Abstract
Acutely, pain is protective. It promotes escape from, and future avoidance of, noxious stimuli through strong and often lifetime associative memories. However, with persistent acute pain or when pain becomes chronic, these memories can promote negative emotions and poor decisions often associated with deleterious behaviors. In this review, we discuss how preclinical studies can provide insights into the relationship between cognition and chronic pain. We also discuss the concept of pain as a cognitive disorder and new strategies for treating chronic pain that emphasize inhibiting the formation of pain memories or promoting “forgetting” of established pain memories.
Keywords: chronic pain, pain theories, memory, extinction
The Importance of Cognition in the Pain Experience
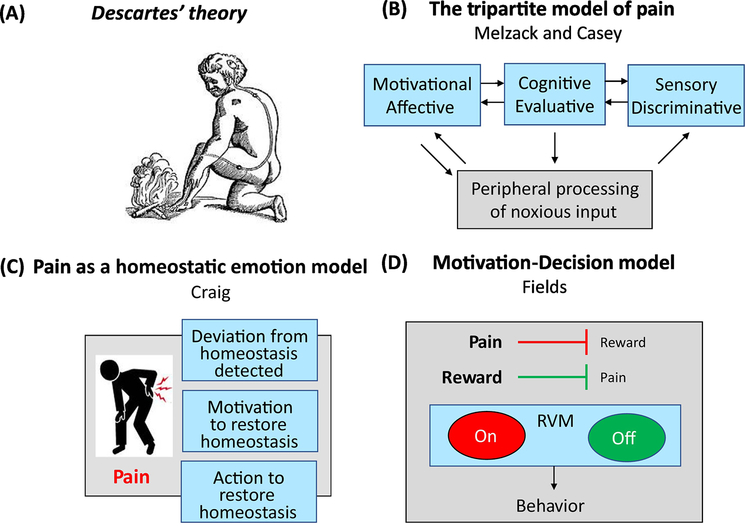
More than 50 years ago, Melzack and Casey proposed a tripartite model of pain composed of sensory/discriminative, affective/motivational and cognitive/evaluative dimensions [1] (see Figure 1 for progression of theories of pain). The power of cognitive therapy has been repeatedly demonstrated in many types of pain. Nevertheless, the relationship between chronic pain (see glossary) and cognition has received relatively insufficient attention, especially in preclinical studies. How pain impacts cognition and conversely, how cognition may impact the expression of chronic pain, remain to be studied in depth. Although animal models cannot fully recapitulate the human experience of pain, the cognitive experience of pain is inherent to survival and likely to be largely evolutionarily conserved (see [2] for review). Studies in animals may therefore allow for detailed mechanistic understanding of the relationship between aspects of pain and cognition. In this review, we discuss the importance of pain-related cognition and how insights from preclinical models can inform our understanding not only of cognitive deficits in chronic pain but the potential causal contribution of cognitive deficits in the maintenance of chronic pain. We then highlight how preclinical studies on the cognitive aspects of pain may provide opportunities for the development of behavioral or pharmacological therapies for chronic pain.
Figure 1: Progression of Theories of Pain.
(A) In the ‘Treatise on Man’ Descartes proposed the first pain pathway [119]. In this, sensory stimuli activate hollow tubules, opening a gate in the brain, allowing the animal spirits to enter the tubules and activate the muscles to move them away from danger. (B) The ‘Tripartite Model’ of Melzack and Casey in the 1960s [1] includes the sensory/discriminative, motivational/affective and cognitive/evaluative dimensions of pain. This epiphany in the understanding of pain, led to a resurgence in interest in the motivational/affective experience, something that had long been neglected after the initial definition of pain as the antithesis of pleasure by ancient Greek philosophers. (C) Craig proposed pain as a “homeostatic emotion”, maintaining the integrity of the body [120]. Acutely in response to pain, autonomic processes protect homeostasis but higher-order motivated behaviors, such as resting injured areas, are also needed to promote healing and aid full restoration of homeostasis. (D) These ideas were expanded further by Fields in the motivation-decision model of pain [19]. In this a subconscious decision is made on what to attend to, based on a cost-benefit analysis. ‘ON’ and ‘OFF’ cells in the rostral ventral medulla (RVM) have been shown to mediate pain according to whether it is to be acted on or not.
The Adaptive Value of Acute Versus Chronic Pain
Acutely, pain is adaptive and serves a protective role. Noxious stimuli either produce, or have potential to, damage the body. Such stimuli seize the individual’s attention and often require an immediate behavioral response. Pain has multiple dimensions including a sensory/discriminative component that provides the alarm to the organism of impending injury, as well as the motivational and affective components that promote actions required for escape and recovery. Embedded in the response to a noxious stimulus is the critical cognitive/evaluative dimension that induces a strong learning experience [3] (see Box 1). These cognitive effects, including memories to evaluate potential threats, can often be seen for the lifetime of an individual, and aid in decisions to avoid behaviors that would result in future injury. Learned pain experiences can also influence perception of future pain experiences [4].
BOX 1: Learning theories in pain:
Multiple models have been proposed to explain the learning that occurs in a pain experience. In Pavlovian and instrumental learning, a cognitive learning system assigns values to create an internal model that is used to form predictions of potentially pain-inducing events which enable higher-level cognitive actions such as decision making, based on expectations. Negative values are often assigned to actions which result in pain [3]. This internal model can be continually updated by any ‘prediction error’ perceived after experience e.g., the action being more or less painful than expected [114]. Whilst such learning applies to all kinds of situations, in the case of pain it has been hypothesized that there is a bias against reducing the expectation of pain because it is evolutionarily more costly to make an error in predicting no, or little pain, as this can result in serious injury [115]. On the other hand, the ‘reinforcement-learning model’ proposes that the direct outcome of an action does not need to be known and subsequent actions depend on the next available prediction [3]. In this model, behaviors that result are not always consistent with the pain aversiveness. Here, pain can be a conditioned stimulus for reward or decreasing the intensity of a painful stimulus can predict the termination of pain and is therefore judged to be far less aversive than an painful stimulus of increasing intensity [3]. The reader is referred to many excellent reviews of learning models of pain [3, 13, 116].
