Abstract
Retinal congenital malformations known as microphthalmia, anophthalmia and coloboma (MAC) are associated with alterations in genes that encode epigenetic proteins that modify chromatin. Here, we review newly discovered functions of such chromatin modifiers in retinal development and discuss the role of epigenetics in MAC in humans and animal models. Further, we highlight how advances in epigenomic technologies provide foundational and regenerative medicine-related insights into blinding disorders. Notably, combining knowledge of epigenetics and pluripotent stem cells (PSCs) is a promising avenue since epigenetic factors cooperate with eye field transcription factors to direct PSC fate -a foundation for congenital retinal disease modeling and cell therapy.
Keywords: stem cells, epigenetics, retina, development, blindness
Epigenetics in Eye Diseases
Epigenetics is a set of reversible, inheritable mechanisms that modulate gene expression programs, but which do not depend on nucleotide sequence. Over time, the field has focused on chemical modifications of chromatin, the regulatory or structure proteins associated with chromatin, and larger-scale dynamics at the level of 3D chromatin structure [1]. Such mechanisms include posttranslational modifications (PTMs) of DNA and histones, and chromatin remodeling in time and space, which lead to altered local accessibility of source genetic material. Epigenetic modifications enable precise tuning of gene expression. Stem cells within an organism harbor identical DNA, thus epigenetic mechanisms underlie acquisition of cell-type specific differentiation during development. For example, DNA methylation plays an important role in repressing transcription and X-chromosome inactivation [2]. Histone PTMs include modifications such as lysine methylation, acetylation, and histone sequence variants [3–5]. Histone PTMs can occur at promoter and enhancer regions of genes, altering accessibility for binding of transcription factor proteins, which regulate gene expression [6]. Histone PTMs are catalyzed by various writer and reader proteins, such as lysine methyltransferases (KMTs) and demethylases (KDM) respectively, and are present in multi-protein complexes which contain non-enzymatic co-factors that, for instance, spatially enable KMT-chromatin interactions [6].
Alterations in genes encoding ubiquitous KMTs, KDMs, and various chromatin-associated or remodeling proteins underlie a variety of congenital eye defects known as MAC: microphthalmia (small eye with disorganized retina), anophthalmia (lack of eye formation), and coloboma (missing tissue component of eye) (Table 1; see Glossary). While MAC is classically linked to mutations in eye field transcription factors (EFTFs) [7], epigenetic regulators that regulate EFTF expression can also lead to these congenital ocular defects (Figure 1 and Table 1).
Table 1.
Genetic Alterations in Epigenetic Genes Associated with MAC
| Gene | Disease | Phenotype | Age-Onset | Prevalence | Underlying Genetics | References |
|---|---|---|---|---|---|---|
| BCOR | Lenz Microphthalmia (Male) Oculofacialcardiodental (OFCD) syndrome (Female) Retinoblastoma (RB) |
Lenz syndrome presents with anophthalmia and microphthalmia, mental retardation and other developmental delays. OFCD presents ocular (congenital cataracts), facial, cardiac and dental defects. Mutations are associated with intraocular tumors. |
Congenital Congenital Congenital to 5 years of age |
Lenz: very rare and identified in only a few families; OFCD: < 1 in 1 million RB: 1 in 16,000 Alterations present in up to 14% of RB1 mutant RBs. |
Lenz: X-linked recessive; OFCD: X-linked dominant; Missense, frameshift, nonsense, and deletion in both syndromes Somatic nonsense mutations in RB |
[33–35] |
| CHD7 | CHARGE syndrome | Microphthalmia and CHARGE syndrome (Coloboma of the eye, Heart defects, Atresia of the choanae, severe Retardation of growth and development, Genetial abnormalities, and Ear abnormalities | Congenital | 1 in 10,000 ~80% of CHARGE diagnosis is associated with CHD7 mutation | Autosomal dominant; Missense, frameshift, nonsense and deletion | [8] |
| KDM6A (UTX) | Kabuki syndrome | Same as KMT2D Kabuki syndrome phenotype | Congenital | 1 in 32,000 2–6% of Kabuki syndrome cases linked to mutations in KDM6A. | X-linked dominant; Frameshift mutation and deletion | [17, 23] |
| KMT2A (MLL1) | Weidemann-Steiner syndrome | Anophthalmia, microphthalmia, short stature, and intellectual disability | Congenital | < 1 in 1 million | Autosomal dominant; Missense, frameshift, nonsense, and deletion | [11] |
| KMT2D (MLL2) | Kabuki syndrome | Abnormal facial features, intellectual disability, postnatal growth deficiency, cardiac and urogenital malformations, cleft palate, and hearing loss | Congenital | 1 in 32,000 55–80% of Kabuki syndrome cases are associated with mutations in KMT2D. | Autosomal dominant; Missense, nonsense, and deletion | [16–22] |
| PRDM13 | North Carolina Macular Dystrophy | Coloboma-like chorioretinal defects in the macula, causing vision loss. | Congenital | < 1 in 1 million | Autosomal dominant; Missense in DNase hypersensitivity site upstream of PRDM13, Duplication | [41,42] |
| SMCHD1 | Bosma arhinia microphthalmia syndroma | Anophthalmia, microphthalmia, craniofacial defects | Congenital | Very rare, fewer than 100 cases described in literature | Autosomal dominant; Missense, nonsense, and deletion | [25–27] |
Figure 1. Chromatin modifying and associated proteins in retinal development and disease.
Broadly expressed epigenetic regulators have been implicated in microphthalmia, anophthalmia, and coloboma. They act as higher order regulators (blue box), together with eye field transcription factors (EFTFs, green box), to govern the proper development of the eye. In one recently reported example in which chromatin associated proteins directly regulate an EFTF gene, WDR5 and p53 co-target the Sox2 on chromatin during retinal organoid differentiation from pluripotent stem cells, a model of retinal development [51]. Indeed, transient Wdr5 inactivation completely inhibited retinal organoid formation from pluripotent stem cells [51].
In this review, we first focus on the role of epigenetic regulators that control early retinogenesis and which are related to MAC in humans and in animal models. Second, we highlight how recent advances in pluripotent stem cell (PSC) derived retinal organoids, epigenomic technologies, and gene-editing enable interrogation of mechanisms that underlie how broadly expressed epigenetic proteins control retinal development and contribute to MAC. Finally, we integrate recent developments in the understanding of epigenetic memory and transcription factor mutations (i.e. TP53) with in PSCs. We discuss the implications of these advances vis-à-vis the safety, speed and efficacy of PSC-based regenerative cell therapies for retinal disease.
Chromatin modifying genes in microphthalmia, anophthalmia, and coloboma
Chromodomain Helicase DNA-binding Protein 7 (CHD7)
Heterozygous mutations in CHD7 lead to CHARGE syndrome (Coloboma of the iris, retina or optic disc, Heart defects, Atresia of the choanae, severe Retardation of growth and development, Genital abnormalities, and Ear abnormalities) [8] (Table 1). However, the epigenetic mechanism whereby haploinsufficiency of CHD7, a nucleosome remodeler, contributes to MAC remains poorly understood. CHD7 contains two chromodomains that facilitate binding to methylated histones, and two helicase domains, a SANT (Swi3, Ada2, N-Cor, and TFIIIB) domain, and two BRK (Brahma and Kismet) domains [9]. CHD7 is believed to modulate transcription through ATP-dependent chromatin remodeling and its genomic distribution parallels that of methylated lysine on histone H3 (H3K4me). CHD7 maps distal to transcription start sites, often within DNase hypersensitive sites, and is located near genes expressed at relatively high levels; CHD7 co-localizes with P300, an enhancer-binding protein, which suggests CHD7 functions in enhancer-mediated transcription [9]. In the embryonic mouse, CHD7 is expressed in the surface ectoderm and the neuroectoderm. Chd7 deletion in the murine embryonic neuroectoderm leads to MAC with reduced lens size and highly dysmorphic, or absent, optic cups, due to improper closure of the optic fissure [10]. In mouse models, it has been found that CHD7 binds and negatively regulates the p53 promoter. Hyperactivation of p53 inappropriately induces target genes and triggers cell-cycle arrest or apoptosis during retinal development, and causes other defects reminiscent of CHARGE syndrome. Strikingly, p53-heterozygosity partially rescued CHARGE-like phenotypes in Chd7-null mice. To enable a better understanding of CHD7 in MAC, as well as its relationship to p53, future studies that analyze CHD7 chromatin targets, its effect on chromatin accessibility, and the role of its ATP-binding motif, SANT and BRK domains (through mutational analysis) on nucleosome remodeling, within developing retinal precursors, from wild-type (WT) and CHD7-mutant primary tissue or PSCs, in TP53 WT and null backgrounds will be needed.
