Abstract
Incubation of food craving is an abstinence-dependent increase in responding for reward-paired cues. Incubation of craving was first reported for rats responding for cocaine-paired cues, and later generalized to several drugs of abuse and for food. Incubation of drug and food craving has been reported in clinical studies as well. Incubation of food craving by rats has been reported for standard chow as well as for high fat and sucrose reinforcers. Parametric and other evaluations of the incubation of food craving reveal manipulations that reduce incubation, including environmental enrichment and pharmacological manipulation of dopamine, glutamate, and endogenous opiates. Several brain regions are likely involved in the effect, including mesolimbic terminals and the central nucleus of the amygdala. Further study of the incubation of food craving could facilitate development of treatments for cravings that precede relapse characteristic of drug and food addictions.
Keywords: abstinence, addiction, craving, drug, food, incubation, relapse
Craving is one criterion for Substance Use Disorder (American Psychiatric Association, 2013) and is closely associated with relapse to drug use following abstinence (Robbins & Ehrman, 1998). As with addiction to drugs of abuse, individuals with disordered eating experience intense cravings leading to relapse to disordered eating, or diet recidivism (Boswell & Kober, 2016). Such poorly constrained consumption of foods, in particular highly palatable foods containing fat and sugar, is contributing to a rise in overweight and obesity (CDC, 2019). A better understanding of craving is necessary to begin to address these serious public health problems.
Craving is often assessed in humans as a subjective “urge” or “desire” (Sayette, Shifmann, Tiffany, Niaura, Martin, & Shadel, 2000). In nonhuman subjects, craving is operationalized as a behavioral response directed towards the reinforcer being studied. Most rodent models of craving are variants of the “reinstatement” procedure (Epstein, Preston, Stewart, & Shaham, 2006; Shalev, Grimm, & Shaham, 2002) where previously suppressed responding is reinstated by presence of a cue previously associated with reinforcement (cue-induced), shock or anxiogenesis (stress-induced), or a sample of the reinforcer (priming-induced). This increase in responding is the objective operational definition of craving in these models. Craving, as such, has been investigated in a variety of ways including application of environmental, pharmacological, and molecular manipulations.
Incubation of Craving
One simple question is how the intensity of craving relates to the length of time since the last reinforced response. Gawin and Kleber (1986) reported that cocaine addicts experience an abstinence-dependent increase in cocaine craving. However, at that time this was the only report of this effect, and it was contrary to what many would expect. Results from subsequent studies conducted years later with cocaine self-administration experienced rats (Fig. 1, Methods detailed below) supported the Gawin and Kleber findings (Grimm, Hope, Wise, & Shaham, 2001; Tran-Nguyen et al., 1998) and have led to development of a large literature reporting on the “incubation of craving” (141 papers found in “incubation of craving” PubMed search since 2001).
Fig. 1.
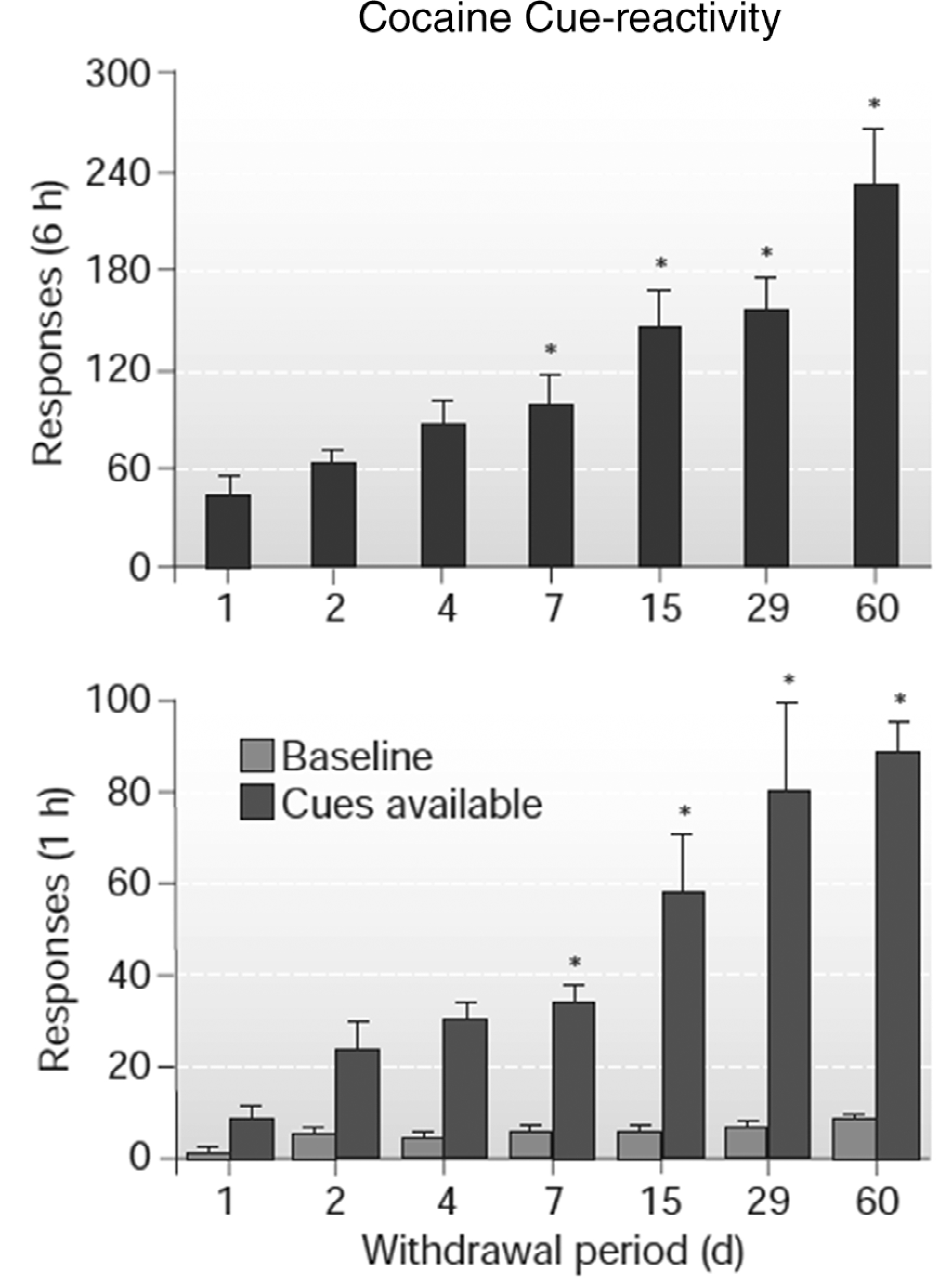
Incubation of cocaine craving in rats. Rats responded in ten 6-hr daily sessions for intravenous cocaine and a tone+light cue. After increasing days of abstinence (withdrawal) rats responded in six 1-hr extinction sessions without the cue (top panel) and a subsequent 1-hr session with response-contingent cue delivery (bottom panel). * Indicates significant difference from Day 1, p < .05. Adapted from Grimm et al., 2001. Copyright 2001 by Springer Nature.
Incubation of craving has subsequently been observed in rodent models for a variety of drugs of abuse (Li, Venniro, & Shaham, 2016) and for foods including sucrose solution (Fig. 2, Methods detailed below), standard, and high-fat chow pellets (Darling, Dingess, Schlidt, Smith, & Brown, 2016; Grimm, Fyall, & Osincup, 2005; Grimm et al., 2003; Krasnova et al., 2014). Since Gawin and Kleber (1986), incubation of craving has been described in the clinical literature again for cocaine (Parvaz, Moeller, & Goldstein, 2016) but also for methamphetamine (Wang et al., 2013), alcohol (Bienkowski et al., 2004), nicotine (Bedi et al., 2011), and heroin (Nava, Caldiroli, Premi, & Lucchini, 2006). In addition, food craving (hunger) was recently found to incubate (Coutinho, Rehfeld, Holst, Kulseng, & Martins, 2018). Overall it appears that behavior directed towards reinforcers across classes (e.g., drug and food) increases over weeks of abstinence, perhaps due to an increase in motivation, although the increase may mediated by several factors that may be more or less pronounced depending on the reinforcer class (e.g., diminished impulse control with a history of cocaine vs. sucrose taking; Burton et al., 2018).
Fig. 2.
