Abstract
Purpose:
Dexamethasone, a uniquely potent corticosteroid, is frequently administered to brain tumor patients to decrease tumor-associated edema, but limited data exist describing how dexamethasone affects the immune system systemically and intratumorally in glioblastoma patients – particularly in the context of immunotherapy.
Experimental Design:
We evaluated the dose-dependent effects of dexamethasone when administered with PD-1 blockade and/or radiotherapy in immunocompetent C57BL/6 mice with syngeneic GL261 and CT-2A glioblastoma tumors. Clinically, the effect of dexamethasone on survival was evaluated in 181 IDH-wildtype glioblastoma patients treated with PD-(L)1 blockade, with adjustment for relevant prognostic factors.
Results:
Despite the inherent responsiveness of GL261 to immune checkpoint blockade, concurrent dexamethasone administration with anti-PD-1 therapy reduced survival in a dose-dependent manner. Concurrent dexamethasone also abrogated survival following anti-PD-1 with or without radiotherapy in immunoresistant CT-2A models. Dexamethasone decreased T lymphocyte numbers by increasing apoptosis, in addition to decreasing lymphocyte functional capacity. Myeloid and NK cell populations were also generally reduced by dexamethasone. Thus, dexamethasone appears to negatively affect both adaptive and innate immune responses. As a clinical correlate, a retrospective analysis of 181 consecutive IDH-wildtype glioblastoma patients treated with PD-(L)1 blockade revealed poorer survival among those on baseline dexamethasone. Upon multivariable adjustment with relevant prognostic factors, baseline dexamethasone administration was the strongest predictor of poor survival (reference no dexamethasone; <2mg HR 2.16, 95%CI: 1.30–3.68, p=0.003; ≥2mg HR 1.97, 95%CI: 1.23–3.16, p=0.005).
Conclusions:
Our preclinical and clinical data indicate that concurrent dexamethasone therapy may be detrimental to immunotherapeutic approaches for glioblastoma patients.
Keywords: Glioblastoma, dexamethasone, corticosteroids, immunotherapy, immune checkpoint blockade
INTRODUCTION
Although inhibition of immune checkpoints such as programmed-death 1 (PD-1) has transformed the treatment of many cancers, some tumors such as glioblastoma have responded poorly as exemplified by recently reported negative phase 3 trials among recurrent (CheckMate-143) and newly-diagnosed glioblastoma patients (CheckMate-498 press release, Bristol Myers Squibb, May 9, 2019) (1). These disappointing results likely reflect multi-faceted mechanisms of immunosuppression exploited by glioblastoma tumors, which are particularly pronounced in older patients (2–4). However, increasing data suggest that exogenous corticosteroid exposure also can limit the therapeutic benefits of immunotherapeutics (e.g. PD-1 and PD-L1 inhibitors) for cancer patients, including those with glioblastoma (5–7). Subgroup analyses of the CheckMate-143 study revealed that baseline dexamethasone use was associated with worse survival among nivolumab recipients than those treated with bevacizumab (1). In a recent clinical trial of immunogene therapy, dexamethasone dose was associated with decreased survival among recurrent malignant glioma patients (8). Likewise, newly-diagnosed glioblastoma patients on dexamethasone failed to generate immune responses following neoantigen vaccination, whereas those not on dexamethasone generated responses to multiple vaccinated neoepitope peptides (9).
Many brain cancer patients receive dexamethasone to treat symptomatic cerebral edema generated by the tumor as well as by standard therapies like external beam radiotherapy (RT) (10). Dexamethasone, which is 5–10 times more potent than other corticosteroids (e.g. prednisone and methylprednisolone), is the steroid of choice for brain cancer patients based on its potency, long half-life, and high brain penetrance (11). Although well known to induce myriad potentially severe, systemic side effects including proximal myopathy, truncal weight gain, hypertension and glucose intolerance, the specific effects of dexamethasone on immune function and response to PD-1 immune checkpoint therapy for glioblastoma tumors have not been well described (12).
Herein we evaluated the impact of dexamethasone administration on response to anti-PD-1 immune checkpoint blockade in the syngeneic immunosensitive GL261 and immunoresistant CT-2A murine glioblastoma models and how dexamethasone affects intratumoral and systemic immune cell populations and functionality. Additionally, we assessed how concurrent dexamethasone administration affected the survival outcomes of 181 IDH-wildtype glioblastoma patients treated with PD-1 or PD-L1 inhibitors (anti-PD-[L]1) in analyses adjusted for patients’ key prognostic factors.
METHODS AND MATERIALS
Cell Lines, Antibodies, and Reagents
Luciferase-transduced GL261 cells (GL261-luc2; PerkinElmer, Inc., Waltham, MA) were expanded and frozen at the same generation. CT-2A cells (obtained from Thomas Seyfried, Boston College) were transduced using firefly luciferase lentiviral particles (CT-2A-luc; Kerafast Inc., Boston, MA). Thawed cells were cultured for up to three passages in Dulbecco’s Modified Eagle Medium supplemented with 10% heat-inactivated fetal calf serum and 100 μg/mL G418 (for GL261-luc2) or 2 μg/mL puromycin (for CT-2A-luc) at 37°C in a humidified incubator maintained at 5% CO2 prior to intracranial implantation, with periodic testing for mycoplasma. Cells were maintained in logarithmic growth phase for all experiments. The 332.8H3 mouse anti-mouse PD-1 monoclonal antibody (IgG1) was generated in the laboratory of Dr. Gordon Freeman and MOPC21 (IgG1; BioXCell, West Lebanon, NH) was used for isotype control (13). The monoclonal antibodies contained less than 2 EU/mg endotoxin protein. Dexamethasone sodium phosphate (4 mg/mL, USP; Fresenius Kabi USA LLC, IL) was diluted with normal saline and injected intraperitoneally (IP) at doses described below.
Intracranial Tumor Cell Inoculation
1×105 GL261-luc2 cells or 0.25×105 CT-2A-luc cells, which are syngeneic in C57BL/6 mice, were resuspended in PBS and injected stereotactically into the right striatum of anesthetized, 7–10 week old, female, albino C57BL/6 mice (Jackson Laboratory; Bar Harbor, ME) using a Hamilton syringe and stereotactic frame (14). Mice were euthanized for either signs of morbidity due to tumor burden or after at least 100 days to terminate the study if healthy appearing. All animal experiments were approved by the Dana-Farber Cancer Institute (DFCI) Animal Care and Use Committee.
In Vivo Treatment and Tumor Assessment
For all studies, mice with enlarging tumor burden defined by increasing bioluminescence signal between days 3 and 6 post-tumor implantation were randomized into control and treatment cohorts. Tumor response assessment was done by quantifying bioluminescence in all animals, as well as MRI in a subset as previously performed (13). Therapeutic anti-PD-1 and isotype controls were administered via IP injection beginning on day 6 after tumor implantation, using 2 dosing regimens. A dose-intensive regimen consisting of a loading dose (500 μg) with repeat injections every 3 days (250 μg/dose) for a total of 6–8 injections was employed to evaluate the effect of dexamethasone in the setting of maximal therapeutic benefit from anti-PD-1 therapy. An abbreviated regimen comprised of only 4 doses (250 μg/dose every 3 days) without a loading dose was also used to examine the effects of dexamethasone when the therapeutic effect of anti-PD-1 was reduced. Control animals received equivalent doses of isotype murine IgG according to the same dosing schedule. Dexamethasone was administered as single agent at 10 mg/kg/day IP and in combination with PD-1 monoclonal antibodies at either low (1 and 2.5 mg/kg/day) or high (10 mg/kg/day) doses on days 6–27. No treatment was administered after day 27 following tumor implantation. Using this treatment schedule, we systematically evaluated anti-tumor activity as measured by bioluminescence imaging, MRI, and overall survival (OS).
Next, we evaluated whether the timing of dexamethasone administration impacted the therapeutic efficacy of inhibitory immune checkpoint blockade. In these experiments anti-PD-1 was initiated on day 6 and administered every 3 days for 8 doses over 27 days and dexamethasone (10 mg/kg/day IP) was administered on days 1–5. We then evaluated the effect of concurrent dexamethasone when added to anti-PD-1 therapy plus fractionated RT in both the GL261 and CT-2A models. Fractionated RT was administered using a X-Rad 225Cx Image Guided Biological Irradiator System (Precision X-Ray; North Branford, CT). Each mouse received a total dose of 10 Gy, delivered as 5 × 2 Gy in 5 consecutive days, using 2 parallel-opposed fields, including an anterior-posterior collimated-field and posterior-anterior collimated-field (Supplemental Methods). Dexamethasone (10 mg/kg) treatment in the combination studies was administered on days 6–16 in these GL261-luc2 studies and days 6–27 in these CT-2A-luc studies.
For re-challenge experiments that assessed immunologic memory responses to tumor, 1×105 GL261 non-luciferase-transduced cells were injected intracranially into the contralateral hemisphere in a cohort of mice that were previously treated and survived over 100 days. A similar tumor cell inoculum was administered to a cohort of treatment naïve mice as a control. Re-challenged mice were followed for a minimum of 128 additional days and received no additional therapy.
