Abstract
Perioperative and surgical management of gastric cancer have been changing as pivotal phase II trials and landmark phase III trials offer new insights to the existing knowledge. The results of many landmark trials have been published or presented in the past year, many of which have changed or will change current clinical practice. For example, FLOT4 has completely changed the regimen of perioperative chemotherapy in Europe. Furthermore, evidence for minimally invasive surgery for clinical Stage I was firmly established by KLASS‐01 and JCOG0912 for distal gastrectomy and CLASS‐02, KLASS‐03, and JCOG1401 for total gastrectomy. Moreover, promising results were provided by CLASS‐01 and KLASS‐02 for locally advanced gastric cancer. For adjuvant chemotherapy, JACCRO GC‐07 (START‐2) has provided a new doublet regimen for pathological Stage III, which is often refractory to chemotherapy. Conversely, JCOG0501 poses a significant challenge for advanced tumors, such as large type 3 and scirrhous (type 4) tumors. In this review, we briefly review recent updates and discuss future perspectives of gastric cancer treatment.
Keywords: adjuvant treatment, cancer of the gastroesophageal junction, gastric cancer, minimally invasive surgery, neoadjuvant treatment, precision medicine
The article reviewed updates in the management of gastric cancer surgery by covering most pivotal recent trials and studies, and then discusses future perspectives of gastric cancer treatment.
![]()
1. INTRODUCTION
Gastric cancer is the 7th leading incident cancer and the 3rd most deadly cancer worldwide after lung cancer and colorectal cancer. According to Global Burden of Diseases, Injury, and Risk Factors Study (GBD), there were more than 1.22 million incident cases and 865 000 people were estimated to have died of gastric cancer in 2017. 1 The data demonstrated that the high‐income Asia Pacific region and East Asia including China showed the highest age‐standardized incidence rates, at 29.5 and 28.6 per 100 000 population, respectively, compared to 15.4 globally. Surprisingly, in 2017, China alone showed almost half of all global incident cases (562 000).
It is also reported in the GBD study that the absolute number of incident cases increased from 864 000 in 1990 to 1 220 000 in 2017, and the absolute number of deaths also increased from 769 000 in 1990 to 865 000 in 2017; these remarkable changes were mostly attributed to a bulk increase in China. In contrast, disability‐adjusted life years (DALY) remained almost constant during the same period. Although the absolute number of incident cases and deaths showed a drastic increase, the age‐standardized incidence and death rates steadily declined year by year. Globally, the age‐standardized incidence rate dropped by 28%, and the age‐standardized death rate and DALY rate fell by 43% and 47%, respectively (Figure 1). This downward trend is particularly prominent in the high‐income Asia Pacific region, where the age‐standardized incidence rate, death rate, and DALY rate plunged by 49%, 57%, and 62%, respectively. Presumably as a consequence, despite the fact that the number of incident cases in this region increased from 117 000 in 1990 to 132 000 in 2017, the number of deaths stayed almost the same, from 66 000 to 68 000.
FIGURE 1.
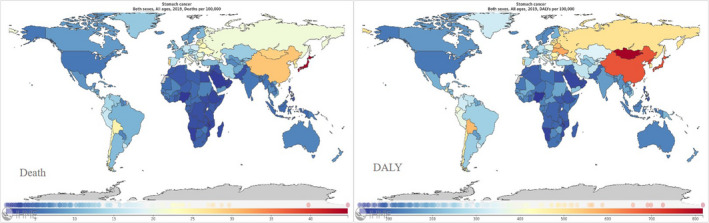
Death (left) and disability‐adjusted life years (DALYs) (right) of stomach cancer per 100 000 population in 2019, for 195 countries and territories. 72
Because the incidence rates and mortality rates of gastric cancer show significant variations among countries, treatment strategies are strikingly diverse from region to region. For example, in Japan and South Korea, where population screening programs have been implemented, many cancers can be detected at an early stage, while locally advanced and metastatic cancers are much more common in other countries where no screening program is available. In this review, we will explore recent updates with their relevant studies in the preoperative treatment strategies in gastric cancer surgery that have changed or will change our practice, with a specific focus on the Asian region.
2. SURGICAL MANAGEMENT OF GASTRIC CANCER
2.1. Minimally invasive surgery for early stage cancer (clinical Stage I)
Minimally invasive gastrectomy by laparoscopic and robotic surgery has gained increased popularity and is now largely accepted among expert communities. As for minimally invasive distal gastrectomy for early cancers, firm evidence based on phase III RCTs in comparison with conventional open surgery is available from Korea and Japan. (Table 1) A JCOG0912 study from Japan in which the primary endpoint was 5‐year relapse‐free survival (RFS) successfully demonstrated non‐inferiority of laparoscopic surgery in the long‐term (hazard ratio [HR], 0.84; 90% confidence interval [CI], 0.56‐1.27 [non‐inferiority margin: 1.54]; P = .008). 2 Likewise, a KLASS‐01 study from Korea also revealed non‐inferiority of laparoscopic surgery with an absolute difference of 0.9% (laparoscopy vs open: 94.2% vs 93.3%, one‐sided 97.5% CI, −1.6% to infinity [non‐inferiority margin: −5%]; P = .64). 3 Taken together with the findings of the two RCTs, laparoscopic distal gastrectomy has been widely accepted as one of the standard treatment options for early gastric cancer.
TABLE 1.
Large randomized controlled trials of minimally invasive distal (total) gastrectomy for early and locally advanced gastric cancer
| Trial name | Country | Subject | Primary endpoint | Open | MIS | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|---|---|---|
| JCOG0912 2 | Japan | cStage I | 5‐year RFS | 94.0% | 95.1% | 0.84 (0.56‐1.27) | .0075 b |
| KLASS‐01 3 | Korea | cStage I | 5‐year OS | 93.3% | 94.2% | 0.93 (‐inf. −1.26) | n/a |
| JLSSG0901 12 | Japan | T2‐T4a and N0‐2 | 5‐year RFS | n/a | n/a | n/a | n/a |
| KLASS‐02 11 | Korea | T2‐T4a and N0‐1 | 3‐year RFS | 81.3% | 80.3% | 1.035 (0.762‐1.406) | .039 b |
| CLASS‐01 9 | China | T2‐T4a and N0‐3 | 3‐year DFS | 77.8% | 76.5% | −1.3% (−6.5%‐inf.) c | n/a |
| STOMACH a , 13 | Netherlands | Resectable GC with NAC | No. of nodes | 43.4 | 41.7 | n/a | .612 |
Abbreviations; DFS, disease‐free survival; GC, gastric cancer; inf., infinity; MIS, minimally invasive surgery; n/a, not available; NAC, neoadjuvant chemotherapy; No,: number; OS, overall survival; RFS, relapse‐free survival.
Total gastrectomy,
P value for non‐inferiority.
Absolute difference with 1‐sided 97.5% CI,
Evidence on minimally invasive total and proximal gastrectomy is also available. Three phase II/III RCTs — CLASS‐02 in China, KLASS‐03 in South Korea, and JCOG1401 in Japan — have evaluated the safety of laparoscopic total and/or proximal gastrectomy for clinical Stage (cStage) I cancer, and demonstrated that both procedures could be safely performed with an acceptable postoperative complication rate. 4 , 5 , 6
Robot‐assisted surgery is a rapidly emerging technology that has the potential to replace laparoscopic surgery. A phase II single‐arm study that evaluated the feasibility of robot‐assisted distal gastrectomy for cStage I/II gastric cancer demonstrated a significant reduction in postoperative pancreatic fistula in comparison with laparoscopic surgery. 7 This was presumably due to several remarkable features of robotic surgery, namely the elimination of hand tremors, and improved manipulation as a result of the robotic wrist, which allows 7 degrees of freedom that are not possible with laparoscopic surgery. However, the cost of robotic surgery is significantly more expensive, which cannot be overlooked in countries where rapidly growing medical expenses have become political and economic issues for the society. 8 Because it is still unclear whether technological innovation will improve short‐ and long‐term outcomes in cancer surgery, the cost‐benefit performance needs to be further clarified before robot‐assisted gastrectomy is more widely accepted.
