Abstract
The aim of this study was to provide definitive reference values for bone mineral density (BMD) and bone turnover markers in the general elderly population. Registered citizens of 50 to 89 years old were targeted for this survey. After random sampling from the resident registry of Obuse town, we established eight groups based on age (50 s, 60 s, 70 s, and 80 s) and gender. A total of 411 people were enrolled. We used a dual-energy x-ray absorptiometry device to measure and evaluate BMD. The bone formation marker bone alkaline phosphatase (BAP) was measured as a bone turnover marker. Bone quality marker pentosidine, and bone resorption markers including urinary total deoxypyridinoline (DPD), tartrate-resistant acid phosphatase 5b (TRACP-5b), 25-hydroxyvitamin D (25[OH]D), and whole parathyroid hormone (PTH) were also measured as bone turnover markers. Sixty-three people (15.3%) were diagnosed as osteoporosis. BMD decreased with age in the femoral neck and total hip. On the other hand, there was no characteristic change with age in the lumber spine. As for bone markers, pentosidine and DPD increased with aging, although 25(OH)D, whole PTH, and BAP showed no characteristic associations with gender and aging. In terms of the relationship between low BMD and bone markers, there was a significant independent association between low BMD and TRACP-5b in females. In conclusions, hip BMD decreased with aging in men and women. However, there was no characteristic decline with aging in the lumbar spine. All bone markers showed no significant independent characteristics associated with age or gender in a multivariate analysis model, except for a significant association between low BMD and TRACP-5b in females. TRACP-5b was a potentially useful marker for the detection of low BMD.
Subject terms: Endocrinology, Health care
Introduction
Bone mineral density (BMD) and bone turnover markers have become widely adopted as evaluation tools for osteoporotic disease1–3. There have been several reports on age-specific changes in BMD and bone turnover markers between men and women4–8, and clarification of age- and gender-specific reference is useful in osteoporosis treatment. Although several reports have attempted to determine reference values for BMD and bone turnover markers9–12, the availability of definitive reference values for BMD and bone turnover markers in the general population are scarce, since earlier studies were based on volunteer cohorts or lumbar disorder patients.
To establish a new population study of the Japanese subjects, we conducted a random sampling from the Obuse Town Registry of Residents to obtain a more representative cohort of the general population with minimal selection bias13–16. This epidemiological study is referred to as the "Obuse Study" after the name of the cooperating municipality of Obuse Town. It is the first study of its kind to provide baseline values for age-specific bone turnover markers in a large cohort study.
The present investigation proposes reference values for BMD and bone turnover markers in the Japanese population using the Obuse study cohort.
Materials and methods
Subjects
The protocols in this study conformed to the ethical guidelines of the 2013 Declaration of Helsinki and STROBE statement. Informed consent was obtained from participants prior to the initiation of the study. Participants were informed about the purposes of the research both verbally and in writing prior to the study, and written informed consent was obtained from all participants. This study was approved by the Institutional Review Board of Shinshu University (study no: 2792). From October 2014 to June 2017, we conducted an epidemiological study of residents (the Obuse Study) as a joint collaboration with a cooperating town office13, 14. Male and female participants between the ages of 50–89 were randomly selected from a pool of 5,352 registrants in the resident registry of a rural town13, 14. Those selected from the registry were asked whether they would be able to undergo a bone density examination, and calls for participation were continued until approximately 50 consenting participants were successfully recruited for each age group and sex13, 14. Four hundred and eleven participants were consequently included in the study, excluding 4 participants with incomplete measurements (Fig. 1)13, 14.
Figure 1.
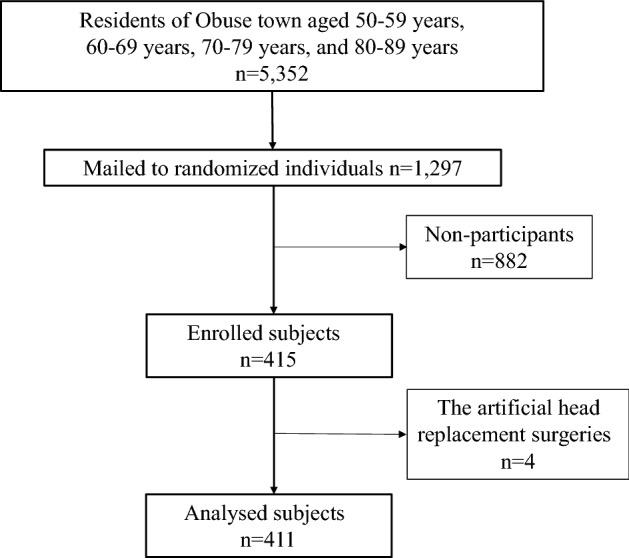
Obuse town resident participant flowchart. 1297 people were randomly sampled from 5352 residents aged between 50 and 89 years in the basic resident registry of Obuse town. A total of 415 people joined the Obuse study cohort, but 4 were excluded due to the artificial head replacement surgeries.