Whether chronic pain has potential benefits to organisms remains uncertain. Hypervigilance, as well as cautious, defensive behaviors to avoid predators [5] have been demonstrated in injured squid [6] and mice [7]. Such studies suggest chronic pain could provide an evolutionary advantage under some circumstances that could also apply to humans. However, chronic pain can interfere with activities that are not harmful and most often do not provide a protective function in humans. Indeed, chronic pain can become a pathology itself that can dominate the daily life of humans leading to cognitive impairment in performance of non-pain related tasks and maladaptive pain memories that can facilitate the maintenance of pain.
Chronic Pain and Cognitive Impairment
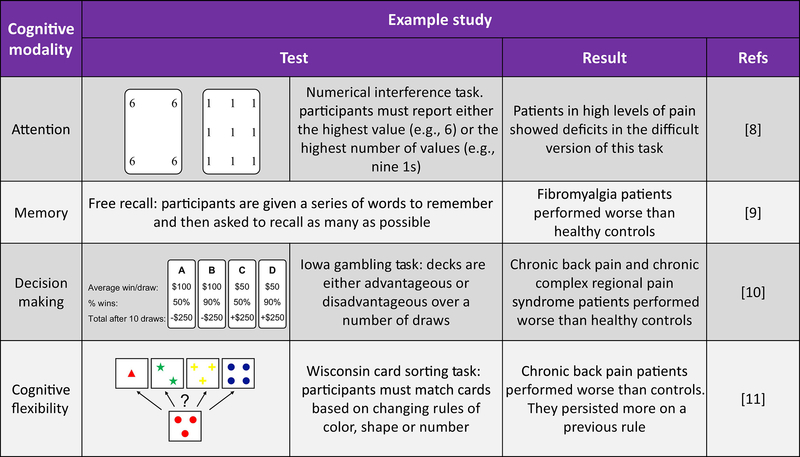
Chronic pain patients often self-report cognitive difficulties and this has been confirmed by clinical studies (for examples see [8–11] and Figure 2). Impairments have been reported in attention, learning and memory, psychomotor ability and executive function (for a review see [12]). Whether or not acute or chronic pain always promotes cognitive impairments remains unclear and could depend on many variables. For example, impairment in attention was only seen at the hardest task difficulty in patients rated as having high, and not low, pain [8]. Therefore, the possible impact of pain-related cognitive impairment in daily life may be the combined consequence of factors including intensity and chronicity of pain, task difficulty, age of the individual, medications being used and others.
Figure 2:
Example studies of cognitive impairments in chronic pain patients.
However, it is clear that in many cases, acute and chronic pain can, and do, influence cognition and conversely that cognitive functions can influence pain (for reviews see [12, 13]). Psychological theory suggests that individuals have limited cognitive resources at any given time, so there is a need for selectivity and prioritization of the many stimuli in the surroundings for attention, learning and higher-order cognitions [14]. Selection of stimuli to attend to, or to learn from, can result from both ‘bottom-up’ and ‘top-down’ mechanisms. Environmental stimuli that are particularly salient, including pain, commonly engage ‘bottom-up’ mechanisms [15]. For example, acutely, it is advantageous for pain to be prioritized, through a “bottom up” mechanism, interrupting other cognition requiring tasks to elicit appropriate behavior to escape from the noxious threat [15]. Similar to acute pain, chronic pain can also seize bottom-up attention and take up a significant proportion of the limited cognitive resource, leaving less available for other cognition requiring tasks [8].
‘Top-down’ mechanisms can be engaged with task-specific stimuli to diminish pain [16]. Focusing cognitive resources away from pain using ‘top-down’ prioritization for other stimuli can result in less seizing of the limited cognitive resource by pain leading to reduced pain perception as in distraction-induced analgesia [16] or reappraisal of pain [17]. In the case of pain reappraisal, for example, imagining a noxious heat stimulus to be less painful produced analgesia and this was mediated by a top-down, fronto-striatal pathway between the ventral medial prefrontal cortex (vmPFC) and nucleus accumbens (NAc) [17]. Sustained top-down executive control to direct attention away from pain and onto other cognitive tasks is required to counter the engulfing of cognitive resources by chronic pain in order to achieve pain relief. However, such sustained top-down control requires effort and consequently increases the cost of cognitive behavior [18]. There is a non-conscious choice on what to attend to, based on a cost-benefit analysis for positive outcomes for the organism [19] and when cost outweighs the benefit, fatigue can lead individuals to disengage from an activity involving effort for diminishing returns [20]. Deficits in the mesolimbic reward system have been observed in chronic pain (for review see [21]), suggesting that there may also be less benefit from engaging in cognitively demanding tasks [18].
In situations in which an individual is not actively trying to distract themselves from pain, ‘top-down’ attention to task related actions is likely to be reduced by pain. Therefore, cognitive deficits associated with chronic pain may result from limited cognitive resources and the interruptive nature of pain, which can additionally promote and sustain chronic pain. For example, patients with chronic pain can suffer from catastrophizing characterized by exaggeration, continued rumination and feelings of helplessness about their pain [22, 23]. Catastrophizing likely involves direction of top-down attention towards any potential pain stimulus with the innate goal to avoid pain-inducing stimuli and situations [15]. As a result, any signal from the body of pain, or generalized similar sensations, may lead to pain-related engagement of top-down, as well as bottom-up, attention away from other cognition requiring tasks [15].