Lysine Methyltransferase 2A (KMT2A), Lysine Methyltransferase 2D (KMT2D), Lysine Demethylase (KDM6A)
Heterozygous KMT2A mutations lead to Wiedemann-Steiner syndrome, a disorder that is linked to short stature, intellectual disability, MAC and other defects of the eye [11] (Table 1). Members of the KMT2/MLL (KMT2: histone-lysine N methyltransferase; MLL: mixed lineage leukemia) family function share the catalytic SET (Su(var)3–9, Enhancer-of-zeste and Trithorax) domain, which “writes” methylation at lysine 4 on histone H3 (H3K4me). This histone modification is associated with transcription [6]. In murine retina, KMT2A is expressed in all mid-gestational neuroblastic cells and persists throughout all layers in adulthood, though it is enriched in the inner retina. Kmt2a inactivation in murine retinal precursors results in decreased proliferation of precursors, altered neuron-to-glia ratio, reduction in horizontal cells, and failure to properly form functional presynaptic terminals in photoreceptors. These alterations contribute to vision loss, as measured by electroretinography (ERG) [12]. Interestingly, there were no differences in retinal H3K4me3 by qualitative immunohistochemistry and semi-quantitative western blotting. This suggests that redundant histone methylation function by other KMT2 family members may compensate for loss of KMT2A; however, loci-specific distribution of KMT2A was not measured, nor was expression of other KMT2 members assessed [12]. Together, these data indicate that KMT2A function in humans and mice is essential for proper retinogenesis. However, heterozygous Kmt2a mutations did not lead to MAC phenotype in mice. The MAC-linked KMT2A heterozygous mutation in Wiedemann-Steiner syndrome is related to a missense variant in an intronic region (intron 8) that is thought to abrogate a canonical splice donor site, and which is predicted to be to a loss-of-function allele. Comparisons that employ induced pluripotent stem cells (iPSC) from KMT2A-mutant patients or base-edited PSCs versus gene-corrected iPSCs, respectively, to generate retinal organoids will be important in assessing the mechanism by which heterozygous KMT2A mutations contribute to MAC in humans.
Mutations in KMT2D contribute to 60–90% of individuals with Kabuki syndrome (KS), which features abnormal facial features, intellectual disability, postnatal growth deficiency, cardiac and urogenital malformations, cleft palate, and hearing loss [13, 14] (Table 1). Further, 38–61% of patients with KS have ocular defects, including corneal staphyloma and retinal coloboma [15]. Thus, the phenotype of KS has been expanded to include MAC [16–18]. More than 150 distinct KMT2D mutations have been described in KS patients [19].KMT2D (a.k.a. MLL2 in humans and MLL4 in mice) monomethylates H3K4 via its catalytic SET domain [20]. In Drosophila, mouse embryonic fibroblasts (MEFs), embryonic stem cells (ESCs), and human cells, KMT2D activates enhancer regions through H3K4me1 to promote embryonic development and differentiation [20]. KMT2D is associated with transcription of target genes by increasing H3K27 acetylation activity of p300 and Pol II binding at enhancers [20, 21]. KMT2D regulates gene expression programs required for heart development via its KMT activity [22]. Mechanistic insights into the relationship between MAC and dysregulated epigenetic function of KMT2D remain unknown; notably, while there are mouse models for Kmt2d loss, there are no reported consequences for the retina.
6–14% of patients with KS carry KDM6A mutations [14].KDM6A (lysine-specific demethylase 6A, a.k.a. UTX) demethylates H3K27me through its JmjC catalytic domain, A megabase-scale deletion encompassing one allele of the X-linked KDM6A gene has been associated with MAC in KS [23] (Table 1). Conditional Kdm6a deletion in murine retina leads to reduced protein kinase C alpha-positive bipolar cells but not MAC [24]. In KS, it will be important to model pathogenic variants in KMT2D and KDM6A linked to MAC in human PSC-derived retinal organoids, since mouse models did not capture the phenotype that is seen in human patients.
Structural Maintenance of Chromosomes Flexible Hinge Domain Containing 1 (SMCHD1)
SMCHD1 is implicated in two distinct disorders: facioscapulohumoral muscular dystrophy (FSHD), which features facial and upper-extremity muscle weakness, and Bosma arrhinia and microphthalmia (BAMS), which is characterized by severe malformations of the human nose, olfactory tract, and eyes [25, 26] (Table 1). SMCHD1 contains two major domains: the N-terminal GHKL (gyrase, Hsp90, histidine kinase, MutL) ATPase motif, and the C-terminus SMC (structural maintenance of chromosomes) domain that has DNA-binding activity [27]. SMCHD1 mediates chromosome compaction of the inactive X chromosome by linking H3K9me3- and H3K27me3-marked domains with X-inactive specific transcript-H3K27me3 [28]. Smchd1 deletion in vivo leads to random X chromosome inactivation (XCI) in the embryo and imprinted XCI in the placenta fail, causing embryonic lethality in female mice in early-mid gestation [29]. Smchd1 deletion also causes disruption of long-range interactions among autosomal HoxB clusters, and other genes in murine neural stem cells [30]. De novo heterozygous SMCHD1 missense mutations, mostly located in coding region of the ATPase domain, are present in individuals with BAMS [25, 26]. One group reported deletion of smchd1 in zebrafish embryos led to MAC but Smchd1 nonsense mutations did not recapitulate this phenotype in mice nor did overexpression of wild-type (WT) and human SMCHD1 missense mutations [26, 29]. However, another team found that overexpression of WT and human SMCHD1 missense mutations, which are associated with increased gain-of-function (GOF) ATPase activity, led to MAC in Xenopus embryos [25]. Future studies that express BAMS-specific mutants in human PSCs during retinal organoid differentiation will be important to better understand how human-specific SMCHD1 alterations lead to BAMS.
BCL-6 co-repressor (BCOR)
BCOR alterations are linked to X-linked dominant oculofaciocardiodental syndrome (OFCD) in females, and X-linked recessive microphthalmia syndrome (Lenz) in males [31]. OFCD comprises of ocular, facial, cardiac and dental defects [31] (Table 1). Lenz syndrome is composed of MAC, mental retardation, and other developmental delays [32]. Nearly 50 distinct BCOR alterations have been identified in OFCD and Lenz syndromes [31]. BCOR alterations are also found in human retinoblastoma, the most common pediatric intraocular cancer [33]. Interestingly, Bcor knockout mice exhibit microphthalmia but not retinoblastoma [34]. BCOR is a ubiquitously expressed transcriptional repressor when associated with BCL-6 [35]. BCOR is a component of the non-canonical polycomb repressive complex 1 (PRC1.1), which comprises KDM2B (H3K36 demethylase), PCGF1, RING1/RNF2 (histone H2A E3 ubiquitin ligase), an SCF ubiquitin E3 ligase and other proteins (but not BCL-6). This BCOR-containing PRC1.1 complex ubiquitinates histone H2A at Lys119, which represses gene expression. BCOR, via PRC1.1, maintains primed pluripotency by repressing mesoderm and endoderm lineage specifying genes in hESCs [36]. Separately BCOR interacts several class I and class II histone deacetylases (HDACs), which also repress transcription at specific loci [35]. To date, BCOR deletion has not been examined in PSC differentiation to retinal organoids, and thus its effects in PSC models of MAC or retinoblastoma remain unknown [37, 38]. This is especially important in retinoblastoma, as mice poorly model human retinoblastoma. For instance RB1 loss is sufficient to generate tumors in humans, but is insufficient for retinoblastoma formation in mice; and the cell-of-origin in mouse and human retinoblastoma are distinct [38, 39]. In the future, it will be important to distinguish whether dysfunction of PRC1.1., HDAC, and/or BCL-6-dependent activity contributes to MAC.
PR-Domain-Containing 13 (PRDM13)
In North Carolina Macular Dystrophy (NCMD), coloboma-like macular chorioretinal defects form [40] (Table 1). NCMD is linked to duplication of PRDM13 as well as multiple variants including alterations in DNase hypersensitivity sites upstream of PRDM13 [41]. PRDM13 is a member of the PRDM family, which harbor an N-terminal PR domain and C-terminal zinc-finger domains [42]. PRDM proteins promote cell proliferation and lineage specification in various cell types [43]. PRDM13 has been reported to harbor histone methyltransferase activity, though its substrate target remains elusive [44]. PRDM13 represses differentiation of excitatory neuron lineages in the dorsal neural tube of the embryonic murine spinal cord [45]. Thus, while the epigenetic regulatory mechanism of how PRDM13 dysfunction triggers NCMD remains unknown, it is possible that PRDM13 overexpression interferes with retinal development through repressive functions reminiscent of its activity in the developing spinal cord. Indeed, overexpression of PRDM13 orthologue in Drosophila causes MAC-like features, but its deletion does not. Prdm13-knockout mice lack Ebf3+ amacrine cells, but retinal development is otherwise normal [46]. Assessing the consequences PRDM13 overexpression (e.g. via PRDM13 duplication) on retinal differentiation from hPSCs or in the retinae of transgenic animal models will be important for future studies.