Bone mineral density and young adult mean measurement
We used a dual-energy x-ray absorptiometry device (GE Prodigy, GE healthcare, Chicago, IL, USA) to measure and evaluate bone mineral density (BMD) and T-score. BMD and T-score were measured at the femoral neck, total hip, and lumbar spine (L2–4). Based on the WHO diagnostic criteria, T-score ≥ − 1 was classified as healthy, − 2.5 < T-score < − 1 as osteopenia, and T-score ≤ − 2.5 as osteoporosis17. Osteoporosis and osteopenia were defined as low BMD.
Assay of bone turnover markers
The bone quality marker pentosidine, as well as the bone resorption marker urinary total deoxypyridinoline (DPD), tartrate-resistant acid phosphatase 5b (TRACP-5b), 25-hydroxyvitamin D (25[OH]D), and whole parathyroid hormone (PTH) were measured as bone turnover markers. Serum pentosidine, TRACP-5b, 25(OH)D, and whole PTH were measured using an enzyme-linked immunosorbent assay kit (SRL, Tokyo, Japan), enzyme immunoassay kit (SRL), electro chemiluminescence immunoassay kit (SRL), and chemiluminescent enzyme immunoassay kit (SRL), respectively. Urine DPD was measured using an enzyme immunoassay kit (SRL). The bone formation marker bone alkaline phosphatase (BAP) was also measured as a bone turnover marker. Serum BAP was measured using a chemiluminescent enzyme immunoassay kit (SRL).
Statistical analysis
The prevalence of osteoporosis was compared for each age group and sex. BMD and bone turnover markers were evaluated for each age and sex using Tukey’s test for comparisons among multiple groups. Multiple logistic regression analysis was applied to determine the association between bone markers and low BMD.
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R software (version 3.5.2; The R Foundation for Statistical Computing, Vienna, Austria).
Results
Of the 415 participants who were randomly sampled from the resident registry, 4 participants who were unable to measure BMD due to artificial hip joint replacement were excluded (Fig. 1). The physical characteristics and functions of the 411 examinees are shown for each age group and sex in Table 1. The interview results regarding participant comorbidities and menopause were shown in Table 2. Thirty-nine (9.5%) people had been treated for osteoporosis. Fifteen (3.6%) people had been treated with hormone therapy. Five (1.2%) people had been treated with steroid. A history of vertebral fracture was observed in 15 subjects (3.6%).
Table 1.
Characteristics of 411 subjects in the Obuse study cohort.
| Age strata (years) | n | Height (cm) | Weight (kg) | BMI (kg/m2) | |
|---|---|---|---|---|---|
| Male | 50–59 | 50 | 171.8 (5.9) | 67.1 (9.0) | 22.7 (2.9) |
| 60–69 | 53 | 166.7 (4.7) | 66.9 (7.7) | 24.1 (2.7) | |
| 70–79 | 55 | 163.2 (4.9) | 60.0 (10.2) | 22.5 (3.4) | |
| 80–89 | 45 | 160.1 (5.6) | 57.5 (8.4) | 22.4 (2.7) | |
| Total | 203 | 165.6 (6.8) | 63.0 (0.98) | 22.9 (0.30) | |
| Female | 50–59 | 47 | 158.1 (4.9) | 55.4 (8.9) | 22.2 (3.8) |
| 60–69 | 61 | 152.8 (5.3) | 52.2 (7.6) | 22.3 (2.7) | |
| 70–79 | 55 | 149.3 (5.6) | 50.7 (7.9) | 22.8 (3.5) | |
| 80–89 | 45 | 144.7 (6.1) | 48.4 (8.1) | 23.1 (3.4) | |
| Total | 208 | 151.3 (7.2) | 51.7 (8.4) | 22.6 (3.3) |
Values represent mean (standard deviation).
BMI, Body Mass Index.
Table 2.
Comorbidities in the study cohort.
| Disease | No. of participants | Prevalence (%) |
|---|---|---|
| Hyperthyroidism | 5 | 1.2 |
| Hyperparathyroidism | 0 | 0 |
| Diabetes mellitus | 52 | 12.7 |
| Paget's disease of bone | 3 | 0.7 |
| Rheumatoid arthritis | 5 | 1.2 |
| Chronic obstructive pulmonary disease | 7 | 1.7 |
| Fracture | 131 | 31.9 |
| Menopause (only female) | 202 | 96.7 |
Sixty-three people (15.3%) were diagnosed as osteoporosis, of which 14 (6.9%) were male and 49 (23.6%) were female. In men and women, diminished hip BMD was seen in the elderly. The decrease in BMD was particularly pronounced in the femoral neck. On the other hand, there was no characteristic change with age in the lumber spine (Table 3).
Table 3.
