Abstract
Interventions focused on utilization of epilepsy surgery can be divided into groups: those that improve patients’ access to surgical evaluation and those that facilitate completion of the surgical evaluation and treatment. Educational intervention, technological innovation, and effective coordination and communication can significantly improve patients’ access to surgery. Patient and public facing, individualized (analog and/or digital) communication can raise awareness and acceptance of epilepsy surgery. Educational interventions aimed at providers may mitigate knowledge gaps using practical and concise consensus statements and guidelines, while specific training can improve awareness around implicit bias. Innovative technology, such as clinical decision making toolkits within the electronic medical record(EMR), machine learning techniques, online decision-support tools, nomograms, and scoring algorithms can facilitate timely identification of appropriate candidates for epilepsy surgery with individualized guidance regarding referral appropriateness, postoperative seizure freedom rate and risks of complication after surgery. There are specific strategies applicable for epilepsy centers’ success: building a multidisciplinary setup, maintaining/tracking volume and complexity of cases, collaborating with other centers, improving surgical outcome with reduced complications, utilizing advanced diagnostics tools, and considering minimally invasive surgical techniques. Established centers may use other strategies, such as multi-stage procedures for multifocal epilepsy, advanced functional mapping with tailored surgery for epilepsy involving the eloquent cortex, and generation of fresh hypotheses in cases of surgical failure. Finally, improved access to epilepsy surgery can be accomplished with policy changes (e.g., anti-discrimination policy, exemption in transportation cost, telehealth reimbursement policy, patient-centered epilepsy care models, pay-per-performance models, affordability and access to insurance, and increased funding for research). Every intervention should receive regular evaluation and feedback driven modification to ensure appropriate utilization of epilepsy surgery.
Keywords: barriers to care, drug-resistant epilepsy, epilepsy, epilepsy surgery, medically resistant epilepsy, underutilization, health communication
Introduction
Despite high-quality evidence including randomized controlled trials (RCT), substantial supporting scientific literature, and well-established guidelines from professional societies, epilepsy surgery remains one of the most underutilized evidence-based treatments in modern medicine.(1–5) In part I of the series, we provided a comprehensive review of barriers to epilepsy surgery. Interventions to improve utilization of epilepsy surgery may address challenges that prevent access to surgical evaluation and prevent patients from completing necessary workup, ultimate completion of the surgery and post-surgical care. In this paper, we explore potential interventional strategies using education, technology, policy-change, and other approaches to empower patients and caregivers with adequate information. Further, we highlight interventions to address gaps in physician knowledge and practice, improve access to comprehensive epilepsy centers, streamline presurgical work-ups, mitigate systemic inequities, and increase research funding with the ultimate goal to reduce the treatment gap surrounding epilepsy surgery. (Figure 1)
Fig. 1.
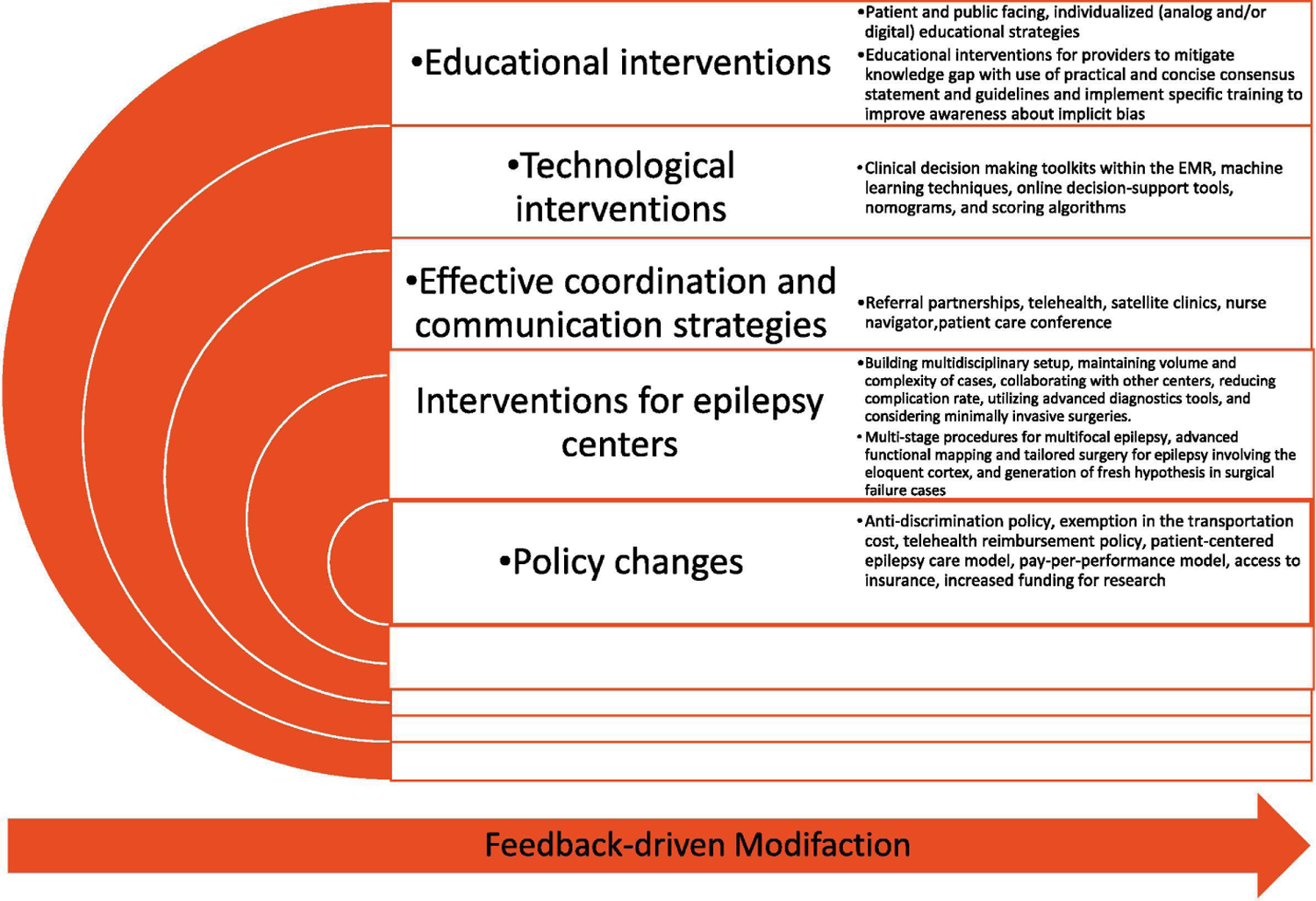
Strategies to overcome epilepsy surgery barriers.
1. Educational Intervention
1.1. Patient-facing educational intervention
1.1.1. Educational strategies
Patients’ fears and misperceptions regarding epilepsy surgery are one of the strongest barriers to pursuing epilepsy surgery (please review Part I: Table 1), and >75% of people with epilepsy desire more information about epilepsy surgery. (6–12) (13) Patient-facing educational strategies (verbal teaching with traditional lectures, audio or videotapes, seminars, module-specific teaching, demonstrations, role playing, etc.) are systematically studied for many chronic conditions such as diabetes, asthma, and cancer, to improve patient and family perspectives about their condition, empowering them in the management of their own disease. A meta-analysis evaluating effectiveness of various teaching strategies in diverse healthcare settings calculated the effect size for most teaching methods as small to moderate [traditional lectures 0.48 (95% CI, 0.29–0.67), discussions 0.34 (95% CI, 0.25–0.43), written materials 0.43 (95% CI, 0.33–0.53), audiotapes 0.43 (95% CI, 0.33–0.53), videotapes 0.41 (95% CI, 0.29–0.53), verbal teaching 0.28 (95% CI, 0.19–0.37)] with the notable exception of large effect size with teaching by demonstration (0.79; 95% CI, 0.55–1.03).(14, 15)
Table 1.
