Abstract
Background.
Differences in socio-environmental exposures influence overall child health, but their association with pediatric CF outcomes is less clear. This observational study investigated the relationship between area-level socioeconomic deprivation, state child health, and CF respiratory outcomes in a national cohort.
Methods.
We assessed relationships between 2015 Area Deprivation Index, a composite measure of socioeconomic disadvantage; 2016 Child Health Index, a state-specific measure of overall child health; and CF respiratory outcomes in the 2016 CF Foundation Patient Registry.
Results.
The sample included 9,934 individuals with CF, aged 6–18 years. In multiple regression analysis adjusted for demographic and clinical covariates, those residing in the worst tertile for area deprivation had 2.8% lower ppFEV1 (95% CI −4.1 to −1.5), 1.2 more IV treatment nights (CI 0.1–2.4), and 20% higher odds of ≥2 pulmonary exacerbations (OR 1.2, CI 1.0–1.5) than best-tertile counterparts. Children with CF in states at the worst tertile for child health had 2.3% lower ppFEV1 (CI −4.5 to −0.2), 2.2 more IV treatment nights (CI 0.5–3.6), and 40% higher odds of ≥2 exacerbations (OR 1.4, CI 1.1–1.8) than best-tertile counterparts. State child health accounted for the association between area deprivation and multiple exacerbations and more IV treatment nights.
Conclusions.
Both area socioeconomic characteristics and state child health play a role in pediatric CF outcomes. The residual association of the state child health with CF outcomes after controlling for area deprivation reflects the ability of state programs to mitigate the effect of poverty.
BACKGROUND
Cystic fibrosis (CF) is the most common life-shortening autosomal recessive genetic disorder among Caucasians and the second most common overall, affecting 1 in 3,500 live births and approximately 30,000 individuals in the United States.1 Much variation in CF outcomes is attributable to non-genetic factors.2 Differences in care patterns may partially explain this variability, but socio-environmental influences, such as local and regional differences in environmental exposures, public health resources, and other social determinants likely play a role as well. 2–4 For example, prior research has reported both local and regional variation in type 2 diabetes incidence among U.S. youth.5 Relationships have been established between area characteristics and childhood BMI,6 childhood diabetes and obesity,7 pediatric cardio-metabolic dysfunction,8 and infant mortality.9 Area deprivation has been associated with pediatric asthma prevalence,10,11 severity,12 and emergency department visits and hospitalizations.13–15 However, the association of area deprivation with CF outcomes has not been explored, although variation of CF outcomes by ZIP code of residence have been noted both in the US and the UK.16–18 Furthermore, while state-level variations in public health have been reported in the US, reflected in metrics such as obesity prevalence, infant mortality, oral health, and overall child well-being,19 this association has likewise never been explored in CF.
This study assessed the relationship between area-level socioeconomic disadvantage measured with the Area Deprivation Index (ADI),20, state-level child health measured with the Child Health Index (CHI),19 and CF-specific respiratory outcomes in a national cohort of children and adolescents with CF, age 6-18.
METHODS
This was a cross-sectional observational study with population-level data for year 2016, obtained from the national CF Foundation Patient Registry (CFFPR).21 The study population included all children and adolescents with CF who at the end of 2016 were between 6 years (the earliest age of reproducible spirometry reported to the CFFPR) and 18 years of age. The study was approved by the Institutional Review Board of the University of Alabama at Birmingham (protocol 300002076).
Measures
Outcome variables.
Lung function measures (forced expiratory volume in 1 second, percent predicted [ppFEV1], calculated using the Global Lung Function Initiative [GLI] reference equations22), and the reported number of pulmonary exacerbations and treatment nights with intravenous (IV) antibiotics, either in the hospital or at home, were obtained from annualized 2016 CFFPR data. The CFFPR requires that ppFEV1 values are obtained from pre-bronchodilator pulmonary function tests. Pulmonary exacerbations are based on physician assessment during the encounter; they were categorized as none or one vs two or more, as done previously.23
Exposure variables.
Area deprivation was assessed with the 2015 Area Deprivation Index (ADI), an existing factor-based composite measure of socioeconomic deprivation (national 1-100 scale), with higher values indicating higher deprivation.20,24 The ADI is constructed from 17 variables in the domains of income, education, employment, and housing quality collected by the 2011-2015 American Community Survey and aggregated to U.S. Census block groups.20,24 Sample-specific ADI scores were calculated using patient residential 5-digit ZIP codes from the CFFPR and employing the 9-digit ZIP code crosswalk built to correspond to Census block groups. Median ADI was computed from all ADI values within each 5-digit ZIP code. ADI values corresponding to ZIP codes from post office boxes, businesses, or large footprint entities were set to missing and excluded from analyses. The ADI scores were then categorized into deciles to create a scale of 1 to 10.
