Abstract
Vaccines developed in high-income countries have been enormously successful in reducing the global burden of infectious diseases, saving perhaps 2.5 million lives per year, but even for successful cases, like the rotavirus vaccine, global implementation may take a decade or more. For unincentivized vaccines, the delays are even more profound, as both the supply of a vaccine from developing country manufacturers and vaccine demand from countries with the high disease burdens have to be generated in order for impact to be manifest. A number of poverty-associated infectious diseases, whose burden is greatest in low-income and middle-income countries, would benefit from appropriate levels of support for vaccine development such as Group A Streptococcus, invasive non-typhoid salmonella, schistosomiasis, shigella, to name a few.
With COVID-19 vaccines we will hopefully be able to provide novel vaccine technology to all countries through a unique collaborative effort, the COVAX facility, led by the World Health Organization (WHO), Gavi, and the Coalition for Epidemic Preparedness Innovations (CEPI). Whether this effort can deliver vaccine to all its participating countries remains to be seen, but this ambitious effort to develop, manufacture, distribute, and vaccinate 60–80% of the world’s population will hopefully be a lasting legacy of COVID-19.
Current Opinion in Immunology 2021, 71:13–20
This review comes from a themed issue on Vaccines
Edited by Sara Cooper and Charles S Wiysonge
For a complete overview see the Issue and the Editorial
Available online 10th April 2021
https://doi.org/10.1016/j.coi.2021.03.009
0952-7915/© 2021 Published by Elsevier Ltd.
Introduction
Vaccines are one of the most successful and cost-effective disease prevention strategies ever implemented [1]. One of the biggest challenges in global health has been securing access to these technologies for people in greatest need. Projections suggest that barely more than half the world’s children will be fully immunized by 2030 [2]. To extend the beneficial impact of vaccines and vaccination on global health, new vaccines for additional diseases as well as improved supply and delivery mechanisms need to be developed, particularly in low-income and middle-income countries (LMIC) [2,3]. While considerable attention has been given to new vaccines for emerging or re-emerging diseases by developing a proactive approach and novel mechanisms such as CEPI, other poverty-related diseases and/or neglected tropical disease vaccines have raised much less attention [4].
Vaccine impact and access
The Global Vaccine Action Plan (GVAP) 2011–2020 endorsed by the World Health Assembly estimated that substantial progress toward GVAP goals could potentially avert 25 million vaccine-preventable deaths by the end of the decade. In its Midterm Review, however, the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) concluded that strenuous efforts by all countries and immunization stakeholders were needed to catch up and achieve GVAP goals (reviewed in Ref. [5]).
Vaccine coverage is an important indicator of the performance of national health and immunization systems (Table 1 ). Although coverage rates of DTP3 reached 85% globally, there were still 19.3 million children who failed to receive basic vaccines. Full Immunization coverage also varies by income group [6], with low-income countries (LIC) achieving just 74% in 2019 while lower middle-income countries (MIC), upper-MIC and high-income countries achieved 84%, 92% and 89–95%, respectively [7]. Further, while immunization coverage rates and new vaccine introductions have been increasing over the past several years, they have now stagnated and risk decline in the midst of the current COVID-19 pandemic [8]. Routine vaccination in MICs lags behind countries with the weakest economies as well as those with ongoing socio-political instability. Unsupported MICs are also forgoing introduction of important new vaccines that have huge impact on death and disease rates (pneumococcal conjugate vaccine, rotavirus vaccine). In countries that transitioned out of Gavi, economic growth not distributed equitably can lead to inequities in access to new vaccines as well [9].
Table 1.
Percentage of global population vaccinated, by antigen, and by year
| 2019 | 2018 | 2017 | 2016 | 2015 | 2000 | 1990 | 1980 | |
|---|---|---|---|---|---|---|---|---|
| BCG | 88 | 89 | 89 | 89 | 87 | 80 | 81 | 15 |
| DTP1 | 90 | 90 | 90 | 90 | 89 | 83 | 88 | 30 |
| DTP3 | 85 | 85 | 86 | 86 | 85 | 72 | 75 | 20 |
| HepB BD | 43 | 41 | 40 | 35 | 35 | 5 | ||
| HepB3 | 85 | 83 | 84 | 84 | 83 | 30 | 1 | |
| Hib 3 | 72 | 71 | 71 | 70 | 63 | 13 | ||
| IPV1 | 82 | 72 | 57 | 46 | 22 | |||
| MCV1 | 85 | 85 | 85 | 85 | 85 | 72 | 73 | 16 |
| MCV2 | 71 | 69 | 68 | 67 | 63 | 18 | ||
| PCV3 | 48 | 46 | 44 | 42 | 37 | |||
| Pol3 | 86 | 85 | 85 | 85 | 85 | 73 | 75 | 21 |
| RCV1 | 71 | 69 | 52 | 48 | 47 | 21 | 8 | 3 |
| rotac | 39 | 34 | 27 | 24 | 22 | |||
| TT2plus | 71 | 72 | 73 | 72 | 70 | 62 | 55 | 9 |
| YFV | 46 | 45 | 44 | 42 | 41 | 11 |
Determinants of vaccine coverage in LMICs vary by vaccine and can be influenced by factors including facility readiness, characteristics of the child, mother’s ability and willingness to vaccinate and awareness, perceptions, and social norms within the community [10]. Community engagement and education are key factors for success [11, 12, 13]. Financing can be a barrier at a national and subnational level, impacting vaccine access, particularly in MICs. Supply side factors are influenced by poor forecasting, planning capacity or ability to procure at affordable prices [14] and challenges in managing budgets and the flow of funds can lead to a lack of product availability or stockouts [10]. Geographical inequities explored in mapping vaccination coverage are greatly influenced by the country vaccine delivery mechanisms. Understanding the spatial variation in vaccination coverage in the context of varying vaccine delivery strategies is important for evaluating the performance of vaccination programs [15].