Bone mineral density and T-score at the femoral neck, proximal femur, and lumbar 1–4, in addition to prevalence of osteoporosis.
| Age strata (years) | N | Femoral neck BMD | Total hip BMD | Lumbar spine BMD | Lumbar spine BMD without vertebral fracture | Femoral neck T-score | Total hip T-score |
Lumbar spine T-score | Number of OP | |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | 50–59 | 50 | 0.90 (0.13) | 0.96 (0.13) | 1.17 (0.18) | 1.17 (0.18) | − 0.39 (1.04) | 0.13 (1.01) | − 0.13 (1.53) | 2 (4.0%) |
| 60–69 | 53 | 0.91 (0.11) | 0.99 (0.13) | 1.28 (0.22) | 1.28 (0.22) | − 0.35 (0.88) | 0.35 (0.99) | 0.76 (1.84) | 0 (0.0%) | |
| 70–79 | 55 | 0.88 (0.12) | 0.97 (0.15) | 1.35 (0.27)a | 1.36 (0.27)a | − 0.51 (0.93) | 0.21 (1.14) | 1.31 (2.28) | 5 (9.1%) | |
| 80–89 | 45 | 0.80 (0.15)abc | 0.87 (0.15)abc | 1.27 (0.29) | 1.28 (0.29) | − 1.12 (1.12) | − 0.59 (1.14) | 0.70 (2.39) | 7 (15.6%) | |
| Total | 203 | 0.88 (0.13) | 0.95 (0.15) | 1.27 (0.25) | 1.27 (0.25) | − 0.58 (1.03) | 0.05 (1.12) | 0.67 (2.08) | 14 (6.9%) | |
| Female | 50–59 | 47 | 0.80 (0.11) | 0.87 (0.13) | 1.09 (0.18) | 1.09 (0.18) | − 0.85 (0.90) | − 0.52 (1.06) | − 0.24 (1.46) | 4 (8.5%) |
| 60–69 | 61 | 0.76 (0.10) | 0.84 (0.11) | 1.02 (0.18) | 1.02 (0.18) | − 1.16 (0.85) | − 0.81 (0.88) | − 0.80 (1.52) | 12 (19.7%) | |
| 70–79 | 55 | 0.72 (0.10)a | 0.80 (0.12)a | 1.03 (0.20) | 1.02 (0.21) | − 1.47 (0.86) | − 1.08 (0.99) | − 0.80 (1.67) | 15 (27.3%) | |
| 80–89 | 45 | 0.67 (0.10)ab | 0.71 (0.10)abc | 1.00 (0.20) | 1.00 (0.20) | − 1.88 (0.80) | − 1.83 (0.84) | − 0.96 (1.67) | 18 (40.0%) | |
| Total | 208 | 0.74 (0.11) | 0.81 (0.13) | 1.04 (0.19) | 1.03 (0.19) | − 1.33 (0.92) | − 1.04 (1.05) | − 0.71 (1.60) | 49 (23.6%) |
Values represent mean (standard deviation).
Values of OP represent number (prevalence).
One female patient aged 70s and 3 female patients aged 80s were excluded due to the artificial head replacement surgeries.
BMD: bone mineral density, OP: osteoporosis.
aSignificantly different (p < 0.05) values from those aged 50–59 years.
bSignificantly different (p < 0.05) values from those aged 60–69 years.
cSignificantly different (p < 0.05) values from those aged 70–79 years.
In men and women, pentosidine and DPD increased with aging. In addition, TRACP-5b increased with age in males. 25(OH)D, whole PTH, and BAP showed no characteristics associated with gender or aging (Table 4). In addition, bone turnover markers, pentosidine, and whole PTH were compared in the presence and absence of OP. Pentosidine was 0.062 ± 0.022 in OP and 0.054 ± 0.025 in non-OP, which was significantly greater in OP (p = 0.028). DPD was 32.2 ± 25.7 in OP and 34.0 ± 25.0 in non-OP, with no significant difference between the two groups (p = 0.64). BAP was 15.9 ± 5.8 in OP and 14.0 ± 4.4 in non-OP, and which was significantly greater in OP (p = 0.030). TRACP-5b was 505 ± 198 in OP and 422 ± 160 in non-OP, significantly greater in OP (p = 0.007). Whole PTH was 24.0 ± 14.7 in OP and 20.7 ± 8.3 in non-OP, and there was no significant difference between the two groups (p = 0.128). 25(OH)D was 21.4 ± 7.6 in OP and 23.7 ± 7.2 in non-OP, with no significant difference between the two groups (p = 0.055).
Table 4.