Flow Cytometry Characterization of Immune Responses
Immune response assessment studies were performed on material obtained from euthanized, tumor-bearing animals on day 16 following a 500 μg anti-PD-1 loading dose on day 6 and 250 μg doses on days 9, 12, and 15 and/or dexamethasone (administered at 10 mg/kg/day IP on days 6–16). For comprehensive profiling of the immune microenvironment by flow cytometry analysis, whole tumor-bearing brain, superficial cervical lymph nodes (cLN), spleen, and thymus were homogenized using enzymatic (1.5 mg/mL collagenase IV, 200 U/mL DNaseI, HBSS with calcium and magnesium) and/or mechanical tissue disaggregation. Red blood cells were removed using a Ficoll gradient (GE Life Sciences). Brain homogenates were resuspended in 25% Percoll Plus (Sigma) and centrifuged (1,500 rpm for 20 minutes, with minimum acceleration and no brake) to remove myelin and isolate leukocytes (13). Samples were split for staining with antibody panels and completely enumerated by flow cytometry. The following antibodies (from Biolegend unless otherwise indicated) were used for flow cytometric analysis: anti-CD45 (30-F11), anti-CD3 (17A2), anti-CD4 (RM4–5), anti-CD8 (53–6.7), anti-CD11b (M1/70), anti-CD80 (16–10A1), anti-CD86 (GL-1), anti-NK1.1 (PK136), anti-Ly6G (1A8), anti-Ly6C (HK1.4), anti-PD-1 (RMP1–30, non-competing epitope to PD-1 treatment monoclonal antibody, eBioscience), anti-PD-L1 (10F.9G2), and anti-CD69 (H1.2F3) Dead cells were excluded using the Zombie NIR fixable viability kit (Biolegend). Following surface staining, cells were processed with the FOXP3 Fixation/Permeabilization Kit (eBioscience). The following antibodies were used for intracellular staining: anti-FOXP3 (FJK-16s, eBioscience) and anti-Ki67 (16A8, Biolegend), and anti-IFNγ (XMG1.2). To assess IFNγ expression, splenocytes were stimulated for intracellular cytokine staining as per the manufacturer’s instructions (Cell Stimulation Cocktail, Invitrogen eBioscience). Additionally, late apoptosis of splenic lymphoid populations was measured (via annexin-V and 7-AAD staining) in non-tumor-bearing mice euthanized at either 1 hour after the first dexamethasone dose or 1 hour after the sixth dexamethasone dose (121 hours after initiation of dexamethasone), including both low (1 mg/kg) or high (10 mg/kg) daily IP dexamethasone dosing and IgG-treated controls for comparison. Acquisition was performed on an LSR Fortessa flow cytometer (BD Biosciences) and analyzed using FlowJo, with the gating strategies displayed in Supplemental Figure S1.
Clinical Cohort
To assess the clinical effect of dexamethasone, we retrospectively identified consecutive glioblastoma patients diagnosed before April 1, 2019 who were evaluated at DFCI and received anti-PD-(L)1 therapy on either a formal clinical trial or a compassionate use basis. Among these patients, 181 had ≥1 month of follow-up after the start of PD-(L)1 blockade, as well as: 1) tumor available at DFCI for an integrated histomolecular diagnosis of IDH-wildtype glioblastoma, W.H.O. grade IV; 2) annotated clinical data; and 3) survival outcome. Pharmacy data and clinic notes were reviewed to identify whether patients were receiving dexamethasone at the time of PD-(L)1 treatment initiation and, if so, the dexamethasone dose (none, <2mg, ≥2mg; Supplemental Table S1). Tumor volumes of interest were manually selected and measured from patients’ contrast-enhancing T1 MRI sequences taken prior to PD-(L)1 treatment. These data were collected under DFCI Institutional Review Board protocol 10–417. OS was estimated using the Kaplan-Meier method from time of PD-(L)1 treatment start to date of death with censorship at the date of last clinical assessment, and comparisons by logrank test. The cut-off for survival data was April 17, 2020. Multivariable Cox regression analysis was performed to adjust OS among the 163 patients with complete annotated data for relevant prognostic factors including age at GBM diagnosis and MGMT promoter methylation status, as well as the following at the time of PD-(L)1 therapy initiation: disease status (newly-diagnosed vs. recurrent), Karnofsky Performance Scale (KPS; ≤70 vs. 80 vs. ≥90), tumor volume (on MRI T1 sequence, post-contrast), and whether a pre-anti-PD-(L)1 treatment gross total resection (GTR) was performed.
Statistical Analysis
For flow cytometry characterization of immune responses, dexamethasone vs. IgG control and concurrent dexamethasone with anti-PD-1 vs. anti-PD-1 alone were prospectively determined to be the comparisons of interests. Complete absolute cell counts from flow cytometry experiments were evaluated using multiple linear regression, including adjustment for data derived from multiple experiments (i.e. batch effect). Data are displayed as mean ± standard error (SE). For visualization, cell counts are normalized to that experiment’s IgG group’s average counts. Apoptosis was evaluated within and between timepoints by two-way ANOVA with Šidák correction for multiple comparisons. Murine OS estimates were determined using Kaplan-Meier methods and were compared using logrank test and Cox regression. Two-sided p values <0.05 were considered statistically significant. Statistical analysis was performed with GraphPad Prism 8 (GraphPad Software, Inc) and STATA (v15.1, IBM).
RESULTS
Concurrent dexamethasone limits the survival benefit of anti-PD-1 monotherapy and combination with radiation therapy in preclinical models
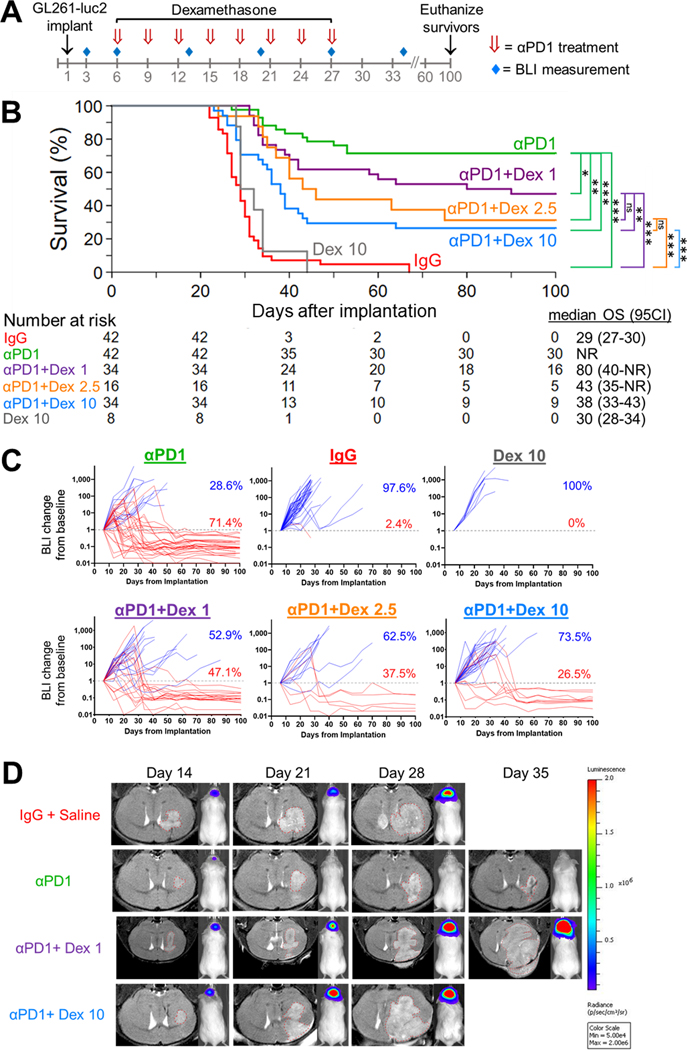
We first evaluated the effect of dose and timing of dexamethasone on the anti-tumor activity of PD-1 blockade. OS was assessed when dexamethasone was concurrently administered at either low (1 or 2.5 mg/kg) or high (10 mg/kg) daily dosing with a dose-intensive anti-PD-1 schedule (Figure 1A). As previously published (13), a majority of mice (71.4%, 95% confidence interval [95%CI]: 55.2–82.7) with growing intracranial GL261-luc2 tumors were effectively cured with anti-PD-1 monotherapy (Figure 1B). In contrast, in anti-PD-1-treated mice who received concurrent dexamethasone, OS decreased in a dose-dependent fashion that was most pronounced at higher dexamethasone doses, although lower doses also decreased OS: OS rates at 100 days were 47.1% (95%CI: 29.8–62.5; p=0.04), 31.3% (95%CI: 11.4–53.7; p=0.008), and 26.5% (95%CI: 13.2–41.8; p<0.001) as concurrent dexamethasone dosage increased from 1 mg/kg to 2.5 mg/kg and 10 mg/kg, respectively (Figure 1B). Mice treated with dexamethasone alone had similarly poor OS compared to control mice treated with IgG (p=0.31). Changes in tumor burden were confirmed by bioluminescence (Figure 1C, Supplemental Figure S2A) and MRI (Figure 1D). Animals surviving long term (i.e. ≥100 days) were re-challenged by implantation of 1×105 GL261 non-luciferase-transduced cells in the contralateral hemisphere. Among those treated with anti-PD-1 alone or anti-PD-1 with concurrent dexamethasone at either 1 mg/kg or 10 mg/kg, 85.7% (6/7), 100% (5/5), and 75.0% (3/4) successfully cleared the re-challenge tumors and survived for at least an additional 128 days (p=0.57), respectively (Supplemental Figure S2B). All challenged naïve control mice died (median OS: 27 days).