The expectation that more and more vaccinations can be administered concomitantly is increasingly unfeasible (Table 2 ). Child health programs are seeking opportunities for new contacts within the first two years of life to deliver vaccines and other services in an integrated way. Moreover, some vaccines must be given at ages outside traditionally scheduled visits to induce protective immunity. New platforms will be required, including for maternal immunization to prevent neonatal diseases (e.g. respiratory syncytial virus, group B streptococcal disease), adolescent immunization (e.g. human papilloma virus, dengue, tuberculosis), and in the second year of life (e.g. malaria), and young adults (HIV) [16], and now all age groups at unprecedented scale for COVID-19 vaccines.
Table 2.
EPI vaccines and variants (per age groups and geographic location), Emerging Infectious Disease vaccines, and development gaps
| EPI vaccines and variants | BCG, OPV/IPV, DPT/DTaP/DT, MMR, HBV, HiB, PCV, Rota, PCV, JE, seasonal flu, VZV, HAV, HPV (2-dose, single dose), OCV, MenACWY, TCV, Yellow Fever, malaria, HEV, dengue |
| EPI vaccine gaps | RSV, CMV, universal seasonal flu, improved TB vaccine, improved malaria, shigella, ETEC, Campylobacter, Staphylococcus aureus, paratyphoid A, invasive Non-Typhoid Salmonella, Group B Streptococcus, Group A Streptococcus, schistosomiasis, other helminthiasis, Chikungunya, plague |
| EID licensed vaccine | Ebola |
| EID vaccine gaps | COVID-19, HIV, pandemic flu (avian, swine), Zika, SARS, MERS, Lassa, Nipah, Marburg, West Nile, Rift Valley |
One of the consequences of the COVID-19 pandemic is its impact on the delivery of health services, including immunizations in LMICs [17]. Preventive mass vaccination campaigns can also inadvertently contribute to COVID-19 spread, and WHO is recommending that these campaigns be carefully considered based on a benefit-risk assessment (WHO/2019-nCoV/immunization_services/2020.1). There will be a need for ‘catch-up’ campaigns, to identify those who missed their immunizations as well as re-establishing community demand. During the last Ebola outbreak, twice as many children died of measles than of Ebola in the Democratic Republic of the Congo [18]. The deaths prevented by sustaining routine childhood immunization in Africa outweigh the excess risk of COVID-19 deaths associated with vaccination clinic visits, especially for the vaccinated children. Routine childhood immunization should be sustained in Africa as much as possible, while considering other factors such as logistical constraints, staff shortages, and reallocation of resources during the COVID-19 pandemic [19].
Vaccine demand
Promising vaccines for diseases that disproportionately affect people in LMICs need help to reach the market [20••]. This requires the development of novel technologies and health and societal economic models able to capture not only the cost–benefit of vaccination, but also its full societal value [21,22]. Estimates of the magnitude of these broader benefits suggest that vaccination has been substantially undervalued [23••].
Implementation gap
Experience provides several examples of vaccines where access has been delayed in the so-called ‘second valley of death’ [24] or the implementation gap, the period between product licensure and public health implementation and impact, particularly due to delays in prequalification of Strategic Advisory Group of Experts (SAGE) recommendations.
Some lessons learned
Following Phase 3 trial results in 2014 [25] the European Medicines Agency issued a positive scientific opinion for RTS,S malaria vaccine in 2015 and WHO recommended pilot implementation to assess the feasibility of administering four doses of required vaccine in children, the vaccine’s role in reducing childhood deaths and severe malaria, and its safety in the context of routine use. It took, however, four years for the world’s first malaria vaccine from the demonstration of RTS,S partial protection against malaria in young children before implementation in three sub-Saharan African countries.
Hepatitis E vaccine (Hecolin®, Xiamen Innovax, China) has >90% efficacy at five years but has not yet been prequalified (PQ); requirements for PQ were not prioritized and additional clinical studies were requested by the SAGE. The absence of PQ and SAGE recommendation have impaired its use in a number of hepatitis E outbreaks, where mortality in pregnant women can be in excess of 40%.
The example of Oral Cholera Vaccine (OCV) is instructive. In 2009 SAGE recommended use of OCV in areas with endemic cholera and consideration for use in areas at risk for cholera outbreaks. Shanchol™ OCV was prequalified in 2011, Euvichol® in 2015, and a Gavi-funded stockpile created in 2013, but only available for outbreaks. Shantha Biotech (Sanofi) would not commit to increase production. WHO announced a global plan to reduce cholera deaths by 90% by 2030, and countries that previously had hidden cholera behind ‘acute watery diarrhea’ were preparing national cholera control plans. In order to achieve a virtuous cycle of supply and demand, manufacturing, funding for clinical development, and regulatory approvals (including WHO PQ) were needed on the supply side, while for demand — disease burden, cost effectiveness, SAGE recommendation, and a global eradication campaign were needed to convince ministries of health (and finance) that there was substantial benefit in procuring and implementing OCV vaccination. For ‘unincentivized’ vaccines funding and coordination are critical to avoid implementation gaps.
