Abstract
Background:
Case-control studies from the early 2000s demonstrated that HPV-related oropharyngeal cancer (HPV-OPC) is a distinct entity associated with number of oral sex partners. Using contemporary data, we investigated novel risk factors (sexual debut behaviors, exposure intensity, and relationship dynamics) and serological markers on odds of HPV-OPC.
Methods:
HPV-OPC patients and frequency-matched controls were enrolled in a multi-center study from 2013–2018. Participants completed a behavioral survey. Characteristics were compared using χ2 for categorical and t-test for continuous variables. Adjusted odds ratios (aOR) were calculated using logistic regression.
Results:
163 HPV-OPC cases and 345 controls were included. Lifetime number of oral sex partners was associated with significantly increased odds of HPV-OPC (>10 partners, odds ratio[OR]=4.3, 95% confidence interval[CI]=2.8–6.7). After adjustment for number of oral sex partners and smoking, younger age at first oral sex (<18 vs.>20 years, aOR=1.8, 95% CI=1.1–3.2) and oral sex intensity (>5 sex-years, aOR=2.8, 95% CI=1.1–7.5) remained associated with significantly increased odds of HPV-OPC. Type of sexual partner such as older partners when a case was younger (OR=1.7, 95% CI=1.1–2.6) or having a partner who had extramarital sex (OR=1.6, 95% CI=1.1–2.4) was associated with HPV-OPC. Seropositivity for antibodies to HPV16 E6 (OR=286, 95% CI=122–670) and any HPV16 E protein (E1,E2,E6,E7; OR=163, 95% CI=70–378) was associated with increased odds of HPV-OPC.
Conclusion:
Number of oral sex partners remains a strong risk factor for HPV-OPC, however timing and intensity of oral sex are novel independent risk factors. These behaviors suggest additional nuances of how and why some individuals develop HPV-OPC.
Keywords: Oropharyngeal neoplasms, sexual behavior, risk factors, papillomaviridae, head and neck cancer
Precis:
In this most comprehensive behavioral case-control study of HPV-related oropharyngeal cancer to date, ever performing oral sex and number of partners remain strong risk factors for HPV-related oropharyngeal cancer. Measures of oral sexual behavior including early age and intensity of exposure are independent risk factors, suggesting these behaviors may explain additional nuances of how and why some people develop HPV-related oropharyngeal cancer.
Introduction
The epidemiology of head and neck cancer has changed dramatically in recent decades. Human papillomavirus (HPV) has driven an increase in incidence of oropharynx cancer (OPC) in the United States and other countries,1,2 which is thought to be explained by trends in oral sexual behavior.3 Case-control studies have demonstrated strong associations between sexual behaviors and odds of HPV-OPC.4–9 However, these studies focused primarily on number of sexual partners without other contextual data about relationship dynamics, intensity of exposure, or order of acts at sexual debut.
It has been hypothesized, though not fully examined, that the sequence of specific sexual behaviors at debut may predispose to HPV infection.10 Depending on site of initial mucosal exposure, serologic response may also differ.11,12 For example, it has been posited that those whose initial exposure to HPV is through vaginal sex have a more robust immune response which decreases chances of subsequent acquisition when exposed to HPV orally. Conversely, exposure to oral HPV without the initial anogenital HPV exposure may increase the risk of oral HPV acquisition, persistence, and HPV-OPC. However, there is little such data to support these hypotheses.
Therefore, we performed a comprehensive contemporaneous examination of sexual and other novel risk factors for HPV-OPC. To better understand the role of other behavioral factors in HPV-OPC risk, we explored differences in sexual behavior, relationship dynamics, and serologic response to HPV between cases of HPV-OPC and controls
Methods
Study Participants
Participants were enrolled in a previously described multicenter case-control study of squamous cell carcinomas called the Papillomavirus Role in Oral cancer Viral Etiology study (PROVE).13 Briefly, cases with newly diagnosed OPC were enrolled between 2013 and 2018 at three National Comprehensive Cancer Network-designated cancer centers: the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital (JHH, Baltimore, MD), University of California San Francisco Helen Diller Family Comprehensive Cancer Center (UCSF, San Francisco, CA) and Tisch Cancer Institute at Icahn School of Medicine at Mount Sinai (MSHS, New York, NY). Cases had incident OPC and no prior history of malignancy (except skin cancer) or systemic chemotherapy. Controls were adult patients from otolaryngology clinics without a chief complaint related to cancer. Consent was obtained from all participants and the study was approved by the institutional review board at each site.
Data Collection
At enrollment, each participant completed a survey, provided a blood sample, and a tumor sample was obtained from cases. Medical record abstraction was performed. The behavioral risk survey was available in Mandarin, Spanish, and English and was administered through computer assisted self-interview (CASI) taken on either a tablet or computer. The survey included detailed questions on lifetime and recent sexual behaviors including number of partners, age of sexual initiation, type and order of sexual acts, partner dynamics, use of substances with sex, and extramarital sex. Data on demographics, substance use, and comorbidities were also collected.
Cases were centrally tested for p16 immunohistochemistry (MTM Laboratories, Heidelberg, Germany) and HPV16 E6/E7 RNA in situ hybridization (ISH; RNAscope®, Advanced Cell Diagnostics, Hayward, CA) at Johns Hopkins and interpreted by a head and neck pathologist (L.M.R.) to determine whether they were HPV-related.12
Specimen Testing
Serum was obtained before initiation of treatment and tested for antibodies to E6 and E7 for oncogenic HPV types 16, 18, 31, 33, 35, 45, 52, and 58 and E1, E2, E4 for HPV types 16 and 18 at the German Cancer Research Center (DKFZ, Heidelberg, Germany). In brief, multiplex serology, an antibody detection method based on glutathione S-transferase (GST) capture ELISA was used in combination with fluorescent bead-based technology.14,15 Each antibody response was considered seropositive or seronegative based upon standardized cutoff values for median fluorescence intensity (MFI).16
Medical record abstraction was performed at the time of diagnosis for tumor site, tumor stage, and nodal stage using American Joint Committee on Cancer (AJCC) 7th edition,17 with additional abstraction later to record primary treatment modality. Data were stored using RedCap (Vanderbilt University, Nashville, TN).
Analysis
This analysis was restricted to HPV-positive cases who answered the “ever” oral sex survey question (97% included) and controls frequency-matched by age (decade of age), sex, race (White Non-Hispanic [NH], Black NH, Hispanic, Asian/Pacific Islander, Other/Multiracial), and study site. HPV-positivity was defined as being p16-positive and ISH-positive (RNA and/or DNA). For this analysis, all available matched controls who answered the survey question on whether they ever performed oral sex were included. Characteristics of cases and matched controls were compared using χ2 for categorical and t-test for continuous variables. Odds ratios (OR) and 95% confidence intervals were calculated using conditional logistic regression. Multivariable models were performed adjusting for tobacco use (pack-years) and number of lifetime oral sex partners (categorical) to understand the independent effect of other sexual risk factors after these two known risk factors were controlled for, and adjusted OR (aOR) was reported. Similar adjusted models were run adjusting for lifetime number of vaginal sex partners instead of oral sex partners to understand the impact of oral sex specifically separate from general sexual exposure.