2.2. Minimally invasive surgery for locally advanced cancer (cStage II–III)
The oncological feasibility of minimally invasive surgery for locally advanced cancer is still debated, whereas the short‐term outcomes have been proven to be comparable or superior to those of open surgery. In Asia, three large phase III RCTs have compared minimally invasive surgery to open surgery: CLASS‐01 in China, KLASS‐02 in Korea, and JLSSG0901 in Japan. The result of CLASS‐01 was first unveiled in 2019, and showed non‐inferiority of laparoscopic surgery as assessed by 3‐year disease‐free survival (laparoscopy vs open: 76.5% vs 77.8%, one‐sided 97.5% CI: −6.5% to infinity [non‐inferiority margin: −10%]). 9 In line with this result, the final result of KLASS‐02 demonstrated that the 3‐year RFS was 80.3% (95% CI: 76.0‐85.0) for laparoscopy and 81.3% (95% CI: 77.0‐85.0, P = .726) for open surgery. 10 , 11 In addition, Cox regression analysis showed a HR of 1.035 (95% CI: 0.762‐1.406, P = .039), concluding that laparoscopic D2 distal gastrectomy could also be an option for locally advanced gastric cancer. The result of JLSSG0901, with the primary endpoint of 5‐year RFS, is not yet available, and is expected to be reported in a few years. 12
From the West, the results of one major RCT from the Netherlands have been reported to date. The STOMACH trial compared minimally invasive total gastrectomy to open after neoadjuvant chemotherapy with the primary endpoint of oncological safety, namely the number of resected lymph nodes and radicality. 13 The trial showed oncological comparability between the two approaches, with 1‐year survival of 90.4% in the open group and 85.5% in the minimally invasive group (P = .70).
According to the results from two large RCTs from Asia, minimally invasive gastrectomy for locally advanced cancer appears to be feasible and promising, but care should be taken when extrapolating the results to general practice. This is particularly true with regards to the generalizability of the findings for several reasons. Firstly, gastrectomy was performed by skilled hands in the trials, and laparoscopic surgery by non‐skilled surgeons might result in different outcomes. Secondly, patients in the trials were highly selected and not all locally advanced tumors were included. For example, type 4 tumors (scirrhous type) affecting the entire stomach might have been avoided in the trials. Thirdly, there may be certain differences in outcomes between upfront laparoscopic surgery and surgery after neoadjuvant chemotherapy (NAC). In the near future, NAC will be a major strategy for locally advanced cancer, to which the results cannot simply be applied. Therefore, when applying these results to clinical practice, we need to consider if laparoscopic surgery is suitable for patients with locally advanced cancer, since the surgical and oncological outcomes may be different even if the surgery is technically possible.
2.3. Sentinel node navigation surgery for cT1N0 cancer
Sentinel node navigation surgery (SNNS) is currently standard practice for breast cancer and melanoma surgery. As for gastric cancer, a single‐arm conceptual study of SNNS has shown its feasibility in gastric cancer, in which cT1N0 gastric tumors up to 4 cm in diameter actually followed the sentinel lymph node theory. 14 In other words, partial resection of the primary tumor with sentinel basin dissection results in comparable outcomes and improved quality of life compared to conventional surgery, thereby taking the place of gastrectomy and adequate lymphadenectomy.
Two trials, one from Japan and the other from Korea, are currently available, in which the long‐term oncological outcomes have been evaluated. In Korea, a phase III RCT, the SENORITA trial, which compared laparoscopic SNNS to conventional gastrectomy with the primary endpoint of 3‐year disease‐free survival (DFS), showed comparable morbidity and mortality rates between the two groups. However, SNNS failed to show non‐inferiority because more recurrence (mainly metachronous gastric cancer) was seen in SNNS (SNNS vs conventional: 91.8% vs 95.5%; HR, 1.901; 95% CI, 0.911‐3.967 [non‐inferiority margin: 2.737]). 15 , 16 However, they concluded that SNNS could be an alternative if rescue surgery is appropriately performed; this was supported by 3‐year overall survival (OS) and disease‐specific survival that were equivalent to those of conventional surgery. In Japan, a single‐center study demonstrated a satisfactory 5‐year survival rate of 98.5%, in addition to a better quality of life as measured by a validated quality of life scale called the PGSAS‐45. 17 , 18 Currently, a multicenter triple‐arm confirmatory study that has been approved by the Japanese authority is under investigation and the result is expected to be available in several years. 19
The significant difference between the two SNNS trials in Korea and Japan is the definition of the “sentinel basin,” which in this context refers to “to what extent lymphatics flow from the primary tumor.” Both studies aimed to minimize the extent of nodal dissection based on the concept of the “sentinel basin.” In the Korean trial, the “sentinel basin” to be dissected refers to a limited area comprising sentinel nodes but not one whole lymphatic station (i.e. whole station 7) that includes sentinel nodes. Conversely, in the Japanese trial, the “sentinel basin” was defined as five distinct lymphatic areas based on their location along the five main arteries to the stomach; consequently, there are lymphatic stations that have to be dissected irrespective of whether they include sentinel nodes or not. Generally speaking, we can say that the Korean study emphasizes quality of life by compromising the extent of dissection, whereas the Japanese study does not compromise radicality in cancer surgery. The ultimate aim of both studies was exactly the same, but careful consideration is necessary when interpreting their results.
One of the important concerns in SNNS is whether the concept can be applied to the state after endoscopic resection. If this is true, non‐curable endoscopic resection could be treated with additional sentinel basin dissection. However, it is unknown whether the lymphatics stay the same after endoscopic resection or not. A multicenter retrospective cohort study was conducted to answer this critical question, and the results suggested that SNNS was still feasible even in the state after endoscopic resection. 20 Another study using in vivo porcine models also demonstrated that the lymphatic flow remained unchanged in most parts of the stomach after endoscopic resection. 21 The number of patients who benefit from SNNS would be considerable if the concept remained feasible even after endoscopic resection.
2.4. Evidence for cancer of the gastroesophageal junction
The incidence of cancer of the gastroesophageal junction (GEJ cancer) has risen remarkably worldwide over the last couple of decades, particularly in the Western hemisphere. 22 , 23 , 24 Meanwhile, the management of GEJ cancer differs considerably among countries, and even among hospitals and surgeons. In some cases, GEJ cancer is managed as a component of esophageal cancer treated with esophagectomy, while other establishments recognize GEJ cancer as a component of gastric cancer and treat it with extended transhiatal gastrectomy. In line with this, no single classification system nor perioperative treatment strategy has been provided thus far. For example, the Nishi classification is a major classification in Japan, while the Siewert classification system is common in the West. Moreover, preoperative chemotherapy is given when GEJ cancer is treated as gastric cancer, whereas chemoradiation therapy may be an option when it is treated as esophageal cancer.
Irrespective of the chosen strategy, one common problem is the extent to which lymph node dissection should be performed. Or rather, vice versa, the extent of dissection may decide the most appropriate procedure. A prospective multicenter nationwide study for locally advanced GEJ cancer was conducted in Japan to answer this question. The study proposed three categories of lymph node stations that should be removed based on the incidence of metastasis. 25 In brief, in cases where clinically positive node(s) in the upper/middle mediastinum are present, whole thoracic lymphadenectomy, including the upper mediastinum, is recommended. Otherwise, the extent of dissection is suggested based on how long the tumor is invading proximally from the GEJ: >4.0 cm, 2.1‐4.0 cm, or <2.0 cm. Lymphadenectomy with the upper mediastinum is recommended when the length of invasion is estimated to be over 4.0 cm.
