responsiveness to cognitive behavioral therapy (CBT) compared to children and adults.21 This susceptibility is associated with age-specific changes in neuroplasticity in the prefrontal cortex. Alterations to the neural regulation of fear and anxiety in adolescence, when paired with early life stress, can lead to a heightened “vulnerability to pathogenic experiences”.21 Yet, the unique molecular expression profiles and limited temporal window of adolescence allows clinicians to develop personalized treatment based on the patient’s genetic profile and age.
As we described above, dopaminergic projections, particularly mesocortical projections, mature during adolescence. Disturbance of this maturation by adverse events during adolescence is experimentally proven to be causal for aberrant animal behavior, such as deficits in information processing, mood control, and psychostimulant hypersensitivity.22 Investigators have elucidated that excess stress hormones, such as glucocorticoids, mediate this disturbance in adolescence.22 By altering the timing of blockade against the activated glucocorticoid receptor signaling, we can determine how the critical period links hypothalamic-pituitary-adrenal (HPA) axis-associated dopaminergic disturbance and aberrant behavior in adulthood.23 In addition, maturation of cortical myelination occurs in adolescence, and disturbances to this maturation process may also underlie abnormal behaviors in adulthood. Among rodents, it has been shown that the two weeks post-weaning may play a crucial role in proper myelination and adult behavior.24
4. Pathological trajectory: from pathogenesis to pathophysiology and final manifestation
Any disease trajectory can be defined as a course from the initial etiology (pathogenesis) to the final clinical manifestation via pathophysiology, in which critical mediators account for the phenotypes25,26 (Fig. 2). In humans, in which every individual is heterogeneous because of genetic variability, the process of pathogenesis is tightly associated with genetic vulnerability and environmental insult in early development. It is possible to postulate that the critical period described above may underlie between the pathogenesis and pathophysiology, in which the biological events of the brain may directly account for the phenotypes.
Fig. 2.
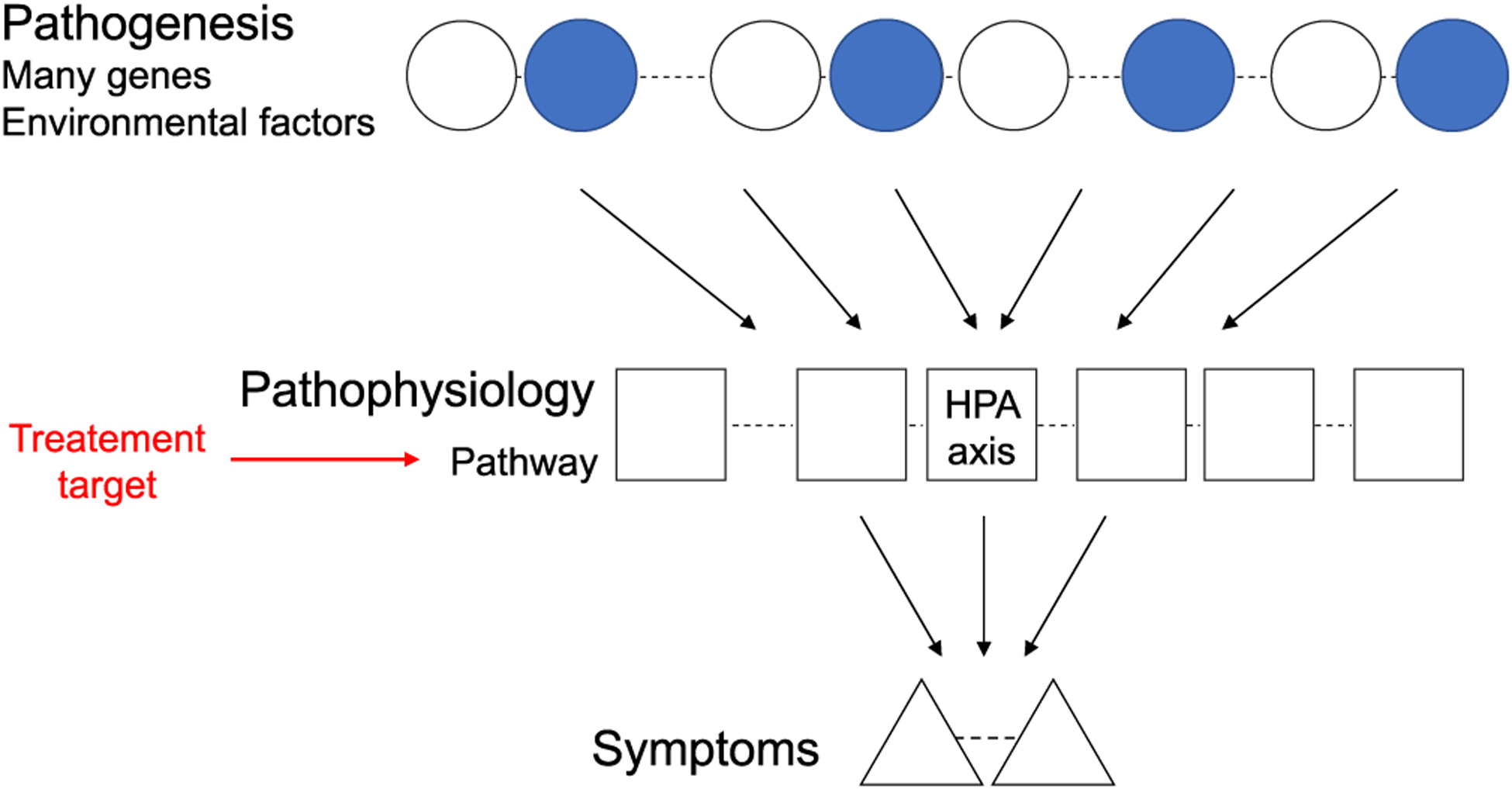
Stress-associated common pathways (pathophysiology) converging from many genetic and environmental factors (pathogeneses). For any disease, there is a trajectory from the initial etiological factors (pathogeneses), including genetic (white circles) and environmental (blue circles) insults, to the clinical manifestations of the disease (symptoms) via common stress-associated pathways (pathophysiology). One of pathways is heavily mediated by the HPA axis, in which its dysregulation can lead to disturbance of brain maturation and subsequent behaviors in young adulthood. These common pathways may be considerd as potential targets for therapeutic intervention and prevention. Adapted from Srivastava and colleagues, with permission from Elsevier.26