Variables were evaluated as both continuous and categorical variables, and alternative cutoffs were examined for categorical variables to explore dose response and ensure consistency of result regardless of selected cutoff (results not shown). Intensity of exposure was illustrated by sex-years, a novel metric defined as number of partners per ten years since sexual debut,18 analogous to how pack-years describes tobacco history and drink-years characterizes alcohol use.19 Never smoking was defined as <1 pack-year. Statistical significance was defined when the two-sided p-value was less than 0.05. The analysis was performed using STATA version 15.1 (College Station, TX).
Results
The study population consisted of 163 incident HPV-OPC cases and 345 matched controls with similar demographic characteristics (Table 1). The majority were male, 50–69 years of age, currently married or living with a partner, and identified as heterosexual. Cases were more likely to have a history of sexually transmitted infection than controls (p=0.003).
Table 1:
Baseline demographics for HPV-OPC cases and controls
| Characteristic | Cases | Controls | p-value |
|---|---|---|---|
| n=163 | n=345 | ||
| Sex | 0.36 | ||
| Male | 85.3% | 82.0% | |
| Female | 14.7% | 18.0% | |
| Age group | 0.10 | ||
| 18–29 | 0.6% | 0.3% | |
| 30–39 | 1.8% | 1.5% | |
| 40–49 | 15.9% | 10.1% | |
| 50–59 | 38.7% | 31.6% | |
| 60–69 | 33.1% | 39.4% | |
| 70–79 | 7.4% | 14.2% | |
| 80–89 | 2.5% | 2.9% | |
| Race | 0.65 | ||
| Non-Hispanic White | 87.1% | 85.5% | |
| Non-Hispanic Black | 6.1% | 8.4% | |
| Other | 6.8% | 6.1% | |
| Study site | 0.14 | ||
| JHU | 68.0% | 76.0% | |
| UCSF | 16.0% | 13.3% | |
| MSHS | 16.0% | 10.7% | |
| Currently married or living with a partner | 0.57 | ||
| No | 22.7% | 25.0% | |
| Yes | 77.3% | 75.0% | |
| Income | 0.74 | ||
| <$15,000 | 5.3% | 2.9% | |
| $15,000–49,999 | 10.6% | 12.2% | |
| $50,000–99,999 | 27.2% | 28.0% | |
| $100,000–199,999 | 36.4% | 37.6% | |
| >$200,000 | 20.5% | 19.3% | |
| Highest degree | 0.35 | ||
| No High school | 4.3% | 2.3% | |
| High school | 17.2% | 15.1% | |
| Some college | 19.0% | 18.3% | |
| College | 34.4% | 31.1% | |
| Graduate | 25.1% | 33.2% | |
| Sexual orientation | 0.34 | ||
| Heterosexual | 95.7% | 93.6% | |
| Homosexual/Bisexual/Other/Not sure | 4.3% | 6.4% | |
| History of sexually transmitted infection | 0.003 | ||
| No | 73.3% | 84.5% | |
| Yes | 26.7% | 15.5% |
Abbreviations: JHU, Johns Hopkins University, UCSF, University of California; MSHS, Mount Sinai Health System
Bolding indicates statistical significance.
Sexual Behaviors
Differences in sexual behaviors between cases and controls are shown in Table 2. Oral sex timing, number, and intensity of partners were each associated with the diagnosis of HPV-OPC. HPV-related OPC cases were more likely than controls to have ever performed oral sex (98.8% vs. 90.4%, p<0.001) and to have performed oral sex at the time of sexual debut (Figure 1A; 33.3% in cases vs. 21.4% in controls, p=0.004; OR 1.8, 95% CI 1.2–2.8). Age of first performing oral sex was significantly younger among HPV-OPC cases than controls (Figure 1B; <18 years vs. >20 years, 37.4% vs. 22.6%, p<0.001; OR 3.1, 95% CI 2.0–5.0). Number of lifetime oral sex partners was higher among those with HPV-OPC (Figure 1C; >10 partners, 44.8% vs. 19.1%, p<0.001; OR 4.3, 95% CI 2.8–6.7). Intensity of oral sexual exposure, measured by sex-years (number of partners per ten years) was significantly higher among cases than controls (Figure 1D; >5 sex-years, 30.8% vs. 11.1%, p<0.001, OR 5.6, 95% CI 3.3–9.6). After adjustment for lifetime number of oral sex partners and tobacco use (pack-years), ever performing oral sex (aOR 4.4, 95% CI 1.1–18.9), early age of first oral sex encounter (<18 years vs. >20 years, aOR 1.8, 95% CI 1.1–3.2) and oral sex intensity (>5 oral sex-years, aOR 2.8, 95% CI 1.1–7.5) each remained significantly associated with increased odds of HPV-OPC.
