Abstract
As evidence emerged supporting noninvasive strategies for coronavirus disease 2019 (COVID-19)–related respiratory distress, we implemented a noninvasive COVID-19 respiratory protocol (NCRP) that encouraged high-flow nasal cannula (HFNC) and self-proning across our healthcare system. To assess safety, we conducted a retrospective chart review evaluating mortality and other patient safety outcomes after implementation of the NCRP protocol (April 3, 2020, to April 15, 2020) for adult patients hospitalized with COVID-19, compared with preimplementation outcomes (March 15, 2020, to April 2, 2020). During the study, there were 469 COVID-19 admissions. Fewer patients underwent intubation after implementation (10.7% [23 of 215]), compared with before implementation (25.2% [64 of 254]) (P < .01). Overall, 26.2% of patients died (24% before implementation vs 28.8% after implementation; P = .14). In patients without a do not resuscitate/do not intubate order prior to admission, mortality was 21.8% before implementation vs 21.9% after implementation. Overall, we found no significant increase in mortality following implementation of a noninvasive respiratory protocol that decreased intubations in patients with COVID-19.
Hypoxemic respiratory failure is a hallmark of severe coronavirus disease 2019 (COVID-19). Initial guidelines favored early mechanical ventilation (MV) over traditional noninvasive strategies, such as high-flow nasal cannula (HFNC) and noninvasive positive pressure ventilation (NIV), based on perceived ineffectiveness and dangers extrapolated from severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) patients.1,2 As COVID-19 progressed, early MV became associated with prolonged ventilator courses and high mortality.3–6 Simultaneously, data emerged that HFNC/NIV and self-proning, could successfully stabilize some COVID-19 patients.7–10 Based on evolving evidence, we implemented a noninvasive COVID-19 respiratory protocol (NCRP) that promoted the early use of HFNC, NIV, and self-proning for hypoxemia in patients with COVID-19, with the intention of avoiding MV in some patients. The protocol was implemented throughout our hospital system, from the Emergency Departments (EDs) to the medical floors and critical care units.
Although preliminary evidence supported the use of HFNC, NIV, and self-proning, the impact of a system-wide noninvasive COVID-19 respiratory protocol on safety has not been well described. The objective of this study was to evaluate patient safety outcomes after implementation of the NCRP, including intubation rate and mortality.
METHODS
Study Design and Setting
We performed a retrospective chart review, adhering to SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, to assess safety outcomes after implementation of the NCRP.11 Baystate Health is a not-for-profit, integrated healthcare system in western Massachusetts composed of four hospitals and one free-standing ED with 980 beds serving over 800,000 people. The Baystate Health IRB determined that this project did not meet criteria for Human Subjects Research.
Selection of Participants
A consecutive sample of adults (≥18 years old) admitted to the hospital with a positive nucleic acid test for SARS-CoV-2 (reverse transcriptase–polymerase chain reaction [RT-PCR]) test via nasopharyngeal swab (Cepheid or Roche Cobas 6800) between March 15, 2020, and April 15, 2020, were included. Participants were identified by either an order for the COVID-19 test with a positive result or a discharge diagnosis of COVID-19. Daily rapid response team (RRT), intensive care unit (ICU), and COVID-19 unit logs were reviewed to ensure all COVID-19 patients were included. Patients with positive tests admitted for reasons unrelated to COVID-19 infections, such as patients in labor, were excluded.
Interventions
At the start of the COVID-19 pandemic, the Baystate Health system adopted a conservative approach to the respiratory management of patients with COVID-19. This approach started with nasal cannula up to 6 L/min or nonrebreather up to 15 L/min. If the patient remained in respiratory distress, intubation was recommended.
Based on emerging evidence, the NCRP was created. The details of the NCRP implementation have been previously described.12 Briefly, over a 4-day period (April 3, 2020, to April 7, 2020), a multidisciplinary team developed, refined, and rapidly implemented a COVID-19 respiratory protocol that encouraged the early use of HFNC, NIV, and self-proning in clinically appropriate patients with hypoxemia and respiratory distress due to COVID-19 prior to intubation across all departments of the Baystate Health system (Appendix 1).
