ABSTRACT
The objective of this study was to highlight the global scientific effort to fight the SARS-CoV-2, addressing the preliminary results of passive immunization through convalescent plasma. We performed a search at the major databases of interventional clinical trial protocols about the transfusion of convalescent plasma in patients with COVID-19, as well as, published articles (n≥25), using the following search strategy: [(COVID-19 OR SARS-CoV-2 OR nCoV-2019) AND (Convalescent plasma OR Plasma exchange) AND (Treatment OR Therapy)]. A total of 24 interventional clinical trial protocols (advanced in phases II-III, III, and IV) were included in this review, as well as three studies that had enough outcomes to evaluate the efficacy of convalescent plasma therapy for patients with COVID-19. All interventional clinical trial protocols applied approximately 500mL of convalescent plasma (from single or more donations) in hospitalized patients, mainly in patients with severe disease associated with standard therapy for COVID-19, and compared to placebo or standard therapy plus specific drugs. Most of interventional clinical trial protocols are multicenter, and the phase IV studies are recruiting at intercontinental centers of North America, Oceania, Europe, but most are recruiting center inside their own county. The three studies published reported similar approach of convalescent plasma intervention with decrease in length of stay, mortality, with less than 4% of adverse events, mainly for treating critical cases with life-threatening disease. All advanced clinical trials focused on convalescent plasma therapy in patients with COVID-19 hospitalized in severe conditions, and the preliminary results provide strong evidence for therapy for the COVID-19 patients.
Keywords: COVID-19; Coronavirus infections; SARS-CoV-2; Betacoronavirus; Immunization; Immunization, passive; Plasma; Convalescent plasma
INTRODUCTION
At the end of 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged, resulting in the coronavirus disease 2019 (COVID-19), which caused an unprecedented health emergency and declared a pandemic by the World Health Organization (WHO) on March 11, 2020.(1) Up to September 2020, this pandemic had affected approximately 28 million and killed roughly 920 thousand people worldwide.(2) In Brazil, more than 5 million cases and more than 130 thousand deaths have occurred so far.(3)
New approaches to the development of immunity transfer were quickly implemented in preclinical studies, and currently interesting results of clinical trials are beginning to emerge, aiming to improve the symptoms of COVID-19, a heterogeneous disease caused by SARS-CoV-2.(2) Simultaneously, different vaccines are being developed and tested for effective disease prevention.(4)
Before SARS-CoV-2, the use of convalescent plasma (CP) had been investigated, with positive outcomes, in outbreaks of other viral infections,(5) such as the pandemic influenza A (H1N1), in 2009,(6) avian influenza A (H5N1),(7) among others. Furthermore, some studies have demonstrated that CP antibodies can limit virus proliferation during the infection and support viral clearance, which is favorable for fast recovery of the disease.(8)
A recent review on plasma therapy in COVID-19 patients reported low frequency of severe adverse event, and improvement in clinical symptoms in some participants after plasma therapy, but the authors judged the risk of reporting bias.(9)
The high or low titers of neutralizing antibodies against COVD-19 can be managed to reduce patient’s symptoms and mortality. There are 24 advanced clinical trials in phases II-III, III, and IV reported in several countries using CP to treat these patients, and answer this question.(10–33) Although passive immunization has been used for over a century to treat infectious diseases, the recent results pose challenges to set the best time of plasma extraction and donor choice, in addition to the cost of this whole procedure. This lack of information empowers the movements of antivaccine and antiplasma groups.(34)
OBJECTIVE
To highlight the global scientific effort in the fight against SARS-CoV-2, addressing passive immunization through convalescent plasma and their preliminary results.
METHODS
A search was performed until 14 September 2020 at ClinicalTrials.gov (https://clinicaltrials.gov/), Chinese Clinical Trial Registry (http://www.chictr.org.cn/abouten.aspx) and EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/) for interventional clinical trials on CP transfusion, in patients with COVID-19, using the following search strategy: [(COVID-19 OR SARS-CoV-2 OR nCoV-2019) AND (Convalescent Plasma OR Plasma Exchange) AND (Treatment OR Therapy)]. Then, the same strategy was used to search for studies in PubMed® and Scopus databases about the efficacy of CP therapy to treat patients with COVID-19.
Inclusion and exclusion criteria
This review included clinical trial protocols (CTP) phases III and IV that addressed the development of therapies based on CP to treat COVID-19 patients by passive immunization, and studies that showed the efficacy of CP therapy applied in more than 25 COVID-19 patients. The reasons for excluding studies were as follows: CTP for observational studies, CTP involving vaccines, and CTP canceled, or not approved, until the searching date in the databases.
Study eligibility, data extraction, data collection, and risk of bias assessment
The study eligibility followed the Preferred. Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.(35)
Data analysis
All results were described and presented using the percentage distribution for all variables analyzed in the tables.