Impact of COVID-19 on routine immunization programs
Some other lessons should be remembered as we implement COVID-19 vaccination worldwide, in adults as well as children. Rotavirus vaccine (RV) is an example. A highly effective vaccine against rotavirus diarrhea was approved in the US in 2006 and was prequalified and recommended by WHO in 2009. By 2015, only 20% of the world’s children had received all three doses of RV (International Vaccine Access Center, 2016); 11 years after its recommendation, in 2020, 60% of children globally had not received three doses of RV. With premium vaccines, like rotavirus, pneumococcal conjugate, and HPV, the greatest number of unvaccinated children live in MIC, countries also hard-hit by COVID-19. COVID-19 vaccines must not suffer the Access Gap and should be globally accessible and equitably distributed [26••,27].
Policy recommendations for new vaccines may only be realized through implementation research to determine how to most effectively ensure widespread use, particularly for populations that have not been a target for vaccination in the past [28•]. Failure to tackle this implementation phase with the same commitment shown to the research and development phase poses substantial risk for vaccines developed for the world’s poorest and most vulnerable people.
Unincentivized vaccines
Unincentivized vaccines are those for which there is little awareness of the target disease among the public, policy makers, and scientists and little perceived incentive for major vaccine manufacturers to engage in vaccine development activities. In addition to vaccines included on a list of WHO Neglected Tropical Diseases, vaccines targeting hepatitis E, Group A Streptococcus (GAS, Streptococcus pyogenes), invasive non-typhoidal Salmonella (iNTS), and Shigella belong to this category.
GAS is responsible for a wide range of acute and chronic clinical manifestations in humans. GAS infections and adverse consequences are estimated to cause about 500 000 annual deaths, in all age ranges, mostly in young adults. Yet, GAS has received little attention in global health programs, and existing tools for prevention are insufficient [29]. In 2018 a World Health Assembly resolution on rheumatic heart disease (RHD), a potential complication of GAS infections, highlighted the need for GAS vaccines to complement control strategies. An R&D roadmap and preferred products characteristics are published [30••] and a Full Value of Vaccine Assessment (FVVA) has been undertaken by the Strep A Vaccine Global Consortium (SAVAC, https://savac.ivi.int/about).
iNTS is another major public health problem in Sub-Saharan Africa [31,32]. The need for vaccines against iNTS is now increasingly being recognized, but there is no iNTS vaccine available for use in humans. A similar FVVA process has been undertaken to accelerate the development of vaccine candidates.
Vaccine supply and delivery
A traditional partnership model consists of research and development with large pharma leading to licensure in a high income country first (e.g. rotavirus vaccine, HPV vaccines). Whether the company continues to supply to high-income and middle-income and Gavi-supported countries is subject to multiple considerations, and global shortfalls in production are often seen (HPV, pneumococcal conjugate, rotavirus). Companies sometime license an aspect of manufacturing (e.g. fill/finish) or the complete manufacturing process to developing country vaccine manufacturers (DCVMs) [33], as recently illustrated for COVID-19 vaccine with the AstraZeneca partnership with Serum Institute of India for the supply of the vaccine to the Indian Government but also to a large number of LMICs (AstraZeneca press release, 6 January 2021).
This model would be sustainable for all parties [34]. However, global access to vaccine technology becomes more of a reality when DCVMs develop similar products and compete in vaccine tender requests from major purchasers (UNICEF, PAHO).
Developing countries vaccine manufacturers and technology transfers
An alternative model envisions accelerating the development of vaccines for LMICs by in- house development or early technology transfer to a DCVM from a research organization (university, product development partnership, etc.). Funding could be derived from the DCVM (such as the Bharat Chikungunya vaccine) or from a funding agency such as the Bill and Melinda Gates Foundation (meningitis A vaccine, oral cholera vaccine, etc.). Licensing would be established between technology owner and the DCVM. The DCVM becomes responsible for the manufacturing and clinical development, perhaps supplemented by the funding partners, followed by regulatory approval for licensure and WHO prequalification (PQ).
The Developing Countries Vaccine Manufacturers Network (DCVMN) was established in 2000 with the mission to increase the availability and affordability of quality vaccines to protect against known and emerging infectious diseases [35]. About 70% of the global EPI vaccine supplies and about 75% procured by UN agencies are produced by DCVMN [36]. Several technology transfers to DCVMs have occurred over the past decades to significantly contribute to global health.
Following an initial collaboration on OCV between Sweden and VABIOTECH in Vietnam, IVI improved the OCV then transferred the technology to several DCVMs including Shantha Biotechnics (Shanchol™), India; EuBiologics (Euvichol®), Republic of Korea; and Incepta (Cholvax), Bangladesh. Shanchol™, Euvichol®, and Euvichol® Plus are WHO-prequalified are the major contributors to the WHO stockpile [37] while Cholvax is marketed in Bangladesh.
The Typhoid Conjugate Vaccine (TCV) candidate developed by IVI consists of Vi-polysaccharide conjugated to DT (Vi-DT). Initially transferred to Shantha Biotechnics, the technology was transferred to SK bioscience (Republic of Korea), PT Bio Farma (Indonesia), and Incepta Vaccines (Bangladesh) [38•]. After promising Phase II results [39], SK bioscience Vi-DT has completed Phase 3 in Nepal and the Philippines, and plans for WHO PQ.