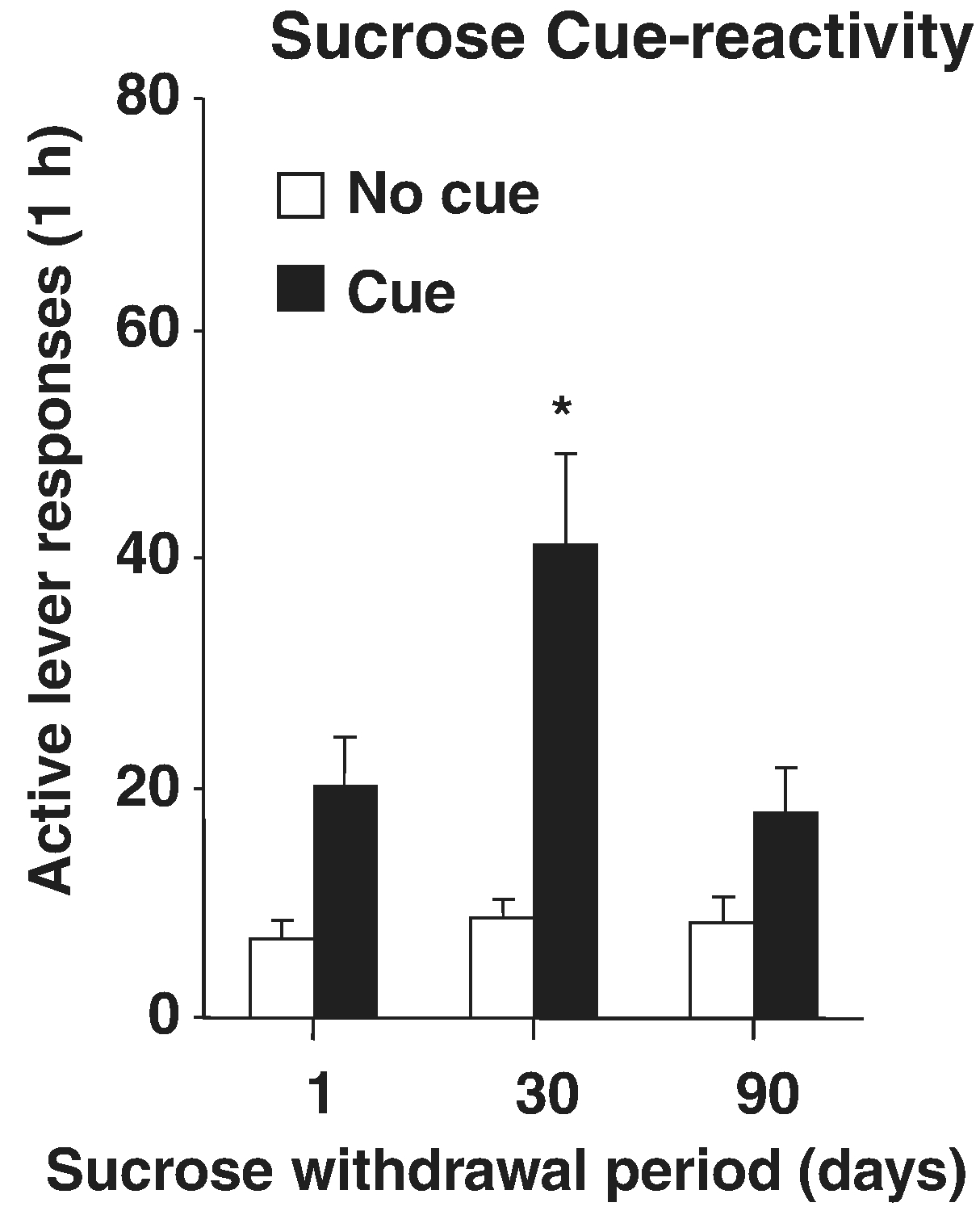
Incubation of sucrose craving in rats. Rats responded in ten 6-hr daily sessions for a liquid drop of sucrose and a tone+light cue. After 1, 30, or 90 days of abstinence (withdrawal) rats responded in six 1-hr extinction sessions without the cue (not shown here) and a subsequent 1-hr session with response-contingent cue delivery (Figure). * Indicates significant difference from Day 1, p < .05. Adapted from “Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving,” by Grimm et al. (2003). Copyright 2003 by the Society for Neuroscience.
The translational value of better understanding incubation of craving is that not only might pharmacotherapies be developed to reduce or even prevent incubation, but addicts and abstainers can be better informed that their recovery may become more difficult as they progress into abstinence. Hopefully this knowledge will also inform treatment protocols and public health perceptions regarding the difficulties individuals face when they turn away from drugs and highly palatable foods.
The purpose of this review is to describe findings in the basic literature regarding the incubation of craving for foods. Our laboratory has focused on sucrose, although we have observed incubation of craving for saccharin (Aoyama, Barnes, & Grimm, 2014) and water (nondeprivedbe rats: Grimm, Ratliff, North, Barnes, & Collins, 2012). Other laboratories have examined incubation of craving for complex foods and fats (e.g., standard or high-fat food pellets; Darling et al., 2016; Dingess et al., 2017; McCue, Kasper, & Hommel, 2019). An exhaustive review of the incubation literature reveals studies of incubation of drug craving that include incubation of food craving as a comparison condition. For example, incubation of sucrose craving is less persistent than incubation of cocaine craving (Grimm et al., 2003). After 90 days of abstinence, sucrose craving returns to the rate following 1 day of abstinence while cocaine craving remains elevated (Grimm et al.). Some mention of these studies will be made here, but the focus will be primarily on incubation of sucrose craving in stand-alone studies. That being stated, it is important to note that comparing drug and food craving directly is difficult as the reinforcing efficacies of foods and drugs vary substantially. In addition, while foods and drugs share the ability to raise synaptic levels of reward-mediating mesolimbic dopamine (Pettit and Justice, 1989; Rada, Avena, & Hoebel, 2005) they differ in effects on other neuronal systems that regulate motivation. For example, sucrose increases, while cocaine decreases, plasma insulin levels (Hallfrisch, Lazar, Jorgensen, & Reiser, 1979; You, Wang, Gardner, & Wise, 2019).
Basic Method to Examine Incubation of Sucrose Craving
Early work from our laboratory parametrically evaluated incubation of sucrose craving using rats. The basic operant procedure we have used is to first allow rats in operant conditioning chambers to respond for a drop of 10% sucrose and a tone+light cue in 10 daily sessions. This training reinforcement contingency is a fixed-ratio 1 with a time out following sucrose delivery (20 or 40 s in duration depending on the study) where a response is recorded but reinforcement is not delivered. This is essentially a fixed-interval schedule; the use of the time-out terminology is carried over from earlier comparison studies where a time-out was necessary to avoid overdose in drug self-administration studies. Testing for craving is responding in the absence of sucrose reinforcement, typically where a response results in presentation of a cue previously associated with reinforcement. To evaluate time-dependent changes in craving, craving (indicated as “cue reactivity”, “responding for the cue”, “cue-induced craving”, or“sucrose seeking” in various publications) is measured the day after the last day of training (early abstinence) or 1 month later (later abstinence) (Fig. 3).
Fig. 3.
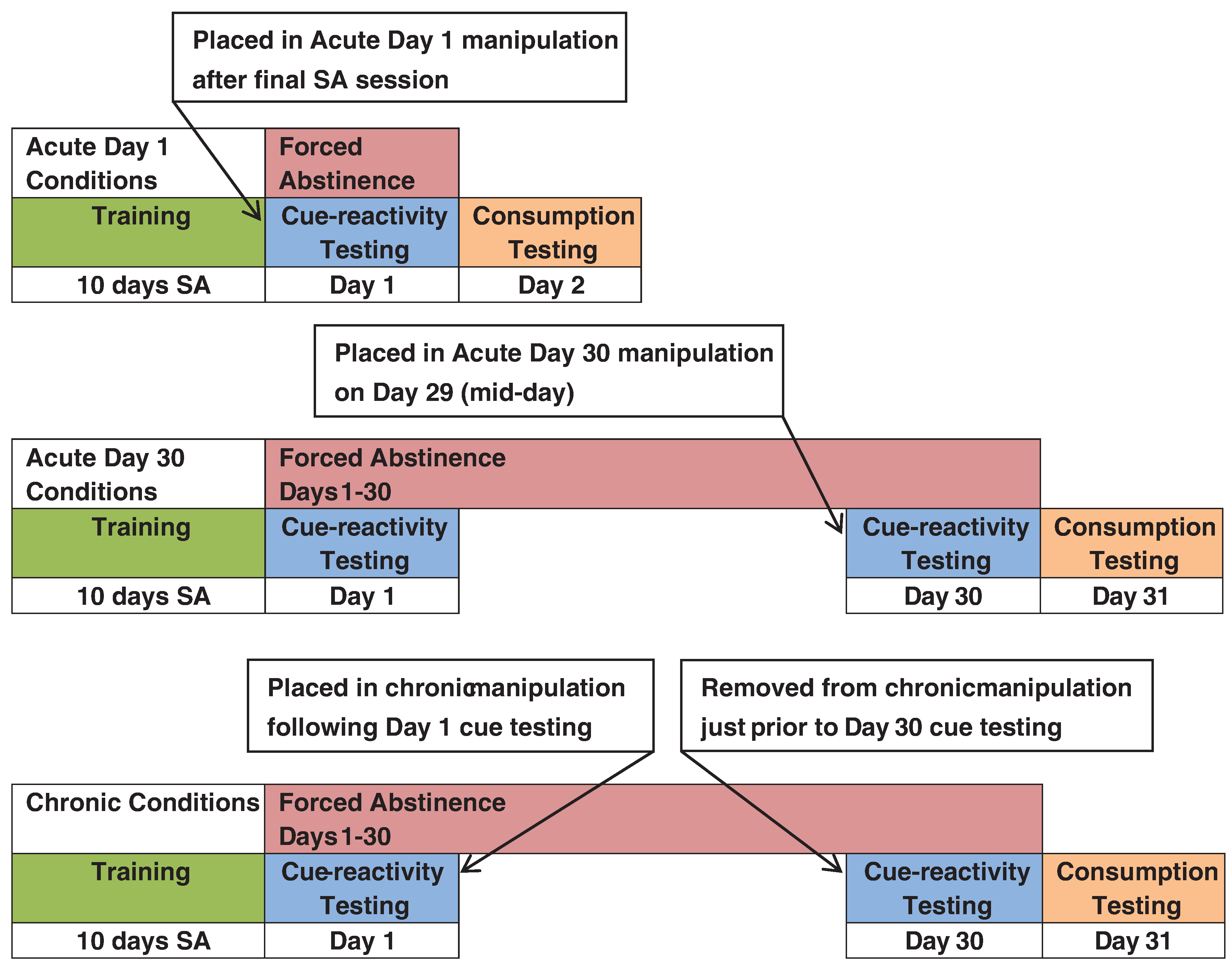
General Method. Adapted from “Brief exposure to novel or enriched environments reduces sucrose cue-reactivity and consumption in rats after 1 or 30 days of forced abstinence from self-administration,” by Grimm et al., 2013. Copyright 2013 by the authors.
