Multiple challenges are faced by patients, clinicians, and researchers in accessing and providing care for rare diseases. Rare diseases, defined as a reported prevalence of <200,000 people in the United States and a prevalence of ≤1 in 2000 people in Europe, can involve any organ system. The majority of the >7000 recognized rare disorders, impacting around 9% of the US population, have a genetic basis and, as such, their effects can be multi-system and may evolve over time and with age. Table 1 lists prevalence of some rare diseases. Although individual rare diseases can be uncommon to encounter, when taken together rare diseases represent a major health burden. Rare diseases are associated with significant economic and financial burdens, both direct and indirect, which are further impacted by whether an approved pharmaceutical treatment is available.1
Table 1.
Estimated Prevalence of Select Rare Diseases per 100,000 People
| Crigler-Najjar syndrome | 1.0 |
| Cystic fibrosis | 7.4 |
| Eosinophilic esophagitis | 40.08 |
| Familial adenomatous polyposis | 6.0 |
| Gastrointestinal stromal tumor | 13.0 |
| Hepatic veno-occlusive disease | 11.0 |
| Hereditary chronic pancreatitis | 0.43 |
| Hirschsprung diseases | 10.9 (birth prevalence) |
| Peutz-Jeghers syndrome | 0.4 |
| Short bowel syndrome | 3.4 |
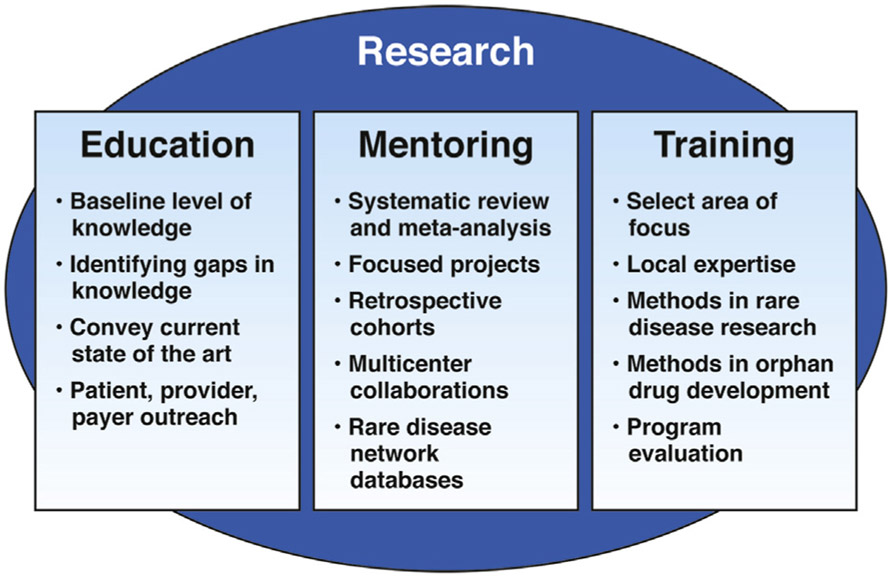
These considerations, as well as the challenges faced by patients (Table 2), underscore the need to advance awareness of rare diseases in the medical community. It is an opportunity for the trainee/junior faculty to harness these challenges into opportunities for their professional growth and learn skill-sets such as engaging non-traditional tools like patient advocacy groups (PAGs), social media and patient community. This review highlights the need to advance research in rare disease and discusses ways to accomplish this goal using education, mentoring and training as the foundations (Figure 1).
Table 2.
Challenges Faced by Patients with Rare Diseases
| Knowledge gap in providers |
| Lengthy, costly, and difficult journey to diagnosis |
| Perceived/real lack of sensitivity in health care team |
| Unaware of/limited access to specialists/subject matter experts |
| Difficult connecting with similar patients |
| Social isolation/societal acceptance |
| School and employment issues |
Figure 1.
Research can be considered an overarching framework to achieve education, mentoring, and training in rare diseases. Each of these facets can, in turn, help to advance knowledge of rare diseases.