Additional ubiquitous chromatin regulators are reported to play key roles in early retinal development (BOX1), but alterations in the corresponding genes have not been linked to human MAC.
Box 1. Essential Roles for Ubiquitous Chromatin Regulators in Retinogenesis.
Lysine demethylase 5B (a.k.a. KDM5B, PLU-1, JARID1B) demethylates H3K4me at several loci, repressing these genes [106]. Murine Kdm5b deletion results in elevated H3K4me3 levels and MAC; conditional retinal inactivation phenocopies loss of NRL, a transcription factor that regulates photoreceptor cell fate and is downstream of KDM5B, which leads to disrupted morphology in photoreceptors [107, 108]. Lysine demethylase 5C (a.k.a. KDM5C, JARID1C) demethylates H3K4me, and kdm5c inhibition in Xenopus laevis led to MAC. kdm5c inhibition led to reduced EFTF mRNAs, such as pax6 and rax [109]. De novo human KDM5B mutations are associated with intellectual disability and congenital heart disease [110], and KDM5C alterations lead to microcephaly and X-linked mental retardation [109], yet such alterations but are not linked to MAC.
Transcription activator Brahma-Related Gene-1 (BRG1), encoded by the SMARCA4 gene, is an ATPase subunit of the SWI/SNF complex involved in chromatin remodeling. Brg1 loss in murine retinae causes microphthalmia due to excessive cell death and prolongation of the cell cycle, and disruption of nucleosome positioning in downregulated genes occurs. Germline SMARCA4 alterations occur in a subset of individuals with Coffin-Siris (which features microcephaly, among other findings) and familial rhabdoid tumor predisposition syndromes, but affected individuals do not exhibit MAC [111, 112].
Chromatin-bound CCCTC-binding factor (CTCF) loops DNA to distal sites bringing widely separated functional elements into close spatial proximity via a cohesion-dependent loop maintenance complex [113], thereby establishing topologically associated domains (TADs) [114]. MAC in animal models can be induced by Ctcf alterations, including Ctcf overexpression and its deletion in embryonic neuroepithelium, due to disruption of CTCF-mediated direct regulation of the EFTF gene Pax6 [115, 116]. CTCF mutations in humanscause of intellectual disability and microcephaly but not MAC [117].
WD Repeat Domain 5 (WDR5), a core subunit of KMT2/MLL complexes [6, 118], interacts with CHD7, CTCF, MYC, OCT4, and facilitates lncRNA binding to chromatin, gene activation, H3K4 methylation, mouse ESC pluripotency, and iPSC reprogramming [51, 119–122]. Wdr5 inactivation inhibits retinal organoid formation from mESCs, and WDR5 interacts with p53; both targeted EFTF gene, Sox2 (Figure 1) [51]. WDR5 point mutations are linked to human congenital heart defects and disrupted speech development, but not MAC [123, 124].
Taken together, it is possible that MAC is not observed in these rare human syndromes due to embryonic lethality. Since the downstream targets of these ubiquitous chromatin regulators converge at EFTFs, future study of these epigenetic proteins will illuminate the poorly understood, earliest layers of molecular control of retinogenesis.
Insights into retinal development and disease through stem cells and epigenomic technologies
Current notions of retinogenesis are based on coordinate expression of a sparse group of EFTFs within the ventral forebrain [47]. A large amount of research has focused on characterizing the precise expression and downstream targets of EFTFs. Yet little is known about epigenetic mechanisms that operate in concert with, or independent of, EFTFs to control retinogenesis (Figure 1). The resulting knowledge gap (1) constrains our understanding of MAC, (2) restrains our appreciation of the functions of the non-coding genome in retinal development and disease, and (3) limits efficiency of retinal differentiation from PSCs for regenerative medicine and disease modeling.
Epigenetic Control of Eye Field Transcription Factors
EFTFs such as SOX2, OTX2, LHX2, RAX/RX, and PAX6, among others, coordinate cell fate and developmental programs in a spatiotemporal-manner during retinogenesis. SOX2 is expressed in neural stem cells and loss of function mutations result in MAC [48]. OTX2 is essential for formation of the rostral head and is expressed in the eye during embryogenesis, and mutations result in otocephaly, a craniofacial malformation that affects the eye [49]. LHX2, RAX and VSX2 are other EFTFs linked to MAC [7]. PAX6, expressed in the inner retina throughout development in mice and humans, is critical for multipotency, proliferation, migration and differentiation of neuroprogenitor cells. As such, mutations in PAX6 lead to craniofacial abnormalities and MAC [50]. How do epigenetic factors control EFTF genes? Our poor understanding in this context is due, in part, to the scarcity of primary eye tissues during the earliest events of retinal development available for study. The advent of methods to produce stem cell-derived retinal organoids has now allowed interrogation of these early events using state-of-the-art epigenomic approaches [51].
Advances in Epigenomic Technologies and their Applications to the Field of Retinal Development
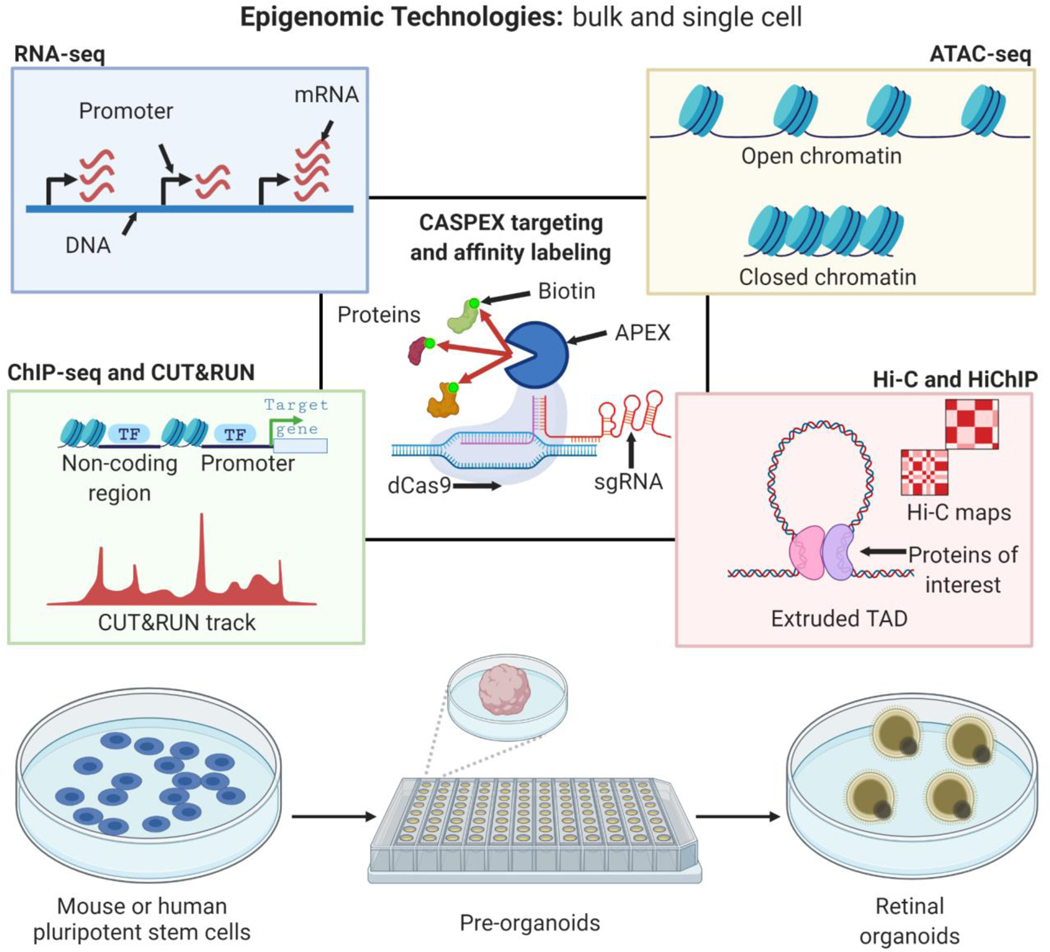
Advances in gene-editing and high-throughput next generation sequencing (NGS) techniques allow spatiotemporal mapping of epigenomic changes at high resolution. These innovations allow interrogation of poorly understood functions of non-coding regions in development and disease [52]. For instance, CRISPR/Cas9-based strategies integrated with epigenomic technologies, using single-cell and bulk approaches, allow a deeper understanding of how alterations in broadly expressed epigenetic regulators govern cell fate specification and disease pathogenesis. These technologies include ATAC-seq (chromatin accessibility), ChIP-seq and CUT&RUN (protein-DNA interactions), Hi-C and Hi-ChIP (protein-directed, chromosome conformation capture of chromatin-chromatin interactions in 3D space), protein-directed proximity labelling identify previously unidentified interactions among epigenetic regulators, EFTFs, and non-coding regions [51, 53–55] (Figure 2).