The Child Health Index (CHI), originally developed by Goldhagen et al. (2005),19 was recreated using 2016 KIDS COUNT data from the Annie E. Casey Foundation for all U.S. states. Missing data for 2016 was substituted with data for 2015. The CHI is a composite score constructed from annual low birth weight, child death rate, teen death rate, and teen birth rate, by state. For ease of interpretation, the CHI scale was transformed so that higher values indicated worse child health, in parallel with the higher ADI values indicating higher deprivation. The CHI scores were then categorized into deciles to create a scale of 1 to 10.
Covariates.
Analyses were adjusted for demographic characteristics: age, sex (Male/Female), and race/ethnicity (non-Hispanic White/Other). Additionally, we adjusted for variables with known variation by state or geographic region: health insurance (Public/Private), CF diagnosis through newborn screening (Yes/No), and P.aeruginosa infection (Yes/No). The health insurance variable was used as a proxy measure of individual socioeconomic status, as done in multiple previous analyses of CFFPR data.25 We did not adjust for factors that do not have known regional variation in this population, such as CFTR genotype or Body Mass Index.
Statistical analysis
We assessed unadjusted and adjusted associations between ADI scores, CHI scores, and CF respiratory outcomes. To examine the effect of ADI and CHI both individually and together, for each outcome we estimated three multivariable models: Model 1 included ADI and covariates; Model 2 included CHI and covariates; Model 3 includes ADI, CHI, and covariates. All three models adjusted for the same covariates (age, sex, race/ethnicity, health insurance, newborn screening diagnosis, and P. aeruginosa). All analyses were clustered by state using robust standard errors.26 In addition to multivariable linear regression with continuous ADI and CHI scales, we performed multivariable logistic regression with ADI and CHI scores transformed into tertiles for ease of interpretation. Sensitivity analyses assessed the extent to which the effect of CHI depends on ADI, and vice versa, for lung function outcomes. Less than 5% of the sample had missing data, therefore listwise deletion was applied. Means comparison tests showed that individuals excluded from analyses due to missing data did not differ from the analyzed sample for clinical outcomes. Statistical tests were two-sided and were performed using a 5% significance level (α = 0.05). Analyses were performed using Stata software, version 15 (College Station, TX: StataCorp LLC).
RESULTS
The study population included 9,934 individuals residing in 50 states. Figure 1 presents a STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) diagram of the study sample. The demographic and clinical characteristics of the cohort, overall and by ADI and CHI top and bottom tertiles, are shown in Table 1. The average age of the cohort was 12.5 years (SD 3.8). Most individuals were White (90.3%) and had public health insurance (53.9%). Less than a third (29.1%) were diagnosed through newborn screening. Mean ppFEV1 was 90.2% (SD 18.6), the mean number of pulmonary exacerbations was 0.6 (range 0-13), and nearly 14% had two or more exacerbations during the year. There was no difference in race, sex, age, or health insurance between those residing in states with worst child health and their counterparts in states with best child health. However, in the most deprived residential areas, there were fewer White non-Hispanic children (86.8% vs 91.5%, p<0.001) and more children with public health insurance (72.0% vs 37.2%, p<0.001) compared to the least deprived residential areas. All clinical characteristics differed significantly by ADI and CHI tertiles, except for newborn screening diagnosis, which was not associated with residential area deprivation but was associated with state child health; specifically, states in the best tertile for child health had higher proportion of CF patients diagnosed through newborn screening than states in the worst tertile (39% vs 24.2%, p<0.001).
Figure 1.