Figure 1. Concurrent dexamethasone reduces the survival benefit of anti-PD-1 therapy in GL261-luc2 glioblastoma mouse models in a dose-dependent manner.
(A) Experimental schema. Anti-PD-1 (αPD1, red arrows) was administered in a dose-intensive schedule, i.e. IP beginning on day 6 (500 μg) followed by 7 additional doses (250 μg/dose) at 3-day intervals, with dexamethasone delivered IP daily from days 6–27. (B) Kaplan-Meier survival estimates of anti-PD-1 therapy without dexamethasone (n=42, data derived from 5 experiments, with 8–10 mice each) and anti-PD-1 therapy with concurrent dexamethasone at 1 mg/kg (n=34, data derived from 4 experiments, with 8–10 mice each), 2.5 mg/kg (n=16, data derived from 2 experiments, with 8 mice each), or 10 mg/kg (n=34, data derived from 4 experiments, with 8–10 mice each), compared to IgG (n=42, data derived from 5 experiments, with 8–10 mice each) and 10 mg/kg dexamethasone-only (n=8, data derived from a single experiment) controls; with comparison by Cox regression. (C) The corresponding longitudinal bioluminescence imaging, displayed as change from baseline (day 6 after implantation, dotted gray line), for mice treated with anti-PD-1 alone (n=42, baseline BLI median 415,600 ph/sec/cm2/sr, interquartile range [IQR] 201,500–981,050) or anti-PD-1 with concurrent 1 mg/kg (n=34, baseline BLI median 434,700 ph/sec/cm2/sr, IQR 246,975–835,500), 2.5 mg/kg (n=16, baseline BLI median 490,950 ph/sec/cm2/sr, IQR 268,800–1,081,875), or 10 mg/kg dexamethasone (n=34, baseline BLI median 367,300 ph/sec/cm2/sr, IQR 227,400–636,675), as compared to IgG control (n=16, baseline BLI median 463,650 ph/sec/cm2/sr, IQR 286,775–1,059,250) and dexamethasone 10 mg/kg only control (n=8, baseline BLI median 159,550 ph/sec/cm2/sr, IQR 143,725–231,700). Tumor response visualized in red and lack of response in blue. (D) Representative longitudinal MRI findings demonstrating increased tumor growth when low (1 mg/kg) or high (10 mg/kg) doses of dexamethasone were co-administered during PD-1 therapy, compared to anti-PD-1 without dexamethasone. Images are obtained serially from the same mice over time. Dotted red line outlines the tumor on coronal MRI plane.
ns, not significant, p≥0.05; *p<0.05; **p<0.01; ***p<0.001; Dex, dexamethasone; BLI, bioluminescence imaging; OS, overall survival; 95CI, 95% confidence interval; NR, not reached.
We then evaluated dexamethasone administered on days 1–5 prior to the initiation of anti-PD-1 therapy on day 6, but not during anti-PD-1 therapy, in the GL261-luc2 glioblastoma model. In contrast to the decreased OS exhibited when dexamethasone was administered concurrently with anti-PD-1, dexamethasone administered prior to, but not during, anti-PD-1 therapy did not alter survival (p=0.64; Supplemental Figure S2C).
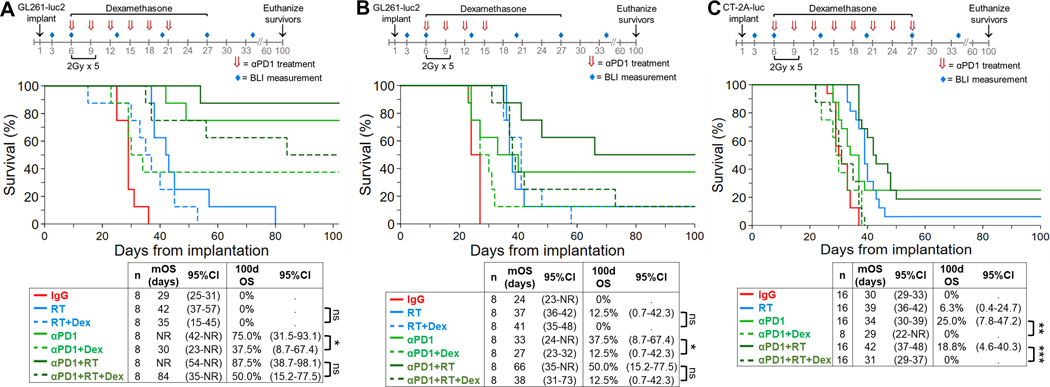
Next, given that RT is standard therapy for glioblastoma patients, we evaluated the effect of concurrent dexamethasone on OS when PD-1 therapy was administered with RT relative to either therapy alone. Initially anti-PD-1 was administered using the dose-intensive schedule to mice with growing intracranial GL261-luc2 tumors (Figure 2A). With this schedule, 75.0% (95%CI: 31.5–93.0) of mice treated with anti-PD-1 monotherapy were long-term survivors (i.e. ≥100 days). As expected, the addition of dexamethasone markedly reduced the median OS benefit of anti-PD-1 therapy (from >100 days [95%CI: 42-not reached] to 30 days [95%CI: 23-not reached], p=0.049) and decreased the long-term survivor rate by half. RT, administered at 2 Gy x 5 daily fractions beginning 6 days after tumor implantation, modestly prolonged median OS compared to isotype controls (42 days, 95%CI: 37–57; vs. 29 days, 95%CI: 25–31; p=0.001). RT added to dose-intensive anti-PD-1 had a nominal effect on survival. However, the addition of dexamethasone to anti-PD-1 plus radiation demonstrated a trend towards decreased median and long-term survival but did not achieve statistical significance (p=0.15).
Figure 2. Concurrent dexamethasone decreases the OS benefit of anti-PD-1 plus RT in syngeneic GL261-luc2 and CT-2A-luc glioblastoma mouse models.
Kaplan-Meier OS estimates are depicted, with comparison by logrank test and Cox regression. (A) To assess concurrent dexamethasone’s effect on a dose-intensive schedule of anti-PD-1 with or without RT in GL261-luc2 mice (n=8/group from a single experiment), anti-PD-1 was administered IP via a loading dose (500 μg) followed by 5 additional doses (250 μg/dose) at 3-day intervals. RT was administered in 2 Gy fractions/day for 5 days beginning on day 6. Dexamethasone was delivered IP daily from days 6–27 at 10 mg/kg. (B) For GL261-luc2 mice (n=8/group from a single experiment), anti-PD-1 (αPD1) was administered IP via an abbreviated dosing schedule every 3 days beginning on day 6 for a total of 4 doses (250 μg/dose). (C) For CT-2A-luc mice (n=8–16/group, derived from two experiments), anti-PD-1 was administered IP via a loading dose (500 μg) followed by 7 additional doses (250 μg/dose) at 3-day intervals. RT was administered in 2 Gy fractions/day for 5 days beginning on day 6. Dexamethasone was delivered IP daily from days 6–27 at 10 mg/kg.
*p<0.05; **p<0.01; ***p<0.001; Dex, dexamethasone; 95CI, 95% confidence interval; NR, not reached
Using the abbreviated anti-PD-1 dosing schedule (Figure 2B), concurrent dexamethasone with anti-PD-1 monotherapy significantly reduced the median OS and the long-term OS rate, from 37.5% (95%CI: 8.7–67.4) to 12.5% (95% CI: 0.1–42.3, p=0.04). Median OS increased from 37 days for RT alone to 66 days for RT plus anti-PD-1, although the net OS improvement for the combination did not achieve significance (p=0.25). However, when dexamethasone was administered with anti-PD-1 plus RT, the survival decreased to the level of RT alone (p=0.78), showing that dexamethasone abrogated the therapeutic benefit of anti-PD-1.
We then repeated this experiment using the CT-2A-luc model, which is known to be less responsive to anti-PD-1 than GL261 (15), using the dose-intensive anti-PD-1 schedule (Figure 2C). Compared to isotype controls, anti-PD-1 exhibited a statistically significant OS benefit with a modest number of long-term survivors (p<0.001). Radiation therapy also moderately improved OS (median of 39 days [95%CI: 36–42], vs. 30 days in isotype controls [95%CI: 29–33]; p<0.001), while the combination of anti-PD-1 plus RT achieved limited additive benefit compared to either modality alone. As in the GL261-luc2 model, the addition of dexamethasone to anti-PD-1 plus RT significantly decreased OS (median 31 days, 95%CI: 29–37) compared to either single-agent anti-PD-1 (median 34 days, 95%CI: 30–39, p=0.003) or the combination of anti-PD-1 with RT (median 42 days, 95CI: 37–48, p<0.001). The addition of dexamethasone was likewise associated with a complete abrogation of the benefits of anti-PD-1 monotherapy on OS (hazard ratio [HR] of adding dexamethasone: 4.34, 95%CI: 1.68–11.21, p<0.002).