Although many studies support the underlying concept of this classification in which the length of invasion should be considered, at the same time there remains debate as to how the GEJ is precisely located in cases with bulky locally advanced cancer. It is often difficult to locate the GEJ clinically, which can result in misclassification of the tumor. Indeed, clinically, Siewert type II tumor often turns out to be histological type I following processing of the specimen. Another concern is the state after neoadjuvant therapy, particularly after neoadjuvant chemoradiation. In many cases, the tumor may be modified considerably, making the assessment much more difficult. Additionally, it remains unknown whether the extent of dissection should be changed if the proximal invasion is shortened. 26 The Japan study will provide clear advances in knowledge of surgery for GEJ cancer, although there remain several issues to be addressed in future work.
2.5. Topics currently open to discussion
2.5.1. Massive peritoneal lavage after gastrectomy
A RCT by Kumamoto University in 2009 demonstrated that “extensive intraoperative peritoneal lavage and intraperitoneal chemotherapy significantly improved the 5‐year OS of locally advanced gastric cancer positive for cytology of peritoneal lavage (CY+)/negative for peritoneal disease (P−).” 27 Extensive lavage relies on the limiting dilution theory, extirpating existing cancer cells by diluting 10 times with 1 L of normal saline (10 L in total), whereby 1010 cancer cells theoretically decrease to only one. Several subsequent RCTs aimed to replicate this promising result with extended inclusion criteria of locally advanced gastric cancer irrespective of the cytological results. However, the results were generally disappointing; none of the studies were able to demonstrate a reduction in peritoneal recurrence nor an improvement in OS by extensive lavage, although one study (SEIPLUS trial in China) demonstrated reduced surgical mortality. 28 , 29 , 30 If limited to patients positive for cytology, the results might have been different; however, there are usually very few patients with CY+/P−, which makes this extent of exclusion unsuitable for phase III RCTs.
2.5.2. Cholecystectomy: take it or leave it?
It is understood that gallstones are common occurrences following gastrectomy, and can cause cholecystitis or even severe cholangitis as a result of small gallstones dropping and getting stuck in the bile duct. In particular, in cases of Roux‐en‐Y anastomosis, it is, if not impossible, difficult to access the bile duct; therefore, for a prophylactic purpose, it is often questioned whether the gallbladder should be removed or not. This question remains part of a long‐standing discussion, and currently, both removing and leaving the gallbladder are common practice.
The PEGASUS‐D study in Korea, in which the efficacy and safety of ursodeoxycholic acid for the prevention of gallstone formation was evaluated, demonstrated a prophylactic effect of ursodeoxycholic acid (300 mg/d or 600 mg/d) on gallstone formation compared to the placebo control at 12 months after surgery; moreover, no severe adverse events caused by drug administration were observed (OR, 0.27; 95% CI, 0.12‐0.62; P = .002 in the 300 mg group, OR, 0.20; 95% CI, 0.08‐0.50; P < .001 in the 600 mg group). 31 Another report from the Italian Research Group for Gastric Cancer (GIRCG) on a RCT of concomitant prophylactic cholecystectomy, The Cholegas Trial, concluded that cholecystectomy has no significant impact on the natural course of patients after gastrectomy over a 5‐year period. 32 However, the Italian study suggests that gallstones are most likely to form 5 years after surgery. In addition, whether the formed gallstones could cause severe issues or not is another matter, although this appeared to be very rare. Therefore, it is unrealistic to administer life‐long ursodeoxycholic acid simply to prevent gallstone formation, especially for such a rare event that may only occur a very long time after gastrectomy; unfortunately, it seems almost impossible to establish evidence by RCTs.
2.5.3. Dissection of splenic hilum nodes for tumors that invade the greater curvature
The JOCG0110 trial concluded that “splenectomy should be avoided as it increases operative morbidity without improving survival.” 33 However, this study included locally advanced proximal gastric adenocarcinoma that did not invade the greater curvature; therefore, it is unclear if splenectomy (splenic hilum nodal dissection, or #10 dissection) was to be performed for tumors invading the greater curvature, e.g. type 4 cancer affecting the whole stomach. Two retrospective studies investigated the efficacy of splenectomy in patients with type 4 cancer, which is recognized as one of the high‐risk groups of splenic hilum nodal metastasis, and concluded that splenic hilum lymph node dissection offers certain survival benefit. 34 , 35 Another study from China evaluated the safety of spleen‐conserving hilar nodal dissection during a minimally invasive technique by pooled analysis, and showed both the safety and feasibility of the procedure. 36 Although both splenectomy and spleen‐conserving dissection for high‐risk groups might improve patient survival, they need to be performed by skilled hands given the complexity of the technique.
2.5.4. Conversion therapy — from unresectable to resectable
Owing to the development of effective chem‐ and molecular‐targeting drugs and their combinations, a small part of cStage IV patients with an initially unresectable or marginally resectable gastric cancer have the chance to undergo surgery with a curative intent after a couple of cycles of chemotherapy, the strategy of which is called “conversion therapy.” With an aim to deal with these patients systematically, a new category has been proposed. 37 In brief, cStage IV is first divided into two types — absence (Category 1/2) or presence (Category 3/4) of macroscopic peritoneal carcinomatosis — and the two are further divided into the following four categories: Category 1 is technically resectable, Category 2 is marginally resectable, Category 3 is potentially unresectable, and Category 4 involves non‐curable metastasis. In accordance with the category, a large retrospective study on conversion therapy, CONVO‐GC‐1, was conducted in China, Korea, and Japan, demonstrating that MST of R0 resected patients was as long as 56.6 months while MST of R1 and R2 was only 25.8 and 21.7 months, respectively. Interestingly, MSTs between the categories did not show a big difference between the categories, suggesting that cStage IV patients could survive if R0 resection could be achieved by conversion therapy. 38 More and more studies have reported that chemotherapy followed by resection of the primary tumor and metastases could be an effective treatment option for cStage IV cancer, as long as R0 could be achieved.
While this new strategy of “conversion therapy” sounds very promising, plenty of issues should be solved or an agreement on these issues should be decided: for example: What regimen is best? What is the best timing for surgery? How can we select appropriate candidates for “R0 resection” which can only be confirmed by thorough pathological inspection after surgery, etc. In addition, it should also be investigated whether conversion therapy is actually superior over chemotherapy alone. In line with the last issue, a large, randomized phase III trial, RENAISSANCE (AIO‐FLOT5), is being conducted in Germany to make evidence for the strategy that compares conversion therapy to chemotherapy alone in patients with limited‐metastatic adenocarcinoma of the stomach and cancer of the gastroesophageal junction. 39
3. NEOADJUVANT TREATMENT AGAINST RESECTABLE LOCALLY ADVANCED GASTRIC CANCER
In Asian countries where early cancers are more common, D2 gastrectomy alone offers favorable survival outcomes in most patients and has long been a standard of care. As a result, NAC has only been indicated for a subset of intractable advanced gastric cancers, namely large type 3 (infiltrative) tumors (<8 cm), type 4 (linitis plastica) tumors, and tumors with bulky metastatic nodes and/or a small number of metastatic nodes in the paraaortic area. The Japan Clinical Oncology Group (JCOG), the largest study group in Japan to conduct phase II/III trials to establish standard treatments for cancers, implemented a phase III RCT evaluating impact of NAC with S‐1/cisplatin (CS) for large type 3 or 4 gastric cancer 40 (Table 2). Although NAC followed by surgery was shown to be safe, contrary to our anticipations, the following report was disappointing in that additional NAC did not demonstrate any survival benefit (3‐year OS: Control vs NAC: 62.4% vs 60.9%, HR: 0.92, 95% CI: 0.68‐1.24, one‐sided P = .284), although a subgroup analysis suggested that NAC might be beneficial for non‐signet ring cell histology. 40 , 41 , 42 Based on this result, NAC for such tumors is not currently recommended in the Japanese gastric cancer treatment guidelines. Another approach using a triplet regimen has also been attempted. The KDOG1001 phase II trial evaluated the feasibility and efficacy of CS plus docetaxel (DCS) for large type 3 tumor, type 4 tumor, and tumors with bulky nodes, and achieved a good histological response rate of 57.5% (including 8% complete response), with acceptable morbidity and mortality rates. 43
TABLE 2.