Creating the demand and ensuring the supply chain
The introduction of vaccines in LMICs has been plagued by a vicious cycle of uncertain demand leading to limited supply, which keeps prices relatively high and, in turn, further uncertainty of demand. Based on experience with accelerating the adoption of Hib, pneumococcal and rotavirus vaccines, a framework for new vaccine adoption was proposed organizing the major steps in the process into a continuum from evidence to policy, implementation and finally access [40].
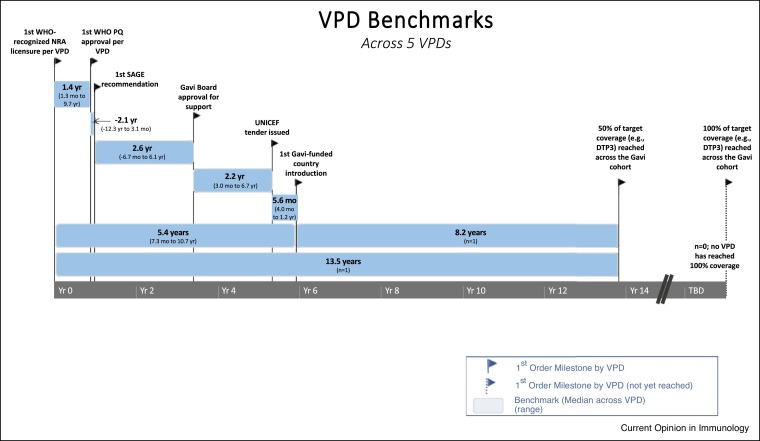
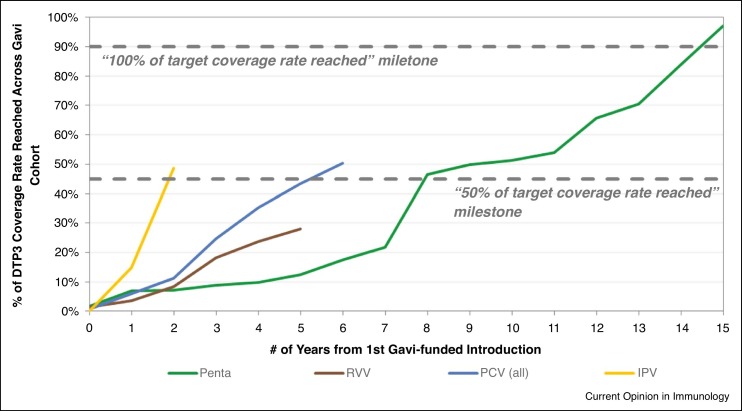
In order to be effectively used in global health settings, assuming they are listed among the Gavi priorities, vaccines must go through several steps, including, WHO PQ enabling procurement by UN agencies, a recommendation by SAGE and position paper by WHO, approval by the Gavi board to release funding support and a tender issued by UNICEF to enable procurement. Additionally, countries must apply for Gavi support, and Gavi may enable procurement for the country and issue a vaccine introduction grant so the country can plan for introduction (Figure 1 ). The process can happen fairly quickly, particularly when outbreaks are a concern or can take several years [27] (Figure 2 ). Timelines vary significantly by product and steps taken to accelerate vaccine introduction [40].
Figure 1.
Timeline and milestones of the supply chain for five vaccine preventable diseases (VPD). Courtesy of International Vaccine Access Center.
Figure 2.
Vaccine timelines and uptake from first Gavi-supported introduction for four Vaccine Preventable Diseases (Penta: pentavalent (diphtheria, tetanus, whooping cough, hepatitis B and Haemophilus influenzae type B) vaccine; RVV: rotavirus vaccine; PCV: pneumococcal conjugate vaccine; IPV: inactivate poliomyelitis virus vaccine). Courtesy of International Vaccine Access Center. Source: World Health Organization. WHO/UNICEF Estimates of National Immunization Coverage for 1980-2016, 2017. https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragedtp3.html.
WHO has developed the Country-led Assessment for Prioritization on Immunization, (CAPACITI), based on the Total System Effectiveness (TSE) framework to assist national policy-makers in prioritizing vaccines. A rotavirus vaccine (RVV) test case was used to compare the decision criteria made by the existing processes for vaccine prioritization and the TSE-pilot model, using Thailand-specific data. The existing decision-making processes in Thailand and TSE offered similar recommendations on the selection of a RVV product and the TSE process was deemed a well-reasoned and step-by-step approach for countries, especially LMIC, to develop a systematic and transparent decision-making process for immunization policy [41].
Presentation and delivery methods, thermostability and cold chain
The addition of new vaccines to the EPI schedule resulted in an increase in the cost and complexity of the immunization supply chain and vaccine delivery. In some cases, approximately half of the costs to vaccinate a child are associated with management of vaccine supply logistics and health care worker administration [42]. Innovative technologies and approaches are needed to simplify vaccine delivery by removing the need for a cold chain, minimizing the packaging footprint, easing administration, and reducing waste [43,44]. For example, technology improvements may include fractional dose for intradermal delivery (inactivated poliovirus vaccine), microarray patches (measles-rubella vaccine), controlled temperature chain allowing storage and distribution of the vaccine out of the traditional 2–8°C cold chain in ambient temperatures up to 40°C, for three days or more, depending on the antigen (meningitis A vaccine) [45]. Another example is the presentation of OCV (Euvichol®) in plastic tubes instead of glass vials that considerably simplified the manufacturing process, reduced the unit cost, storage and administration of OCV and significantly contributed to increase the WHO stockpile [37].