Bone markers and 25-hydroxyvitamin D.
| Age strata (years) | Pentosidine | DPD | 25(OH)D | TRACP-5b | Whole PTH | BAP | |
|---|---|---|---|---|---|---|---|
| Male | 50–59 | 0.05 (0.01) | 3.3 (0.8) | 25.2 (6.0) | 312.2 (88.3) | 21.8 (8.1) | 11.9 (2.4) |
| 60–69 | 0.05 (0.02) | 3.7 (1.2) | 22.9 (5.4) | 380.8 (144.0) | 19.8 (7.7) | 13.5 (3.8) | |
| 70–79 | 0.06 (0.02)a | 3.9 (1.1)a | 29.3 (7.5)ab | 448.7 (198.4)a | 20.7 (7.7) | 13.6 (4.7) | |
| 80–89 | 0.07 (0.02)abc | 5.2 (1.7)abc | 22.0 (5.6)c | 489.7 (194.8)ab | 22.0 (12.1) | 13.3 (3.7) | |
| Total | 0.06 (0.02) | 4.0 (1.4) | 25.0 (6.8) | 406.2 (174.2) | 21.0 (8.9) | 13.1 (3.8) | |
| Female | 50–59 | 0.04 (0.01) | 5.9 (1.2) | 22.0 (6.5) | 416.1(130.9) | 22.6 (11.0) | 15.2 (4.5) |
| 60–69 | 0.05 (0.02) | 5.3 (1.3) | 20.6 (6.2) | 478.5 (141.7) | 20.1 (7.7) | 16.1 (5.1) | |
| 70–79 | 0.06 (0.05)a | 5.4 (2.0) | 25.0 (9.1)b | 490.1 (167.4) | 22.2 (12.3) | 15.5 (5.4) | |
| 80–89 | 0.06 (0.02)a | 6.2 (2.5)b | 19.2 (6.0)c | 433.8 (167.9) | 21.0 (10.5) | 14.1 (5.3) | |
| Total | 0.06 (0.03) | 5.7 (1.8) | 21.8 (7.4) | 457.6 (154.8) | 21.4 (10.4) | 15.3 (5.1) |
Values represent mean (standard deviation).
DPD: deoxypyridinoline, 25(OH)D: 25-hydroxyvitamin D, TRACP-5b: tartrate-resistant acid phosphatase 5b, PTH: parathyroid hormone, BAP: bone alkaline phosphatase.
aSignificantly different (p < 0.05) values from those aged 50–59 years.
bSignificantly different (p < 0.05) values from those aged 60–69 years.
cSignificantly different (p < 0.05) values from those aged 70–79 years.
Relevant factors selected by the unifactorial analysis were subjected to multiple logistic regression analysis with gender. The results showed that when considering low BMD as a dependent variable, bone markers were not associated significantly with low BMD in males (Table 5). However, there was a significant independent association between low BMD and TRACP-5b in females (Table 6).
Table 5.
Independent association between low BMD and bone markers in males.
| Factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| Odds ratio (95%CI) | p value | Odds ratio (95%CI) | p value | |
| Age | 1.20 (0.91–1.60) | 0.20 | ||
| Pentosidine | 1.11 (0.84–1.47) | 0.47 | ||
| DPD | 1.40 (1.04–1.87) | 0.026 | 1.18 (0.84–1.67) | 0.34 |
| 25(OH)D | 0.86 (0.65–1.14) | 0.30 | ||
| TRACP-5b | 1.51 (1.11–2.05) | 0.0079 | 1.38 (0.97–1.97) | 0.072 |
| Who1e PTH | 1.32 (0.98–1.77) | 0.064 | ||
| BAP | 1.08 (0.82–1.41) | 0.60 | ||
CI: confidence interval, DPD: deoxypyridinoline, 25(OH)D: 25-hydroxyvitamin D, TRACP-5b: tartrate-resistant acid phosphatase 5b, PTH: parathyroid hormone, BAP: bone alkaline phosphatase.
Table 6.
Independent association between low BMD and bone markers in females.
| Factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| Odds ratio (95%CI) | p value | Odds ratio (95%CI) | p value | |
| Age | 2.23 (1.54–3.21) | 0.00002 | 1.90 (1.28–2.83) | 0.0015 |
| Pentosidine | 1.94 (1.01–3.70) | 0.045 | 1.36 (0.70–2.66) | 0.366 |
| DPD | 1.35 (0.93–1.95) | 1.95 | ||
| 25(OH)D | 0.74 (0.54–0.99) | 0.048 | 0.74 (0.53–1.03) | 0.075 |
| TRACP-5b | 1.56 (1.11–2.20) | 0.011 | 1.57 (1.07–2.31) | 0.021 |
| Who1e PTH | 1.26 (0.85–1.85) | 0.25 | ||
| BAP | 1.25 (0.90–1.75) | 0.19 | ||
CI: confidence interval, DPD: deoxypyridinoline, 25(OH)D: 25-hydroxyvitamin D, TRACP-5b: tartrate-resistant acid phosphatase 5b, PTH: parathyroid hormone, BAP: bone alkaline phosphatase.