Skills, tools, and strategies for healthcare professionals
| Skills | • Skill to teach the patient self-management skill, including medication adherence, identification of seizure triggers, documentation and reporting of seizures, adherence to seizure action plan, and healthy living • Skill to motivate the patient to gain information and confidence so that they can make informed decisions about their health • Skill to improve different communication methods, including face-to-face (probably the best way to communicate), telephone, and electronic communications • Skill to empathetic understanding of the patient – how they connect with family, what support systems are in place, and how their environment impacts their care. • Skill regarding understanding of the implicit bias and its role in the treatment decision • Skill to assess if the person is taking in the information presented to them and what stressors are in the way. Paying attention to the patient’s worries and fears and try to dispel them • Skill to get the person engaged in conversation and find ways that the patient feels comfortable talking about fear and anxiety related to epilepsy surgery • Skill to establish a relationship with the patient • Skill to be aware of nonverbal messages when delivering verbal communication, including gestures, body language |
| Tools | • Visual tools usually supplement verbal education • Use a question list so that patients can ask questions and providers can answer them • Use the teach-back method/Ask patients to repeat information in their own words • Provide information in several different ways (analog and digital) to make sure the patient understands the concept and access that in a later time • Audiotapes of patient consultations can be effective for patient recall of verbal education • Regular practice of the communication skills to use them effectively and structured skill development exercises may be helpful for providers. |
| Strategies during the patient encounter | • Determine the patient’s barriers to health literacy. Assessing the ability to learn may include interview or observation • Ask patients to state their goals of epilepsy care to begin a discussion • Find out what the patient already knows before providing information; ask, “What do you already know about epilepsy surgery?” • Present the most important information first • Emphasize one to three key points • Focus on one issue at a time. • Present the information in logical blocks |
The preferred formats, timing and delivery of information and counselling regarding epilepsy surgery, and the outcomes following educational interventions have been investigated in a limited fashion.(16) A RCT showed significantly greater decrease in anxiety and depression levels in 100 pre-surgical patients with provision of more information (experiential information by showing two separate video interviews with one male and one female who had undergone temporal lobe surgery for epilepsy, as well as information about different diagnostic tests). Individuals identified as monitors (a particular coping or disposition style associated with more anxiety, information-need, and preference toward problem-focused strategies) had greater improvement than individuals who prefer distancing and/or avoidance-type strategies.(17) Providing detailed information can make >50% of patients and family members more favorable toward epilepsy surgery.(18) Besides traditional teaching tools, a prospective and randomized clinical study showed superiority of a new mixed reality tool (“VSI Patient Education” running on HoloLens® glasses) over the traditional 3-dimensional rubber brain model to educate patients before epilepsy surgery.(19) Patients could comprehend and imagine the procedure better using the new tool. Subsequently, they felt significantly less anxiety about epilepsy surgery and recommended the new tool as a preferred patient education tool. Similar effect was noted among family members.
Availability of both analog (i.e., written information in the physician’s office, handouts, large chart in the examination and waiting rooms) and digital (downloadable apps or URLs of relevant websites, webinars, podcasts) tools can be beneficial, as can combining teaching strategies with verbal instruction (especially if a patient has trouble recalling oral information with memory impairment) with other educational strategies.(20–21) Studies from other fields demonstrate structured (compared to ad hoc instruction), patient-specific, and culturally appropriate (especially for minority groups) interventions can be more efficient, delivered in either group or individual settings. (15, 20–22)(23) Patient-specific risks and benefit analysis provided in easily understandable terms can be extremely valuable, particularly if the patient and family develop insight regarding how life might be different post-surgery. Moreover, emphasis on enhanced abilities after epilepsy surgery rather than disability (maintaining data driven realism) is necessary to invoke a powerful effect of emotions on the attitudes and decision making surrounding surgical therapy. This is the key for many patients with intractable epilepsy as they may be unintentionally discouraged about surgery by improper use of language (example, epilepsy surgery as a last resort option) during early encounters with their providers. Structured instruments can assess misconceptions and anxiety related to surgery and can be pivotal to provide individualized counselling early after diagnosis. (24, 25) Additional considerations should be given regarding educational interventions in young patients with epilepsy: information given both with and without the caregivers, provision of a quiet room for teaching without distraction, and use of electronic media formats (the Internet, social networking).(26) Decisional aids and option listing may facilitate surgical decision making, especially in patients with baseline memory and cognitive difficulties.(27, 28) Online forums, chats, patient testimonial videos, peer-to-peer support programs, and social media posts can also provide needed support, information, and resources for epilepsy surgery candidates.
1.1.2. Self-management and stigma reduction interventions
Surgical options are more frequently denied by patients with comorbid psychiatric diseases.(11) Although epilepsy surgery-specific education is a directed way to approach patient anxiety related to surgery, individual patient self-management and stigma reduction interventions in the early stages of epilepsy may generate broader acceptance of surgical therapy by eliminating barriers to mental health care. Multiple non-pharmacological, evidence-based programs are readily available to provide self-management support in People with epilepsy (PWE). (29)(26) With high self-management skills, they may be more willing to pursue epilepsy surgery as intractable seizures become disabling and prevent them from taking full control of their lives. They also can use one of the many available smartphone applications to record seizure history, track changes over time, and share the content with their health care team. In a closed loop healthcare system, these data can be fed into the system to promote faster pathways to epilepsy surgery.
1.2. Society or community facing educational strategies
Society or community level intervention can be distinctly advantageous to raise public awareness of epilepsy by reducing lack of knowledge, misinformation, and stigmatizing attitudes and behaviors.(30–33) Although we lack understanding of the effect of public education specific to epilepsy surgery, the Institute of Medicine (US) Committee on Public Health Dimensions of the Epilepsies recommended several potential strategies to promote public knowledge about epilepsy, including improving coverage and depiction of the epilepsies in the media with specific programs for journalists as well as writers and producers in the entertainment industry, engaging people with epilepsy and their families in public awareness efforts, coordinating public awareness efforts(including social media campaigns) and developing shared messaging, and ensuring that all campaigns include rigorous formative research.(34) Public educational efforts may be more effective if tailored to intended audience demographics and consider issues related to limited health literacy.
1.3. For referring providers
1.3.1. Medical Education, Communication Training, Residency Education
Despite significant knowledge gap among providers(Part I:Table 2), the impact of educational intervention to promote referral to comprehensive epilepsy centers has not been systematically studied.(35, 36) Healthcare professionals including epilepsy specialists may also need to use certain strategies for effective communication, shared decision-making, and care planning.(Table 1) Early exposure to the patient-centered communication training may be beneficial during residency training, along with high quality education, training, and exposure related to surgical therapy. Interested trainees may further pursue dedicated epilepsy surgery fellowships afterwards, including development of further expertise in the use and interpretation of various diagnostic tools. An important positive step in the US was establishment of the new subspecialty board certification in epilepsy by the American Board of Psychiatry and Neurology (ABPN) which helped establish a core curriculum for accredited fellowships and standardized training in epilepsy. Additional resources may include education programs (example, the J. Kiffin Penry epilepsy education programs), mini-fellowships or appointment of specialists from experienced centers with visiting professorship.
1.3.2. Locally adapted consensus guideline, educational campaign, conferences, Telementoring programs
Although there is unequivocal need for provider education, strategies that would be enthusiastically embraced among all providers are unclear. In general, physicians can easily receive comprehensive information about epilepsy surgery via free online resources, peer-reviewed journals, or textbooks, but widespread implementation might be restricted due to time constraints in a busy clinical practice. Practical, pragmatic, and concise consensus statements and guideline (with accompanying structured referral sheet, reflecting local circumstances and barriers) can be useful if they are widely disseminated through different outlets to penetrate all levels of the healthcare system. (37) National, regional, or statewide educational campaigns targeting providers can be employed to improve awareness about epilepsy surgery as a standard of care rather than an experimental option. Interested neurologists also can receive epilepsy surgery specific education by attending various international, national, regional, or local educational conferences, where they are exposed to views and opinions of expert leaders from various epilepsy surgery centers and from other providers who have successfully adopted practice to increase epilepsy surgery in their centers. Telementoring programs such as ECHO (Extension for Community Healthcare Outcomes) can be utilized to link primary care providers, neurologists, and epilepsy specialists in an interactive teleconference format to expand access to epilepsy education and training.(38–40)
1.3.3. Implicit(unconscious) bias training
No data as yet specifically measures the effects of implicit bias in epilepsy surgery referrals or surgery triage. However, since racial disparities has been demonstrated to restrict access to epilepsy surgery(Part I: Systemic inequities in access), it stands to reason that research and provider-facing strategies in this area are needed to insure that equity to access for care is present.(41, 42)(43)(44, 45)
2. Digital tools for education, decision support, and prognostic estimation
2.1. Online tool for referral facilitation
One barrier to epilepsy surgery is recognition of ideal candidates, particularly in primary and secondary care centers. Online decision-support tools may help physicians without specialized epilepsy training to identify individual patients for referral to a comprehensive epilepsy center. Roberts et al. reported use of an online tool, Canadian Appropriateness of Epilepsy Surgery (CASES) tool, to facilitate appropriate referral for epilepsy surgery.(46) This evidence-based, clinical decision tool uses several variables to determine referral appropriateness with relative ease and excellent concordance with the clinical judgment of epilepsy specialists.(47)
2.2. Nomogram and scoring algorithms
Besides referral facilitation, other statistical tools such as nomogram or scoring algorithms have been developed to estimate likelihood of seizure freedom after surgery, which can be particularly helpful to facilitate preoperative individualized patient counseling and provide better estimation of outcomes compared to individual epilepsy characteristics. Multiple patient characteristics contribute to individualized surgical outcome prediction as shown by multiple statistical tools [eg, modified seizure freedom score (m-SFS), The Epilepsy Surgery Grading Scale, and Epilepsy Surgery Nomogram (ESN)].(48–50) Prediction of seizure outcomes at 2 or 5 years after surgery using these m-SFS and ESN were non inferior to prediction by expert epileptologists.(51) Moreover, there was a moderate statistical difference favoring the m-SFS over clinicians. Beyond seizure-outcome, there are several multivariate models that help predict complications such as verbal memory and post-operative naming decline.(52) (53)
2.3. Tools and screen prompts embedded in EMR and machine learning techniques
Recently, there has been exponential growth in the use of EMR, which exceed paper records in ability to track patient information over time. Epilepsy toolkits can be designed within the EMR to facilitate structured clinical documentation and support clinical decision making by prompting for appropriate referral for epilepsy surgery.(54) Matykiewicz et al. demonstrated that information-theoretic and machine learning techniques (natural language processing) can be effectively used to process text data from clinical notes for earlier identification of candidates for epilepsy surgery.(55) There are several other preliminary studies that demonstrated that various machine learning techniques (using clinical, demographic, neuropsychological, or structural and functional connectivity data from invasive EEG) can predict individualized surgical outcomes in epilepsy.(56)
3. Interventions for Comprehensive Epilepsy Programs
After presurgical evaluation, strategies to prevent patient attrition prior to surgery are important. Use of an algorithmic and streamlined approach to preoperative workup and access to necessary diagnostic tools and expertise locally or within a network of other epilepsy centers may increase the likelihood of completed evaluations. For certain patient populations, the decision to offer epilepsy surgery may rely heavily on the experience of the referral center. Thus, recognizing neglected populations and connecting them to expertise within a referral network may improve access to surgical therapy.