Table 2:
Sexual behavior of HPV-OPC cases vs. controls 1
| Characteristic | Cases | Controls | p-value | OR (95%CI) | aOR (95% CI)* |
|---|---|---|---|---|---|
| Oral Sex Behaviors | |||||
| Ever performed oral sex | <0.001 | ||||
| No | 9.6% | Ref | Ref | ||
| Yes | 90.4% | 8.5 (2.0–35.9) | 4.4 (1.1–18.9) | ||
| Oral sex at debut | 0.004 | ||||
| No | 78.6% | Ref | Ref | ||
| Yes | 21.4% | 1.8 (1.2–2.8) | 1.4 (0.9–2.2) | ||
| Age at first oral sex encounter (years) | <0.001 | ||||
| >20 | 50.1% | Ref | Ref | ||
| 18–20 | 27.3% | 2.5 (1.6–4.0) | 2.1 (1.3–3.5) | ||
| <18 | 22.6% | 3.1 (2.0–5.0) | 1.8 (1.1–3.2) | ||
| Oral sex partners | <0.001 | ||||
| 0-≤5 | 66.7% | Ref | N/A | ||
| >5-≤10 | 14.2% | 2.5 (1.4–4.3) | |||
| >10 | 19.1% | 4.3 (2.8–6.7) | |||
| Oral Sex-Years (# partners/10 years since sexual debut) | <0.001 | ||||
| 0–1 | 56.1% | Ref | Ref | ||
| >1–5 | 32.9% | 2.5 (1.6–4.0) | 1.7 (0.8–3.5) | ||
| >5 | 11.1% | 5.6 (3.3–9.6) | 2.8 (1.1–7.5) | ||
| Other Sexual Behaviors | |||||
| Vaginal sex at debut | 0.003 | ||||
| No | 22.0% | Ref | Ref | ||
| Yes | 78.0% | 0.5 (0.4–0.8) | 0.7 (0.5–1.2) | ||
| Vaginal sex partners | <0.001 | ||||
| 0-≤5 | 46.5% | Ref | Ref | ||
| >5-≤10 | 20.4% | 2.7 (1.5–4.9) | 2.7 (1.4–5.2) | ||
| >10 | 33.2% | 5.1 (3.1–8.3) | 3.2 (1.7–6.0) | ||
| Vaginal sex-years(# partners/10 years since sexual debut) | <0.001 | ||||
| 0–1 | 36.1% | Ref | Ref | ||
| >1–5 | 46.1% | 3.8 (2.1–7.0) | 3.5 (1.8–6.8) | ||
| >5 | 17.8% | 9.3 (4.9–17.6) | 5.7 (2.6–12.5) | ||
| Deep kissing partners | <0.001 | ||||
| 0-≤5 | 28.3% | Ref | Ref | ||
| >5-≤10 | 25.7% | 1.8 (0.9–3.7) | 1.7 (0.8–3.5) | ||
| >10 | 46.0% | 4.9 (2.7–9.0) | 2.6 (1.3–5.3) | ||
| Casual partners | <0.001 | ||||
| 0–1 | 38.6% | Ref | Ref | ||
| 2–10 | 36.8% | 2.3 (1.4–3.8) | 1.6 (1.0–2.8) | ||
| >10 | 24.6% | 3.0 (1.8–5.1) | 1.2 (0.6–2.2) | ||
| Relationship Dynamics | |||||
| Extramarital sex by either partner | 0.002 | ||||
| No | 59.8% | Ref | Ref | ||
| Yes | 36.0% | 1.6 (1.1–2.4) | 1.0 (0.6–1.5) | ||
| I suspect my partner did | 4.2% | 3.4 (1.6–7.5) | 3.6 (1.6–8.3) | ||
| When <age 23, had partner >10 years older | 0.01 | ||||
| No | 80.0% | Ref | Ref | ||
| Yes | 20.0% | 1.7 (1.1–2.6) | 1.1 (0.7–1.8) |
Adjusted for number of lifetime oral sex partners and pack-years of smoking
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; Ref, reference group
Bolding indicates statistical significance with p<0.05.
Figure 1:
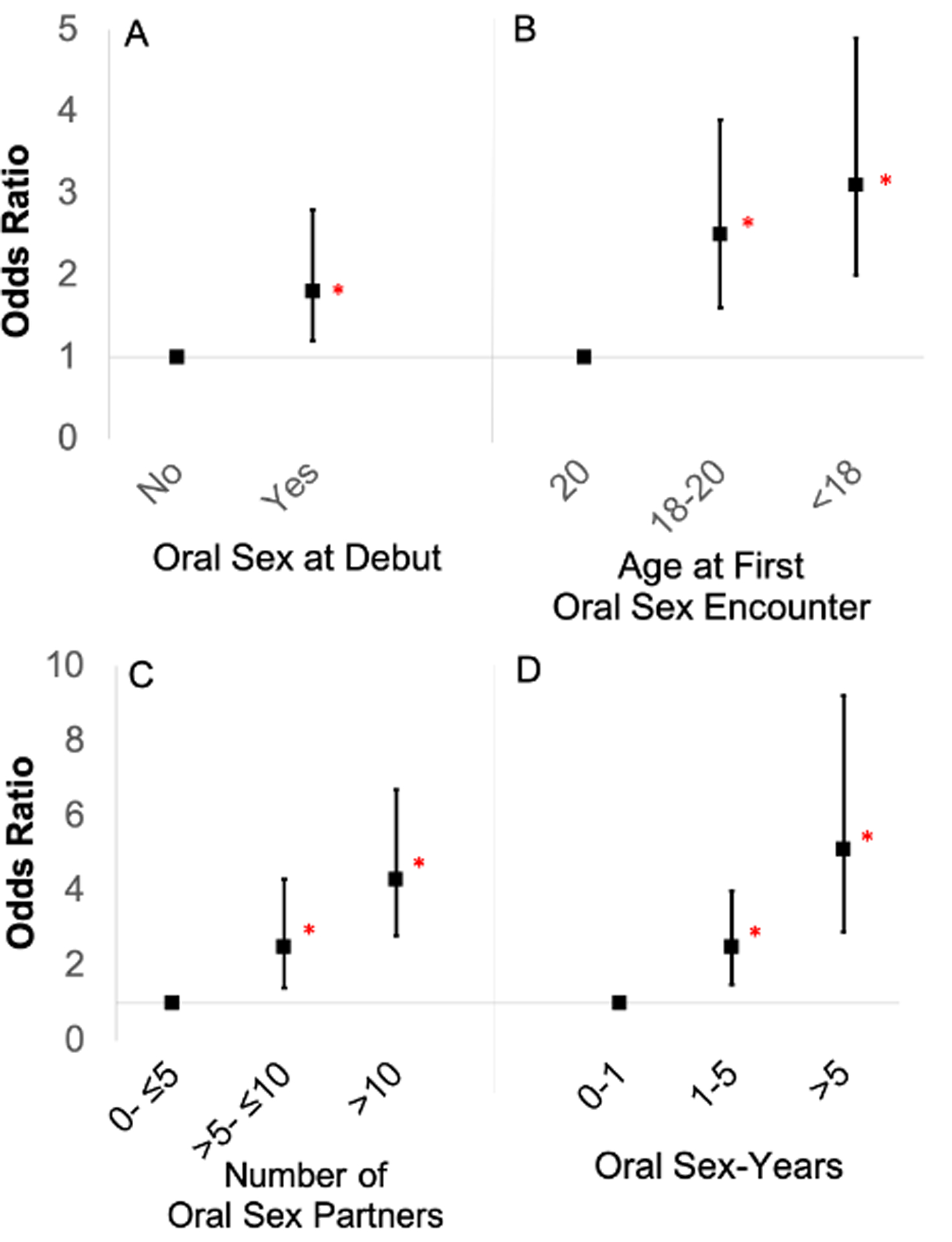
Black squares represent unadjusted odds ratios of HPV-OPC for case subjects versus controls. Vertical lines represent 95% confidence intervals. Red asterisks represent statistical significance. Odds ratios and 95% confidence intervals were derived from conditional logistic regression analysis for case-control comparison. A: Odds of HPV-OPC if a participant reported performing oral sex at sexual debut. B: Dose-response relationship for age of first oral sex encounter. C: Dose-response relationship of number for people performed oral sex on in lifetime. D: Dose-response relationship for oral sex-years, a measure of intensity of oral sexual partners defined as number of partners performed oral sex on per ten years since sexual debut.