We then ask how disturbances to brain maturation during the critical period can lead to chronic dysfunction. If the critical period is restricted to a specific developmental time frame for the manifestation of a devastating phenotype, then why does biological influence during the critical period produce sustained behavioral changes later in life, and what is the mechanism underlying the disease trajectory from the biological changes during the critical period to the persistent symptomatic manifestations? One possible answer may be that such a devastating event in this short time frame may fundamentally alter the developmental program. Nevertheless, its underlying molecular mechanism is unclear. However, new experimental evidence has indicated that biological influence during the critical period may affect the epigenetic landscape on the chromosome, which may result in the irreparable and long-lasting changes. For example, dysregulation of the HPA axis during the critical period affects dopaminergic neurons long-term through alterations to the epigenetic hallmarks on genes such as tyrosine hydrolase (Th), brain-derived neurotrophic factor (Bdnf), and FK506 binding protein 5 (Fkbp5).23
5. Future prospective: potential application of preclinically defined critical period to clinical settings
As described above, many preclinical studies have recently evidenced that there are critical periods in adolescence, and that disturbance to biological processes during these sensitive developmental time frames may account for abnormal adult behaviors. At least in part, epigenetic mechanisms during the critical period may underlie such late adolescent/young adult onset and long-lasting behavioral abnormalities.27 How can we utilize this knowledge from preclinical studies to better the treatment and prevention of patients with late adolescent/young adult onset psychiatric disorders?
Interventions with aberrant epigenetic processes are currently one of the leading topics in translational neuroscience. For example, experimental drugs such as histone deacetylase (HDAC) inhibitors have been tested using animal models for Alzheimer’s, in which epigenetic implications have been suggested for the memory disturbances.28 Nevertheless, in general, epigenetic intervention as a clinical application is challenging, because we still do not know of a way to intervene the epigenetic change for a specific subset of genes without interfering with entire genes. One noteworthy idea is to utilize the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Cas9 system to target an exact set of epigenetic changes, but this is only currently utilized at the animal level.29 Therefore, a more realistic way to address the critical period and its devastating effect on adult behavior may be to take a prophylactic approach. If we can define the critical period for certain types of adult phenotypes associated with adverse events in early life, we may define certain groups of patients who may possibly have a higher vulnerability to such stressors. As a result, we can guide them to avoid such stressors prior to the critical period, circumventing the deteriorating event altogether and protecting them from the long-lasting brain dysfunction and behavioral deficits. By focusing on the intersection of environment, biology, and lifestyle, we need personalized prevention, treatment, and care for all of us.