3.1. Progressive expertise and collaborative network
The expertise to perform epilepsy surgery varies among centers, as does the complexity of patients. Newly established epilepsy centers may evaluate seemingly less complex cases (i.e. tumor-associated lesional epilepsy) in the beginning to gain experience. After gaining adequate expertise, epilepsy centers may continue with a minimum number of evaluations, including complex cases, consistently to maintain necessary skills.(57) The International League Against Epilepsy (ILAE) has recommended annual performance of 20–50 surgeries to maintain proficiency in epilepsy surgery.(58) However, the Austrian, German, and Swiss working group of presurgical diagnosis and treatment correctly pointed out that the volume requirement may need adjustment depending on the catchment area of the epilepsy surgery center.(59) Thus, maintaining skills necessary to most effectively carry out epilepsy surgery may require a network model. Formation of collaborative networks and partnership among epilepsy centers may allow access to rare expertise and expensive diagnostic tools, while providing opportunity for comparative effectiveness research across the collaborative. For example, the European Task Force on Childhood Epilepsy Surgery, established in 2004, provided a platform to discuss complex surgical cases two times per year among epilepsy surgery centers from at least nine different countries.(60) From that collaboration, the TimeToStop study (a retrospective cohort study) was completed that report early AED withdrawal after epilepsy surgery does not affect long-term seizure outcome in children.(61) The surgery subgroup of the Pediatric Epilepsy Research Consortium (PERC), a network of U.S. Pediatric Epilepsy Centers, developed a multicenter collaborative database collecting common data elements on every patient evaluated for epilepsy surgery, including epilepsy characteristics, components of evaluation, surgical procedures performed, and outcome. Besides standardizing common data elements and investigating the relative value of differing decision trees, approaches, surgical techniques, this collaboration may facilitate referral of surgical candidates, standardization of evaluation algorithms and treatment, and comparative effectiveness of different therapeutic pathways.
3.2. Referral strategy
Given the complexities of surgical decision making, the pursuit of candidacy is strongly influenced by the availability of collective expertise and experience regarding epilepsy surgery. There is reservation about offering epilepsy surgery in certain groups of patients-even in epilepsy centers-depending on the patient and epilepsy characteristics (extremes of ages, seizure onset zone approximating eloquent cortex, multifocal seizures, patients with intellectual disabilities or psychiatric comorbidities) due to perceptions of poor surgical outcome. Although some reluctance in undertaking epilepsy surgery evaluation might be valid (particularly in the less experienced centers), many accomplished centers can competently break these barriers in surgical decision making. There has been a demographic shift of the presenting patient population from the relatively straight-forward evaluation (i.e., mesial temporal lobe epilepsy) to more complex epilepsy substrates over time.(62–67) Appropriate referral should be considered in these cases following a standardized guideline. Besides existing referral guidelines (NAEC in the US, European Reference Network in Europe, and Children’s Epilepsy Surgery Service(CESS) in England, ILAE Pediatric Epilepsy Surgery Task Force recently published criteria for two levels of care in pediatric epilepsy surgery centers through a modified Delphi Process and can guide patient referral from Level 1 to Level 2 centers.(68) As suggested in this guideline, Level 1 centers may provide epilepsy surgery for discrete lesions including hippocampal sclerosis or lobectomy/lesionectomy for lesions not involving the eloquent cortex in children age 9 years and older. However, level 2 centers, with expanded standards for personnel and technology, can provide surgical treatment across the age span and in children with more diverse range of epilepsy etiologies, including with normal MRI or ill‐defined, multilobar, hemispheric, or multifocal MRI lesions.
3.3. Risk minimization practices for specific populations
Although there are severe negative developmental consequences to not offering surgical therapy in infants with catastrophic epilepsies, many young children are denied surgery due to lack of expertise and fear of complications.(69, 70) Although lack of local expertise can be addressed with formation of strategic referral partnerships, special emphasis should be given to the increased risk of surgery in this age group. These advanced referral centers should be equipped with suitable critical care expertise (careful attention to blood loss, meticulous operative technique, early transfusion of autologous blood products or cell saver blood, and postoperative close monitoring in an ICU setting for potential complications) to minimize complications. Improved safety outcomes may also encourage future referral for surgery early in the disease process.(71, 72) Similar attention is needed towards complication rate when surgery is offered for older adults with intractable epilepsy. Although general complication rates of epilepsy surgery are comparable to younger populations, special consideration should be given regarding cardiovascular risk factors, risk of subdural hematoma (may be secondary to age associated decreased cerebral volume), and risk of decline in confrontation naming.(73) Special operative strategies are necessary for surgery in close approximation to eloquent areas of the brain.(74, 75) The surgical planning may involve careful and detailed functional mapping of the critical areas. Other strategies are available for complex cases including awake surgery, tailored resection, and resection in combination with multiple subpial transections (MST) or responsive neurostimulation (RNS).(76) Functional mapping may be challenging in young patients, and centers with expertise in pediatric functional mapping as well as emerging technologies like high-gamma mapping, may serve as nodal centers for referring such challenging patients.
3.4. Strategies for multifocal or widespread epileptogenic zone
Multifocal seizures have been considered as a relative contraindication for epilepsy surgery, however, both curative and palliative surgical options are available for these patients. For example, a surgical technique consisting of a 3 stage, bilateral, invasive intracranial monitoring has been utilized in tuberous sclerosis complex (TSC)- a condition associated with multiple tubers and multiple seizure foci.(77) (78) Patients with catastrophic hemispheric epilepsy syndromes and frequent seizures can become seizure-free after anatomic or functional hemispherectomy. Centers with adequate expertise also can perform corpus callosotomy, a palliative surgical option, for patients with intractable drop seizures, especially in patients with Lennox-Gastaut syndrome (LGS).(79)
3.5. Choosing appropriate outcome
Patients with intellectual disabilities and epilepsy frequently have higher seizure burden but rarely undergo epilepsy surgery (2–15% of the total surgical patients).(80) Although the intelligence quotient( IQ) level was noted to be an independent predictor of seizure freedom in a population-based epilepsy surgery series from Sweden, up to 37% of patients in the 50–69 IQ range and 22% of patients in the <50 IQ were seizure free at 2 year follow up.(80) This underscores the importance of not excluding patients with intellectual disability from epilepsy surgery, particularly in the presence of an obvious epileptogenic lesion in the MRI. More importantly, improvement in IQ has been noted after epilepsy surgery which can be correlated with individual’s ability to live and socialize independently.
3.6. Early psychological interventions
Psychiatric comorbidities are particularly prevalent in patients with intractable epilepsy. Early involvement of psychiatrists, psychologists, and neuropsychologists is important for prompt management of psychologic issues, which can help patients decide about epilepsy surgery. Detailed assessment by mental health providers, early in the process, also allows better understanding of patient’s expectation from the epilepsy surgery.
