Abstract
Aim
Randomized controlled trials (RCT) are the gold standard in surgical research, and case‐matched studies, such as studies with propensity score matching, are expected to serve as an alternative to RCT. Both study designs have been used to investigate the potential superiority of laparoscopic surgery to open surgery for rectal cancer, but it remains unclear whether there are any differences in the findings obtained using these study designs. We aimed to examine similarities and differences between findings from different study designs regarding laparoscopic surgery for rectal cancer.
Methods
Systematic review and meta‐analyses. A comprehensive literature search was conducted using PubMed, Scopus, and Cochrane. RCT, case‐matched studies, and cohort studies comparing laparoscopic low anterior resection and open low anterior resection for rectal cancer were included. In total, 8 short‐term outcomes and 3 long‐term outcomes were assessed. Meta‐analysis was conducted stratified by study design using a random‐effects model.
Results
Thirty‐five studies were included in this review. Findings did not differ between RCT and case‐matched studies for most outcomes. However, the estimated treatment effect was largest in cohort studies, intermediate in case‐matched studies, and smallest in RCT for overall postoperative complications and 3‐year local recurrence.
Conclusion
Findings from case‐matched studies were similar to those from RCT in laparoscopic low anterior resection for rectal cancer. However, findings from case‐matched studies were sometimes intermediate between those of RCT and unadjusted cohort studies, and case‐matched studies and cohort studies have a potential to overestimate the treatment effect compared with RCT.
Keywords: case‐matched study, low anterior resection, randomized controlled trial, rectal cancer
This article reports on similarities and differences in findings of randomized controlled trials (RCTs), case‐matched studies, and cohort studies, as revealed by meta‐analyses of previously published studies on laparoscopic low anterior resection (LAR) for rectal cancer. Results of case‐matched studies were often similar to those of RCTs in terms of outcomes of laparoscopic LAR for rectal cancer. However, case‐matched studies occasionally overestimate the effects of interventions compared to RCTs.

1. INTRODUCTION
Observational studies inherently include confounding factors that can affect their results, and several differences have been noted between the findings of randomized controlled trials (RCT) and those of observational studies 1 . For adequate comparison of interventions in observational studies, matching methods are frequently used in surgical research and the most common method is propensity score matching 2 . First introduced by Rosenbaum et al, propensity score matching is expected to be an alternative to RCT 3 , 4 , which are currently considered the gold standard in surgical research investigating the treatment effect of an intervention. Although it may be favorable to conduct RCT, this is often difficult to do for practical and ethical reasons in surgical research 2 . In addition, RCT generally require substantial resources including time, money, and collaboration among diverse specialists in order to ensure patient safety, data correctness, and standardization of interventions.
Recently, nationwide databases such as the National Clinical Database (NCD) and the National Database (NDB) have become available, despite several restrictions on their use 5 , 6 . If findings from case‐matched studies are similar to those from RCT, case‐matched studies using a nationwide database may supersede RCT. There are methodological differences between RCT and case‐matched studies such as patient selection and adjustment for confounding factors 7 , 8 . However, it remains unclear whether there are differences in findings between RCT and case‐matched studies.
We aimed to investigate similarities and differences in findings between RCT, case‐matched studies, and cohort studies regarding laparoscopic low anterior resection (LAR) for rectal cancer. LAR is defined as a procedure representing the performance of surgery in NCD 5 . These study designs have been used to investigate the potential superiority of laparoscopic LAR to open LAR for rectal cancer, which is of major interest to surgeons.
2. METHODS
We conducted a systematic review and meta‐analyses.
2.1. Eligibility
Studies in which laparoscopic LAR was compared with open LAR for rectal cancer were eligible. When multiple surgical procedures were included in a study, studies in which over 70% of patients underwent LAR were included. Small studies that included less than 50 patients for each intervention group were excluded. Study design was restricted to RCT, case‐matched study, or cohort study. Both prospective and retrospective studies were included, and the method of randomization or matching was not restricted. The language was restricted to English.
2.2. Outcome measures
Short‐term outcomes were the incidence of postoperative overall complications, the incidence of anastomotic leakage, mortality, reoperation rate, length of stay, operative time, estimated blood loss, and rate of positive circumferential resection margins. Long‐term outcomes were 3‐year overall survival (OS), 3‐year disease‐free survival (DFS), and 3‐year local recurrence rate (LRR).
2.3. Literature search and study selection
A comprehensive literature search was conducted on June 12, 2019, using PubMed, Scopus, and Cochrane Central Register of Controlled Trials. The search terms used were “rectal cancer”, “anterior resection”, “laparoscopy”, “open”, and related terms (Appendix S1). Duplications were excluded by checking the names of study authors, publication year, and study characteristics such as study design, setting, and period. Two review authors (NH and YF) independently screened the titles and abstracts of studies identified by literature search, and then assessed the full texts of potential eligible articles. Disagreement was resolved by discussion.
2.4. Data extraction
The same authors (NH and YF) also independently extracted data from the included studies; the data included study design and setting, number and characteristics of patients, surgical procedure, and short‐ and long‐term outcomes. Each double‐checked the extracted data for the other, and any discordance between them was resolved by discussion. For cohort studies, unadjusted data were extracted to assess the results without adjusting for confounding factors.
2.5. Statistical analysis
Data synthesis was carried out using Review Manager 5.3 (Cochrane Collaboration Software, Nordic Cochrane Centre). A random‐effects model was used for all meta‐analyses because of presumed heterogeneity in the surgical quality of LAR across the included studies. The Mantel‐Haenszel method was used for dichotomous variables and inverse‐variance weighting was applied for continuous variables. Risk ratio (RR) with 95% confidence interval (CI) was used for dichotomous variables in a meta‐analysis. Risk difference (RD) was applied instead of RR when a rare outcome was assessed. Mean difference (MD) with 95% CI was used for continuous variables when a single measure was included in a meta‐analysis. The median with range was converted to mean with standard difference (SD) by the method of Hozo et al. 9 A two‐sided P‐value <.05 was considered statistically significant.