Education
A core foundational method to enhance the study of rare diseases is exposing learners to these conditions during their medical education journey. The platforms include didactic lectures in the medical school curriculum, case-based studies, small group discussions, and the consideration of rare diseases when generating differential diagnoses at the bedside. The breadth and depth of lectures and formal teaching sessions on rare diseases should evolve from medical school curriculum to fellowship training (Table 3). Hands-on care of patients with rare diseases promotes medical training and helps learners to gain insights into fundamental pathophysiologic principles, acquire the instinct to recognize unique disease states, consider rare diseases in uncommon clinical scenarios, and understand that, although rare diseases affect a limited number of individuals, they may impact a disproportionate amount of health care resources.2 Establishing the diagnosis of a rare disease can involve ongoing testing and potential treatment of more common conditions as a “rule out.”
Table 3.
A Checklist of Tools to Self-advance: from Medical Student to Faculty
| Didactic lectures |
| Case-based studies |
| Small group discussions |
| Differential diagnosis at bedside |
| Hands-on care in inpatient and clinic settings |
| Attend rare diseases conferences |
| Harness nontraditional research methods |
| Engage patient advocacy groups |
| Network and collaborate with rare disease researchers through consortia |
| Apply for mentoring/internship programs via professional organizations |
| Enhance engagement in research projects |
| Be exposed to centers of excellence, expert consensus opinions, and clinical guidelines |
| Access various web-based resources |
| Create databases |
In addition to on-site patient-centered and didactic teaching, program directors/mentors should enable learners to attend rare disease conferences, engage PAGs, and work with rare disease researchers at the local institution or at a distant institution as an elective. One tool to support this could be training/electives grants offered by various national organizations; professional gastrointestinal diseases societies have mentor–mentee programs, summer internships, etc. These programs could also serve as portals for faculty with interests in rare diseases to engage prospective learners, especially if at same institution, and involve them in clinics, research projects, multi-disciplinary conferences etc. To facilitate knowledge and care of patients, learners could access tools such as centers of excellence, expert consensus opinions, and clinical guidelines that help provide evidence-based care. It is important to be familiar with PAGs and multicenter consortia such as Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR), a multicenter collaboration in the National Center for Advancing Translational Sciences’ (NCATS) Rare Diseases Clinical Research Network (RDCRN) which is managed in collaboration with several institutes of the National Institutes of Health (NIH).3
In addition to local resources, other tools to accomplish physician education include online webinars such as the CEGIR-driven web-based lecture series on eosinophilic GI disorders (EGIDs) and rare diseases research.4 Other sources of information include the Genetic and Rare Diseases Information Center through NCATS, which includes information for health care professionals such as International Classification of Diseases coding of rare diseases, and caring for patients with rare diseases; the Toolkit supported by the Office of Rare Diseases Research (ORDR) at NCATS; and National Organization of Rare Disorders (NORD), which provides an online physician guide for clinicians on how to facilitate the timely diagnosis and treatment of specific rare disorders.
Training and Mentoring
Engagement of trainees or junior faculty in rare diseases research is important, both for their career development and for advancing knowledge in the field. Because it may not be practical for someone at this career stage to conduct a large-scale prospective research project, identifying appropriate and feasible studies is key, as is leveraging existing resources. The mentoring and training model from CEGIR can provide some practical examples in the GI field, although similar models can be found in other areas. Survey studies using Internet-based platforms are generally straightforward to conduct and can yield important insights and preliminary data on under examined questions. In CEGIR, investigators used this approach to study variability in upper GI tract biopsy practices during endoscopy as way to establish best practices.5 Another study used a survey approach for needs assessment in patients with EGIDs, leveraging social media, email lists, and websites maintained by collaborating PAGs.6
Collaboration with PAGs is crucial and facilitates access to rare disease populations in many fields. Using retrospective data from multiple centers to answer focused questions is also a strategy to engage mentees with feasible projects. For example, investigators collected data from 6 sites within CEGIR to compile what became the largest study of the non-esophageal EGIDs yet to be published and were able to assess diagnostic trends over time, presenting symptoms, and treatment outcomes. Similarly, CEGIR investigators were able to assess the accuracy of billing codes for eosinophilic colitis to understand difficulties with identifying and enrolling this very rare EGID subtype. CEGIR has provided opportunities for trainees and junior faculty to write review articles with which they are able to familiarize themselves with the literature in the field, gain experience compiling a publication, and being able to establish a track record of scholarship.