Concurrent dexamethasone administration decreases intratumoral and systemic immune effector cell populations
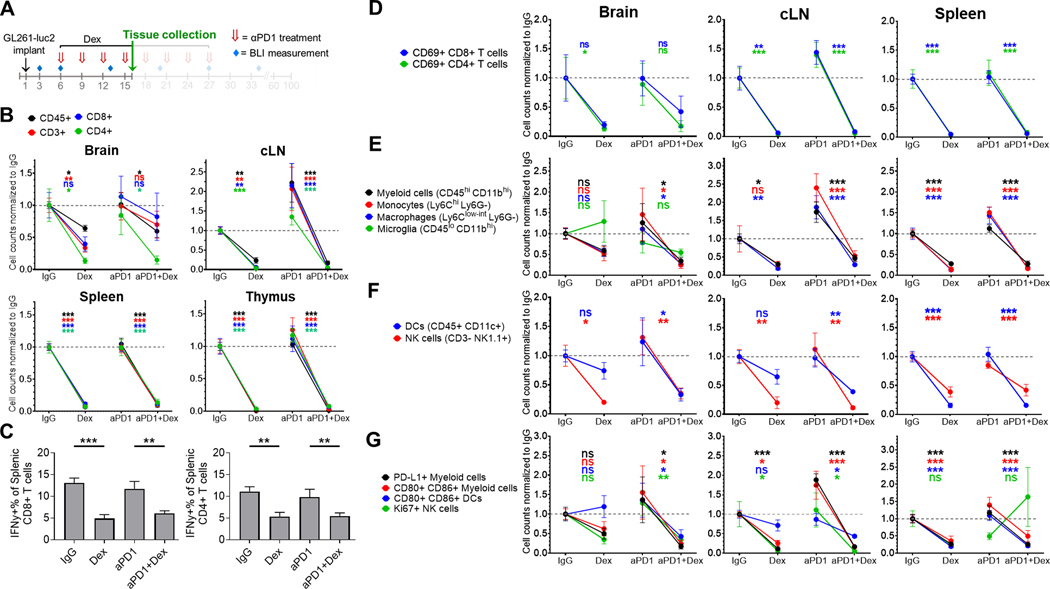
To investigate the mechanisms by which concurrent dexamethasone administration limits anti-PD-1 therapeutic benefit, we used flow cytometry to quantify the adaptive and innate immune cell populations isolated from intracranial tumor, cLNs, and spleen. Concurrent dexamethasone (10 mg/kg) administration significantly decreased the numbers of CD45+, CD3+, CD4+, and CD8+ cells from the cLN, spleen, and thymus independent of anti-PD-1 therapy (Figure 3A-B). These patterns were also observed in some brain tumor infiltrating lymphocyte (TIL) populations as well. Dexamethasone also reduced regulatory CD4+ FOXP3+ T cells among TILs, cLNs, and spleens, with a significant reduction of the CD8 to CD4+ FOXP3+ ratio in cLNs compared to IgG control (Supplemental Figure S3A-B). Decreased CD8+, CD4+ FOXP3-, and CD4+ FOXP3+ T cells were also observed in the tumor-immune microenvironment by visualization with multiplexed immunofluorescence (CyCIF) staining (Supplemental Figure S4, Supplemental Methods).
Figure 3. Concurrent dexamethasone negatively affects intratumoral and systemic adaptive and innate immune cell populations in the GL261-luc2 glioblastoma mouse model.
(A) Experimental schema. Tissue was collected at day 16 of a dose-intensive regimen of anti-PD-1, in which anti-PD-1 (αPD1) was administered IP beginning on day 6 (500 μg loading dose) followed by 3 additional doses (250 μg) at 3-day intervals, with dexamethasone (10 mg/kg) administered IP on days 6–16. Tissue (n=4–8/group, derived from two experiments) was harvested on day 16 and analyzed by flow cytometry. Immune cell counts were evaluated by multiple linear regression, normalized to the corresponding IgG control group’s mean count (displayed as dashed gray line), and displayed as mean ± SE. (B) Differences in CD45+ leukocytes and CD45+ CD3+ lymphocytes, including CD4+ and CD8+ T cells between treatment groups. (C) Percentage of splenic IFNγ+ CD4+ and CD8+ lymphocytes by treatment group. (D) Change in the number of early activated CD69+ T cells by site for each treatment group. Additionally, differences between treatment groups in innate immune cells including (E) myeloid cells (CD45hi CD11bhi), macrophages (Ly6Clo-int Ly6G-), monocytes (Ly6Chi Ly6G-), and microglia (in the brain, CD45lo CD11bhi), (F) dendritic cells (DCs; CD45+ CD11c+) and NK cells (CD45+ CD3- NK1.1+); as well as (G) activated (CD80+ CD86+) myeloid cells and DCs, PD-L1+ myeloid cells, and Ki67+ NK cells were analyzed.
cLN, cervical lymph node; Dex, dexamethasone; ns, not significant, p≥0.05; *p<0.05; **p<0.01; ***p<0.001
To evaluate the effect of dexamethasone on T cell function, we assayed IFNγ cytokine expression in ex vivo stimulated splenic T cells from tumor-bearing mice by intracellular cytokine staining. Concurrent dexamethasone significantly decreased the percentage of IFNγ-positive CD4+ and CD8+ T cells (Figure 3C). We then evaluated the effect of dexamethasone on T cell activation as assessed by expression of the early activation marker CD69. Dexamethasone, either alone or concurrent with anti-PD-1, reduced the number of CD69+ CD4+ and CD69+ CD8+ cells, particularly in the cLN and spleen (Figure 3D).
Regarding innate immunity, dexamethasone significantly decreased intratumoral natural killer (NK) cells (CD45+ CD3- NK1.1+), while other myeloid populations (e.g. tumor-associated macrophages, monocytes, dendritic cells [DCs]) trended downward particularly if dexamethasone was added to anti-PD-1 (Figure 3E-F). Most innate immune populations were also consistently decreased in cLN and spleen following dexamethasone. Similarly, declines in PD-L1+ myeloid cells (CD45hi CD11bhi), activated myeloid cells (CD45hi CD11bhi CD80+ CD86+), and mature DCs (CD45+ CD11c+ CD86+ CD80+) were observed with dexamethasone administration (Figure 3G).
Dexamethasone induces lymphocyte apoptosis
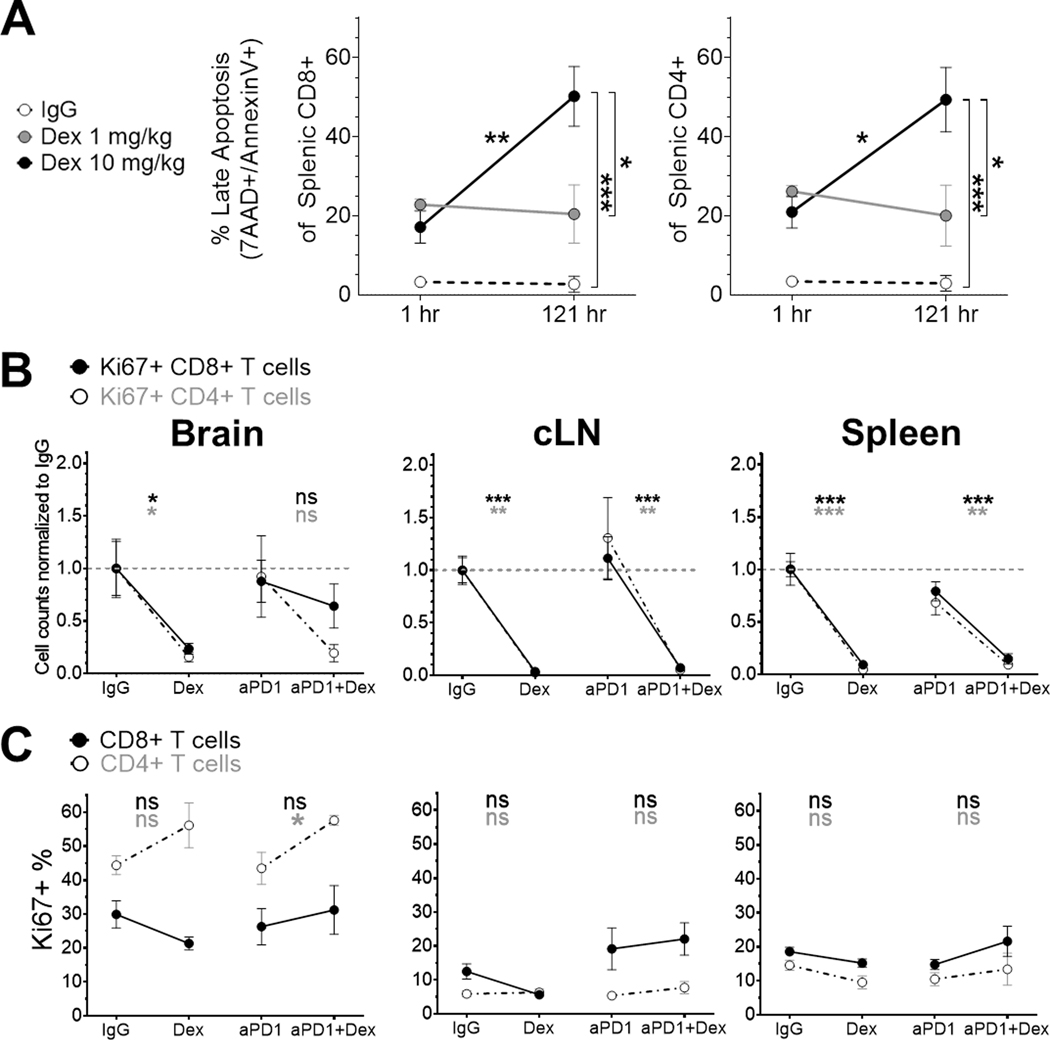
We then investigated how dexamethasone quantitatively decreases lymphocyte levels. Dexamethasone increased the percentage of splenic CD4+ and CD8+ T cells expressing late apoptosis markers (7-AAD+ annexin-V+) as early as 1 hour after either low (1 mg/kg) or high (10 mg/kg) doses (Figure 4A). With continued daily dexamethasone dosing for 6 days, the percentage of late apoptotic CD8+ and CD4+ T cells remained stable following low dosing and significantly increased following high dosing (adjusted p=0.005 for CD8+ T cells and adjusted p=0.03 for CD4+ T cells). Dexamethasone significantly decreased the absolute counts of Ki67+ CD4+ and CD8+ T cells from cLN and spleen, as well as Ki67+ TILs in the absence of anti-PD-1 (Figure 4B); however, dexamethasone did not reduce the proportion of CD4+ and CD8+ T cells that were proliferative (Figure 4C). These data show that dexamethasone reduces T lymphocytes at least in part by inducing apoptosis.
