Abstract
BACKGROUND
We evaluated to see if the algorithmic approach of pulmonary embolism (PE) [Wells’ score, followed by D-dimer test and computed tomography pulmonary angiography (CTPA)] is appropriately followed in teaching hospitals of Shiraz, Iran.
METHODS
From October 2012 to October 2013, we prospectively calculated Wells’ score for all patients who underwent CTPA with clinical suspicion to PE; patients with low probability who had not checked the D-dimer or had low level of D-dimer were considered as non-adherent to the guideline and those with high level of D-dimer or high probability of Wells’ score were labeled as adherent to the PE guideline. CTPA scans were independently reported by two radiologists.
RESULTS
During study period, 364 patients underwent CTPA to rule out PE, of which 125 (34.3%) had Wells’ score > 4 (high probable risk) and 239 had Wells’ score ≤ 4. Amongst low probable risk patients (Wells’ score ≤ 4), only 32 patients had undergone the D-dimer test (23 patients had high level of D-dimer). Based on the algorithmic approach, patients with suspected PE, patients with high probability (125 patients), and patients with low probability with elevated D-dimer level (23 patients) were considered as adherent to the PE guideline; consequently, the total adherence to PE guideline was 148 out of 364 (40.6%).
CONCLUSION
We followed the algorithmic approach guideline in about 40.0% of cases; however, we should pay more attention to the algorithmic approach in patients with suspected PE.
Keywords: Pulmonary Embolism, Diagnostic Imaging, Tomography, Blood, Spiral Computed Tomography
Introduction
Pulmonary embolism (PE), which occurs when a blood clot occludes the main or branches of pulmonary artery, is a life-threating disorder of cardiovascular system.1-3 Swift diagnosis is vital to start timely and effective therapy, but nonspecific clinical and laboratory findings make the diagnosis difficult, leading to increased mortality rate.4,5 Angiography has been the gold standard method for diagnosis of PE,6 but since it is invasive, computed tomography pulmonary angiography (CTPA) scan is being used to make a definite diagnosis.7
The approach to suspected cases should be initiated by scoring systems (e.g., Wells); the D-dimer test is the second step in low probability cases and CTPA scan for high probability cases. The Wells’ score and Geneva score are developed to help physicians for early detection and treatment of PE, according to some important key points in patients’ history and physical examination. Many studies have shown that a normal CTPA can safely exclude the diagnosis of PE;8-11 hence, its usage has increased, especially by physicians in the emergency wards. Due to some complications, such as radiation-induced carcinogenesis, anaphylaxis, and contrast-induced nephropathy (CIN), and also high cost of CTPA, minimizing this method is recommended.12 We conducted this study to assess the adherence of our physicians to the algorithmic approach in patients suspicious of PE.
Materials and Methods
From October 2012 to October 2013, we prospectively enrolled all consecutive patients who had been scheduled for CTPA, based on their treating physicians’ decision, in two major referral hospitals (Namazi and Faghihi Hospitals with 750 and 363 active beds, respectively), Shiraz, Iran.13 Patients scheduled for CTPA to find diseases other than PE, e.g., pulmonary artery aneurysm (PAA) or arteriovenous malformation (AVM), were excluded from the study. Then, we recorded all items of Wells’ score in a prepared form, such as demographic criteria, arterial blood gases (ABGs), electrocardiography (ECG), D-dimer, echocardiography, and lower extremities color Doppler sonography (CDS) for each patient. Based on standard algorithmic approach to patients with suspected PE, we calculated Wells’ score for all participants independent from treating physician’s decision who had ordered the CTPA. Patients with low probability who had not checked D-dimer or had low level of D-dimer were considered as none-adherent to the guideline and those with high level of D-dimer or high probability of the Wells’ score were labeled as adherent to PE guideline. The following variables and their assigned scores (in brackets) were used to calculate the Wells’ score: Clinical symptoms of deep vein thrombosis (DVT) (3.0), no alternative diagnosis (3.0), heart rate (HR) > 100 beats per minute (bpm) (1.5), immobilization or surgery in the previous four weeks (1.5), previous DVT/PE (1.5), hemoptysis (1.0), and malignancy (1.0).14 In Wells’ criteria, values greater than 4 are considered as high probable limit of the suspected PE.15 CTPA scans were taken by GE Bright Speed 16-slice computed tomography (CT) scanner machine (Milwaukee, WI, USA) and independently reported by two radiologists; in case of disagreement, a third radiologist’s opinion was considered.
This study was approved by the local Ethics Committee of Shiraz University of Medical Sciences, Shiraz (IR.SUMS.REC.1390.S5893).