Discussion
In the present cohort study, we were able to calculate mean BMD and bone turnover markers by age and sex for the elderly aged 50 years and older according to the Japanese population ratio in more than 400 subjects randomly selected from a rural town registry in Japan. We were able to create a cohort that more accurately reflects the general population in comparison to traditional population studies that recruited active volunteers. Another feature of this study was the uniform distribution of age and gender ratios between 50 and 89 years old, as a result of collecting about 50 physical examination participants by age and gender. This uniform distribution is advantageous for making accurate statistical comparisons between men and women and between age groups.
In this study, significantly diminished BMD was seen in the elderly at the femoral neck and total hip in males and females. The BMD of the femoral neck, total hip, and lumber spine were comparable with previous studies in our country and elsewhere after accounting for gender and age (Table 7). Previous studies have demonstrated that BMD decreased with aging18, 19, and this study obtained the same results as have been described in the literature.
Table 7.
Mean BMD values in previous reports.
| Study | Country | Sex | Number | Age (years) | Femoral neck | Total hip | Lumber spine |
|---|---|---|---|---|---|---|---|
| Lee, 2019 | Korea | Male | 244 | Age > 65 | 0.78 (0.007) | 0.87 (0.008) | 0.94 (0.005) |
| Female | 319 | 0.56 (0.005) | 0.70 (0.005) | 0.73 (0.008) | |||
| Schacht, 2019 | Denmark | Male | 98 | 69.0 (6.0) | 0.95 (0.18) | 1.10 (0.24) | 1.31 (0.26) |
| Female | 86 | 70.0 (5.8) | 0.83 (0.19) | 0.88 (0.24) | 1.13 (0.25) | ||
| Fuggle, 2018 | England and Wales | Male | 194 | 64.4 (2.5) | 1.03 (0.14) | 1.06 (0.15) | |
| Female | 171 | 66.5 (2.7) | 0.89 (0.13) | 0.95 (0.17) | |||
| Fujiwara, 2003 | Japan | Male | 763 | 62.9 (9.8) | 0.73 (0.11) | 0.98 (0.16) | |
| Female | 1593 | 65.4 (9.8) | 0.62 (0.11) | 0.82 (0.11) | |||
| Our study | Japan | Male | 203 | 69.5 (11.2) | 0.88 (0.13) | 0.95 (0.15) | 1.27 (0.25) |
| Female | 208 | 70.0 (11.0) | 0.74 (0.11) | 0.81 (0.13) | 1.04 (0.19) |
Values represent mean (standard deviation).
Vertebral fracture should be considered as an influence on lumbar BMD, but vertebral fracture in this study was only 3.6%, and we do not think it has a significant influence on the results of lumbar BMD. We also performed an analysis of lumbar BMD without the subject of vertebral fractures, and the values were similar (Table 3). All bone markers in this study were within the standard value for males and females in each generation10, 12. In men and women, pentosidine and DPD increased with age. TRACP-5b increased with aging in males. However, other markers showed no association with age. In the current study, subjects were randomly selected from a rural Japanese town; thus, subjects may be healthy. Aging may exert little influence on bone markers, while the prevalence of osteoporosis and osteopenia increased with aging in both males and females.
In terms of the association between low BMD and bone markers, there was a significant association between low BMD and TRACP-5b in females. TRACP-5b is a bone resorption marker that is not affected by renal dysfunction and has a low diurnal variability20, 21. Thus, TRACP-5b has been considered a useful marker. TRACP-5b was inversely correlated with BMD in females22. Furthermore, TRACP-5b has been described to be associated with increased fracture risk in elderly females23, 24. TRACP-5b could be a potential marker to predict fractures. In this study, TRACP-5b was related to low BMD in randomly selected female residents in the area. High bone resorption may be a factor for low BMD in female residents. TRACP-5b may be a marker which was useful for the detection of low BMD.
Several reports have shown that diabetic patients have a higher risk of fractures, even though they do not have decreased BMD25–29. On the other hand, pentosidine has been found to increase in DM patients30. Pentosidine, one of the advanced glycation end products, is a marker of bone quality (matrix) and is associated with fragility fractures independently of bone density31, and can be associated with the pathogenesis of bone fragility in patients with DM. The most common comorbidity in this cohort was DM. There may have been some effect on the pentosidine levels. However, since the purpose of our study was to present data from a population close to the general population, not volunteers, we believe it is meaningful to present data including subjects with DM.
For females, previous studies reported large differences between pre- and postmenopausal serum bone turnover markers concentrations32, 33. During the menopausal transition, bone turnover markers increase due to increased osteoclast activity, which is caused by decreased estrogen levels34. In terms of BMD, it was reported that the degree of decline with age was greater in women than in men4, 6. On the other hand, Hannan et al. reported that men have higher bone mineral density than women, but there is no difference in the degree of bone mineral density loss with age6.