Other factors might also influence the relationship between chronic pain and cognition. Functional and structural changes in the brain induced by chronic pain could diminish available cognitive resources. Functional magnetic resonance imaging has shown that there is an overlap in brain regions activated by chronic pain and cognitive tasks (for review see [12]). Therefore, pain might reduce functional availability for cognition in specific circuits [12]. The anterior cingulate cortex (ACC) is vital for the affective qualities of pain [24] and for cognitive control, which is required for sustaining goal-directed behavior (for review see [25]). Differential activation of the ACC by an acute pain stimulus or by sustained attention was seen in patients with chronic pain, compared to healthy controls [26]. Some studies have suggested that chronic pain may lead to structural changes such as loss of grey matter in the brain [27, 28]. While the concept of chronic pain as neurodegenerative disease remains uncertain due to confounding interpretations, e.g., observed, purported loss of grey matter may instead be reduced cerebral blood flow [29], possible neuronal loss could underlie pain-related cognitive deficits. Loss of grey matter particularly in areas linked with top-down cognitive control such as the dorsolateral PFC (dlPFC) [28] could lead to reductions in available cognitive resources.
Preclinical studies of Chronic Pain and Cognitive Impairment
In addition to the factors mentioned above, cognitive deficits in chronic pain patients may also arise from effects of medications such as analgesics, or drugs used to treat co-morbid disorders such as major depressive disorder (MDD). The effects of drugs on the brain, particularly after chronic use, are often difficult to account for in clinical studies thus confounding conclusions on the specific effects of pain on cognition. Whilst some variability has been reported in the preclinical literature [30–32], substantial research has shown that rodent models of pain develop deficits in all types of cognitions tested including: attention [33, 34], learning and memory [30, 31, 35–37] as well as higher order executive functions such as decision making [38, 39] and cognitive flexibility [31, 40–42]. These findings suggest the utility of preclinical models in exploring mechanisms that may be relevant to the relationship of pain and behavior. Following a painful stimulus, a decision must be made on whether to attend to or suppress pain and direct behavior to other, more important actions. The Motivation-Decision model of pain [19] (Figure 1) has provided insights into the neural mechanisms underlying action selection in circumstances of pain and competing motivations. In this model if pain is more important than other motivations, it is facilitated. In contrast, if there is a more important competing motivation, such as hunger or a reward, pain is inhibited. In a study where rats were trained to receive a highly salient pleasurable reward of chocolate each day, the response to an unexpected painful stimulus was reduced by expectation of reward [43]. Interestingly, this may correspond to cognition dependent behavior. In a two lever choice task, a rodent model of neuropathic pain showed an impairment in cognitive flexibility when the ‘profitable’ lever was reversed [40]. However, the animals performed better in the food-rewarded task of cognitive flexibility under food-deprivation, as opposed to when food was provided ad libitum in their home cage [40]. This suggests that because food became more important, more cognitive resources were made available for food-related stimuli.
In this model, implementation of the neural ‘decision’ may ultimately take place at the level of the rostral ventromedial medulla (RVM) [19], where two main classes of pain modulatory cells termed ‘ON’ (i.e., net pain facilitation cells) and ‘OFF’ (i.e., net pain inhibition cells) have been identified [44]. These cells project to the spinal cord to either positively or negatively modulate the output of the ascending noxious signal from the dorsal horn [45]. As proposed in the ‘Gate Control Theory of Pain’ [46], the descending pain pathway provides the link from the mind to the ‘sensory apparatus’ that allows appropriate action selection in response to painful stimuli. This assessment can take into account learning and memories from past experiences that involve the hippocampus, amygdala, striatum and other brain regions (for review see [47]). Modulation of pain from these descending pathways also involve connections to the midbrain periaqueductal gray (PAG) that sends projections to the RVM [48].
Less is known, however, about the motivation-decision model in relation to chronic pain. For the ‘decision’ to be made, there must be information on the current homeostatic state related to pain as well as the potential value of rewards, including pain relief, that can be influenced by competing states such as hunger. This information may be provided, in part, through the mesolimbic dopamine pathway, particularly the NAc and the ventral tegmental area (VTA) [19]. The mesolimbic dopamine signaling seems to be disordered in chronic pain, but details remain to be clarified. As noted, cognitive fatigue, due to increase in ‘cost’ and decrease in benefit due to disordered reward perception (for review see [21]), can also contribute to cognitive deficits in chronic pain [18]. In a progressive-ratio task, in which a greater number of lever presses or nose-pokes are required for reward, an inflammatory and a neuropathic mouse model of pain had a lower ‘break-point’ at which these animals gave up [49] suggestive of an increased cost-benefit ratio. Analgesics have been shown to reliably increase dopamine levels in the NAc in both neuropathic and incisional pain rat models [50] and produce conditioned place preference (CPP) [50, 51]. This is suggestive of the strong motivational effect of relief of the aversive qualities of chronic pain and may explain why pain can seize cognitive resources from other tasks.
Rodent models of pain can also provide a more in-depth analysis of changes in key cognitive areas which may underlie a reduction in limited cognitive resources. For instance, decreased neurogenesis, the formation of new neurons, has been reported in rodent models of chronic pain in the dentate gyrus of the hippocampus [52–55]. The hippocampus is the brain region most synonymous with learning and memory and heightened neurogenesis has been shown to improve learning (for review see [56]). Therefore, a pain-related deficit in neurogenesis could also be causal in learning deficits in chronic pain. Furthermore, long term potentiation (LTP), the fundamental process for memory formation in the hippocampus, is also reduced in both neuropathic [57] and migraine models of pain [58], the latter of which was associated with spatial learning and memory deficits [58]. There are also morphological and functional changes in the PFC in rodent models of chronic pain which may reduce cognitive capacity. In both arthritis and neuropathic animal models of pain, decreased excitatory activity has been reported in the mPFC [38, 59], which may be related to reduced glutamatergic transmission [59]. Some of the decreased activity in the mPFC in an arthritis model of chronic pain may be due to hyperactivity of the basolateral amygdala (BLA) induced inhibition of the mPFC [60]. Blockade of the heightened BLA activity increased mPFC activity and also ameliorated a decision-making deficit in a rodent gambling task [38, 60]. A reduction in length of axon initial segment in layer 5/6 of the infralimbic area of the mPFC has been seen in a neuropathic model of pain [42]. In these animals a deficit in cognitive flexibility was seen, alleviated by metformin, which increased the length of the axon initial segment, suggesting this could be causal in the cognitive flexibility impairment [42]. In addition to impaired decision making and cognition, these neural changes could also facilitate the maintenance of pain (discussed below).