In early studies we evaluated craving as both responding in extinction conditions for several hours (response produces neither the reinforcer nor the cue) followed by reintroduction of cue deliveries (cue-induced reinstatement), but in later studies we measured craving as responding during a modified extinction procedure where responding produces the cue and the sound of the infusion pump, but no sucrose is delivered. This approach allowed us to reduce the duration of the test session to make it more amenable to behavioral pharmacology and molecular biology studies. In either case, when the cue is available its delivery is contingent upon a lever press because we found that contingent cue presentations produce the most robust responding (Grimm and See, 2000). In addition, in some studies we include measurement of incubation of sucrose self-administration (“taking”). Taking (“Consumption Testing” in Fig. 3) is simply placing subjects back into training conditions in which responding results in delivery of sucrose and the sucrose-paired cue and measuring rate of responding for sucrose delivered as during training. As with sucrose craving, sucrose taking incubates (Fig. 4).
Fig. 4.
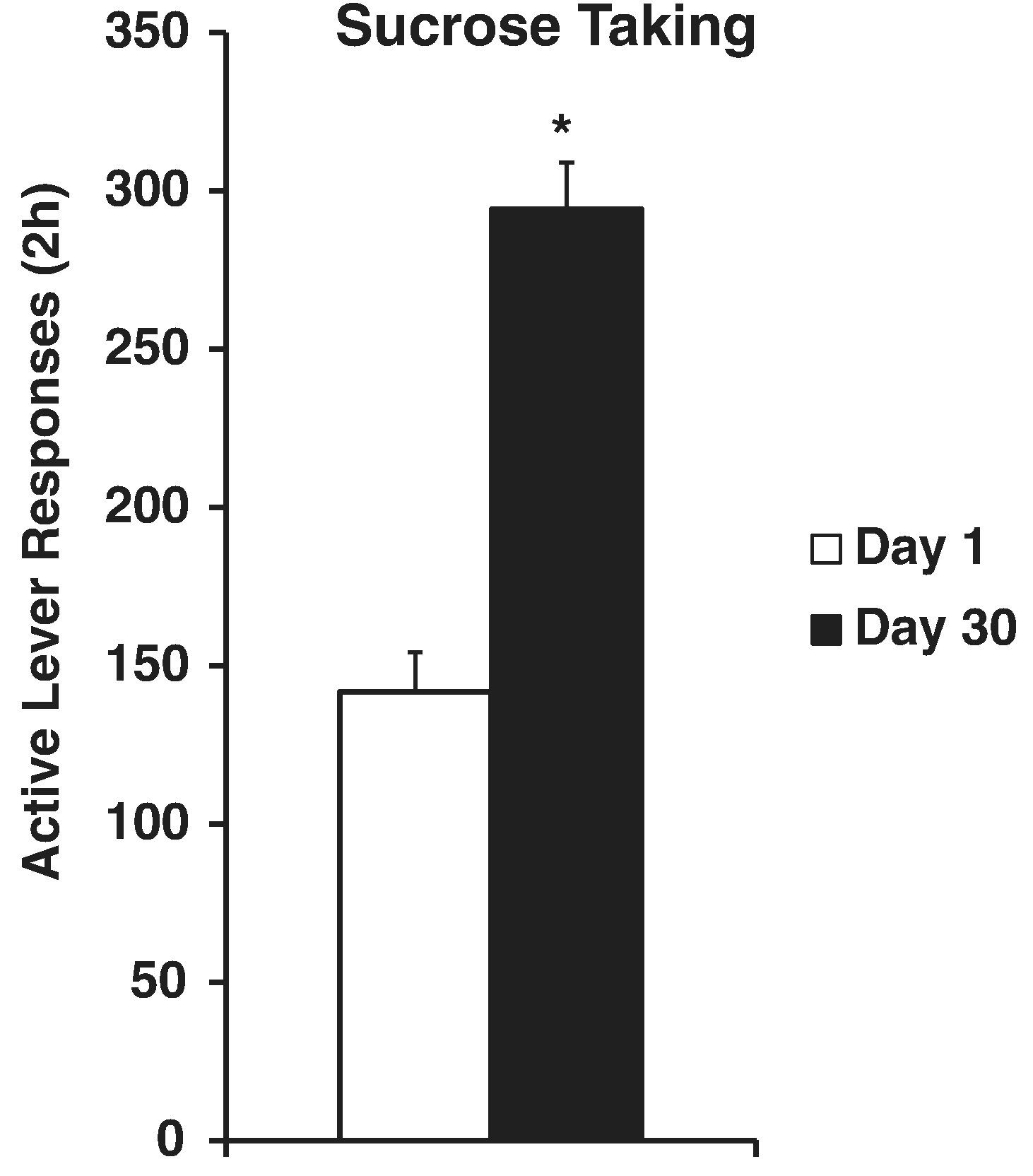
Incubation of sucrose taking in rats. Rats responded in ten 2-hr daily sessions for a liquid drop of sucrose and a tone+light cue. After 1 or 30 days of abstinence rats responded in a 2-hr extinction session with the cue (not shown here). The next day rats responded in a 2-hr session with the same reinforcement contingencies as during training. * Indicates significant difference from Day 1, p < .05. Adapted from Grimm et al., 2013. Copyright 2013 by the authors.
Initial Findings
Our first parametric evaluation revealed that incubation of sucrose craving was difficult to alter. One manipulation in this set of experiments was to sate rats with sucrose by providing unlimited access to sucrose for an extended period prior to a craving test. Our hypothesis was that satiation would counter the increased sucrose-directed responding observed with incubation. In this first study (Grimm et al., 2005), and replicated later with a slightly different procedure (Harkness, Wells, Webb, & Grimm, 2016), either the afternoon of the 10th training session (early abstinence), or 29 days later (later abstinence) a bottle of 10% sucrose replaced home cage drinking water. Rats avidly consumed bottled sucrose and this reduced sucrose craving 17 h later, but only at the early abstinence time point (Fig. 5).
Fig. 5.