3.7. Repeat Surgery-Generation of fresh hypothesis for cases with failed surgery
There are few strategies to improve utilization of repeat surgery in cases of previously failed surgery. A comprehensive reassessment of clinical features including seizure semiology with repeat neurophysiological study with or without repeat neuroimaging may be helpful to understand the cause of the surgical failure. Reasons for failure include incomplete localization of the epileptogenic network, incomplete resection, or activation of a new epileptogenic focus due to the surgical process itself or due to patient’s inherent tendency to develop new epileptogenic foci based on genetic expression. If a fresh hypothesis can be generated with a detailed assessment of the clinical data and further testing, a successful second operation with long-term seizure control can be achieved in carefully selected patients. Yardi et al. reported that Engel I outcome was achieved in 42 and 33% of patients after 1 or ≥2 failed epilepsy surgeries, respectively.(81)
3.8. Presurgical diagnostic workup
Presurgical diagnostic workup can be time-consuming for many patients and, depending on the etiologies, complex. Lack of standardized presurgical testing pathways and clearly stated time goals to complete these can be significant barriers to epilepsy surgery.
3.8.1. Brain MRI
Among all the diagnostic tools, a quality brain MRI is the most important, and a potential epileptogenic lesion visible in the MRI represents the core of the surgical strategy. Higher magnetic field strength (1.5 T vs. 3 T vs. 7T), availability of expert radiologists, dedicated epilepsy protocol sequences (eg, “essential 6” sequence, ILAE standardized best-practice neuroimaging sequences) and access to MRI post processing techniques can be helpful. (82) (83) (84) (85) (86) (87) Re-review of the initial negative MRI should be done after completion of other testing such as EEG, functional scans or MEG with particular focus on the suspected epileptogenic zone.
3.8.2. Automation, sophisticated image analysis, machine learning
Automated quantitative assessment of subclinical data may be valuable in ‘MRI-negative’ patients with underlying hippocampal sclerosis and focal cortical dysplasia. Volumetry, automated FLAIR signal analysis, and T2 relaxometry are particularly useful in the detection of subtle hippocampal atrophy; automation of the process can significantly curtail the time and labor constraints. Several sophisticated image analysis instruments (double-inversion recovery, arterial spin labelling, novel diffusion imaging methods using neurite orientation dispersion, density imaging, diffusion kurtosis imaging) can increase sensitivity for the detection of focal cortical dysplasia and compliment visual reading of the MRI. Wide dissemination of validated and readily available technologies can be utilized for detection of definitive epileptogenic lesions for successful surgery. Machine learning techniques also can help with detection of lesions in brain MRI with excellent accuracy. Hong et al showed that an automated classifier relying on surface-based features of morphology and intensity could detect focal cortical dysplasia (FCD) in patients with extratemporal MRI-negative epilepsy with 74% sensitivity and 100% specificity.(88) Ahmed et al. also showed that an automated quantitative morphometric approach correctly identified 14 out of 24 MRI negative FCD lesions.(89)
3.8.3. Strategies for successful long term video EEG monitoring
Long term video EEG monitoring to capture habitual seizure is the next most important step in the presurgical workup but may be skipped in certain situations, i.e. when simple seizure semiology perfectly matches with the detected MRI lesion or the interictal EEG abnormality is highly concordant with localization and lateralization information available from the clinical semiology and brain MRI. However, most centers prefer ictal recording to rule out psychogenic nonepileptic seizures. Hemispheric surgeries and palliative surgeries also may not require ictal localization. Increased utilization of epilepsy surgery might occur if this step could be skipped in appropriate patients due to high cost and difficulties with prolonged inpatient stays. Many patients need long term monitoring but are prematurely discharged prior to capturing habitual seizures which may adversely impact surgical candidacy. Some strategies can be useful to minimize duration of inpatient admission with higher likelihood of capturing essential EEG data: planned admission when the patient is having frequent seizures, aggressive but careful withdrawal of AEDs, and use of provocative factors such as sleep deprivation.
3.8.4. Use of tools for nonlesional cases or patients with extensive/multifocal abnormalities
Successful determination of the potential epileptogenic zone is an essential step of epilepsy surgery. Thus focal epilepsy with nonlesional or nonobvious MRI abnormality or extensive/multifocal abnormalities without clear delineation of the epileptogenic lesion may require functional imaging for localization of epileptogenic lesion. The benefit of these modalities is potential avoidance of invasive monitoring in highly selected cases by providing concordant data and raising confidence in localization. Jayakar et al reported a longitudinal cohort of 102 children with nonlesional epilepsy among which 22 underwent excisional surgery based on integrated data from clinical exam, video EEG, and metabolic scanning without extra operative invasive monitoring.(90) Although value and cost-effectiveness of these modalities have not been firmly established, optimal presurgical evaluation is based on judgement of physicians and culture of the institute.(91, 92) PET and SPECT are the most commonly used optional studies.(93, 94) Although not widely available, magnetoencephalography (MEG) may disclose epileptogenic lesions not detected by the MRI and increase the potential surgical pool.(95) There are other developing tools, such as EEG-fMRI and electrical source imaging using high density EEG that, individually or in combination, may map irritative zones accurately in a head model. Multimodal 3D imaging data integration with semi-automated computer-assisted planning software may also facilitate identification of seizure onset zone and early propagation zone with attractive visualization. There are emerging consensual practical guidelines regarding use of newer diagnostic tools for universal application.(96) However, further research is needed to find appropriate roles of these techniques in the presurgical algorithm.(97) Many of these are redundant with concordant MRI and ictal/interictal video EEG data, but can be particularly valuable to generate a hypothesis for intracranial EEG implantation in more complex nonlesional cases. Due to the availability of a plethora of diagnostic tools, it is becoming more prevalent to find modest concordance between tests to generate a reasonable hypothesis to be tested by invasive monitoring.(98)
3.8.5. Minimally invasive surgical approaches
One particular reason for underutilization of epilepsy surgery is great fear about open brain surgery, both during invasive evaluation and resection. Approximately a third of epilepsy surgery candidates need intracranial monitoring and popularization of stereo EEG can help accomplish widespread epilepsy network mapping with relative ease compared to traditional grid/strip methods. New minimally invasive surgical approaches have been specifically developed such as stereotactic radiosurgery and laser-thermoablation that can be valuable for a large subset of patients with anxiety about open craniotomy and surgery.(99) Laser interstitial thermal therapy (LITT) can be particularly useful for mesial temporal lobe epilepsy, small cortical malformations, cavernous malformation, deeper small lesions like periventricular heterotopia and hypothalamic hamartoma.(100). Gupta et al. reported a heterogeneous group of 35 patients who underwent LITT therapy with 44% enjoying Engel I outcome during >12 months follow up.(101)
4. Access, communication, and coordination barriers
4.1. Development of surgical epilepsy center
Access to epilepsy surgery is limited in resource poor settings where surgical options are available only in select government hospitals with long waiting periods or private corporate hospitals in large urban areas with exorbitant costs that most citizens cannot afford. Moreover, most centers in resource-limited settings lack adequate expertise and advanced diagnostic tools for the facility to perform intracranial monitoring. However, epilepsy surgery centers can be established even in resource-poor settings with gradual expansion of the patient base.(102) A recent study reported that 382 patients underwent resective surgery over a 5-year period in West China despite lack of SEEG, SPECT, and many patients prematurely discontinuing workup due to expense. Jukkarwala et al. reported the development of a low cost epilepsy surgery center in India with an initial investment of USD 15,000–20,000.(103) The combined cost of presurgical evaluation and surgery was USD 860–1650, which is 10% of the cost routinely incurred per surgery in developed countries. The authors utilized several strategies that can be adopted by other developing centers: 1. involve trained epileptologists for the initiation of the program 2. spread awareness about epilepsy surgery in the community 3. in-house training of EEG technologists and nurses 4. identify most suitable candidates (based on clinical history, EEG, and MRI) who would not require time consuming and lengthy presurgical evaluation 5. subsidize the expenses for poor patients 6. outsource selected cases requiring expert neuropsychiatrists or radiologists 7. referral of difficult cases to the more established centers.
