1. Introduction
Pancreatic transplantation has become a standard of care for complicated type 1 diabetes therapy. In the United States in 2015, there were approximately 1000 patients awaiting pancreas transplant, with the percentage of active listings at 65%, the highest in decades.1 Solid organ pancreata can be transplanted individually, after a kidney transplant (pancreas-after-kidney [PAK]), or with a simultaneous pancreas-kidney (SPK) transplant. As diabetes is cited as a major, in- creasing public health burden,2 pancreas transplant has been recommended by the American Diabetes Association and other national guidelines as an accepted treatment, particularly when coupled with end-stage renal disease.3 Benefits to pancreas transplantation, SPK, and PAK are well described, having both improvements in mortality when compared to those on the waiting list4 and better overall glycemic control, reducing number of hypoglycemic episodes compared to those on insulin regimens.5 In addition, pancreas transplantation has been shown to delay secondary complications of diabetes, such as cardiovascular disease6 and nervous system complications.7
Despite these demonstrated advantages for pancreatic transplantation, only approximately 10% of available organs are recovered from donors after brain death (DBD). Additionally, there has been an overall decline in pancreatic transplantation over the past decade.8 In pancreatic transplantation, inconsistent donor management and organ acceptance practices are pervasive. Potentially contributing to this lack of consistency in donor management is the fact that the current risk-adjustment models used to predict both organ procurement organization (OPO) donor pancreas utilization and transplant center graft survival models lack detailed donor critical care data.
In an effort to increase standardization and data collection, several OPOs have collaborated to develop a checklist of critical care endpoints to guide the bedside care of potential organ donors. These endpoints are also known as donor management goals (DMGs), and they represent normal hemodynamic, respiratory, renal, acid-base, and endocrine parameters for an organ donor. Multiple studies have shown improvements in both organ utilization rates9–13 and recipient graft outcomes14–16 when these goals are met.
The link between optimal management of the potential organ donor after brain death (DBD) and pancreatic graft utilization and function has not yet been explored in the literature, and we sought to further elucidate this relationship using a deceased organ donor database containing demographic and critical care data at 4 time points during donor management. Given anecdotal reports of insulin requirements being used as criteria for pancreatic acceptance or denial, we also sought to determine the relationship between insulin dose and pancreatic usage and function.
2. Methods
2.1. Study Design
A prospective observational study of all donors after brain death (DBD) from 10 organ procurement organizations (OPOs) in United Network for Organ Sharing (UNOS) Regions 1,3, 4, 5, and 6 (covering Oregon, California, Nevada, Utah, New Mexico, Arizona, Georgia, Massachusetts, and Texas) was performed from July 2013 to September 2015 on 2862 DBDs. Among DBDs, only standard criteria donors (SCDs) were examined, as pancreata are rarely transplanted from expanded criteria donors (ECDs). After removing pediatric donors, donors involved in other studies, and donors with incomplete insulin data, the total number of donors analyzed was 1819. Of these 1819 donors, 238 pancreata were transplanted, for a transplantation rate of 13.0%, consistent with national averages.
2.2. Data Collection and Outcome Measures
Donor demographics and critical care data were collected prospectively through use of the UNOS Donor Management Goals Registry Web Portal (https://nationaldmg.org). These data were entered remotely by OPOs managing the donor and were collected at 4 standardized time points: at the time of referral, authorization for donation, allocation of organs for transplantation, and prior to leaving the intensive care unit for organ recovery. There are nine critical care endpoints in the DMG Bundle. The current DMGs utilized by participating OPOs are listed in Table 1. The Bundle is considered “Met” when any 7 of the 9 parameters are achieved. When a value is not recorded or present, it is counted as “Not Met.” Critical care values are recoded over the course of donor management, and Figure 1 illustrates the general timeline of a potential organ donor in the ICU, delineating when the DMGs are measured. The process begins with a neurologic injury, at which point, OPOs are often contacted and a referral is made to the OPO for imminent brain death when clinical triggers are met. If regression to brain death occurs, the patient’s family is approached by the OPO for authorization for donation, and if this happens, the OPO takes over management of the donor after he or she has been determined brain dead. Approximately 12-18 hours later, organ offers are being made, and this time point is noted as “Allocation.” Finally, on call to the operating room for organ recovery, terminal values are recorded, and this time point is noted as “Prior to OR” These “Prior to OR” measurements are often taken on call to the OR for organ recovery, and usually less than an hour passes be- tween the recording of these terminal values and actual start of the case. The entire process of donor management by the OPO occurs from “Authorization” through “Prior to OR”.
TABLE 1.
Donor management goals
| Donor management goal | Parameter |
|---|---|
| Mean arterial pressure (mm Hg) | 60–110 |
| Central venous pressure (mm Hg) | 4–12 |
| Left ventricular ejection fraction (%) | ≥50 |
| Low-dose vasopressors, numbera | ≤1 and low-dose |
| Arterial blood gas, pH | 7.3–7.5 |
| PaO2:FiO2 ratio | ≥300 |
| Serum sodium (mEq/L) | ≤155 |
| Urine output (mL/kg/h over 4 h) | ≥0.5 |
| Glucose (mg/dL) | ≤180 |
| Bundle Metb | Any 7/9 |
Abbreviation(s): FiO2, fraction of inspired oxygen; PaO2 partial pressure of oxygen.
“Low-dose vasopressors” is defined as dopamine ≤ 10 μg/kg/min, phenylephrine ≤ 1 μg/kg/min, or norepinephrine ≤ 0.2 μg/kg/min
“Bundle Met” is defined as meeting any 7 of the 9 DMGs.