Figure 2. Advances in next generation sequencing technologies enable epigenomic interrogation of retinal development.
Dissection of function of chromatin-related proteins (Figure 1) during retinal development is facilitated by bulk and single cell state-of-the-art technologies such as ATAC-seq, RNA-seq, Hi-C, Hi-ChIP, ChIP-seq, CUT&RUN, and CASPEX. Many of these tools allow for low cell inputs, which make them amenable to better understanding the transition of functional role of these proteins during pluripotent stem cells to pre-organoids, and pre-organoids to retinal organoids, in which many intermediate cell types arise transiently and at low cell numbers.
Recent examples of this integrative approach are instructive. Various teams of investigators have profiled and compared human fetal retina (non-diseased) with human PSC-derived retinal organoids. Such studies have revealed significant transcriptomic similarities and cell-type compositions across primary murine (fetal, adult) and human fetal retina and PSC-derived organoids at various stages of maturation [53, 56–58]. The integration of ChIP-seq, RNA-seq, and ATAC-seq in mouse photoreceptors and bipolar cells have revealed cis-regulatory architecture that govern enhancer function and reveal function of other non-coding regions that are implicated in hereditary retinal diseases [59–61]. A similar study integrated bulk ATAC-seq and RNA-seq to track changes in chromatin accessibility and transcriptome landscapes. footprinting analysis of accessible DNA sequences, identified DNA motifs associated with key transcription factors and constructed transcription regulatory networks that were found to be similar between primary human fetal and stem cell-derived retinal organoids [62]. ChIP-seq analyses of developing human and mouse retinaidentified distinct, species-specific bivalent chromatin modifications (H3K4me3 and H3K27me3), indicating cross-species differences in epigenetic control of retinogenesis [62]. ATAC-seq analysis of retinal organoids derived from iPSCs from NRL-mutant patients with enhanced S-cone syndrome (a congenital form of blindness due to cell fate disruption of rod versus cone photoreceptors) revealed a putative novel regulator of human cone photoreceptor development, MEF2C [63]. Further, integrated ATAC-seq, RNA-seq, and Hi-C identifed bipolar neuron-specific super-enhancer of the EFTF gene VSX2, whose deletion in mice led to absence of bipolar neurons [64]. Together, the integration of epigenomic techniques with PSC-biology revealed mechanistic insights into how developmental alterations in chromatin accessibility, histone modifications, and non-coding regions of the genome (e.g. enhancers) contribute to MAC.
Implications for Regenerative Medicine
The ability to generate all somatic cell lineages is a defining property of PSCs. Because humans cannot regenerate retinal cells, PSC-derived retinal cells have emerged as the basis for cell-therapy strategies. PSC-based therapies have been deployed for degenerative diseases that affect various organs, including the retina and the cornea [65–72] (Figure 3). The potential for PSC-based strategies to replace diseased or dead cells for retinal disorders is vast: age-related macular degeneration (AMD), diabetic retinopathy, glaucoma, optic neuropathies and hereditary retinopathies are degenerative diseases responsible for virtually all causes of irreversible blindness, affecting ~315M patients worldwide [73–76].
Figure 3: Sources of pluripotent stem cells, and their differentiation to clinically important retinal cells in regenerative medicine for vision disorders.
Pluripotent stem cells can be derived from blastocysts (embryonic stem cells), somatic fetal and postnatal tissues (induced pluripotent stem cells). There is evidence that induced pluripotent stem cells carry epigenetic memory from the epigenome of the original somatic cell type, which biases the pluripotent stem cell to preferentially toward the lineage of the parental somatic cell type. Some pluripotent stem cell lines acquire alterations in the TP53 gene, and gain-of-function TP53 is known in cancer cells to bind distinct chromatin targets relative to wild-type TP53. Retinal organoids formed from pluripotent stem cells contained the various types of retinal cells, including clinically relevant cells like retinal ganglion cells (which die in glaucoma) and photoreceptors and retinal pigment epithelium (which die in age-related macular degeneration). In pluripotent stem cell-based cell therapy trials, clinically relevant retinal cells types are transplanted into the eye.
TP53 mutations and Epigenetic Memory in Pluripotent Stem Cells Used in Translational Research and Clinical Trials
Still, the mechanisms by which broadly expressed epigenetic factors cooperate with ubiquitous embryonic transcription factors to direct PSC fate remain largely unexplored. This knowledge gap has clinical implications (see Clinician’s Corner). Increased activity of the ubiquitous embryonic transcription factor p53 (encoded by TP53 in humans) contributes to MAC in mice and humans in disorders such as Fanconi anemia and CHARGE syndrome [77–79]. In the reverse case, ~5% of ESCs carry inactivating p53 mutations [80–82]. TP53 inactivation and gain-of-function (GOF) mutations occur during culture of hPSCs [80]. These mutations have occurred in H1 and H9 hESC lines, commonly used in laboratories, and in some retinal and spinal cord cell therapy trials [67, 71, 80, 83–87]. In mESCs, p53 directly interacts with WDR5 and that they both target EFTF gene Sox2 during retinogenesis (Figure 1). Transient WDR5 inhibition triggers p53-dependent mESC misspecification towards the mesoderm lineage under conditions that normally induce retinal fate [51]. In cancer cell lines, p53 GOF mutants bind to and upregulate chromatin regulatory genes that are distinct from those targeted by WT p53 [88]. GOF p53 mutants contribute to genome-wide increases in histone methylation and acetylation, which alters chromatin structure [89, 90]. Yet there is little known how TP53 mutations re-wire the 3D genome and derange retinal differentiation. These data highlight the importance of assessing the genomic integrity of PSCs at a higher resolution than the current standard of karyotyping.
Clinician’s Corner.
Alterations in chromatin regulatory genes are linked to congenital diseases that also include microphthalmia, anophthalmia and coloboma. Yet pathologic mechanisms are not yet clear, and genetically engineered animal models do not recapitulate many ocular features found in corresponding human diseases.
PSC-based therapies have been deployed for degenerative diseases that affect the retina, spinal cord, brain, and heart [65–71]. The potential for PSC-based strategies to replace diseased or dead cells for degenerative retinal disorders is vast: AMD, diabetic retinopathy, glaucoma, optic neuropathies and hereditary retinopathies.
Directed differentiation protocols generate human RPE, photoreceptors, and other retinal cell types from hPSCs. Once generated in vitro under good manufacturing practices, PSC-derived retinal cells can be cultured in a monolayer (RPE) or multilayer (organoid tissue containing photoreceptors) fragments, in suspension or as sheets over biosynthetic scaffolds. A retinal surgeon will perform vitrectomy using three microports to enter the eye. Following removal of vitreous, a small-diameter cannula injects cells subretinally in individuals with AMD or particular hereditary retinal dystrophies. Follow-up examinations are required to assess safety and function using a wide variety of functional (e.g. visual acuity testing, microperimetry) and structural imaging technologies (e.g. optical coherence tomography).
The use of PSC-derived retinal organoids for disease modeling, drug screening and cell therapies remains limited for three major reasons: (i) In disease modeling, somatic cells from diseased individuals are reprogrammed to a pluripotent state. Reprograming, and prolonged PSC culture, can select for mutations in cancer-associated genes, like TP53. Growth advantage of mutant TP53 PSCs may dominate wild-type PSCs prior to downstream applications. (ii) Further, various PSC lines, even if derived from the same person, generate retinal tissues at variable efficiency. One contributing reason, and which should be kept in mind when selecting PSC lines for therapy, for variable efficiency is due to epigenetic memory, a phenomenon in which a PSC tends to differentiate to somatic cell types from which PSC were originally derived through reprogramming. (iii) Moreover, even in PSCs that form retinal organoids with relatively high efficiency, it takes many months to generate mature, disease-relevant retinal cells like photoreceptors, and they form at low efficiencies, with limited maturation. The elucidation of epigenetic mechanisms that control expression of retinogenic genes and consequences of TP53 mutations on 3D chromosome architecture in retinal organoids could enable therapeutic use of small molecule drugs that alter epigenetic pathways that may lead to safer, faster and more cost-effective strategies in regenerative medicine.