Other sexual behaviors were also associated with diagnosis of HPV-OPC. Odds of HPV-OPC increased with higher number of lifetime vaginal sex partners and deep kissing partners (each p<0.001, Table 2). Number of vaginal sex-years was also associated with HPV-OPC (p<0.001). Both number of vaginal sex partners and vaginal sex-years were associated with HPV-OPC after adjustment for smoking (pack-years) and number of lifetime oral sex partners. Models that adjusted for number of vaginal sex partners showed the associations with oral sexual behaviors all remained significant and were not explained by number of vaginal sex partners.
We examined odds of HPV-OPC in terms of relationship dynamics and characterized partner type. Increased number of casual sex partners was associated with odds of HPV-OPC (Figure 2A; >10 casual partners, 37.5% vs. 24.6%, p<0.001; OR 3.0, 95% CI 1.8–5.1). Extramarital sex (Figure 2B; 43.9% vs. 36.0%, p=0.002; OR 1.6, 95% CI 1.1–2.4) and suspicion that a partner had extramarital sex (10.8% vs. 4.2%, p=0.002; OR 3.4, 95% CI 1.6–7.5) were each associated with increased odds of HPV-OPC. Increased odds of HPV-OPC were also observed for those who had a sexual partner at least ten years older when the participant was younger than age 23 (Figure 2C; 30.0% vs. 20.0%, p=0.01; OR 1.7, 95% CI=1.1–2.6). Once number of lifetime oral sex partners and smoking were included in the model, only suspicion of extramarital sex remained associated with HPV-OPC. Associations were similar when explored only among men or only among women (Supplemental Table 3), although there was insufficient power to explore the associations among women due to limited numbers.
Figure 2:
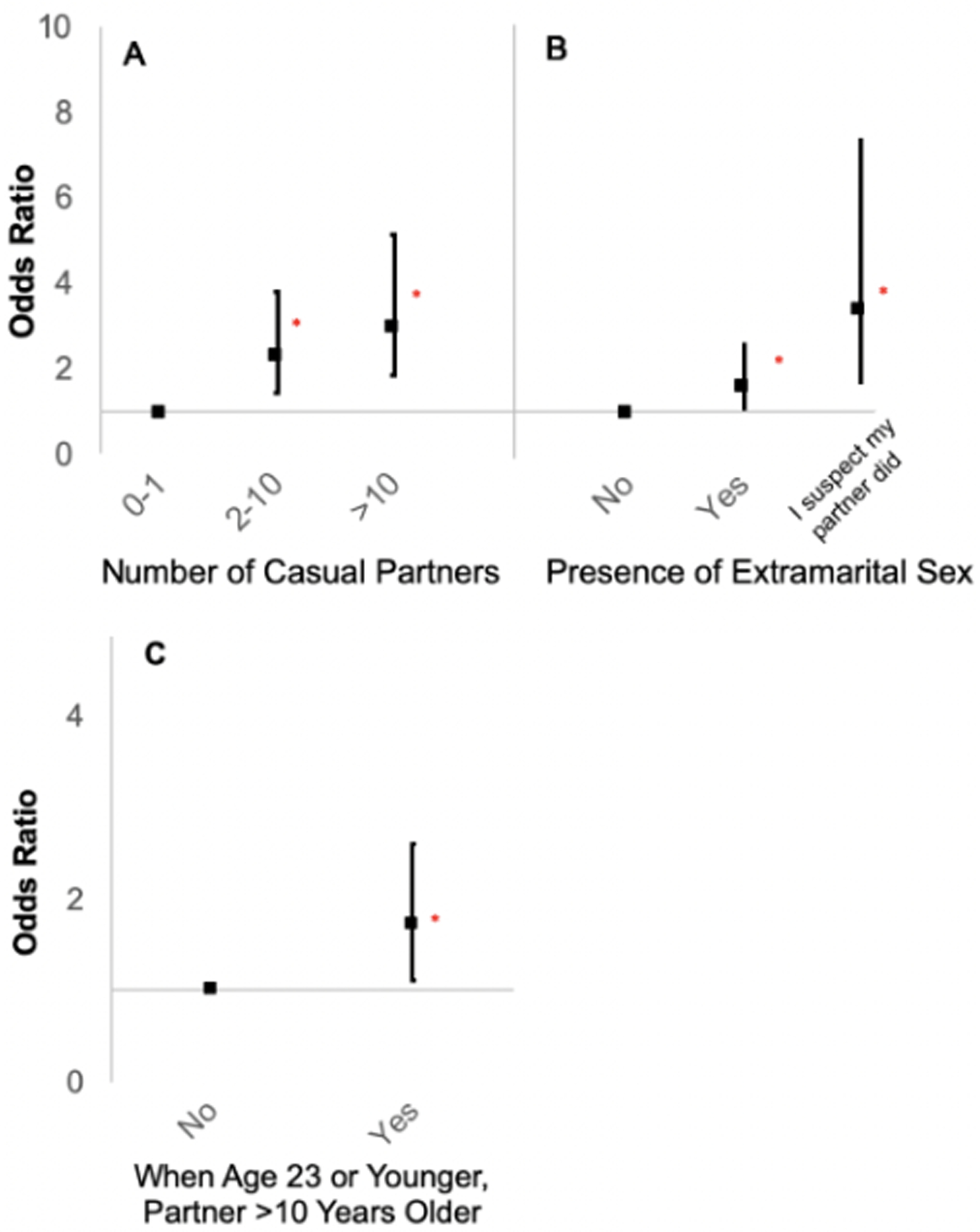
Black squares represent unadjusted odds ratios of HPV-OPC for case subjects versus controls. Vertical lines represent 95% confidence intervals. Red asterisks represent statistical significance. Odds ratios and 95% confidence intervals were derived from conditional logistic regression analysis for case-control comparison. A: Dose-response relationship of number of casual sex partners and risk of HPV-OPC. B: Odds of HPV-OPC by presence or suspicion of extramarital sex C: Odds of HPV-OPC when a participant reported having a sexual partner greater than 10 years older when they were under 23 years old
Substance Use
Substance use was also examined. There was no association between ever or current cigarette use, alcohol use, pack-years of smoking, or drink-years and odds of HPV-OPC (Table 3). Some types of drug use were associated with increased odds of HPV-OPC including ever marijuana use (p=0.001), ever cocaine use (p=0.01) and joint-years of smoking marijuana (p=0.01). However, after adjustment for oral sex behaviors, these associations did not remain significant.