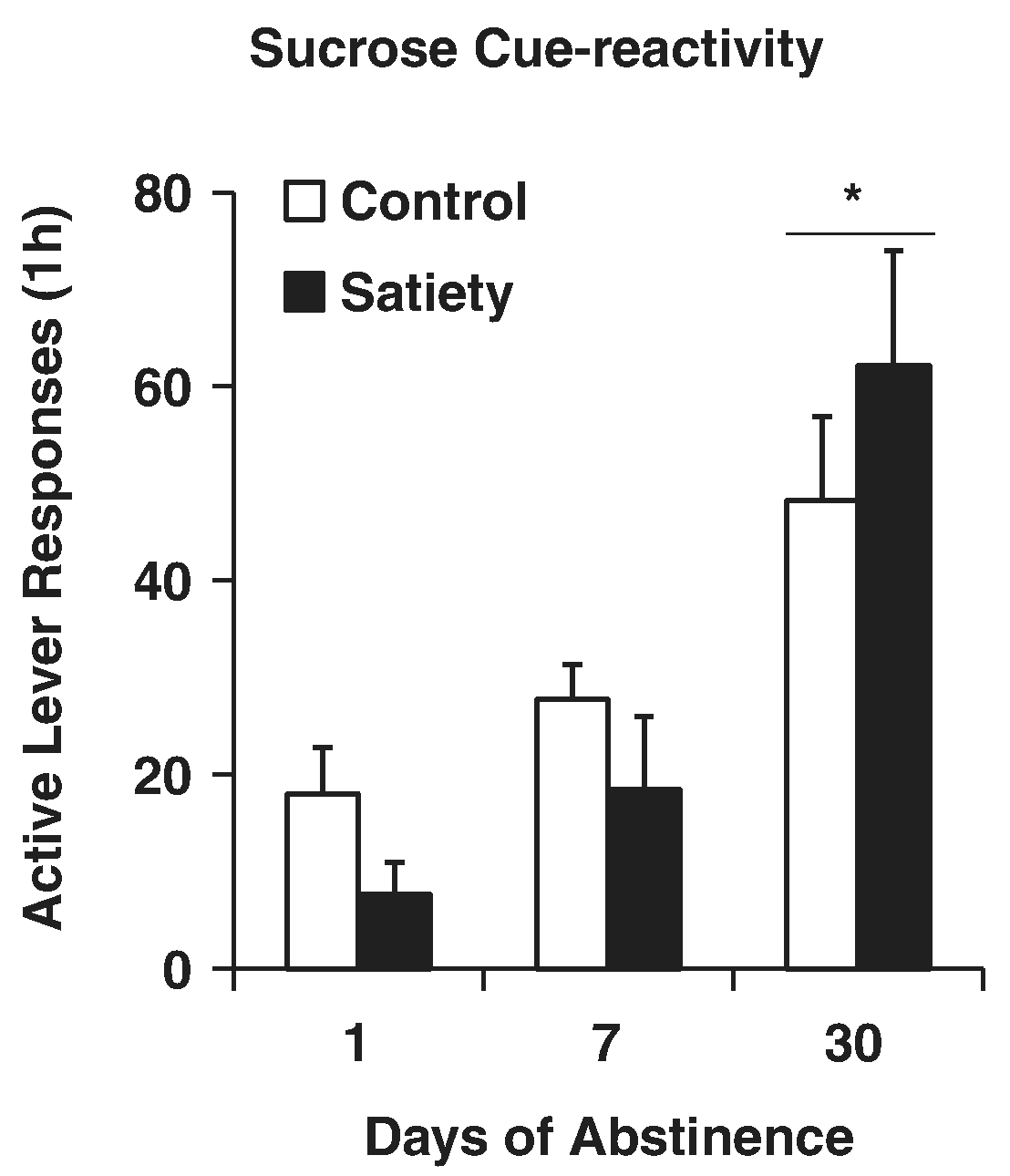
Incubation of sucrose craving is not attenuated by sucrose satiation. Rats responded in ten 6-hr daily sessions for a liquid drop of sucrose and a tone+light cue. Either the afternoon of the 10th day of training, or 29 days later, rats were provided a bottle of 10% sucrose in the home cage until immediately before cue-reactivity testing the next day. During the next day testing, rats responded in six 1-hr extinction sessions without the cue (not shown here) and a subsequent 1-hr session with response-contingent cue delivery (Figure). * Indicates significant difference from Day 1, p < .05. Adapted from Grimm et al., 2005. Copyright 2004 by Elsevier Inc. Reprinted with permission.
Although sucrose “satiety” was not effective at reducing incubation of sucrose craving, subsequent studies revealed means to reduce incubation. These include learning, environmental, and pharmacological manipulations. Molecular biology studies have also provided a means to identify structures and molecular targets for further study.
Learning Manipulation
LiCl-mediated sucrose taste aversion effectively transferred to the sucrose-paired cue after 1 month, but not 1 day of abstinence (Harkness, Webb, & Grimm, 2010). Briefly, rats were trained using self-administration procedures outlined above. For the 2 days immediately after training, or starting 28 days later, rats received injections of illness-inducing LiCl paired with sucrose access in a novel location. The next day the rats responded for the sucrose-paired cue and the day after that, for sucrose itself. As stated above, sucrose craving was significantly reduced after LiCl-sucrose pairing only for rats that had the pairing in late abstinence (Fig. 6). Responding for sucrose itself was very low for all rats that had received LiCl-sucrose pairings. These results could mean that the association between sucrose and the tone+light cue, that is the memory of the relevance of the cue, becomes more flexible with incubation (Harkness et al., 2010).
Fig. 6.
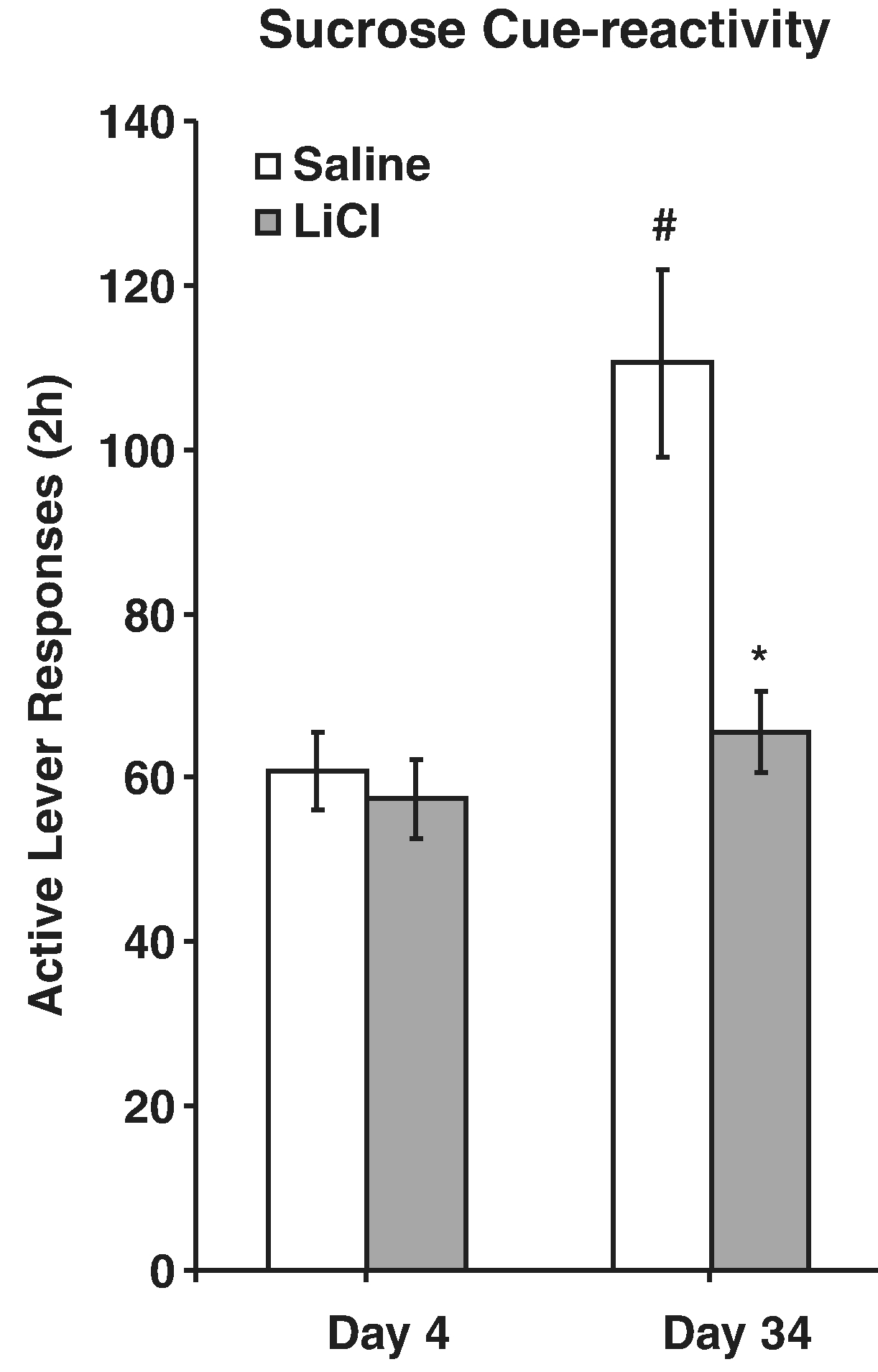
Incubation of sucrose craving is attenuated by recent devaluation of sucrose with LiCl. Rats responded in ten 2-hr daily sessions for a liquid drop of sucrose and a tone+light cue. Either the next 3 days, or days 31–33 of abstinence, sucrose was paired with LiCl in a novel environment. The next day rats were tested for sucrose cue-reactivity by allowing them to respond in extinction conditions including the tone+light cue. # Indicates significant difference from Day 1 Saline control, * indicates significant difference from Day 30 Saline control, p’s < .05. Adapted from Harkness et al., 2010. Copyright 2009 by Springer-Verlag. Reprinted with permission.
Environmental Manipulation
We found that placing rats into a large, novel environment either overnight (acute) or for nearly a month (chronic) reduced sucrose craving and taking (Grimm et al., 2008; Grimm et al., 2013) (Fig. 7). For our studies, this environmental enrichment (EE) consists of placing three or four rats into a large, multilevel environment with hiding places and novel items (pet toys). These are rotated every M, W, F for chronic studies. Whether EE disrupts incubation will require further study. EE was found to reduce incubated cocaine craving (Thiel, Painter, Pentkowski, Mitroi, Crawford, & Neisewander, 2012), yet the low level of craving behavior was significantly greater than an early-abstinence EE comparison indicating that some amount of incubation persists. This was also the case with our studies, for example, comparing responding of rats that were enriched just prior to craving testing on Day 1 versus Day 30 of abstinence (EE Acute conditions, Fig. 7). That being said, EE reduces incubated craving levels for either cocaine or sucrose to near or below levels in early abstinence. We have hypothesized that EE reduces craving by creating a negative behavioral contrast between the EE and the self-administration contexts (Grimm et al., 2019). While the comparison is still speculative, there may be some shared features of the EE-mediated contrast and successive negative contrast observed as a decrease in sucrose intake with an incentive downshift from a high to low sucrose concentration (Genn, Ahn, & Phillips, 2004).