The first transplantation of iPSC-derived tissue into the human body was for a patient with AMD1. This phase I-like trial, conducted in Japan, was a non-randomized and non-blinded trial and tested safety of autologous iPSC-derived RPE subretinal transplantation. In the first patient, the intervention appeared safe at 1 year but the study was not powered for efficacy [91]. Moreover, the second patient did not receive an autologous iPSC-RPE graft due to the presence of DNA copy number deletions that arose during the process of reprogramming the patient’s fibroblasts to iPSCs [91]. However, it is difficult to predict the efficiency of an iPSC line toward retinal differentiation. Recent reports have suggested that the epigenome of the original cell type from which the iPSC line was derived plays a role in efficiency of PSC differentiation to particular cell types, a phenomenon known as epigenetic memory. For instance, iPSCs reprogrammed from murine rod photoreceptors and human retinal pigment epithelial cells (RPE) differentiate to retinal organoids and RPE, respectively, more readily than do those derived from fibroblasts [92, 93]. Enriched CTCF binding at photoreceptor genes and the H3K27me3 levels on the EFTF gene Meis1 correlated to higher efficiency of iPSCs toward retinal differentiation [92, 94]. Yet mechanisms underlying PSC epigenetic memory and retinogenesis remains incompletely understood. Indeed, the production of hPSC-derived retinal organoids remains inefficient, expensive, and requires several months of culture [95]. Thus, a deeper knowledge of epigenetic memory may impact the use of PSC-derived retinal organoids for therapy, drug screening, and disease modeling.
Concluding Remarks
Recent advances in MAC disease-related gene discoveries, epigenomics, PSC-derived retinal organoids, and regenerative medicine have begun to scratch the surface of the crucial and wide-ranging roles of epigenetic proteins in retinal development, disease, and therapeutics. Specifically, we put a subset of MAC diseases in context with genetic alterations that encode proteins with chromatin-associated functions. Further, we highlighted examples that integrate progress in PSC and retinal organoid fields with that of gene-editing, NGS and epigenomic technologies. Synthesizing these efforts revealed how newly discovered functions of these proteins during the earliest stages of retinogenesis can be used with PSC-derived retinal organoids, to amplify embryonic tissues that are traditionally difficult to secure, and impact our understanding, and potential treatment of, blinding disorders in the young and old.
Still, questions remain. A major knowledge gap limits our understanding of the epigenetic mechanisms that contributes to congenital blinding retinal diseases (see Outstanding Questions). PSC-derived retinal organoids allow isolation of enough primary retinal tissues needed for large-scale epigenomic analyses that reveal how alterations in genes that code for chromatin modifying proteins causally affect function of EFTFs, the classic mediators of retinal development and whose dysfunction is implicated in MAC. Recent advances in epigenomic technologies, along with CRISPR/Cas9-based strategies, allow identification of novel protein-protein, protein-chromatin and chromatin-chromatin interactions within 3D genomic architecture, and single-cell resolution of the global transcriptome and chromatin accessibility landscape. Future studies that integrate these technologies with PSC-derived retinal cells will provide foundational insights into how ubiquitously expressed epigenetic regulators control cell fate determination mechanisms and interface with non-coding genomic regions associated with congenital blinding diseases. Emerging areas of epigenetics research such as the modulation of RNA transcript stability by chemical modifications like methylation, a.k.a. epitranscriptomics, and its relationship to retinal cell lineage determination, will further inform this important translational field of research [96]. In addition, small molecule drugs that target epigenetic pathways, such as EZH2 inhibition to inhibit retinoblastoma growth, or other chromatin-modifying pharmacologic agents that promote retinal differentiation from PSCs, or even alter MAC phenotypes in congenital diseases linked to genetic alterations in epigenetic genes discussed above is a potential avenue for future therapeutics [97–99]. Finally, the integration of these strategies will also be important for improving the fidelity by which PSC-derived RPE model structural features and allow functional interrogation of variants associated with age-related retinal degenerative diseases like AMD and other acquired retinal diseases [100, 101]. By comparing epigenomic differences between iPSC-derived neural retina and RPE with corresponding primary retinal cells from AMD eyes (isolated from autopsy), as well as iPSC-derived retinal cells from those with AMD, it may be possible to identify epigenetic features of “aging,” such as changes in chromatin modifications at particular loci. One then might use such epigenetic features of aging as a readout to test for exogenous agents (biologics, drugs including those that target epigenetic pathways, etc) that recapitulate or reverse “retinal aging in a dish” of iPSC or other stem cell derived RPE or photoreceptors [102].
Outstanding Questions.
What are the mechanisms by which ubiquitously expressed chromatin modifying genes, direct cell type specific transcription factors expressed at a specific time and place (e.g. EFTFs), control retinal development and contribute to congenital blinding diseases?
What are the EFTF-independent mechanisms by which broadly expressed epigenetic protein regulate retinal development and disease?
How can stem cell-derived organoids and epigenomic technologies best be integrated to probe the functions of “epigenetic memory,” and of non-coding regions that affect the 3D genome and whose alterations are related to blinding hereditary diseases?
Given advances in sequencing technologies, what should the current standard by which research laboratories and biopharmaceutical industry evaluate genomic integrity, including status of TP53, of pluripotent stem cell lines for research applications and clinical trials?
Since TP53 gain-of-function mutations alter the epigenome in cancer cells such that there are genome-wide changes of histone methylation and acetylation, how do similar alterations affect the epigenome of human pluripotent stem cell undergoing retinogenesis, and how do such changes alter the risk-benefit ratio of using stem cell-based cell therapies in clinical trials?
As pluripotent stem cell-derived retinal organoids model events of embryonic retinogenesis, they are useful for modeling congenital diseases like microphthalmia. How could this technology, with or without stem cell intermediates (e.g. through direct reprogramming) be used to model phenotypic, genetic, and epigenetic aspects of blinding, age-related degenerative diseases like macular degeneration, in which onset of disease is 60–80 years after birth?
The traditional focus of epigenetics is chemical modifications to chromatin and resultant changes in gene expression. How do similar changes to RNA, a.k.a. epitranscriptomics, affect retinal development and disease?
PSC lines used for regenerative medicine trials acquire mutations in the transcription factor TP53 [80]. Further, GOF p53 leads to genome-wide increases in histone methylation and acetylation that enable growth advantages for cancer cell growth [88]. During PSC retinogenesis, WDR5 associates with p53 to target the EFTF gene Sox2 [51]. Future studies that map GOF P53 on 3D genomic architecture during retinogenesis will deepen our understanding of the effects of TP53 mutations. In addition, a better understanding of the molecular determinants underlying epigenetic memory may allow future selection of particular cell types that, when reprogrammed to induced pluripotent stem cell lines, enable enhanced efficiency toward retinal differentiation, which would lead to more cost-effective and potentially safer, stem cell-derived retinal cell therapies [64, 92, 94, 103]. An enhanced understanding of the epigenetic mechanisms that govern retinogenesis in PSC may accelerate the use of stem cell-based therapies and other regenerative strategies, including in situ conversion of host glial cells to functional retinal cells [104] and direct reprogramming of fibroblasts to photoreceptors without stem cell intermediates [105].
Supplementary Material
Highlights.
Surprisingly, many ubiquitious chromatin-modifying regulators control retinal development. They are linked to congenital blinding diseases such as microphthalmia.
Disruption of retinal cell fate occurs via alterations in catalytic activity of chromatin modifiers, inactivation of non-catalytic co-factors that comprise non-canonical epigenetic complexes, and temporal interference of protein-transcription factor (TF) interactions that engage chromatin.
Advances in pluripotent stem cell (PSC)-derived retinal organoids and epigenomic technologies have enabled a deeper understanding of the causal role of chromatin accessibility and modifications, and non-coding regions of the genome in microphthalmia.
Such advances provide translational insights into the roles of epigenetic memory and TF (e.g. TP53) mutations in source PSCs used for cell therapies in age-related blinding diseases.
Acknowledgements.
We apologize to those whose work could not be included in this review article, owing to space limitations. The authors would like to thank Agamjot Sangotra for her discussions and input on the content of this work. This work was supported in part by NIH R01EY030989 (to R.C.R.), NIH P30CA046592 (to the University of Michigan Comprehensive Cancer Center); NIH T32GM113900 (to the University of Michigan Molecular and Cellular Pathology Ph.D. Program in support of B.W.B.), the Research to Prevent Blindness (to the University of Michigan Kellogg Eye Center and R.C.R.), A. Alfred Taubman Medical Research Institute Leslie and Abigail Wexner Emerging Scholar Award (to R.C.R.), Grossman Research Fund (to R.C.R.), Leonard G. Miller Professorship and Ophthalmic Research Fund at the Kellogg Eye Center (to R.C.R.), Barbara Dunn Research Fund (to R.C.R.), Roz Greenspon Research Fund (to R.C.R.), Beatrice & Reymont Paul Foundation (to R.C.R.), and March Hoops to Beat Blindness (to R.C.R.).