Pivotal randomized controlled trials of perioperative chemotherapy for locally advanced gastric cancer
| Trial name | Country | NAC | Adjuvant | Primary endpoint | Control | Intervention | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|
| JCOG0501 42 | Japan | CS | S‐1 | 3‐year OS | 62.4% | 60.9% | 0.916 (0.679‐1.236) | .28 |
| PRODIGY 45 | Korea | DOS | S‐1 | 3‐year PFS | 60.2% | 66.3% | 0.70 (0.52‐0.95) | .023 |
| RESONANCE 46 | China | SOX | SOX | 3‐year DFS | n/a | n/a | n/a | n/a |
| FLOT4‐AIO 47 | Germany | FLOT | FLOT | OS (MST) | 50 months | 35 months | 0.77 (0.63‐0.94) | .012 |
Abbreviations: CI, confidence interval; CS, S‐1 + cisplatin; DFS, disease‐free survival; DOS, docetaxel + oxaliplatin+S‐1; FLOT, fluorouracil + leucovorin+oxaliplatin + docetaxel; MST, mean survival time; n/a, not available; OS, overall survival; PFS, progression‐free survival; SOX, S‐1 + oxaliplatin.
NAC for cStage III has also been under investigation. The COMPASS‐D phase II trial, with a two‐by‐two factorial design, evaluated the significance of NAC by comparing two vs four cycles of CS vs DCS. The results of a 3‐year follow‐up, demonstrated that four cycles of DCS offered the best OS of 71.9%, and thus has been chosen as a promising regimen in an upcoming phase III trial. 44 Furthermore, in 2019, a phase III RCT from South Korea, the PRODIGY Trial, compared NAC to upfront D2 gastrectomy. The results demonstrated a prolonged progression‐free survival (PFS) of NAC with S‐1/oxaliplatin/docetaxel (DOS) for locally advanced cancer of the stomach or the gastroesophageal junction (HR, 0.70; 95% CI, 0.52‐0.95; P = .023), but there was no significant difference in OS between the two groups (HR, 0.84; 95% CI, 0.60‐1.19; P = .338). 45 In China, a phase III study using S‐1 plus oxaliplatin (SOX) as NAC for pathological Stage (pStage) IIA‐IIIC, the RESONANCE Trial, showed that 67.8% and 23.6% of patients receiving NAC showed pathological efficacy and complete response, respectively, with no significant differences in short‐term surgical outcomes. 46
In Europe, the FLOT4 trial by the German group FLOT‐AIO revealed a phenomenal result in the perioperative treatment of gastric cancer. 47 They had historically used a triplet regimen with epirubicin and cisplatin plus either fluorouracil or capecitabine (ECF/ECX) based on the MAGIC trial, and an equivalent regimen replacing cisplatin with oxaliplatin (EOX). 48 , 49 However, a new regimen using the docetaxel‐based triplet FLOT (fluorouracil plus leucovorin, oxaliplatin and docetaxel) resulted in a meaningful improvement of 5‐year OS by 9% (HR, 0.77; 95% CI, 0.63‐0.94; P = .012) and median overall survival time (MST) by 15 months without increasing significant adverse events; consequently, this new regime took the place of ECF/ECX/EOX as a standard treatment for gastric cancer.
In Asian countries, where D2 gastrectomy has been recognized as the most effective treatment for resectable gastric cancer, the most critical issue is yet to be conclusive: which patients should be indicated for NAC in the first place? This is the starting point since appropriate patient selection is the most important issue in successful trials. With regard to optimal regimens, considering the above results, triplet regimens are likely to be promising. Still, their efficacy needs to be validated, particularly regarding OS, in phase III trials.
4. POSTOPERATIVE ADJUVANT CHEMOTHERAPY
The efficacy of adjuvant chemo/chemoradiation therapy has been widely accepted based on landmark trials: Intergroup 0116 (INT0116) in the United States, FLOT4 in Europe, and ACTS‐GC in Japan, and CLASSIC in China. 47 , 50 , 51 , 52 However, there remain outstanding issues, including determination of the optimal number of cycles between survival benefit and tolerability, whether additional radiation therapy is beneficial, along with the optimal drug combination.
Since a large RCT (ACTS‐GC) comparing D2 gastrectomy alone to surgery plus adjuvant S‐1 for 1 year (eight cycles) for pStage II/III demonstrated prolonged survival with adjuvant S‐1 administration, surgery plus S‐1‐based adjuvant therapy has been a major treatment strategy not only in Japan but also in China and Korea. 51 Adjuvant chemotherapy using S‐1 alone for 1 year worked well, with a HR of 0.669 (95% CI: 0.540‐0.828); however, two issues remained unsolved.
One, was the duration of administration for pStage II patients. While S‐1 showed a striking improvement in survival for pStage II (HR: 0.509, 95% CI: 0.338‐0.765), S‐1 for 1 year was a difficult treatment, with 34.2% of patients in the trial having stopped due to adverse events or other reasons, and 42.4% having had reduced doses of S‐1. Therefore, there was some hope that S‐1 for half a year for Stage II patients might work as well as S‐1 for one year, with less burden of adverse effects and better adherence to chemotherapy. JCOG conducted a phase III trial (JCOG1104) for pStage II patients who were less likely to experience recurrence, and compared S‐1 for half a year (four cycles) to 1 year (eight cycles) (Table 3). Surprisingly, at the first planned interim analysis, the point estimate of the HR for half a year was 2.52 (95% CI: 1.11‐5.77), which met the prespecified threshold (HR: 1.37) for early termination. 53 Moreover, the HR for the updated 3‐year RFS was 1.84 (95% CI: 0.93‐3.63) with a RFS of 89.8% in the half‐a‐year group and 93.1% in the 1‐year group. Therefore, S‐1 for 1 year is still recommended for pStage II patients.
TABLE 3.
Landmark trials for adjuvant chemo/chemoradiation therapy for locally advanced gastric cancer
| Trial name | Adjuvant | Experimental | Outcome | Control | Experimental | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|---|---|---|
| START‐2 a , 54 | S‐1 alone | DS | 3‐year RFS | 50% | 66% | 0.632 (0.400‐0.998 b ) | <.001 |
| JCOG1104 53 | S‐1 for 1 year | S‐1 for 6 months | RFS (3‐year) | 93.1% | 89.8% | 1.84 (0.93‐3.63) | n/a |
| ARTIST‐2 57 | S‐1 alone |
SOX SOXRT |
DFS (3‐year) | 65% |
78% 73% |
0.617 (n/a) 0.686 (n/a) |
.0157 .0572 |
| CRITICS 58 | ECF/ECX/EOX | Additional RT 45Gy | OS | 43 months | 37 months | 1.01 (0.84‐1.22) | .90 |
Abbreviations: CI, confidence interval; DFS, disease‐free survival; DS, docetaxel + cisplatin; ECF, epirubicin + cisplatin+fluorouracil; ECX, epirubicine + cisplatin+capecitabine; EOX, epirubicine + oxaliplatin+capecitabine; n/a, not available; OS, overall survival; RFS, relapse‐free survival; RT, radiation therapy; SOX, S‐1 + oxaliplatin.