Figure 1:
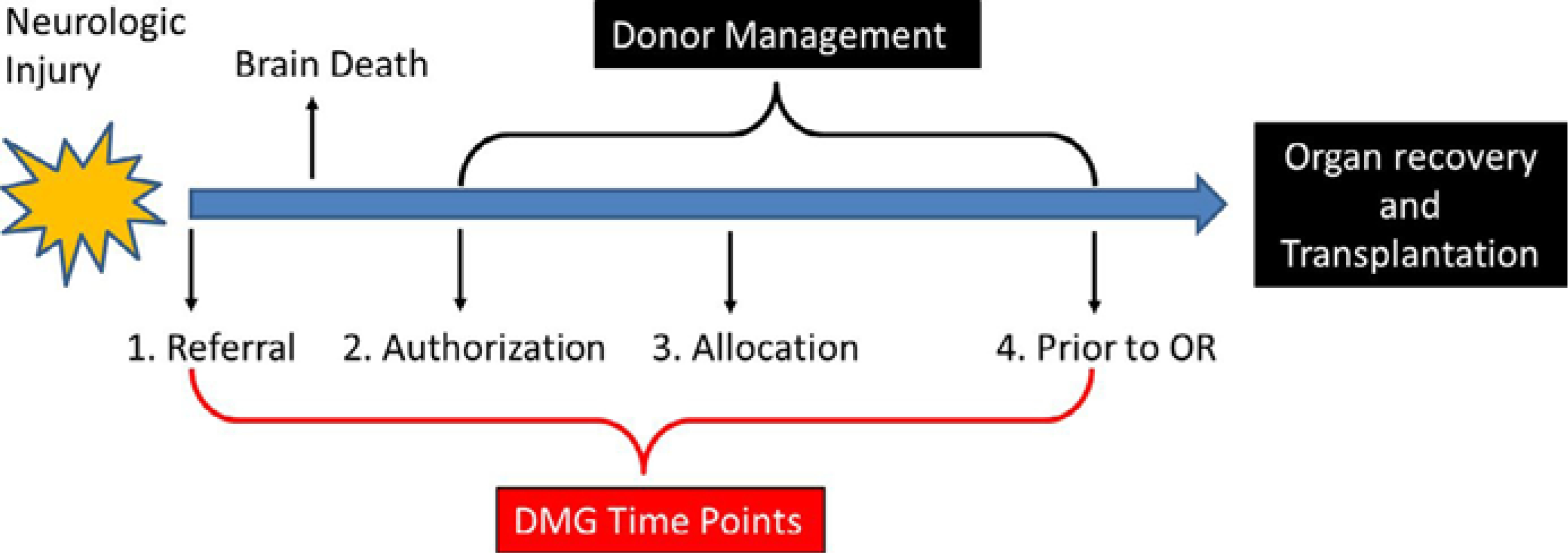
Donor management timeline
There were two primary outcomes for this study, pancreatic utilization/transplantation and recipient graft survival. Independent predictors of each outcome measure were determined using the statistical methods described in the next section. We hypothesized that higher insulin doses in the donor would predict decreased pancreas graft utilization, but these higher doses would not affect recipient graft survival.
2.3. Statistical Analysis
A two-part analysis was performed to identify predictors of pancreatic transplantation. First, a univariate analysis was conducted to assess demographic and critical care elements associated with pancreas graft acceptance for transplantation. Categorical variables were analyzed using Pearson’s chi-square test, and continuous variables were analyzed using independent samples t test.
Variables with a P < 0.05 on univariate analysis were then included in multivariable logistic regression models to determine independent predictors of utilization. For multivariable analysis, when multiple time points were significant on univariate analysis, time points closest to Allocation were preferentially used, given that this is when organ offers are being made and accepted. In addition, categorical data were preferentially used over continuous variable data, as it produces more relatable odds ratios. Lastly, inherently related values were run in separate models. For this study, we ran two multivariable analyses for both utilization and survival, one that contained the individual DMG elements and one which contained the variable “Bundle Met,” defined as meeting seven of the nine individual DMG elements. Variables with a P < 0.05 on multivariable analysis were considered independent predictors of pancreatic transplantation.
To determine independent predictors of pancreas graft survival, we also performed univariate and multivariate analyses. Only donors whose pancreata were accepted for transplantation were included in these analyses. Again, for multivariable analysis, categorical data were preferentially used over continuous variable data, time points closest to Allocation were used, given this being the closest time point to when organ offers are being made, and inherently related values were run in separate models. For those variables with a P < 0.05 on univariate analysis, Cox regression analysis were performed to deter- mine independent predictors of pancreatic graft survival.
Statistical analysis was performed with Stata version 25.0 for Windows (StataCorp). Within the text and tables, values are reported as mean ± standard deviation (SD) or percent frequency (%) unless stated otherwise.
3. Results
There were 1819 DBDs with complete data that met all of the inclusion criteria. A total of 238 pancreatic transplants were performed, for a transplantation rate of 13.1%. A total of 198 of these pancreatic transplants were simultaneous pancreas-kidney (SPK) and 40 were either pancreas-after-kidney (PAK) transplants or pancreas trans- plant alone (PTA). The average age of donors was 37.0 ± 11.6 years and 63% were male. For the 238 pancreata transplanted, survival rate was 91.6% with mean time to follow up of 192 ± 156 days. The mean number of OTPD for donors with a pancreas transplanted was 5.9 ± 1.1, and for those donors that did not have a pancreas trans- planted, the mean OTPD was 3.4 ± 1.6 (P < 0.001).
Univariate analysis of categorical donor variables associated with pancreatic transplantation is displayed in Table 2. This table displays the percentages of pancreata transplanted when DMG elements are met or not met. Achieving the DMGs for ejection fraction, pH, PaO2:FiO2 ratio, urine output, and low-dose vasopressors at various time points were significantly associated with pancreatic transplantation. Additionally, the Bundle being met at all time points was also significantly associated with pancreas graft utilization. Regarding demographic variables, cause of death being anoxia was the only notable donor characteristic associated with pancreatic transplantation. Table 3 displays continuous donor variables associated with pancreatic transplantation. Of note, lower donor age, weight, BMI, HgbA1c, lactate level, serum glucose, and insulin dose were associated with pancreatic trans- plantation on univariate analysis.
TABLE 2.
Proportion of pancreata transplanted when donor categorical variables were met or not met (n = 1819)
| Demographics | Met | Not met | P | |||||||||
| % Pancreata transplanted (n = 238) when demographic categorical variable met/not met | ||||||||||||
| Male gender | 14.0 | 11.6 | 0.15 | |||||||||
| Blood type O | 14.2 | 12.0 | 0.17 | |||||||||
| Cause of death (anoxia) | 9.2 | 15.1 | <0.001 | |||||||||
| Referral | Authorization | Allocation | Prior to OR | |||||||||
| DMG variable | Met | Not met | P | Met | Not met | P | Met | Not met | P | Met | Not met | P |
| % pancreata transplanted (n = 238) when DMG categorical variable met/not met | ||||||||||||
| MAP 60–110 mm Hg | 12.9 | 14.0 | 0.62 | 12.8 | 16.4 | 0.20 | 13.2 | 12.3 | 0.77 | 13.0 | 14.9 | 0.55 |
| CVP 4–12 mm Hg | 15.9 | 12.8 | 0.30 | 18.1 | 11.4 | <0.001 | 14.9 | 11.5 | 0.03 | 16.4 | 10.0 | <0.001 |
| EF ≥ 50% | 4.2 | 14.1 | <0.001 | 9.6 | 13.8 | 0.049 | 14.8 | 11.6 | 0.04 | 15.0 | 8.9 | <0.001 |
| ABG pH 7.3–7.5 | 13.3 | 12.8 | 0.74 | 13.8 | 10.8 | 0.11 | 12.9 | 8.2 | 0.01 | 13.8 | 6.1 | 0.003 |
| PaO2:FiO2 ratio ≥ 300 | 16.4 | 10.6 | <0.001 | 16.8 | 10.4 | <0.001 | 18.1 | 8.6 | <0.001 | 18.0 | 6.4 | <0.001 |
| Sodium ≤ 155 mEq/L | 13.1 | 12.9 | 0.95 | 13.2 | 12.8 | 0.84 | 13.3 | 12.2 | 0.55 | 12.9 | 13.8 | 0.68 |
| Glucose ≤ 180 mg/dL | 12.2 | 14.2 | 0.21 | 14.0 | 10.2 | 0.04 | 14.8 | 9.6 | 0.002 | 14.3 | 8.6 | 0.003 |
| UOP ≥ 0.5 mL/kg/h | 14.0 | 8.3 | 0.01 | 14.8 | 5.0 | <0.001 | 14.9 | 4.9 | <0.001 | 14.3 | 7.8 | 0.002 |
| Low-dose vasopressors | 12.9 | 13.5 | 0.76 | 11.9 | 15.2 | 0.047 | 13.7 | 9.6 | 0.06 | 13.7 | 6.7 | 0.02 |
| DMG Bundle Met | 13.0 | 7.3 | 0.21 | 18.1 | 11.7 | <0.001 | 18.5 | 8.3 | <0.001 | 16.5 | 6.4 | <0.001 |
Note: “Low-dose vasopressors” is defined as ≤ 1 and dopamine ≤ 10 μg/kg/min, phenylephrine ≤ 1 μg/kg/min, or norepinephrine ≤ 0.2 μg/kg/min.