RESULTS
Study selection
After applying the search strategies in the databases, 170 CTP were identified (153 protocols at ClinicalTrials.gov, 14 at Chinese Clinical Trial Registry, and three at the EU Clinical Trials Register). The search strategy used the PRISMA.(35)
Based on established inclusion and exclusion criteria, of 170 protocols identified, 146 clinical trials were excluded after screening (130 protocols were phases I and II and 16 were observational), remaining 24 protocols selected from these databases. In total, 24 CTP were included in the present work for passive immunization for COVID-19 through CP therapy.(10–13,15–33)
Of the selected studies published in the databases mentioned above, only three studies had enough data that allow statistical analysis of the outcomes, to evaluate the CP therapy efficacy due to the number of patients with COVID-19 (n≥25).(36–38)
Overview of clinical trial protocols for passive immunization for COVID-19
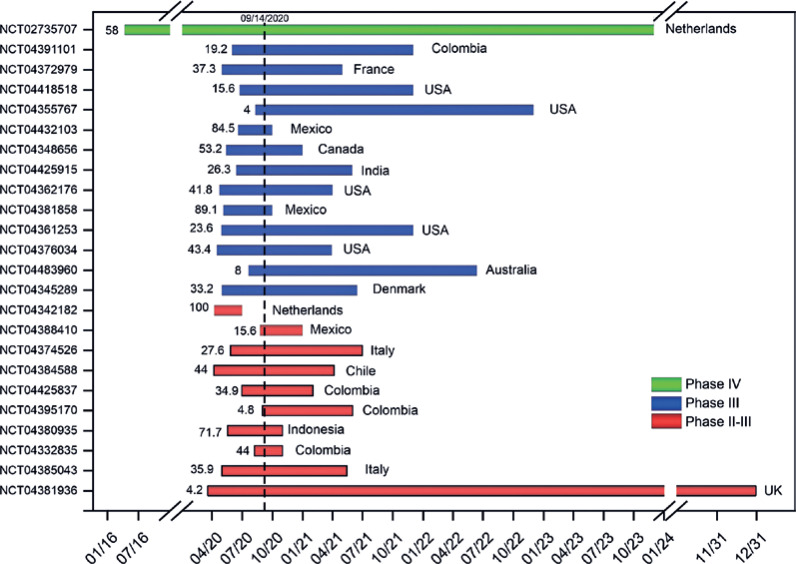
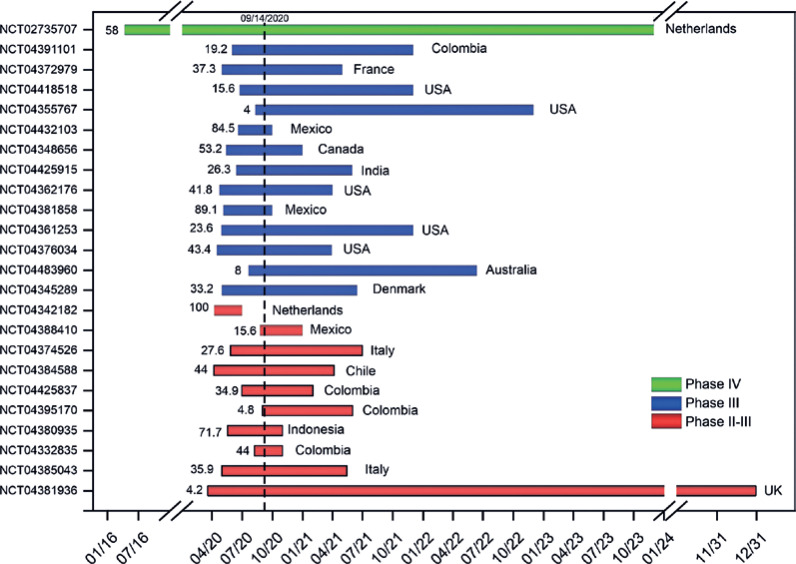
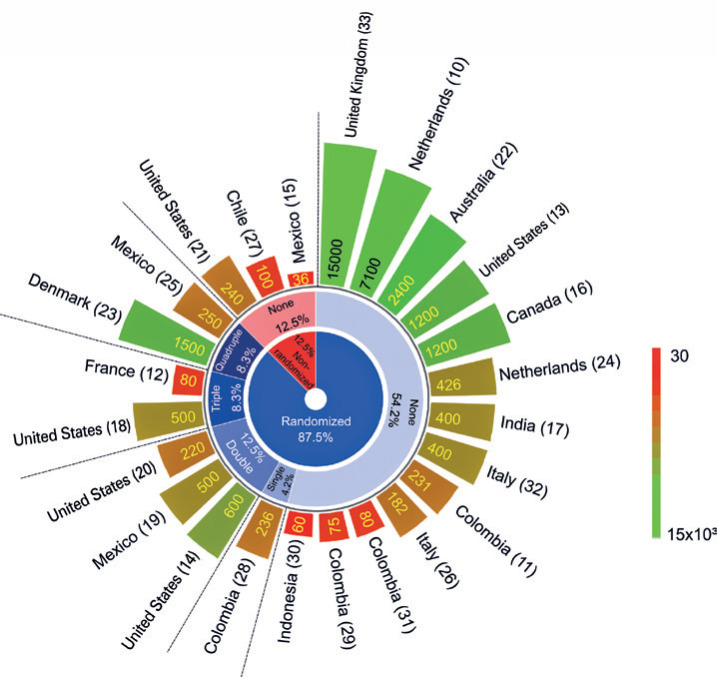
Of 24 more advanced clinical trials on CP therapy for COVID-19 inpatients, only one (4.2%) was in phase IV, with 58% of study progress rate (SPR), recruiting individuals in different countries by Netherlands sponsor (green bar of figure 1).(10) The phase III studies (54.2%),(11–13,15–19) almost half of CTP had more than 40% of SPR, mainly in Mexico(15,19) and the United States (blue bars of figure 1),(18,21) and the phase II-III studies (41.7%),(24–33) one CTP has 100% of SPR in the Netherlands,(24) and almost half of CTP have more than 40% of SPR (red bars of figure 1), as shown in figure 1 and table 1.
Figure 1. Analysis of the study progress rate percentile of clinical trial protocols on convalescent plasma therapy for hospitalized patients of COVID-19 distributed by their phases (green bar: phase IV; blue bars: phase III; and red bars: phase II-III) and the corresponding sponsor country.