3. RESULTS
3.1. Characteristics of included studies
The comprehensive literature search identified 3229 articles. Of these, 1368 duplications were removed. Screening was conducted for 1861 articles by checking the titles and abstracts for the potential to be included in this review. After screening, the full text of 106 articles was checked to assess whether they met the inclusion criteria. Finally, 35 studies (40 articles) were included in this review (Figure 1) 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 . Nine articles were reported from 4 RCT and were treated as 4 studies 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 . Included studies comprised 5 RCT, 10 case‐matched studies, and 20 cohort studies. Four studies were conducted internationally and the remaining 31 were reported from 11 countries. One case‐matched study was prospective and the remaining 9 were retrospective. Among cohort studies, 1 was prospective and 19 studies were retrospective (Table 1).
Figure 1.
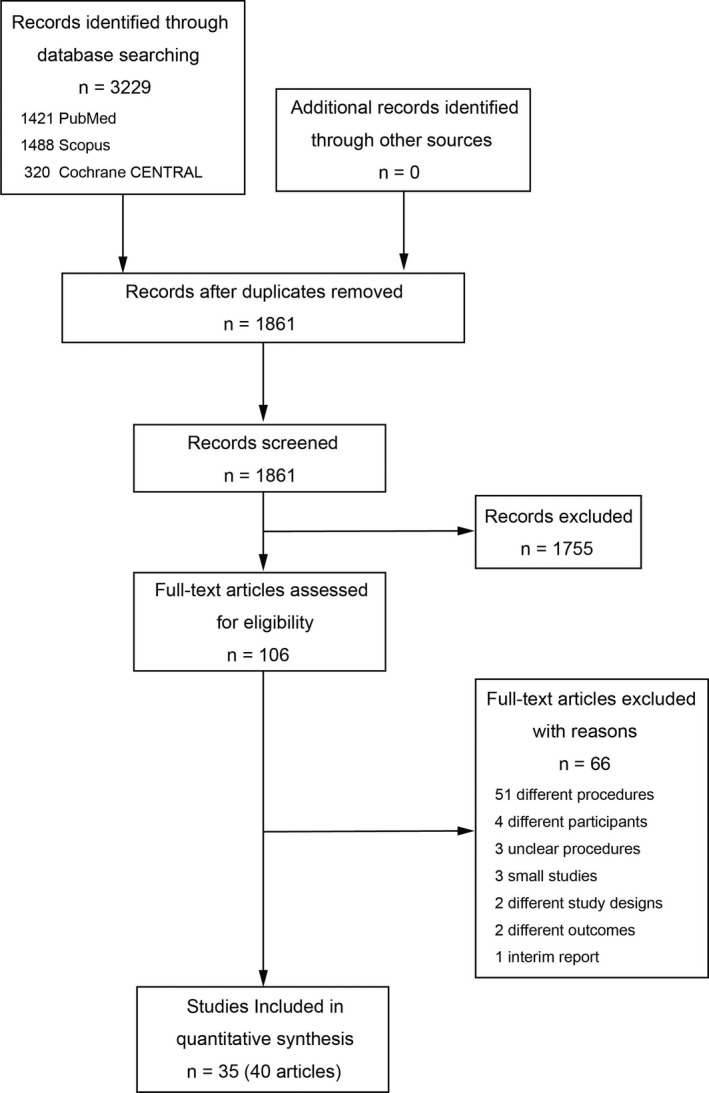
Flow diagram of study selection Central Register of Controlled Trials (CENTRAL)
Table 1.
Patient characteristics
| Study | Setting |
Prospective/ Retrospective |
Study period | Surgical procedures | Patients, n | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Institution | Start | End | Laparoscopy | Open | ||||||||||||
| Randomized controlled trials | |||||||||||||||||
| ACOSOG 10 , 11 | International | Multi | Prospective | 2008/10 | 2013/9 | LAR, APR, HP, TC | 240 | 222 | |||||||||
| ALaCaRT 12 , 13 | International | Multi | Prospective | 2010/3 | 2014/11 | LAR, APR | 238 | 235 | |||||||||
| COLAR II 14 , 15 , 16 | International | Multi | Prospective | 2004/1 | 2010/5 | AR, APR, HP | 699 | 345 | |||||||||
| COREAN 17 , 18 | Korea | Multi | Prospective | 2006/4 | 2009/8 | LAR, APR | 170 | 170 | |||||||||
| Braga 2007 19 | Italy | Single | Prospective | NR | NR | LAR, APR | 83 | 85 | |||||||||
| Case‐matched studies | |||||||||||||||||
| da Luz Moreira 2011 20 | USA | Single | Retrospective | 1992 | 2008 | LAR, APR | 91 | 91 | |||||||||
| de’Angelis 2017 21 | International | Multi | Retrospective | 2005/1 | 2015/12 | LAR, APR | 52 | 52 | |||||||||
| Guo 2015 22 | China | Single | Retrospective | 2007/4 | 2013/12 | LAR, APR | 191 | 191 | |||||||||
| Katsuno 2016 23 | Japan | Multi | Prospective | 2010/6 | 2013/2 | LAR | 209 | 209 | |||||||||
| Koulas 2009 24 | Greece | Single | Retrospective | 1998/10 | 2006/12 | AR, ISR, APR | 57 | 60 | |||||||||
| Manchon‐Walsh 2019 25 | Spain | Multi | Retrospective | 2011 | 2012 | AR,APR, HP | 842 | 517 | |||||||||
| Milone 2017 26 | Italy | Multi | Retrospective | 2009/1 | 2015/12 | AR, APR | 242 | 235 | |||||||||
| Nussbaum 2015 27 | USA | Multi | Retrospective | 2010 | 2011 | LAR | 6430 | 6430 | |||||||||
| Park 2013 28 | Korea | Single | Retrospective | 2003/1 | 2008/11 | AR, ISR, APR | 406 | 406 | |||||||||
| Tayar 2018 29 | Brazil | Single | Retrospective | 2008 | 2012 | LAR | 50 | 50 | |||||||||
| Cohort studies | |||||||||||||||||
| Allaix 2016 30 | Italy | Single | Retrospective | 1994/4 | 2005/8 | AR, APR | 153 | 154 | |||||||||
| Anthuber 2003 31 | Germany | Single | Retrospective | 1996/1 | 2002/3 | AR, APR, HP | 101 | 334 | |||||||||
| Du 2017 32 | China | Single | Retrospective | 2015/1 | 2017/1 | AR | 80 | 70 | |||||||||
| Kellokumpu 2012 33 | Finland | Single | Retrospective | 1999/1 | 2006/12 | HAR, LAR, APR | 100 | 91 | |||||||||
| Kim 2015 34 | Korea | Single | Retrospective | 2002/1 | 2011/12 | AR | 131 | 176 | |||||||||
| Laurent 2009 35 | France | Single | Retrospective | 1994/1 | 2006/12 | AR, APR, HP | 238 | 233 | |||||||||
| Law 2009 36 | China | Single | Retrospective | 2000/6 | 2006/12 | AR, APR, HP | 111 | 310 | |||||||||
| Lee 2013 37 | Korea | Single | Retrospective | 2001/6 | 2008/12 | LAR | 80 | 80 | |||||||||
| Li 2010 38 | China | Multi | Prospective | 2005/6 | 2007/6 | AR, APR | 65 | 70 | |||||||||
| Li 2011 39 | China | Single | Retrospective | 2000/1 | 2005/6 | AR, APR | 113 | 123 | |||||||||