Additionally, one-on-one mentoring relationships are invaluable; CEGIR mentees conduct important formative research, including identifying biomarkers, developing new models for study of disease pathogenesis, and using novel devices to characterize disease severity. To facilitate these efforts and learner growth, CEGIR has a training core directed toward learners and exposes learners to resources outside of local institution. These strategies need not be limited to GI or EGIDs research, but could be applied to any rare disease to develop a program of research (Table 3). Moreover, recognizing that rare diseases may have a serious impact on patients and the health care system, journal editors may consider publishing case reports on rare diseases to highlight knowledge of these disorders to practitioners and students.
How to Start Research and to Collaborate in Rare Diseases
Trainees’ and learners’ interests in rare disease, once initiated via exposures through formal didactic sessions and other informal methods, should evolve to research endeavors. Rare diseases research requires a nontraditional skill set, including using a multidisciplinary team beyond physicians. As highlighted, owing to the wide geographic distribution ofthese patients, it helps to collaborate with multiple sites and be creative and innovative with study designs. Besides case reports and case series, other possibilities include meta-analysis, database mining, systematic reviews, and prospective longitudinal studies. The creation of a database at the local institution or via a collaborative process facilitates research in rare diseases, as does familiarity with processes such as orphan drug development. The Orphan Drug Act grants special orphan designation status to drugs, both small molecules and biologics, for rare diseases and provides various development incentives, including tax credits, because innovative treatment strategies for rare diseases can be costly.
New investigators have extensive opportunities for research activities in rare diseases. Some unmet needs include the characterization of the natural history of rare diseases and conditions, identification of genotypic and phenotypic subpopulations, development and/or validation of clinical outcome measures, biomarkers and/or companion diagnostics, and therapy and device product development; treatments are available for only approximately 600 of the overall 7000 rare diseases.
To succeed, it is essential to use the strengths of academia, government, industry, and venture philanthropy to develop collaborative efforts. These efforts can lead to participation in research consortia and international networks using federated platforms of data gathering and sharing. Increasing advances in medical bioinformatics with interoperability of information resources and access to big datasets and electronic health records will expand research opportunities such as to identify pathways and possible interventions for rare diseases. In fact, technological advances have facilitated community partnerships through tools such as PAGs and social media networks. It is, however, imperative to recognize and understand the cultural, ethical, legal, and social issues related to data gathering and sharing from diverse populations.
Positioning of PAGs and Other Organizations
There are substantial benefits to engaging PAGs and patient community in research activities. Investigators can contribute to PAGs and build relationships by volunteering for activities, such as PAGs’ medical, scientific, and patient advisory boards. Investigators can also enable and empower their patients to initiate local support groups. Many of these efforts are directed to support research and new investigator training programs, which can benefit the learner, and to translate research results to patient communities. PAGs help to recruit for patient registries, biospecimen repositories, natural history studies, and various stages of clinical studies and trials. Additionally, PAGs can identify patient-driven needs assessment in areas of research and key outcomes that are important to patients themselves.6 Patients are a powerful tool to mobilize the community via social media and nonmedical interactions.
The strengths of PAGs is exemplified by their role in the enactment of the Orphan Drug Act. PAGs have been invaluable for awareness generation and fundraising; >280 PAGs form NORD, which serves as a central repository of information and efforts in rare diseases care. Often families acquire greater expertise in their rare condition than most of their health care providers, and this empowerment has carried fundamental advancements in enhancing their lives. In DGAT1 deficiency, for example, unremitting diarrhea from birth can necessitate dependence on total parenteral nutrition and early death. As children were identified by genetic testing, and families connected through online media, their collective experiences contributed to the identification of tailored dietary fat restriction as central to disease management. This citizen science outpaced any individual academic center, because the feedback and modifications occurred in real-time with a collaborative patient and family base.2
The RDCRN has 21 consortia studying >190 diseases, and works to improve rare disease understanding and awareness, in both the patient and medical communities. The consortiums have provided multicenter collaborations and cross-institutional mentorships that have accelerated the research of early stage investigators and mobilized the necessary resources for robust clinical research. The International Rare Diseases Research Consortium (IRDiRC) was formed in 2011 by the NIH and the European Commission to advance study of rare diseases and foster international collaboration. Orphanet, established in France in 1997, has grown to a consortium of >40 countries around the globe and among other things maintains the Orphanet rare disease nomenclature (ORPHAnumber).