Table 1. Study design, arms, interventions, and study time progress.
| Identity number |
Phase | Patient features |
Intervention by arm | CP dose (day) | Age range (years) |
Start date |
Completion date |
Progress (%) |
Recruitment status |
|---|---|---|---|---|---|---|---|---|---|
| NCT02735707(10) | IV | Severe acute respiratory illness and severe community acquired pneumonia | Corticosteroid versus antibiotics versus macrolide versus Influenza antiviral versus LPV/Rit versus HydChl versus IFN-β1a versus Anak versus Tmab versus Smab versus Vit C versus heparin versus simvastatin versus CP versus ST | 1 or 2 units within 48 hours | >18 | 4/11/16 | 12/1/23 | 58 | Recruitingz |
| NCT04391101(11) | III | Severe patients treated in ICU | CP associated with ST versus ST | 400-500mL | >18 | 6/1/20 | 12/1/21 | 19.2 | Not yet recruiting |
| NCT04372979(12) | III | Early care of hospitalized patients | CP associated with ST versus SP associated with ST | 200-230mL | 18-80 | 5/1/20 | 5/1/21 | 37.3 | Not yet recruiting |
| NCT04418518(13) | III | Early care for patients hospitalized | CP associated with ST versus ST | 500mL of single-donor or 2 units of 250mL from 1-2 donations | 18-70 | 6/24/20 | 12/1/21 | 15.6 | Recruiting |
| NCT04355767(14) | III | Severe/critical hospitalized patients | CP (antibodies titers ≥1:160) versus placebo | CP: 1-unit versus placebo: saline with multivitamin | >18 | 8/11/20 | 12/1/22 | 4.0 | Not yet recruiting |
| NCT04432103(15) | III | Severe/critical hospitalized patients | CP from IgG (severe patients versus critical patients) associated with ST | NR | >19 | 6/19/20 | 9/30/20 | 84.5 | Not yet recruiting |
| NCT04348656(16) | III | Early care for patients hospitalized | CP versus ST | 500mL of single-donor or 2 units of 250mL from 1-2 donations | >20 | 5/14/20 | 12/31/20 | 53.2 | Recruiting |
| NCT04425915(17) | III | Severe/critical hospitalized patients | CP associated with ST versus ST | 2 doses of 250mL on consecutive day started by day 3 of symptom onset | >21 | 6/14/20 | 5/30/21 | 26.3 | Recruiting |
| NCT04362176(18) | III | Patients hospitalized or in ICU | Pathogen reduced CP versus placebo | CP: 500mL within 12 hours (day 0) versus placebo: 250mL of Lactate Ringers associated with multivitamins (day 1) | >22 | 4/24/20 | 4/1/21 | 41.8 | Recruiting |
| NCT04381858(19) | III | Severe respiratory failure with invasive mechanical ventilation | CP (antibodies titers >1:164) versus HIg | CP: 400mL (2 units) versus HIg: 0.3g/kg/day (5 doses) | 16-18 | 5/6/20 | 9/30/20 | 89.1 | Recruiting |
| NCT04361253(20) | III | Patients hospitalized | CP (high-titer) versus SP (FFP or FP 24) | HT-CP: 2 doses of 250mL of single donor within 24 hours; FFP: 2 units of 200-275mL | >1 | 04/30/20 | 12/1/21 | 23.6 | Recruiting |
| NCT04376034(21) | III | Mild, moderate and severe/critical severity | CP associated with ST versus ST | Adult: 200 to 250mL; children: 10mL/kg; 2 units severe patients or critical condition | >30 days | 4/16/20 | 3/30/21 | 43.4 | Recruiting |
| NCT04483960(22) | III | No severe patients | LPV/Rit versus HydChl versus CP | 1 unit on day 1 and day 2 | >18 | 7/21/20 | 6/12/22 | 8.0 | Recruiting |
| NCT0434528 9(23) | III | Mild, moderate and severe/critical hospitalized patients | CP associated with ST (Smab versus baricitinib versus HydChl) versus ST associated with injective placebo | CP: (twice 300mL) and single dose of 600mL; placebo: (twice 300mL) and single dose of 600mL IV saline oral placebo: three times/day (7 days) | >18 | 5/1/20 | 6/15/21 | 33.2 | Recruiting |
| NCT04342182(24) | II-III | Hospitalized patients | CP associated with ST versus ST | 300mL (according to the Erasmus MC KIS protocol) | >18 | 4/8/20 | 7/1/20 | 100 | Recruiting |
| NCT04388410(25) | II-III | Hospitalized patients with severe disease or at risk for severe disease. | CP versus placebo | CP: 2 units of 200mL within 24-72 hours); placebo: 200mL of saline | >18 | 8/25/20 | 12/31/20 | 15.6 | Not yet recruiting |
| NCT04374526(26) | II-III | Hospitalized patients | CP associated with ST versus ST | 200mL/day for 3 days | >65 | 5/27/20 | 6/30/21 | 27.6 | Recruiting |
| NCT04384588(27) | II-III | Oncological and non-oncological patients with severe disease | CP associated with ST versus ST | 1 or more units | >15 | 4/7/20 | 4/6/21 | 44.0 | Recruiting |
| NCT04425837(28) | II-III | Hospitalized patients at high risk of severe disease or in ICU | CP (antibodies titers of ≥1:160) associated with ST versus ST | 2 doses of 200mL in a day | >18 | 7/1/20 | 2/1/21 | 34.9 | Not yet recruiting |
| NCT04395170(29) | II-III | Early care for patients hospitalized | CP associated with ST versus anti-COVID-19 HIg versus ST | CP: 200-250mL (day 1 and 3); 10% IgG solution: 50mL (patient ≥50kg); 1 mL/Kg (patient <50Kg), on days 1 and 3 | >18 | 9/1/20 | 6/1/21 | 4.8 | Not yet recruiting |
| NCT04380935(30) | II-III | Hospitalized patients in ICU (using mechanical ventilation) | CP associated with ST versus ST | NR | >18 | 5/18/20 | 10/31/20 | 71.7 | Not yet recruiting |
| NCT04332835(31) | II-III | Moderate and severe/critical severity | CP associated with ST versus HyChl associated with ST | 250mL on days 1 and 2 | 18-60 | 8/8/20 | 10/31/20 | 44.0 | Not yet recruiting |
| NCT04385043(32) | II-III | Severe/critical hospitalized patients | CP associated with ST versus ST | NR | 18-60 | 5/1/20 | 5/15/21 | 35.9 | Recruiting |
| NCT04381936(33) | II-III | Hospitalized patients at high-risk of severe disease | LPV/Ritversus corticosteroid versus HyChl versus Azi versus Tmab associated with ST versus CP associated with ST | 275mL±75mL on days 1 and 2 | all | 3/19/20 | 12/1/31 | 4.2 | Recruiting |
Passive immunotherapy occurs through the infusion of plasma from convalescent individuals, hence the use of the term convalescent plasma which can also be called hyperimmune plasma or ABO-compatible convalescent plasma.