Cognition and Maintenance of Chronic Pain
Chronic Pain as a Persistence of Memory
It has increasingly become clear that some forms of chronic pain may be considered a disorder of cognition itself. Therefore, not treating the cognitive disorder may also make it impossible to adequately relieve chronic pain. Apkarian and colleagues have suggested that ‘chronic pain is a persistence of the memory of pain and/or the inability to extinguish the memory of pain evoked by an initial inciting injury’[61]. Supporting this possibility, case studies have been reported in which severe amnesia alleviated chronic pain [62] (Box 2). As discussed above, pain can form a lasting memory. Whilst touching a hotplate promotes a strong association that leads to adaptive behaviors that can last a lifetime, chronic pain patients may instead form unhelpful associations with non-painful events. For example, chronic pain patients may form associative memories between pain and their environment or between pain and common tasks such as walking, that may reinforce pain-related learning [61]. As the individual doesn’t perceive this consciously, or it may take place in an unavoidable environment such as their own home, this may lead to the frequent reactivation of the memory and hence persistence of pain after the physical injury is healed [61]. The fear-avoidance model postulates that chronic pain patients may exhibit avoidance of pain-associated stimuli even past healing, possibly because the consequences of lack of caution could be graver than increased vigilance [63]. This avoidance may maintain the idea that there is something to fear, preventing the extinction of the pain association [63]. Furthermore, even when the memory is extinguished and pain is in remission, environmental stimuli associated with pain may trigger reactivation and perception of pain [61]. This could be similar to relapse of addiction where specific environmental triggers may lead to drug taking [64, 65] as well as to the resiliency to full extinction that is often seen in fear conditioning paradigms, in which extinguished fear associations often return [66].
BOX 2: Forgetting Pain: Clinical Case Studies.
Amnesia, or memory loss, can occur after events such as injury or disease, traumatic experiences, substance abuse or even in the normal process of aging. Retrograde amnesia often involves loss of the most recent memories and not those from years prior, although this may also occur over time.
One of the most famous cases of this type of amnesia is patient H.M. who underwent bilateral resection of the uncus, amygdala, anterior hippocampus, and parahippocampal gyrus for treatment of intractable epilepsy [117]. After the surgery, he was unable to form new memories. Interestingly, H.M. would not report pain to an intense thermal stimulus, despite an intact spinothalamic pathway and peripheral nociceptors [118]. Whether this is related to a deficit in memory or lack of aversion due to amygdala lesion is uncertain.
Interestingly, in the case of chronic pain, in two patients amnesia seemed to essentially be a miracle cure for their pain [62]. Prior to their amnesia, both had been on high levels of opioids alongside having tried other methods of pain relief, both pharmacological and behavioral. The first patient was hospitalized for her pain for a year. During this time, she was witnessed to have at least 5 seizures and after one episode she couldn’t remember anything about her period of hospitalization but had normal long-term memories and cognitive functioning. To test her cognitive functioning, she was weaned off her opioid medications. To the physicians’ surprise, she showed minimal withdrawal and substantially reduced pain. Pain was still substantially reduced six months on.
The second patient had a history of low back pain and sciatica [62]. Seemingly everything had been tried to ameliorate his pain, from nerve injections, steroids, implantation of an intrathecal morphine pump, psychotherapy and drugs usually used for neuropathic pain such as gabapentin and antidepressants. A period of time after a motorcycle accident, the man suffered severe amnesia despite his brain magnetic resonance imaging scan apparently being normal. Again, for cognitive testing, the opioid dosages were significantly reduced and he did not complain of further pain. Over the next two years he started to regain some memories and have mild back and leg pain but did not request further opioid treatment.
Conceptualization of chronic pain as a cognitive disorder may also help to explain why an injury that will result in transient acute pain in most people can lead to chronic pain in others, a question that remains one of the greatest mysteries of pain research. Increased vulnerability to the development of chronic pain in some populations of people may result from reduced extinction and increased resilience of the pain memory. For instance, older individuals are more likely to have chronic pain [67] and they have been shown to exhibit reduced extinction of fear memories [68] potentially due to reduced cognitive flexibility [69]. Also, chronic pain can be comorbid with many other disorders including major depressive disorder (MDD) [70], generalized anxiety disorder (GAD) [71] and post-traumatic stress disorder (PTSD) [72]. In vulnerable individuals with MDD, for example, the bias towards pain learning over forgetting may be further amplified, due to greater attention, interpretation and distress associated with negative experiences and thus heightened motivation to remember and avoid negative experiences in the future. The comorbidity with PTSD could be indicative of a pain-induced deficit in extinction processes, promoting increased likelihood of PTSD through lack of fear extinction or the converse, extinction deficits in PTSD could promote chronic pain.
It should be noted that traumatic life events can result not only in physical pain but also in emotional turmoil, which may also affect cognition. Initially, emotional turmoil may compete for cognitive space with the physical pain of an acute injury, but aversive emotional memories may extend beyond injury healing. It has been hypothesized that in some individuals, diverting cognitive resources to acute physical pain is preferred to constantly present emotional pain memories [73], potentially leading to behavior such as self-inflicted harm (e.g., cutting) [74]. The subconscious realization that emotional pain is partially relieved by physical pain could lead to the emergence of a chronic pain condition to help mask emotional conditions [73]. This may explain the reported success of treating some pain disorders that have strong links to psychological states such as fibromyalgia [75] and irritable bowel syndrome [76] with emotional awareness and expression therapy, which focuses on encouraging awareness and expression of avoided emotions.