Fig. 7.
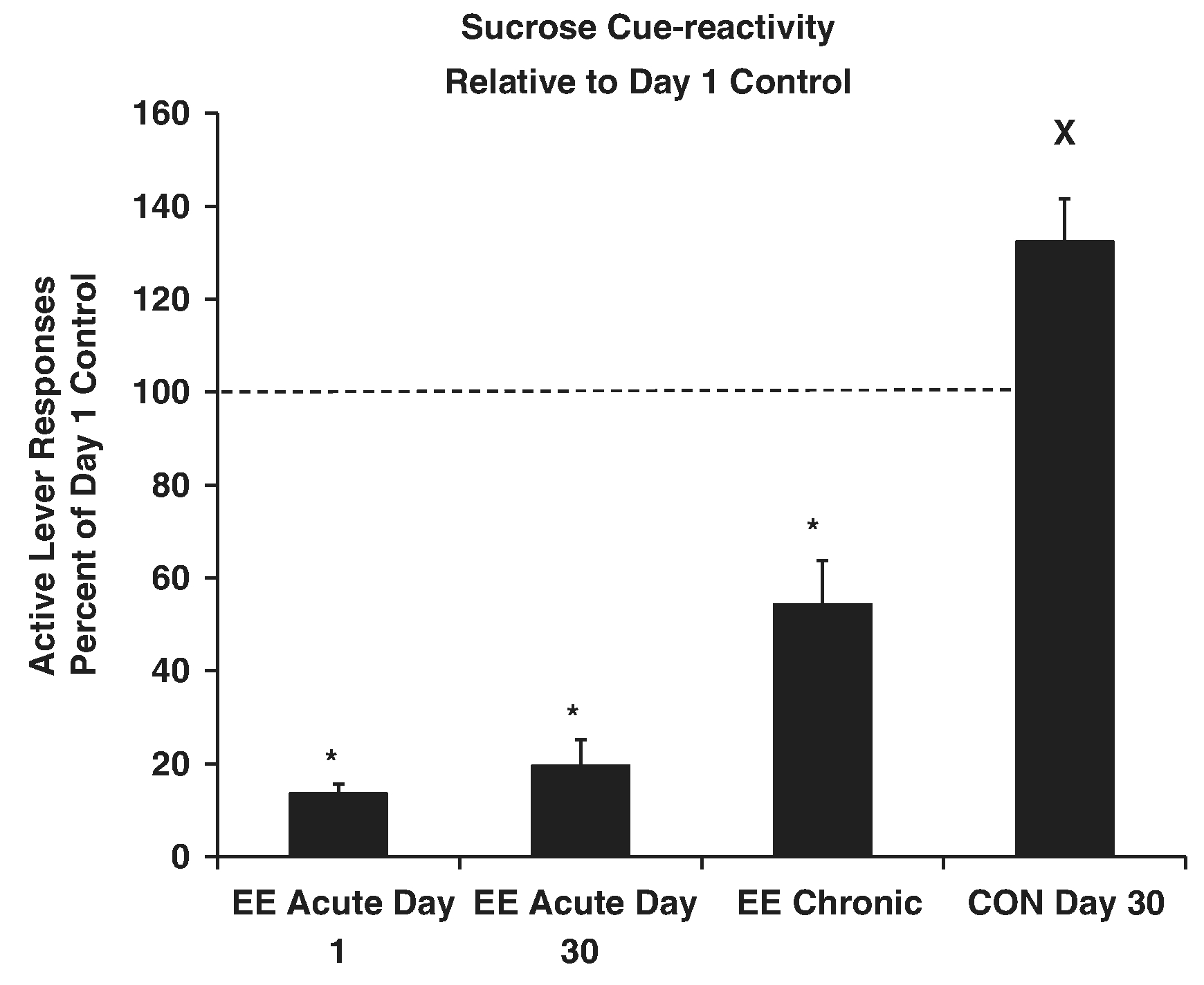
Incubation of sucrose craving is attenuated by Environmental Enrichment. Rats responded in ten 2-hr daily sessions for a liquid drop of sucrose and a tone+light cue. For acute EE, rats were placed into EE the afternoon of the 10th day of training or the afternoon of the 29th day of abstinence. Testing occurred the next day (Day 1 or Day 30 of abstinence). For chronic EE, rats were placed into EE from the afternoon of the 10th day of training until testing on Day 30 of abstinence. Rats were tested for sucrose cue-reactivity by allowing them to respond in extinction conditions including the tone+light cue. Control (CON) Day 30 was significantly greater than CON Day 1 responding (not shown on Figure). Data in this figure have been transformed to represent percent of the Day 1 abstinence control average responding. *Indicates significant difference from CON Day 30, X indicates significant difference from EE Acute Day 1, p’s < .05. Adapted from Grimm et al., 2013. Copyright 2013 by the authors.
Pharmacological Manipulations
We have also evaluated incubation of sucrose craving by targeting several neurotransmitter systems and, as noted above, some drugs have abstinent-dependent effects on craving behavior. In our first study, we simply challenged rats with a range of doses of the indirect dopamine agonist cocaine just before a craving test. Cocaine increased sucrose craving, but the dose-effect curve was shifted to the right for rats tested in later versus early abstinence. We interpreted this to be due to an abstinence-dependent change in function of the brain dopamine system (Grimm et al., 2006). This interpretation was based on comparing the established roles for brain dopamine subsystems (e.g., nigrostriatal, mesolimbic, mesocortical) with the apparent increased motivation to respond for reward-paired cues observed with incubation of craving. These systems are involved in a range of behaviors that sustain responding directed at rewarding stimuli, including initiation of movement, habit, reward, and motivation (Wise, 2013). To focus in on what aspect(s) of the dopamine system are most relevant, we challenged rats with the dopamine D1 receptor antagonist SCH23390 just before a craving test. D1 antagonism reduced later abstinence responding to levels seen in early abstinence, effectively averting incubation (Fig. 8). Importantly, doses that reduced responding did not necessarily decrease general locomotion. These results indicate that dopamine signaling specific to behavior directed towards a sucrose cue is altered during protracted abstinence. This is supported by our subsequent finding of an abstinence-dependent increase in dopamine D1 agonist-enhanced sucrose craving (Glueck, Ginder, Hyde, North, & Grimm, 2017). The D1 antagonism effect with incubation was not found to be specific to the nucleus accumbens core or shell (mesolimbic dopamine terminals) however (Grimm et al., 2011), indicating that changes in other brain regions and/or neurotransmitter systems mediate incubation of sucrose craving.
Fig. 8.
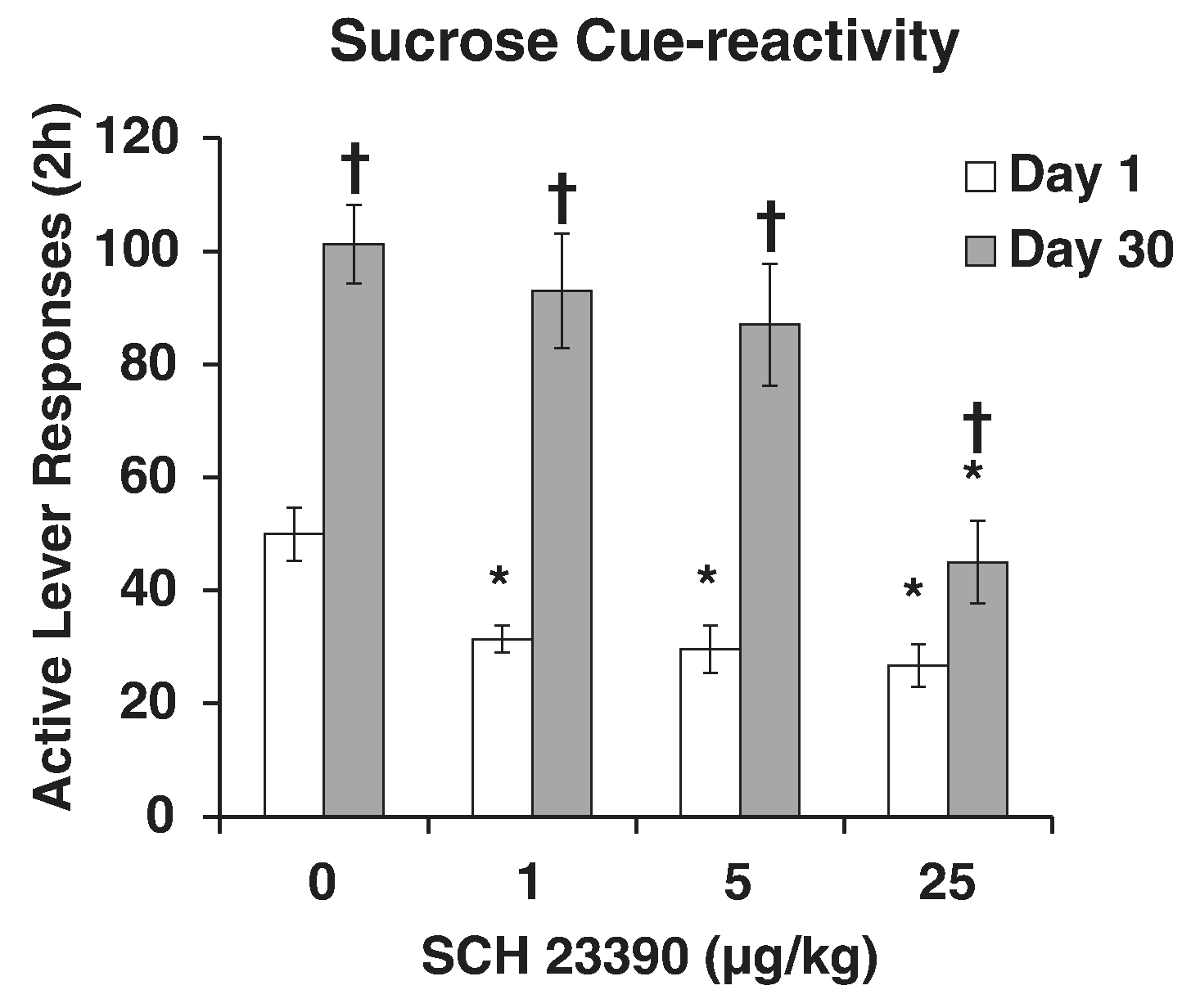
Incubation of sucrose craving is attenuated by a dopamine D1 receptor antagonist. Rats responded in ten 2-hr daily sessions for a liquid drop of sucrose and a tone +light cue. Rats were tested for sucrose cue-reactivity by allowing them to respond in extinction conditions including the tone+light cue either 1 or 30 days into sucrose abstinence. Fifteen minutes before testing, rats were injected with saline or one of three doses of SCH23390. * Indicates significant difference from saline on that day of abstinence, † indicates significant difference from Day 1 at that dose, p’s < .05. Adapted from Grimm et al., 2011. Copyright 2011 by Springer-Verlag. Reprinted with permission.