CP: convalescent plasma; LPV: lopinavir; Rit: ritonavir; HydChl: hydroxychloroquine; IFN-β1a: interferon-β1a; Anak: anakinra; Tmab: tocilizumab; Smab: sarilumab; Vit C: vitamin C; ST: standard therapy; ICU: intensive care unit; SP: standard plasma; IgG: immunoglobulin G; NR: not report; HIg: human immunoglobulin; FFP: fresh frozen plasma; FP24: plasma frozen within 24 hours after phlebotomy; HT-CP: high-titer convalescent plasma; IV: intravenous; Azi: azithromycin.
Convalescent plasma intervention
The CP intervention applied in all CTP was for inpatients with different degrees of disease impairment, especially for severe cases admitted to the ICU (58.3%) with or without invasive mechanical ventilation, and all patients received the ST for COVID-19. The intervention arms applied in these inpatients normally compared the CTP of plasma therapy plus ST versus only ST associated or not with some selected drugs, such as corticosteroid, antibiotics, antimalarials (hydroxychloroquine), anticoagulants, human immunoglobulin, antiviral drugs, among others.(10,22,23,29,31,33) The CP therapy was applied mainly as single dose, with different volume of transfusion (45.8%), and the volume more frequently used was 500mL, in 20.8%,(13,16,18,39) following by doses of 200 to 250mL in 8.3%,(12,21) 400mL in 8.3%,(19,39) 300mL in 4.2%,(24) and 600mL in 4.2%.(23) In the cases using more than one dose, the volume was two doses of 250mL,(13,16,17,20,29,31,33) used in most of CTP, following by 2x200mL(19,25,28,29,33) and 2x300mL.(23,33) Plasma doses were derived from a single donor,(13,16,20,23) or until two different donors in some CTP.(13,16) Almost all CTP test the intervention in individuals aged over 18 years, with exception of two CTP (Table 1).
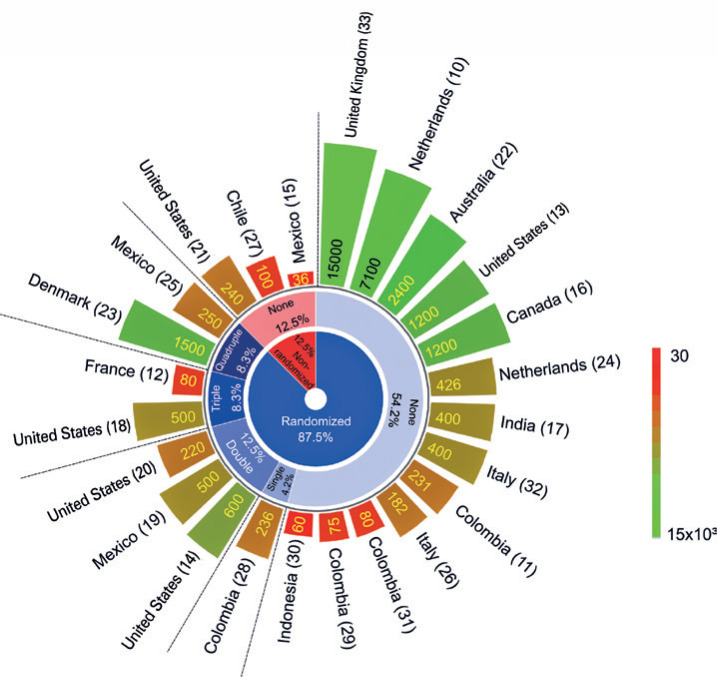
Regarding study design characteristics of these CTP, figure 2 shows the intervention was mostly (87.5%) randomized and few CTP used some type of masking (33.3%), such as: 4.2% single blinding (participant), 12.5% double-blinding (participant and outcome evaluator), 8.3% triple-blinding (participant, care provider and outcome evaluator), and 8.3% quadruple-blinding (participant, care provider, investigator and outcome evaluator). Although 12.5% of CTP have not adopted any technique used to minimize the bias in allocations and blinding, keeping it open-label. The estimated enrollment of clinical trial in phase IV is 7,100 individuals,(10) in phase III from 36 to 2,400 individuals,(11–23) and phase II-III from 60 to 15,000.(24–33) The number of volunteers estimated in each protocol was represented by the color scale bar in figure 2.
Figure 2. Study design of clinical trials of passive immunization against COVID-19 (plasma therapy), distributed inside out by the different types of allocation (randomized or not), masking (none, single, double, triple, and quadruple blinding), estimated enrollment (varied from 36 to 15,000 individuals), and study countries. The color scale bar represents the number of volunteers estimated in each protocol.