JACCRO‐GC 07.
99.99% confidence interval.
The other issue was that the survival benefit was unsatisfactory for patients with more advanced disease, with HRs of 0.791 (95% CI: 0.520‐1.205) for pStage IIIB, suggesting that more intensified chemotherapy is necessary to improve survival for patients with advanced cancer. To challenge this issue, a START‐2 trial comparing S‐1 plus docetaxel (DS) to S‐1 alone was conducted for pStage III after curative surgery. The second interim analysis was performed, in which the superiority of DS over S‐1 for 3‐year RFS as the primary endpoint was demonstrated (HR: 0.632; 99.99% CI, 0.400‐0.998; P < .001). 54 Based on this result, the safety monitoring committee recommended early termination of the trial. Although 5‐year OS is currently being analyzed, with results expected in the near future, DS is currently one of the standard regimens for pStage III as well as SOX and capecitabine plus oxaliplatin (XELOX/CapeOX), which have been demonstrated to be effective by the two phase II trials: G‐SOX and J‐CLASSIC, respectively. 55 , 56
In Korea, a subgroup analysis of the preceding trial named ARTIST showed a potential benefit of adjuvant radiation therapy for node‐positive patients after D2 gastrectomy. Consequently, the subsequent ARTIST 2 trial was conducted to compare adjuvant S‐1 monotherapy for 1 year to S‐1 for 1 year plus oxaliplatin for half a year (SOX) or SOX plus radiation of 45Gy (SOXRT) in pStage II/III node‐positive patients. However, the trial was stopped early because of benefit, and a preliminary result was presented with significant HRs for DFS (0.617 for S‐1 vs SOX [P = .016] and 0.686 for S‐1 vs SOXRT [P = .057]), with no significant difference between SOX and SOXRT (HR: 0.910, P = .667). 57 This result suggests that radiation therapy has a limited effect on local recurrence in cases where adequate D2 gastrectomy is performed; thus, SOX or SOXRT is recommended for pStage II/III in Korea.
In Europe, before FLOT became the mainstay for treatment, perioperative chemotherapy using ECF/ECX/EOX was a popular strategy for locally advanced gastric cancer; this was based on the MAGIC trial that showed both tumor downsizing and downstaging with a significant survival benefit in comparison with surgery alone. However, a potential benefit of adjuvant radiation therapy (total: 45 Gy; 1.8 Gy/fraction, 25 times) was investigated in combination with postoperative chemotherapy in a trial named CRITICS; this was on the basis of a promising result of the Intergroup 0116 trial in the United States, where postoperative fluorouracil (FU) monotherapy in combination with FU‐based chemoradiotherapy improved OS compared with surgery alone in patients with locally advanced and radically resected gastric cancer. However, the CRITICS trial concluded no survival benefit of additional radiation therapy in terms of OS, with an MST of 43 months (95% CI: 31‐57) in the chemotherapy group and 37 months (95% CI: 30‐48) in the chemoradiotherapy group (HR, 1.01; 95% CI, 0.84‐1.22; P = .90). As a result, preoperative chemotherapy with adequate surgery can be considered the backbone of resectable gastric cancer treatment. 58
The article on the CRITICS trial also addressed a successor phase II trial named CRITICS‐II, which focused more on preoperative treatment strategies. 59 One of the rationales of the trial was that many patients from Europe and North America are less tolerable, that is, are less likely to be able to receive planned postoperative therapy. For example, in the CRITICS trial, more than 90% of the trial patients could receive the recommended dose intensities of preoperative chemotherapy drugs, whereas only approximately 50% of patients could receive the recommended dose intensities postoperatively. Another rationale related to the fact that the survival benefit likely comes from tumor downsizing and increased resectability due to the effect of preoperative treatment. In these countries, many gastric tumors grow in the upper third or gastroesophageal junction and need to be treated by (extended) total gastrectomy, in which the tolerability of postoperative chemotherapy would be relatively low.
5. FUTURE PERSPECTIVE IN GASTRIC CANCER TREATMENT: A POSSIBLE TAILORED TREATMENT
By and large, two strategic directions in gastric cancer surgery can be considered in the future. For early cancer, a “truly” minimally invasive approach is necessary with the aid of navigation surgery like SNNS whereby the extent of resection of the stomach can be minimized and tailored. For locally advanced cancer, D2 gastrectomy remains the standard of care for the time being, and thus it is vital to improve survival by combining chemotherapy including cytotoxic drugs, molecular‐targeting agents and immune checkpoint inhibitors (ICIs), and radiation with surgical therapy.
The efficacy of molecular‐targeting agents and ICIs has been demonstrated by many trials for advanced/recurrent gastric cancer. 60 , 61 Following these encouraging results, several regimens of cytotoxic agents plus molecular‐targeting drugs or ICIs are currently being tested in the neoadjuvant and adjuvant settings: for example, the TRIGGER study (JCOG1301‐C) in Japan comparing neoadjuvant chemotherapy vs chemotherapy plus trastuzumab as neoadjuvant treatment in patients positive for human epidermal growth factor receptor 2 (HER2); the INNOVATION trial in Europe and Korea comparing chemotherapy alone vs chemotherapy plus trastuzumab vs chemotherapy plus trastuzumab plus pertuzumab in the neoadjuvant and adjuvant settings for HER2‐positive gastric and gastroesophageal junction adenocarcinoma; the ATTRACTION‐5 trial in Japan comparing chemotherapy vs chemotherapy plus nivolumab in the adjuvant setting for locally advanced gastric cancer undergoing curative surgery; the VESTIGE trial in Europe comparing adjuvant chemotherapy vs immunotherapy with nivolumab and low‐dose ipilimumab for high‐risk resected gastric or esophageal adenocarcinoma; and the KEYNOTE‐585 trial, an international study comparing chemotherapy vs pembrolizumab plus chemotherapy in the neoadjuvant or adjuvant setting for locally advanced gastric or gastroesophageal junction adenocarcinoma. 62 , 63 , 64 , 65 In the era of precision medicine, though surgery plus cytotoxic agents will remain as the mainstay of treatment for locally advanced cancer, these combinations, which are optimized according to molecular and immune profile of each patient, will be standard of care in the neoadjuvant and adjuvant settings.
Different from the conventional one‐drug‐fits‐all strategy, the molecular‐targeting therapy and immunotherapy require more information concerning tumor characteristics in order to optimize therapeutic regimens and exploit their efficacy. In other words, molecular, metabolomic, and gene expression profiling will play a critical role in future gastric cancer treatment where more and more new promising drugs are expected to come out. Therefore, there is an urgent need to identify novel biomarkers that are able to predict responses to chemotherapy/radiation and/or that are associated with survival outcomes.
Several recent studies concerning metabolomic or gene expression analysis have offered encouraging results in gastric cancer treatment. With regard to biomarkers of poor outcomes, a study from Memorial Sloan Kettering Cancer Center explored genomic underpinnings of early recurrence and subsequent poor survival in patients with early gastric cancer. The team analyzed patients with early gastric cancer with poor survival using a targeted exome capture‐based next‐generation sequencing assay. The results revealed a distinct genomic profile, in which co‐occurring hotspot mutations and loss of heterozygosity in TP53 (TP53MUT/LOH) were enriched in patients with T1‐2 N0 gastric cancer who experienced recurrence and death within 5 years after curative resection, whereas it was neither prognostic nor enriched in patients with locally advanced or metastatic cancer. 66 Another study investigated early peritoneal recurrence using metabolomic analysis, which is another approach to explore the mechanism of cancer progression. 67 By using capillary electrophoresis time‐of‐flight mass spectrometry (CE‐TOFMS), a low level of b‐alanine, which has been suggested to be involved in cancer proliferation and metastasis, was found to be an independent predictor of peritoneal recurrence as well as an independent prognostic factor. In the near future, validation studies using larger cohorts are expected to ensure these promising results.