SARS-CoV-2 vaccines — learning curve
Until end of 2019, the vaccine world followed its usual cycle of discovery, development, and delivery. Then came an unexpected and unprecedented worldwide public health threat that disrupted lives, economies and society at large. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China. Exposure to SARS-CoV-2 results in a range of COVID-19 clinical outcomes, from asymptomatic infection to severe acute respiratory distress and death [46]. On 30 January 2020, WHO declared the outbreak a public health emergency of international concern, and on 12 March 2020, a pandemic.
Considerable mitigation measures were undertaken by some countries and have resulted in reductions (for a period of time) of the number of infections and deaths [47,48]. The pressure of the pandemic on economies, health systems, and populations highlighted the need for the rapid development of safe and effective vaccines, as a critical strategy in containing this pandemic.
Developers, manufacturers, regulators, and public health authorities have been confronted to a series of new paradigms including development of unprecedented number of vaccines simultaneously, extensive use of global contract manufacturing and licensing to scale up production, and global cooperation via COVAX. Will these be legacies for other global health vaccines or fall into oblivion once the pandemic has recessed?
Several vaccine manufacturers have recently announced that their SARS-CoV-2 vaccine candidates were safe and efficacious against COVID-19. Hopefully promising results with other vaccines will soon follow as billions of doses will be needed worldwide over the next years. However, the true global health impact of these vaccines remains to be demonstrated through scale-up, systems to manage cold chain, vaccine acceptance, and the ability to demonstrate long-term safety, among other things.
COVAX partners' (Gavi, CEPI, WHO) fair allocation mechanism defines quantities each country will receive when a COVID-19 vaccine becomes available [49], and the WHO SAGE Values Framework provides guidance on how a country might prioritize doses received. At least in the first phase of allocations, each country will receive a proportional amount of their population beginning at 3% going up to 20%. The first priority includes frontline health and social care workers who are at high risk of disease and support critical infrastructure in the country. Older adults and those who have underlying conditions are next.
The distribution of COVID-19 vaccines will be a task of unprecedented magnitude and scale, involving logistical task forces, in particular for cold chain and vaccine administration (some vaccines will require ultra-cold chain storage and delivery which many countries are not set up to handle), coordination to ensure just-in-time supply and follow-up for a second dose, accountability, and communication.
Concerns about delivery include transitioning a system largely set up for children (the extended program on immunizations (EPI) to a universal, staged immunization program. Older adults generally do not access vaccination systems, and mechanisms and infrastructure established for women and children go for vaccination need to be expanded or supplemented [28•]. Ebola provides some important lessons learned, although we also need to acknowledge that a vaccine program of this magnitude has never been attempted. We must also consider that dialogue around speed of development and the use of novel unlicensed technologies have also caused some to be suspicious of new vaccines. Vaccine hesitancy and misinformation represent substantial obstacles to achieving coverage and community immunity [50,51]. Differences in intentions of the public to receive COVID-19 vaccines ranged from almost 90% (China) to less than 55% (Russia) [52•].
Effectiveness, virus variants, and safety surveillance
The major reason for conducting vaccine effectiveness assessments is to make sure a vaccine protects people from getting a disease under real-world conditions, outside of the strict setting of clinical trials, modulated by the effectiveness of non-pharmaceutical interventions [53]. Vaccine effectiveness assessments can also provide important information about how well a vaccine is working in groups of people not included or not well represented in clinical trials. Surveillance of circulating SARS-COV-2 variants in vaccinated and non-vaccinated populations is also critical to assess whether vaccine-induced immune responses are still countering the virus [54•].
Unanswered questions around safety and longer-term adverse events will require continued follow-up of vaccine trial participants and as monitoring of adverse events following immunization (AEFI) after vaccines are introduced [55••]. Key lessons could be learnt from introduction of new vaccines in response to previous pandemic and epidemic emergencies (H1N1, Ebola). Infrastructure, pharmacovigilance capacity and plan for safety of COVID-19 vaccines should be in place in all countries before new vaccines are introduced and continued post introduction. Sophisticated systems are needed to track and trace on a global scale who was immunized with which COVID-19 vaccine product, where and when. Public concerns about the safety of the novel vaccines and rumors that can arise during the current and future pandemics and the need for program managers to be ready to address them through appropriate vaccine safety pharmacovigilance and communication strategies.
Conclusion
Vaccines developed in high-income countries have been enormously successful in reducing the global burden of infectious diseases, saving perhaps 2.5 million lives per year, but even for successful cases, like the rotavirus vaccine, global implementation may take a decade or more. For unincentivized vaccines, the delays are even more profound, as both the supply of a vaccine from DCVMNs and vaccine demand from countries with the high disease burdens have to be generated in order for impact to be manifest. A number of poverty-associated infectious diseases, whose burden is greatest in LMICs, would benefit from appropriate levels of support for vaccine development — GAS, iNTS, schistosomiasis, shigella, to name a few.