We, and others, have examined some of these other regions and/or neurotransmitter systems in relation to incubation of sucrose craving. Thus far, we have found that an opiate antagonist (naloxone) selectively reduces incubated sucrose craving (Grimm, Manaois, Osincup, Wells, & Buse, 2007). Glutamate neurotransmission is another target of interest. LY379268 (mGluR2/3 agonist) disrupts incubation of sucrose craving (systemic and intra-central amygdala) (Uejima, Bossert, Poles, & Lu, 2007). These LY379268 results are of particular interest for addiction in general, as similar effects were observed with incubation of cocaine craving (Lu, Uejima, Gray, Bossert, & Shaham, 2007).
Molecular Studies
Molecular biology measures provide a means to further reveal brain site signaling-cascades related to incubation of food craving. As yet, there are only a handful of studies relating molecular markers with incubation of food craving. In our first studies, incubation of sucrose and cocaine craving were compared, and whereas there were some findings related to a history of cocaine self-administration, there were no abstinent-dependent changes in dopamine level regulating proteins tyrosine hydroxylase or dopamine transporter (Grimm, Shaham, & Hope, 2002). Nor were there sucrose craving incubation-related changes in neuroplasticity-related brain derived neurotrophic factor protein levels (Grimm et al., 2003) in several brain regions examined. Similarly, Swinford-Jackson, Anastasio, Fox, Stutz, and Cunningham (2016) observed decreased serotonin 2C receptors in the prefrontal cortex along with incubation of cocaine, but not sucrose, craving, and microdialysis measures of ventromedial prefrontal cortex dopamine were associated with incubation of cocaine, but not sucrose, craving (Shin et al., 2016). Serotonin 2C receptors have been associated with appetite (Halford & Harrold, 2012). Finally, incubation of sucrose craving was not associated with changes in cortical (prelimbic, infralimbic, orbitofrontal) perineuronal net densities (Slaker, Barnes, Sorg, & Grimm, 2016). These densities are hypothesized to be recruited to “stabilize” synapses and have been related to memory formation and retention (Slaker et al., 2018). In contrast to these negative effects, using a molecular manipulation, McCue et al. (2019) reported that knockdown of neuromedin U receptor 2 in the paraventricular nucleus of the hypothalamus prevents incubation of high-fat food craving. Neuromedin is a neuropeptide involved in many behaviors, including feeding (Smith, Kasper, Ara, Anastasio, & Hommel, 2019; Vallöf, Kalafateli, & Jerlhag, 2019).
“Mapping” studies have revealed more details regarding the neuronal substrates of incubation of food craving. Although Fos immunoreactivity (IR), a measure of recent neuronal activation, was not associated with incubation of chocolate-flavored pellets craving (Noye Tuplin & Holahan, 2019), we observed Fos IR “incubate” in regions involved in several aspects of reward processing (prelimbic, infralimbic, anterior cingulate, somatosensory cortices, dorsolateral striatum, core and shell of the nucleus accumbens, and central amygdala) (Grimm et al., 2016). In contrast to the negative findings with brain-derived neurotrophic factor and perineuronal nets described above, we recently reported abstinence-dependent changes in DARPP32 signaling (phosphorylation at Threonine 34) in ventral tegmental area, dorsolateral and dorsomedial striatum, and orbitofrontal cortex (Grimm et al., 2018). These are regions involved in reward, habit, and reward valuation (Wise, 2013). DARPP32 is a signaling cascade molecule that can serve to integrate dopamine and glutamate receptor stimulation to accelerate or brake neuronal plasticity (Walaas, Hemmings, Greengard, & Nairn, 2011). Figure 9 depicts representative results from our Fos and DARPP32 studies demonstrating incubation of Fos IR and phosphorylation of DARPP32 at its threonine 34 in the dorsolateral striatum, a region involved in habit formation.
Fig. 9.
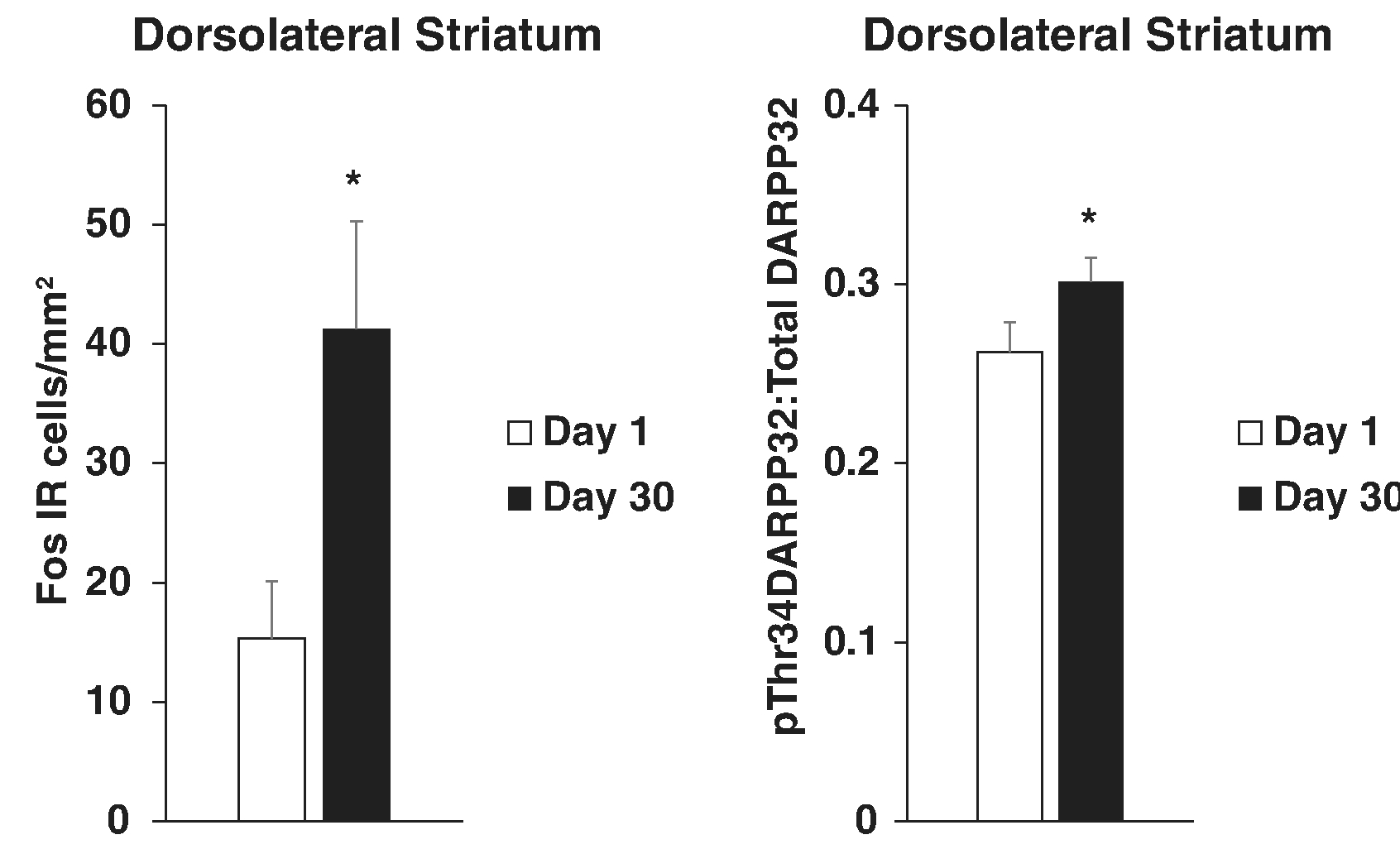
Incubation of sucrose craving is associated with elevated dopamine-related neuronal signaling in several brain regions including the dorsolateral striatum. Rats responded in ten 2-hr daily sessions for a liquid drop of sucrose and a tone+light cue. Rats were tested for sucrose cue-reactivity by allowing them to respond in extinction conditions including the tone+light cue either 1 or 30 days into sucrose abstinence (DARPP32 study also included “no test” controls). Brains were examined after testing for Fos IR (left panel) or levels of total and threonine 34 phosphorylated DARPP32 (right panel). * Indicates significant difference from Day 1, p < .05. Left panel adapted from Grimm et al., 2016. Copyright 2015 by Springer-Verlag Berlin Heidelberg. Reprinted with permission. Right panel adapted from Grimm et al., 2018. Copyright 2018 by Springer Nature under the terms of the Creative Commons, creativecommons.org/licenses/by/4.0/.