Bone turnover markers levels are high in early puberty and then decline faster in girls than in boys35–37. In young adults, bone turnover markers levels are higher in males than in females and then decline faster in females, reaching their lowest levels in the 40 s in females and in the 50 s in males38. PINP and CTX-I increase at menopause and then remain higher than premenopausal levels39, 40. In contrast, in older men, CTX-I and PINP levels are generally stable or only slightly elevated past age 7041.
There is a limitation in this study. Although the research design reduces the sampling bias by adopting random sampling from the resident register, we may not have been able to control for all potential biases as a result of the low participation rate.
Conclusions
A characteristic feature of this study was the collection of participants with age ranging from 50 to 89 years by a randomly sampling from the resident register.
Therefore, this research was designed to create a cohort that more accurately reflects common residents. BMD decreased with aging in the femoral neck and total hip. On the other hand, there was no characteristic change with aging in the lumber spine. Furthermore, all bone markers except for pentosidine and DPD showed no significant independent characteristics associated with age or gender in a multivariate analysis model. However, a significant association between low BMD and TRACP-5b in females was observed; therefore, high bone resorption may be a factor for low BMD in female residents.
Author contributions
R.T., M.U., M.N., T.S., and, Y.N. wrote the main and revised manuscript texts, and R.T., M.U., and S.I. prepared Fig. 1 and Tables 1, 2, 3, 4, 5, 6, 7. N.S., S.I., J.T., and H.K. gave supervision to this study. All authors reviewed the manuscript.
Funding
This work was supported by a grant from the Japan Orthopaedics and Traumatology Research Foundation, Inc. (no. 339), project research funds from the Japanese Orthopaedic Association, a research fund from the Japanese Society for Musculoskeletal Medicine, and a research fund from The Nakatomi Foundation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ryosuke Tokida and Masashi Uehara.
References
- 1.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman SL, Calderon AD. The utility and limitations of FRAX: A US perspective. Curr. Osteoporos. Rep. 2010;8:192–197. doi: 10.1007/s11914-010-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glendenning P, Chubb SAP, Vasikaran S. Clinical utility of bone turnover markers in the management of common metabolic bone diseases in adults. Clin. Chim. Acta. 2018;481:161–170. doi: 10.1016/j.cca.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Krall EA, Dawson-Hughes B, Hirst K, Gallagher JC, Sherman SS, Dalsky G. Bone mineral density and biochemical markers of bone turnover in healthy elderly men and women. J. Gerontol. A Biol. Sci. Med. Sci. 1997;52:M61–M67. doi: 10.1093/gerona/52A.2.M61. [DOI] [PubMed] [Google Scholar]
- 5.Jones G, Nguyen T, Sambrook P, Kelly PJ, Eisman JA. Progressive loss of bone in the femoral neck in elderly people: Longitudinal findings from the Dubbo osteoporosis epidemiology study. BMJ. 1994;309:691–695. doi: 10.1136/bmj.309.6956.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannan MT, Felson DT, Anderson JJ. Bone mineral density in elderly men and women: Results from the Framingham osteoporosis study. J. Bone Miner. Res. 1992;7:547–553. doi: 10.1002/jbmr.5650070511. [DOI] [PubMed] [Google Scholar]
- 7.Blunt BA, Klauber MR, Barrett-Connor EL, Edelstein SL. Sex differences in bone mineral density in 1653 men and women in the sixth through tenth decades of life: The Rancho Bernardo Study. J. Bone Miner. Res. 1994;9:1333–1338. doi: 10.1002/jbmr.5650090903. [DOI] [PubMed] [Google Scholar]
- 8.Sherman SS, Tobin JD, Hollis BW, Gundberg CM, Roy TA, Plato CC. Biochemical parameters associated with low bone density in healthy men and women. J. Bone Miner. Res. 1992;7:1123–1130. doi: 10.1002/jbmr.5650071003. [DOI] [PubMed] [Google Scholar]
- 9.Callegari ET, Gorelik A, Garland SM, Chiang CY, Wark JD. Bone turnover marker reference intervals in young females. Ann. Clin. Biochem. 2017;54:438–447. doi: 10.1177/0004563216665123. [DOI] [PubMed] [Google Scholar]