Bold, used in multivariable model.
Abbreviation(s): ABG, arterial blood gas; CVP, central venous pressure; DMG, donor management goal; EF, ejection fraction; FiO2, fraction of inspired oxygen; MAP, mean arterial pressure; PaO2, partial pressure of oxygen; UOP, urine output.
TABLE 3.
Continuous donor variables associated with pancreatic utilization (n = 1819), stratified by time point
| Referral |
Authorization |
Allocation |
Prior to OR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Transplanted (n = 238) | Not transplanted (n = 1581) | P | Transplanted (n = 238] | Not transplanted (n = 1581) | P | Transplanted (n = 238) | Not transplanted (n = 1581) | P | Transplanted (n = 238) | Not transplanted (n = 1581) | P |
| Demographics | ||||||||||||
| Age (y) | 27 ± 6 | 39 ± 11 | <0.001 | – | – | – | – | – | – | – | – | – |
| Weight (kg) | 73 ± 15 | 84 ± 22 | <0.001 | – | – | – | – | – | – | – | – | – |
| Height (cm) | 173 ± 10 | 171 ± 10 | 0.011 | – | – | – | – | – | – | – | – | – |
| BMI (kg/m2) | 24 ± 4 | 29 ± 7 | <0.001 | – | – | – | – | – | – | – | – | – |
| HgbA1c (%) | 5.3 ± 0.4 | 5.8 ± 1.4 | <0.001 | – | – | – | – | – | – | – | – | – |
| Lipase (U/L) | – | – | – | – | – | – | – | – | – | 78 ± 164 | 83 ± 137 | 0.78 |
| Amylase (U/L) | – | – | – | – | – | – | – | – | – | 115 ± 140 | 145 ± 168 | 0.03 |
| ICU laboratory values and treatments | ||||||||||||
| Lactate (mmol) | 5.1 ± 4.3 | 7.2 ± 11.3 | 0.01 | 1.9 ± 1.1 | 2.8 ± 4.0 | <0.001 | 1.9 ± 1.1 | 2.8 ± 4.0 | 0.28 | 1.8 ± 2.8 | 2.4 ± 4.5 | 0.18 |
| Creatinine (mg/dL) | 1.0 ± 0.6 | 1.4 ± 1.4 | <0.001 | 1.0 ± 0.5 | 1.7 ± 1.7 | <0.001 | 1.0 ± 0.8 | 1.7 ± 1.8 | <0.001 | 1.0 ± 0.6 | 1.8 ± 1.9 | <0.001 |
| Insulin (units/h) | – | – | – | – | – | – | 1.4 ± 2.0 | 2.1 ± 3.8 | <0.001 | 2.7 ± 6.2 | 3.5 ± 5.6 | 0.03 |
| DMGs | ||||||||||||
| MAP (mm Hg) | 86 ± 20 | 85 ± 20 | 0.47 | 85 ± 16 | 84 ± 16 | 0.23 | 86 ± 16 | 88 ± 16 | 0.09 | 87 ± 16 | 89 ± 14 | 0.06 |
| CVP (mm Hg) | 7.5 ± 3.5 | 8.8 ± 4.2 | 0.11 | 9.2 ± 5.5 | 7.8 ± 3.7 | 0.02 | 8.5 ± 3.7 | 9.8 ± 4.7 | 0.001 | 8.8 ± 3.1 | 10.1 ± 5.2 | <0.001 |
| EF (%) | 49 ± 16 | 52 ± 17 | 0.49 | 52 ± 14 | 53 ± 16 | 0.73 | 57 ± 12 | 58 ± 13 | 0.69 | 59 ± 10 | 59 ± 11 | 0.40 |
| ABG, pH | 7.3 ± 0.2 | 7.3 ± 0.1 | 0.12 | 7.4 ± 0.1 | 7.4 ± 0.1 | 0.85 | 7.4 ± 0.1 | 7.4 ± 0.1 | 0.95 | 7.4 ± 0.1 | 7.4 ± 0.1 | 0.92 |
| PaO2:FiO2 ratio | 313 ± 158 | 274 ± 146 | 0.001 | 310 ± 134 | 267 ± 134 | <0.001 | 347 ± 121 | 284 ± 131 | <0.001 | 397 ± 133 | 393 ± 141 | <0.001 |
| Sodium (mEq/L) | 143 ± 7 | 143 ± 8 | 0.92 | 150 ± 11 | 150 ± 10 | 0.62 | 147 ± 10 | 148 ± 9 | 0.05 | 148 ± 8 | 148 ± 8 | 0.83 |
| Glucose (mg/dL) | 195 ± 89 | 194 ± 95 | 0.86 | 151 ± 59 | 158 ± 76 | 0.11 | 156 ± 45 | 170 ± 57 | <0.001 | 149 ± 33 | 159 ± 55 | <0.001 |
| UOP (mL/kg/h) | 3.1 ± 3.0 | 2.3 ± 2.0 | <0.001 | 2.5 ± 2.2 | 1.7 ± 1.7 | <0.001 | 2.3 ± 1.9 | 1.8 ± 1.8 | <0.001 | 2.2 ± 2.2 | 1.7 ± 1.6 | 0.01 |
| Low-dose vasopressorsa, No. | 0.5 ± 0.7 | 0.6 ± 00 | 0.41 | 0.8 ± 0.8 | 0.8 ± 0.7 | 0.95 | 0.3 ± 0.6 | 0.5 ± 0.6 | 0.003 | 0.1 ± 0.3 | 0.3 ± 0.5 | <0.001 |
| Total DMG | 5.2 ± 1.4 | 5.1 ± 1.3 | 0.40 | 5.7 ± 1.4 | 5.4 ± 1.3 | <0.001 | 6.9 ± 1.2 | 6.2 ± 1.4 | <0.001 | 7.6 ± 1.0 | 6.9 ± 1.4 | <0.001 |
Note: Bold, used in multivariable model. Values expressed as Mean ± Standard Deviation.