Measurements
A chart review was performed using a structured data collection form (Appendix 2). The data collection form was piloted by three physician-researchers. Data abstraction was performed by 16 clinicians. Abstractors were practicing emergency providers and hospitalists and were blinded to the study outcomes. Abstractors received a 1-hour training and abstracted data from at least five charts in parallel with investigators. An additional 10% of charts were double abstracted to calculate interrater reliability for five variables determined a priori.
To validate the capture of outcomes of interest, we triangulated data sources by cross-referencing the monthly RRT log, the ICU list, all orders for HFNC, and RRT activations. Data abstraction occurred from April 21, 2020, to April 30, 2020. Patients who were still hospitalized after April 30,2020, were followed until hospital discharge, ending July 1, 2020.
Outcomes and Analysis
The primary outcome was mortality, defined as the proportion of deaths by admissions during the post–NCRP implementation period (April 3, 2020, to April 15, 2020), compared with the preimplementation period (March 15, 2020, to April 2, 2020). Deaths were stratified by patient code status (do not resuscitate/do not intubate [DNR/DNI] established prior to admission vs Full Code or presumed Full Code). Mortality outcomes were evaluated using one-sided Fisher exact tests.
To assess whether the protocol led to an increase in the use of the interventions and a decrease in intubations, we compared the use of proning, HFNC, NIV, and intubation before the protocol was implemented and with use after. Intubation rates were analyzed using interrupted time series (piecemeal regression), without adjustments, using a cut point of April 2, 2020.
Secondary outcomes included unexpected cardiac arrests, ICU transfers and consultations, and RRT activations during the postimplementation period, compared with the preimplementation period. Secondary outcomes were evaluated using standard chi-square tests (χ2). Additional descriptive outcomes included use of the NCRP, overall and by components, and in-hospital rates of MV.
RESULTS
From March 15, 2020, through April 15, 2020, there were 469 patients with COVID-19 admitted to the four hospitals of the Baystate Health system. Patients had an average age of 70 years (SD, 16.4), 241 (52%) were female, and 336 (72%) spoke English as their primary language. Most patients, 405 (86.4%), required supplemental oxygen upon being admitted to the hospital (Table 1).
TABLE 1.
Characteristics of Patients Admitted to the Healthcare System With COVID-19
| N | Preimplementationa
|
Postimplementationb
|
||
|---|---|---|---|---|
| 254 | 215 | |||
| Age, mean, SD, y | 68.6 | 16.2 | 71.6 | 16.6 |
|
| ||||
| BMI, mean, SD, kg/m2 | 30.8 | 6.5 | 30.1 | 7.0 |
|
| ||||
| Gender (No., %) | ||||
| Male | 129 | 50.8 | 99 | 46.0 |
| Female | 125 | 49.2 | 116 | 54.0 |
|
| ||||
| Race, No., % | ||||
| White | 230 | 90.6 | 185 | 86.0 |
| Black | 15 | 5.9 | 17 | 7.9 |
|
| ||||
| Ethnicity, No., % | ||||
| Hispanic | 33 | 13.0 | 42 | 19.5 |
| Russian | 59 | 23.2 | 14 | 6.5 |
|
| ||||
| Medical history, No., % | ||||
| Chronic pulmonary disease | 86 | 33.9 | 77 | 35.8 |
| Diabetes | 28 | 11 | 17 | 7.9 |
| Congestive heart failure | 83 | 32.7 | 63 | 29.3 |
|
| ||||
| Elixhauser index, mean, SDc | 15 | 11 | 15.9 | 11 |
|
| ||||
| Initial vital signs, mean, SD | ||||
| Temperature, °F | 99.7 | 1.7 | 99.7 | 1.8 |
| Heart rate | 93 | 20.2 | 93 | 21.3 |
| Respiratory rate | 23 | 5.9 | 23 | 5.5 |
| O2 saturation | 94 | 6.6 | 94 | 5.9 |
| Received supplemental O2, No., % | 217 | 85.4 | 188 | 87.4 |
|
| ||||
| Admit Location, No., % | ||||
| Floor | 199 | 78.3 | 165 | 76.7 |
| Intermediate care/ICU | 55 | 21.7 | 50 | 23.3 |
|
| ||||
| Discharge disposition, No., % | ||||
| Home | 145 | 57.1 | 91 | 42.3 |
| Died | 61 | 24.0 | 62 | 28.8 |
| SNF, Rehab, LTC, or other facility | 48 | 18.9 | 62 | 28.8 |
|
| ||||
| Length of stay, mean, SD, d | 10 | 9.4 | 8.4 | 8.6 |
Preimplementation period included March 15, 2020, to April 2, 2020.