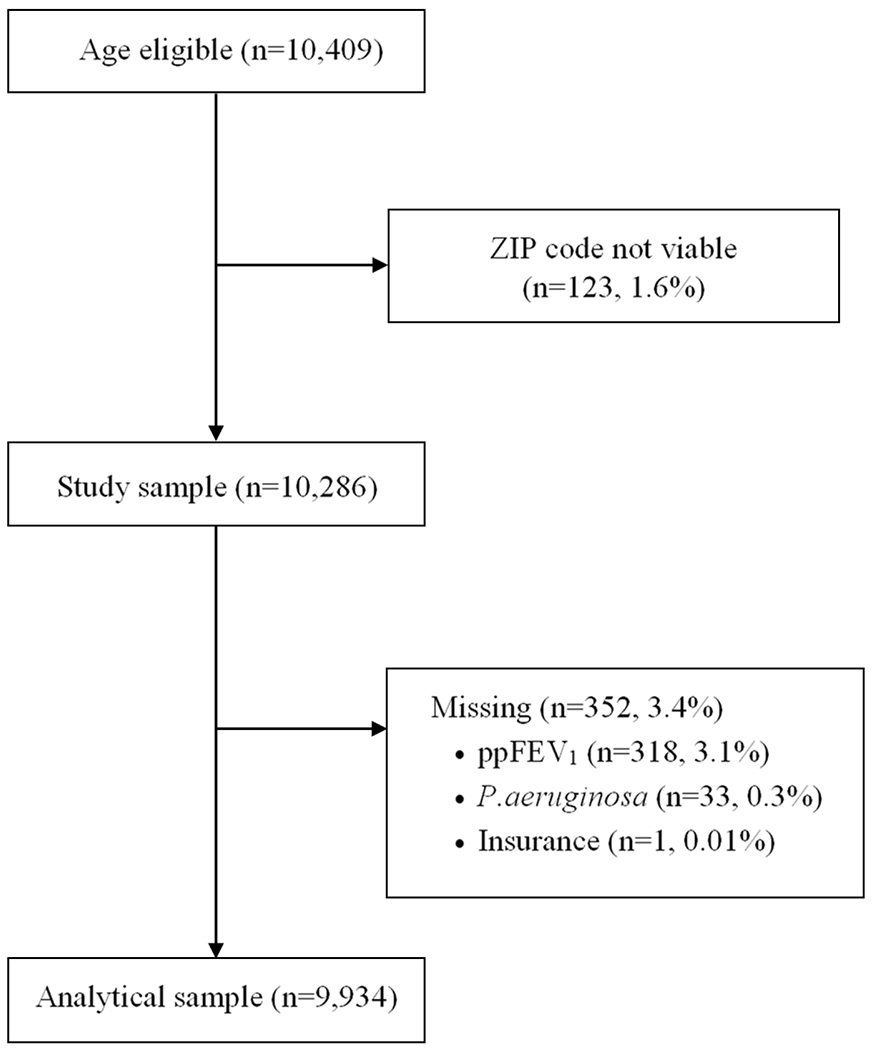
STROBE diagram of study population
Table 1.
Characteristics of the CF sample: overall and by Area Deprivation Index and Child Health Index tertiles (N=9,934)
| Characteristics | Total | Area Deprivation Index (ADI) | Child Health Index (CHI) | ||
|---|---|---|---|---|---|
| Low deprivation 1st tertile | High deprivation 3rd tertile | Best health 1st tertile | Worst health 3rd tertile | ||
| Demographic | |||||
| Age (range 6-18) | 12.5 (3.8) | 12.6 (3.8) | 12.5 (3.7) | 12.5 (3.8) | 12.5 (3.7) |
| Female, % | 49.3 | 49.5 | 51.1 | 49.2 | 49.6 |
| White, % | 90.3 | 91.5 | 86.8*** | 89.8 | 90.5 |
| Public health insurance, % | 53.9 | 37.2 | 72.0*** | 52.0 | 60.2 |
| Clinical | |||||
| ppFEV1 (range 15-149) | 90.2 (18.6) | 91.7 (18.4) | 87.4 (19.2)*** | 92.1 (18.5) | 89.0 (18.7)*** |
| Pulmonary exacerbations (range 0-13) | 0.6 (1.1) | 0.5 (0.3) | 0.7 (1.2)*** | 0.5 (1.0) | 0.7 (1.2)*** |
| ≥2 pulmonary exacerbations, % | 13.5 | 10.8 | 16.2*** | 11.0 | 16.0*** |
| IV treatment nights (range 0-272) | 8.0 (18.0) | 6.39 (15.2) | 9.3 (19.0)*** | 6.5 (16.4) | 9.3 (18.8)*** |
| Newborn screening diagnosis, % | 29.1 | 30.4 | 27.0 | 39.0 | 24.2** |
| P. aeruginosa, % | 34.4 | 31.7 | 37.1*** | 31.0 | 34.4* |
All values are mean (SD) unless otherwise noted.
Boldface indicates statistical significance of the difference between the 1st and 3rd tertile of ADI and CHI: *p<0.05, **p<0.01, ***p<0.001; two-tailed tests.
Figure 2 shows the distribution of U.S. states by state CHI deciles and sample-specific ADI deciles. Eight states (Alabama, Arkansas, Kentucky, Louisiana, Mississippi, Oklahoma, South Carolina, and West Virginia) ranked in the bottom two deciles on both area deprivation and state child health. The raw CHI scores of all states, with mean ADI scores of the state CF sample, are presented in supplementary Table 1.
Figure 2.