Abbreviation(s): ABG, arterial blood gas; BMI, body mass index; CVP, central venous pressure; DMG, donor management goal; EF, ejection fraction; FiO2, fraction of inspired oxygen; HgA1c, hemoglobin A1C; MAP, mean arterial pressure; PaO2, partial pressure of oxygen; UOP, urine output.
“Low-dose vasopressors” is defined as dopamine ≤ 10 μg/kg/min, phenylephrine ≤ 1 μg/kg/min, or norepinephrine ≤ 0.2 μg/kg/min.
HgbA1c measurements were further broken into quartiles and analyzed for their effects on pancreatic transplantation. Quartile 1 (≤5.2%) and quartile 2 (5.3%-5.4%) showed levels associated with pancreatic usage, consistent with a mean value for acceptance of 5.3% (Table 3). If the donor’s HgbA1c fell within quartiles 3 (5.5%-5.7%) and 4 (≥5.8%), there was an association with not trans- planting the pancreas. These results can be seen in Figure 2.
Figure 2:
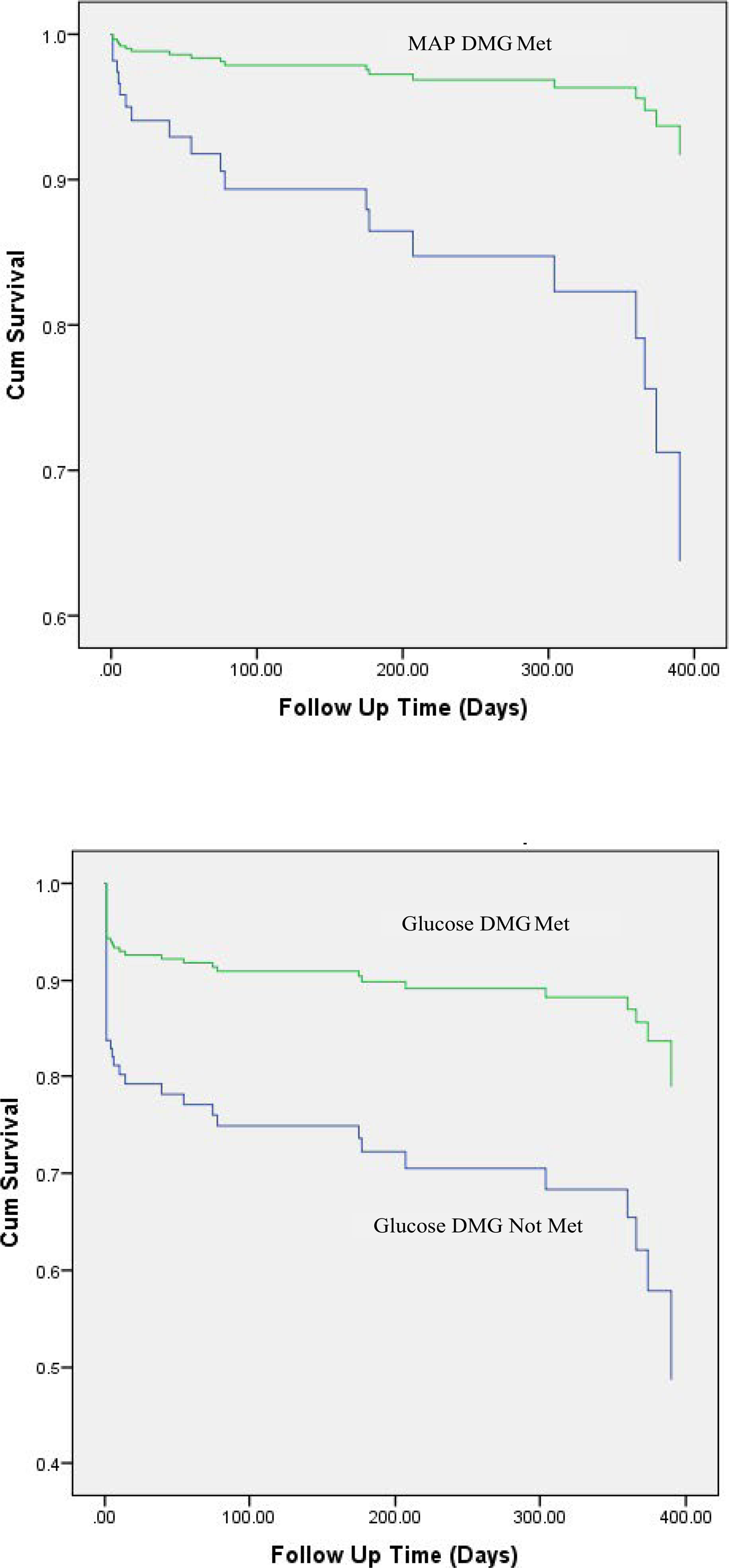
Kaplan-Meir curves for pancreatic graft survival, stratified for independent predictors of survival
We then used multivariable regression to look for independent predictors of pancreatic transplantation. On this analysis, age, HgbA1c, and an EF ≥ 50% at allocation all proved to be independent predictors of pancreatic utilization. Results of multivariable regression are displayed in Table 4. Though trending toward significance, insulin use was not independently predictive of pancreatic transplantation.
TABLE 4.
Predictors of pancreatic utilization using multivariable logistic regression
| Variable | Odds ratio | 95% confidence interval | P |
|---|---|---|---|
| Age/y | 0.89 | 0.83–0.95 | <0.001 |
| BMI (per kg/m2) | 1.02 | 0.93–1.11 | 0.72 |
| HgbA1c (per %) | 0.07 | 0.01–0.34 | 0.001 |
| Creatinine (per mg/dL) | 0.43 | 0.040–0.68 | 0.53 |
| Amylase (per U/L) | 1.00 | 0.11–1.09 | 0.11 |
| Lactate (per mmol)* | 0.93 | 0.67–1.30 | 0.69 |
| Insulin (per units/h) | 0.70 | 0.49–1.01 | 0.06 |
| COD Anoxia | 0.56 | 0.20–1.59 | 0.27 |
| Donor management goals achieved at allocation | |||
| CVP 4–12 mm Hg | 0.76 | 0.26–2.24 | 0.61 |
| LVEF ≥ 50% | 3.29 | 1.10–9.85 | 0.03 |
| ABG, pH 7.3–7.5 | 1.56 | 0.24–10.33 | 0.65 |
| PaO2:FiO2 ratio ≥ 300 | 0.89 | 0.32–2.50 | 0.82 |
| Glucose ≤ 180 mg/dL | 1.50 | 0.51–4.40 | 0.47 |
| UOP ≥ 0.5 mL/kg/h for 4 h | 5.33 | 0.48–58.7 | 0.17 |
Note: Hosmer and Lemeshow = 0.83, 0.56
“Low-dose vasopressors” is defined as one or fewer of the following: dopamine ≤ 10 μg/kg/min, phenylephrine ≤ 1 μg/kg/min, or norepinephrine ≤ 0.2 μg/kg/min; “Bundle Met” is defined as meeting any 7 of the 9 DMGs.