4.2. Referral partnerships, satellite clinics, and telehealth
There must be coordinated effort to improve communication between epilepsy centers and referring providers, who should be involved in the decision-making process and updated regularly during the evaluation process. There also should be every effort to return patients to the referring provider after the surgery or completion of inconclusive workup. Complex surgical cases can be referred to highly specialized centers to remove surgical barriers. Establishment of a hub (full array of services) and spoke (limited services) two-tier model can be particularly useful in resource-limited settings. Vadera et al. discussed utilization of strategic hospital partnerships between a university program with two other epilepsy centers with limited surgical capabilities.(104) The authors reported significant increase in the number of epilepsy surgery cases (both simple and complex cases) after developing the partnership. Besides improving access and surgical volume, there was financial gain for both parties: increasing revenue from the presurgical workup and the increased surgical volume in the referring institutes and the surgical center, respectively. Moreover, referring physicians could remain active in decision making and the patient returned back to them postoperatively. The surgical partnership model does not need to be geographically restricted and can be broadly implemented to improve access to an experienced surgical team. Satellite clinics and telemedicine also can offer a solution for travel restriction in patients with epilepsy.(105) Telehealth also can be helpful in areas with health professional shortage or with limited access to comprehensive epilepsy programs that offer epilepsy surgery.(106)
4.3. Multidisciplinary setup, epilepsy care-coordinator/nurse navigator, patient care conference
Neurologists, epilepsy specialists, and neurosurgeons need to work together discussing options and risk/benefit of surgery with patients as a team in a multidisciplinary setting. Epilepsy surgery program coordinators or managers can help schedule and bundle presurgical studies, especially for patients travelling from a distance. Hill et al. showed significant increase (32–96% growth) in the epilepsy surgery evaluation within a short time frame after employment of an epilepsy surgery manager.(107) Composite process change efforts, such as increasing frequency of patient care conference, faster scheduling of diagnostic tests, dedicated epilepsy surgery clinic, and employment of a nurse navigator have been reported to increase surgical volume significantly (a study reported a 37% yearly increase of surgical volume with reducing average evaluation time by 96 days).(108) Additionally, using language concordant epilepsy-care coordinators can help patients with limited English proficiency navigate the convoluted journey to epilepsy surgery. However, easy access to medical interpreters throughout the care process would be more practical solutions for most programs.
5. Policies and programs
Public policies can support epilepsy research, increase access to epilepsy surgery, and mitigate systemic inequities in the access of surgery. Community advocacy groups, lay patient and professional organizations can advocate and promote policies at the state and federal levels to facilitate epilepsy surgery evaluation.(109)
5.1. Policies to increase access and optimize payment models
Improvement in the access to epilepsy surgery can be achieved through the proper use of government and health-care policies (e.g., anti-discrimination policy, sick leave policy). Improved public transportation at reduced costs for people undergoing epilepsy surgery evaluation can be particularly advantageous as most patients with intractable epilepsy would not be allowed to drive due to seizure-free restriction. Advocacy groups also can propose legislative changes to the congress regarding various issues, such as current payment model. Neurologists and epileptologists spend an enormous amount of time in the care of complex epilepsy patients for counselling, evaluating diagnostic tests, and presenting in the multispecialty case conference. Current fee-for service models may not capture all these efforts, and a patient-centered epilepsy care model that stratifies payment amount based on complex care may be more suitable. Similarly, pay-per-performance type models (for example, adherence to AAN guideline regarding epilepsy surgery referral) can also be promoted in appropriate settings.
5.2. Health care coverage and workforce diversity
Epilepsy surgery is not equally distributed among races and socioeconomic status and improvement in health care coverage with insurance may reduce disparities in accessing surgical treatment. For example, socioeconomic disparities with underrepresentation of poor in the surgical cohort may be less prevalent in countries with universal health care system as noted in one recent study from Ontario, Canada.(110) Large scale favorable change in the government policies (including insurance approval for presurgical workup) can be beneficial. A favorable change was demonstrated in a recent National Inpatient Sample analysis with no racial discrepancies to access surgical care after the introduction of the Affordable Care act.(111) Additionally, disparities related to racial and cultural factors can be minimized with appropriate policies and programs to increase in bilingual physicians and expansion of racial and ethnic diversity among physicians.
5.3. Increased funding for epilepsy research
Policy change with preferential governmental funding in research related to epilepsy surgery is urgently needed. Although epilepsy is the fourth most common neurological disorder with immense public health impact, research related to epilepsy remains underfunded compared to other major neurological disorders such as Alzheimer disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, Parkinson disease, and stroke. Meador et al reported that relative NIH funding is 1.7 times greater for stroke and staggering 61.1 times greater in ALS research than epilepsy.(112) This disparity in research funding can’t be explained by the overall impact of these diseases on patients. This disparity in governmental funding is more profound in research related to epilepsy surgery, which doesn’t have pharmaceutical or medical-device industry backup. Given extreme difficulty in the recruitment process for surgical clinical trials, other research methods (comparative effectiveness trials, observational research, big data sources from multi-institutional registries) should be employed to answer many unknown questions regarding use of epilepsy surgery. Particular emphasis may be given to the following areas: structured instruments to assess patients’ perceptions regarding epilepsy surgery and appropriate behavioral intervention methods, optimum diagnostic algorithm for presurgical workup, long-term postsurgical outcome data for extratemporal epilepsies, and chronic outcome and safety data after minimally invasive surgeries.
Conclusion
In this review, we comprehensively discuss various interventional strategies that may help address challenges encountered during pre-surgical evaluation to the completion of epilepsy surgery. Solving the underutilization of epilepsy surgery involves educational interventions for patients/families, community, and referring providers, use of technological advancements, and creation of a standardized system in the comprehensive epilepsy center. Moreover, other potential solutions will involve increasing healthcare workforce diversity, reducing barriers based on racial and socioeconomic disparity (insurance options), developing more epilepsy centers wherever there is need, increasing collaboration between epilepsy centers with different technological and human (skills and expertise) resources, and increasing research funding for epilepsy. However, there should be a robust system in place to measure relevant outcomes (cost effectiveness, impact on Disability-Adjusted Life Year) in real time to determine if interventions or initiatives are effective and permit adaptability based on constructive feedback. This type of feedback-driven modification may allow critical reflection of the improvement process and further refinement and optimization of the program to ensure all potential surgical candidates receive high-quality care.
Highlights.
Multifaceted interventions are necessary to tackle underutilization of epilepsy surgery
Educational interventions can be useful for both patients and providers
Targeted coordination and communication strategies promote efficient evaluation
Innovative technology and supportive Policies improve access to epilepsy surgery
Specific strategies for epilepsy centers ensure appropriate surgical evaluation
Acknowledgement:
The authors wish to acknowledge the member institutions of the PERC Epilepsy Surgery group for their continued support to increase the utilization and understanding of pediatric epilepsy surgery
DS receives research support from TRI, UAMS through the CTSA of the NIH (UL1 TR003107). RA receives research support from NIH NINDS R01 NS115929 and Procter Foundation (Procter Scholar Award 2018–2021).