| Li 2015 40 | China | Single | Retrospective | 2003/1 | 2008/12 | AR, APR | 129 | 152 | |||||||||
| Liu 2016 41 | China | Single | Retrospective | 2011 | 2013 | AR, APR | 84 | 65 | |||||||||
| Mohamed 2014 42 | China | Single | Retrospective | 2000/1 | 2011/12 | AR, APR, HP | 470 | 593 | |||||||||
| Pan 2016 43 | China | Single | Retrospective | 2009/1 | 2012/12 | AR, APR | 85 | 102 | |||||||||
| Staudacher 2007 44 | Italy | Single | Retrospective | 1998/1 | 2005/9 | AR | 108 | 79 | |||||||||
| Strouch 2013 45 | USA | Single | Retrospective | 2005/1 | 2011/6 | LAR | 75 | 75 | |||||||||
| Wu 2018 46 | China | Single | Retrospective | 2009/1 | 2013/12 | LAR, APR, HP | 277 | 614 | |||||||||
| Yang 2013 47 | China | Single | Retrospective | 2010/5 | 2012/5 | LAR, APR | 87 | 90 | |||||||||
| Zhang 2019 48 | China | Single | Retrospective | 2008/1 | 2011/12 | AR, CAA | 112 | 116 | |||||||||
| Zhou 2014 49 | China | Single | Retrospective | 2005/1 | 2008/1 | LAR, APR | 57 | 65 | |||||||||
Abbreviations: APR, abdominoperineal resection; AR, anterior resection; CAA, colo‐anal anastomosis; HAR, high anterior resection; HP, Hartmann’s procedure; ISR, intersphincteric resection; LAR, low anterior resection; NR, not reported; TC, total colectomy.
3.2. Short‐term outcomes
3.2.1. Incidence of overall postoperative complications
Twenty‐four studies with 9881 patients including 4 RCT with 2012 patients, 6 case‐matched studies with 2939 patients, and 14 cohort studies with 4930 patients reported on the incidence of overall complications and were included in a meta‐analysis stratified by study design.
There were no significant differences between laparoscopic LAR and open LAR in RCT (RR 1.00, 95% CI 0.89‐1.12, P = .95) and case‐matched studies (RR 0.91, 95% CI 0.83‐1.01, P = .07). However, laparoscopic LAR had a significantly lower incidence of overall postoperative complications than open LAR in cohort studies (RR 0.76, 95% CI 0.67‐0.87, P < .001) (Table 2, Figure 2).
Table 2.
Summary of meta‐analyses based on study designs
| Outcomes | Measures | Randomized controlled trials | Case‐matched studies | Cohort studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Patients |
Point estimation |
95% CI | Study | Patients |
Point estimation |
95% CI | Study | Patients |
Point estimation |
95% CI | ||
| n | n | n | n | n | n | ||||||||
| Short‐term | |||||||||||||
| Postoperative overall complications | RR | 4 | 2012 | 1.00 | 0.89, 1.12 | 6 | 2939 | 0.91 | 0.83, 1.01 | 14 | 4930 | 0.76 | 0.67, 0.87 |
| Anastomotic leakage | RR | 5 | 2144 | 1.11 | 0.78, 1.57 | 6 | 1986 | 1.05 | 0.79, 1.39 | 16 | 5126 | 0.98 | 0.77, 1.24 |
| Mortality | RD | 5 | 2487 | −0.00 | −0.01, 0.00 | 7 | 15 799 | −0.00 | −0.01, 0.00 | 12 | 4645 | −0.00 | −0.01, −0.00 |
| Reoperation | RR | 4 | 2012 | 1.07 | 0.71, 1.61 | 4 | 1745 | 1.21 | 0.67, 2.19 | 8 | 3291 | 0.82 | 0.60, 1.13 |
| Length of stay | MD | 2 | 630 | −1.83 | −5.74, 2.07 | 7 | 3056 | −2.96 | −4.50, −1.42 | 8 | 2059 | −2.15 | −3.38, −0.91 |
| Operative time | MD | 3 | 970 | 48.13 | 38.11, 58.14 | 3 | 1298 | −6.32 | −60.17, 47.53 | 10 | 2480 | 13.88 | −1.16, 28.92 |
| Estimated blood loss | MD | 2 | 630 | −116.84 | −234.90, 1.22 | 3 | 1298 | −64.79 | −93.98, −35.60 | 9 | 1680 | −79.71 | −108.05, −51.37 |
| Positive CRMs | RR | 4 | 2163 | 1.25 | 0.81, 1.92 | 4 | 14 644 | 0.75 | 0.65, 0.85 | 6 | 1679 | 1.28 | 0.80, 2.04 |
| Long‐term | |||||||||||||
| 3‐year OS | RR | 3 | 1834 | 1.02 | 0.98, 1.06 | 6 | 2956 | 1.05 | 0.99, 1.10 | 11 | 4367 | 1.06 | 1.00, 1.12 |
| 3‐year DFS | RR | 4 | 2296 | 1.03 | 0.97, 1.09 | 4 | 1480 | 0.99 | 0.94, 1.05 | 6 | 1858 | 0.98 | 0.95, 1.01 |
| 3‐year LRR | RR | 4 | 2002 | 1.05 | 0.66, 1.68 | 2 | 994 | 0.70 | 0.39, 1.25 | 7 | 3243 | 0.64 | 0.47, 0.88 |
Abbreviations: CI, confidence interval; CRM, circumferential resection margin; DFS, disease‐free survival; LRR, local recurrence rate; MD, mean difference; OS, overall survival; RD, risk difference; RR: risk ratio.