Funding Opportunities
The prospective researcher should familiarize himself or herself with various funding sources in the public and private sectors. In addition to hard dollars, soft support such as reaching out to patient communities via PAGs is an invaluable and important resource stream. Table 4 lists several of the research resources available to investigate rare diseases and orphan products. The Food and Drug Administration Clinical Trials Grants Program from Office of Orphan Product Development deserves special mention. The Office of Orphan Product Development administers extramural grants programs and provides funding for clinical research that tests the safety and efficacy of drugs, biologics, medical devices and medical foods in rare diseases. The Orphan Products Grants Program has been supporting clinical trial research since 1983 and has facilitated the marketing approval of >60 products.
Table 4.
Rare Diseases Funding Resources
| Rare Diseases Clinical Research Network (RDCRN) program at NCATS https://ncats.nih.gov/rdcrn/about |
| Therapeutics for Rare and Neglected Diseases (TRND) program. https://ncats.nih.gov/trnd/about |
| Genetics and Rare Diseases Information Center (GARD) https://ncats.nih.gov/gard |
| National Organization of Rare Diseases (NORD) https://rarediseases.org/for-clinicians-and-researchers/research-opportunities/research-grant-program/. |
| Rare Diseases Registry Program (RaDaR) Program https://ncats.nih.gov/radar |
| FDA Clinical Trials Grants Program from Office of Orphan Product Development (https://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/officeofscienceandhealthcoordination/ucm2018190.htm) |
| International Rare Diseases Research Consortium http://www.irdirc.org/ |
| Patient advocacy groups |
Conclusions
The effects of rare diseases are felt at multiple levels of society, including patients, their families, and health care providers; research investigators; employers; schools; bio-pharmaceutical and medical devices industry; and government research, regulatory, and health care services agencies attempting to respond to the many needs of the community. Rare diseases afford early career gastroenterologists opportunities for discovery in unexamined territories and accelerate career advancement.
We have provided the learner reasons for delving into rare diseases, how education, training, and mentoring can be incorporated into learning, how research can guide these efforts, and practical tools on how to conduct research. It is imperative to pursue opportunities for collaboration and coordination of initiatives, research projects, and fundraising efforts through research networks, public-private partnerships, and PAGs. As a community, we should not only enable our learners, but also empower them to harness the willingness and ability of individuals with rare diseases to participate in clinical trials, patient registries, biospecimen repositories, natural history studies, and other research efforts.
Acknowledgments
Funding
ESD and SKG are supported in part by the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) (U54 AI117804), part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including American Partnership For Eosinophilic Disorders (APFED), Campaign Urging Research for Eosinophilic Disease (CURED), and Eosinophilic Family Coalition (EFC).
Footnotes
Conflicts of interest
The authors have made the following disclosures: SCG and RGS have no conflicts to disclose. ESD has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; is a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Biorasi, Calypso, Celgene/Receptos, Enumeral, EsoCap, Gossamer Bio, GSK, Regeneron, Robarts, Salix, and Shire; and has received educational grants from Allakos, Banner, and Holoclara. SKG is a Consultant for Abbott, Adare, Allakos, Celgene, Gossamer Bio, QOL, and UpToDate; and has received research funding from Shire.
References
- 1.Angelis A, Tordrup D, Kanavos P. Socio-economic burden of rare diseases: a systematic review of cost of illness evidence. Health Policy 2015;119:964–979. [DOI] [PubMed] [Google Scholar]
- 2.Picoraro JA, Gupta SK. Rare diseases enrich our field. AGA Perspectives. April 2019. Available from: http://agaperspectives.gastro.org/rare-diseases-enrich-our-field/#.XV70rOhKhPY. Accessed August 22, 2019. [Google Scholar]
- 3.Gupta SK, Falk GW, Aceves SS, et al. Consortium of Eosinophilic Gastrointestinal Disease Researchers: advancing the field of eosinophilic GI disorders through collaboration. Gastroenterology 2019;4:838–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Consortium of Eosinophilic Gastrointestinal Disease Researchers. Education videos. Available: www.rarediseasesnetwork.org/cms/cegir/Learn-More/Educational-Videos. Accessed August 22, 2019.
- 5.Dellon ES, Collins MH, Bonis PA, et al. Substantial variability in biopsy practice patterns among gastroenterologists for suspected eosinophilic gastrointestinal disorders. Clin Gastroenterol Hepatol 2016; 14:1842–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiremath G, Kodroff E, Strobel MJ, et al. Individuals affected by eosinophilic gastrointestinal disorders have complex unmet needs and frequently experience unique barriers to care. Clin Res Hepatol Gastroenterol 2018; 42:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]