Rodent Models and Maintenance of Chronic Pain through Persistent Memory
It is difficult to determine in patient populations if extinction deficits are pre-existing in vulnerable patients or brought on in the acute stages of pain. It is well recognized that a memory of pain in a particular environment leads to an aversion of this environment in rodents [51, 77], suggesting that these animals also have the capacity for strong associative memories. Supporting a role for pain in reducing extinction abilities, impaired extinguishing of fear-related conditioned behavior was observed in an animal model of neuropathic pain [55]. Furthermore, a recent study showed that naïve animals that failed to extinguish a fear memory before surgery, had similar levels of nerve-injury induced sensory features of pain (i.e., allodynia, pain from a normally innocuous stimulus) but higher scores on affective qualities of pain [78]. These animals were also those more prone to develop anxiety and depression-like behavior [78].
Preclinical studies suggest that memories are extinguished not by forgetting, but by new learning [79, 80]. Why chronic pain may promote deficits in extinction remains uncertain. Of particular interest in this case is neurogenesis. It is widely accepted that a reduction in neurogenesis in chronic pain conditions may underlie the comorbidities of cognitive impairment and MDD [52, 53]. Lower levels of neurogenesis favor a state of remembering over new learning [81]. Under chronic pain conditions, decreased neurogenesis may be producing a situation which is preferable for remembering pain and not for extinction.
Extinction may also be impaired in chronic pain due to changes in the PFC. The infralimbic area of the PFC has been shown to accelerate extinction learning [82] through inhibition of the CeA [83]. In a rodent model of arthritis, decreased inhibition of the CeA by the infralimbic cortex has been shown [84] suggesting impaired extinction mechanisms may be promoting maintenance of chronic pain. Further evidence for the role of the CeA in chronic pain maintenance has been shown by increased activity in the right hemisphere’s CeA after induction of chronic pain [85]. Interestingly, preclinical investigations have shown that there is an unexplained lateralization of the CeA on chronic pain, increased activity of the right CeA promotes pain, whilst some evidence suggests that the left CeA may have a converse, protective effect [86]. Little is known as to how this occurs and the role of the lateralization in chronic pain maintenance and how this may reduce extinction of pain memories.
Treating Chronic Pain as an Inability to ‘Forget’
If chronic pain is viewed as persistence or failure to extinguish pain memories, then future treatments could be tailored to address this. Acutely, on the initial emergence of ongoing pain after an injury, it would be beneficial to prevent the formation of memories that could lead to chronic pain, whilst in established chronic pain the focus would likely need to be on the formation of new pain-free memories to extinguish the persistent memory of chronic pain. How extinction occurs is debated. Pain may be reduced by ‘unlearning’ i.e. learning that pain is no longer associated with a conditioned stimulus or, in contrast, forming another association with another stimulus or context that predicts the absence of pain (for review see [66]). Preventing formation or extinction of pain memories might be achieved either through psychological or pharmacological strategies or both.
At present, there are few pharmacological therapies that focus on the cognitive element of chronic pain, but some mechanistic strategies deserve future evaluation. On the initial emergence of persistent acute pain, prevention of LTP, a fundamental synaptic mechanism for induction of a memory [87] could be achieved through antagonist effects at glutamatergic N-Methyl-D-aspartate (NMDA) receptors. Ketamine, a non-competitive NMDA channel blocker, works at least in part, through this mechanism [88]. Ketamine has been shown to reduce persistent post-surgical pain [89] and to be effective for pain-related co-morbid disorders including MDD [90]. Another option is the newly emerging concept of inhibiting protein translation, with specific translation inhibitors that can also prevent LTP [91]. Inhibitors of translation have been shown to produce analgesic effects in rodent models of persistent pain [92–94]. These include translation inhibitors that act on the mammalian target of rapamycin [93] and more recently on downstream effectors in this pathway such as eukaryotic translation initiation factor 4E [95, 96], which may have less side effects [95]. Although most often attributed to reduced increases in peripheral neuronal sensitivity [94], analgesic effects of translation inhibitors have also been shown with microinjection into discrete brain areas such as the insular cortex [97], so there could be dual central and peripheral nervous system modes of action. Interestingly, drugs that are in current use for the treatment of chronic pain, including gabapentinoids and opioids, impair memory [98, 99]. Therefore, they might promote their beneficial effects initially by reducing pain memory formation. In chronic pain, these medications can also prevent reconsolidation of pain associated memories during a labile period, when the person is re-exposed to the previously pain-associated stimulus. Reducing reconsolidation has been shown to reduce expression of that particular memory (for review see [100]).
When pain has become chronic, the focus may need to be on extinguishing the memory of pain, instead of forgetting, by establishing new pain-free memories. This could involve reappraisal of pain through psychological therapies. Cognitive behavioral therapy (CBT) and mindfulness promote reappraisal of chronic pain [101, 102], which provides a different, less aversive, interpretation of the pain experience, and this has been shown to provide pain relief in the short term [103]. Long-term consequences of CBT may aid ‘top-down’ cognitive processes in diverting cognitive resources from pain. CBT is associated with decreased connectivity in brain circuits associated with catastrophizing [104] and increased grey matter in the prefrontal and parietal cortices [105], both of which may reduce the ability of pain to seize cognitive resources. Acceptance and commitment therapy (ACT) promotes accepting pain and not avoiding it, so the individual can focus on other life goals [106]. ACT has been reported to decrease the duration of post-operative pain after orthopedic surgery [107] perhaps reducing the fear-avoidance element that can prolong pain after injury has healed. As noted above, emotional awareness and expression therapy could decrease preference for physical pain as a distractor from emotional turmoil [73].