Figure 4. Concurrent dexamethasone increases apoptosis of CD4+ and CD8+ T cells in the GL261-luc2 glioblastoma mouse model.
(A) Late apoptosis was evaluated by 7-AAD+ and annexin-V+ staining in non-tumor-bearing mouse spleens (n=3/group, from a single experiment) either 1 hour after the first dexamethasone dose or 1 hour after the sixth daily dexamethasone dose. Apoptosis differences were tested by two-way ANOVA with post-test correction. Cell counts normalized to the corresponding IgG control group’s mean count (B) and percent (C) of proliferating CD4+ and CD8+ T cells were evaluated by Ki67 staining, using the same dosing schema and analyses as Figure 3 (n=4–8/group, derived from two experiments).
cLN, cervical lymph node; Dex, dexamethasone; hr, hour; ns, not significant, p≥0.05; *p<0.05; **p<0.01; ***p<0.001
Dexamethasone decreases the adjusted survival among glioblastoma patients undergoing anti-PD-(L)1 therapy
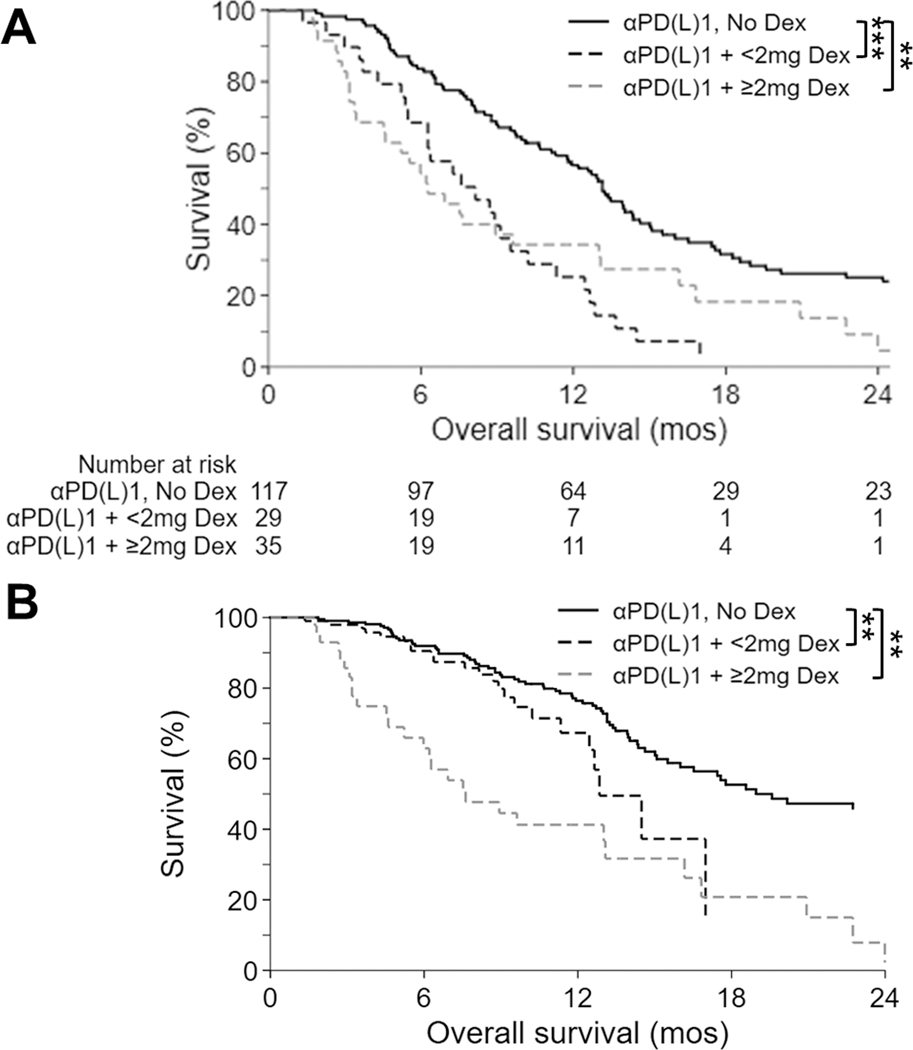
To examine the influence of dexamethasone on the clinical activity of anti-PD-(L)1 therapy among glioblastoma patients, we analyzed the OS of 181 consecutive, IDH-wildtype glioblastoma patients at our institution who were treated with anti-PD-(L)1 therapy, including 75.7% (n=137) at recurrence and 24.3% (n=44) in the newly-diagnosed setting. The median follow-up from diagnosis of these patients was 22.1 months (interquartile range: 15.3–30.7 months) and 153 (84.5%) have died. Baseline dexamethasone, either at <2mg daily (n=29, 16.0%) or ≥2mg daily (n=35, 19.3%), significantly decreased unadjusted median OS to 8.1 months (95%CI: 5.5–9.5; p<0.001) and 6.3 months (95%CI: 4.5–9.6; p=0.001), respectively, from 13.1 months (95%CI: 11.3–14.6) for those not on baseline dexamethasone (n=117, 64.6%; Figure 5A). The detrimental effect of baseline dexamethasone persisted in multivariable analyses adjusted for disease setting (newly-diagnosed vs. recurrent), patient age, MGMT promoter methylation status, KPS, tumor volume at anti-PD-(L)1 initiation, and extent of resection (Figure 5B, Table 1). Baseline dexamethasone use was the strongest identified negative risk factor for OS. Even after multivariable adjustment, baseline dexamethasone eliminated the survival benefit when administered at either lower (i.e. <2mg daily; hazard ratio [HR] 2.16, 95%CI: 1.30–3.60, p=0.003) or higher doses (i.e. ≥2mg daily; HR 1.97, 95%CI: 1.23–3.16, p=0.005) compared to no baseline dexamethasone. Similar results with dexamethasone were observed in our preclinical GL261 (Figures 1 and 2) and CT-2A (Figure 2) murine models. As expected, multivariable analysis also identified newly-diagnosed patients, younger patients, and MGMT promoter methylated tumors as having improved OS.
Figure 5. Baseline dexamethasone is associated with decreased OS among glioblastoma patients receiving anti-PD-(L)1 therapy, irrespective of dexamethasone dose.
Kaplan-Meier OS estimates for 181 IDH-wildtype glioblastoma patients treated with anti-PD-(L)1 therapy, who were either on ≥2mg (dashed gray line), <2mg (dashed black line), or no (solid black line) baseline dexamethasone are depicted; including both (A) unadjusted analyses (n=181) and (B) analyses adjusted (by a Cox regression model; n=163) for relevant prognostic factors including disease setting (newly-diagnosed vs. recurrent), patient age, MGMT promoter methylation, KPS and tumor volume prior to anti-PD-(L)1 initiation, and extent of resection.
**p<0.01; ***p<0.001; Dex, dexamethasone; mos, months
Table 1.