Glossary
- Anophthalmia
a developmental birth defect characterized by absence of one or both eyes
- ATAC-seq (Assay for Transposase-Accessible Chromatin using sequencing)
a profiling technique that uses hyperactive transposase Tn5 to integrate and fragment (tagmentation) regions of open chromatin, which allows mapping of the chromatin accessibility landscape genome-wide using a relatively low number of cells
- Coloboma
a congenital defect resulting in missing tissue components of the eye or eyelid. Colobomas may be isolated or associated with syndromic finding that include systemic malformations outside ocular tissues. In this review, we refer to the optic fissure closure defect of coloboma, which segmentally affects some or all of the iris, choroid, retina, and optic nerve
- ChIP-seq (Chromatin Immunoprecipitation followed by DNA sequencing)
a technique that profiles DNA targets of a protein of interest by cross-linking that protein to DNA, chromatin fragmentation, immunoprecipitation of cross-linked protein-DNA, unlinking protein, DNA purification and sequencing
- CUT&RUN (Cleavage Under Targets and Release Using Nuclease)
a technique in which DNA targets of a protein of interest are mapped by using an antibody against the protein, cleaved using a micrococcal nuclease, and DNA-protein complexes are mapped by paired-end DNA sequencing. This is a more sensitive technique that requires less biological input material than ChIP-seq
- Electroretinography
a technique used in human and mammalian models whereby electrodes are placed on the ocular surface, or near the ocular surface, allowing electrical activity of specific retinal cells in response to light to be measured
- Epigenetic memory
features of the epigenome which are inherited during cellular mitosis, meiosis, and reprogramming. In the context of reprogramming somatic cells to induced pluripotent stem cells, epigenetic memory refers to bias of differentiation toward cell type of the parental somatic cell
- Epigenome
chemical modifications of DNA and histones within a given cell of an organism
- Eye Field Transcription Factors (EFTFs)
highly conserved regulatory transcription factors that orchestrate ocular development. Expression of these factors enables ectopic eyes outside the nervous system
- Genomic integrity
the status of mutations, copy number alterations, and chromosomal abnormalities in a given cell or cell line
- Hi-C
a chromosome conformation capture-based technique that maps chromatin-chromatin interactions in 3D space following DNA cross-linking, restriction digestion, intramolecular ligation, reversal of cross-links, and high-throughput sequencing
- Hi-ChIP (Hi-C with ChIP)
a technique that uses in situ crosslinking, nuclei isolation, Hi-C contact generation, ChIP, reversal of crosslinking, DNA isolation, biotin capture of Hi-C contacts, tagmentation, and high-throughput sequencing to detect protein-directed, chromatin-chromatin interactions in 3D space
- Microphthalmia
a birth defect in which one or both eyes are abnormally small, and which is usually associated with disorganized retinal structure due to aberrant retinal development
- Pluripotent stem cells
pluripotent stem cells have the capacity to self-renew and differentiate to any somatic cell type. Embryonic stem cells are derived from the inner cell mass of the blastocyst. Induced pluripotent stem cells are derived from somatic cells such as fibroblasts through reprogramming
- Protein-directed proximity labelling
a technique that uses a labeling enzyme to biotinylate biomolecules such as nuclei acids and proteins, proximal to a protein of interest. In a method depicted in Figure 2, a catalytically dead RNA-guided nuclease (dCas9) is fused to an engineered peroxidase APEX2, which allows targeting specific genomic loci, and biontinylation of proteins nearby this locus. After cell lysis, biotinylated proteins are pulled down with streptavidin-coated beads, and identified with proteomic methods like liquid chromatography with tandem mass spectrometry
- Reprogramming
in the context of induced pluripotent stem cells, reprogramming refers to the overexpression of pluripotency factors in somatic cells, such as OCT4, SOX2, KLF4, C-MYC (so-called Yamanaka factors), which converts them into pluripotent stem cells
- Retinal organoid
pluripotent stem cell-derived, three-dimensional anlage of retinal neuroepithelium. With prolonged culture, such organoids can generate functional photoreceptors
- Topologically associated domains (TADs)
extruded DNA loops formed by CTCF that form transcriptionally active regions demarcated from heterochromatin
Footnotes
RESOURCES
Registered at University Hospital Medical Information Network Clinical Trials Registry [UMIN-CTR] number UMIN000011929 https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000013279&type=summary&language=E
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Felsenfeld G, A brief history of epigenetics. Cold Spring Harb Perspect Biol, 2014. 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csankovszki G, Nagy A, and Jaenisch R, Synergism of Xist RNA, DNA methylation, and histone hypoacetylation in maintaining X chromosome inactivation. J Cell Biol, 2001. 153(4): p. 773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noberini R. and Bonaldi T, Epigenetic drug target deconvolution by mass spectrometry-based technologies. Nat Struct Mol Biol, 2019. 26(10): p. 854–857. [DOI] [PubMed] [Google Scholar]
- 4.Pal S. and Tyler JK, Epigenetics and aging. Sci Adv, 2016. 2(7): p. e1600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nacev BA, et al. , The expanding landscape of ‘oncohistone’ mutations in human cancers. Nature, 2019. 567(7749): p. 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao RC and Dou Y, Hijacked in cancer: the KMT2 (MLL) family of methyltransferases. Nat Rev Cancer, 2015. 15(6): p. 334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plaisancie J, et al. , Genetics of anophthalmia and microphthalmia. Part 1: Non-syndromic anophthalmia/microphthalmia. Hum Genet, 2019. 138(8–9): p. 799–830. [DOI] [PubMed] [Google Scholar]
- 8.Vissers LE, et al. , Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet, 2004. 36(9): p. 955–7. [DOI] [PubMed] [Google Scholar]
- 9.Schnetz MP, et al. , CHD7 targets active gene enhancer elements to modulate ES cell-specific gene expression. PLoS Genet, 2010. 6(7): p. e1001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gage PJ, Hurd EA, and Martin DM, Mouse Models for the Dissection of CHD7 Functions in Eye Development and the Molecular Basis for Ocular Defects in CHARGE Syndrome. Invest Ophthalmol Vis Sci, 2015. 56(13): p. 7923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strom SP, et al. , De Novo variants in the KMT2A (MLL) gene causing atypical Wiedemann-Steiner syndrome in two unrelated individuals identified by clinical exome sequencing. BMC Med Genet, 2014. 15: p. 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brightman DS, et al. , MLL1 is essential for retinal neurogenesis and horizontal inner neuron integrity. Sci Rep, 2018. 8(1): p. 11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng SB, et al. , Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat Genet, 2010. 42(9): p. 790–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyake N, et al. , MLL2 and KDM6A mutations in patients with Kabuki syndrome. Am J Med Genet A, 2013. 161A(9): p. 2234–43. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka R, et al. , Congenital corneal staphyloma as a complication of Kabuki syndrome. Am J Med Genet A, 2012. 158A(8): p. 2000–2. [DOI] [PubMed] [Google Scholar]
- 16.McVeigh TP, Banka S, and Reardon W, Kabuki syndrome: expanding the phenotype to include microphthalmia and anophthalmia. Clin Dysmorphol, 2015. 24(4): p. 135–9. [DOI] [PubMed] [Google Scholar]
- 17.Bogershausen N, et al. , An unusual presentation of Kabuki syndrome with orbital cysts, microphthalmia, and cholestasis with bile duct paucity. Am J Med Genet A, 2016. 170(12): p. 3282–3288. [DOI] [PubMed] [Google Scholar]
- 18.Chen YH, et al. , Rare ocular features in a case of Kabuki syndrome (Niikawa-Kuroki syndrome). BMC Ophthalmol, 2014. 14: p. 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokitsu-Nakata NM, et al. , Analysis of MLL2 gene in the first Brazilian family with Kabuki syndrome. Am J Med Genet A, 2012. 158A(8): p. 2003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang SP, et al. , A UTX-MLL4-p300 Transcriptional Regulatory Network Coordinately Shapes Active Enhancer Landscapes for Eliciting Transcription. Mol Cell, 2017. 67(2): p. 308–321 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorighi KM, et al. , Mll3 and Mll4 Facilitate Enhancer RNA Synthesis and Transcription from Promoters Independently of H3K4 Monomethylation. Mol Cell, 2017. 66(4): p. 568–576 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ang SY, et al. , KMT2D regulates specific programs in heart development via histone H3 lysine 4 di-methylation. Development, 2016. 143(5): p. 810–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindgren AM, et al. , Haploinsufficiency of KDM6A is associated with severe psychomotor retardation, global growth restriction, seizures and cleft palate. Hum Genet, 2013. 132(5): p. 537–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umutoni D, et al. , H3K27me3 demethylase UTX regulates the differentiation of a subset of bipolar cells in the mouse retina. Genes Cells, 2020. 25(6): p. 402–412. [DOI] [PubMed] [Google Scholar]