Figure 2.
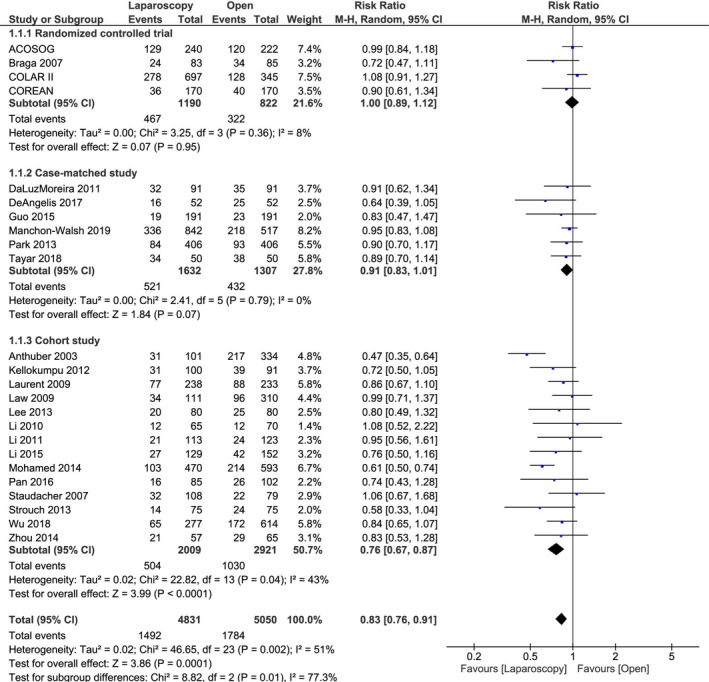
Results of meta‐analysis stratified by study design: Incidence of postoperative overall complications
3.2.2. Incidence of anastomotic leakage
In total, 27 studies with 9256 patients including 5 RCT with 2144 patients, 6 case‐matched studies with 1986 patients, and 16 cohort studies with 5126 patients reported on the incidence of anastomotic leakage and were included in a meta‐analysis stratified by study design.
There were no significant differences between laparoscopic LAR and open LAR in all three types of study design; RCT (RR 1.11, 95% CI 0.78‐1.57, P = .57), case‐matched studies (RR 1.05, 95% CI 0.79‐1.39, P = .74), and cohort studies (RR 0.98, 95% CI 0.77‐1.24, P = .88) (Table 2, Figure S1).
3.2.3. Mortality
Twenty‐four studies with 22 931 patients including 5 RCT with 2487 patients, 7 case‐matched studies with 15 799 patients, and 12 cohort studies with 4645 patients reported on mortality and were included in a meta‐analysis stratified by study design.
There were no significant differences between laparoscopic LAR and open LAR in RCT (RD −0.00, 95% CI −0.01 to 0.00, P = .53). In contrast, laparoscopic LAR showed significantly lower mortality than open LAR in case‐matched studies (RD −0.00, 95% CI −0.01 to −0.00, P = .03), and cohort studies (RD −0.01, 95% CI −0.01 to −0.00, P = .04) (Table 2, Figure S2).
3.2.4. Reoperation rate
A total of 16 studies with 7048 patients including 4 RCT with 2012 patients, 4 case‐matched studies with 1745 patients, and 8 cohort studies with 3291 patients reported on reoperation rate and were included in a meta‐analysis stratified by study design.
No significant differences were noted between laparoscopic LAR and open LAR in all three types of study design: RCT (RR 1.07, 95% CI 0.71‐1.61, P = .76), case‐matched studies (RR 1.21, 95% CI 0.67‐2.19, P = .53), and cohort studies (RR 0.82, 95% CI 0.60‐1.13, P = .22) (Table 2, Figure S3).
3.2.5. Length of stay
Seventeen studies with 5745 patients including 2 RCT with 630 patients, 7 case‐matched studies with 3056 patients, and 8 cohort studies with 2059 patients reported on length of stay and were included in a meta‐analysis stratified by study design.
There were no significant differences between laparoscopic LAR and open LAR in RCT (MD −1.83, 95% CI −5.74 to 2.07, P = .36). Conversely, laparoscopic LAR had significantly shorter length of stay than open LAR in case‐matched studies (MD −2.96, 95% CI −4.50 to −1.42, P < .001), and cohort studies (MD −2.15, 95% CI −3.38 to −0.91, P < .001) (Table 2, Figure S4).
3.2.6. Operative time
In total, 16 studies with 4748 patients including 3 RCT with 970 patients, 3 case‐matched studies with 1298 patients, and 10 cohort studies with 2480 patients reported on operative time and were included in a meta‐analysis stratified by study design.
Laparoscopic LAR had longer operative time than open LAR in RCT (MD 48.13, 95% CI 38.11 to 58.14, P < .001). In contrast, there were no significant differences between laparoscopic LAR and open LAR in case‐matched studies (MD −6.32, 95% CI −60.17 to 47.53, P = .82) and cohort studies (MD 13.88, 95% CI −1.16 to 28.92, P = .07) (Table 2, Figure S5).
3.2.7. Estimated blood loss
Fourteen studies with 3608 patients including 2 RCT with 630 patients, 3 case‐matched studies with 1298 patients, and 9 cohort studies with 1680 patients reported on estimated blood loss and were included in a meta‐analysis stratified by study design.