With COVID-19 vaccines we will hopefully be able to provide novel vaccine technology to all countries through a unique collaborative effort led by WHO, Gavi, and CEPI. Whether COVAX can deliver vaccine to all its participating countries remains to be seen, but this ambitious effort to develop, manufacture, distribute, and vaccinate 60–80% of the world’s population will hopefully be a lasting legacy of COVID-19.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
References
- 1.Plotkin S.A., Plotkin S.L. The development of vaccines: how the past led to the future. Nat Rev Microbiol. 2011;9:889–893. doi: 10.1038/nrmicro2668. [DOI] [PubMed] [Google Scholar]
- 2.Orenstein W.A., Seib K., Graham-Rowe D., Berkley S. Contemporary vaccine challenges: improving global health one shot at a time. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3009848. [DOI] [PubMed] [Google Scholar]
- 3.Guignard A., Praet N., Jusot V., Bakker M., Baril L. Introducing new vaccines in low- and middle-income countries: challenges and approaches. Expert Rev Vaccines. 2019;18:119–131. doi: 10.1080/14760584.2019.1574224. [DOI] [PubMed] [Google Scholar]
- 4.Hotez P.J. Ten failings in global neglected tropical diseases control. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daugherty M.A., Hinman A.R., Cochi S.L., Garon J.R., Rodewald L.E., Nowak G., McKinlay M.A., Mast E.E., Orenstein W.A. The Global Vaccine Action Plan - insights into its utility, application, and ways to strengthen future plans. Vaccine. 2019;37:4928–4936. doi: 10.1016/j.vaccine.2019.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Restrepo-Mendez M.C., Barros A.J., Wong K.L., Johnson H.L., Pariyo G., Franca G.V., Wehrmeister F.C., Victora C.G. Inequalities in full immunization coverage: trends in low- and middle-income countries. Bull World Health Organ. 2016;94:794–805B. doi: 10.2471/BLT.15.162172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . Full Child Immunization Coverage Estimates by World Bank Income Group. WHO; Geneva, Switzerland: 2020. Global health observatory. [Google Scholar]
- 8.World Health Organization . WHO; Geneva, Switzerland: 2020. WHO and UNICEF Warn of a decline in Vaccinations During COVID-19. [Google Scholar]
- 9.Berkley S. Vaccination lags behind in middle-income countries. Nature. 2019;569:309. doi: 10.1038/d41586-019-01494-y. [DOI] [PubMed] [Google Scholar]
- 10.Phillips D.E., Dieleman J.L., Lim S.S., Shearer J. Determinants of effective vaccine coverage in low and middle-income countries: a systematic review and interpretive synthesis. BMC Health Serv Res. 2017;17:681. doi: 10.1186/s12913-017-2626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usman S., Bologna L., Stamidis K.V. The CORE Group Partners Project in North East Nigeria: community engagement strategies to combat skepticism and build trust for vaccine acceptance. Am J Trop Med Hyg. 2019;101:68–73. doi: 10.4269/ajtmh.19-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demissie S.D., Kozuki N., Olorunsaiye C.Z., Gebrekirstos P., Mohammed S., Kiapi L., Chantler T., Karafillakis E., Landegger J. Community engagement strategy for increased uptake of routine immunization and select perinatal services in north-west Ethiopia: a descriptive analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization and International Initiative for Impact Evaluation . WHO; Geneva, Switzerland: 2017. An Evidence Map of Social, Behavioural and Community Engagement Interventions for Reproductive, Maternal, Newborn and Child Health. [Google Scholar]
- 14.Mihigo R., Okeibunor J., Cernuschi T., Petu A., Satoulou A., Zawaira F. Improving access to affordable vaccines for middle-income countries in the african region. Vaccine. 2019;37:2838–2842. doi: 10.1016/j.vaccine.2019.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Utazi C.E., Thorley J., Alegana V.A., Ferrari M.J., Takahashi S., Metcalf C.J.E., Lessler J., Cutts F.T., Tatem A.J. Mapping vaccination coverage to explore the effects of delivery mechanisms and inform vaccination strategies. Nat Commun. 2019;10 doi: 10.1038/s41467-019-09611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien K.L., Binka F., Marsh K., Abramson J.S. Mind the gap: jumping from vaccine licensure to routine use. Lancet. 2016;387:1887–1889. doi: 10.1016/S0140-6736(16)30394-4. [DOI] [PubMed] [Google Scholar]
- 17.Hogan A.B., JB, Sherrard-Smith E., et al. Imperial College; London: 2020. The Potential Impact of the COVID-19 Epidemic on HIV, TB and Malaria in Low- and Middle-Income Countries. [Google Scholar]
- 18.Nelson R. COVID-19 disrupts vaccine delivery. Lancet Infect Dis. 2020;20:546. doi: 10.1016/S1473-3099(20)30304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbas K., Procter S.R., van Zandvoort K., Clark A., Funk S., Mengistu T., Hogan D., Dansereau E., Jit M., Flasche S., et al. Routine childhood immunisation during the COVID-19 pandemic in Africa: a benefit-risk analysis of health benefits versus excess risk of SARS-CoV-2 infection. Lancet Glob Health. 2020;8:e1264–e1272. doi: 10.1016/S2214-109X(20)30308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Kaslow D.C., Black S., Bloom D.E., Datla M., Salisbury D., Rappuoli R. Vaccine candidates for poor nations are going to waste. Nature. 2018;564:337–339. doi: 10.1038/d41586-018-07758-3. [DOI] [PubMed] [Google Scholar]; Red flag article — promising immunizations for diseases that affect mostly people in low-income and middle-income countries need help getting to market.