The continuous variables with normal distribution were presented as mean and standard deviation (SD) and non-normal variables as median and interquartile range (IQR). The categorical variables were reported as numbers and percentages. The means of two normally-distributed continuous variables were compared by independent samples t-test. Mann-Whitney U test was used to compare means of two groups of variables not normally distributed. The frequencies of categorical variables were compared using chi-square test. The normality of distribution of continuous variables was tested by one-sample Kolmogorov-Smirnov test (K-S test). The data were analyzed by SPSS software (version 11.5, SPSS Inc., Chicago, IL, USA). A P-value of < 0.050 was considered to be statistically significant.
Results
During the study, 364 patients in the age range of 18-100 years were included. The average age of patients with PE was 56.8 ± 22.0 years and that of patients without PE was 55.2 ± 19.0 years (P = 0.565) (Table 1).
Table 1.
Age and gender in patients suspicious to pulmonary embolism (PE)
| PE risk factors | Proven PE (n = 85) | Without PE (n = 279) | P |
|---|---|---|---|
| Age (year) (mean ± SD) | 56.8 ± 22.0 | 55.2 ± 19.0 | 0.565* |
| Gender (female) [n (%)] | 33 (38.8) | 144 (51.6) | 0.047** |
Student’s t-test
Chi-square test
PE: Pulmonary embolism; SD: Standard deviation
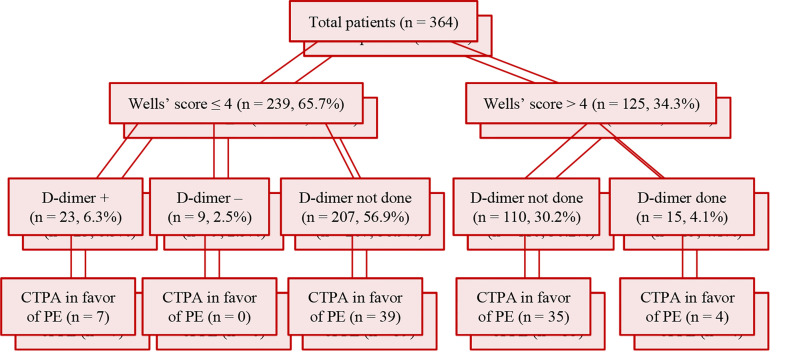
As shown in figure 1, during the study period, 364 patients underwent CTPA for suspected PE, of whom 125 (34.3%) had Wells’ score > 4 (high probable risk). Among low probable risk patients (Wells’ score < 4), only 32 patients had undergone D-dimer test (23 patients had high level of D-dimer). Considering patients who underwent CTPA, the total adherence to algorithmic approach in patients with suspected PE was 148 (125 high-risk patients plus 23 low-risk patients with positive D-dimer) out of 364 (40.6%).
Figure 1.
The adherence to algorithmic approach to pulmonary embolism (PE) in patients who underwent computed tomography pulmonary angiography (CTPA), Shiraz, Iran, 2012-2013
The level of D-dimer was not measured in 207 out of 239 low-risk patients. The serum level of D-dimer was inappropriately checked in 15 out of 125 patients with Wells’ score greater than 4.
Table 2 shows that “clinical DVT”, “hemoptysis” and “no alternative diagnosis” were significantly higher in the PE group (P < 0.050).
Table 2.
The frequency of Wells’ score items and requested para-clinical data in patients suspicious to pulmonary embolism (PE)
| PE risk factors | Proven PE (n = 85) [n (%)] | Without PE (n = 279) [n (%)] | P* |
|---|---|---|---|
| Previous DVT/PE | 10 (11.7) | 31 (11.1) | 0.846 |
| Surgery | 25 (29.4) | 63 (22.6) | 0.197 |
| Immobilization | 24 (28.2) | 59 (21.1) | 0.185 |
| Clinical DVT | 31 (36.5) | 64 (22.9) | 0.016 |
| No alternative diagnosis | 47 (55.3) | 81 (29.1) | 0.001 |
| Hemoptysis | 12 (14.1) | 16 (5.7) | 0.018 |
| Cancer | 15 (17.6) | 60 (21.5) | 0.540 |
| Tachycardia (HR > 100/min) | 32 (37.6) | 98 (35.1) | 0.699 |
| Requested para-clinical | |||
| ABG analysis | 69 (81.2) | 231 (82.8) | 0.746 |
| ECG | 80 (94.1) | 273 (97.8) | 0.138 |
| Echocardiography | 66 (77.6) | 203 (72.7) | 0.400 |
| D-dimer | 11 (12.9) | 36 (12.9) | 0.999 |
| CDS | 47 (55.3) | 104 (37.3) | 0.004 |
Chi-square test
DVT: Deep vein thrombosis; PE: Pulmonary embolism; HR: Heart rate; ABG: Arterial blood gases; ECG: Electrocardiography; CDS: Color Doppler sonography
The final report of the CTPA scan was in favor of PE in 85 patients (23.0%).