Funding:
Debopam Samanta is supported by the Translational Research Institute (TRI), grant UL1 TR003107 through the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The other authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
References
- 1.Engel J Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: A randomized trial. JAMA. 2012;307(9):922–30. Available from: https://pubmed.ncbi.nlm.nih.gov/22396514https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4821633/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311–8. Available from: 10.1056/nejm200108023450501. [DOI] [PubMed] [Google Scholar]
- 3.Dwivedi R, Ramanujam B, Chandra PS, Sapra S, Gulati S, Kalaivani M, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377(17):1639–47. Available from: 10.1056/nejmoa1615335. [DOI] [PubMed] [Google Scholar]
- 4.Cross JH, Jayakar P, Nordli D, Delalande O, Duchowny M, Wieser HG, et al. Proposed criteria for referral and evaluation of children for epilepsy surgery: Recommendations of the subcommission for pediatric epilepsy surgery. Epilepsia. 2006;47(6):952–9. Available from: 10.1111/j.1528-1167.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- 5.Engel J, Wiebe S, French J, Sperling M, Williamson P, Spencer D, et al. Practice parameter: Temporal lobe and localized neocortical resections for epilepsy. Neurology. 2003;60(4):538–47. Available from: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 6.Catenoix H, Feutrier C, Taffin F, Peverelli R, Rodot M, Robinson P, et al. Epilepsy surgery: A therapeutic patient education program. Rev Neurol. 2018;174(10):726–30. [DOI] [PubMed] [Google Scholar]
- 7.Hrazdil C, Roberts JI, Wiebe S, Sauro K, Vautour M, Hanson A, et al. Patient perceptions and barriers to epilepsy surgery: Evaluation in a large health region. Epilepsy & Behavior. 2013;28(1):52–65. [DOI] [PubMed] [Google Scholar]
- 8.Ladino LD, Benjumea-Cuartas V, Diaz-Marin DM, Lopez-Gonzalez R, Orozco-Hernandez JP, Bedoya-Rodriguez P, et al. Patients’ perceptions of and attitudes towards epilepsy surgery: Mistaken concepts in colombia. Rev Neurol. 2018;67(1):6–14. [PubMed] [Google Scholar]
- 9.Swarztrauber K, Dewar S, Engel J Jr. Patient attitudes about treatments for intractable epilepsy. Epilepsy & Behavior. 2003;4(1):19–25. [DOI] [PubMed] [Google Scholar]
- 10.Prus N, Grant AC. Patient beliefs about epilepsy and brain surgery in a multicultural urban population. Epilepsy & Behavior. 2010;17(1):46–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson CT, Noble E, Mani R, Lawler K, Pollard JR. Epilepsy surgery: Factors that affect patient decision-making in choosing or deferring a procedure. Epilepsy research and treatment. 2013;2013. [DOI] [PMC free article] [PubMed]
- 12.Henning O, Alfstad KA, Nakken KO, Lossius MI. A call for better information about epilepsy: The patients’ perspective—An online survey. Seizure. 2019;69:173–9. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann LK, Welter E, Berg AT, Perzynski AT, Van Doren JR, Sajatovic M. Epilepsy misconceptions and stigma reduction: Current status in western countries. Epilepsy & Behavior. 2016;60:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman AJ, Cosby R, Boyko S, Hatton-Bauer J, Turnbull G. Effective teaching strategies and methods of delivery for patient education: A systematic review and practice guideline recommendations. Journal of Cancer Education. 2011;26(1):12–21. [DOI] [PubMed] [Google Scholar]
- 15.Theis SL, Johnson JH. Strategies for teaching patients: A meta-analysis. Clinical Nurse Specialist CNS. 1995;9(2). [DOI] [PubMed] [Google Scholar]
- 16.Choi H, Pargeon K, Bausell R, Wong JB, Mendiratta A, Bakken S. Temporal lobe epilepsy surgery: What do patients want to know? Epilepsy & Behavior. 2011;22(3):479–82. [DOI] [PubMed] [Google Scholar]
- 17.Andrewes D, Camp K, Kilpatrick C, Cook M. The assessment and treatment of concerns and anxiety in patients undergoing presurgical monitoring for epilepsy. Epilepsia. 1999;40(11):1535–42. [DOI] [PubMed] [Google Scholar]
- 18.Erba G, Messina P, Pupillo E, Beghi E, Group TOP. Acceptance of epilepsy surgery in the pediatric age—what the parents think and what the doctors can do. Epilepsy & Behavior. 2013;29(1):112–20. [DOI] [PubMed] [Google Scholar]
- 19.House PM, Pelzl S, Furrer S, Lanz M, Simova O, Voges B, et al. Use of the mixed reality tool “VSI patient education” for more comprehensible and imaginable patient educations before epilepsy surgery and stereotactic implantation of DBS or stereo-EEG electrodes. Epilepsy Res. 2020;159:106247. [DOI] [PubMed] [Google Scholar]
- 20.Yankova Z Patients’ knowledge of patient controlled analgesia (PCA) and their experience of postoperative pain relief: A review of the impact of structured preoperative education. Journal of Advanced Perioperative Care. 2008;3(3). [Google Scholar]
- 21.McCallum GB, Morris PS, Brown N, Chang AB. Culture‐specific programs for children and adults from minority groups who have asthma. Cochrane Database of Systematic Reviews. 2017(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khunti K, Camosso‐Stefinovic J, Carey M, Davies MJ, Stone MA. Educational interventions for migrant south asians with type 2 diabetes: A systematic review. Diabetic Med. 2008;25(8):985–92. [DOI] [PubMed] [Google Scholar]
- 23.Yankova Z Patients’ knowledge of patient controlled analgesia (PCA) and their experience of postoperative pain relief: A review of the impact of structured preoperative education. Journal of Advanced Perioperative Care. 2008;3(3). [Google Scholar]
- 24.Steinbrenner M, Kowski AB, Holtkamp M. Referral to evaluation for epilepsy surgery: Reluctance by epileptologists and patients. Epilepsia. 2019;60(2):211–9. Available from: 10.1111/epi.14641. [DOI] [PubMed] [Google Scholar]
- 25.Erba G, Messina P, Pupillo E, Beghi E, OPTEFF Group. Acceptance of epilepsy surgery among adults with epilepsy—what do patients think? Epilepsy & Behavior. 2012;24(3):352–8. [DOI] [PubMed] [Google Scholar]
- 26.Lewis SA, Noyes J, Mackereth S. Knowledge and information needs of young people with epilepsy and their parents: Mixed-method systematic review. BMC pediatrics. 2010;10(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toerien M, Shaw R, Reuber M. Initiating decision‐making in neurology consultations:’recommending’versus ‘option‐listing’and the implications for medical authority. Sociol Health Illn. 2013;35(6):873–90. [DOI] [PubMed] [Google Scholar]
- 28.Dewar SR, Pieters HC. Perceptions of epilepsy surgery: A systematic review and an explanatory model of decision-making. Epilepsy & Behavior. 2015;44:171–8. [DOI] [PubMed] [Google Scholar]
- 29.Chaytor N, Ciechanowski P, Miller JW, Fraser R, Russo J, Unutzer J, et al. Long-term outcomes from the PEARLS randomized trial for the treatment of depression in patients with epilepsy. Epilepsy & Behavior. 2011;20(3):545–9. [DOI] [PubMed] [Google Scholar]
- 30.Owens S, Sirven JI, Shafer PO, Fishman J, Wild I, Findley M, et al. Innovative approaches reaching underserved and rural communities to improve epilepsy care: A review of the methodology of the connectors project. Epilepsy & Behavior. 2019;90:273–83. [DOI] [PubMed] [Google Scholar]
- 31.Mogal Z, Aziz H. Epilepsy treatment gap and stigma reduction in pakistan: A tested public awareness model. Epilepsy & Behavior. 2020;102:106637. [DOI] [PubMed] [Google Scholar]
- 32.Kobau R, Price P. Knowledge of epilepsy and familiarity with this disorder in the US population: Results from the 2002 HealthStyles survey. Epilepsia. 2003;44(11):1449–54. [DOI] [PubMed] [Google Scholar]
- 33.Price P, Kobau R, Buelow J, Austin J, Lowenberg K. Improving understanding, promoting social inclusion, and fostering empowerment related to epilepsy: Epilepsy foundation public awareness campaigns—2001 through 2013. Epilepsy & Behavior. 2015;44:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strawbridge LM, Schultz AM, Liverman CT, England MJ. Epilepsy across the spectrum: Promoting health and understanding. National Academies Press; 2012. [PubMed] [Google Scholar]
- 35.Hakimi AS, Spanaki MV, Schuh LA, Smith BJ, Schultz L. A survey of neurologists’ views on epilepsy surgery and medically refractory epilepsy. Epilepsy & Behavior. 2008;13(1):96–101. Available from: 10.1016/j.yebeh.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Kumlien E, Mattsson P. Attitudes towards epilepsy surgery: A nationwide survey among swedish neurologists. Seizure. 2010;19(4):253–5. [DOI] [PubMed] [Google Scholar]
- 37.Fountain NB, Van Ness PC, Swain-Eng R, Tonn S, Bever CT. Quality improvement in neurology: AAN epilepsy quality measures: Report of the quality measurement and reporting subcommittee of the american academy of neurology. Neurology. 2011;76(1):94–9. [DOI] [PubMed] [Google Scholar]
- 38.Project ECHO for epilepsy and neurology epilepsy foundation [homepage on the Internet]. [cited 10/1/2020].
- 39.Providing epilepsy care to patients in need project ECHO neurology advisor [homepage on the Internet]. [cited 10/1/2020]. Available from: https://www.neurologyadvisor.com/topics/epilepsy/providing-epilepsy-care-to-patients-in-need-project-echo/.