With regard to the response to chemotherapy/radiation, some studies using patient cohorts in RCTs have been reported. There are two additional studies from the CLASSIC trial conducted in China, South Korea, and Taiwan. The first one investigated whether microsatellite instability (MSI) status and programed‐cell death ligand 1 (PD‐L1) expression were predictors of prognosis and response to chemotherapy for patients with pStage II/III gastric cancer. 68 As a result, MSI‐high (MSI‐H) status was observed in 6.8%, 2.7% of which were positive for tumor PD‐L1, and 28.4% were positive for stromal PD‐L1 (sPD‐L1). Interestingly, adjuvant chemotherapy significantly improved DFS in the microsatellite stable (MSS) group but not in the MSI‐H group. Moreover, in the MSS group, sPD‐L1‐negative patients had significant survival benefit from adjuvant chemotherapy compared with surgery alone. This phenomenon regarding MSI status is supported by another study that evaluated MSI status with individual patient data meta‐analysis of the MAGIC, CLASSIC, ARTIST, and ITACA‐S trials. 69 In brief, the analysis demonstrated that patients with MSI‐low/MSS gastric cancer benefited from chemotherapy plus surgery, while those with MSI‐high status did not. As a result, the study suggested that MSI status could serve as a robust prognostic marker. The second study from Korea explored biologically relevant candidate genes using exploratory bioinformatics analyses of gastric cancer transcriptome datasets. In this study, four classifier genes, GZMB (granzyme B), WARS (tryptophanyl‐tRNA synthetase), SFRP4 (secreted frizzled‐related protein 4), and CDX1 (caudal‐type homeobox 1), were found to be predictive of chemotherapy‐response benefit for patients with resectable pStage II/III gastric cancer. 70 In Japan, where S‐1 is one of the key drugs in gastric cancer treatment, a study investigated biomarkers associated with response to adjuvant therapy using S‐1 by comprehensive gene expression analysis. The results identified 147 upregulated and 192 downregulated differentially expressed genes in patients who benefited from S‐1 adjuvant chemotherapy after curative resection. 71 Interestingly, upregulated genes showed significant enrichment in immune‐related genes by gene ontology analysis; thus, they concluded that patients who were likely to benefit from S‐1 adjuvant chemotherapy could be predicted by evaluating tumor immune activation.
6. CONCLUSION
We have explored pivotal studies that have changed or will change the current clinical practice of gastric cancer treatment. Overall, minimally invasive surgery for cStage I is already part of the standard of care, while still in development for locally advanced cancer. In addition to minimally invasive surgery, more individualized surgery like SNNS will be necessary for cStage I. For locally advanced cancer, although considerable effort has been made to improve survival by surgery, it remains important to develop a treatment strategy combining NAC, surgery, and adjuvant therapy. Moreover, metabolomic or gene expression profiling will be an essential component of the next‐generation of therapy in an era of precision medicine; however, it seems that there is still a paucity of data, and many studies need to be validated to bring these results into clinical practice.
DISCLOSURE
Funding: The authors received no specific funding for this work.
Conflict of Interest: The authors declare no conflict of interests associated with this article.
Irino T, Matsuda S, Wada N, Kawakubo H, Kitagawa Y. Essential updates 2019/2020: Perioperative and surgical management of gastric cancer. Ann Gastroenterol Surg. 2021;5:162–172. 10.1002/ags3.12438
REFERENCES
- 1. Etemadi A, Safiri S, Sepanlou SG, Ikuta K, Bisignano C, Shakeri R, et al. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, et al. Survival outcomes after laparoscopy‐assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non‐inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5(2):142–51. [DOI] [PubMed] [Google Scholar]
- 3. Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long‐term survival among patients with stage I gastric cancer: the KLASS‐01 randomized clinical trial. JAMA Oncol. 2019;5(4):506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Katai H, Mizusawa J, Katayama H, Kunisaki C, Sakuramoto S, Inaki N, et al. Single‐arm confirmatory trial of laparoscopy‐assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer. 2019;22(5):999–1008. [DOI] [PubMed] [Google Scholar]
- 5. Hyung WJ, Yang HK, Han SU, Lee YJ, Park JM, Kim JJ, et al. A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi‐center phase II clinical trial, KLASS 03. Gastric Cancer. 2019;22(1):214–22. [DOI] [PubMed] [Google Scholar]
- 6. Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, et al. Laparoscopic versus open total gastrectomy for clinical stage I gastric cancer: Morbidity and mortality results from a prospective randomized multicenter controlled trial (CLASS02). J Clin Oncol. 2020;38(4_suppl):378. [Google Scholar]
- 7. Uyama I, Suda K, Nakauchi M, Kinoshita T, Noshiro H, Takiguchi S, et al. Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: a multi‐institutional prospective single‐arm study. Gastric Cancer. 2019;22(2):377–85. [DOI] [PubMed] [Google Scholar]
- 8. Shibasaki S, Suda K, Obama K, Yoshida M, Uyama I. Should robotic gastrectomy become a standard surgical treatment option for gastric cancer? Surg Today. 2020;50(9):955–65. [DOI] [PubMed] [Google Scholar]
- 9. Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Effect of laparoscopic vs open distal gastrectomy on 3‐year disease‐free survival in patients with locally advanced gastric cancer: the CLASS‐01 randomized clinical trial. JAMA. 2019;321(20):1983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, et al. Short‐term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS‐02‐RCT). Ann Surg. 2019;270(6):983–91. [DOI] [PubMed] [Google Scholar]
- 11. Hyung WJ, Yang H‐K, Park Y‐K, Lee H‐J, An JY, Kim W, et al. Long‐term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: the KLASS‐02‐RCT randomized clinical trial. J Clin Oncol. 2020;38(28):3304–13. [DOI] [PubMed] [Google Scholar]
- 12. Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, et al. A multi‐institutional, prospective, phase II feasibility study of laparoscopy‐assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J Surg. 2015;39(11):2734–41. [DOI] [PubMed] [Google Scholar]
- 13. van der Wielen N, Straatman J, Daams F, Rosati R, Parise P, Weitz J, et al. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial. Gastric Cancer. 2021;24(1):258–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31(29):3704–10. [DOI] [PubMed] [Google Scholar]
- 15. An JY, Min JS, Hur H, Lee YJ, Cho GS, Park YK, et al. Laparoscopic sentinel node navigation surgery versus laparoscopic gastrectomy with lymph node dissection for early gastric cancer: short‐term outcomes of a multicentre randomized controlled trial (SENORITA). Br J Surg. 2020;107(11):1429–39. [DOI] [PubMed] [Google Scholar]
- 16. Ryu KW, Kim YW, Min JS, An JY, Yoon HM, Eom BW, et al. Laparoscopic sentinel node navigation surgery versus laparoscopic standard gastrectomy with lymph node dissection in early gastric cancer: results of postoperative morbidity and mortality from a multicenter randomized controlled trial (SENORITA trial). J Clin Oncol. 2018;36(15_suppl):e16043. [Google Scholar]