Abbreviation(s): ABG, arterial blood gas; BMI, body mass index; COD, cause of death; CVP, central venous pressure; FiO2, fraction of inspired oxygen; LVEF, left ventricular ejection fraction; PaO2, partial pressure of oxygen; UOP, urine output.
Met at authorization rather than allocation;
Separate models run for inherently related variables (DMG Bundle in one model, individual elements in another model).
For graft survival, categorical elements associated with graft survival on univariate analysis were achieving the MAP DMG at referral as well as achieving the EF and glucose DMGs prior to organ recovery (Table 5). For continuous donor demographic elements, lower age and lower hemoglobin A1C were associated with survival of pancreatic grafts. In addition, values for MAP and pH at referral were noted to be associated on univariate analysis (Table 6). When analyzing continuous and categorical data with a Cox multivariable regression model, lower age, achieving the MAP DMG at the referral time point, and achieving the glucose DMG at the prior to organ recovery time point were all found to be independent predictors of pancreatic graft survival (Table 7). When stratifying transplanted pancreata by binary independent predictors, we can see the survival of the pancreatic grafts as a function of meeting the MAP DMG at the referral time point, and the glucose DMG at the prior to organ recovery time point in Figure 3.
TABLE 5.
Association of categorical variables with proportion of pancreas grafts functioning at most recent follow-up (n = 238, mean duration of follow-up = 192 ± 156 d)
| Demographics | Met | Not met | P | |||||||||
| % Pancreata survival (n = 218) when demographic variable met/not met | ||||||||||||
| Blood type O | 81.3 | 89.1 | 0.03 | |||||||||
| Male gender | 83.1 | 88.5 | 0.28 | |||||||||
| Cause of death (anoxia) | 87.5 | 84.1 | 0.53 | |||||||||
| Referral | Authorization | Allocation | Prior to OR | |||||||||
| DMG variable | Met | Not met | P | Met | Not met | P | Met | Not met | P | Met | Not met | P |
| % Pancreata survival (n = 218) when DMG variable met/not met | ||||||||||||
| MAP 60–110 mm Hg | 87.4 | 72.5 | 0.02 | 84.9 | 84.6 | 0.97 | 85.4 | 78.9 | 0.45 | 84.1 | 94.4 | 0.24 |
| CVP 4–12 mm Hg | 87.0 | 84.7 | 0.77 | 86.9 | 83.8 | 0.52 | 86.7 | 82.7 | 0.39 | 88.9 | 78.7 | 0.03 |
| EF ≥ 50% | 62.5 | 85.7 | 0.07 | 79.3 | 85.6 | 0.37 | 86.5 | 83.0 | 0.46 | 84.0 | 88.2 | 0.45 |
| ABG pH 7.3–7.5 | 82.6 | 88.8 | 0.20 | 86.3 | 79.2 | 0.22 | 83.9 | 95.2 | 0.17 | 84.1 | 100 | 0.15 |
| PaO2:FiO2 ratio ≥ 300 | 83.7 | 86.2 | 0.59 | 83.5 | 86.5 | 0.52 | 82.6 | 89.2 | 0.18 | 84.7 | 85.7 | 0.85 |
| Sodium ≤ 155 mEq/L | 84.2 | 93.8 | 0.31 | 85.1 | 84.4 | 0.90 | 83.9 | 89.1 | 0.37 | 85.4 | 82.5 | 0.65 |
| Glucose ≤ 180 mg/dL | 81.3 | 88.7 | 0.11 | 85.1 | 83.7 | 0.82 | 84.5 | 86.0 | 0.79 | 87.6 | 70.0 | 0.03 |
| UOP ≥ 0.5 mL/kg/h | 84.6 | 87.5 | 0.71 | 85.1 | 81.3 | 0.68 | 84.7 | 87.5 | 0.76 | 84.4 | 88.5 | 0.59 |
| Low-dose vasopressors | 83.2 | 89.2 | 0.25 | 82.3 | 88.7 | 0.18 | 84.9 | 84.6 | 0.97 | 85.1 | 80.0 | 0.66 |
| DMG Bundle Met | 76.1 | 87.0 | 0.06 | 86.1 | 84.3 | 0.73 | 84.2 | 86.3 | 0.67 | 83.5 | 80.0 | 0.69 |
Note: “Low-dose vasopressors” is defined as ≤ 1 and dopamine ≤ 10 μg/kg/min, phenylephrine ≤ 1 μg/kg/min, or norepinephrine ≤ 0.2 μg/kg/min; DMG, donor management goal; Bold, used in multivariable model.
Abbreviation(s): ABG, arterial blood gas; CVP, central venous pressure; EF, ejection fraction; Fio2, fraction of inspired oxygen; MAP, mean arterial pressure; PaO2, partial pressure of oxygen; UOP, urine output.
TABLE 6.
Association of continuous variables with proportion of pancreas grafts functioning at most recent follow-up (n = 238, mean duration of follow-up = 192 ± 156 d)
| Referral |
Authorization |
Allocation |
Prior to OR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Graft survival (n = 218) | Graft failure (n = 20) | P | Graft survival (n = 218) | Graft failure (n = 20) | P | Graft survival (n = 218) | Graft failure (n = 20) | P | Graft survival (n = 218) | Graft failure (n = 20) | P |
| Demographics | ||||||||||||
| Age (y) | 26 ± 6 | 32 ± 11 | 0.03 | – | – | – | – | – | – | – | – | – |
| Weight (kg) | 73 ± 15 | 71 ± 14 | 0.66 | – | – | – | – | – | – | – | – | – |
| Height (cm) | 173 ± 10 | 171 ± 11 | 0.59 | – | – | – | – | – | – | – | – | – |
| BMI (kg/m2) | 24 ± 4 | 24 ± 4 | 0.94 | – | – | – | – | – | – | – | – | – |
| HgbA1c (%) | 5.3 ± 0.4 | 5.1 ± 0.2 | 0.18 | – | – | – | – | – | – | – | – | – |
| Lipase (U/L) | – | – | – | – | – | – | – | – | – | 5.3 ± 0.4 | 5.1 ± 0.2 | 0.18 |
| Amylase (U/L) | – | – | – | – | – | – | – | – | – | 79 ± 165 | 81 ± 154 | 0.94 |
| ICU laboratory values and treatments | ||||||||||||
| Lactate (mmol) | 4.9 ± 4.2 | 6.4 ± 6.0 | 0.47 | 1.8 ± 1.1 | 2.3 ± 1.6 | 0.27 | 2.5 ± 4.1 | 2.8 ± 2.3 | 0.77 | 1.6 ± 1.9 | 3.9 ± 7.4 | 0.35 |