Table 3:
Comparison of substance use among HPV-OPC cases and controls
| Characteristic | Cases | Controls | p-value | OR (95%CI) | aOR (95%CI)* |
|---|---|---|---|---|---|
| Ever Substance Use | |||||
| Cigarette Smoking | 0.19 | ||||
| Never | 54.5% | 60.7% | Ref | Ref | |
| Ever | 45.5% | 39.3% | 1.3 (0.8–1.9) | 1.2 (0.8–1.8) | |
| Current Smoking | 0.77 | ||||
| No | 92.6% | 93.3% | Ref | Ref | |
| Yes | 7.4% | 6.7% | 1.1 (0.5–2.4) | 1.0 (0.4–6.7) | |
| Alcohol Use | 0.51 | ||||
| Never | 2.2% | 3.3% | Ref | Ref | |
| Ever | 97.8% | 96.7% | 1.5 (0.4–5.6) | 1.5 (0.4–5.7) | |
| Marijuana Use | 0.001 | ||||
| Never | 23.3% | 37.5% | Ref | Ref | |
| Ever | 76.7% | 62.5% | 2.0 (1.3–3.0) | 1.4 (0.9–2.2) | |
| Cocaine Use | 0.01 | ||||
| Never | 70.1% | 79.9% | Ref | Ref | |
| Ever | 29.9% | 20.1% | 1.7 (1.1–2.6) | 1.2 (0.8–2.0) | |
| Pack-years of smokers | 0.42 | ||||
| 0-<1 | 54.5% | 60.7% | Ref | Ref | |
| 1-<10 | 14.1% | 11.8% | 1.3 (0.7–2.4) | 1.2 (0.6–2.2) | |
| 10+ | 31.4% | 27.5% | 1.3 (0.8–2.0) | 1.2 (0.8–1.9) | |
| Drink-years | 0.69 | ||||
| 0 | 2.6% | 2.9% | Ref | Ref | |
| >0-<10 | 8.0% | 6.7% | 1.9 (0.4–8.1) | 1.8 (0.4–8.4) | |
| 10+ | 90.1% | 90.4% | 1.6 (0.4–5.8) | 1.5 (0.4–6.0) | |
| Joint-years of marijuana | 0.01 | ||||
| 0 | 50.7% | 69.7% | Ref | Ref | |
| >0-<10 | 12.0% | 8.7% | 1.9 0.8–4.7) | 1.2 (0.4–3.1) | |
| 10+ | 37.3% | 21.6% | 2.4 (1.3–4.3) | 1.6 (0.8–3.1) |
Adjusted for number of lifetime oral sex partners
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; Ref, reference group
Bolding indicates statistical significance.
Serum Biomarkers
HPV serum antibodies were compared among cases and controls (Table 4). Seropositivity for HPV16 E6 was extremely common among cases (93.6%) and rare among controls (6.4%). HPV16 E6 seropositivity (OR 286, 95% CI 122–670) and seropositivity for any HPV16 E protein (E1, E2, E6, or E7; OR 163, 95% CI 70–378) were associated with a 280-fold and 160-fold increase in odds of HPV-OPC, respectively. HPV16 L1 antibodies were associated with a 34-fold increase in odds of HPV-OPC (OR 34, 95% CI 18–60).
Table 4:
HPV seroprevalence and odds of HPV-OPC among cases and controls
| Antibody Seroprevalence | Cases | Controls | p-value | OR (95% CI) |
|---|---|---|---|---|
| n=146 | n=304 | |||
| HPV16 E6 | <0.001 | |||
| Negative | 4.8% | 95.2% | Ref | |
| Positive | 93.6% | 6.4% | 286 (122–670) | |
| HPV16 E7 | <0.001 | |||
| Negative | 30.1% | 93.1% | Ref | |
| Positive | 69.9% | 6.9% | 31 (18–55) | |
| Any HPV 16 E protein (E1, E2, E6, E7) | <0.001 | |||
| Negative | 4.8% | 89.1% | Ref | |
| Positive | 95.2% | 10.9% | 163 (70–378) | |
| HPV 16 L1 | <0.001 | |||
| Negative | 30.8% | 93.8% | Ref | |
| Positive | 69.2% | 6.3% | 34 (18–60) | |
| Number of HPV types seropositive for: | ||||
| Number seropositive for E6 | <0.001 | |||
| 0 | 5.5% | 89.4% | Ref | |
| 1–2 | 74.0% | 10.2% | 118 (52–265) | |
| ≥3 | 20.6% | 0.3% | 1020 (123–8436) | |
| Number seropositive for E7 | <0.001 | |||
| 0 | 21.2% | 84.5% | Ref | |
| 1–2 | 19.9% | 15.1% | 5.2 (2.8–9.5) | |
| ≥3 | 58.9% | 0.3% | 712 (95–5301) | |
| Number seropositive for L1 | <0.001 | |||
| 0 | 26.0% | 74.3% | Ref | |
| 1–2 | 31.5% | 17.1% | 5.3 (3.1–8.9) | |
| ≥3 | 42.5% | 8.6% | 14 (8–25) |
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; Ref, reference group; HPV, human papillomavirus
Bolding indicates statistical significance.
Next, the number of HPV types individuals were seropositive to were considered for anti-E6, E7, and L1 (Table 4). In addition to the strong association of HPV16 E6 seropositivity, overall odds increased with E6 seropositivity to number of HPV types (1–2 vs. 0 types, OR 118, 95% CI 52–265) and had further increased odds of HPV-OPC in those with ≥3 vs. 0 types (OR 1020, 95% CI 123–8436). Similarly, odds of HPV-OPC increased with number of HPV types seropositive for anti-E7 (≥3 vs. 0 types, OR 712, 95% CI 95–5301) and anti-L1 antibodies (≥3 vs. 0 types, OR 14 95% CI 8–25).
HPV16 E6 Seropositive Controls
Of interest, there were 9 controls with HPV16 E6 seropositivity. Reflective of all the controls, a majority were male (n=7, 77.8%) and non-Hispanic white (n=7, 77.8%), with a median age of 59 years (IQR 54.6–65). They presented for otologic or laryngological evaluation and none had any known past or current medical history of any HPV-related cancer (anal, cervical, or penile). One reported a non-melanoma skin cancer. Compared to HPV16 E6 seronegative controls, these participants reported statistically similar sexual behaviors.
Discussion
HPV-related oropharyngeal cancer is now widely recognized as a distinct disease entity.1,7,20,21 Since the time HPV was first suggested as having a causal role in OPC, several case-control studies have pointed to sexual behavior, specifically oral sexual behavior10,22–24 as a risk factor for this malignancy. While lifetime number of oral sex partners, a surrogate for oral exposure5 to HPV, is known to be associated with risk for HPV-OPC, this is the first study to demonstrate that other contextual factors such as the timing and intensity of oral sex are also associated with diagnosis of HPV-OPC. These findings underscore the importance of oral sex as a risk factor for HPV-OPC and that the association between oral sex and HPV-OPC is independent of general sexual exposure.