- 40.Zhou C, Crawford A, Serhal E, Kurdyak P, Sockalingam S. The impact of project ECHO on participant and patient outcomes: A systematic review. Academic Medicine. 2016;91(10):1439–61. [DOI] [PubMed] [Google Scholar]
- 41.Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: How doctors may unwittingly perpetuate health care disparities. Journal of general internal medicine. 2013;28(11):1504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burneo JG, Black L, Knowlton RC, Faught E, Morawetz R, Kuzniecky RI. Racial disparities in the use of surgical treatment for intractable temporal lobe epilepsy. Neurology. 2005;64(1):50–4. Available from: 10.1212/01.wnl.0000150829.89586.25. [DOI] [PubMed] [Google Scholar]
- 43.Nathan CL, Gutierrez C. FACETS of health disparities in epilepsy surgery and gaps that need to be addressed. Neurology: Clinical Practice. 2018;8(4):340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teal CR, Gill AC, Green AR, Crandall S. Helping medical learners recognise and manage unconscious bias toward certain patient groups. Med Educ. 2012;46(1):80–8. [DOI] [PubMed] [Google Scholar]
- 45.Marcelin JR, Siraj DS, Victor R, Kotadia S, Maldonado YA. The impact of unconscious bias in healthcare: How to recognize and mitigate it. J Infect Dis. 2019;220(Supplement_2):S62–73. [DOI] [PubMed] [Google Scholar]
- 46.Roberts JI, Hrazdil C, Wiebe S, Sauro K, Hanson A, Federico P, et al. Feasibility of using an online tool to assess appropriateness for an epilepsy surgery evaluation. Neurology. 2014. September 2;83(10):913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lukmanji S, Altura KC, Rydenhag B, Malmgren K, Wiebe S, Jetté N. Accuracy of an online tool to assess appropriateness for an epilepsy surgery evaluation–A population-based swedish study. Epilepsy Res. 2018;145:140–4. [DOI] [PubMed] [Google Scholar]
- 48.Jehi L, Yardi R, Chagin K, Tassi L, Russo GL, Worrell G, et al. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: A retrospective analysis. Lancet Neurol. 2015. March;14(3):283–90. [DOI] [PubMed] [Google Scholar]
- 49.Garcia Gracia C, Yardi R, Kattan MW, Nair D, Gupta A, Najm I, et al. Seizure freedom score: A new simple method to predict success of epilepsy surgery. Epilepsia. 2015;56(3):359–65. [DOI] [PubMed] [Google Scholar]
- 50.Dugan P, Carlson C, Jetté N, Wiebe S, Bunch M, Kuzniecky R, et al. Derivation and initial validation of a surgical grading scale for the preliminary evaluation of adult patients with drug‐resistant focal epilepsy. Epilepsia. 2017;58(5):792–800. [DOI] [PubMed] [Google Scholar]
- 51.Gracia CG, Chagin K, Kattan MW, Ji X, Kattan MG, Crotty L, et al. Predicting seizure freedom after epilepsy surgery, a challenge in clinical practice. Epilepsy & Behavior. 2019;95:124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doherty C, Nowacki AS, Pat McAndrews M, McDonald CR, Reyes A, Kim MS, et al. Predicting mood decline following temporal lobe epilepsy surgery in adults. Epilepsia. 2021. [DOI] [PMC free article] [PubMed]
- 53.Busch RM, Hogue O, Kattan MW, Hamberger M, Drane DL, Hermann B, et al. Nomograms to predict naming decline after temporal lobe surgery in adults with epilepsy. Neurology. 2018. December 4;91(23):e2144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narayanan J, Dobrin S, Choi J, Rubin S, Pham A, Patel V, et al. Structured clinical documentation in the electronic medical record to improve quality and to support practice‐based research in epilepsy. Epilepsia. 2017;58(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matykiewicz P, Cohen KB, Holland KD, Glauser TA, Standridge SM, Verspoor KM, et al. In: Earlier identification of epilepsy surgery candidates using natural language processing. Proceedings of the 2013 workshop on biomedical natural language processing; ; 2013. p. 1–9. [Google Scholar]
- 56.Abbasi B, Goldenholz DM. Machine learning applications in epilepsy. Epilepsia. 2019;60(10):2037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arzimanoglou A, Cross JH, Gaillard WD, et al. Pediatric epilepsy surgery. 1st ed. Montrouge, France: John Libbey Eurotext,; 2016. [Google Scholar]
- 58.Binnie CD, Polkey CE, International League Against Epilepsy. Commission on neurosurgery of the international league against epilepsy (ILAE) 1993–1997: Recommended standards. Epilepsia. 2000. October;41(10):1346–9. [DOI] [PubMed] [Google Scholar]
- 59.Rosenow F, Bast T, Czech T, Feucht M, Hans VH, Helmstaedter C, et al. Revised version of quality guidelines for presurgical epilepsy evaluation and surgical epilepsy therapy issued by the austrian, german, and swiss working group on presurgical epilepsy diagnosis and operative epilepsy treatment. Epilepsia. 2016;57(8):1215–20. [DOI] [PubMed] [Google Scholar]
- 60.Boshuisen K, Arzimanoglou A, Cross JH, Uiterwaal CS, Polster T, van Nieuwenhuizen O, et al. Timing of antiepileptic drug withdrawal and long-term seizure outcome after paediatric epilepsy surgery (TimeToStop): A retrospective observational study. The Lancet Neurology. 2012;11(9):784–91. [DOI] [PubMed] [Google Scholar]
- 61.Lamberink HJ, Boshuisen K, Otte WM, Geleijns K, Braun KP, TimeToStop Study Group, et al. Individualized prediction of seizure relapse and outcomes following antiepileptic drug withdrawal after pediatric epilepsy surgery. Epilepsia. 2018;59(3):e28–33. [DOI] [PubMed] [Google Scholar]
- 62.Jehi L, Friedman D, Carlson C, Cascino G, Dewar S, Elger C, et al. The evolution of epilepsy surgery between 1991 and 2011 in nine major epilepsy centers across the united states, germany, and australia. Epilepsia. 2015;56(10):1526–33. Available from: https://pubmed.ncbi.nlm.nih.gov/26250432https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5082694/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF. Epilepsy surgery trends in the united states, 1990–2008. Neurology. 2012;78(16):1200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barba C, Specchio N, Guerrini R, Tassi L, De Masi S, Cardinale F, et al. Increasing volume and complexity of pediatric epilepsy surgery with stable seizure outcome between 2008 and 2014: A nationwide multicenter study. Epilepsy & Behavior. 2017;75:151–7. [DOI] [PubMed] [Google Scholar]
- 65.Barba C, Cross JH, Braun K, Cossu M, Klotz KA, De Masi S, et al. Trends in pediatric epilepsy surgery in europe between 2008 and 2015: Country‐, center‐, and age‐specific variation. Epilepsia. 2020;61(2):216–27. [DOI] [PubMed] [Google Scholar]
- 66.Cloppenborg T, May TW, Blümcke I, Grewe P, Hopf LJ, Kalbhenn T, et al. Trends in epilepsy surgery: Stable surgical numbers despite increasing presurgical volumes. Journal of Neurology, Neurosurgery & Psychiatry. 2016;87(12):1322–9. Available from: 10.1136/jnnp-2016-313831. [DOI] [PubMed] [Google Scholar]
- 67.Schiltz NK, Koroukian SM, Lhatoo SD, Kaiboriboon K. Temporal trends in pre-surgical evaluations and epilepsy surgery in the U.S. from 1998 to 2009. Epilepsy Res. 2013;103(2–3):270–8. Available from: https://pubmed.ncbi.nlm.nih.gov/22858308https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3496828/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gaillard WD, Jette N, Arnold ST, Arzimanoglou A, Braun KP, Cukiert A, et al. Establishing criteria for pediatric epilepsy surgery center levels of care: Report from the ILAE pediatric epilepsy surgery task force. Epilepsia. 2020. [DOI] [PubMed]
- 69.Gröppel G, Dorfer C, Dressler A, Mühlebner A, Porsche B, Czech T, et al. Epilepsy surgery in infants. Wien Klin Wochenschr. 2018;130(9–10):341–8. [DOI] [PubMed] [Google Scholar]
- 70.Loddenkemper T, Holland KD, Stanford LD, Kotagal P, Bingaman W, Wyllie E. Developmental outcome after epilepsy surgery in infancy. Pediatrics. 2007;119(5):930–5. [DOI] [PubMed] [Google Scholar]
- 71.Wyllie E, Comair YG, Kotagal P, Raja S, Ruggieri P. Epilepsy surgery in infants. Epilepsia. 1996;37(7):625–37. [DOI] [PubMed] [Google Scholar]
- 72.Gowda S, Salazar F, Bingaman WE, Kotagal P, Lachhwani DL, Gupta A, et al. Surgery for catastrophic epilepsy in infants 6 months of age and younger. Journal of Neurosurgery: Pediatrics. 2010;5(6):603–7. [DOI] [PubMed] [Google Scholar]
- 73.Punia V, Abdelkader A, Busch RM, Gonzalez‐Martinez J, Bingaman W, Najm I, et al. Time to push the age limit: Epilepsy surgery in patients 60 years or older. Epilepsia open. 2018;3(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rolston JD, Englot DJ, Knowlton RC, Chang EF. Rate and complications of adult epilepsy surgery in north america: Analysis of multiple databases. Epilepsy Res. 2016;124:55–62. Available from: https://pubmed.ncbi.nlm.nih.gov/27259069https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5260847/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rolston J Surgical strategies for epilepsy in eloquent areas. J Epilepsy. 2016;2(103):2472,0895.1000103. [Google Scholar]
- 76.Benifla M, Otsubo H, Ochi A, Snead OC, Rutka JT. Multiple subpial transections in pediatric epilepsy: Indications and outcomes. Child’s Nervous System. 2006;22(8):992–8. [DOI] [PubMed] [Google Scholar]