Multivariable Cox regression analysis of the effect of baseline dexamethasone on overall survival in glioblastoma patients treated with anti-PD-(L)1
| Multivariable Cox regression | ||||
|---|---|---|---|---|
| n | HR | 95%CI | p value | |
| Dexamethasone at αPD-(L)1 baseline | ||||
| None | 105 | Referent | ||
| <2mg Dex | 25 | 2.16 | (1.30–3.60) | 0.003 |
| ≥2mg Dex | 33 | 1.97 | (1.23–3.16) | 0.005 |
| Age at diagnosis (yr) | ||||
| <45 | 27 | Referent | ||
| 45–54 | 41 | 1.37 | (0.75–2.52) | 0.31 |
| 55–64 | 58 | 1.95 | (1.10–3.45) | 0.02 |
| ≥65 | 37 | 2.19 | (1.16–4.14) | 0.02 |
| Disease setting | ||||
| Recurrent | 120 | Referent | ||
| Newly-diagnosed | 43 | 0.45 | (0.29–0.70) | <0.001 |
| KPS at αPD-(L)1 baseline | ||||
| ≤70 | 29 | 0.89 | (0.52–1.53) | 0.68 |
| 80 | 53 | Referent | ||
| ≥90 | 81 | 0.93 | (0.61–1.43) | 0.75 |
| MGMT promoter status | ||||
| Unmethylated | 93 | Referent | ||
| Methylated | 56 | 0.48 | (0.32–0.72) | <0.001 |
| Partially methylated | 14 | 1.54 | (0.80–2.95) | 0.19 |
| Tumor volume at αPD-(L)1 baseline | ||||
| Lowest tertile | 40 | 0.71 | (0.43–1.18) | 0.18 |
| Middle tertile | 44 | Referent | ||
| Highest tertile | 45 | 1.30 | (0.81–2.09) | 0.28 |
| n/a | 34 | 1.20 | (0.68–2.11) | 0.54 |
| GTR prior to αPD-(L)1 | ||||
| No | 88 | Referent | ||
| Yes | 75 | 0.82 | (0.57–1.18) | 0.29 |
HR, hazard ratio; CI, confidence interval; KPS, Karnofsky Performance Scale; GTR, gross total resection
DISCUSSION
Immune checkpoint blockade has transformed the treatment of many cancers. Immune-related side effects of immune checkpoint inhibitors are often treated with corticosteroids such as prednisone and methylprednisolone. While some studies show that the use of corticosteroids does not compromise therapeutic benefit, other studies indicate that they may weaken efficacy (16,17). Accumulating data also support the idea that baseline corticosteroid use or administration early in the course of immune checkpoint therapy may be detrimental. For example, baseline corticosteroid use portends poorer outcome among patients with advanced non-small cell lung cancer following immune checkpoint blockade, while advanced melanoma patients who received corticosteroids within 7 weeks of initiating CTLA-4 blockade had worse outcomes compared to patients who received corticosteroids at a later time point, particularly among those with a low mutational burden tumor (5–7).
Patients with primary as well as metastatic secondary tumors of the central nervous system are frequently prescribed dexamethasone – a potent anti-inflammatory agent used to treat symptomatic cerebral edema induced by the tumor or its treatment – for prolonged periods. Recent multivariable analyses indicate that corticosteroid use portends a worse survival for newly-diagnosed glioblastoma patients that is independent of established prognostic factors such as degree of resection and baseline performance status (18,19). The mechanism underlying decreased survival among corticosteroid-treated brain tumor patients remains to be clarified, but the suppressive effects of corticosteroids on immune function and anti-tumor immune responses are likely a contributing factor. Glioblastoma patients on dexamethasone exhibit notable lymphopenia, including lower levels of circulating CD4+ and CD8+ T cells compared to glioblastoma patients not on dexamethasone or age-matched normal donors (20,21).
To better understand the effects of dexamethasone therapy on anti-PD-(L)1 therapy, we replicated the dexamethasone dosing and administration schedules administered to glioblastoma patients using the immunocompetent, syngeneic glioblastoma GL261 and CT-2A murine models. Our group and others have previously demonstrated that the GL261 model responds favorably to anti-PD-1 therapy, most likely due to its inherent immunogenicity and high tumor mutational burden (13,22,23). The GL261 model has been appropriately criticized as being unrepresentative of human glioblastoma tumors, which typically exhibit low immunogenicity and mutational burden, creating an immunologically “cold” tumor microenvironment (24,25). Despite the heightened immunogenicity of the GL261 model, we find that concurrent dexamethasone limits the therapeutic benefit of anti-PD-1 immune checkpoint blockade even at low doses. We also noted that the timing of dexamethasone appears to be relevant. Dexamethasone administered concurrently with anti-PD-1 exerted a detrimental effect on survival in our murine GBM models, whereas dexamethasone administered prior to initiation of anti-PD-1 did not – although this difference may have reflected the short half-life of dexamethasone in mice and the reduced exposure associated with the pre-anti-PD-1 dexamethasone administration schedule. Additionally, the effects of concurrent dexamethasone were dose-dependent, with high dexamethasone dose levels (10 mg/kg) reducing the long-term survival rate by half as compared to low dose levels (1 mg/kg).
Because radiation therapy is an established cornerstone of glioblastoma therapy, we evaluated the effect of concurrent dexamethasone on the survival associated with anti-PD-1 therapy when combined with fractionated radiation therapy using a schedule analogous to that used to treat human patients. We deliberately employed a radiation schedule that prolongs survival but fails to cure most tumor-bearing mice, as this is the typical effect of radiation in glioblastoma patients. Using a dosing schedule of 2 Gy daily for 5 days, we observed a modest survival benefit for both the GL261-luc2 and CT-2A-luc syngeneic glioblastoma models. When dexamethasone was concurrently administered, there was a trend towards decreased survival from PD-1 blockade combined with RT; although these analyses were not powered to detect an additive effect of PD-1 blockade with RT. Of note, concurrent dexamethasone did not appear to affect memory T cell responses based on our demonstration that long-term survivors were capable of rejecting tumor re-challenges, regardless of whether the mice had received concurrent dexamethasone – a finding that recapitulates what has previously been reported in both intracranial and subcutaneous tumor models (7,26). Although studies from both tumor and viral preclinical settings found that memory T cells are sensitive to glucocorticoid-induced apoptosis, recent work indicates that concurrent corticosteroids, through suppression of critical fatty acid metabolism pathways, selectively diminish and impair the low-affinity, but not the high-affinity memory CD8+ T cells (7,27,28). Together these data suggest that for highly immunogenic tumors, high-affinity anti-tumor memory T cell populations may persist despite corticosteroid-induced lymphopenia at the time of immune checkpoint blockade therapy. Further study of the effects of corticosteroids on T cell memory responses are warranted.
We then investigated the mechanisms underlying the attenuated survival associated with the addition of dexamethasone to anti-PD-1 therapy in our syngeneic glioblastoma models. In our experiments, concurrent dexamethasone markedly decreased overall CD3+ T lymphocyte counts, including CD4+ and CD8+ T cells, isolated from the tumor, draining cervical lymph nodes, spleen, and thymus. Our findings corroborate those of a recent study in which the number of peripheral blood CD4+ and CD8+ T cells were reduced when dexamethasone was administered to GL261-luc bearing mice (26). Similar to other tumor models, we observed that the same dose of corticosteroids can exert differential effects on T cells depending on whether they reside in peripheral or intratumoral compartments – with greater lymphodepletion generally displayed by the peripheral compartments (29). We found that the mechanism of T cell depletion associated with concurrent dexamethasone dosing involved, at least in part, inducing apoptosis of CD4+ and CD8+ T cells beginning as early as 1 hour after dexamethasone initiation, even at relatively low dexamethasone dose levels. This effect persisted through five days after dexamethasone initiation and was increased with repeated higher dexamethasone doses. Additionally, we investigated the effect of dexamethasone on lymphocyte proliferation and found that dexamethasone reduced the absolute numbers of proliferative T cells, although the proportion of surviving T cells that were proliferative did not decrease. Depending on their dosing and timing, corticosteroids have been found to have varied suppressive and supportive effects on lymphocyte proliferation (29–31). Additionally, distinct immune cell types and states have been shown to exhibit differential sensitivity to dexamethasone, which may underlie the differences that we observed in CD4+ and CD8+ TILs and peripheral T cells. For example, in in vitro cultures of human T cells, dexamethasone impaired proliferation of naive T cells – but not memory T cells – by upregulating expression of CTLA-4 in naïve T cells. Inhibition of CTLA-4, but not PD-1, was then able to partially restore proliferation (27).
In addition to quantitative effects on immune cell subsets, concurrent dexamethasone dosing also impacted functional capacity. We observed that dexamethasone decreased the ability of splenic CD4+ and CD8+ T cells to generate IFNγ responses and decreased the number of CD4+ and CD8+ cells expressing the early activation marker CD69 that were isolated from cLN, spleen, and intracranial tumor. We also evaluated the effect of dexamethasone on innate immunity and noted decreases in most myeloid subsets and NK cells, as well as decreased levels of activated (CD80+ CD86+) myeloid and dendritic cells. In order to comprehensively profile the immune microenvironment irrespective of response to anti-PD-1 therapy, entire tumor-bearing tissues were evaluated, therefore it is possible that endogenous extratumoral immune cells were included in our analyses. However, their contribution is expected to be marginal due to the brain’s unique immunological niche, which is characterized by a relative paucity of lymphocytes, non-microglial myeloid cells, and dendritic cells (32).