- 25.Gordon CT, et al. , De novo mutations in SMCHD1 cause Bosma arhinia microphthalmia syndrome and abrogate nasal development. Nat Genet, 2017. 49(2): p. 249–255. [DOI] [PubMed] [Google Scholar]
- 26.Shaw ND, et al. , SMCHD1 mutations associated with a rare muscular dystrophy can also cause isolated arhinia and Bosma arhinia microphthalmia syndrome. Nat Genet, 2017. 49(2): p. 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkie AO, Many faces of SMCHD1. Nat Genet, 2017. 49(2): p. 176–178. [DOI] [PubMed] [Google Scholar]
- 28.Nozawa RS, et al. , Human inactive X chromosome is compacted through a PRC2-independent SMCHD1-HBiX1 pathway. Nat Struct Mol Biol, 2013. 20(5): p. 566–73. [DOI] [PubMed] [Google Scholar]
- 29.Blewitt ME, et al. , SmcHD1, containing a structural-maintenance-of-chromosomes hinge domain, has a critical role in X inactivation. Nat Genet, 2008. 40(5): p. 663–9. [DOI] [PubMed] [Google Scholar]
- 30.Jansz N, et al. , Smchd1 regulates long-range chromatin interactions on the inactive X chromosome and at Hox clusters. Nat Struct Mol Biol, 2018. 25(9): p. 766–777. [DOI] [PubMed] [Google Scholar]
- 31.Ragge N, et al. , Expanding the phenotype of the X-linked BCOR microphthalmia syndromes. Hum Genet, 2019. 138(8–9): p. 1051–1069. [DOI] [PubMed] [Google Scholar]
- 32.Temtamy SA, Ismail SI, and Meguid NA, Lenz microphthalmia syndrome: three additional cases with rare associated anomalies. Genet Couns, 2000. 11(2): p. 147–52. [PubMed] [Google Scholar]
- 33.Afshar AR, et al. , Next-Generation Sequencing of Retinoblastoma Identifies Pathogenic Alterations beyond RB1 Inactivation That Correlate with Aggressive Histopathologic Features. Ophthalmology, 2020. 127(6): p. 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamline MY, et al. , OFCD syndrome and extraembryonic defects are revealed by conditional mutation of the Polycomb-group repressive complex 1.1 (PRC1.1) gene BCOR. Dev Biol, 2020. 468(1–2): p. 110–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huynh KD, et al. , BCoR, a novel corepressor involved in BCL-6 repression. Genes Dev, 2000. 14(14): p. 1810–23. [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, et al. , A Non-canonical BCOR-PRC1.1 Complex Represses Differentiation Programs in Human ESCs. Cell Stem Cell, 2018. 22(2): p. 235–251 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng X, et al. , Characterization of human-induced pluripotent stem cells carrying homozygous RB1 gene deletion. Genes Cells, 2020. 25(7): p. 510–517. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, et al. , Human embryonic stem cell-derived organoid retinoblastoma reveals a cancerous origin. Proc Natl Acad Sci U S A, 2020. 117(52): p. 33628–33638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ajioka I, et al. , Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell, 2007. 131(2): p. 378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Small KW, et al. , Multimodal Imaging and Functional Testing in a North Carolina Macular Disease Family: Toxoplasmosis, Fovea Plana, and Torpedo Maculopathy Are Phenocopies. Ophthalmol Retina, 2019. 3(7): p. 607–614. [DOI] [PubMed] [Google Scholar]
- 41.Small KW, et al. , North Carolina Macular Dystrophy Is Caused by Dysregulation of the Retinal Transcription Factor PRDM13. Ophthalmology, 2016. 123(1): p. 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mona B, et al. , Repression by PRDM13 is critical for generating precision in neuronal identity. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vervoort M, et al. , Evolution of Prdm Genes in Animals: Insights from Comparative Genomics. Mol Biol Evol, 2016. 33(3): p. 679–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanotel J, et al. , The Prdm13 histone methyltransferase encoding gene is a Ptf1a-Rbpj downstream target that suppresses glutamatergic and promotes GABAergic neuronal fate in the dorsal neural tube. Dev Biol, 2014. 386(2): p. 340–57. [DOI] [PubMed] [Google Scholar]
- 45.Chang JC, et al. , Prdm13 mediates the balance of inhibitory and excitatory neurons in somatosensory circuits. Dev Cell, 2013. 25(2): p. 182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodson NB, et al. , Prdm13 is required for Ebf3+ amacrine cell formation in the retina. Dev Biol, 2018. 434(1): p. 149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuber ME, et al. , Specification of the vertebrate eye by a network of eye field transcription factors. Development, 2003. 130(21): p. 5155–67. [DOI] [PubMed] [Google Scholar]
- 48.Bakrania P, et al. , SOX2 anophthalmia syndrome: 12 new cases demonstrating broader phenotype and high frequency of large gene deletions. Br J Ophthalmol, 2007. 91(11): p. 1471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuo I, et al. , Mouse Otx2 functions in the formation and patterning of rostral head. Genes Dev, 1995. 9(21): p. 2646–58. [DOI] [PubMed] [Google Scholar]
- 50.Glaser T, et al. , PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet, 1994. 7(4): p. 463–71. [DOI] [PubMed] [Google Scholar]
- 51.Li Q, et al. , p53 Integrates Temporal WDR5 Inputs during Neuroectoderm and Mesoderm Differentiation of Mouse Embryonic Stem Cells. Cell Rep, 2020. 30(2): p. 465–480 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burgess DJ, Epigenomics: Deciphering non-coding variation with 3D epigenomics. Nat Rev Genet, 2016. 18(1): p. 4. [DOI] [PubMed] [Google Scholar]
- 53.Sridhar A, et al. , Single-Cell Transcriptomic Comparison of Human Fetal Retina, hPSC-Derived Retinal Organoids, and Long-Term Retinal Cultures. Cell Rep, 2020. 30(5): p. 1644–1659 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mumbach MR, et al. , HiChIP: efficient and sensitive analysis of protein-directed genome architecture. Nat Methods, 2016. 13(11): p. 919–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myers SA, et al. , Discovery of proteins associated with a predefined genomic locus via dCas9-APEX-mediated proximity labeling. Nat Methods, 2018. 15(6): p. 437–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cowan CS, et al. , Cell Types of the Human Retina and Its Organoids at Single-Cell Resolution. Cell, 2020. 182(6): p. 1623–1640 e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mo A, et al. , Epigenomic landscapes of retinal rods and cones. Elife, 2016. 5: p. e11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim JW, et al. , Recruitment of Rod Photoreceptors from Short-Wavelength-Sensitive Cones during the Evolution of Nocturnal Vision in Mammals. Dev Cell, 2016. 37(6): p. 520–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corbo JC, et al. , CRX ChIP-seq reveals the cis-regulatory architecture of mouse photoreceptors. Genome Res, 2010. 20(11): p. 1512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White MA, et al. , Massively parallel in vivo enhancer assay reveals that highly local features determine the cis-regulatory function of ChIP-seq peaks. Proc Natl Acad Sci U S A, 2013. 110(29): p. 11952–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murphy DP, et al. , Cis-regulatory basis of sister cell type divergence in the vertebrate retina. Elife, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie H, et al. , Chromatin accessibility analysis reveals regulatory dynamics of developing human retina and hiPSC-derived retinal organoids. Sci Adv, 2020. 6(6): p. eaay5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kallman A, et al. , Investigating cone photoreceptor development using patient-derived NRL null retinal organoids. Commun Biol, 2020. 3(1): p. 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norrie JL, et al. , Nucleome Dynamics during Retinal Development. Neuron, 2019. 104(3): p. 512–528 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartz SD, et al. , Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet, 2012. 379(9817): p. 713–20. [DOI] [PubMed] [Google Scholar]
- 66.da Cruz L, et al. , Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol, 2018. 36(4): p. 328–337. [DOI] [PubMed] [Google Scholar]
- 67.Kashani AH, et al. , A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med, 2018. 10(435). [DOI] [PubMed] [Google Scholar]
- 68.Lebkowski J, GRNOPC1: the world’s first embryonic stem cell-derived therapy. Interview with Jane Lebkowski. Regen Med, 2011. 6(6 Suppl): p. 11–3. [DOI] [PubMed] [Google Scholar]
- 69.Cyranoski D, Trials of embryonic stem cells to launch in China. Nature, 2017. 546(7656): p. 15–16. [DOI] [PubMed] [Google Scholar]
- 70.Menasche P, et al. , Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J Am Coll Cardiol, 2018. 71(4): p. 429–438. [DOI] [PubMed] [Google Scholar]
- 71.Manley NC, et al. , Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cells: Preclinical Efficacy and Safety in Cervical Spinal Cord Injury. Stem Cells Transl Med, 2017. 6(10): p. 1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cyranoski D, Woman is first to receive cornea made from ‘reprogrammed’ stem cells. Nature, 2019. [DOI] [PubMed] [Google Scholar]
- 73.Parmeggiani F, Clinics, epidemiology and genetics of retinitis pigmentosa. Curr Genomics, 2011. 12(4): p. 236–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yau JW, et al. , Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care, 2012. 35(3): p. 556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong WL, et al. , Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health, 2014. 2(2): p. e106–16. [DOI] [PubMed] [Google Scholar]