Postimplementation period included April 3, 2020, to April 15, 2020.
val Walraven modification of the Elixhauser Index
Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit; LTC, long-term care; O2, oxygen; SD, standard deviation; SNF, skilled nursing facility.
Postimplementation Mortality
Overall, 123 (26.2%) patients died during the study period. In the preimplementation cohort, 24% (61 of 254) of patients died, compared with 28.8% (62 of 215) in the postimplementation cohort (one-sided Fisher exact, P = .14). Excluding patients with an established DNR/DNI prior to admission, 21.8% (48 of 220) patients died in the preimplementation period vs 21.9% (35 of 160) patients after implementation of the NCRP (Table 2).
TABLE 2.
Rates of NCRP, Intubation, and Death
| All patients | Preimplementationa
|
Postimplementationb
|
P valuec | ||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| 254 (100%) | 215 (100%) | ||||||
| Received any NCRP Interventiond | 38 | 15.0 | 76 | 35.3 | <.01 | ||
| Received HFNC | 14 | 5.5 | 53 | 24.7 | <.01 | ||
| Proning attempted | 19 | 7.5 | 49 | 22.8 | <.01 | ||
| Received NIV | 11 | 4.3 | 11 | 5.0 | .69 | ||
|
| |||||||
| Intubated | 64 | 25.2 | 23 | 10.7 | <.01 | ||
|
| |||||||
| Died | 61 | 24.0 | 62 | 28.8 | .14 | ||
|
| |||||||
| DNR/DNI before admission | 34 (13.4%) | 55 (25.6%) | |||||
|
| |||||||
| Received any NCRP intervention | 5 | 14.3 | 19 | 33.9 | |||
|
| |||||||
| Intubated | 0 | 0 | 0 | 0 | |||
|
| |||||||
| Died | 13 | 38.2 | 27 | 49.1 | |||
|
| |||||||
| Full code on admission | 220 (86.6%) | 160 (74.4%) | |||||
|
| |||||||
| Received any NCRP intervention | 33 | 15.1 | 57 | 35.8 | |||
|
| |||||||
| Changed to DNR/DNI during hospitalization | 70 | 40 | 68 | 42.8 | |||
|
| |||||||
| Intubated | 64 | 29.1 | 23 | 14.4 | |||
|
| |||||||
| Died | 48 | 21.8 | 35 | 21.9 | |||
Preimplementation period included March 15, 2020, to April 2, 2020.
Postimplementation period included April 3, 2020, to April 15, 2020.
Calculated with use of one-sided Fisher exact test.
Some patients received a combination of NCRP interventions, so totals will not add up to the “Received any NCRP intervention” row.
Abbreviations: DNR/DNI, do not resuscitate/do not intubate; HFNC, high-flow nasal canula; NIV, noninvasive ventilation (continuous or bilevel positive airway pressure); NCRP, noninvasive COVID-19 respiratory protocol.
Secondary Safety Outcomes
There was no increase in RRT activations (preimplementation, 16.5% [42 of 254], vs postimplementation, 11.6% [25 of 215]; χ2 P= 0.17) or ICU consultations (preimplementation, 18.1% [47 of 254], vs postimplementation, 16.3% [35 of 215]; χ2 P= 0.52). ICU transfers decreased in the postimplementation period (preimplementation, 26.8% [68 of 254], vs postimplementation, 13.5% [29 of 215], χ2 P < .001). There was one unexpected cardiac arrest documented in the postimplementation period, compared to none before implementation.