Younger age at oral sex debut is independently associated with increased odds of HPV-OPC. While early oral sex debut may be a surrogate for either riskier sexual behavior or higher lifetime potential exposure to HPV, it remained significant after adjusting for total number of both oral and vaginal sex partners, suggesting early oral sex may be capturing a different aspect of risk. Changing behavioral norms have shifted the population average toward an earlier age of sexual debut,25 and younger generations are more likely to perform oral sex at sexual debut.3 The finding that earlier age of oral sex may increase odds of HPV-OPC suggests this societal change in behavior could be one reason for the increasing incidence of HPV-OPC.26
The finding that timing of oral sexual behavior is associated with HPV-OPC led us to investigate other variables at sexual initiation, specifically order of acts at sexual debut. This is the first study to examine sequence of acts at sexual debut and association with risk of HPV-OPC. It has previously been speculated that order of sexual acts at debut might in part explain increasing HPV-OPC rates,10 but there has been no data to support this hypothesis. The rationale underlying this hypothesis is that first genital exposure to HPV results in a robust immune response, generating sufficient immunity when HPV is introduced orally. We found that cases were more likely to have performed oral sex at debut. Conversely, controls were more likely to have performed solely vaginal sex as first sexual act. Our behavioral data therefore support the theory that when first exposure to HPV is via oral mucosa without preceding genital exposure, a weaker immune response is produced and infection is more likely to persist.10,11
Another potential component of HPV-OPC risk described in this analysis is a novel measure of sexual intensity (measured by sex-years, a calculation of the number of oral sex partners per 10 years across different ages).18 Similar to how pack-years describes tobacco history and drink-years characterizes alcohol use,19 the new metric of sex-years characterizes cumulative sexual exposure over time as a surrogate for potential exposure to HPV. Our data suggest that higher concentration and intensity of partner exposure is an independent predictor of HPV-OPC risk, as evidenced by an association of increased sex-years with HPV-OPC even after adjusting for number of oral sex partners.
This paper also illustrates, to our knowledge for the first time, that partner behaviors and relationship dynamics may influence risk of HPV-OPC development. Cases of HPV-OPC were more likely to report history of casual partners and extramarital sex, similar to what has been reported in cervical cancer.27,28 These findings also support the concept of sexual networks, which have been investigated in HIV and infectious disease epidemiological studies.29 A sexual network is a collection of dyads linked by sexual contact who share a similar risk profile for social and behavioral norms.30 Although this data may be colored by recall bias, it suggests that an individual’s risk of HPV-OPC is impacted by not just their own practices, but by their partner’s behaviors and exposures.
Age-disparate relationships have also been associated with risk of cervical cancer,31 HIV infection,32 and other sexually transmitted infections.33 An increased risk of HPV-OPC was observed in those who had a significantly older sexual partner at a young age. This finding may reflect the hypothetically broader viral exposure burden which the older partner shares with the younger partner. It may also be a surrogate for power imbalance in a relationship or non-consensual sex, although the association was no longer significant after adjustment for number of vaginal sex partners.
Previous studies have found an independent association between marijuana and HPV-OPC.8,20,34 In this analysis, marijuana use was associated with HPV-OPC in univariate analysis, but after adjustment this association was lost, consistent with other studies.35–39 The inconsistent data concerning marijuana and its association with HPV-OPC highlights the potential confounding between drug use and sexual practices34,40 and the evolving trends of casual marijuana use.41–43
Consistent with prior literature, seropositivity for antibodies to early HPV16 oncoproteins E6 and E7 was associated with diagnosis of HPV-OPC. E6 oncoprotein seroprevalence in this study was similar to other studies,24,44,45 although some prospective cohort studies reported lower seroprevalence.16,46 While the presence of E1, E2, and E4 have been reported in a prospective cohort,47 in this case-control analysis we evaluate the breadth of early oncoproteins. A combined biomarker consisting of anti-HPV16 E1, E2, E6 and E7 antibodies has been previously suggested as a marker for HPV-OPC risk,47 and our findings would support this; almost all cases were positive for HPV16 E1, E2, E6, or E7. However, 10% of controls were seropositive to at least one E protein, suggesting low specificity for this combined biomarker.
Seropositivity for HPV16 E6 has high specificity for HPV-OPC, and seroprevalence is rare in individuals without cancer.48,49 Prior studies have reported HPV16 E6/E7 seroprevalence rates from <1%16,49 to 4%5 in non-cancer controls. While HPV16 L1 seroconversion occurs soon after infection and denotes past exposure, development of E6/E7 antibodies occurs after initiation of carcinogenesis and therefore indicates conversion to a malignant state.50 The HPV16 E6 seroprevalence observed in nine controls is therefore notable, and may herald later diagnosis with HPV-OPC or another HPV-driven malignancy (anal cancer).49
This report is the first to show that seropositivity for increasing number of distinct HPV types is associated with greater risk of HPV-OPC. We also examined the composition of HPV types in those who were seropositive for antibodies to more than one HPV E6 type. Seropositivity for HPV16 and HPV33 anti-E6 together was common, and antibodies to these two HPV types were identified together in 81.7% of cases. However, the antibody cross-reactivity of phylogenetically-related HPV types likely explains these findings47,51,52 as it is rare that multiple infections cause HPV-OPC (<5%).53 HPV16 drives 85–90% of HPV-OPC,53–55 while HPV33 is responsible for 3–5% of cases.56,57 The association we see with increasing number of HPV types and HPV-OPC diagnosis may rather reflect the antibody levels of the causative HPV type which thereby increases risk of cross-reactivity.
This study has strengths and limitations. This is a multi-institutional study with frequency-matched controls, centrally tested HPV tumor and blood biomarkers, and detailed behavioral data collected via a confidential computer assisted self interview (CASI). As with all self-reported behaviors, we cannot rule out the potential for recall bias or misreporting.
Even with this limitation, this data adds novel context and depth to our understanding of HPV-OPC. As HPV-OPC incidence in the United States continues to rise,58 these findings have important public health implications and inform epidemiological understanding of head and neck cancer. While this detailed contextual understanding of HPV-OPC risk does not have direct implications for current disease detection or screening, we illustrate the complexity of the association between sexual practices and risk of oropharyngeal cancer, and these novel risk factors may contribute to identification of cohorts enriched for oral HPV.59
Conclusion
In conclusion, this study provides the most comprehensive behavioral picture of HPV-related oropharyngeal cancer to date. Ever performing oral sex and number of partners remain strong risk factors for HPV-OPC. Measures of sexual behavior including timing (age) of oral sex and intensity of exposure (partners per 10 years) are independent risk factors for HPV-OPC, suggesting these behaviors may explain additional nuances of how and why some people develop HPV-OPC.