- 77.Weiner HL, Carlson C, Ridgway EB, Zaroff CM, Miles D, LaJoie J, et al. Epilepsy surgery in young children with tuberous sclerosis: Results of a novel approach. Pediatrics. 2006;117(5):1494–502. [DOI] [PubMed] [Google Scholar]
- 78.Arya R, Tenney JR, Horn PS, Greiner HM, Holland KD, Leach JL, et al. Long-term outcomes of resective epilepsy surgery after invasive presurgical evaluation in children with tuberous sclerosis complex and bilateral multiple lesions. Journal of Neurosurgery: Pediatrics. 2015;15(1):26–33. [DOI] [PubMed] [Google Scholar]
- 79.Samanta D Management of lennox-gastaut syndrome beyond childhood: A comprehensive review. Epilepsy & Behavior. 2020:107612. [DOI] [PubMed]
- 80.Malmgren K, Olsson I, Engman E, Flink R, Rydenhag B. Seizure outcome after resective epilepsy surgery in patients with low IQ. Brain. 2008;131(2):535–42. [DOI] [PubMed] [Google Scholar]
- 81.Yardi R, Morita‐Sherman ME, Fitzgerald Z, Punia V, Bena J, Morrison S, et al. Long‐term outcomes of reoperations in epilepsy surgery. Epilepsia. 2020;61(3):465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wellmer J, Quesada CM, Rothe L, Elger CE, Bien CG, Urbach H. Proposal for a magnetic resonance imaging protocol for the detection of epileptogenic lesions at early outpatient stages. Epilepsia. 2013;54(11):1977–87. [DOI] [PubMed] [Google Scholar]
- 83.Bernasconi A, Cendes F, Theodore WH, Gill RS, Koepp MJ, Hogan RE, et al. Recommendations for the use of structural magnetic resonance imaging in the care of patients with epilepsy: A consensus report from the international league against epilepsy neuroimaging task force. Epilepsia. 2019;60(6):1054–68. [DOI] [PubMed] [Google Scholar]
- 84.Phal PM, Usmanov A, Nesbit GM, Anderson JC, Spencer D, Wang P, et al. Qualitative comparison of 3-T and 1.5-T MRI in the evaluation of epilepsy. Am J Roentgenol. 2008;191(3):890–5. [DOI] [PubMed] [Google Scholar]
- 85.Knake S, Triantafyllou C, Wald LL, Wiggins G, Kirk GP, Larsson PG, et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: A prospective study. Neurology. 2005;65(7):1026–31. [DOI] [PubMed] [Google Scholar]
- 86.Wang I, Oh S, Blümcke I, Coras R, Krishnan B, Kim S, et al. Value of 7T MRI and post‐processing in patients with nonlesional 3T MRI undergoing epilepsy presurgical evaluation. Epilepsia. [DOI] [PMC free article] [PubMed]
- 87.Von Oertzen J, Urbach H, Jungbluth S, Kurthen M, Reuber M, Fernandez G, et al. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. Journal of Neurology, Neurosurgery & Psychiatry. 2002;73(6):643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hong S, Kim H, Schrader D, Bernasconi N, Bernhardt BC, Bernasconi A. Automated detection of cortical dysplasia type II in MRI-negative epilepsy. Neurology. 2014;83(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmed B, Brodley CE, Blackmon KE, Kuzniecky R, Barash G, Carlson C, et al. Cortical feature analysis and machine learning improves detection of “MRI-negative” focal cortical dysplasia. Epilepsy & Behavior. 2015;48:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jayakar P, Dunoyer C, Dean P, Ragheb J, Resnick T, Morrison G, et al. Epilepsy surgery in patients with normal or nonfocal MRI scans: Integrative strategies offer long‐term seizure relief. Epilepsia. 2008;49(5):758–64. [DOI] [PubMed] [Google Scholar]
- 91.Mouthaan BE, Rados M, Barsi P, Boon P, Carmichael DW, Carrette E, et al. Current use of imaging and electromagnetic source localization procedures in epilepsy surgery centers across europe. Epilepsia. 2016;57(5):770–6. [DOI] [PubMed] [Google Scholar]
- 92.Mouthaan BE, Rados M, Boon P, Carrette E, Diehl B, Jung J, et al. Diagnostic accuracy of interictal source imaging in presurgical epilepsy evaluation: A systematic review from the E-PILEPSY consortium. Clinical Neurophysiology. 2019;130(5):845–55. [DOI] [PubMed] [Google Scholar]
- 93.Knowlton RC. The role of FDG-PET, ictal SPECT, and MEG in the epilepsy surgery evaluation. Epilepsy & Behavior. 2006;8(1):91–101. Available from: 10.1016/j.yebeh.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 94.Rathore C, Kesavadas C, Ajith J, Sasikala A, Sarma SP, Radhakrishnan K. Cost-effective utilization of single photon emission computed tomography (SPECT) in decision making for epilepsy surgery. Seizure. 2011;20(2):107–14. Available from: 10.1016/j.seizure.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 95.Mohamed IS, Bouthillier A, Bérubé A, Cossette P, Finet P, Saint-Hilaire J, et al. The clinical impact of integration of magnetoencephalography in the presurgical workup for refractory nonlesional epilepsy. Epilepsy & Behavior. 2018;79:34–41. Available from: 10.1016/j.yebeh.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 96.Jayakar P, Gaillard WD, Tripathi M, Libenson MH, Mathern GW, Cross JH. Diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia. 2014;55(4):507–18. Available from: 10.1111/epi.12544. [DOI] [PubMed] [Google Scholar]
- 97.Hotan GC, Struck AF, Bianchi MT, Eskandar EN, Cole AJ, Westover MB. Decision analysis of intracranial monitoring in non-lesional epilepsy. Seizure. 2016;40:59–70. Available from: https://pubmed.ncbi.nlm.nih.gov/27348062https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967015/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jehi L, Friedman D, Carlson C, Cascino G, Dewar S, Elger C, et al. The evolution of epilepsy surgery between 1991 and 2011 in nine major epilepsy centers across the united states, germany, and australia. Epilepsia. 2015;56(10):1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schiltz NK, Vaca GF. Epidemiologist’s view: Addressing the epilepsy surgery treatment gap with minimally-invasive techniques. Epilepsy Res. 2018;142:179–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Remick M, McDowell MM, Gupta K, Felker J, Abel TJ. Emerging indications for stereotactic laser interstitial thermal therapy in pediatric neurosurgery. International Journal of Hyperthermia. 2020;37(2):84–93. [DOI] [PubMed] [Google Scholar]
- 101.Gupta K, Cabaniss B, Kheder A, Gedela S, Koch P, Hewitt KC, et al. Stereotactic MRI‐guided laser interstitial thermal therapy for extratemporal lobe epilepsy. Epilepsia. 2020;61(8):1723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li W, Hao N, Liu W, An D, Yan B, Li J, et al. The experience of the multidisciplinary team in epilepsy management from a resource‐limited country. Epilepsia Open. 2019;4(1):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jukkarwala A, Baheti NN, Dhakoji A, Salgotra B, Menon G, Gupta A, et al. Establishment of low cost epilepsy surgery centers in resource poor setting. Seizure. 2019;69:245–50. [DOI] [PubMed] [Google Scholar]
- 104.Vadera S, Chan AY, Mnatsankanyan L, Sazgar M, Sen-Gupta I, Lin J, et al. Strategic hospital partnerships: Improved access to care and increased epilepsy surgical volume. Neurosurgical Focus. 2018;44(5):E9. Available from: 10.3171/2018.1.focus17683. [DOI] [PubMed] [Google Scholar]
- 105.Kissani N, Lengané YTM, Patterson V, Mesraoua B, Dawn E, Ozkara C, et al. Telemedicine in epilepsy: How can we improve care, teaching, and awareness? Epilepsy & Behavior. 2020;103:106854. [DOI] [PubMed] [Google Scholar]
- 106.Mahabadi SM, Fehr C, Wu A, Hernandez-Ronquillo L, Rizvi SA, Tellez-Zenteno JF. Evaluation of wait times for assessment and surgical intervention according the geographic area of residence in the province of saskatchewan, canada. Seizure. 2020. [DOI] [PubMed]
- 107.Hill CE, Raab J, Roberts D, Lucas T, Pollard J, Kheder A, et al. Addressing barriers to surgical evaluation for patients with epilepsy. Epilepsy & Behavior. 2018;86:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Drees C, Sillau S, Brown M, Abosch A. Preoperative evaluation for epilepsy surgery: Process improvement. Neurology.Clinical practice. 2017;7(3):205–13. Available from: https://pubmed.ncbi.nlm.nih.gov/30107011https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6081968/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mifsud J, Brodie MJ. Lay epilepsy organizations: The key for research funding? Epilepsy & Behavior. 2018;81:123–4. Available from: 10.1016/j.yebeh.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 110.Burneo JG, Shariff SZ, Liu K, Leonard S, Saposnik G, Garg AX. Disparities in surgery among patients with intractable epilepsy in a universal health system. Neurology. 2016;86(1):72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sharma K, Kalakoti P, Henry M, Mishra V, Riel-Romero RM, Notarianni C, et al. Revisiting racial disparities in access to surgical management of drug-resistant temporal lobe epilepsy post implementation of affordable care act. Clin Neurol Neurosurg. 2017;158:82–9. [DOI] [PubMed] [Google Scholar]
- 112.Meador KJ, French J, Loring DW, Pennell PB. Disparities in NIH funding for epilepsy research. Neurology. 2011;77(13):1305–7. Available from: https://pubmed.ncbi.nlm.nih.gov/21947534https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3265048/. [DOI] [PMC free article] [PubMed] [Google Scholar]