- 21.Rappuoli R., Pizza M., Del Giudice G., De Gregorio E. Vaccines, new opportunities for a new society. Proc Natl Acad Sci U S A. 2014;111:12288–12293. doi: 10.1073/pnas.1402981111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gessner B.D., Kaslow D., Louis J., Neuzil K., O’Brien K.L., Picot V., Pang T., Parashar U.D., Saadatian-Elahi M., Nelson C.B. Estimating the full public health value of vaccination. Vaccine. 2017;35:6255–6263. doi: 10.1016/j.vaccine.2017.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Bloom D.E., Fan V.Y., Sevilla J.P. The broad socioeconomic benefits of vaccination. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aaj2345. [DOI] [PubMed] [Google Scholar]; Evaluating vaccination programs according to their broad socioeconomic benefits, beyond their health benefits, to address vaccine underutilization and weak incentives for vaccine innovation.
- 24.Butler D. Translational research: crossing the valley of death. Nature. 2008;453:840–842. doi: 10.1038/453840a. [DOI] [PubMed] [Google Scholar]
- 25.Olotu A., Fegan G., Wambua J., Nyangweso G., Leach A., Lievens M., Kaslow D.C., Njuguna P., Marsh K., Bejon P. Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. N Engl J Med. 2016;374:2519–2529. doi: 10.1056/NEJMoa1515257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Kim J.H. SARS-CoV-2 vaccine development, access, and equity. J Exp Med. 2020;217 doi: 10.1084/jem.20201288. [DOI] [PMC free article] [PubMed] [Google Scholar]; Concise, sharp and incisive opinion paper on COVID-19 vaccine 'make it, prove it, use it' framework.
- 27.Luthra K., Zimmermann A.J., Vasudevan P., Privor-Dumm L. Pneumococcal conjugate vaccine introduction and uptake timelines for Gavi supported countries. International Symposium on Pneumococci and Pneumococcal Disease; Melbourne, Australia; 2018. [Google Scholar]
- 28•.Privor-Dumm L.A., Poland G.A., Barratt J., Durrheim D.N., Deloria Knoll M., Vasudevan P., Jit M., Bonvehi P.E., Bonanni P. International Council on Adult Immunization: a global agenda for older adult immunization in the COVID-19 era: a roadmap for action. Vaccine. 2020 doi: 10.1016/j.vaccine.2020.06.082. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; Proposed framework for delivering routine and pandemic vaccines to adult populations.
- 29.Macleod C.K., Bright P., Steer A.C., Kim J., Mabey D., Parks T. Neglecting the neglected: the objective evidence of underfunding in rheumatic heart disease. Trans R Soc Trop Med Hyg. 2019;113:287–290. doi: 10.1093/trstmh/trz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Vekemans J., Gouvea-Reis F., Kim J.H., Excler J.L., Smeesters P.R., O’Brien K.L., Van Beneden C.A., Steer A.C., Carapetis J.R., Kaslow D.C. The path to group A streptococcus vaccines: World Health Organization research and development technology roadmap and preferred product characteristics. Clin Infect Dis. 2019;69:877–883. doi: 10.1093/cid/ciy1143. [DOI] [PMC free article] [PubMed] [Google Scholar]; Example of the proposed research and development pathway of an unicentivized vaccine targeting a neglected disease.
- 31.Marks F., von Kalckreuth V., Aaby P., Adu-Sarkodie Y., El Tayeb M.A., Ali M., Aseffa A., Baker S., Biggs H.M., Bjerregaard-Andersen M., et al. Incidence of invasive salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study. Lancet Glob Health. 2017;5:e310–e323. doi: 10.1016/S2214-109X(17)30022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J.H., Mogasale V., Im J., Ramani E., Marks F. Updated estimates of typhoid fever burden in sub-Saharan Africa. Lancet Glob Health. 2017;5:e969. doi: 10.1016/S2214-109X(17)30328-5. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization . World Health Organization; Geneva, Switzerland: 2011. Increasing Access to Vaccines Through Technology Transfer and Local Production. [Google Scholar]
- 34.Rappuoli R., Black S., Bloom D.E. Vaccines and global health: in search of a sustainable model for vaccine development and delivery. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aaw2888. [DOI] [PubMed] [Google Scholar]
- 35.Pagliusi S., Leite L.C., Datla M., Makhoana M., Gao Y., Suhardono M., Jadhav S., Harshavardhan G.V., Homma A. Developing Countries Vaccine Manufacturers Network: doing good by making high-quality vaccines affordable for all. Vaccine. 2013;31(Suppl. 2):B176–B183. doi: 10.1016/j.vaccine.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 36.Jadhav S., Gautam M., Gairola S. Role of vaccine manufacturers in developing countries towards global healthcare by providing quality vaccines at affordable prices. Clin Microbiol Infect. 2014;20(Suppl. 5):37–44. doi: 10.1111/1469-0691.12568. [DOI] [PubMed] [Google Scholar]
- 37.Shaikh H., Lynch J., Kim J., Excler J.L. Current and future cholera vaccines. Vaccine. 2020;38(Suppl. 1):A118–A126. doi: 10.1016/j.vaccine.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 38•.Syed K.A., Saluja T., Cho H., Hsiao A., Shaikh H., Wartel T.A., Mogasale V., Lynch J., Kim J.H., Excler J.L., et al. Review on the recent advances on typhoid vaccine development and challenges ahead. Clin Infect Dis. 2020;71:S141–S150. doi: 10.1093/cid/ciaa504. [DOI] [PMC free article] [PubMed] [Google Scholar]; Illustration of the successful outcome of technology transfer to developing country manufacturers for typhoid conjugate vaccines.