Related to the findings with DARPP32, other laboratories have reported plasticity-related changes correlating with incubation of food craving. Specifically, incubation of sucrose craving was associated with a decreased glutamate AMPA/NMDA receptor ratio in the nucleus accumbens (a mesolimbic dopamine terminal region, noted above; Counotte, Schiefer, Shaham, & O’Donnell, 2014), while incubation of standard or high-fat pellet craving was associated with food-type specific changes in nucleus accumbens dendritic spine density and AMPA receptors (Dingess et al., 2017).
Overall these findings reveal several brain regions that could be involved in the incubation of sucrose craving and/or incubation of food craving in general. Glutamate in the central nucleus of the amygdala is the only region so far demonstrated through both mapping and functional studies to be necessary for the incubation of sucrose craving (Grimm et al., 2016; Roura-Martinez et al., 2019; Uejima et al., 2007). Dopamine receptors, in particular the D1 receptor, along with the neuropeptide neuromedin and the signaling-cascade protein DARPP32 are also related to incubation of sucrose craving. Further studies are required to further characterize what may be a complex interplay of plasticity of brain regions and molecular signals that mediate the incubation of food craving. Designs of such studies might incorporate the functional approach of microinjecting compounds to agonize or antagonize a receptor of interest (e.g., akin to the D1 antagonist study described above) or alter expression of a particular protein that might be important in the incubation process.
Summary and Conclusions
In summary, craving for both drug and food reinforcers measured as responding for drug or food-paired cues incubates over abstinence, as does responding for sucrose itself. The incubation of responding for sucrose cues, incubation of sucrose craving, is a robust response that persists even if rats have been sated with sucrose. Incubation of sucrose craving can be reduced through learning (LiCl-mediated taste aversion), environmental enrichment, and various pharmacological manipulations. Several brain regions known to be involved in habit, reward, and motivation are associated with the incubation of sucrose/food craving assessed with plasticity markers including Fos, dendritic spine changes, and AMPA/NMDA ratios, as are neurotransmitters dopamine and glutamate, neuromodulator neuromedin U, and the signaling cascade molecule DARPP32.
To further the translational value of this work, future studies are required to evaluate the generality of incubation across reinforcers, including foods containing various sugar and fat combinations (Naleid et al., 2008). In addition, investigations including comparisons of sex and age are warranted. One salient feature of incubation research is the critical role of time (abstinence). In addition to changes in motivation, as described above, incubation may be driven by abstinence-dependent changes in memory and perhaps stress-responsivity. These time-dependent changes may overlap to some extent with those mediating the saccharin elation and ethanol deprivation effects (Pinel & Huang, 1976) and the recently described incubation of fear (Pajser, Limoges, Long, & Pickens, 2019). It is possible that findings from detailed examination of the incubation of food craving will inform treatment strategies for addictions in general, but especially for disordered eating including diet recidivism.
Acknowledgments
J.W. Grimm’s research team’s contributions were supported by NIH NIDA DA016285(1–5) and the State of Washington.
References
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington DC: Author. [Google Scholar]
- Aoyama K, Barnes J, & Grimm JW (2014). Incubation of saccharin craving and within-session changes in responding for a cue previously associated with saccharin. Appetite, 72, 114–122. 10.1016/j.appet.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, & de Wit H (2011). Incubation of cue-induced cigarette craving during abstinence in human smokers. Biological Psychiatry, 69 (7), 708–711. 10.1016/j.biopsych.2010.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L, … Kostowski W. (2004). Time-dependent changes in alcohol-seeking behaviour during abstinence. European Neuropsychopharmacology, 14 (5), 355–360. 10.1016/j.euroneuro.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Boswell RG, & Kober H (2016). Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obesity Reviews, 17(2), 159–177. 10.1111/obr.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AC, Bissonette GB, Vazquez D, Blume EM, Donnelly M, Heatley KC, … Roesch MR. (2018). Previous cocaine self-administration disrupts reward expectancy encoding in ventral striatum. Neuropsychopharmacology, 43(12), 2350–2360. 10.1038/s41386-018-0058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2019). Overweight and obesity. Retrieved from www.cdc.gov/obesity October 9, 2019.
- Counotte DS, Schiefer C, Shaham Y, & O’Donnell P (2014). Time-dependent decreases in nucleus accumbens AMPA/NMDA ratio and incubation of sucrose craving in adolescent and adult rats. Psychopharmacology, 231(8), 1675–1684. 10.1007/s00213-013-3294-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho SR, Rehfeld JF, Holst JJ, Kulseng B, & Martins C (2018). Impact of weight loss achieved through a multidisciplinary intervention on appetite in patients with severe obesity. American Journal of Physiology: Endocrinology and Metabolism, 315(1), E91–E98. 10.1152/ajpendo.00322.2017 [DOI] [PubMed] [Google Scholar]
- Darling RA, Dingess PM, Schlidt KC, Smith EM, & Brown TE (2016). Incubation of food craving is independent of macronutrient composition. Scientific Reports, 6, 30900. 10.1038/srep30900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingess PM, Darling RA, Derman RC, Wulff SS, Hunter ML, Ferrario CR, & Brown TE (2017). Structural and functional plasticity within the nucleus accumbens and prefrontal cortex associated with time-dependent increases in food cue-seeking behavior. Neuropsychopharmacology, 42(12), 2354–2364. 10.1038/npp.2017.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, & Shaham Y (2006). Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology, 189(1), 1–16. 10.1007/s00213-006-0529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH, & Kleber HD (1986). Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Archives of General Psychiatry, 43(2), 107–113. 10.1001/archpsyc.1986.01800020013003 [DOI] [PubMed] [Google Scholar]
- Genn RF, Ahn S, & Phillips AG (2004). Attenuated dopamine efflux in the rat nucleus accumbens during successive negative contrast. Behavioral Neuroscience, 118(4), 869–873. 10.1037/0735-7044.118.4.869 [DOI] [PubMed] [Google Scholar]
- Glueck E, Ginder D, Hyde J, North K, & Grimm JW (2017). Effects of dopamine D1 and D2 receptor agonists on environmental enrichment attenuated sucrose cue reactivity in rats. Psychopharmacology, 234 (5), 815–825. 10.1007/s00213-016-4516-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Barnes JL, Koerber J, Glueck E, Ginder D, Hyde J, & Eaton L (2016). Effects of acute or chronic environmental enrichment on regional Fos protein expression following sucrose cue-reactivity testing in rats. Brain Structure & Function, 221(5), 2817–2830. 10.1007/s00429-015-1074-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Buse C, Manaois M, Osincup D, Fyall A, & Wells B (2006). Time-dependent dissociation of cocaine dose-response effects on sucrose craving and locomotion. Behavioural Pharmacology, 17(2), 143–149. 10.1097/01.fbp.0000190686.23103.f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, & Osincup DP (2005). Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiology and Behavior, 84 (1), 73–79. 10.1016/j.physbeh.2004.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Glueck E, Ginder D, Hyde J, North K, & Jiganti K (2018). Sucrose abstinence and environmental enrichment effects on mesocorticolimbic DARPP32 in rats. Scientific Reports, 8(1), 13174. 10.1038/s41598-018-29625-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Harkness JH, Ratliff C, Barnes J, North K, & Collins S (2011). Effects of systemic or nucleus accumbens-directed dopamine D1 receptor antagonism on sucrose seeking in rats. Psychopharmacology, 216(2), 219–233. 10.1007/s00213-011-2210-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, & Shaham Y (2001). Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature, 412(6843), 141–142. 10.1038/35084134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hyde J, Glueck E, North K, Ginder D, Jiganti K, … Hovander D. (2019). Examining persistence of acute environmental enrichment-induced anti-sucrose craving effects in rats. Appetite, 139, 50–58. 10.1016/j.appet.2019.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, & Shaham Y (2003). Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. Journal of Neuroscience, 23(3), 742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Manaois M, Osincup D, Wells, Buse C. (2007). Naloxone attenuates incubated sucrose craving in rats. Psychopharmacology, 194(4), 537–544. 10.1007/s00213-007-0868-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, & Harkness JH (2008). Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behavioural Pharmacology, 19(8), 777–785. 10.1097/FBP.0b013e32831c3b18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Ratliff C, North K, Barnes J, & Collins S (2012). Nicotine increases sucrose self-administration and seeking in rats. Addiction Biology, 17(3), 623–633. 10.1111/j.1369-1600.2012.00436.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, & See RE (2000). Contingent access to stimuli associated with cocaine self-administration is required for reinstatement of drug-seeking behavior. Psychobiology, 28(3), 383–386. [Google Scholar]