There were no significant differences between laparoscopic LAR and open LAR in RCT (MD −116.84, 95% CI −234.90 to 1.22, P = .05). However, laparoscopic LAR had significantly less estimated blood loss than open LAR in case‐matched studies (MD −64.79, 95% CI −93.98 to −35.60, P < .001) and cohort studies (MD −79.71, 95% CI −108.05 to −51.37, P < .001) (Table 2, Figure S6).
3.2.8. Rate of positive circumferential resection margins
Fourteen studies with 18 486 patients including 4 RCTs with 2163 patients, 4 case‐matched studies with 14 644 patients, and 6 cohort studies with 1679 patients reported on the rate of positive circumferential resection margins and were included in a meta‐analysis stratified by study design.
There were no significant differences between laparoscopic LAR and open LAR in RCT (RR 1.25, 95% CI 0.81‐1.92, P = .31) and cohort studies (RR 1.28, 95% CI 0.80‐2.04, P = .30). However, laparoscopic LAR had a significantly lower rate of positive circumferential resection margins than open LAR in case‐matched studies (RR 0.75, 95% CI 0.65‐0.85, P < .001) (Table 2, Figure S7).
3.3. Long‐term outcomes
3.3.1. Three‐year OS
A total of 20 studies with 9157 patients including 3 RCT with 1834 patients, 6 case‐matched studies with 2956 patients, and 11 cohort studies with 4367 patients reported on the 3‐year OS and were included in a meta‐analysis stratified by study design.
There were no significant differences between laparoscopic LAR and open LAR in RCT (RR 1.02, 95% CI 0.98‐1.06, P = .28) and case‐matched studies (RR 1.05, 95% CI 0.99‐1.10, P = .09). In contrast, laparoscopic LAR had a significantly higher 3‐year OS rate than open LAR in cohort studies (RR 1.06, 95% CI 1.00‐1.12, P = .04) (Table 2, Figure S8).
3.3.2. Three‐year DFS
Fourteen studies with 5634 patients including 4 RCT with 2296 patients, 4 case‐matched studies with 1480 patients, and 6 cohort studies with 1858 patients reported on the 3‐year DFS rate and were included in a meta‐analysis stratified by study design.
There were no significant differences between laparoscopic LAR and open LAR in all three types of study design: RCT (RR 1.03, 95% CI 0.97‐1.09, P = .28), case‐matched studies (RR 0.99, 95% CI 0.94‐1.05, P = .76), and cohort studies (RR 0.98, 95% CI 0.95‐1.01, P = .24) (Table 2, Figure S9).
3.3.3. Three‐year LRR
In total, 13 studies with 6239 patients including 4 RCT with 2002 patients, 2 case‐matched studies with 994 patients, and 7 cohort studies with 3243 patients reported on the 3‐year LRR and were included in a meta‐analysis stratified by study design.
There were no significant differences between laparoscopic LAR and open LAR in RCT (RR 1.05, 95% CI 0.66‐1.68, P = .82) and case‐matched studies (RR 0.70, 95% CI 0.39‐1.25, P = .23). By contrast, laparoscopic LAR had a significantly lower 3‐year LRR than open LAR in cohort studies (RR 0.64, 95% CI 0.47‐0.88, P = .007) (Table 2, Figure 3).
Figure 3.
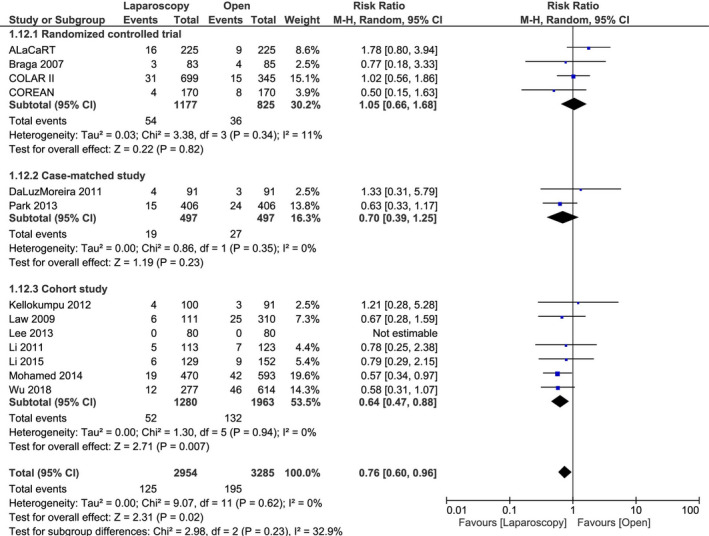
Results of meta‐analysis stratified by study design: 3‐year local recurrence rate
4. DISCUSSION
This systematic review and meta‐analysis including 35 studies that compared laparoscopic LAR with open LAR for rectal cancer showed some similarities and differences in findings from the different study designs, particularly RCT and case‐matched studies. Results from cohort studies were used as a reference for unadjusted results potentially affected by confounding factors. We conducted a meta‐analysis for 11 outcomes and found similarities for all outcomes other than operative time and the rate of positive circumferential resection margins between RCT and case‐matched studies. Cohort studies without covariate adjustment tended to overestimate the treatment effect of laparoscopic LAR in the incidence of postoperative overall complications, 3‐year OS, and 3‐year LRR.
Short‐term outcomes were not significantly different between laparoscopic LAR and open LAR in both RCT and case‐matched studies for the incidence of postoperative overall complications, the incidence of anastomotic leakage, and reoperation rate. Meanwhile, significant differences were noted in case‐matched studies but not in RCT regarding mortality, length of stay, and estimated blood loss. However, we deemed these three outcomes similar between RCT and case‐matched studies based on the distribution of 95% CI in forest plots. Thus, the findings of RCT were similar to those of case‐matched studies in terms of 9 short‐term outcomes. However, the number of patients was smaller in RCT than in case‐matched studies and the 95% CI of RCT were wider than those of case‐matched studies in length of stay and estimated blood loss. In addition, the two outcomes can be influenced by the retrospective nature of case‐matched studies. There remains potential difference between RCT and case‐matched studies in these outcomes. Operative time of laparoscopic LAR was significantly longer in RCT but not in case‐matched studies and the rate of positive circumferential resection margins was significantly lower in case‐matched studies but not in RCT. Case‐matched studies might overestimate the treatment effect of laparoscopic LAR in terms of operative time and the rate of positive circumferential resection margins. No significant differences in any long‐term outcomes were noted between laparoscopic LAR and open LAR in both RCT and case‐matched studies. Thus, RCT were similar to case‐matched studies in terms of the majority of outcomes.