NCRP Protocol Implementation
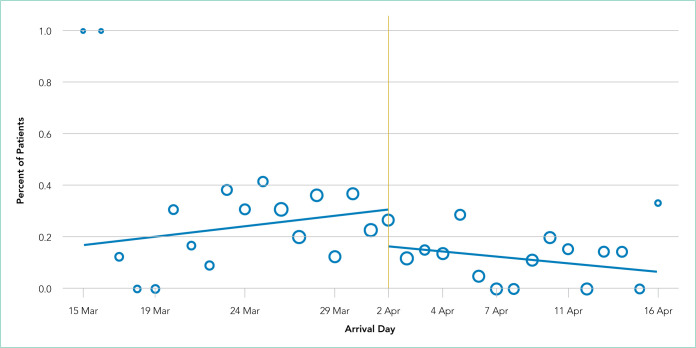
After implementation, the proportion of patients using HFNC increased from 5.5% (14 of 254) to 24.7% (53 of 215), and self-proning increased from 7.5% (19 of 254) to 22.8% (49 of 215). The proportion of patients who were intubated (MV) decreased from 25.2% (64 of 254) to 10.7% (23 of 215) (χ2 P < .01). Interrupted time series analysis demonstrated an immediate reduction in the proportion of patients intubated after the intervention (incident rate ratio, 0.44; 95% CI, 0.23-0.83; P = .012) (Figure). The median time from admission to MV was longer in the postimplementation period patients (postimplementation, 1.4 days; interquartile range, 0.21-2.9; vs preimplementation, 0.66 days; IQR 0.23-1.69).
FIG.
Interrupted Time Series Analysis of Intubation Rates by Date of Arrival. Circle size represents relative number of patients seen each day. The slope of the preimplementation intubation rate increased about 2% per day (incidence rate ratio (IRR), 1.015; P = .54) whereas after the implementation of the noninvasive COVID-19 respiratory protocol, the slope decreased about 2% per day (IRR, 0.977; P = .56). Although there was a significant change in level after implementation, (IRR, 0.44; 95% CI, 0.23-0.83; P = .012), the slopes prior to and after the protocol implementation were not statistically different (P = .41).
Interrater Reliability
Interrater reliability for variables chosen a priori was k = 1.0 for self-proning, k = 1.0 for intubation, k = 0.95 for discharge disposition, k = 0.94 for nasal cannula, and k = 0.74 for HFNC.
DISCUSSION
The rapid spread of SARS-CoV-2 led to early recommendations based on minimal data. As evidence emerged, hospitals were forced to adapt to protect patients and medical providers. As a healthcare system, we incorporated emerging evidence to rapidly implement a noninvasive respiratory treatment protocol. Aware of the methodological problems in evaluating the NCRP itself, we integrated best practices of quality improvement to examine multiple patient safety outcomes after NCRP implementation. We found the rate of intubation decreased with no significant increase in mortality, ICU transfers, RRT activations, or unexpected deaths after the implementation of the NCRP.
Although we were unable to measure all confounders and changes that co-occurred during the study period, initial vital signs, age, BMI, past medical history, and use of oxygen were similar between the pre- and postimplementation cohorts. Further, there were many constants worth noting. First, COVID-19 respiratory protocols were highly regulated to ensure patient safety and minimize COVID-19 transmission. Second, there were no new nonrespiratory treatments or medications during the study. Third, although the COVID-19 hospital census rose during the study, it never overwhelmed resources; there was no rationing of clinical care.
The nonsignificant increase in mortality in the postimplementation period was limited to patients with an established DNR/DNI prior to admission. Established DNR/DNI patients were largely from skilled nursing facilities that were disproportionally impacted in the postimplementation period through clustered outbreaks of COVID-19 in our region, which likely contributed to the increased mortality.13
Additionally, despite decreased MV rates in the postimplementation period, we did not find a concurrent decrease in mortality. We do not believe this is a failure of noninvasive treatments. Rather, the increased proportion of DNR/DNI patients, combined with increased nursing home outbreaks in the postimplementation period likely influenced mortality. The postimplementation decreases in ICU transfers and RRT activations supports this hypothesis.
Finally, it is worth nothing that, although the goal of decreasing intubations was to improve patient care and decrease mortality, a decrease in intubations alone, without a change in mortality, may be important because mechanical ventilation has been associated with increased morbidity, such as posttraumatic stress disorder.14
Taken together, the post–NCRP implementation period appears to have been safe for patients, compared to the pre-implementation period’s protocol. Future research may help understand the impact of specific noninvasive interventions on COVID-19–related MV and mortality.