Global research network in clinical trial protocol
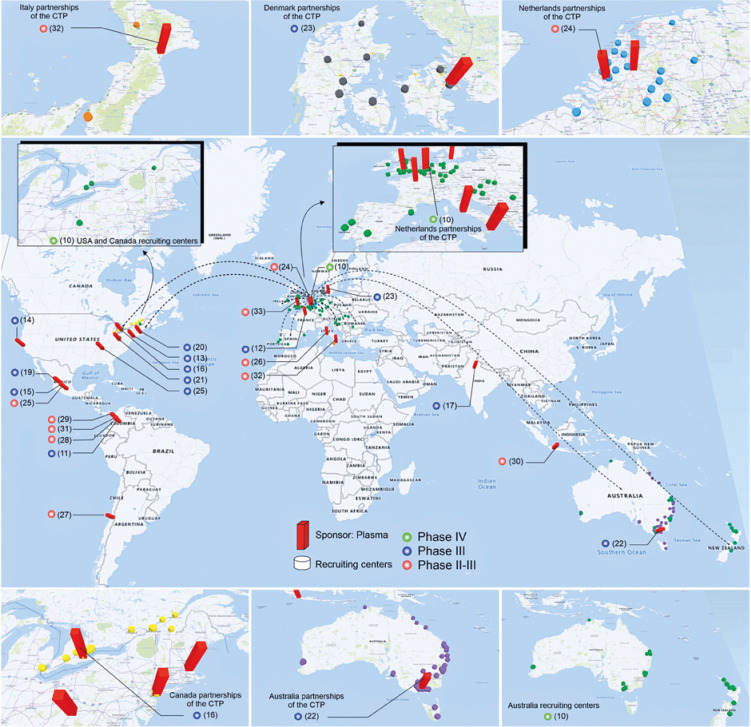
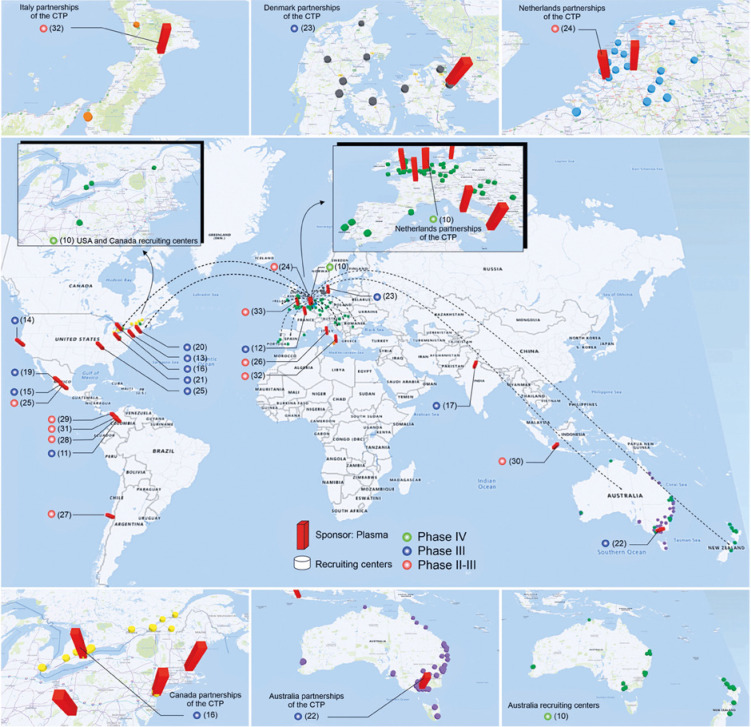
Among the CP multicenter CTP,(10,12,13,16,17,22–24,26,30,32) the CTP phase IV(10) is the only one with intercontinental collaborations represented by the dashed black lines on the world map (Figure 3), with 87 recruitment centers (green cylinders) distributed in North America, Europe, and Oceania. The CTP phase III also have collaborations among countries and involve 48 recruitment centers (yellow cylinders) in the USA and Canada.(16) The other multicenter CTP involve a varied number of recruitment centers within the same country, such as: Australia with 79 centers (purple cylinders),(22) the Netherlands with 18 centers (blue cylinders),(24) Denmark with 12 centers (dark gray cylinders),(23) Italy with six centers (orange cylinders),(32) highlighted in figure 3, with the enlarged image for better visualization of the collaboration centers, and others with approximately three centers.(12,17,26,30) The single center CTP concentrate mainly in North America(15,18–21) and South America.(11,27–29,31)
Figure 3. The global distribution of clinical trials by phase (circles) and the centers carrying out research on COVID-19 plasma therapy (red bar) and their recruitment centers (cylinders). The main centers of each continent are highlighted in the enlarged image around the central map. Phase IV is indicated by green circle; phase III by blue circle, and phases II-III by red circle. The intercontinental collaborations of the clinical trial protocols at phase IV are represented by the dashed black lines on the world map.
Plasma therapy outcome in COVID-19 patients
The three studies that evaluate the efficacy of plasma therapy also prioritize the evaluation of COVID-19 in severely ill patients or those in life-threatening situation, such as the advanced clinical trials mentioned above. The CP therapy intervention varied a lot among studies, without a consensus about the best CP pattern of application. Regarding the results, the studies showed a reduction by 53% in disease severity (not needing intensive care),(38) 26% in length of hospital stay,(36) and from 35% to 50% in mortality rate,(36–38) reporting adverse effects in less than 4% of patients after treatment with CP in different doses and volumes associated with standard therapy for COVID-19,(36–38) as shown in table 2.
Table 2. Studies that evaluation the efficacy of plasma therapy in COVID-19 patients.
| References | n/CP sample | Antibody titer | CP dose (mL) |
Viral charge (x10 ^/dL) |
Previous treatments | Hospitalization (variation) |
Adverse events (n) |
Mortality rate reduction |
|---|---|---|---|---|---|---|---|---|
| Abolghasemi et al.(36) | 189/115 CP versus 74 ST | Plasma antibody titer cut off index >1.1 | 2x500mL | NI | ST + antiviral (LPV/Rit), HydChl and anti-inflammatory agent | 9.54 days CP versus 12.88 days ST CP reduced length of stay by ~26% | ~1% CP Transient mild fever and chills following infusion of the plasma (n=1) | 14.8% CP versus 24.3% ST ~40% |
| Li et al.(37) | 103/52 CP (23 severe + 29 life-threatening) versus 51 ST (22 severe + 29 life-threatening) | 1:350 (6 donors) | 4-13mL/kg | Reduction in severe patients: 44.7% (24 hours), 68.1% (48 hours) and 87.2% (72 hours) virus-free Life-threatening patients: 53.8% (24 hours); 73.1 (48 hours) and 84.6% (72 hours) virus-free | Antiviral (41/46; 89.1%); antibacterial (38/46; 82.6%); Chinese herbal medicine (26/46; 56.5%); steroids (21/46 45.7%); antifungal (15/46; 32.6%); HIg (13/46; 28.3%); interferon (12/46; 26.1%) | Severe: 32.00 (26.00-40.00); life-threatening: indeterminate (46.00-indeterminate) | ~4% CP Chills and rashes within 2 hours (n=1); shortness of breath, cyanosis and severe dyspnea within 6 hours (n=1) | 15.7% CP versus 24.0% ST ~35% |
| Xia et al.(38) | 1,568/138 | Not significantly higher in rapid responders than in moderate responders | 200-1,200mL | 20/25 (80%) became virus-free after 14 days | NI | 2.4% CP versus 5.1% ST CP reduced admitted to the ICU by ~53% | ~2% CP Pruritus or erythema during transfusion (n=3) | 2.2% CP versus 4.1% ST ~50% |
CP: convalescent plasma; ST: standard treatment; NI: not informed; LPV: lopinavir; Rit: ritonavir; HydChl: hydroxychloroquine; HIg: human immunoglobulin; ICU: intensive care unit.