In accordance with our study findings, a recent study utilizing the GL261-luc model also demonstrated that dexamethasone administered for 5 days concurrently with anti-PD-1 therapy was associated with decreased survival, including fewer long-term survivors, compared to mice who did not receive dexamethasone (26). However, protracted dexamethasone administration before, during, and after PD-1 dosing did not appear to impact survival in that study. In contrast to our study, a subset of tumor-bearing mice treated with dexamethasone alone remained long-term survivors, whereas we found that all mice treated with dexamethasone monotherapy succumbed to progressive tumor with comparable survival to that of untreated controls. The discordance between our results and the findings of the previous study may reflect the different anti-PD-1 antibodies and dosing schedules, different tumor cell inocula, and different sources of dexamethasone.
To evaluate whether our preclinical findings are clinically relevant, we retrospectively evaluated the survival outcome among 181 consecutive IDH-wildtype glioblastoma patients treated at our institution with anti-PD-(L)1 therapy. We found that patients who were not on baseline dexamethasone had an improved OS compared to those on dexamethasone, independent of key prognostic factors like disease setting, age, MGMT promoter methylation status, KPS, tumor size, or extent of resection prior to anti-PD-(L)1 treatment. Additionally, the detrimental effects of dexamethasone were independent of dexamethasone dose: both lower (<2mg) and higher (≥2mg) doses were associated with worse OS following anti-PD-(L)1 treatment. Our analysis, however, was not powered to assess OS differences between dose levels. Due to the retrospective and heterogenous nature of our data, our results require prospective validation in a randomized controlled trial, but they are consistent with the planned subgroup analyses of a recent randomized phase 3 study in which recurrent glioblastoma patients treated with nivolumab had poorer survival if they were on baseline dexamethasone compared to those who were not (1). This result supports our preclinical and clinical data indicating that dexamethasone contributes to limit the therapeutic benefit of immune checkpoint blockade among glioblastoma patients. Our findings have salient implications for ongoing and planned clinical trials that are evaluating combinations of immunotherapeutic agents, including checkpoint inhibitors, with other therapeutic agents for glioblastoma patients; as well as for patients with a spectrum of brain metastasis types, where immune checkpoint inhibitors are part of standard-of-care management and corticosteroids are often indicated (33–35). Further evaluation of the effect of dexamethasone and other corticosteroids on patients’ outcomes for such immunotherapy treatments for glioblastoma and oncology in general is warranted.
Conclusions
In conclusion, we demonstrate that the concurrent systemic administration of dexamethasone diminishes the survival benefits associated with PD-1 immune checkpoint blockade in both anti-PD-1-resistant (CT-2A) and anti-PD-1-responsive (GL261) immunocompetent, syngeneic glioblastoma models in a dose-dependent manner. Although the GL261 model recapitulates some of the cell-of-origin and histopathologic features of human glioblastoma tumors, its marked mutational load and intrinsic immunogenicity lead to an overestimation of therapeutic benefit from immune checkpoint blockade relative to what has been observed among glioblastoma patients (23,24,36). The heightened sensitivity of this model to immune checkpoint blockade makes it all the more striking that concurrent dexamethasone administration – even at relatively low doses – attenuated the therapeutic benefit of PD-1 inhibition. These findings were also replicated in our experiments with the more clinically-relevant, immunoresistant CT-2A syngeneic glioblastoma model; where concurrent dexamethasone abrogated the survival benefits associated with either PD-1 monotherapy or anti-PD-1 administered along with fractionated RT. Our findings are consistent with preclinical studies demonstrating that concurrent dexamethasone also diminished anti-tumor immune responses and therapeutic benefits associated with Delta24-RGD oncolytic virus therapy (37). We further demonstrated that concurrent dexamethasone led to quantitative and qualitative/functional decreases in both adaptive and innate immune effector cells. Our data attribute decreased lymphocyte levels to increased apoptosis associated with dexamethasone administration. Among IDH-wildtype glioblastoma patients undergoing anti-PD-(L)1 treatment, we demonstrate that baseline dexamethasone use is associated with poorer survival even after adjustment for disease setting, age, tumor size, tumor resection, MGMT promoter methylation, and patient’s performance status. Our data also support recent findings that older GBM patients have worse survival than younger patients following immune checkpoint therapy (4).
Taken together, our results further support accumulating concerns that corticosteroids can be detrimental to immunotherapy for oncology patients: dexamethasone therapy, which is typically used to treat symptomatic cerebral edema in glioblastoma patients, limits the therapeutic benefit of immune checkpoint blockade. Careful evaluation of dexamethasone use is warranted for neuro-oncology patients undergoing immunotherapy clinical trials. Our preclinical analyses also indicate that the detrimental effect of dexamethasone appears to be dose dependent, suggesting that the lowest possible dose should be used for patients where the concurrent use of dexamethasone is unavoidable. Evaluation of alternative approaches to treat symptomatic cerebral edema such as inhibition of vascular permeability induced by vascular endothelial growth factor merit further study as strategies to limit dexamethasone exposure among glioblastoma patients receiving immunotherapy.
Supplementary Material
Statement of translational relevance.
Increasing data indicate that corticosteroids can exert a detrimental effect on immunotherapy for oncology patients. Dexamethasone, a uniquely potent corticosteroid, is frequently administered to glioblastoma patients to decrease tumor-associated edema, but limited data exist describing how it affects systemic and intratumoral immune activity – particularly in the context of immunotherapy. We demonstrate that concurrent dexamethasone administration, even at a low dose, limits the therapeutic benefit of anti-PD-1 therapy both in mouse glioblastoma models and in a retrospective cohort of 181 IDH-wildtype glioblastoma patients. Mechanistically, dexamethasone decreased intratumoral T cells and systemic levels of T cells, natural killer cells, and myeloid cells, while qualitatively impairing lymphocyte function. The mechanism of T cell depletion included induction of apoptosis. These findings indicate that dexamethasone hinders both adaptive and innate immune responses and that its administration should be carefully assessed among glioblastoma patients undergoing immunotherapy clinical trials.
ACKNOWLEDGEMENTS
We gratefully acknowledge the following organizations for funding support: The Jennifer Oppenheimer Cancer Research Initiative; The Ben and Catherine Ivy Foundation; Hope It’s A Beach Thing; and the Pan Mass Challenge (Erica’s Entourage and CRUS11TOUR). Funding support was also from the NIH: P01CA236749 (D. Reardon, E. Chiocca, A. Sharpe, A. Anderson, G. Freeman, and P. Gokhale), K12CA090354 (J. Iorgulescu), P50CA101942 (A. Sharpe and G. Freeman), P01AI39671 (A. Sharpe), U54CA224088 (G. Baker, P. Sorger, and A. Sharpe), R01CA203873 (E Chiocca), P01CA069246 (E Chiocca), P01CA163205 (E. Chiocca), R01NS110942 (E. Chiocca), P01336749 (E. Chiocca), P50CA165962 (K. Ligon), and R01CA229400 (A. Anderson). J. Iorgulescu acknowledges support from the Conquer Cancer Foundation, Friends of Dana-Farber Cancer Institute, and Brigham Research Institute (NextGen award). We thank Min Wu for assistance in generating CT-2A luciferase-transduced cells, and Drs. Geoffrey Young, Lei Qin, Xin Chen, and Jing Li for assistance in evaluation of patients’ radiographic imaging.
Funding support: We gratefully acknowledge the following organizations for funding support: The Jennifer Oppenheimer Cancer Research Initiative; The Ben and Catherine Ivy Foundation; Hope It’s A Beach Thing; and the Pan Mass Challenge (Erica’s Entourage and CRUS11TOUR). Funding support was also from the NIH: P01CA236749 (D. Reardon, E. Chiocca, A. Sharpe, A. Anderson, G. Freeman, and P. Gokhale), K12CA090354 (J. Iorgulescu), P50CA101942 (A. Sharpe and G. Freeman), P01AI39671 (A. Sharpe), U54CA224088 (G. Baker, P. Sorger, and A. Sharpe), R01CA203873 (E Chiocca), P01CA069246 (E Chiocca), P01CA163205 (E. Chiocca), R01NS110942 (E. Chiocca), P01336749 (E. Chiocca), P50CA165962 (K. Ligon), and R01CA229400 (A. Anderson). J. Iorgulescu acknowledges support from the Conquer Cancer Foundation, Friends of Dana-Farber Cancer Institute, and Brigham Research Institute (NextGen award).
David A. Reardon has received research support from: Acerta Pharmaceuticals; Agenus; Celldex; EMD Serono; Incyte; Inovio; Midatech; Omniox; Tragara. He has received compensation for advisory board participation from: Abbvie; Advantagene; Agenus; Amgen; Bayer; Bristol-Myers Squibb; Celldex; DelMar; EMD Serono; Genentech/Roche; Imvax; Inovio; Merck; Merck KGaA; Monteris; Novocure; Oncorus; Oxigene; Regeneron; Stemline; Taiho Oncology, Inc.