Extinction is not forgetting but development of new memories which are in direct contrast with the old, either replacing the old memories over time or forming a parallel memory suggesting the old memory is relevant only in certain contexts [66]. Cognitive enhancers may promote extinction, especially if used during low pain periods. These have a range of mechanisms of actions including glutamatergic agonism, noradrenaline reuptake inhibition and stimulants (e.g. amphetamine-like drugs) (for review see [108]). Recent reports found a cognitive enhancer that is an NMDA receptor partial agonist, reduced pain in a rodent model and in chronic low back pain patients although these studies did not directly test cognition [109, 110]. Further aid in promoting extinction could result from specifically enhancing cognitive flexibility. High neurogenesis is associated with good cognitive flexibility [111] and current antidepressants have been shown to increase neurogenesis [112], with more specific drugs for the upregulation of neurogenesis in clinical trials for depression [113].
Concluding Remarks
Whilst our understanding of the importance of the cognitive/evaluative component of pain has dramatically increased in recent years, it is still the relatively ignored component of Melzack and Casey’s tripartite model [1] especially in preclinical research. This may be one factor that has contributed to the relative failure to discover new therapies for the treatment of chronic pain.
Here we have discussed how we can ‘reverse-translate’ psychological models of cognitive deficits in chronic pain to rodent models, to understand further how they can occur and lead to pharmacological treatments. We also addressed the idea that chronic pain may result from a persistence of now aberrant pain memories. Many uncertainties and questions remain to be explored experimentally. For example, do impairments in extinction cause chronic pain or do they develop to maintain it (see Outstanding Questions). Such questions can be challenging to answer in chronic pain patients but may be addressed in preclinical models, which allow investigation of the effects of pain itself without confounding factors on the memory machinery. With greater understanding of the concept of pain as a cognitive disorder, new therapies could be developed either preventing the abberrant formation of pain memory after surgeries or extinguishing it once the pain becomes chronic.
Outstanding Questions.
What causes cognitive deficits in chronic pain? Is there cognitive impairment in all chronic pain patients? Are cognitive deficits observed in some chronic pain patients caused by distraction of pain seizing cognitive resources?
Is chronic pain a ‘vicious circle’ such that chronic pain causes deficits in cognitive processes required for extinction and so is unable to be ‘extinguished’ itself.
Would an intervention to prevent ‘learning’ of pain be efficacious in reducing the development of chronic pain after surgery or injury?
Would cognitive enhancers (during a “low pain situation”- see above) that don’t reduce the sensation of pain acutely, induce ‘extinction’ of chronic pain?
Are cognitive deficits in chronic pain reversible with time or therapy?
Do existing chronic pain treatments work, at least in part, through cognitive mechanisms resulting from an increased extinction of pain memories?
Highlights.
Pain is a strong cognitive experience, seizing attention in the moment and driving learning and memory to avoid the painful stimulus again.
Whilst acute pain has a protective role, in most cases chronic pain does not. Chronic pain is associated with cognitive impairment, which may be due to pain using up a significant proportion of limited cognitive resources.
Chronic pain has been hypothesized to be an inability to forget or extinguish pain memories, suggesting it can be associated with impairment of cognition.
Current chronic pain therapies may act through extinction of pain memories instead of/as well as their effects on sensory systems.
ACKNOWLEDGEMENTS
Supported by grants from the National Institues on Health - R01 DA 041809 (F.P.) and P01 DA041307 (F.P., E.N.).
Glossary
- Chronic pain
pain that lasts for more than 3–6 months or beyond the period of healing
- Cognitive Flexibility
the ability to change actions/thinking in response to a change in outcome
- Extinction
Reduction in response to a conditioned stimulus when the unconditioned stimulus is omitted. Represents new learning rather than forgetting
- Neuropathic Pain
pain caused by a lesion or disease of the somatosensory nervous system
- Noxious stimulus
a stimulus that is damaging or threatens damage to tissues
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Melzack R and Casey KL (1968) Sensory, Motivational, and Central Control Determinants of Pain A New Conceptual Model, Charles C. Thomas. [Google Scholar]
- 2.Mogil JS (2019) Mice are people too: Increasing evidence for cognitive, emotional and social capabilities in laboratory rodents. Canadian Psychology/Psychologie canadienne 60 (1), 14–20. [Google Scholar]
- 3.Seymour B (2019) Pain: A Precision Signal for Reinforcement Learning and Control. Neuron 101 (6), 1029–1041. [DOI] [PubMed] [Google Scholar]
- 4.Reicherts P et al. (2016) Psychological Placebo and Nocebo Effects on Pain Rely on Expectation and Previous Experience. J Pain 17 (2), 203–14. [DOI] [PubMed] [Google Scholar]
- 5.Walters ET and Williams ACC (2019) Evolution of mechanisms and behaviour important for pain. Philos Trans R Soc Lond B Biol Sci 374 (1785), 20190275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crook RJ et al. (2014) Nociceptive sensitization reduces predation risk. Curr Biol 24 (10), 1121–5. [DOI] [PubMed] [Google Scholar]
- 7.Lister KC et al. (2020) Chronic pain produces hypervigilance to predator odor in mice. Curr Biol 30 (15), R866–R867. [DOI] [PubMed] [Google Scholar]
- 8.Eccleston C (1994) Chronic pain and attention: a cognitive approach. Br J Clin Psychol 33 ( Pt 4), 535–47. [DOI] [PubMed] [Google Scholar]
- 9.Park DC et al. (2001) Cognitive function in fibromyalgia patients. Arthritis Rheum 44 (9), 2125–33. [DOI] [PubMed] [Google Scholar]