DISCUSSION
In the absence of effective treatment for patients with COVID-19, many studies have sought alternatives to treat patients and enhance patient’s immune defense, such as the use of CP therapy. The recovery trial(40) provides evidence to support some treatments (for instance, dexamethasone) and improve immunity in critical condition cases, and this trial uses CP therapy as one therapeutic arm. However, many aspects of this therapy are still being explored, such as a timeout/collection interval for COVID-19, or immunoglobulin G/immunoglobulin M (IgG/IgM) titers from donors, the therapy clinical improvement and efficacy in critical or non-critical patients and adverse effects. Among 170 CTP identified, only 24 CTP were in advanced phase (III/IV) with 33,000 individuals, concentrated in the regions of America, showing the pivotal questions on efficient use of the CP still uncertain or fragile to justify an increased use in critical or non-critical hospital care. In addition, no country, including the United States, has licensed CP as a treatment for COVID-19. The Food and Drug Administration (FDA) judged eligible for wider use under an emergency use authorization,(41) although other countries have granted approval for use on an individual patient basis.
One of the first CP clinical trials that analyzed 103 patients with severe and life-threatening COVID-19 (median age 70 years),(37) showed no statistically significance in clinical improvement after 28 days or reduced mortality. However, there was evidence of notable therapeutic effects and possible antiviral activity in group of 60 to 80-year-old patients, at the final stage of the disease course, after 14 days of symptoms, using only units with a very high antibody titers (IgG over 1:50) specific for spike (S)- and receptor-binding domain (RBD). Another study on CP therapy in severe COVID-19 patients(42) showed significant improvement of clinical symptoms, with an increase in oxyhemoglobin saturation after the third day of transfusion, reduction of pulmonary lesions, amelioration of routine laboratory criteria, and pulmonary function accompanied by rapid neutralization of viremia, using 200mL of CP derived from recently recovered donors with the neutralizing antibody titers between 1:160-640, approximately 16.5 days after onset of symptoms, associated to standard care and antiviral agents. Only three CTP(14,19,28) mentioned the antibody titers used in the CP therapy (over 1:160), and the studies by Abolghasemi et al.,(36) and Li et al.,(37) also reported the use of antibody titers above 1.1 in CP therapy associated with clinical improvement and reduction in mortality.
To reduce the variability in therapeutic response of patients, the WHO recommends some care and standardization in the selection of CP donors.(43) Eligibility criterion in relation to donor age does not vary widely: 18-67 years.(36,44) The donors were patients who recovered from COVID-19 and showed no detection of SARS-CoV-2 by real-time Quantitative polymerase chain reaction (qRT-PCR) or any related symptoms after a period that varied among studies. In one study, donors could have recovered after one week, and the short recovery period might have contributed to the death of 5 out of 6 patients.(45) Longer recovery period allowed reports of therapeutic efficacy. This period could be 10 days, with collection performed twice, with a difference of 24 hours,(46) at least 14 days,(36,47) and more than two weeks.(36,42,44) In some cases, qRT-PCR from nasopharyngeal swabs must be tested negative twice, and an interval of 24 hours between tests.(36,42)
Regarding the quantification of antibodies, S-RBD-specific IgG titers vary from donor to donor. One study demonstrated that ten out of 25 collected plasma displayed the titer of 1:450, 6/25 1:350, while in the others vary from 1:1 to 1:150.(44) Most of studies employed a CP volume of roughly 500mL, in a single dose or divided into two doses, derived from a single donor,(12,16,20,23) or two different donors.(12,16) Therefore, this lack of standardization regarding donor selection, quality control of the CP, and recipient patients could explain the varied therapeutic effects.