We deemed there to be no difference between RCT and case‐matched studies in terms of all outcomes other than operative time and the rate of positive circumferential resection margins. However, we found that the estimated treatment effect of laparoscopic LAR tended to be larger in case‐matched studies compared with RCT but smaller in case‐matched studies compared with cohort studies in the incidence of overall complications and 3‐year LRR according to the distribution of 95% CI in forest plots. RCT can adjust for all confounding factors, and case‐matched studies can adjust for measurable confounding factors only. In this review, cohort studies were not adjusted for any confounding factors because we extracted unadjusted data from cohort studies. Therefore, the difference in the 95% CI between study designs might be due to the difference in adjustments for confounding factors.
Propensity score matching is a representative matching method that was first reported in 1983 3 . Propensity score matching is now widely used, but has several attendant problems such as insufficient reporting of the details of covariant selection, rate of missing covariates, and matching methods 50 , 51 . Although there are methodological differences between RCT and case‐matched studies such as patient selection and residual confounding, it remains unclear whether findings of RCT differ from those of case‐matched studies 7 , 8 . Kuss et al reported that outcomes of RCT were similar to those of case‐matched studies in cardiac surgery 52 . According to Dahabreh et al, case‐matched studies potentially overestimate treatment effects in acute coronary syndrome 53 . In addition, there are currently no reports on the similarities and differences between RCT and case‐matched studies in gastrointestinal surgery. In this study, we dealt with LAR for rectal cancer and showed there to be differences between study designs in some outcomes. It remains unclear whether similar results are found in other gastrointestinal surgeries. Further studies are needed to elucidate the similarities and differences in outcomes between study designs in other gastrointestinal surgeries.
This review highlights two outcomes, the incidence of overall postoperative complications and 3‐year LRR, that could be influenced by the adjustment for covariates. The incidence of overall postoperative complications often comprises multiple complications, some of which are sometimes evaluated subjectively, especially in retrospective cohort studies. The subjective assessment of postoperative complications could explain the difference between study designs in terms of outcomes. Nevertheless, local recurrence is usually evaluated objectively by using imaging modalities such as computed tomography and magnetic resonance imaging. Thus, study design can influence both short‐ and long‐term study outcomes, whether the evaluation is subjective or objective. Case‐matched studies and cohort studies are useful in surgical research because it is often difficult to conduct RCT. However, it is necessary to carefully consider the impact of study design on study outcomes.
The strength of the present review is the large number of studies analyzed to comparatively investigate similarities and differences in findings between RCT and case‐matched studies. This review included 35 studies and investigated numerous outcomes including both short‐ and long‐term outcomes for a single comparison. However, this study has some limitations. The number of studies and patients differed between study designs, with a tendency to be lower in RCT and higher in cohort studies. Also, this review included published data only and did not assess study quality. In this review, we showed the similarities and differences in terms of outcomes among RCT, case‐matched studies, and cohort studies on laparoscopic LAR for rectal cancer. Although further study is required in order for the results of this review to be generalizable, we hope these results help clinicians to better interpret the results of surgical research.
In conclusion, findings from case‐matched studies were similar to those of RCT in laparoscopic LAR for rectal cancer. However, findings of case‐matched studies were sometimes intermediate between those of RCT and unadjusted cohort studies, and case‐matched studies and cohort studies had a potential to overestimate the treatment effect compared with RCT.
DISCLOSURE
Funding: This review was supported by a grant from Kondou Kinen Medical Foundation.
Conflicts of interest: The authors declare no conflicts of interest for this study.
Author Contribution: All authors contributed to the study concept and design. Literature search and data collection were performed by NH and YF. Statistical analysis was conducted by NH and checked by the other authors. The first draft of manuscript was written by NH and all authors commented on previous version of the manuscript. All authors read and approved the final.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Fig S7
Fig S8
Fig S9
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
This review was supported by a grant from Kondou Kinen Medical Foundation.