Supplementary Material
Funding:
National Institute of Dental and Craniofacial Research (P50 DE019032) and the National Institute on Deafness and Other Communication Disorders (1K23DC014758)
Footnotes
Conflicts of Interest: Dr. Waterboer serves on advisory boards for MSD (Merck Sharp & Dohme)
References:
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(36):4550–4559. doi: 10.1200/JCO.2013.50.3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Souza G, Cullen K, Bowie J, Thorpe R, Fakhry C. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PloS One. 2014;9(1):e86023. doi: 10.1371/journal.pone.0086023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen F, Yan L, Liu F, et al. Oral human papillomavirus infection, sexual behaviors and risk of oral squamous cell carcinoma in southeast of China: A case-control study. J Clin Virol. 2016;85:7–12. doi: 10.1016/j.jcv.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 5.D’Souza G, Kreimer AR, Viscidi R, et al. Case–Control Study of Human Papillomavirus and Oropharyngeal Cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497 [DOI] [PubMed] [Google Scholar]
- 6.Farsi NJ, El-Zein M, Gaied H, et al. Sexual behaviours and head and neck cancer: A systematic review and meta-analysis. Cancer Epidemiol. 2015;39(6):1036–1046. doi: 10.1016/j.canep.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 7.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025 [DOI] [PubMed] [Google Scholar]
- 8.Heck JE, Berthiller J, Vaccarella S, et al. Sexual behaviours and the risk of head and neck cancers: a pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int J Epidemiol. 2010;39(1):166–181. doi: 10.1093/ije/dyp350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laprise C, Madathil SA, Schlecht NF, et al. Increased risk of oropharyngeal cancers mediated by oral human papillomavirus infection: Results from a Canadian study. Head Neck. 2019;41(3):678–685. doi: 10.1002/hed.25436 [DOI] [PubMed] [Google Scholar]
- 10.Rettig E, Kiess AP, Fakhry C. The role of sexual behavior in head and neck cancer: implications for prevention and therapy. Expert Rev Anticancer Ther. 2015;15(1):35–49. doi: 10.1586/14737140.2015.957189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu B, Viscidi RP, Wu Y, et al. Seroprevalence of Human Papillomavirus (HPV) Type 6 and 16 Vary by Anatomic Site of HPV Infection in Men. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2012;21(9):1542–1546. doi: 10.1158/1055-9965.EPI-12-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Windon MJ, Waterboer T, Hillel AT, et al. Sex differences in HPV immunity among adults without cancer. Hum Vaccines Immunother. 2019;15(7–8):1935–1941. doi: 10.1080/21645515.2019.1568157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Souza G, Westra WH, Wang SJ, et al. Differences in the Prevalence of Human Papillomavirus (HPV) in Head and Neck Squamous Cell Cancers by Sex, Race, Anatomic Tumor Site, and HPV Detection Method. JAMA Oncol. 2017;3(2):169–177. doi: 10.1001/jamaoncol.2016.3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holzinger D, Wichmann G, Baboci L, et al. Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma. Int J Cancer. 2017;140(12):2748–2757. doi: 10.1002/ijc.30697 [DOI] [PubMed] [Google Scholar]
- 15.Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51(10):1845–1853. doi: 10.1373/clinchem.2005.052381 [DOI] [PubMed] [Google Scholar]
- 16.Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of Human Papillomavirus Antibodies and Risk of Subsequent Head and Neck Cancer. J Clin Oncol. 2013;31(21):2708–2715. doi: 10.1200/JCO.2012.47.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming ID. AJCC Cancer Staging Manual. Fifth Edition. Lippincott-Raven; 1997. https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC5thEdCancerStagingManual.pdf [Google Scholar]
- 18.Fakhry C, Waterboer T, Westra WH, et al. Distinct biomarker and behavioral profiles of human papillomavirus-related oropharynx cancer patients by age. Oral Oncol. 2020;101:104522. doi: 10.1016/j.oraloncology.2019.104522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anantharaman D, Abedi-Ardekani B, Beachler DC, et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int J Cancer. 2017;140(9):1968–1975. doi: 10.1002/ijc.30608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709 [DOI] [PubMed] [Google Scholar]
- 21.van Houten VMM, Snijders PJF, van den Brekel MWM, et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer. 2001;93(2):232–235. doi: 10.1002/ijc.1313 [DOI] [PubMed] [Google Scholar]
- 22.Chancellor JA, Ioannides SJ, Elwood JM. Oral and oropharyngeal cancer and the role of sexual behaviour: a systematic review. Community Dent Oral Epidemiol. 2017;45(1):20–34. doi: 10.1111/cdoe.12255 [DOI] [PubMed] [Google Scholar]
- 23.Schnelle C, Whiteman DC, Porceddu SV, Panizza BJ, Antonsson A. Past sexual behaviors and risks of oropharyngeal squamous cell carcinoma: a case-case comparison. Int J Cancer. 2017;140(5):1027–1034. doi: 10.1002/ijc.30519 [DOI] [PubMed] [Google Scholar]
- 24.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497 [DOI] [PubMed] [Google Scholar]
- 25.Finer LB. Trends in premarital sex in the United States, 1954–2003. Public Health Rep Wash DC 1974. 2007;122(1):73–78. doi: 10.1177/003335490712200110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittekindt C, Wagner S, Bushnak A, et al. Increasing Incidence rates of Oropharyngeal Squamous Cell Carcinoma in Germany and Significance of Disease Burden Attributed to Human Papillomavirus. Cancer Prev Res Phila Pa. 2019;12(6):375–382. doi: 10.1158/1940-6207.CAPR-19-0098 [DOI] [PubMed] [Google Scholar]
- 27.Biswas LN, Manna B, Maiti PK, Sengupta S. Sexual risk factors for cervical cancer among rural Indian women: a case-control study. Int J Epidemiol. 1997;26(3):491–495. doi: 10.1093/ije/26.3.491 [DOI] [PubMed] [Google Scholar]
- 28.Franceschi S, Plummer M, Clifford G, et al. Differences in the risk of cervical cancer and human papillomavirus infection by education level. Br J Cancer. 2009;101(5):865–870. doi: 10.1038/sj.bjc.6605224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hickson DA, Mena LA, Wilton L, et al. Sexual Networks, Dyadic Characteristics, and HIV Acquisition and Transmission Behaviors Among Black Men Who Have Sex With Men in 6 US Cities. Am J Epidemiol. 2017;185(9):786–800. doi: 10.1093/aje/kww144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amirkhanian YA. Social Networks, Sexual Networks and HIV Risk in Men Who Have Sex with Men. Curr HIV/AIDS Rep. 2014;11(1):81–92. doi: 10.1007/s11904-013-0194-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baussano I, Diaz M, Tully S, et al. Effect of age-difference between heterosexual partners on risk of cervical cancer and human papillomavirus infection. Papillomavirus Res. 2017;3:98–104. doi: 10.1016/j.pvr.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregson S, Nyamukapa CA, Garnett GP, et al. Sexual mixing patterns and sex-differentials in teenage exposure to HIV infection in rural Zimbabwe. Lancet Lond Engl. 2002;359(9321):1896–1903. doi: 10.1016/S0140-6736(02)08780-9 [DOI] [PubMed] [Google Scholar]