Acknowledgements
Parts of the content were presented by M.N. at the symposium “Novel approaches leading next generation of pharmacology” on March 10th, 2018. We thank Ms. Yukiko Y. Lema and Mr. Travis E. Faust for organizing the figures in the manuscript and critical reading of the manuscript, respectively. A.S. and M.N. receive research funding from the National Institute of Health [MH-069853 (A.S.), MH-084018 (A.S.), MH-085226 (A.S.), MH-088753 (A.S.), MH-092443 (A.S.), MH-094268 Silvio O. Conte center (A.S.), MH-105660 (A.S.), DA-040127 (A.S. and M.N), K99MH-094408 (M.N.)], Brain & Behavior Research Foundation (A.S. and M.N.), Stanley (A.S.), RUSK (A.S.), S-R Foundations (A.S.), Maryland Stem Cell Research Fund, Japan Society for the Promotion of Science [22007152 (M.N.) and 23-639 (M.N.)], and Japan Science and Technology Agency Precursory Research for Embryonic Science and Technology JPMJPR14M6 (M.N.).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Steinberg L, Morris AS. Adolescent development. Annu Rev Psychol. 2001;52:83–110. [DOI] [PubMed] [Google Scholar]
- 2.Dahl RE, Allen NB, Wilbrecht L, Suleiman AB. Importance of investing in adolescence from a developmental science perspective. Nature. 2018;554:441–450. [DOI] [PubMed] [Google Scholar]
- 3.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. [DOI] [PubMed] [Google Scholar]
- 4.Lee FS, Heimer H, Giedd JN, et al. Mental health. Adolescent mental health–opportunity and obligation. Science. 2014;346(6209):547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemeroff CB. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron. 2016;89(5):892–909. [DOI] [PubMed] [Google Scholar]
- 7.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325(7374):1212–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. [DOI] [PubMed] [Google Scholar]
- 9.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatr. 2000;57(7):637–648. [DOI] [PubMed] [Google Scholar]
- 10.Neniskyte U, Gross CT. Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nat Rev Neurosci. 2017;18(11):658–670. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358(3):383–400. [DOI] [PubMed] [Google Scholar]
- 12.Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci. 2000;20(23):8780–8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cerebr Cortex. 2007;17(5):1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2(10):859–861. [DOI] [PubMed] [Google Scholar]
- 15.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. [DOI] [PubMed] [Google Scholar]
- 16.Uhlhaas PJ, Singer W. Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol Psychiatr. 2015;77(12):1001–1009. [DOI] [PubMed] [Google Scholar]
- 17.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–113. [DOI] [PubMed] [Google Scholar]
- 18.Hubel DH, Wiesel TN. Effects of monocular deprivation in kittens. Naunyn-Schmiedebergs Archiv fur experimentelle Pathologie und Pharmakologie. 1964;248:492–497. [DOI] [PubMed] [Google Scholar]
- 19.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. [DOI] [PubMed] [Google Scholar]
- 20.Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5(7):521–531. [DOI] [PubMed] [Google Scholar]
- 21.Casey BJ, Glatt CE, Lee FS. Treating the developing versus developed brain: translating preclinical mouse and human studies. Neuron. 2015;86(6):1358–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niwa M, Jaaro-Peled H, Tankou S, et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339(6117):335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niwa M, Lee RS, Tanaka T, Okada K, Kano S, Sawa A. A critical period of vulnerability to adolescent stress: epigenetic mediators in mesocortical dopaminergic neurons. Hum Mol Genet. 2016;25(7):1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337(6100):1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landek-Salgado MA, Faust TE, Sawa A. Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Mol Psychiatr. 2016;21(1):10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava R, Faust T, Ramos A, Ishizuka K, Sawa A. Dynamic changes of the mitochondria in psychiatric illnesses: new mechanistic insights from human neuronal models. Biol Psychiatry. 2018;83(9):751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–367. [DOI] [PubMed] [Google Scholar]
- 28.Graff J, Rei D, Guan JS, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483(7388):222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao HK, Hatanaka F, Araoka T, et al. In Vivo target gene activation via CRISPR/cas9-mediated trans-epigenetic modulation. Cell. 2017;171(7), 1495–1507 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32(9):485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]