- 10.Nishizawa Y, Ohta H, Miura M, Inaba M, Ichimura S, Shiraki M, Takada J, Chaki O, Hagino H, Fujiwara S, Fukunaga M, Miki T, Yoshimura N. Guidelines for the use of bone metabolic markers in the diagnosis and treatment of osteoporosis (2012 edition) J. Bone Miner. Metab. 2013;31:1–15. doi: 10.1007/s00774-012-0392-y. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Lv F, Zhang Z, Deng W, Li Y, Deng Z, Jiang Y, Wang O, Xing X, Xu L, Xia W. Establishment of a normal reference value of parathyroid hormone in a large healthy Chinese population and evaluation of its relation to bone turnover and bone mineral density. Osteoporos. Int. 2016;27:1907–1916. doi: 10.1007/s00198-015-3475-5. [DOI] [PubMed] [Google Scholar]
- 12.Nishizawa Y, Nakamura T, Ohata H, Kushida K, Gorai I, Shiraki M, Fukunaga M, Hosoi T, Miki T, Nakatsuka K, Miura M, Committee on the guidelines for the use of biochemical markers of bone turnover in osteoporosis: Japan Osteoporosis Society Guidelines on the use of biochemical markers of bone turnover in osteoporosis (2001) J. Bone Miner. Metab. 2001;19:338–344. doi: 10.1007/s007740170002. [DOI] [PubMed] [Google Scholar]
- 13.Uehara M, Takahashi J, Ikegami S, Tokida R, Nishimura H, Sakai N, Kato H. Sagittal spinal alignment deviation in the general elderly population: A Japanese cohort survey randomly sampled from a basic resident registry. Spine J. 2019;19:349–356. doi: 10.1016/j.spinee.2018.06.346. [DOI] [PubMed] [Google Scholar]
- 14.Tokida R, Uehara M, Ikegami S, Takahashi J, Nishimura H, Sakai N, Kato H. The association between sagittal spinal alignment and physical function in a Japanese cohort sampled from an older general population: A Japanese cohort survey randomly sampled from a basic resident registry. J. Bone Joint Surg. Am. 2019;101:1698–1706. doi: 10.2106/JBJS.18.01384. [DOI] [PubMed] [Google Scholar]
- 15.Uehara M, Takahashi J, Ikegami S, Tokida R, Nishimura H, Kuraishi S, Sakai N, Kato H. Impact of diffuse idiopathic skeletal hyperostosis on sagittal spinal alignment in the general elderly population: A Japanese cohort survey randomly sampled from a basic resident registry. JBJS Open Access. 2019;4:e0062.1–6. doi: 10.2106/JBJS.OA.18.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikegami S, Takahashi J, Uehara M, Tokida R, Nishimura H, Sakai A, Kato H. Physical performance reflects cognitive function, fall risk, and quality of life in community-dwelling older people. Sci. Rep. 2019;9:12242. doi: 10.1038/s41598-019-48793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 18.Iki M, Fujita Y, Tamaki J, Kouda K, Yura A, Kadowaki E, Sato Y, Moon JS, Okamoto N, Kurumatani N, Study Group for Functioning Capacity and Quality of Life in Elderly Japanese (Fujiwara-kyo Study Group) Design and baseline characteristics of a prospective cohort study for determinants of osteoporotic fracture in community-dwelling elderly Japanese men: The Fujiwara-kyo osteoporosis risk in men (FORMEN) study. BMC Musculoskelet. Disord. 2009;24:165. doi: 10.1186/1471-2474-10-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimura N, Kinoshita K, Danjoh S, Takijiri T, Morioka S, Kasamatsu T, Sakata K, Hashimoto T. Bone loss at the lumbar spine and the proximal femur in a rural Japanese community, 1990–2000: The Miyama Study. Osteoporos. Int. 2002;13:803–808. doi: 10.1007/s001980200111. [DOI] [PubMed] [Google Scholar]
- 20.Yamada S, Inaba M, Kurajoh M, Shidara K, Imanishi Y, Ishimura E, Nishizawa Y. Utility of serum tartrate-resistant acid phosphatase (TRACP5b) as a bone resorption marker in patients with chronic kidney disease: Independence from renal dysfunction. Clin. Endocrinol. 2008;69:189–196. doi: 10.1111/j.1365-2265.2008.03187.x. [DOI] [PubMed] [Google Scholar]
- 21.Halleen JM. Tartrate-resistant acid phosphatase 5B is a specific and sensitive marker of bone resorption. Anticancer Res. 2003;23:1027–1029. [PubMed] [Google Scholar]
- 22.Qin YJ, Zhang ZL, Zhang H, Hu WW, Liu YJ, Hu YQ, Li M, Gu JM, He JW. Age-related changes of serum tartrate-resistant acid phosphatase 5b and the relationship with bone mineral density in chinese women. Acta Pharmacol. Sin. 2008;29:1493–1498. doi: 10.1111/j.1745-7254.2008.00890.x. [DOI] [PubMed] [Google Scholar]
- 23.Gerdhem P, Ivaska KK, Alatalo SL, Halleen JM, Hellman J, Isaksson A, Pettersson K, Väänänen HK, Akesson K, Obrant KJ. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J. Bone Miner. Res. 2004;19:386–393. doi: 10.1359/JBMR.0301244. [DOI] [PubMed] [Google Scholar]