- 76.Tham YC, et al. , Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology, 2014. 121(11): p. 2081–90. [DOI] [PubMed] [Google Scholar]
- 77.Tsilou ET, et al. , Ocular and orbital manifestations of the inherited bone marrow failure syndromes: Fanconi anemia and dyskeratosis congenita. Ophthalmology, 2010. 117(3): p. 615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bergman JE, et al. , CHD7 mutations and CHARGE syndrome: the clinical implications of an expanding phenotype. J Med Genet, 2011. 48(5): p. 334–42. [DOI] [PubMed] [Google Scholar]
- 79.Bowen ME, et al. , The Spatiotemporal Pattern and Intensity of p53 Activation Dictates Phenotypic Diversity in p53-Driven Developmental Syndromes. Dev Cell, 2019. 50(2): p. 212–228 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Merkle FT, et al. , Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature, 2017. 545(7653): p. 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmid P, et al. , Expression of p53 during mouse embryogenesis. Development, 1991. 113(3): p. 857–65. [DOI] [PubMed] [Google Scholar]
- 82.Sabapathy K, et al. , Regulation of ES cell differentiation by functional and conformational modulation of p53. EMBO J, 1997. 16(20): p. 6217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyer JS, et al. , Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A, 2009. 106(39): p. 16698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaewkhaw R, et al. , Transcriptome Dynamics of Developing Photoreceptors in Three-Dimensional Retina Cultures Recapitulates Temporal Sequence of Human Cone and Rod Differentiation Revealing Cell Surface Markers and Gene Networks. Stem Cells, 2015. 33(12): p. 3504–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lowe A, et al. , Intercellular Adhesion-Dependent Cell Survival and ROCK-Regulated Actomyosin-Driven Forces Mediate Self-Formation of a Retinal Organoid. Stem Cell Reports, 2016. 6(5): p. 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DeMott K. NEI awards prize for progress toward developing lab-made retinas: 3-D Retina Organoid Challenge 2020 encourages design of next-generation models to study blinding diseases and test therapies. 2018. [Google Scholar]
- 87.Hays D. Five research teams will develop new models for eye disease research: NEI Audacious Goals Initiative funds projects to accelerate the development of regenerative treatments for blindness. 2018. [Google Scholar]
- 88.Zhu J, et al. , Gain-of-function p53 mutants co-opt chromatin pathways to drive cancer growth. Nature, 2015. 525(7568): p. 206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boettiger AN, et al. , Super-resolution imaging reveals distinct chromatin folding for different epigenetic states. Nature, 2016. 529(7586): p. 418–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Otterstrom J, et al. , Super-resolution microscopy reveals how histone tail acetylation affects DNA compaction within nucleosomes in vivo. Nucleic Acids Res, 2019. 47(16): p. 8470–8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mandai M, et al. , Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N Engl J Med, 2017. 376(11): p. 1038–1046. [DOI] [PubMed] [Google Scholar]
- 92.Hiler D, et al. , Quantification of Retinogenesis in 3D Cultures Reveals Epigenetic Memory and Higher Efficiency in iPSCs Derived from Rod Photoreceptors. Cell Stem Cell, 2015. 17(1): p. 101–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim K, et al. , Epigenetic memory in induced pluripotent stem cells. Nature, 2010. 467(7313): p. 285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang L, et al. , Retinal Cell Type DNA Methylation and Histone Modifications Predict Reprogramming Efficiency and Retinogenesis in 3D Organoid Cultures. Cell Rep, 2018. 22(10): p. 2601–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miltner AM and La Torre A, Retinal Ganglion Cell Replacement: Current Status and Challenges Ahead. Dev Dyn, 2019. 248(1): p. 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kadumuri RV and Janga SC, Epitranscriptomic Code and Its Alterations in Human Disease. Trends Mol Med, 2018. 24(10): p. 886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khan M, et al. , Characterization and pharmacologic targeting of EZH2, a fetal retinal protein and epigenetic regulator, in human retinoblastoma. Lab Invest, 2015. 95(11): p. 1278–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan N, et al. , Postnatal onset of retinal degeneration by loss of embryonic Ezh2 repression of Six1. Sci Rep, 2016. 6: p. 33887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Avedschmidt SE, et al. , The Targetable Epigenetic Tumor Protein EZH2 is Enriched in Intraocular Medulloepithelioma. Invest Ophthalmol Vis Sci, 2016. 57(14): p. 6242–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith EN, et al. , Human iPSC-Derived Retinal Pigment Epithelium: A Model System for Prioritizing and Functionally Characterizing Causal Variants at AMD Risk Loci. Stem Cell Reports, 2019. 12(6): p. 1342–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boles NC, et al. , Epigenomic and Transcriptomic Changes During Human RPE EMT in a Stem Cell Model of Epiretinal Membrane Pathogenesis and Prevention by Nicotinamide. Stem Cell Reports, 2020. 14(4): p. 631–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saini JS, et al. , Nicotinamide Ameliorates Disease Phenotypes in a Human iPSC Model of Age-Related Macular Degeneration. Cell Stem Cell, 2017. 20(5): p. 635–647 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buchholz DE, et al. , Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med, 2013. 2(5): p. 384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yao K, et al. , Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature, 2018. 560(7719): p. 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mahato B, et al. , Pharmacologic fibroblast reprogramming into photoreceptors restores vision. Nature, 2020. 581(7806): p. 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Benevolenskaya EV, Histone H3K4 demethylases are essential in development and differentiation. Biochem Cell Biol, 2007. 85(4): p. 435–43. [DOI] [PubMed] [Google Scholar]
- 107.Hao H, et al. , Transcriptional regulation of rod photoreceptor homeostasis revealed by in vivo NRL targetome analysis. PLoS Genet, 2012. 8(4): p. e1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Albert M, et al. , The histone demethylase Jarid1b ensures faithful mouse development by protecting developmental genes from aberrant H3K4me3. PLoS Genet, 2013. 9(4): p. e1003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim Y, et al. , Physiological effects of KDM5C on neural crest migration and eye formation during vertebrate development. Epigenetics Chromatin, 2018. 11(1): p. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ji W, et al. , De novo damaging variants associated with congenital heart diseases contribute to the connectome. Sci Rep, 2020. 10(1): p. 7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsurusaki Y, et al. , Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nat Genet, 2012. 44(4): p. 376–8. [DOI] [PubMed] [Google Scholar]
- 112.Schneppenheim R, et al. , Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am J Hum Genet, 2010. 86(2): p. 279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hansen AS, et al. , CTCF and cohesin regulate chromatin loop stability with distinct dynamics. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Narendra V, et al. , CTCF establishes discrete functional chromatin domains at the Hox clusters during differentiation. Science, 2015. 347(6225): p. 1017–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li T, Lu Z, and Lu L, Regulation of eye development by transcription control of CCCTC binding factor (CTCF). J Biol Chem, 2004. 279(26): p. 27575–83. [DOI] [PubMed] [Google Scholar]
- 116.Watson LA, et al. , Dual effect of CTCF loss on neuroprogenitor differentiation and survival. J Neurosci, 2014. 34(8): p. 2860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen F, et al. , Three additional de novo CTCF mutations in Chinese patients help to define an emerging neurodevelopmental disorder. Am J Med Genet C Semin Med Genet, 2019. 181(2): p. 218–225. [DOI] [PubMed] [Google Scholar]
- 118.Liu Y, et al. , Structural insights into trans-histone regulation of H3K4 methylation by unique histone H4 binding of MLL3/4. Nat Commun, 2019. 10(1): p. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang YW, et al. , Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. Elife, 2014. 3: p. e02046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ang YS, et al. , Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell, 2011. 145(2): p. 183–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thomas LR, et al. , Interaction with WDR5 promotes target gene recognition and tumorigenesis by MYC. Mol Cell, 2015. 58(3): p. 440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schulz Y, et al. , CHARGE and Kabuki syndromes: a phenotypic and molecular link. Hum Mol Genet, 2014. 23(16): p. 4396–405. [DOI] [PubMed] [Google Scholar]
- 123.Zaidi S, et al. , De novo mutations in histone-modifying genes in congenital heart disease. Nature, 2013. 498(7453): p. 220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eising E, et al. , A set of regulatory genes co-expressed in embryonic human brain is implicated in disrupted speech development. Mol Psychiatry, 2019. 24(7): p. 1065–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.