The D-dimer tests were requested in only 12.9% (32 low-risk patients and 15 high-risk patients) (Table 2), and it was positive in 100% of patients with PE and 55.0% of patients without PE (Table 3, P = 0.009).
Table 3.
The comparison of the results of D-dimer test, electrocardiography (ECG), echocardiography, and the Doppler ultrasound of lower extremities in patients suspicious to pulmonary embolism (PE)
| Requested tests | Proven PE [n (%)] | Without PE [n (%)] | P* | |
|---|---|---|---|---|
| D-dimer (n = 47) | High level | 11/11 (100) | 20/36 (55.5) | 0.009 |
| Normal | 0 (0) | 16/36 (44.5) | ||
| ECG (n = 353) | In favor of PE | 51/80 (63.7) | 123/273 (45.1) | 0.003 |
| Normal | 29/80 (36.3) | 150/273 (54.9) | ||
| Echocardiography (n = 269) | In favor of PE | 16/66 (24.2) | 23/203 (11.3) | 0.015 |
| Normal | 50/66 (75.8) | 180/203 (88.7) | ||
| CDS (n = 151) | In favor of PE | 25/47 (53.2) | 31/104 (29.8) | 0.010 |
| Normal | 22/47 (46.8) | 73/104 (70.2) |
Chi-square test
PE: Pulmonary embolism; ECG: Electrocardiography; CDS: Color Doppler sonography
The ECGs were requested for 353 patients (Table 2), majority of whom had normal ECG (51.0%). Amongst those who had ECG findings in favor of PE, the most common abnormality was sinus tachycardia.
Table 3 shows the comparison of the results of D-dimer test, ECG, echocardiography, and the Doppler ultrasound of lower extremities in patients suspicious to PE with those without PE in CTPA.
The echocardiography was done for 269 patients. Echocardiographic findings in favor of PE [increased right ventricle (RV) size, decreased RV function, tricuspid regurgitation, McConnell’s sign] were seen in 39 patients (14.5%); 16 out of 66 (24.0%) patients with PE and 23 out of 203 (11.0%) patients without PE had these findings (P = 0.015) (Table 3).
Amongst those who underwent CDS of both lower extremities (151 patients), 56 had DVT; 25 (53.2%) with proven PE in CTPA and 31 (29.8%) patients without PE (P = 0.010) (Table 3).
Discussion
When used appropriately, CTPA has a major role in the diagnostic approach in patients with suspected PE, but the procedure complications such as CIN and radiation-induced carcinogenesis should be considered.
Considering patients who underwent CTPA in our teaching hospitals, the total adherence to algorithmic approach in patients suspicious of PE was 40.6%. In this study, we evaluated the CT scans of 364 patients suspicious of PE, of which only 23.0% were in favor of PE. Based on previous studies, the prevalence of suspicious cases for PE were 2-3 in every 1000 people,16,17 which 33.0% of them had PE, while our study showed that 23.0% had PE; hence, it seems that we are overusing CTPA. Our relatively low adherence to PE approach guideline could be partly explained by economic and audit issues in our centers. Quantitative D-dimer is not available at all time and the audit of CTPA requests is not regularly performed. On the other hand, performing CTPA merely takes few hours and that is why our physicians and residents have tendency to use CTPA as an available and theoretically highly accurate option. In the real life situation, the residents and physicians in emergency wards are under tremendous pressure. We must take into account their high stress and anxiety in missing a case with PE. As an administrative solution, adding the prerequisites of the Wells’ score value and D-dimer results in the CTPA requests can increase the adherence to the guideline.
Wells’ score as a risk stratification questionnaire has an item such as “Is the possibility of other differential diagnoses less than PE?” which is highly dependent on the user experience and clinical judgment; hence, the total score could vary. Nevertheless, if the Wells’ score of ≤ 4 is considered as low probable for PE, 65.0% of our patients should be evaluated based on their D-dimer level rather than undergoing CTPA as the first diagnostic approach. However, we underuse D-dimer test as a first modality of diagnosis in patients with low probable PE and it was requested in only 12.0% of the patients.