- 17. Isozaki H, Matsumoto S, Murakami S. Survival outcomes after sentinel node navigation surgery for early gastric cancer. Ann Gastroenterol Surg. 2019;3(5):552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okubo K, Arigami T, Matsushita D, Sasaki K, Kijima T, Noda M, et al. Evaluation of postoperative quality of life by PGSAS‐45 following local gastrectomy based on the sentinel lymph node concept in early gastric cancer. Gastric Cancer. 2020;23(4):746–53. [DOI] [PubMed] [Google Scholar]
- 19. Kamiya S, Takeuchi H, Fukuda K, Kawakubo H, Takahashi N, Mitsumori N, et al. A multicenter non‐randomized phase III study of sentinel node navigation surgery for early gastric cancer. Jpn J Clin Oncol. 2020. 10.1093/jjco/hyaa179 [DOI] [PubMed] [Google Scholar]
- 20. Mayanagi S, Takahashi N, Mitsumori N, Arigami T, Natsugoe S, Yaguchi Y, et al. Sentinel node mapping for post‐endoscopic resection gastric cancer: multicenter retrospective cohort study in Japan. Gastric Cancer. 2020;23(4):716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nohara K, Goto O, Takeuchi H, Sasaki M, Maehata T, Yahagi N, et al. Gastric lymphatic flows may change before and after endoscopic submucosal dissection: in vivo porcine survival models. Gastric Cancer. 2019;22(4):723–30. [DOI] [PubMed] [Google Scholar]
- 22. Steevens J, Botterweck AA, Dirx MJ, van den Brandt PA, Schouten LJ. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur J Gastro Hepatol. 2010;22(6):669–78. [DOI] [PubMed] [Google Scholar]
- 23. Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Sem Radiat Oncol. 2013;23(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bartel M, Brahmbhatt B, Bhurwal A. Incidence of gastroesophageal junction cancer continues to rise: analysis of surveillance, epidemiology, and end results (SEER) database. J Clin Oncol. 2019;37(4_suppl):40. [Google Scholar]
- 25. Kurokawa Y, Takeuchi H, Doki Y, Mine S, Terashima M, Yasuda T, et al. Mapping of lymph node metastasis from esophagogastric junction tumors: a prospective nationwide multicenter study. Ann Surg. 2019. 10.1097/SLA.0000000000003499 [DOI] [PubMed] [Google Scholar]
- 26. Mitchell KG, Ikoma N, Nelson DB, Maru DM, Erasmus JJ, Weston BR, et al. Mediastinal nodal involvement after neoadjuvant chemoradiation for Siewert II/III adenocarcinoma. Ann Thorac Surg. 2019;108(3):845–51. [DOI] [PubMed] [Google Scholar]
- 27. Kuramoto M, Shimada S, Ikeshima S, Matsuo A, Yagi Y, Matsuda M, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009;250(2):242–6. [DOI] [PubMed] [Google Scholar]
- 28. Guo J, Xu A, Sun X, Zhao X, Xia Y, Rao H, et al. Combined surgery and extensive intraoperative peritoneal lavage vs surgery alone for treatment of locally advanced gastric cancer: the SEIPLUS randomized clinical trial. JAMA Surg. 2019;154(7):610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Misawa K, Mochizuki Y, Sakai M, Teramoto H, Morimoto D, Nakayama H, et al. Randomized clinical trial of extensive intraoperative peritoneal lavage versus standard treatment for resectable advanced gastric cancer (CCOG 1102 trial). Br J Surg. 2019;106(12):1602–10. [DOI] [PubMed] [Google Scholar]
- 30. So JBY, Ji J, Han SU, Terashima M, Li G, Kim H‐H, et al. Extensive peritoneal lavage after curative gastrectomy for gastric cancer study (EXPEL): an international multicenter randomized controlled trial. J Clin Oncol. 2020;38(4_suppl):279. [Google Scholar]
- 31. Lee SH, Jang DK, Yoo MW, Hwang SH, Ryu SY, Kwon OK, et al. Efficacy and safety of ursodeoxycholic acid for the prevention of gallstone formation after gastrectomy in patients with gastric cancer: the PEGASUS‐D randomized clinical trial. JAMA Surg. 2020;155(8):703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bencini L, Marchet A, Alfieri S, Rosa F, Verlato G, Marrelli D, et al. The Cholegas trial: long‐term results of prophylactic cholecystectomy during gastrectomy for cancer‐a randomized‐controlled trial. Gastric Cancer. 2019;22(3):632–9. [DOI] [PubMed] [Google Scholar]
- 33. Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. 2017;265(2):277–83. [DOI] [PubMed] [Google Scholar]
- 34. Kano Y, Ohashi M, Ida S, Kumagai K, Makuuchi R, Sano T, et al. Therapeutic value of splenectomy to dissect splenic hilar lymph nodes for type 4 gastric cancer involving the greater curvature, compared with other types. Gastric Cancer. 2020;23(5):927–36. [DOI] [PubMed] [Google Scholar]
- 35. Hayashi T, Yoshikawa T, Kamiya A, Date K, Wada T, Otsuki S, et al. Is splenectomy for dissecting splenic hilar lymph nodes justified for scirrhous gastric cancer? Gastric Cancer. 2020;23(5):922–6. [DOI] [PubMed] [Google Scholar]
- 36. Zhong Q, Chen QY, Xu YC, Zhao G, Cai LS, Li GX, et al. Reappraise role of No. 10 lymphadenectomy for proximal gastric cancer in the era of minimal invasive surgery during total gastrectomy: a pooled analysis of 4 prospective trial. Gastric Cancer. 2021;24(1):245–57. [DOI] [PubMed] [Google Scholar]
- 37. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19(2):329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Terashima M, Yoshida K, Rha SY, Bae JM, Li G, Katai H, et al. ,Ji J et al.: International retrospective cohort study of conversion therapy for stage IV gastric cancer 1 (CONVO‐GC‐1). J Clin Oncol. 2018;36(15_suppl):4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Al‐Batran SE, Goetze TO, Mueller DW, Vogel A, Winkler M, Lorenzen S, et al. The RENAISSANCE (AIO‐FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited‐metastatic adenocarcinoma of the stomach or esophagogastric junction ‐ a phase III trial of the German AIO/CAO‐V/CAOGI. BMC Cancer. 2017;17(1):893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Terashima M, Iwasaki Y, Mizusawa J, Katayama H, Nakamura K, Katai H, et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S‐1 plus cisplatin for type 4 or large type 3 gastric cancer, the short‐term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer. 2019;22(5):1044–52. [DOI] [PubMed] [Google Scholar]
- 41. Katai H, Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, et al. Subgroup analysis of JCOG0501 phase III study to confirm superiority of additional neoadjuvant chemotherapy with S‐1 plus cisplatin to D2 gastrectomy with S‐1 adjuvant chemotherapy for resectable type IV or large type III gastric cancer. J Clin Oncol. 2019;37(4_suppl):110. [Google Scholar]
- 42. Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, et al. Gastrectomy with or without neoadjuvant S‐1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open‐label, phase 3, randomized controlled trial. Gastric Cancer. 2020. 10.1007/s10120-020-01136-7 [DOI] [PubMed] [Google Scholar]
- 43. Hosoda K, Azuma M, Katada C, Moriya H, Mieno H, Ishido K, et al. A phase II study of neoadjuvant chemotherapy with docetaxel, cisplatin, and S‐1, followed by gastrectomy with D2 lymph node dissection for high‐risk advanced gastric cancer: results of the KDOG1001 trial. Gastric Cancer. 2019;22(3):598–606. [DOI] [PubMed] [Google Scholar]