Limitations
Given the urgency of COVID-19 treatment, the NCRP was designed as a quality improvement initiative rather than a prospective trial. Issues of selection bias and confounding limit our ability to evaluate the effect of the NCRP itself. Additionally, unmeasured patient and provider factors may have influenced outcomes. For example, increased provider knowledge and experience treating COVID-19 may have improved outcomes over time, and unmeasured patient characteristics may have been different in the pre-and postimplementation groups. Finally, our study was limited to a single healthcare system, which may limit generalizability
That said, the objective of our study was to evaluate patient safety outcomes of the NCRP, an important first step while other hospital systems continue to confront increasing rates of COVID-19 and must decide on appropriate respiratory management. To that end, our enrollment captured 469 COVID-19 admissions across four diverse hospitals without obvious differences in initial measured covariates. Further, the strict protocolization of respiratory treatments, the evaluation of multiple safety outcomes, and the complete patient follow-up all support the conclusion that NCRP in the postimplementation period did not increase adverse patient outcomes. Further studies are needed to determine the efficacy of the NCRP protocol itself.
CONCLUSION
In our health system, patients with COVID-19 did not experience a significant increase in mortality, RRT activations, or ICU admissions despite decreased rates of MV after implementation of a respiratory protocol that encouraged early noninvasive management of COVID-19 respiratory distress.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge Elizabeth Coray, Joseph Lahey, Richard Gabor, Cheryl Greenstein, Sarah Badach, Marie Boutin, Adrienne Wurl, Anthony Kitchen, Michelle Holton, Matthew Shapiro, Eleanor Ragone, Nageshwar Jonnalagadda, Ryan Flynn, Raghuveer Rakasi, and Jasmine Paadam.
Footnotes
Find additional supporting information in the online version of this article.
Disclosures: The authors reported no conflicts of interest. All authors had access to the data and played a role in the drafting of the manuscript.
Funding: Dr Soares is supported by a K08 from National Institute of Drug Abuse (1K08DA045933-01). Dr E Schoenfeld is supported by a K08 from Agency for Healthcare Research and Quality (5K08HS025701-02). Dr Westafer is supported by a K12 from the National Heart, Lung, and Blood Institute (1K12HL138049-01). Dr Visintainer is supported by a grant from NHLBI (R01HL134674). Dr Tidswell is supported by NHLBI (U01HL122989-01).
References
- 1.Brown CA, 3rd, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020;1(2):80–84. doi: 10.1002/emp2.12063. doi: 10.1002/emp2.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389–397. doi: 10.7326/m13-2486. doi: 10.7326/m13-2486. [DOI] [PubMed] [Google Scholar]
- 3.Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560–1564. doi: 10.1164/rccm.202004-1163le. doi: 10.1164/rccm.202004-1163le. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/s0140-6736(20)31189-2. doi: 10.1016/s0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farfel JM, Franca SA, Sitta Mdo C, Filho WJ, Carvalho CR. Age, invasive ventilatory support and outcomes in elderly patients admitted to intensive care units. Age Ageing. 2009;38(5):515–520. doi: 10.1093/ageing/afp119. doi: 10.1093/ageing/afp119. [DOI] [PubMed] [Google Scholar]
- 7.Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375–378. doi: 10.1111/acem.13994. doi: 10.1111/acem.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. doi: 10.1186/s13613-020-00650-2. doi: 10.1186/s13613-020-00650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. doi: 10.1186/s13613-020-00653-z. doi: 10.1186/s13613-020-00653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (standards for quality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986–992. doi: 10.1136/bmjqs-2015-004411. doi: 10.1136/bmjqs-2015-004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westafer LM, Elia T, Medarametla V, Lagu T. A transdiciplinary COVID-19 early respiratory intervention protocol: an implementation story. J Hosp Med. 2020;15(6):372–374. doi: 10.12788/jhm.3456. doi: 10.12788/jhm.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.COVID-19 Response Reporting. [Accessed July 20, 2020]; Mass.gov. https://www.mass.gov/info-details/covid-19-response-reporting#covid-19-daily-dashboard-
- 14.Shaw RJ, Harvey JE, Bernard R, Gunary R, Tiley M, Steiner H. Comparison of short-term psychological outcomes of respiratory failure treated by either invasive or non-invasive ventilation. Psychosomatics. 2009;50(6):586–591. doi: 10.1176/appi.psy.50.6.586. doi: 10.1176/appi.psy.50.6.586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.