Hoshino N, Fukui Y, Hida K, Obama K. Similarities and differences between study designs in short‐ and long‐term outcomes of laparoscopic versus open low anterior resection for rectal cancer: A systematic review and meta‐analysis of randomized, case‐matched, and cohort studies. Ann Gastroenterol Surg.2021;5:183–193. 10.1002/ags3.12409
REFERENCES
- 1. Shikata S, Nakayama T, Noguchi Y, Taji Y, Yamagishi H. Comparison of effects in randomized controlled trials with observational studies in digestive surgery. Ann Surg. 2006;244:668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adamina M, Guller U, Weber WP, Oertli D. Propensity scores and the surgeon. Br J Surg. 2006;93:389–94. [DOI] [PubMed] [Google Scholar]
- 3. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrica. 1983;70:41–55. [Google Scholar]
- 4. Reiffel JA. Propensity‐score matching: optimal, adequate, or incomplete? J Atr Fibrillation. 2018;11:2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hasegawa H, Takahashi A, Kakeji Y, Ueno H, Eguchi S, Endo I, et al. Surgical outcomes of gastroenterological surgery in Japan: Report of the National Clinical Database 2011–2017. Ann Gastroenterol Surg. 2019;3:426–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakayama T, Imanaka Y, Okuno Y, Kato G, Kuroda T, Goto R, et al. Analysis of the evidence‐practice gap to facilitate proper medical care for the elderly: investigation, using databases, of utilization measures for National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB). Environ Health Prev Med. 2017;22:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D'Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 8. Ali MS, Groenwold RH, Belitser SV, Pestman WR, Hoes AW, Roes KCB, et al. Reporting of covariate selection and balance assessment in propensity score analysis is suboptimal: a systematic review. J Clin Epidemiol. 2015;68:112–21. [DOI] [PubMed] [Google Scholar]
- 9. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, et al. Effect of laparoscopic‐assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314:1346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA, et al. Disease‐free Survival and Local Recurrence for Laparoscopic Resection Compared With Open Resection of Stage II to III Rectal Cancer: Follow‐up Results of the ACOSOG Z6051 Randomized Controlled Trial. Ann Surg. 2019;269(4):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stevenson ARL, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, et al. Effect of laparoscopic‐assisted resection vs open resection on pathological outcomes in rectal cancer: The ALaCaRT randomized clinical trial. JAMA. 2015;314:1356–63. [DOI] [PubMed] [Google Scholar]
- 13. Stevenson ARL, Solomon MJ, Brown CSB, Lumley JW, Hewett P, Clouston AD, et al. Disease‐free Survival and Local Recurrence After Laparoscopic‐assisted Resection or Open Resection for Rectal Cancer: The Australasian Laparoscopic Cancer of the Rectum Randomized Clinical Trial. Ann Surg. 2019;269:596–602. [DOI] [PubMed] [Google Scholar]
- 14. van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WCJ, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short‐term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–8. [DOI] [PubMed] [Google Scholar]
- 15. Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MHGM, de Lange‐de Klerk ESM, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. NEJM. 2015;372:1324–32. [DOI] [PubMed] [Google Scholar]
- 16. Petersson J, Koedam TW, Bonjer HJ, Andersson J, Angenete E, Bock D, et al. Bowel Obstruction and Ventral Hernia After Laparoscopic Versus Open Surgery for Rectal Cancer in A Randomized Trial (COLOR II). Ann Surg. 2019;269:53–7. [DOI] [PubMed] [Google Scholar]
- 17. Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim D‐W, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short‐term outcomes of an open‐label randomised controlled trial. Lancet Oncol. 2010;11:637–45. [DOI] [PubMed] [Google Scholar]
- 18. Jeong SY, Park JW, Nam BH, Kim S, Kang S‐B, Lim S‐B, et al. Open versus laparoscopic surgery for mid‐rectal or low‐rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open‐label, non‐inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767–74. [DOI] [PubMed] [Google Scholar]
- 19. Braga M, Frasson M, Vignali A, Zuliani W, Capretti G, Di Carlo V. Laparoscopic resection in rectal cancer patients: outcome and cost‐benefit analysis. Dis Colon Rectum. 2007;50:464–71. [DOI] [PubMed] [Google Scholar]
- 20. da Luz MA, Mor I, Geisler DP, Remzi FH, Kiran RP. Laparoscopic resection for rectal cancer: a case‐matched study. Surg Endosc. 2011;25:278–83. [DOI] [PubMed] [Google Scholar]
- 21. de’Angelis N, Landi F, Vitali GC, Memeo R, Martínez‐Pérez A, Solis A, et al. Multicentre propensity score‐matched analysis of laparoscopic versus open surgery for T4 rectal cancer. Surg Endosc. 2017;31:3106–21. [DOI] [PubMed] [Google Scholar]
- 22. Guo C, Zhang Z, Ren B, Men X. Comparison of the long‐term outcomes of patients who underwent laparoscopic versus open surgery for rectal cancer. J Buon. 2015;20:1440–6. [PubMed] [Google Scholar]
- 23. Katsuno H, Shiomi A, Ito M, Koide Y, Maeda K, Yatsuoka T, et al. Comparison of symptomatic anastomotic leakage following laparoscopic and open low anterior resection for rectal cancer: a propensity score matching analysis of 1014 consecutive patients. Surg Endosc. 2016;30:2848–56. [DOI] [PubMed] [Google Scholar]
- 24. Koulas SG, Pappas‐Gogos G, Spirou S, Roustanis E, Tsimogiannis KE, et al. Evaluations of laparoscopic proctocolectomy versus traditional technique in patients with rectal cancer. JSLS. 2009;13:564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manchon‐Walsh P, Aliste L, Biondo S, Espin E, Pera M, Targarona E, et al. A propensity‐score‐matched analysis of laparoscopic vs open surgery for rectal cancer in a population‐based study. Colorectal Dis. 2019;21:441–50. [DOI] [PubMed] [Google Scholar]
- 26. Milone M, Elmore U, Vignali A, Mellano A, Gennarelli N, Manigrasso M, et al. Pulmonary complications after surgery for rectal cancer in elderly patients: Evaluation of laparoscopic versus open approach from a multicenter study on 477 consecutive cases. Gastroenterol Res Pract. 2017;2017:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nussbaum DP, Speicher PJ, Ganapathi AM, Englum BR, Keenan JE, Mantyh CR, et al. Laparoscopic versus open low anterior resection for rectal cancer: results from the national cancer data base. J Gastrointest Surg. 2015;19:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park SJ, Choi GS, Jun SH, Park SY, Kim HJ. Long‐term outcomes after laparoscopic surgery versus open surgery for rectal cancer: a propensity score analysis. Ann Surg Oncol. 2013;20:2633–40. [DOI] [PubMed] [Google Scholar]