The PE positive rate for CTPA in our study (23.0%) was higher than Costantino et al. study (10.0%),18 but less than Perrier et al. study (26.0%).19 After excluding suboptimal or poor technique CTPA, the negative CTPA studies can be explained with an alternative cause for dyspnea, such as pneumonia, pulmonary edema, pleural effusion, or atelectasis.11
The D-dimer assay is very sensitive, but non-specific screening test for venous thromboembolism (VTE) has an important role in diagnostic approach to PE.20 The proper administration of the D-dimer in patients with low to intermediate risk for PE can reduce 26.0% of CTPA.21
Abcarian et al. evaluated the necessity of computed tomography (CT) scan based on quantitative D-dimer results. Out of the 426 patients who had both CT and D-dimer test, 82 patients had less than 0.4 μg/ml of serum D-dimer level and no sign of embolism in their pulmonary CT scan. Thus, it was concluded that if this test was done quantitatively, it would result in high negative predictive value (NPV).22 Unfortunately, in our center, the D-dimer is not done quantitatively; hence, its predictive value is reduced even in the few occasions when it is requested. Nevertheless, in patients who underwent the test, the D-dimer test results were positive in all patients with PE and in 55.0% of those who did not have PE.
According to the previous studies, the ECG changes are useful, but they have limited sensitivity.23 We observed the ECG findings in favor of RV strain only in 49.0% of the patients.
Previous studies showed that 25.0% of patients with PE had the echocardiographic findings supporting the dilated RV, and we also found that 24.0% of patients had findings in favor of PE, which is more than patients without PE (P = 0.015). Considering different studies, the sensitivity of the echocardiographic findings of PE is 60%-70%; consequently, its negative results cannot lead to rejection of PE diagnosis.24 Thus, as a diagnostic modality, echocardiography in patients with suspected PE with stable hemodynamic state is not recommended.24 On the other hand, the absence of echocardiographic signs of RV volume overload in high-risk patients with hypotension could reject the PE as a reason of shock.25 In the present study, the echocardiographic findings of PE were found in 39 out of 269 patients who underwent the procedure; CTPA revealed the evidence of emboli in 16 (24.0%) cases, which can partly be due to lower experience of our cardiology residents as the doer of echocardiography.
As it was shown in the previous studies,19,26 the analysis of lower extremities CDS detected statistically significant higher rate of DVT in patients with PE. In our study, 25 out of 47 (53.0%) patients with PE who underwent CDS had evidence of DVT.
Limitations: Our study was conducted only in two tertiary hospitals. The echocardiography and CDS were done by different doers, and the D-dimer tests were checked qualitatively.
Conclusion
Considering the limitations of the study, we concluded that we underused the Wells’ score and D-dimer and overused CTPA in our approach to patients with suspected PE. We should pay more attention to the algorithmic approach in patients with suspected PE.
Acknowledgments
The authors wish to thank the Deputy Chancellor of Shiraz University of Medical Sciences for financial support. This manuscript is derived from the residency thesis (project number: 90-5893) of Dr. S. Ahsant, in fulfillment of the requirements for the degree of specialty in internal medicine, Shiraz University of Medical Sciences. The authors wish to thank Mr. H. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Stein PD, Kayali F, Olson RE. Estimated case fatality rate of pulmonary embolism, 1979 to 1998. Am J Cardiol. 2004;93(9):1197–9. doi: 10.1016/j.amjcard.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 2.Limbrey R, Howard L. Developments in the management and treatment of pulmonary embolism. Eur Respir Rev. 2015;24(137):484–97. doi: 10.1183/16000617.00006614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069k. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 4.Bauld RA, Patterson C, Naylor J, Rooms M, Bell D. Deep vein thrombosis and pulmonary embolism in the military patient. J R Army Med Corps. 2015;161(3):288–95. doi: 10.1136/jramc-2015-000502. [DOI] [PubMed] [Google Scholar]
- 5.Fedullo PF, Tapson VF. Clinical practice. The evaluation of suspected pulmonary embolism. N Engl J Med. 2003;349(13):1247–56. doi: 10.1056/NEJMcp035442. [DOI] [PubMed] [Google Scholar]