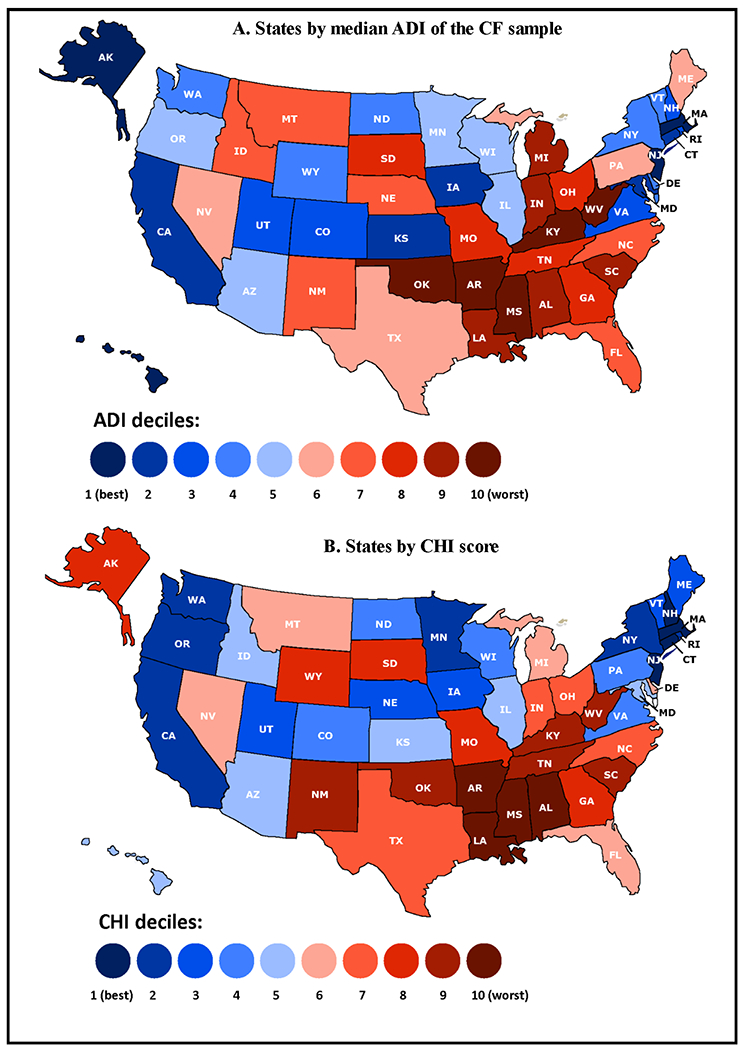
States by (A). Median Area Deprivation Index (ADI) of the CF sample; (B). State Child Health Index (CHI)
In multiple linear regression adjusted for age, sex, race/ethnicity, health insurance, newborn screening diagnosis, and P. aeruginosa, each decile increase in local area deprivation (ADI scale 1-10) was associated with 0.4% decrease in ppFEV1 (p=0.003) and 0.3 more IV treatment nights (p=0.001). Similarly, each decile increase in state CHI (scale 1-10) was associated with 0.3% decrease in ppFEV1 (p=0.044) and 0.3 more IV treatment nights (p=0.003) (supplementary Table 2).
Table 2 shows results from nested multiple regression models of lung function (ppFEV1) and IV treatment nights adjusted for age, sex, race/ethnicity, health insurance, newborn screening, and P. aeruginosa (graph representations in supplementary Figure 1). For ease of interpretation, ADI and CHI scores are shown in tertiles. In Model 1 (ADI and covariates), CF patients residing in the most deprived areas had 2.8% lower ppFEV1 (95% CI −4.1 to −1.5, p<0.001) and 1.2 more IV treatment nights (95% CI 0.1 to 2.4, p<0.001) than those residing in the least deprived areas. In Model 2 (CHI and covariates), CF patients residing in states with worst overall child health had 2.3% lower ppFEV1 (95% CI −4.4 to −0.2, p<0.05) and 2.2 more IV treatment nights (95% CI 0.6 to 3.7, p<0.01) than counterparts in states with best child health. In the full Model 3 (both ADI and CHI plus covariates), area deprivation and overall child health remained significantly associated with FEV1 and IV treatment nights but partially accounted for each other’s role as demonstrated by the reduction in magnitude of ADI coefficients (Model 1 to Model 3) and CHI coefficients (Model 2 to Model 3). The effect sizes of ADI and CHI, both individually and together, were larger than the effect sizes of age, sex, race/ethnicity, and newborn screening diagnosis; only health insurance and P. aeruginosa had larger effects than ADI and CHI (data not shown).
Table 2.
Multiple regression models of CF outcomes clustered by state (N=9,934)
| ppFEV1 | IV Nights | |||||
|---|---|---|---|---|---|---|
| Model 1a β (95% CI) |
Model 2b β (95% CI) |
Model 3c β (95% CI) |
Model 1a β (95% CI) |
Model 2b β (95% CI) |
Model 3c β (95% CI) |
|
| Area Deprivation Index (ADI), tertiles | ||||||
| ADI 1 (least disadvantaged) |
0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||
| ADI 2 | −0.41 (1.3, 0.5) | - | 0.09 (-0.6, 0.8) | 1.39** (0.5, 2.3) | − | 0.97* (0.1, 1.8) |
| ADI 3 (most disadvantaged) |
−2.78*** (−4.1, −1.5) | - | −1.95** (−3.1, −0.8) | 1.23* (0.1, 2.4) | − | 0.53 (−0.7, 1.7) |
| Child Health Index (CHI), tertiles | ||||||
| CHI 1 (best child health) |
0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||
| CHI 2 | - | −2.64* (−4.8, −0.5) | −2.32* (−4.4, −0.2) | - | 1.72** (−0.8, 2.7) | 1.55** (0.6, 2.5) |
| CHI 3 (worst child health) | - | −2.31* (−4.4, −0.2) | −1.85 (−3.9, 0.2) | - | 2.17** (0.6, 3.7) | 1.95* (0.3, 3.6) |
Model 1: ADI alone;
Model 2: CHI alone;
Model 3 (full): both ADI and CHI
All models adjusted for age, sex, race/ethnicity, health insurance, newborn screening, and P.aeruginosa.