- 10.Apkarian AV et al. (2004) Chronic pain patients are impaired on an emotional decision-making task. Pain 108 (1–2), 129–36. [DOI] [PubMed] [Google Scholar]
- 11.Tamburin S et al. (2014) Cognition and emotional decision-making in chronic low back pain: an ERPs study during Iowa gambling task. Front Psychol 5, 1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriarty O et al. (2011) The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol 93 (3), 385–404. [DOI] [PubMed] [Google Scholar]
- 13.Wiech K (2016) Deconstructing the sensation of pain: The influence of cognitive processes on pain perception. Science 354 (6312), 584–587. [DOI] [PubMed] [Google Scholar]
- 14.Fisher J et al. (2018) The limited capacity model of motivated mediated message processing: Taking stock of the past. Annals of the International Communication Association 42. [Google Scholar]
- 15.Legrain V et al. (2009) A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain 144 (3), 230–2. [DOI] [PubMed] [Google Scholar]
- 16.Birnie KA et al. (2017) Mechanisms of distraction in acute pain perception and modulation. Pain 158 (6), 1012–1013. [DOI] [PubMed] [Google Scholar]
- 17.Woo CW et al. (2015) Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol 13 (1), e1002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Damme S et al. (2018) Tired of pain? Toward a better understanding of fatigue in chronic pain. Pain 159 (1), 7–10. [DOI] [PubMed] [Google Scholar]
- 19.Fields HL (2006) A Motivation-Decision Model of Pain: The Role of Opioids, IASP Press. [Google Scholar]
- 20.Thorndike EL Mental fatigue. Psychol Rev 7, 466–82. [Google Scholar]
- 21.Serafini RA et al. (2020) The Mesolimbic Dopamine System in Chronic Pain and Associated Affective Comorbidities. Biol Psychiatry 87 (1), 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards RR et al. (2011) Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol 7 (4), 216–24. [DOI] [PubMed] [Google Scholar]
- 23.Racine M et al. (2016) The reciprocal associations between catastrophizing and pain outcomes in patients being treated for neuropathic pain: a cross-lagged panel analysis study. Pain 157 (9), 1946–1953. [DOI] [PubMed] [Google Scholar]
- 24.Johansen JP et al. (2001) The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A 98 (14), 8077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shackman AJ et al. (2011) The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12 (3), 154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buffington AL et al. (2005) Acute and persistent pain modulation of attention-related anterior cingulate fMRI activations. Pain 113 (1–2), 172–84. [DOI] [PubMed] [Google Scholar]
- 27.Fritz HC et al. (2016) Chronic Back Pain Is Associated With Decreased Prefrontal and Anterior Insular Gray Matter: Results From a Population-Based Cohort Study. J Pain 17 (1), 111–8. [DOI] [PubMed] [Google Scholar]
- 28.Apkarian AV et al. (2004) Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 24 (46), 10410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pomares FB et al. (2017) Histological Underpinnings of Grey Matter Changes in Fibromyalgia Investigated Using Multimodal Brain Imaging. J Neurosci 37 (5), 1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren WJ et al. (2011) Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology 36 (5), 979–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moriarty O et al. (2016) Impaired recognition memory and cognitive flexibility in the rat L5–L6 spinal nerve ligation model of neuropathic pain. Scandinavian Journal of Pain 10, 61–73. [DOI] [PubMed] [Google Scholar]
- 32.Grégoire S et al. (2012) Study of emotional and cognitive impairments in mononeuropathic rats: effect of duloxetine and gabapentin. Pain 153 (8), 1657–63. [DOI] [PubMed] [Google Scholar]
- 33.Pais-Vieira M et al. (2009) Sustained attention deficits in rats with chronic inflammatory pain. Neurosci Lett 463 (1), 98–102. [DOI] [PubMed] [Google Scholar]
- 34.Higgins GA et al. (2015) Enduring attentional deficits in rats treated with a peripheral nerve injury. Behav Brain Res 286, 347–55. [DOI] [PubMed] [Google Scholar]
- 35.Low LA et al. (2012) Nerve injury causes long-term attentional deficits in rats. Neurosci Lett 529 (2), 103–7. [DOI] [PubMed] [Google Scholar]
- 36.Leite-Almeida H et al. (2009) The impact of age on emotional and cognitive behaviours triggered by experimental neuropathy in rats. Pain 144 (1–2), 57–65. [DOI] [PubMed] [Google Scholar]
- 37.Grégoire S et al. (2014) Monoarthritis-induced emotional and cognitive impairments in rats are sensitive to low systemic doses or intra-amygdala injections of morphine. Eur J Pharmacol 735, 1–9. [DOI] [PubMed] [Google Scholar]
- 38.Ji G et al. (2010) Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 30 (15), 5451–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pais-Vieira M et al. (2009) Cognitive impairment of prefrontal-dependent decision-making in rats after the onset of chronic pain. Neuroscience 161 (3), 671–9. [DOI] [PubMed] [Google Scholar]
- 40.Cowen SL et al. (2018) Chronic pain impairs cognitive flexibility and engages novel learning strategies in rats. Pain 159 (7), 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leite-Almeida H et al. (2014) Asymmetric c-fos expression in the ventral orbital cortex is associated with impaired reversal learning in a right-sided neuropathy. Mol Pain 10, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiers S et al. (2018) Neuropathic Pain Creates an Enduring Prefrontal Cortex Dysfunction Corrected by the Type II Diabetic Drug Metformin But Not by Gabapentin. J Neurosci 38 (33), 7337–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dum J et al. (1983) Activation of hypothalamic beta-endorphin pools by reward induced by highly palatable food. Pharmacol Biochem Behav 18 (3), 443–7. [DOI] [PubMed] [Google Scholar]
- 44.Fields HL et al. (1995) Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol 74 (4), 1742–59. [DOI] [PubMed] [Google Scholar]
- 45.Heinricher MM et al. (1989) Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Mot Res 6 (4), 427–39. [DOI] [PubMed] [Google Scholar]