- 33.Begley E, Crosby RA, DiClemente RJ, Wingood GM, Rose E. Older partners and STD prevalence among pregnant African American teens. Sex Transm Dis. 2003;30(3):211–213. doi: 10.1097/00007435-200303000-00006 [DOI] [PubMed] [Google Scholar]
- 34.Marks MA, Chaturvedi AK, Kelsey K, et al. Association of marijuana smoking with oropharyngeal and oral tongue cancers: Pooled analysis from the INHANCE Consortium. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2014;23(1):160–171. doi: 10.1158/1055-9965.EPI-13-0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berthiller J, Amy Lee Y, Boffetta P, et al. Marijuana smoking and the risk of head and neck cancer: pooled analysis in the INHANCE Consortium. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2009;18(5):1544–1551. doi: 10.1158/1055-9965.EPI-08-0845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashibe M, Morgenstern H, Cui Y, et al. Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based case-control study. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2006;15(10):1829–1834. doi: 10.1158/1055-9965.EPI-06-0330 [DOI] [PubMed] [Google Scholar]
- 37.Llewellyn CD, Johnson NW, Warnakulasuriya KA. Risk factors for squamous cell carcinoma of the oral cavity in young people--a comprehensive literature review. Oral Oncol. 2001;37(5):401–418. doi: 10.1016/s1368-8375(00)00135-4 [DOI] [PubMed] [Google Scholar]
- 38.Rosenblatt KA, Daling JR, Chen C, Sherman KJ, Schwartz SM. Marijuana use and risk of oral squamous cell carcinoma. Cancer Res. 2004;64(11):4049–4054. doi: 10.1158/0008-5472.CAN-03-3425 [DOI] [PubMed] [Google Scholar]
- 39.Sidney S, Quesenberry CP, Friedman GD, Tekawa IS. Marijuana use and cancer incidence (California, United States). Cancer Causes Control CCC. 1997;8(5):722–728. doi: 10.1023/a:1018427320658 [DOI] [PubMed] [Google Scholar]
- 40.Parks KA, Collins RL, Derrick JL. The influence of marijuana and alcohol use on condom use behavior: findings from a sample of young adult female bar drinkers. Psychol Addict Behav J Soc Psychol Addict Behav. 2012;26(4):888–894. doi: 10.1037/a0028166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grucza RA, Agrawal A, Krauss MJ, Cavazos-Rehg PA, Bierut LJ. Recent Trends in the Prevalence of Marijuana Use and Associated Disorders in the United States. JAMA Psychiatry. 2016;73(3):300–301. doi: 10.1001/jamapsychiatry.2015.3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasin DS. US Epidemiology of Cannabis Use and Associated Problems. Neuropsychopharmacology. 2018;43(1):195–212. doi: 10.1038/npp.2017.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miech R, Koester S. Trends in U.S., Past-Year Marijuana Use from 1985–2009; An Age-Period-Cohort Analysis. Drug Alcohol Depend. 2012;124(3):259–267. doi: 10.1016/j.drugalcdep.2012.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holzinger D, Wichmann G, Baboci L, et al. Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma. Int J Cancer. 2017;140(12):2748–2757. doi: 10.1002/ijc.30697 [DOI] [PubMed] [Google Scholar]
- 45.Lang Kuhs KA, Kreimer AR, Trivedi S, et al. Human papillomavirus 16 E6 antibodies are sensitive for human papillomavirus-driven oropharyngeal cancer and are associated with recurrence. Cancer. 2017;123(22):4382–4390. doi: 10.1002/cncr.30966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kreimer AR, Johansson M, Yanik EL, et al. Kinetics of the Human Papillomavirus Type 16 E6 Antibody Response Prior to Oropharyngeal Cancer. J Natl Cancer Inst. 2017;109(8). doi: 10.1093/jnci/djx005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Waterboer T, Haddad RI, et al. Human papillomavirus (HPV) 16 antibodies at diagnosis of HPV-related oropharyngeal cancer and antibody trajectories after treatment. Oral Oncol. 2017;67:77–82. doi: 10.1016/j.oraloncology.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(21):2708–2715. doi: 10.1200/JCO.2012.47.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lang Kuhs KA, Anantharaman D, Waterboer T, et al. Human Papillomavirus 16 E6 Antibodies in Individuals Without Diagnosed Cancer: A Pooled Analysis. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2015;24(4):683–689. doi: 10.1158/1055-9965.EPI-14-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kreimer AR, Brennan P, Lang Kuhs KA, et al. Human papillomavirus antibodies and future risk of anogenital cancer: a nested case-control study in the European prospective investigation into cancer and nutrition study. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(8):877–884. doi: 10.1200/JCO.2014.57.8435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michael KM, Waterboer T, Sehr P, et al. Seroprevalence of 34 Human Papillomavirus Types in the German General Population. PLoS Pathog. 2008;4(6). doi: 10.1371/journal.ppat.1000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Combes J-D, Pawlita M, Waterboer T, et al. Antibodies against high-risk human papillomavirus proteins as markers for invasive cervical cancer. Int J Cancer. 2014;135(10):2453–2461. doi: 10.1002/ijc.28888 [DOI] [PubMed] [Google Scholar]
- 53.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319–1331. doi: 10.1016/S1470-2045(14)70471-1 [DOI] [PubMed] [Google Scholar]
- 54.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi: 10.1002/ijc.30716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walline HM, Komarck C, McHugh JB, et al. High-risk human papillomavirus detection in oropharyngeal, nasopharyngeal, and, oral cavity cancers: Comparison of multiple methods. JAMA Otolaryngol-- Head Neck Surg. 2013;139(12):1320–1327. doi: 10.1001/jamaoto.2013.5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlander A-LF, Larsen CG, Jensen DH, et al. Continuing rise in oropharyngeal cancer in a high HPV prevalence area: A Danish population-based study from 2011 to 2014. Eur J Cancer. 2017;70:75–82. doi: 10.1016/j.ejca.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 57.Schache AG, Powell NG, Cuschieri KS, et al. HPV-Related Oropharynx Cancer in the United Kingdom: An Evolution in the Understanding of Disease Etiology. Cancer Res. 2016;76(22):6598–6606. doi: 10.1158/0008-5472.CAN-16-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dyne EAV. Trends in Human Papillomavirus–Associated Cancers — United States, 1999–2015. MMWR Morb Mortal Wkly Rep. 2018;67. doi: 10.15585/mmwr.mm6733a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Souza G, McNeel TS, Fakhry C. Understanding personal risk of oropharyngeal cancer: risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(12):3065–3069. doi: 10.1093/annonc/mdx535 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