Boldface indicates statistical significance: ***p<0.001, **p<0.01, *p<0.05, two-tailed tests.
We also performed sensitivity analyses to assess the extent of interaction between CHI and ADI (supplementary Table 3). Interaction terms indicated that children with CF who resided in areas of worst deprivation in states with worst child health had more IV treatment nights than their advantaged counterparts (7.8 vs.7.5; p=0.005). Meanwhile, children who resided in areas of least deprivation in states with best child health had 1% higher ppFEV1 than their disadvantaged counterparts (90% vs. 89%; p=0.008).
Figure 3 shows the odds of having ≥2 pulmonary exacerbations by ADI and CHI tertiles after adjusting for age, sex, race/ethnicity, health insurance, newborn screening, and P. aeruginosa. Children with CF residing in the worst tertile for area deprivation had approximately 20% higher odds of multiple pulmonary exacerbations (OR 1.23, 95% CI 1.0 to 1.54, p<0.05) than counterparts in the best tertile (Figure 3A). Those residing in states in the worst tertile for child health had approximately 40% higher odds of multiple pulmonary exacerbations (OR 1.38, 95% CI 1.1 to 1.8, p<0.01) than counterparts in the best tertile (Figure 3B). When ADI and CHI were placed together in a model, ADI was no longer significant, indicating that its association with increased pulmonary exacerbations is accounted for by CHI (Figure 3C).
Figure 3. Odds of two or more exacerbations by Area Deprivation Index (ADI) and Child Health Index (CHI) (N=9,934).
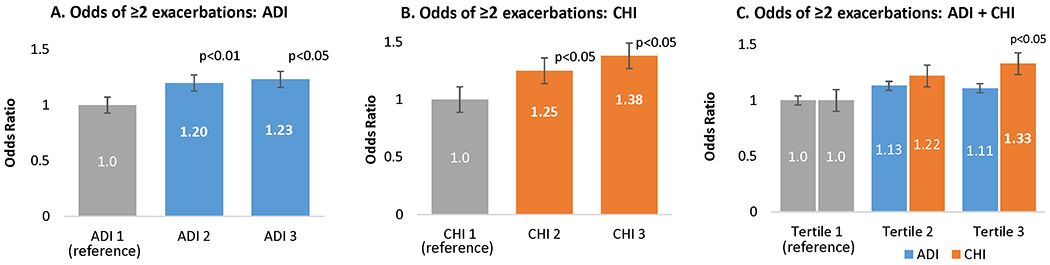
Reference category: ADI 1 (tertile 1, least deprivation), CHI 1 (tertile 1, best child health).
All models adjusted for age, sex, race/ethnicity, health insurance, newborn screening, and P. aeruginosa.
DISCUSSION
Using demographic and clinical data from a population-based CF patient registry, we assessed the association between residential area socioeconomic deprivation, state-level child health, and respiratory outcomes (lung function, pulmonary exacerbations, and IV treatment nights) in patients with CF who were 6-18 years old at the end of 2016. This is the first report that applies the Area Deprivation Index (ADI) to CF Foundation Patient Registry data.
Our findings suggest that the respiratory health of pediatric CF patients parallels the overall health of the general child population. State and area-level characteristics play a role for CF clinical outcomes. Although mechanism of these associations are multifactorial, our findings suggest that differences in public health infrastructure and social determinants of health are clearly involved.
Eight states ranked at the bottom on both socioeconomic deprivation and overall child health, all in the Southern U.S. The same region also has increased rates of adult chronic disease (obesity, diabetes, high blood pressure, coronary heart disease, myocardial infarction, stroke, chronic kidney disease, cancer, arthritis, asthma, chronic obstructive pulmonary disease, and depression) as well as worse health-related behaviors (smoking, physical activity, and fruit and vegetable consumption).27 These data highlight that childhood residency has health impact well into adulthood.
Children raised in worse socioeconomic circumstances have worse health.28,29 Children with CF are not an exception when exposed to the health risks of deprivation and social disadvantage. Prior research suggests that the socioeconomic disparity in CF outcomes has a very early onset.18,30 Inequalities in ppFEV1 by Medicaid status are evident at the earliest measurement of lung function (6 years of age) and widen only slightly over time.17 Furthermore, there is evidence that the nutritional and anthropometric status of children with CF is correlated with their socioeconomic status.18,25,31 It is unsurprising that early-life deprivation sets children with CF on a trajectory toward diminished health just as it does for children in the general population.