- 24.Ivaska KK, Gerdhem P, Väänänen HK, Akesson K, Obrant KJ. Bone turnover markers and prediction of fracture: A prospective follow-up study of 1040 elderly women for a mean of 9 years. J. Bone Miner. Res. 2010;25:393–403. doi: 10.1359/jbmr.091006. [DOI] [PubMed] [Google Scholar]
- 25.Moseley KF. Type 2 diabetes and bone fractures. Curr. Opin. Endocrinol. Diabetes Obes. 2012;19:128–135. doi: 10.1097/MED.0b013e328350a6e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz AV, Sellmeyer DE. Diabetes, fracture, and bone fragility. Curr. Osteoporos. Rep. 2007;5:105–111. doi: 10.1007/s11914-007-0025-x. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, Donaldson MG, Cauley JA, Harris TB, Koster A, Womack CR, Palermo L, Black DM, Study of Osteoporotic Fractures (SOF) Research Group. Osteoporotic Fractures in Men (MrOS) Research Group. Health, Aging, and Body Composition (Health ABC) Research Group Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305:2184–2192. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR, Study of Osteoporotic Features Research Group Older women with diabetes have an increased risk of fracture: A prospective study. J. Clin. Endocrinol. Metab. 2001;86:32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 29.de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: The Rotterdam Study. Osteoporos. Int. 2005;16:1713–1720. doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 30.Choi YJ, Ock SY, Jin Y, Lee JS, Kim SH, Chung Y. Urinary pentosidine levels negatively associates with trabecular bone scores in patients with type 2 diabetes mellitus. Osteoporos. Int. 2018;29:907–915. doi: 10.1007/s00198-017-4359-7. [DOI] [PubMed] [Google Scholar]
- 31.Nakano M, Nakamura Y, Suzuki T, Miyazaki A, Takahashi J, Saito M, Shiraki M. Pentosidine and carboxymethyl-lysine associate differently with prevalent osteoporotic vertebral fracture and various bone markers. Sci. Rep. 2020;10:22090. doi: 10.1038/s41598-020-78993-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ardawi MS, Maimani AA, Bahksh TA, Rouzi AA, Qari MH, Raddadi RM. Reference intervals of biochemical bone turnover markers for Saudi Arabian women: A cross-sectional study. Bone. 2010;47:804–814. doi: 10.1016/j.bone.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Iki M, Akiba T, Matsumoto T, Nishino H, Kagamimori S, Kagawa Y, Yoneshima H, JPOS Study Group Reference database of biochemical markers of bone turnover for the Japanese female population. Japanese Population-based Osteoporosis (JPOS) Study. Osteoporos. Int. 2004;15:981–991. doi: 10.1007/s00198-004-1634-1. [DOI] [PubMed] [Google Scholar]
- 34.Al-Azzawi F. The menopause and its treatment in perspective. Postgrad. Med. J. 2001;77:292–304. doi: 10.1136/pmj.77.907.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez L, Guañabens N, Peris P, Vidal S, Ros I, Monegal A, Bedini JL, Deulofeu R, Pons F, Muñoz-Gomez J, Ballesta AM. Usefulness of biochemical markers of bone turnover in assessing response to the treatment of Paget's disease. Bone. 2001;29:447–452. doi: 10.1016/S8756-3282(01)00592-0. [DOI] [PubMed] [Google Scholar]
- 36.Garnero P, Fledelius C, Gineyts E, Serre CM, Vignot E, Delmas PD. Decreased beta-isomerization of the C-terminal telopeptide of type I collagen alpha 1 chain in Paget's disease of bone. J. Bone Miner. Res. 1997;12:1407–1415. doi: 10.1359/jbmr.1997.12.9.1407. [DOI] [PubMed] [Google Scholar]
- 37.Orford N, Cattigan C, Brennan SL, Kotowicz M, Pasco J, Cooper DJ. The association between critical illness and changes in bone turnover in adults: A systematic review. Osteoporos. Int. 2014;25:2335–2346. doi: 10.1007/s00198-014-2734-1. [DOI] [PubMed] [Google Scholar]
- 38.Kitareewan W, Boonhong J, Janchai S, Aksaranugraha S. Effects of the treadmill walking exercise on the biochemical bone markers. J. Med. Assoc. Thai. 2011;94(Suppl 5):S10–S16. [PubMed] [Google Scholar]
- 39.Naylor K, Eastell R. Bone turnover markers: Use in osteoporosis. Nat. Rev. Rheumatol. 2012;8:379–389. doi: 10.1038/nrrheum.2012.86. [DOI] [PubMed] [Google Scholar]
- 40.Garnero P, Borel O, Delmas PD. Evaluation of a fully automated serum assay for C-terminal cross-linking telopeptide of type I collagen in osteoporosis. Clin. Chem. 2001;47:694–702. doi: 10.1093/clinchem/47.4.694. [DOI] [PubMed] [Google Scholar]
- 41.Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R. Bone turnover in prolonged critical illness: Effect of vitamin D. J. Clin. Endocrinol. Metab. 2003;88:4623–4632. doi: 10.1210/jc.2003-030358. [DOI] [PubMed] [Google Scholar]