- 6.Kluetz PG, White CS. Acute pulmonary embolism: Imaging in the emergency department. Radiol Clin North Am. 2006;44(2):259–71. doi: 10.1016/j.rcl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Estrada YMR, Oldham SA. CTPA as the gold standard for the diagnosis of pulmonary embolism. Int J Comput Assist Radiol Surg. 2011;6(4):557–63. doi: 10.1007/s11548-010-0526-4. [DOI] [PubMed] [Google Scholar]
- 8.Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: Management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135(2):98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 9.Ghanima W, Almaas V, Aballi S, Dorje C, Nielssen BE, Holmen LO, et al. Management of suspected pulmonary embolism (PE) by D-dimer and multi-slice computed tomography in outpatients: An outcome study. J Thromb Haemost. 2005;3(9):1926–32. doi: 10.1111/j.1538-7836.2005.01544.x. [DOI] [PubMed] [Google Scholar]
- 10.den Exter PL, Klok FA, Huisman MV. Diagnosis of pulmonary embolism: Advances and pitfalls. Best Pract Res Clin Haematol. 2012;25(3):295–302. doi: 10.1016/j.beha.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Green DB, Raptis CA, Alvaro HG, Bhalla S. Negative computed tomography for acute pulmonary embolism: Important differential diagnosis considerations for acute dyspnea. Radiol Clin North Am. 2015;53(4):789–99. doi: 10.1016/j.rcl.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Ward MJ, Sodickson A, Diercks DB, Raja AS. Cost-effectiveness of lower extremity compression ultrasound in emergency department patients with a high risk of hemodynamically stable pulmonary embolism. Acad Emerg Med. 2011;18(1):22–31. doi: 10.1111/j.1553-2712.2010.00957.x. [DOI] [PubMed] [Google Scholar]
- 13.Masoompour SM, Petramfar P, Farhadi P, Mahdaviazad H. Five-Year Trend Analysis of Capacity Utilization Measures in a Teaching Hospital 2008-2012. Shiraz E-Med J. 2015;16(2):e211. [Google Scholar]
- 14.Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: Increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416–20. [PubMed] [Google Scholar]
- 15.McLenachan CJ, Chua O, Chan BSH, Vecellio E, Chiew AL. Comparison of Wells and YEARS clinical decision rules with D-dimer for low-risk pulmonary embolus patients. Intern Med J. 2019;49(6):739–44. doi: 10.1111/imj.14138. [DOI] [PubMed] [Google Scholar]
- 16.Donkers-van Rossum AB. Diagnostic strategies for suspected pulmonary embolism. Eur Respir J. 2001;18(3):589–97. doi: 10.1183/09031936.01.00248601. [DOI] [PubMed] [Google Scholar]
- 17.Gutte H, Mortensen J, Jensen CV, Johnbeck CB, von der Recke P, Petersen CL, et al. Detection of pulmonary embolism with combined ventilation-perfusion SPECT and low-dose CT: Head-to-head comparison with multidetector CT angiography. J Nucl Med. 2009;50(12):1987–92. doi: 10.2967/jnumed.108.061606. [DOI] [PubMed] [Google Scholar]
- 18.Costantino MM, Randall G, Gosselin M, Brandt M, Spinning K, Vegas CD. CT angiography in the evaluation of acute pulmonary embolus. AJR Am J Roentgenol. 2008;191(2):471–4. doi: 10.2214/AJR.07.2552. [DOI] [PubMed] [Google Scholar]
- 19.Perrier A, Roy PM, Sanchez O, Le Gal G, Meyer G, Gourdier AL, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352(17):1760–8. doi: 10.1056/NEJMoa042905. [DOI] [PubMed] [Google Scholar]
- 20.Kelly J, Rudd A, Lewis RR, Hunt BJ. Plasma D-dimers in the diagnosis of venous thromboembolism. Arch Intern Med. 2002;162(7):747–56. doi: 10.1001/archinte.162.7.747. [DOI] [PubMed] [Google Scholar]
- 21.Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: Sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425–30. doi: 10.2214/AJR.08.2186. [DOI] [PubMed] [Google Scholar]
- 22.Abcarian PW, Sweet JD, Watabe JT, Yoon HC. Role of a quantitative D-dimer assay in determining the need for CT angiography of acute pulmonary embolism. AJR Am J Roentgenol. 2004;182(6):1377–81. doi: 10.2214/ajr.182.6.1821377. [DOI] [PubMed] [Google Scholar]
- 23.Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J. 2005;25(5):843–8. doi: 10.1183/09031936.05.00119704. [DOI] [PubMed] [Google Scholar]
- 24.Hull RD, Hirsh J, Carter CJ, Raskob GE, Gill GJ, Jay RM, et al. Diagnostic value of ventilation-perfusion lung scanning in patients with suspected pulmonary embolism. Chest. 1985;88(6):819–28. doi: 10.1378/chest.88.6.819. [DOI] [PubMed] [Google Scholar]
- 25.Kucher N, Luder CM, Dornhofer T, Windecker S, Meier B, Hess OM. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J. 2003;24(4):366–76. doi: 10.1016/s0195-668x(02)00476-1. [DOI] [PubMed] [Google Scholar]
- 26.Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: The task force for the diagnosis and management of acute pulmonary embolism of the European society of cardiology (ESC). Eur Heart J. 2008;29(18):2276–315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]