Some possible adverse effects with the use of CP can be avoided, such as CP free of antigens, which could cause transfusion-related acute lung injury (TRALI), such as human leukocyte antigens that protect the embryo.(36) In a multicenter clinical trial, the use of CP was not allowed in pregnant women, aiming to prevent TRALI.(36)
The severity of patient’s disease transfused with CP varied from mild, moderate, severe to critical. A CTP with severe patients divided the study groups in severe acute respiratory illness and severe community acquired pneumonia.(10) Another CTP compared the effects of the treatment in oncological and non-oncological COVID-19 patients.(27) Admission in the intensive care unit is related in some CTP,(11,18,28,30) although it is presumed that it is applied to all severe patients. In some CTP, the CP treatment was compared to other treatments, such as corticoids, antibiotics, monoclonal antibodies, and anti-viral drugs.(10,22,23,33)
Some published results allowed evaluation of different parameters concerning the efficacy of treatment. In a study in which CP was applied, 6 out of 17 patients required mechanical ventilation, mainly elderly patients.(47) In a multicenter study, the mortality rate was 14.8% of the patients (n=115).(36) Similar results were found in another multicenter study (15.7%).(36) Another investigation reported a mortality rate of only 2.2%.(38) A study employed this therapy in patients with hypertension, diabetes or cardiovascular disease, but it was not clear the effect of these comorbidities in the CP treatment effect.(36)
The transfusion of CP therapy for COVID-19 must follow some pre-established conditions, such as availability of a population of donors who have recovered from the disease and can donate convalescent serum; blood banks to process serum donations; availability of assays, including serological tests, to detect SARS-CoV-2 in serum and virological assays to measure viral neutralization; laboratory support for virology to carry out these tests; and standardization of phase and condition of COVID-19 patient.(48)
The main limitations of the multicenter studies were the reduced number of patients in the control groups compared to the treatment group, usually due to lack of blood group CP match, and concomitant or previous use of another treatment.(36) Another limitation is the lack of standard protocols and training for the study staff, as well as diversity in patients monitoring.(49) In turn, the main limitation of our study is the impossibility of carrying out a meta-analysis, due to the lack of a robust number of studies reporting conclusive therapeutic effects of this modality, such as decrease in SARS-CoV-2 titers. However, few articles published on multicenter studies demonstrated that CP could be a promising therapeutic modality.
CONCLUSION
Currently, there are no reliable therapeutic options for critically-ill COVID-19 patients. Based on the few consolidated multicenter clinical data results available, we concluded the convalescent plasma therapy studies provided relevant results in severe/critical cases of COVID-19 patients, reducing length of hospital stay, disease severity, and mortality, with low frequency of adverse events in a considerable number of patients. However, it is not possible to state, in a conclusive fashion, about the real relevance of this treatment, considering the lack of data that enable a robust statistics analysis, such as a meta-analysis.
ACKNOWLEDGEMENTS
Lionel Fernel Gamarra was supported by the Sistema Nacional de Laboratórios em Nanotecnologias (SisNANO) 2.0 (Conselho Nacional de Desenvolvimento Científico e Tecnológico/Ministério da Ciência, Tecnologia, Inovações e Comunicações, process 442539/2019-3).
REFERENCES
- 1.1. Ge H, Wang X, Yuan X, Xiao G, Wang C, Deng T, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020; 39(6):1011-9. [DOI] [PMC free article] [PubMed]
- 2.2. Worldometer. COVID-19 Pandemic Data Update 2020. COVID-19 Coronavirus pandemic. United States: Worldometer; 2020 [cited 2020 Oct 26]. [Last update posted Oct 26, 2020]. Available from: https://www.worldometers.info/coronavirus/
- 3.3. Worldmeter. COVID-19 Pandemic Data Update. 2020. Brazil: Worldometer; 2020 [cited 2020 Sep 18]. [Last update posted Sep 18, 2020]. Available from: https://www.worldometers.info/coronavirus/country/brazil/
- 4.4. Rego GN, Nucci MP, Alves AH, Oliveira FA, Marti LC, Nucci LP, et al. Current clinical trials protocols and the global effort for immunization against SARS-CoV-2. Vaccines (Basel). 2020;8(3):E474. Review. [DOI] [PMC free article] [PubMed]
- 5.5. Wong HK, Lee CK. Pivotal role of convalescent plasma in managing emerging infectious diseases. Vox Sang. 2020;115(7):545-7. [DOI] [PMC free article] [PubMed]
- 6.6. Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447-56. [DOI] [PMC free article] [PubMed]
- 7.7. Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357(14):1450-1. [DOI] [PubMed]
- 8.8. Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44-6. [DOI] [PMC free article] [PubMed]
- 9.9. Valk SJ, Piechotta V, Chai KL, Doree C, Monsef I, Wood EM, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;5(5):CD013600. Review. [DOI] [PMC free article] [PubMed]
- 10.10. ClinicalTrials.gov. Randomized, embedded, multifactorial adaptive platform trial for community- acquired pneumonia (REMAP-CAP). Bethesda: National Library of Medicine; 2016 [cited 2020 Oct 28]. [First posted Apr 13, 2016; Last update posted Oct 12, 2020]. Available from: https://ClinicalTrials.gov/show/NCT02735707
- 11.11. ClinicalTrials.gov. Convalescent plasma for patients with COVID-19: a pilot study. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted Apr 2, 2020; Last update posted Aug 17, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04332380
- 12.12. ClinicalTrials.gov. Efficacy of convalescent plasma therapy in the early care of COVID-19 patients (PLASCOSSA). Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted May 4, 2020; Last update posted Oct 26, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04372979
- 13.13. ClinicalTrials.gov. A trial of CONvalescent plasma for hospitalized adults with acute COVID-19 respiratory illness (CONCOR-1). Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted June 5, 2020; Last update posted July 7, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04418518
- 14.14. ClinicalTrials.gov. Convalescent plasma in outpatients with COVID-19 (C3PO). Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted Apr 21, 2020; Last update posted Sep 29, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04355767
- 15.15. ClinicalTrials.gov. Treatment of severe and critical COVID-19 pneumonia with convalescent plasma. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted June 16, 2020; Last update posted June 17, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04432103
- 16.16. ClinicalTrials.gov. CONvalescent plasma for hospitalized adults with COVID-19 respiratory illness (CONCOR-1). Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted Apr 16, 2020; Last update posted Oct 20, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04348656
- 17.17. ClinicalTrials.gov. Efficacy of convalescent plasma therapy in patients with COVID-19. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted June 11, 2020; Last update posted Sep 3, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04425915