- 44. Hayashi T, Yoshikawa T, Sakamaki K, Nishikawa K, Fujitani K, Tanabe K, et al. Primary results of a randomized two‐by‐two factorial phase II trial comparing neoadjuvant chemotherapy with two and four courses of cisplatin/S‐1 and docetaxel/cisplatin/S‐1 as neoadjuvant chemotherapy for advanced gastric cancer. Ann Gastroenterol Surg. 2020;4(5):540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang YK, Yook JH, Park YK, Kim YW, Kim J, Ryu MH, et al. Phase III randomized study of neoadjuvant chemotherapy (CT) with docetaxel(D), oxaliplatin(O) and S‐1(S) (DOS) followed by surgery and adjuvant S‐1, vs surgery and adjuvant S‐1, for resectable advanced gastric cancer (GC) (PRODIGY). Ann Oncol. 2019;30:v876. [Google Scholar]
- 46. Wang X, Li S, Xie T, Lu Y, Guo X, Lin C. Early results of the randomized, multicenter, controlled evaluation of S‐1 and oxaliplatin as neoadjuvant chemotherapy for Chinese advanced gastric cancer patients (RESONANCE Trial). J Clin Oncol. 2020;38(4_suppl):280. [Google Scholar]
- 47. Al‐Batran S‐E, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro‐oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57. [DOI] [PubMed] [Google Scholar]
- 48. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. [DOI] [PubMed] [Google Scholar]
- 49. Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36–46. [DOI] [PubMed] [Google Scholar]
- 50. Noh SH, Park SR, Yang H‐K, Chung HC, Chung I‐J, Kim S‐W, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5‐year follow‐up of an open‐label, randomised phase 3 trial. Lancet Oncol. 2014;15(12):1389–96. [DOI] [PubMed] [Google Scholar]
- 51. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five‐year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S‐1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–93. [DOI] [PubMed] [Google Scholar]
- 52. Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Updated analysis of SWOG‐directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30(19):2327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yoshikawa T, Terashima M, Mizusawa J, Nunobe S, Nishida Y, Yamada T, et al. Four courses versus eight courses of adjuvant S‐1 for patients with stage II gastric cancer (JCOG1104 [OPAS‐1]): an open‐label, phase 3, non‐inferiority, randomised trial. Lancet Gastroenterol Hepatol. 2019;4(3):208–16. [DOI] [PubMed] [Google Scholar]
- 54. Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC‐07, a randomized controlled trial. J Clin Oncol. 2019;37(15):1296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S‐1 with cisplatin plus S‐1 in chemotherapy‐naïve patients with advanced gastric cancer. Ann Oncol. 2015;26(1):141–8. [DOI] [PubMed] [Google Scholar]
- 56. Fuse N, Bando H, Chin K, Ito S, Yoshikawa T, Tsuburaya A, et al. Adjuvant capecitabine plus oxaliplatin after D2 gastrectomy in Japanese patients with gastric cancer: a phase II study. Gastric Cancer. 2017;20(2):332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park SH, Zang DY, Han B, Ji JH, Kim TG, Oh SY, et al. ARTIST 2: Interim results of a phase III trial involving adjuvant chemotherapy and/or chemoradiotherapy after D2‐gastrectomy in stage II/III gastric cancer (GC). J Clin Oncol. 2019;37(15_suppl):4001. [Google Scholar]
- 58. Cats A, Jansen EPM, van Grieken NCT, Sikorska K, Lind P, Nordsmark M, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open‐label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):616–28. [DOI] [PubMed] [Google Scholar]
- 59. Slagter AE, Jansen EPM, van Laarhoven HWM, van Sandick JW, van Grieken NCT, Sikorska K, et al. CRITICS‐II: a multicentre randomised phase II trial of neo‐adjuvant chemotherapy followed by surgery versus neo‐adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo‐adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer. BMC Cancer. 2018;18(1):877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kang Y‐K, Boku N, Satoh T, Ryu M‐H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro‐oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO‐4538‐12, ATTRACTION‐2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. The Lancet. 2017;390(10111):2461–71. [DOI] [PubMed] [Google Scholar]
- 61. Shitara K, Bang Y‐J, Iwasa S, Sugimoto N, Ryu M‐H, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2‐positive gastric cancer. N Engl J Med. 2020;382(25):2419–30. [DOI] [PubMed] [Google Scholar]
- 62. Kataoka K, Tokunaga M, Mizusawa J, Machida N, Katayama H, Shitara K, et al. A randomized Phase II trial of systemic chemotherapy with and without trastuzumab followed by surgery in HER2‐positive advanced gastric or esophagogastric junction adenocarcinoma with extensive lymph node metastasis: Japan Clinical Oncology Group study JCOG1301 (Trigger Study). Jpn J Clin Oncol. 2015;45(11):1082–6. [DOI] [PubMed] [Google Scholar]
- 63. Wagner AD, Grabsch HI, Mauer M, Marreaud S, Caballero C, Thuss‐Patience P, et al. EORTC‐1203‐GITCG ‐ the "INNOVATION"‐trial: effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II‐intergroup trial of the EORTC‐Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI‐Cancer group. BMC Cancer. 2019;19(1):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Terashima M, Kim YW, Yeh TS, Chung HC, Chen JS, Boku N, et al. ATTRACTION‐05 (ONO‐4538‐38/BMS CA209844): a randomized, multicenter, double‐blind, placebo‐ controlled Phase 3 study of Nivolumab (Nivo) in combination with adjuvant chemotherapy in pStage III gastric and esophagogastric junction (G/EGJ) cancer. Ann Oncol. 2017;28:v266–v267. [Google Scholar]
- 65. Bang YJ, Van Cutsem E, Fuchs CS, Ohtsu A, Tabernero J, Ilson DH, et al. KEYNOTE‐585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019;15(9):943–52. [DOI] [PubMed] [Google Scholar]
- 66. Datta J, Da Silva EM, Kandoth C, Song T, Russo AE, Hernandez JM, et al. Poor survival after resection of early gastric cancer: extremes of survivorship analysis reveal distinct genomic profile. Br J Surg. 2020;107(1):14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kaji S, Irino T, Kusuhara M, Makuuchi R, Yamakawa Y, Tokunaga M, et al. Metabolomic profiling of gastric cancer tissues identified potential biomarkers for predicting peritoneal recurrence. Gastric Cancer. 2020;23(5):874–83. [DOI] [PubMed] [Google Scholar]
- 68. Choi YY, Kim H, Shin SJ, Kim HY, Lee J, Yang HK, et al. Microsatellite instability and programmed cell death‐ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the CLASSIC randomized controlled study. Ann Surg. 2019;270(2):309–16. [DOI] [PubMed] [Google Scholar]
- 69. Pietrantonio F, Miceli R, Raimondi A, Kim YW, Kang WK, Langley RE, et al. Individual patient data meta‐analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37(35):3392–400. [DOI] [PubMed] [Google Scholar]
- 70. Cheong J‐H, Yang H‐K, Kim H, Kim WH, Kim Y‐W, Kook M‐C, et al. Predictive test for chemotherapy response in resectable gastric cancer: a multi‐cohort, retrospective analysis. Lancet Oncol. 2018;19(5):629–38. [DOI] [PubMed] [Google Scholar]
- 71. Nakamura K, Hatakeyama K, Furukawa K, Fujiya K, Kamiya S, Hikage M, et al. Prediction of S‐1 adjuvant chemotherapy benefit in stage II/III gastric cancer treatment based on comprehensive gene expression analysis. Gastric Cancer. 2020;23(4):648–58. [DOI] [PubMed] [Google Scholar]
- 72. Global Burden of Disease 2019 [internet]. GBD2019 Home: Institute for Health Metrics and Evaluation, Seattle, WA [Accessed 1st Dec 2020. Available from: http://www.healthdata.org/gbd/2019