Gordon Freeman has served on advisory boards for Roche, Bristol-Myers-Squibb, Xios, Origimed, Triursus, iTeos, NextPoint, IgM, and Jubilant. He has patents/pending royalties with Roche, Merck MSD, Bristol-Myers-Squibb, Merck KGA, Boehringer-Ingelheim, AstraZeneca, Dako, Leica, Mayo Clinic, and Novartis.
Arlene Sharpe has served on advisory boards for CoStim, is on advisory boards for Surface Oncology, Elstar, SQZ Biotechnologies, Selecta, Elpiscience, and Monopteros, has research funding from Novartis, Roche, Ipsen, Quark and Merck, and is a consultant for Novartis. She has patents/pending royalties from Roche and Novartis.
E. Antonio Chiocca is currently an advisor to Advantagene Inc., Alcyone Biosciences, Insightec, Inc., DNAtrix Inc, Immunomic Therapeutics, Sangamo Therapeutics, Seneca Therapeutics and has equity interest in DNAtrix, Immunomic Therapeutics, Seneca Therapeutics; he has also advised Oncorus, Merck, Tocagen, Ziopharm, Stemgen, NanoTx., Ziopharm Oncology, Cerebral Therapeutics, Genenta. Merck, Janssen, Karcinolysis, Shanghai Biotech. He has received research support from NIH, US Department of Defense, American Brain Tumor Association, National Brain Tumor Society, Alliance for Cancer Gene Therapy, Neurosurgical Research Education Foundation, Advantagene, NewLink Genetics and Amgen. He also is a named inventor on patents related to oncolytic HSV1 and noncoding RNAs.
Ana C. Anderson is on the scientific advisory boards for Tizona Therapeutics, Compass Therapeutics, Zumutor Biologics, and Astellas Global Pharma.
Keith L. Ligon is a co-founder of Travera LLC and is on scientific advisory boards of IntegraGen and RareCyte, and is a consultant for BMS, BroadBranch, and receives research support from Bristol Myers Squibb, Amgen, Novartis, Deciphera, Tragara, X4, CrownBio, and Lilly.
Peter K. Sorger is on the Board of Directors or SAB for RareCyte, Glencoe Software, NanoString and Applied BioMath.
Footnotes
Prior Presentation: none.
Conflict of interest statement: None of the other authors have any conflict of interest with the subject matter or materials discussed in the manuscript.
REFERENCES
- 1.Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma. JAMA Oncol. 2020;e201024. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro-Oncol. 2015;17 Suppl 7:vii9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perng P, Lim M. Immunosuppressive Mechanisms of Malignant Gliomas: Parallels at Non-CNS Sites. Front Oncol. 2015;5:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ladomersky E, Zhai L, Lauing K, Bell A, Xu J, Kocherginsky M, et al. Advanced Age Increases Immunosuppression in the Brain and Decreases Immunotherapeutic Efficacy in Subjects with Glioblastoma. Clin Cancer Research. 2020. doi: 10.1158/1078-0432.CCR-19-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36:2872–8. [DOI] [PubMed] [Google Scholar]
- 6.Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune Checkpoint Inhibitor Outcomes for Patients With Non–Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J Clin Oncol. 2019;37:1927–34. [DOI] [PubMed] [Google Scholar]
- 7.Tokunaga A, Sugiyama D, Maeda Y, Warner AB, Panageas KS, Ito S, et al. Selective inhibition of low-affinity memory CD8+ T cells by corticosteroids. J Exp Med. 2019;216:2701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiocca EA, Yu JS, Lukas RV, Solomon IH, Ligon KL, Nakashima H, et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: Results of a phase 1 trial. Sci Transl Med. 2019;11:eaaw5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kostaras X, Cusano F, Kline GA, Roa W, Easaw J. Use of dexamethasone in patients with high-grade glioma: a clinical practice guideline. Curr Oncol Tor Ont. 2014;21:e493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth P, Happold C, Weller M. Corticosteroid use in neuro-oncology: an update. Neuro-Oncol Pract. 2015;2:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reardon DA, Gokhale PC, Klein SR, Ligon KL, Rodig SJ, Ramkissoon SH, et al. Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic, Immunocompetent Model. Cancer Immunol Res. 2016;4:124–35. [DOI] [PubMed] [Google Scholar]
- 14.Maes W, Van Gool SW. Experimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 model. Cancer Immunol Immunother. 2011;60:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakashima H, Alayo QA, Penaloza-MacMaster P, Freeman GJ, Kuchroo VK, Reardon DA, et al. Modeling tumor immunity of mouse glioblastoma by exhausted CD8+ T cells. Sci Rep. 2018;8:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res. 2016;22:886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124:3706–14. [DOI] [PubMed] [Google Scholar]
- 18.Pitter KL, Tamagno I, Alikhanyan K, Hosni-Ahmed A, Pattwell SS, Donnola S, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139:1458–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shields LBE, Shelton BJ, Shearer AJ, Chen L, Sun DA, Parsons S, et al. Dexamethasone administration during definitive radiation and temozolomide renders a poor prognosis in a retrospective analysis of newly diagnosed glioblastoma patients. Radiat Oncol. 2015;10:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafson MP, Lin Y, New KC, Bulur PA, O’Neill BP, Gastineau DA, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro-Oncol. 2010;12:631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chitadze G, Flüh C, Quabius ES, Freitag-Wolf S, Peters C, Lettau M, et al. In-depth immunophenotyping of patients with glioblastoma multiforme: Impact of steroid treatment. Oncoimmunology 2017; 6: e1358839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johanns TM, Ward JP, Miller CA, Wilson C, Kobayashi DK, Bender D, et al. Endogenous Neoantigen-Specific CD8 T Cells Identified in Two Glioblastoma Models Using a Cancer Immunogenomics Approach. Cancer Immunol Res. 2016;4:1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Touat M, Li YY, Boynton AN, Spurr LF, Iorgulescu JB, Bohrson CL, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutledge WC, Kong J, Gao J, Gutman DA, Cooper LAD, Appin C, et al. Tumor-Infiltrating Lymphocytes in Glioblastoma Are Associated with Specific Genomic Alterations and Related to Transcriptional Class. Clin Cancer Res. 2013;19:4951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maxwell R, Luksik AS, Garzon-Muvdi T, Hung AL, Kim ES, Wu A, et al. Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. Oncoimmunology. 2018;7:e1500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giles AJ, Hutchinson M-KND, Sonnemann HM, Jung J, Fecci PE, Ratnam NM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar D, Sehrawat S. Divergent Effects of a Transient Corticosteroid Therapy on Virus-Specific Quiescent and Effector CD8+ T Cells. Front Immunol. 2019;10:1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aston WJ, Hope DE, Cook AM, Boon L, Dick I, Nowak AK, et al. Dexamethasone differentially depletes tumour and peripheral blood lymphocytes and can impact the efficacy of chemotherapy/checkpoint blockade combination treatment. Oncoimmunology. 2019;8(11):e1641390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianchi M, Meng C, Ivashkiv LB. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc Natl Acad Sci U S A. 2000;97(17):9573–9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franchimont D, Galon J, Vacchio MS, Fan S, Visconti R, Frucht DM, et al. Positive effects of glucocorticoids on T cell function by up-regulation of IL-7 receptor alpha. J Immunol. 2002;168(5):2212–2218. [DOI] [PubMed] [Google Scholar]
- 32.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18(2):123–131. [DOI] [PubMed] [Google Scholar]
- 33.Medikonda R, Dunn G, Rahman M, Fecci P, Lim M. A review of glioblastoma immunotherapy. J Neurooncol. 2020; 10.1007/s11060-020-03448-1. [DOI] [PubMed] [Google Scholar]
- 34.Parakh S, Park JJ, Mendis S, Rai R, Xu W, Lo S, et al. Efficacy of anti-PD-1 therapy in patients with melanoma brain metastases. Br J Cancer. 2017;116(12):1558–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. [DOI] [PubMed] [Google Scholar]
- 36.Candolfi M, Curtin JF, Nichols WS, Muhammad AG, King GD, Pluhar GE, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleijn A, Kloezeman J, Treffers-Westerlaken E, Fulci G, Leenstra S, Dirven C, et al. The in vivo therapeutic efficacy of the oncolytic adenovirus Delta24-RGD is mediated by tumor-specific immunity. PloS One. 2014;9:e97495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin J-R, Izar B, Wang S, Yapp C, Mei S, Shah PM, et al. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. eLife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin J-R, Fallahi-Sichani M, Sorger PK. Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nat Commun. 2015;6:8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng T, Thorn K, Schroeder T, Wang L, Theis FJ, Marr C, et al. A BaSiC tool for background and shading correction of optical microscopy images. Nat Commun. 2017;8:14836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. ArXiv150504597 Cs [Internet]. 2015. [cited 2020 Jun 18]; Available from: http://arxiv.org/abs/1505.04597 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.