| Creatinine (mg/dL) | 1.0 ± 0.7 | 1.0 ± 0.2 | 0.54 | 1.0 ± 0.5 | 0.9 ± 0.3 | 0.57 | 1.0 ± 0.8 | 1.0 ± 0.6 | 0.93 | 1.0 ± 0.6 | 1.1 ± 0.5 | 0.55 |
| Insulin (units/h) | – | – | – | – | – | – | 1.4 ± 2.1 | 1.3 ± 1.2 | 0.69 | 2.7 ± 6.4 | 1.9 ± 2.1 | 0.61 |
| DMGs | ||||||||||||
| MAP (mm Hg) | 85 ± 17 | 86 ± 15 | 0.74 | 85 ± 16 | 86 ± 15 | 0.78 | 86 ± 16 | 88 ± 17 | 0.63 | 86 ± 12 | 87 ± 16 | 0.75 |
| CVP (mm Hg) | 7.7 ± 4.0 | 8.1 ± 2.9 | 0.81 | 7.8 ± 3.8 | 8.0 ± 2.7 | 0.89 | 8.6 ± 3.7 | 7.3 ± 3.5 | 0.25 | 8.9 ± 3.0 | 7.6 ± 4.0 | 0.16 |
| EF (%) | 47 ± 16 | 65 ± 10 | 0.34 | 51 ± 14 | 65 ± 10 | <0.001 | 54 ± 12 | 57 ± 15 | 0.98 | 58 ± 11 | 60 ± 8 | 0.59 |
| ABG, pH | 7.3 ± 0.2 | 7.4 ± 0.1 | 0.03 | 7.4 ± 0.1 | 7.4 ± 0.1 | 0.83 | 7.4 ± 0.1 | 7.4 ± 0.1 | 0.99 | 7.4 ± 0.1 | 7.4 ± 0.05 | 0.75 |
| PaO2:FiO2 ratio | 310 ± 161 | 344 ± 123 | 0.26 | 311 ± 134 | 302 ± 132 | 0.78 | 344 ± 122 | 369 ± 105 | 0.39 | 400 ± 135 | 379 ± 109 | 0.50 |
| Sodium (mEq/L) | 143 ± 7 | 140 ± 3 | 0.001 | 150 ± 11 | 149 ± 9 | 0.81 | 147 ± 10 | 146 ± 8 | 0.46 | 148 ± 8 | 147 ± 7 | 0.76 |
| Glucose (mg/dL) | 196 ± 92 | 185 ± 57 | 0.60 | 151 ± 59 | 141 ± 56 | 0.45 | 156 ± 44 | 152 ± 46 | 0.69 | 147 ± 32 | 162 ± 41 | 0.12 |
| UOP (mL/kg/h) | 3.0 ± 3.1 | 3.9 ± 2.3 | 0.21 | 2.5 ± 2.3 | 2.3 ± 1.4 | 0.69 | 2.3 ± 1.9 | 2.5 ± 2.0 | 0.69 | 2.2 ± 2.2 | 2.9 ± 2.2 | 0.17 |
| Low-dose vasopressorsa, No. | 0.6 ± 0.7 | 0.3 ± 0.6 | 0.13 | 0.8 ± 0.8 | 0.7 ± 0.7 | 0.64 | 0.3 ± 0.6 | 0.3 ± 0.4 | 0.49 | 0.1 ± 0.3 | 0.1 ± 0.2 | 0.16 |
| Total DMG | 5.2 ± 1.4 | 5.6 ± 1.3 | 0.24 | 5.7 ± 1.4 | 5.8 ± 1.3 | 0.93 | 6.9 ± 1.3 | 7.0 ± 1.1 | 0.83 | 7.6 ± 1.1 | 7.7 ± 1.3 | 0.85 |
Note: Bold, used in multivariable model. Values expressed as Mean ± Standard Deviation.
Abbreviation(s): ABG, arterial blood gas; BMI, body mass index; CVP, central venous pressure; DMG, donor management goal; EF, ejection fraction; FiO2, fraction of inspired oxygen; HgA1c, hemoglobin A1C; MAP, mean arterial pressure; PaO2, partial pressure of oxygen; UOP, urine output.
“Low-dose vasopressors” is defined as dopamine ≤ 10 μg/kg/min, phenylephrine ≤ 1 μg/kg/min, or norepinephrine ≤ 0.2 μg/kg/min.
TABLE 7.
Cox regression analysis for pancreas graft survival (n = 238, mean duration of follow-up = 192 ± 156 d)
| Hazard ratio | 95% confidence interval | P | |
|---|---|---|---|
| Variable | |||
| Age/y | 0.93 | 1.03–1.12 | <0.001 |
| Blood type O | 0.78 | 0.46–3.67 | 0.64 |
| Referral ABG, pH (per mol/L) | 0.45 | 0.65–4.23 | 0.10 |
| Referral sodium (per mEq/L) | 1.08 | 0.85–1.16 | 0.11 |
| Referral MAP 60–110 mm Hg | 0.28 | 1.67–7.46 | 0.001 |
| Prior to OR CVP 4–12 mm Hg | 0.55 | 0.30–1.91 | 0.78 |
| Prior to OR glucose ≤ 180 mg/dL | 0.27 | 1.79–7.81 | <0.001 |
Note: AUC: 0.76.
“DMG Bundle Met” is defined as meeting 7 of the 9 DMGs.
Abbreviation(s): CVP, central venous pressure; DMG, donor management goal; MAP, mean arterial pressure; OR, organ recovery.
Figure 3:
HgbA1c quartiles and associations with pancreatic transplantation. Percentages expressed as percentage of total pancreata transplanted (n = 238) or not transplanted (n= 1581)
4. Discussion
Transplantation of the pancreas is a common therapeutic procedure for complicated type 1 diabetes, although its frequency has been declining over the last decade. Transplantation of islet cells has likely contributed to this decline; however, several studies have shown superior results with simultaneous pancreas kidney transplant or pancreas after kidney transplants.17 This study aimed to address potential modifiable donor variables that could affect transplantation rates as well as graft outcomes in order to offer potential steps for intervention to improve pancreatic transplantation. We found that lower donor age, lower donor HgbA1c levels, and achieving the EF DMG at allocation were independent predictors of pancreatic transplantation. After analyzing data from pancreata that had been transplanted, we found that achieving the DMG for MAP at the referral time point and the DMG for glucose at the prior to organ recovery time point were independent predictors of graft survival. Of note, insulin dose was neither a predictor of pancreas graft utilization nor survival.
Multiple studies have shown that aggressive donor management can result in improved outcomes, in terms of both organ yield and organ function.9–11,14,18 Both kidney14 and hepatic16 grafts have been studied and analyzed for predictors of usage and function, and we have attempted to expand this work to help identify modifiable fac- tors in the donor that affect pancreas graft acceptance as well as survival in the recipient. With just over 1000 pancreas and kidney/ pancreas transplants performed in 2017 per OPTN data, it is import- ant to both optimize the number of available organs for transplant and create an environment in which good critical care can improve outcomes from these rare grafts.