One’s socioeconomic context is associated with differential exposure to chronic stressors,32 a primary mechanism of socioeconomic inequalities in health.33–35 However, research on these exposures in the context of CF is scarce, and little is known about the specific pathways leading from social disadvantage to worse CF outcomes. Macpherson et al.36 report that children with CF who are cared for by single mothers have worse health outcomes than children with dual caregivers. There is also evidence that social disadvantage is associated with a higher prevalence of depressive symptoms and lower quality of life.37,38 Increased exposure to environmental tobacco smoke has also been implicated.39 Improved understanding of these mechanisms will require increased inclusion of socioeconomic measures in CF patient registries and medical records, as well as linking of patient registry data with area-level measures of social and environmental exposures.
Regional variations in CF demographics, insurance, pathogens, medication use, and co-morbidities have been reported previously. For example, the South has the highest proportion of patients with Methicillin-resistant Staphylococcus aureus, P. aeruginosa, and non-tuberculous mycobacterium.40 Although mean regional CF mortality rates are not statistically different among regions, the South has the highest mortality rates in each age grouping,40 which parallel the greater mortality and morbidity among the general population in that region.19 Our study adds to this literature by reporting a significant association between area-level socioeconomic deprivation, overall child health on a state level, and pediatric CF outcomes. It is important to note that the effects sizes of ADI and CHI were larger than the effect sizes age, sex, race/ethnicity, and newborn screening diagnosis. For example, the adjusted effects of ADI and CHI on ppFEV1 are larger than the average annual loss of lung function in pediatric CF.41–43 As well, the 23-38% increase in the adjusted odds of ≥2 pulmonary exacerbations is greater than the effect of air pollution23 and comparable to the effect of some established cystic fibrosis therapies.44 Finally, we should note that the contextual effects of ADI and CHI were observed after accounting for individual-level factors, such as socioeconomic status (indicated by health insurance type), P.aeruginosa infection, and demographic and clinical characteristics.
Area deprivation appears to provide a partial explanation for the association between overall child health and CF outcomes. However, the residual association of overall child health with CF outcomes after controlling for area deprivation reflects the ability of state programs to ameliorate the effect of poverty. Further research should investigate the effect of specific public health policy measures (e.g., policies related to tobacco control, Medicaid and state child health insurance programs, food assistance and school meal programs, housing, and air and water quality monitoring, among others) on both overall child health and CF-specific health outcomes.
While our study is the first one to use the ADI in analysis of pediatric CF outcomes, it has limitations. As the CFFPR collects only residential ZIP codes rather than full addresses, ADI scores were calculated at that level of geography. This is an inferior method of data aggregation45 resulting “in relatively large geographic zones with linkages that can lead to less precise estimates, especially in areas in which concentrated poverty abuts more wealthy regions.”46 Even with this imprecision, significant associations between ADI and CF outcomes were observed. Future research should use smaller-area measures for more precise estimates.
We did not adjust CF outcomes for the size, performance, or other characteristics of the pediatric CF programs in each state. Currently there are more than 120 accredited CF centers and 50 affiliate programs at teaching and community hospitals across the country. Although the CF Foundation maintains high accreditation standards, CF care and outcomes across centers and programs seem to vary. Access to and quality of CF care may therefore be an unmeasured confounder of CF outcomes in this analysis.
CONCLUSION
Survival in CF has improved dramatically over the past decades, but variations in disease outcomes persist. Our study highlights significant regional disparities in the health of children with CF and points to potential mechanisms responsible for such inequities. Improvements in the social, economic, and living environment and public health infrastructure will likely also benefit CF outcomes. Population-level policy, system, and environmental interventions may be more impactful than individual patient-level interventions. Research on the social determinants of CF health is therefore fundamental for CF health equality, as it may provide rationale for community interventions to reduce regional disparities in pulmonary health.
Supplementary Material
Acknowledgments:
The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. Additionally, we thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CF Foundation Patient Registry.
Funding: This study was supported by a grant from the National Institutes of Health (P30DK72482).