- Grimm JW, Shaham Y, & Hope BT (2002). Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behavioural Pharmacology, 13(5–6), 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Weber R, Barnes J, Koerber J, Dorsey K, & Glueck E (2013). Brief exposure to novel or enriched environments reduces sucrose cue-reactivity and consumption in rats after 1 or 30 days of forced abstinence from self-administration. PloS One, 8(1), e54164. 10.1371/journal.pone.0054164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford JC, & Harrold JA (2012). 5-HT(2C) receptor agonists and the control of appetite. Handbook of Experimental Pharmacology, 209, 349–356. 10.1007/978-3-642-24716-3_16 [DOI] [PubMed] [Google Scholar]
- Hallfrisch J, Lazar F, Jorgensen C, & Reiser S (1979). Insulin and glucose responses in rats fed sucrose or starch. American Journal of Clinical Nutrition, 32(4), 787–793. 10.1093/ajcn/32.4.787 [DOI] [PubMed] [Google Scholar]
- Harkness JH, Webb S, & Grimm JW (2010). Abstinence-dependent transfer of lithium chloride-induced sucrose aversion to a sucrose-paired cue in rats. Psychopharmacology, 208(4), 521–530. 10.1007/s00213-009-1755-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness JH, Wells J, Webb S, & Grimm JW (2016). Extended exposure to environmental cues, but not to sucrose, reduces sucrose cue reactivity in rats. Learning & Behavior, 44(1), 59–66. 10.3758/s13420-015-0190-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM, … Cadet JL. (2014). Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology, 39(8), 2008–2016. 10.1038/npp.2014.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Venniro M, & Shaham Y (2016). Translational research on incubation of cocaine craving. JAMA Psychiatry, 73(11), 1115–1116. 10.1001/jamapsychiatry.2016.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, & Shaham Y (2007). Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biological Psychiatry, 61(5), 591–598. 10.1016/j.biopsych.2006.04.011 [DOI] [PubMed] [Google Scholar]
- McCue DL, Kasper JM, & Hommel JD Incubation of feeding behavior is regulated by neuromedin U receptor 2 in the paraventricular nucleus of the hypothalamus. Behavioural Brain Research, 359, 763–770. 10.1016/j.bbr.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naleid AM, Grimm JW, Kessler DA, Sipols AJ, Aliakbari S, Bennett JL, … Figlewicz DP. (2008). Deconstructing the vanilla milkshake: the dominant effect of sucrose on self-administration of nutrient-flavor mixtures. Appetite, 50(1), 128–138. 10.1016/j.appet.2007.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava F, Caldiroli E, Premi S, & Lucchini A (2006). Relationship between plasma cortisol levels, withdrawal symptoms and craving in abstinent and treated heroin addicts. Journal of Addictive Diseases, 25(2), 9–16. 10.1300/J069v25n02_02 [DOI] [PubMed] [Google Scholar]
- Noye Tuplin EW, & Holahan MR (2019). Exploring time-dependent changes in conditioned place preference for food reward and associated changes in the nucleus accumbens. Behavioural Brain Research, 361, 14–25. 10.1016/j.bbr.2018.12.031 [DOI] [PubMed] [Google Scholar]
- Pajser A, Limoges A, Long C, & Pickens CL (2019). Individual differences in voluntary alcohol consumption are associated with conditioned fear in the fear incubation model. Behavioural Brain Research, 362, 299–310. 10.1016/j.bbr.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, & Goldstein RZ (2016). Incubation of cue-Induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry, 73(11), 1127–1134. 10.1001/jamapsychiatry.2016.2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, & Justice JB Jr. (1989). Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacology, Biochemistry and Behavior, 34(4), 899–904. 10.1016/0091-3057(89)90291-8 [DOI] [PubMed] [Google Scholar]
- Pinel JP, & Huang E (1976). Effects of periodic withdrawal on ethanol and saccharin selection in rats. Physiology and Behavior, 16(6), 693–698. 10.1016/0031-9384(76)90238-9 [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, & Hoebel BG (2005). Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience, 134(3), 737–744. 10.1016/j.neuroscience.2005.04.043 [DOI] [PubMed] [Google Scholar]
- Robbins SJ, & Ehrman RN (1998). Cocaine use is associated with increased craving in outpatient cocaine abusers. Experimental and Clinical Psychopharmacology, 6(2), 217–224. [DOI] [PubMed] [Google Scholar]
- Roura-Martinez D, Ucha M, Orihuel J, Ballesteros-Yanez I, Castillo CA, Marcos A, … Higuera-Matas A. (2019). Central nucleus of the amygdala as a common substrate of the incubation of drug and natural reinforcer seeking. Addiction Biology. 10.1111/adb.12706 [DOI] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, & Shadel WG (2000). The measurement of drug craving. Addiction, 95 Suppl 2, S189–210. 10.1080/09652140050111762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, & Shaham Y (2002). Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacological Reviews, 54(1), 1–42. [DOI] [PubMed] [Google Scholar]
- Shin CB, Serchia MM, Shahin JR, Ruppert-Majer MA, Kippin TE, & Szumlinski KK (2016). Incubation of cocaine-craving relates to glutamate over-flow within ventromedial prefrontal cortex. Neuropharmacology, 102, 103–110. 10.1016/j.neuropharm.2015.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaker M, Barnes J, Sorg BA, & Grimm JW (2016). Impact of environmental enrichment on perineuronal nets in the prefrontal cortex following early and late abstinence from sucrose self-administration in rats. PloS One, 11(12), e0168256. 10.1371/journal.pone.0168256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaker ML, Jorgensen ET, Hegarty DM, Liu X, Kong Y, Zhang F, … Sorg BA. (2018). Cocaine exposure modulates perineuronal nets and synaptic excitability of fast-spiking interneurons in the medial prefrontal cortex. eNeuro, 5(5). 10.1523/ENEURO.0221-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AE, Kasper JM, Ara 13, Anastasio NC., & Hommel JD. (2019). Binge-type eating in rats is facilitated by neuromedin U receptor 2 in the nucleus accumbens and ventral tegmental area. Nutrients, 11 (2). 10.3390/nu11020327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinford-Jackson SE, Anastasio NC, Fox RG, Stutz SJ, & Cunningham KA (2016). Incubation of cocaine cue reactivity associates with neuroadaptations in the cortical serotonin (5-HT) 5-HT2C receptor (5-HT2CR) system. Neuroscience, 324, 50–61. 10.1016/j.neuroscience.2016.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Painter MR, Pentkowski NS, Mitroi D, Crawford CA, & Neisewander JL (2012). Environmental enrichment counters cocaine abstinence-induced stress and brain reactivity to cocaine cues but fails to prevent the incubation effect. Addiction Biology, 17(2), 365–377. 10.1111/j.1369-1600.2011.00358.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, & Neisewander JL (1998). Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology, 19 (1), 48–59. 10.1016/S0893-133X(97)00205-4 [DOI] [PubMed] [Google Scholar]
- Uejima JL, Bossert JM, Poles GC, & Lu L (2007). Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behavioural Brain Research, 181(2), 292–296. 10.1016/j.bbr.2007.04.019 [DOI] [PubMed] [Google Scholar]
- Vallöf D, Kalafateli AL, & Jerlhag E (2019). Brain region-specific neuromedin U signalling regulates alcohol-related behaviours and food intake in rodents. Addiction Biology, e12764. 10.1111/adb.12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas SI, Hemmings HC Jr., Greengard P, & Nairn AC (2011). Beyond the dopamine receptor: regulation and roles of serine/threonine protein phosphatases. Frontiers in Neuroanatomy, 5, 50. 10.3389/fnana.2011.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Shi J, Chen N, Xu L, Li J, Li P, … Lu L. (2013). Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PloS One, 8(7), e68791. 10.1371/journal.pone.0068791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (2013). Dual roles of dopamine in food and drug seeking: the drive-reward paradox. Biological Psychiatry, 73(9), 819–826. 10.1016/j.biopsych.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Wang B, Gardner EL, & Wise RA (2019). Cocaine and cocaine expectancy increase growth hormone, ghrelin, GLP-1, IGF-1, adiponectin, and corticosterone while decreasing leptin, insulin, GIP, and prolactin. Pharmacology, Biochemistry and Behavior, 176, 53–56. 10.1016/j.pbb.2018.11.001 [DOI] [PubMed] [Google Scholar]