Predicting graft utilization, especially in relatively rare pancreatic grafts, may be difficult and subject to many biases from varied sources. In 2010, the pancreas donor risk index (PDRI) was developed as a way to identify primarily donor factors associated with pancreatic allograft failure and as a way to inform organ acceptance practice.19 It consists of 8 donor factors (age, sex, race, height, BMI, serum creatinine, cause of death, and donor being of DCDD status) and 2 transplant factors (including CIT and type of transplant—SPK, PAK, or PTA). We chose not to examine the PDRI for this population, as many of the factors used in the PDRI calculation were already included in our analysis, where we focused on potentially modifiable donor factors. Additionally, not all transplant centers use this metric in evaluating pancreatic donors and other pretransplant screening tools such as Pre-Procurement Pancreas Allocation Suitability Score (P-PASS), which contains the variables of age, BMI, intensive care unit stay, preexistent cardiac arrest, serum sodium, serum amylase or lipase, and the use of vasopressive agents, have been found to be advantageous in certain populations.20 Other composite models containing many of these variables have been studied21; however, we chose not to introduce a new scoring system for these grafts. In addition, due to the fact that OPOs do not have agency over the critical care of DCDDs, our study was limited to brain-dead donors, which would make the PDRI model less relevant for this group of donors. In this data set, precise or individual reasons for an organ being accepted or declined for transplantation by transplant centers are unknown; however, we have attempted to identify donor factors which may affect these decisions. Unmodifiable donor factors that we found as independent predictors of pancreatic utilization were donor age and hemoglobin A1C. The only modifiable donor critical parameter that predicted pancreas graft utilization was an EF ≥ 50% at organ allocation, and we believe this result to be due to the fact that pancreata are typically accepted from the youngest and healthiest subset of donors.
Defining pancreatic graft failure has gone through multiple evolutions throughout the brief history of the therapy. Typically, graft failure is defined as the need for exogenous insulin after transplant, but this may underestimate the role of insulin insensitivity and may not represent complete absence of beta cell function. Several groups have examined factors predictive of graft function in recipients, including Dean et al, who found multiple recipient factors as predictive of insulin need post-transplant.22 Multiple reports have examined the donor immune function as a contributor to graft dysfunction and failure; however, few have examined the impact of the critical care of the donor after declaration of brain death on successful transplantation. One report has examined traditional donor and recipient factors and found im- paired recipient early glucose tolerance being independently associated with poor long-term graft survival.23 However, only donor age, type (DCDD vs DBD), and BMI were used in their model. In this study, we present the first analysis of donor critical care data that may affect pancreatic graft survival. Modifiable parameters that predicted graft survival were achieving a MAP 60-110 mm Hg at the beginning of donor management and a glucose ≤180 mg/ dL prior to organ recovery. These donor factors contributing to graft survival likely representing an overall hemodynamic and physiologic stability of the donor, which can contribute to graft success. We also must consider that this analysis of recipient survival is limited to those donors whose pancreata were accepted for transplantation, a more homogenous cohort than the overall pool of donors. These grafts are biased toward optimal characteristics, which diminishes the ability to discriminate across a range of variables. For example, BMI, historically found as a contributor to graft survival, did not significantly affect survival in this population, likely as a result of few grafts being selected from those with higher BMIs. This notion is confirmed with the univariate data on selection (Table 3) showing higher BMIs not being selected for transplant. This overall homogeny is a limitation of all studies that compare donor factors predicting organ utilization with those that affect recipient outcomes.
Islet cell transplantation has also been examined as a therapy for diabetes and offers a less invasive strategy for achieving glucose homeostasis. Despite this potential benefit, data have shown the frequent need for multiple islet cell transplants and overall inferior outcomes compared to pancreas transplantation, though this gap is closing.24 One proposed allocation strategy for islet allocation is to preferentially use them from obese donors, whose organs have been shown to have worse outcomes after pancreatic transplantation than nonobese donors.25,26 Despite the technical issues associated with graft failure cited in these studies, we were unable to find an independent association between donor BMI and pancreatic trans- plantation or survival in our study of DBDs.
One of the assumed influences on pancreatic graft function is donor insulin requirements. Anecdotal reports exist of trans- plant centers turning down pancreata for transplantation based on donor insulin requirements. Given this bias that insulin use in the donor portends a worse clinical outcome in the recipient, we hypothesized that greater insulin use during donor management would predict decreased graft utilization. However, being that insulin insensitivity may simply be a bystander of the inflammatory physiologic milieu that accompanies brain death and does not necessarily represent impaired function of the pancreas, we hypothesized that insulin dose would not have an impact on graft survival. One group recently demonstrated this phenomenon in a COHORT, finding that in addition to normoglycemia, DBDs on high-dose insulin therapies also had increases in anti-inflammatory cytokines and decreases in pro-inflammatory cytokines.27 Our results demonstrated a significant association between lower insulin dose and pancreas utilization on univariate analysis, but these trends did not remain significant when placed in multivariable models. In addition, graft function was not affected by increasing dosages of insulin in the donor. However, independent of insulin requirements, our results did illustrate the impact of normoglycemia, as meeting the terminal DMG value for glucose (≤180 mg/dL) was an independent predictor of graft survival.
A limitation of this donor-derived data set is that we are only able to analyze donor factors affecting transplantation rates and success. Many groups have addressed recipient factors and how they affect pancreatic graft function; however, these are not included in our current study. Additionally, though the area under the curve for our Cox regression model is 0.76, we recognize that with a model built on 20 events, fewer degrees of freedom may result in a more reliable model. We also recognize that one potential future investigation would be to examine the Bundle over time and how it affects pancreatic acceptance and transplantation. As seen in a previous study,28 positive Bundle status change does result in more OTPD; however, this metric has not been applied yet to individual organs. Future studies should include examining Bundle status change over time for pancreata, a larger number of subjects, and should examine both donor and recipient factors in the same model. In addition, future studies will address the length of time donor management occurs, which has not been carefully studied in pancreatic grafts. Though a recent publication examined longer lengths of donor management, and showed improvements in heart, lung, and overall OTPD with >20 hours of donor manage- ment,29 these metrics have yet to be applied specifically to pancreatic grafts and are outside of the scope of our current study.
This study presents a prospective analysis of 1819 brain-dead organ donors with 238 pancreata transplanted, of which 91% were still functioning after an average of over 6 months. The modifiable donor predictors of graft utilization and survival included hemodynamic parameters as well as serum glucose. Insulin dose in the donor did not predict graft acceptance or survival. Agencies that create risk-adjustment models that aim to examine transplant center performance should investigate whether adding donor critical care parameters improves model performance.
ACKNOWLEDGEMENTS
Support for work related to this manuscript obtained from a grant from the Laura and John Arnold Foundation.
Footnotes
CONFLICT OF INTEREST
Authors have no commercial interests to disclose.