References
- 1.Foundation CF. Cystic Fibrosis Foundation Patient Registry, 2017 Annual Data Report. Bethesda, Maryland: 2018. [Google Scholar]
- 2.Oates GR, Schechter MS. Socioeconomic Status and Health Outcomes: Cystic Fibrosis as a Model. Expert Rev Respir Med. 2016;10(9):967–977. [DOI] [PubMed] [Google Scholar]
- 3.Collaco JM, Blackman SM, McGready J, Naughton KM, Cutting GR. Quantification of the Relative Contribution of Environmental and Genetic Factors to Variation in Cystic Fibrosis Lung Function. J Pediatr. 2010;157(5):802–807 e801–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor GT, Quinton HB, Kahn R, et al. Case-Mix Adjustment for Evaluation of Mortality in Cystic Fibrosis. Pediatric pulmonology. 2002;33(2):99–105. [DOI] [PubMed] [Google Scholar]
- 5.Liese AD, Lawson A, Song HR, et al. Evaluating Geographic Variation in Type 1 and Type 2 Diabetes Mellitus Incidence in Youth in Four Us Regions. Health Place. 2010;16(3):547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll-Scott A, Gilstad-Hayden K, Rosenthal L, et al. Disentangling Neighborhood Contextual Associations with Child Body Mass Index, Diet, and Physical Activity: The Role of Built, Socioeconomic, and Social Environments. Soc Sci Med. 2013;95:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodd C, Sharma AK. Prevalence of Overweight and Obesity in Canadian Children, 2004 to 2013: Impact of Socioeconomic Determinants. Paediatr Child Health. 2017;22(3):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams AD, Shenassa E, Slopen N, Rossen L. Cardiometabolic Dysfunction among U.S. Adolescents and Area-Level Poverty: Race/Ethnicity-Specific Associations. J Adolesc Health. 2018;63(5):546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirai AH, Sappenfield WM, Kogan MD, et al. Contributors to Excess Infant Mortality in the U.S. South. Am J Prev Med. 2014;46(3):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salmond C, Crampton P, Hales S, Lewis S, Pearce N. Asthma Prevalence and Deprivation: A Small Area Analysis. J Epidemiol Community Health. 1999;53(8):476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claudio L, Stingone JA, Godbold J. Prevalence of Childhood Asthma in Urban Communities: The Impact of Ethnicity and Income. Ann Epidemiol. 2006;16(5):332–340. [DOI] [PubMed] [Google Scholar]
- 12.Bryant-Stephens T Asthma Disparities in Urban Environments. J Allergy Clin Immunol. 2009;123(6):1199–1206; quiz 1207–1198. [DOI] [PubMed] [Google Scholar]
- 13.Largent J, Nickerson B, Cooper D, Delfino RJ. Paediatric Asthma Hospital Utilization Varies by Demographic Factors and Area Socio-Economic Status. Public Health. 2012;126(11):928–936. [DOI] [PubMed] [Google Scholar]
- 14.Liu SY, Pearlman DN. Hospital Readmissions for Childhood Asthma: The Role of Individual and Neighborhood Factors. Public Health Rep. 2009;124(1):65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nkoy FL, Stone BL, Knighton AJ, et al. Neighborhood Deprivation and Childhood Asthma Outcomes, Accounting for Insurance Coverage. Hosp Pediatr. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Schechter MS, McColley SA, Silva S, et al. Association of Socioeconomic Status with the Use of Chronic Therapies and Healthcare Utilization in Children with Cystic Fibrosis. J Pediatr. 2009;155(5):634–U667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor GT, Quinton HB, Kneeland T, et al. Median Household Income and Mortality Rate in Cystic Fibrosis. Pediatrics. 2003;111(4):e333–339. [DOI] [PubMed] [Google Scholar]
- 18.Taylor-Robinson DC, Smyth RL, Diggle PJ, Whitehead M. The Effect of Social Deprivation on Clinical Outcomes and the Use of Treatments in the Uk Cystic Fibrosis Population: A Longitudinal Study. Lancet Respir Med. 2013;1(2):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldhagen J, Remo R, Bryant T 3rd, et al. The Health Status of Southern Children: A Neglected Regional Disparity. Pediatrics. 2005;116(6):e746–753. [DOI] [PubMed] [Google Scholar]
- 20.Area Deprivation Index. University of Wisconsin School of Medicine and Public Health; 2018. Available at: https://www.neighborhoodatlas.medicine.wisc.edu/.