- 39.Capeding M.R., Sil A., Tadesse B.T., Saluja T., Teshome S., Alberto E., Kim D.R., Park E.L., Park J.Y., Yang J.S., et al. Safety and immunogenicity of Vi-DT conjugate vaccine among 6-23-month-old children: phase II, randomized, dose-scheduling, observer-blind study. EClinicalMedicine. 2020;27 doi: 10.1016/j.eclinm.2020.100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine O.S., Hajjeh R., Wecker J., Cherian T., O’Brien K.L., Knoll M.D., Privor-Dumm L., Kvist H., Nanni A., Bear A.P., et al. A policy framework for accelerating adoption of new vaccines. Hum Vaccin. 2010;6:1021–1024. doi: 10.4161/hv.6.12.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rattanavipapong W., Kapoor R., Teerawattananon Y., Luttjeboer J., Botwright S., Archer R.A., Giersing B., Hutubessy R.C.W. Comparing 3 approaches for making vaccine adoption decisions in Thailand. Int J Health Policy Manag. 2020;9:439–447. doi: 10.15171/ijhpm.2020.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Portnoy A., Ozawa S., Grewal S., Norman B.A., Rajgopal J., Gorham K.M., Haidari L.A., Brown S.T., Lee B.Y. Costs of vaccine programs across 94 low- and middle-income countries. Vaccine. 2015;33(Suppl 1):A99–A108. doi: 10.1016/j.vaccine.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 43.Chen D., Zehrung D. Desirable attributes of vaccines for deployment in low-resource settings. J Pharm Sci. 2013;102:29–33. doi: 10.1002/jps.23352. [DOI] [PubMed] [Google Scholar]
- 44.Kristensen D.D., Bartholomew K., Villadiego S., Lorenson K. What vaccine product attributes do immunization program stakeholders value? Results from interviews in six low- and middle-income countries. Vaccine. 2016;34:6236–6242. doi: 10.1016/j.vaccine.2016.10.057. [DOI] [PubMed] [Google Scholar]
- 45.Giersing B.K., Modjarrad K., Kaslow D.C., Moorthy V.S., WHO Product Development for Vaccine Advisory Committee Report from the World Health Organization’s Product Development for Vaccines Advisory Committee (PDVAC) meeting, Geneva, 7-9th Sep 2015. Vaccine. 2016;34:2865–2869. doi: 10.1016/j.vaccine.2016.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schunemann H.J. C-SURGEs: physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chinazzi M., Davis J.T., Ajelli M., Gioannini C., Litvinova M., Merler S., Pastore Y.P.A., Mu K., Rossi L., Sun K., et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368:395–400. doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization . WHO; Geneva, Switzerland: 2020. Fair Allocation Mechanism for COVID-19 Vaccines Through the COVAX Facility. [Google Scholar]
- 50.Larson H.J., Jarrett C., Eckersberger E., Smith D.M., Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine. 2014;32:2150–2159. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 51.Lane S., MacDonald N.E., Marti M., Dumolard L. Vaccine hesitancy around the globe: analysis of three years of WHO/UNICEF Joint Reporting Form data-2015-2017. Vaccine. 2018;36:3861–3867. doi: 10.1016/j.vaccine.2018.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Lazarus J.V., Ratzan S.C., Palayew A., Gostin L.O., Larson H.J., Rabin K., Kimball S., El-Mohandes A. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27:225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Survey of COVID-19 vaccine in 19 countries flagging the governance and cultural differences.
- 53.Haug N., Geyrhofer L., Londei A., Dervic E., Desvars-Larrive A., Loreto V., Pinior B., Thurner S., Klimek P. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat Hum Behav. 2020;4:1303–1312. doi: 10.1038/s41562-020-01009-0. [DOI] [PubMed] [Google Scholar]
- 54•.Dearlove B., Lewitus E., Bai H., Li Y., Reeves D.B., Joyce M.G., Scott P.T., Amare M.F., Vasan S., Michael N.L., et al. A SARS-CoV-2 vaccine candidate would likely match all currently circulating variants. Proc Natl Acad Sci U S A. 2020;117:23652–23662. doi: 10.1073/pnas.2008281117. [DOI] [PMC free article] [PubMed] [Google Scholar]; Providing the basis for future molecular epidemiology studies of circulating SARS-CoV-2 variants post vaccination.
- 55••.Haynes B.F., Corey L., Fernandes P., Gilbert P.B., Hotez P.J., Rao S., Santos M.R., Schuitemaker H., Watson M., Arvin A. Prospects for a safe COVID-19 vaccine. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abe0948. [DOI] [PubMed] [Google Scholar]; Emphasising on vaccine safety: Rigorous clinical trial design and post-licensure surveillance should provide a reliable strategy to identify adverse events, including the potential for enhanced severity of COVID-19 disease, following vaccination.