- 18.18. ClinicalTrials.gov. Passive immunity trial of the nation for COVID-19 (PassItOnII). Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted Apr 24, 2020; Last update posted Aug 27, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04362176
- 19.19. ClinicalTrials.gov. Convalescent plasma vs human immunoglobulin to treat COVID-19 pneumonia. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted May 11, 2020; Last update posted May 12, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04381858
- 20.20. ClinicalTrials.gov. Evaluation of SARS-CoV-2 (COVID-19) antibody-containing plasma therapy (ESCAPE). Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted Apr 24, 2020; Last update posted May 18, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04361253
- 21.21. ClinicalTrials.gov. Convalescent plasma collection and treatment in pediatrics and adults. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted May 6, 2020; Last update posted May 6, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04376034
- 22.22. ClinicalTrials.gov. Australasian COVID-19 trial (ASCOT) [Internet]. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted July 23, 2020; Last update posted July 23, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04483960
- 23.23. ClinicalTrials.gov. Efficacy and safety of novel treatment options for adults with COVID-19 pneumonia. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted Apr 14, 2020; Last update posted July 31, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04345289
- 24.24. ClinicalTrials.gov. Convalescent plasma as therapy for Covid-19 severe SARS-CoV-2 disease (CONCOVID Study). Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted Apr 10, 2020; Last update posted May 18, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04342182
- 25.25. ClinicalTrials.gov. Safety and efficacy of convalescent plasma transfusion for patients with COVID-19 (EPCOvid-1). Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted May 14, 2020; Last update posted Aug 26, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04388410
- 26.26. ClinicalTrials.gov. Early transfusIon of convalescent plasma in elderly COVID-19 patients. to prevent disease progression. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted May 5, 2020; Last update posted Oct 6, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04374526
- 27.27. ClinicalTrials.gov. COVID19-convalescent plasma for treating patients with active symptomatic COVID 19 infection (FALP-COVID). Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted May 12, 2020; Last update posted May 12, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04384588
- 28.28. ClinicalTrials.gov. Effectiveness and safety of convalescent plasma in patients with high-risk COVID-19. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted June 11, 2020; Last update posted June 11, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04425837
- 29.29. ClinicalTrials.gov. Convalescent plasma (PC) and human intravenous anti-COVID-19 immunoglobulin (IV anti COVID-19 IgG) in patients hospitalized for COVID-19. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28] [First posted May 20, 2020; Last update posted July 10, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04395170
- 30.30. ClinicalTrials.gov. Effectiveness and safety of convalescent plasma therapy on COVID-19 patients with acute respiratory distress syndrome. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28] [First posted May 8, 2020; Last update posted Aug 18, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04380935
- 31.31. ClinicalTrials.gov. Convalescent plasma for patients with COVID-19: a randomized, open label, parallel, controlled clinical study. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted Apr 3, 2020; Last update posted Sep 2, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04332835
- 32.32. ClinicalTrials.gov. Hyperimmune plasma in patients with COVID-19 severe infection. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted May 12, 2020; Last update posted May 12, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04385043
- 33.33. ClinicalTrials.gov. Randomised evaluation of COVID-19 therapy. Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted May 11, 2020; Last update posted Sep 29, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04381936
- 34.34. Wadman M. Antivaccine forces gaining online. Science. 2020;368(6492):699. [DOI] [PubMed]
- 35.35. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed]
- 36.36. Abolghasemi H, Eshghi P, Cheraghali AM, Imani Fooladi AA, Bolouki Moghaddam F, Imanizadeh S, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apheresis Sci. 2020;59(5):102875. [DOI] [PMC free article] [PubMed]
- 37.37. Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: a Randomized Clinical Trial. JAMA. 2020;324(5):460-70. Erratum in: JAMA. 2020;324(5):519. [DOI] [PMC free article] [PubMed]
- 38.38. Xia X, Li K, Wu L, Wang Z, Zhu M, Huang B, et al. Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood. 2020;136(6):755-9. [DOI] [PMC free article] [PubMed]
- 39.39. ClinicalTrials.gov. Convalescent plasma for the treatment of severe SARS-CoV-2 (COVID-19). Bethesda: National Library of Medicine; 2020 [cited 2020 Oct 28]. [First posted May 18, 2020; Last update posted May 20, 2020]. Available from: https://ClinicalTrials.gov/show/NCT04391101
- 40.40. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexametasona em pacientes hospitalizados com Covid-19. N Engl J Med. 2021;384(8):693-704.
- 41.41. Estcourt LJ, Roberts DJ. Convalescent plasma for covid-19. BMJ. 2020; 370:m3516. [DOI] [PubMed]
- 42.42. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490-6. [DOI] [PMC free article] [PubMed]
- 43.43. World Health Organization (WHO). Blood products and related biologicals. Blood Regulators Network (BRN). Position paper on use of convalescent plasma, serum or immune globulin concentrates as an element in response to an emerging virus. Geneva: WHO; 2017 [cited 2020 Oct 26]. Available from: https://www.who.int/bloodproducts/brn/2017_BRN_PositionPaper_ConvalescentPlasma.pdf
- 44.44. Salazar E, Perez KK, Ashraf M, Chen J, Castillo B, Christensen PA, et al. Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-90. [DOI] [PMC free article] [PubMed]
- 45.45. Zeng QL, Yu ZJ, Gou JJ, Li GM, Ma SH, Zhang GF, et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020 Jun;222(1):38-43. [DOI] [PMC free article] [PubMed]
- 46.46. Olivares-Gazca JC, Priesca-Marín JM, Ojeda-Laguna M, Garces-Eisele J, Soto-Olvera S, Palacios-Alonso A, et al. Infusion of convalescent plasma is associated with clinical improvement in critically ill patients with covid-19: a pilot study. Rev Invest Clin. 2020;72(3):159-64. [DOI] [PubMed]
- 47.47. Erkurt MA, Sarici A, Berber İ, Kuku İ, Kaya E, Özgül M. Life-saving effect of convalescent plasma treatment in covid-19 disease: clinical trial from eastern Anatolia. Transfus Apheresis Sci. 2020;59(5):102867. [DOI] [PMC free article] [PubMed]
- 48.48. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545-8. [DOI] [PMC free article] [PubMed]
- 49.49. Joyner MJ, Wright RS, Fairweather D, Senefeld JW, Bruno KA, Klassen SA, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7. [DOI] [PMC free article] [PubMed]