- 29. Tayar DO, Ribeiro U Jr, Cecconello I, Magalhaes TM, Simoes CM, Auler JOC Jr. Propensity score matching comparison of laparoscopic versus open surgery for rectal cancer in a middle‐income country: short‐term outcomes and cost analysis. Clinicoecon Outcomes Res. 2018;10:521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allaix ME, Giraudo G, Ferrarese A, Arezzo A, Rebecchi F, Morino M. 10‐Year Oncologic Outcomes After Laparoscopic or Open Total Mesorectal Excision for Rectal Cancer. World J Surg. 2016;40:3052–62. [DOI] [PubMed] [Google Scholar]
- 31. Anthuber M, Fuerst A, Elser F, Berger R, Jauch KW. Outcome of laparoscopic surgery for rectal cancer in 101 patients. Dis Colon Rectum. 2003;46:1047–53. [DOI] [PubMed] [Google Scholar]
- 32. Du B, Zhang W, Yang X, Wu D, Lv Y, Wang T. Clinical efficacy of laparoscopic minimally invasive surgery for rectal cancer and its effect on anorectal dynamics. Biomed Res (India). 2017;28:7665–8. [Google Scholar]
- 33. Kellokumpu IH, Kairaluoma MI, Nuorva KP, Kautiainen HJ, Jantunen IT. Short‐ and long‐term outcome following laparoscopic versus open resection for carcinoma of the rectum in the multimodal setting. Dis Colon Rectum. 2012;55:854–63. [DOI] [PubMed] [Google Scholar]
- 34. Kim JH, Ahn BK, Park SJ, Park MI, Kim SE, Baek SU, et al. Long‐term outcomes of laparoscopic versus open surgery for rectal cancer: a single‐center retrospective analysis. Korean J Gastroenterol. 2015;65:273–82. [DOI] [PubMed] [Google Scholar]
- 35. Laurent C, Leblanc F, Wutrich P, Scheffler M, Rullier E. Laparoscopic versus open surgery for rectal cancer: long‐term oncologic results. Ann Surg. 2009;250:54–61. [DOI] [PubMed] [Google Scholar]
- 36. Law WL, Poon JTC, Fan JKM, Lo SH. Comparison of outcome of open and laparoscopic resection for stage II and stage III rectal cancer. Ann Surg Oncol. 2009;16:1488–93. [DOI] [PubMed] [Google Scholar]
- 37. Lee SD, Park SC, Park JW, Kim DY, Choi HS, Oh JH. Laparoscopic versus open surgery for stage I rectal cancer: long‐term oncologic outcomes. World J Surg. 2013;37:646–51. [DOI] [PubMed] [Google Scholar]
- 38. Li J, Chen R, Xu YQ, Wang X‐C, Zheng S, Zhang S‐Z, et al. Impact of a laparoscopic resection on the quality of life in rectal cancer patients: results of 135 patients. Surg Today. 2010;40:917–22. [DOI] [PubMed] [Google Scholar]
- 39. Li S, Chi P, Lin H, Lu X, Huang Y. Long‐term outcomes of laparoscopic surgery versus open resection for middle and lower rectal cancer: an NTCLES study. Surg Endosc. 2011;25:3175–82. [DOI] [PubMed] [Google Scholar]
- 40. Li S, Jiang F, Tu J, Zheng X. Long‐term oncologic outcomes of laparoscopic versus open surgery for middle and lower rectal cancer. PLoS One. 2015;10:e0135884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu Y, Lu XM, Tao KX, Ma J‐H, Cai K‐L, Wang L‐F, et al. Anatomical basis and clinical research of pelvic autonomic nerve preservation with laparoscopic radical resection for rectal cancer. J Huazhong Univ Sci Technolog Med Sci. 2016;36:211–4. [DOI] [PubMed] [Google Scholar]
- 42. Mohamed ZK, Law WL. Outcome of tumor‐specific mesorectal excision for rectal cancer: The impact of laparoscopic resection. World J Surg. 2014;38:2168–74. [DOI] [PubMed] [Google Scholar]
- 43. Pan R, Zheng S, Cai W, Wang Z, Zheng M. Retrospective study on the effect of laparoscopic and open total mesorectal excision for middle/low T3 rectal cancer. Int J Cli Exp Med. 2016;9:21708–15. [Google Scholar]
- 44. Staudacher C, Vignali A, Saverio DP, Elena O, Andrea T. Laparoscopic vs. open total mesorectal excision in unselected patients with rectal cancer: impact on early outcome. Dis Colon Rectum. 2007;50:1324–31. [DOI] [PubMed] [Google Scholar]
- 45. Strouch MJ, Zhou G, Fleshman JW, Birnbaum EH, Hunt SR, Mutch MG. Time to initiation of postoperative chemotherapy: an outcome measure for patients undergoing laparoscopic resection for rectal cancer. Dis Colon Rectum. 2013;56:945–51. [DOI] [PubMed] [Google Scholar]
- 46. Wu QB, Deng XB, Zhang XB, Kong LH, Zhou ZG, Wang ZQ. Short‐term and long‐term outcomes of laparoscopic versus open surgery for low rectal cancer. J Laparoendos Adv Surg Tech. 2018;28:637–44. [DOI] [PubMed] [Google Scholar]
- 47. Yang Q, Xiu P, Qi X, Yi G, Xu L. Surgical margins and short‐term results of laparoscopic total mesorectal excision for low rectal cancer. JSLS. 2013;17:212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang Z, Rao B, Sun Z, Zhang N. Comparison of perioperative clinicopathologic outcome and postoperative survival of laparoscopic and open sphincter‐sparing surgery in patients with rectal cancer: A retrospective study. Journal of BUON. 2019;24:464–9. [PubMed] [Google Scholar]
- 49. Zhou T, Zhang G, Tian H, Liu Z, Xia S. Laparoscopic rectal resection versus open rectal resection with minilaparotomy for invasive rectal cancer. J Gastrointest Oncol. 2014;5:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McMurry TL, Hu Y, Blackstone EH, Kozower BD. Propensity scores: Methods, considerations, and applications in the Journal of Thoracic and Cardiovascular Surgery. J Thorac Cardiovasc Surg. 2015;150:14–9. [DOI] [PubMed] [Google Scholar]
- 51. Lonjon G, Porcher R, Ergina P, Fouet M, Boutron I. Potential Pitfalls of Reporting and Bias in Observational Studies With Propensity Score Analysis Assessing a Surgical Procedure: A Methodological Systematic Review. Ann Surg. 2017;265:901–9. [DOI] [PubMed] [Google Scholar]
- 52. Kuss O, Legler T, Börgermann J. Treatments effects from randomized trials and propensity score analyses were similar in similar populations in an example from cardiac surgery. J Clin Epidemiol. 2011;64:1076–84. [DOI] [PubMed] [Google Scholar]
- 53. Dahabreh IJ, Sheldrick RC, Paulus JK, Chung M, Varvarigou V, Jafri H, et al. Do observational studies using propensity score methods agree with randomized trials? A systematic comparison of studies on acute coronary syndromes. Eur Heart J. 2012;33:1893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Fig S7
Fig S8
Fig S9
Supplementary Material
Supplementary Material