- 21.Knapp EA, Fink AK, Goss CH, et al. The Cystic Fibrosis Foundation Patient Registry. Design and Methods of a National Observational Disease Registry. Ann Am Thorac Soc. 2016;13(7):1173–1179. [DOI] [PubMed] [Google Scholar]
- 22.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-Ethnic Reference Values for Spirometry for the 3–95-Yr Age Range: The Global Lung Function 2012 Equations. Eur Respir J. 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of Ambient Air Pollution on Pulmonary Exacerbations and Lung Function in Cystic Fibrosis. Am J Respir Crit Care Med. 2004;169(7):816–821. [DOI] [PubMed] [Google Scholar]
- 24.Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible - the Neighborhood Atlas. N Engl J Med. 2018;378(26):2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The Association of Socioeconomic Status with Outcomes in Cystic Fibrosis Patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–1337. [DOI] [PubMed] [Google Scholar]
- 26.Rogers WH. Regression Standard Errors in Clustered Samples. In: Stata Technical Bulletin Reprints. Vol 3. College Station, TX: Stata Press; 2013:88–94. [Google Scholar]
- 27.Oates GR, Jackson BE, Partridge EE, Singh KP, Fouad MN, Bae S. Sociodemographic Patterns of Chronic Disease: How the Mid-South Region Compares to the Rest of the Country. Am J Prev Med. 2017;52(1S1):S31–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spencer N, Thanh TM, Louise S. Low Income/Socio-Economic Status in Early Childhood and Physical Health in Later Childhood/Adolescence: A Systematic Review. Matern Child Health J. 2013;17(3):424–431. [DOI] [PubMed] [Google Scholar]
- 29.Schreier HM, Chen E. Socioeconomic Status and the Health of Youth: A Multilevel, Multidomain Approach to Conceptualizing Pathways. Psychol Bull. 2013;139(3):606–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Britton LJ, Oates GR, Oster RA, et al. Risk Stratification Model to Detect Early Pulmonary Disease in Infants with Cystic Fibrosis Diagnosed by Newborn Screening. Pediatr Pulmonol. 2016;51(11):1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor-Robinson D, Whitehead M, Diggle P, Smyth R. The Effect of Social Deprivation on Weight in the Uk Cystic Fibrosis Population. J Epidemiol Community Health. 2011;65:A389–A389. [Google Scholar]
- 32.Turner RJ, Wheaton B, Lloyd DA. The Epidemiology of Social Stress. Am Sociol Rev. 1995;60(1):104–125. [Google Scholar]
- 33.Evans GW. A Multimethodological Analysis of Cumulative Risk and Allostatic Load among Rural Children. Dev Psychol. 2003;39(5):924–933. [DOI] [PubMed] [Google Scholar]
- 34.Lupie SJ, King S, Meaney MJ, McEwen BS. Can Poverty Get under Your Skin? Basal Cortisol Levels and Cognitive Function in Children from Low and High Socioeconomic Status. Dev Psychopathol. 2001;13(3):653–676. [DOI] [PubMed] [Google Scholar]
- 35.Roubinov DS, Hagan MJ, Boyce WT, Adler NE, Bush NR. Family Socioeconomic Status, Cortisol, and Physical Health in Early Childhood: The Role of Advantageous Neighborhood Characteristics. Psychosom Med. 2018;80(5):492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macpherson C, Redmond AO, Leavy A, McMullan M. A Review of Cystic Fibrosis Children Born to Single Mothers. Acta Paediatr. 1998;87(4):397–400. [DOI] [PubMed] [Google Scholar]
- 37.Schechter MS, Cruz I, Blackwell LS, Quittner AL. Risk Factors for Anxiety and Depression in Cystic Fibrosis. Pediatr Pulmonol. 2010:109–110. [Google Scholar]
- 38.Quittner AL, Schechter MS, Rasouliyan L, Haselkorn T, Pasta DJ, Wagener JS. Impact of Socioeconomic Status, Race, and Ethnicity on Quality of Life in Patients with Cystic Fibrosis in the United States. Chest. 2010;137(3):642–650. [DOI] [PubMed] [Google Scholar]
- 39.Oates GR, Baker E, Rowe SM, et al. Tobacco Smoke Exposure and Socioeconomic Factors Are Independent Predictors of Pulmonary Decline in Pediatric Cystic Fibrosis. J Cyst Fibros. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kopp BT, Nicholson L, Paul G, Tobias J, Ramanathan C, Hayes D Jr. Geographic Variations in Cystic Fibrosis: An Analysis of the U.S. Cf Foundation Registry. Pediatr Pulmonol. 2015;50(8):754–762. [DOI] [PubMed] [Google Scholar]
- 41.Cogen J, Emerson J, Sanders DB, et al. Risk Factors for Lung Function Decline in a Large Cohort of Young Cystic Fibrosis Patients. Pediatr Pulmonol. 2015;50(8):763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical Use of Ibuprofen Is Associated with Slower Fev1 Decline in Children with Cystic Fibrosis. Am J Respir Crit Care Med. 2007;176(11):1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D, Keogh R, Clancy JP, Szczesniak RD. Flexible Semiparametric Joint Modeling: An Application to Estimate Individual Lung Function Decline and Risk of Pulmonary Exacerbations in Cystic Fibrosis. Emerg Themes Epidemiol. 2017;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhatt JM. Treatment of Pulmonary Exacerbations in Cystic Fibrosis. Eur Respir Rev. 2013;22(129):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grubesic TH, Matisziw TC. On the Use of Zip Codes and Zip Code Tabulation Areas (Zctas) for the Spatial Analysis of Epidemiological Data. Int J Health Geogr. 2006;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durfey SNM, Kind AJH, Gutman R, et al. Impact of Risk Adjustment for Socioeconomic Status on Medicare Advantage Plan Quality Rankings. Health Aff (Millwood). 2018;37(7):1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