REFERENCES
- 1.Kandaswamy R, Stock PG, Gustafson SK, et al. OPTN/SRTR 2015 annual data report: pancreas. Am J Transplant. 2017;17(Suppl 1):117–173. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM. Diabetes: advances in diagnosis and treatment. JAMA. 2015;314(10):1052–1062. [DOI] [PubMed] [Google Scholar]
- 3.Amiel SA, Pursey N, Higgins B, Dawoud D, Guideline DG. Diagnosis and management of type 1 diabetes in adults: summary of updated NICE guidance. BMJ. 2015;351:h4188. [DOI] [PubMed] [Google Scholar]
- 4.Rana A, Gruessner A, Agopian VG, et al. Survival bene- fit of solid-organ transplant in the United States. JAMA Surg. 2015;150(3):252–259. [DOI] [PubMed] [Google Scholar]
- 5.Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK. Hypoglycemia and risk of cardiovascular disease and all-cause mor- tality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 2015;38(2):316–322. [DOI] [PubMed] [Google Scholar]
- 6.Perseghin G, Fiorina P, De Cobelli F, et al. Cross-sectional assessment of the effect of kidney and kidney-pancreas transplantation on resting left ventricular energy metabolism in type 1 diabetic- uremic patients: a phosphorous-31 magnetic resonance spectroscopy study. J Am Coll Cardiol. 2005;46(6):1085–1092. [DOI] [PubMed] [Google Scholar]
- 7.Fiorina P, Vezzulli P, Bassi R, et al. Near normalization of metabolic and functional features of the central nervous system in type 1 diabetic patients with end-stage renal disease after kidney-pancreas transplantation. Diabetes Care. 2012;35(2):367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratta RJ, Gruessner AC, Odorico JS, Fridell JA, Gruessner RW. Pancreas transplantation: an alarming crisis in confidence. Am J Transplant. 2016;16(9):2556–2562. [DOI] [PubMed] [Google Scholar]
- 9.Franklin GA, Santos AP, Smith JW, Galbraith S, Harbrecht BG, Garrison RN. Optimization of donor management goals yields increased organ use. Am Surg. 2010;76(6):587–594. [DOI] [PubMed] [Google Scholar]
- 10.Hagan ME, McClean D, Falcone CA, Arrington J, Matthews D, Summe C. Attaining specific donor management goals increases number of organs transplanted per donor: a quality improvement project. Prog Transplant. 2009;19(3):227–231. [DOI] [PubMed] [Google Scholar]
- 11.Malinoski DJ, Daly MC, Patel MS, Oley-Graybill C, Foster CE 3rd, Salim A. Achieving donor management goals before deceased donor procurement is associated with more organs transplanted per donor. J Trauma. 2011;71(4):990–995; discussion 6. [DOI] [PubMed] [Google Scholar]
- 12.Malinoski DJ, Patel MS, Daly MC, Oley-Graybill C, Salim A, UNOS Region 5 DMG Workgroup. The impact of meeting donor management goals on the number of organs transplanted per donor: results from the United Network for Organ Sharing Region 5 prospective donor management goals study. Crit Care Med. 2012;40(10):2773–2780. [DOI] [PubMed] [Google Scholar]
- 13.Patel MS, Zatarain J, De La Cruz S, et al. The impact of meeting donor management goals on the number of organs transplanted per expanded criteria donor: a prospective study from the UNOS Region 5 Donor Management Goals Workgroup. JAMA Surg. 2014;149(9):969–975. [DOI] [PubMed] [Google Scholar]
- 14.Malinoski DJ, Patel MS, Ahmed O, et al. The impact of meeting donor management goals on the development of delayed graft function in kidney transplant recipients. Am J Transplant. 2013;13(4):993–1000. [DOI] [PubMed] [Google Scholar]
- 15.Patel MS, Niemann CU, Sally MB, et al. The impact of hydroxyethyl starch use in deceased organ donors on the development of delayed graft function in kidney transplant recipients: a propensity- adjusted analysis. Am J Transplant. 2015;15(8):2152–2158. [DOI] [PubMed] [Google Scholar]
- 16.Bloom MB, Raza S, Bhakta A, et al. Impact of deceased organ donor demographics and critical care end points on liver transplantation and graft survival rates. J Am Coll Surg. 2015;220(1):38–47. [DOI] [PubMed] [Google Scholar]
- 17.Gerber PA, Pavlicek V, Demartines N, et al. Simultaneous islet-kidney vs pancreas-kidney transplantation in type 1 diabetes mellitus: a 5 year single centre follow-up. Diabetologia. 2008;51(1):110–119. [DOI] [PubMed] [Google Scholar]
- 18.Salim A, Martin M, Brown C, Rhee P, Demetriades D, Belzberg H. The effect of a protocol of aggressive donor management: Implications for the national organ donor shortage. J Trauma. 2006;61(2):429–433; discussion 33-5. [DOI] [PubMed] [Google Scholar]
- 19.Axelrod DA, Sung RS, Meyer KH, Wolfe RA, Kaufman DB. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant. 2010;10(4):837–845. [DOI] [PubMed] [Google Scholar]
- 20.Smigielska K, Skrzypek P, Czerwinski J, et al. Usefulness of pancreas donor risk index and pre-procurement pancreas allocation suitability score: results of the polish national study. Ann Transplant. 2018;23:360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finger EB, Radosevich DM, Dunn TB, et al. A composite risk model for predicting technical failure in pancreas transplantation. Am J Transplant. 2013;13(7):1840–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dean PG, Kudva YC, Larson TS, Kremers WK, Stegall MD. Posttransplant diabetes mellitus after pancreas transplantation. Am J Transplant. 2008;8(1):175–182. [DOI] [PubMed] [Google Scholar]
- 23.Mittal S, Nagendran M, Franklin RH, Sharples EJ, Friend PJ, Gough SC. Postoperative impaired glucose tolerance is an early predictor of pancreas graft failure. Diabetologia. 2014;57(10):2076–2080. [DOI] [PubMed] [Google Scholar]
- 24.Niclauss N, Morel P, Berney T. Has the gap between pancreas and islet transplantation closed? Transplantation. 2014;98(6):593–599. [DOI] [PubMed] [Google Scholar]
- 25.Humar A, Ramcharan T, Kandaswamy R, Gruessner RW, Gruessner AG, Sutherland DE. The impact of donor obesity on outcomes after cadaver pancreas transplants. Am J Transplant. 2004;4(4):605–610. [DOI] [PubMed] [Google Scholar]
- 26.Berney T, Johnson PR. Donor pancreata: evolving approaches to organ allocation for whole pancreas versus islet transplantation. Transplantation. 2010;90(3):238–243. [DOI] [PubMed] [Google Scholar]
- 27.Aljiffry M, Hassanain M, Schricker T, et al. Effect of insulin therapy using hyper-insulinemic normoglycemic clamp on inflammatory response in brain dead organ donors. Exp Clin Endocrinol Diabetes. 2016;124(5):318–323. [DOI] [PubMed] [Google Scholar]
- 28.Patel MS, De La Cruz S, Sally MB, Groat T, Malinoski DJ. Active donor management during the hospital phase of care is associated with more organs transplanted per donor. J Am Coll Surg. 2017;225(4):525–531. [DOI] [PubMed] [Google Scholar]
- 29.Christmas AB, Bogart TA, Etson KE, et al. The reward is worth the wait: a prospective analysis of 100 consecutive organ donors. Am Surg. 2012;78(3):296–299. [PubMed] [Google Scholar]