- 46.Melzack R and Wall PD (1965) Pain mechanisms: a new theory. Science 150 (3699), 971–9. [DOI] [PubMed] [Google Scholar]
- 47.Atlas LY and Wager TD (2012) How expectations shape pain. Neurosci Lett 520 (2), 140–8. [DOI] [PubMed] [Google Scholar]
- 48.Heinricher MM et al. (2009) Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev 60 (1), 214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz N et al. (2014) Chronic pain. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 345 (6196), 535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie JY et al. (2014) Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: a potential biomarker of efficacy. Pain 155 (8), 1659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navratilova E et al. (2012) Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A 109 (50), 20709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dellarole A et al. (2014) Neuropathic pain-induced depressive-like behavior and hippocampal neurogenesis and plasticity are dependent on TNFR1 signaling. Brain Behav Immun 41, 65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dimitrov EL et al. (2014) Anxiety- and depression-like behavior and impaired neurogenesis evoked by peripheral neuropathy persist following resolution of prolonged tactile hypersensitivity. J Neurosci 34 (37), 12304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egorova E et al. (2019) Hippocampal Neurogenesis in Conditions of Chronic Stress Induced by Sciatic Nerve Injury in the Rat. Cells Tissues Organs 207 (1), 58–68. [DOI] [PubMed] [Google Scholar]
- 55.Mutso AA et al. (2012) Abnormalities in hippocampal functioning with persistent pain. J Neurosci 32 (17), 5747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deng W et al. (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11 (5), 339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kodama D et al. (2007) Altered hippocampal long-term potentiation after peripheral nerve injury in mice. Eur J Pharmacol 574 (2–3), 127–32. [DOI] [PubMed] [Google Scholar]
- 58.Zhang M et al. (2020) Cognitive impairment in a classical rat model of chronic migraine may be due to alterations in hippocampal synaptic plasticity and N-methyl-D-aspartate receptor subunits. Mol Pain 16, 1744806920959582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly CJ et al. (2016) Reduced Glutamatergic Currents and Dendritic Branching of Layer 5 Pyramidal Cells Contribute to Medial Prefrontal Cortex Deactivation in a Rat Model of Neuropathic Pain. Front Cell Neurosci 10, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiritoshi T et al. (2016) Rescue of Impaired mGluR5-Driven Endocannabinoid Signaling Restores Prefrontal Cortical Output to Inhibit Pain in Arthritic Rats. J Neurosci 36 (3), 837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Apkarian AV (2008) Pain perception in relation to emotional learning. Curr Opin Neurobiol 18 (4), 464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi DS et al. (2007) Sudden amnesia resulting in pain relief: the relationship between memory and pain. Pain 132 (1–2), 206–10. [DOI] [PubMed] [Google Scholar]
- 63.Vlaeyen JW et al. (2016) The fear-avoidance model of pain. Pain 157 (8), 1588–9. [DOI] [PubMed] [Google Scholar]
- 64.McNally GP (2014) Extinction of drug seeking: Neural circuits and approaches to augmentation. Neuropharmacology 76 Pt B, 528–32. [DOI] [PubMed] [Google Scholar]
- 65.Khoo SY et al. (2017) How contexts promote and prevent relapse to drug seeking. Genes Brain Behav 16 (1), 185–204. [DOI] [PubMed] [Google Scholar]
- 66.Dunsmoor JE et al. (2015) Rethinking Extinction. Neuron 88 (1), 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blyth FM et al. (2001) Chronic pain in Australia: a prevalence study. Pain 89 (2–3), 127–34. [DOI] [PubMed] [Google Scholar]
- 68.Battaglia S et al. (2018) Context-dependent extinction of threat memories: influences of healthy aging. Sci Rep 8 (1), 12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giller F and Beste C (2019) Effects of aging on sequential cognitive flexibility are associated with fronto-parietal processing deficits. Brain Struct Funct 224 (7), 2343–2355. [DOI] [PubMed] [Google Scholar]
- 70.Bair MJ et al. (2003) Depression and pain comorbidity: a literature review. Arch Intern Med 163 (20), 2433–45. [DOI] [PubMed] [Google Scholar]
- 71.Csupak B et al. (2018) A population-based examination of the co-occurrence and functional correlates of chronic pain and generalized anxiety disorder. J Anxiety Disord 56, 74–80. [DOI] [PubMed] [Google Scholar]
- 72.Kind S and Otis JD (2019) The Interaction Between Chronic Pain and PTSD. Curr Pain Headache Rep 23 (12), 91. [DOI] [PubMed] [Google Scholar]
- 73.Lane RD et al. (2018) Biased Competition Favoring Physical Over Emotional Pain: A Possible Explanation for the Link Between Early Adversity and Chronic Pain. Psychosom Med 80 (9), 880–890. [DOI] [PubMed] [Google Scholar]
- 74.Reitz S et al. (2015) Incision and stress regulation in borderline personality disorder: neurobiological mechanisms of self-injurious behaviour. Br J Psychiatry 207 (2), 165–72. [DOI] [PubMed] [Google Scholar]
- 75.Lumley MA et al. (2017) Emotional awareness and expression therapy, cognitive behavioral therapy, and education for fibromyalgia: a cluster-randomized controlled trial. Pain 158 (12), 2354–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thakur ER et al. (2017) Emotional awareness and expression training improves irritable bowel syndrome: A randomized controlled trial. Neurogastroenterol Motil 29 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.King T et al. (2009) Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci 12 (11), 1364–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ji G et al. (2018) Fear extinction learning ability predicts neuropathic pain behaviors and amygdala activity in male rats. Mol Pain 14, 1744806918804441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quirk GJ and Mueller D (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33 (1), 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goodman J and Packard MG (2019) There Is More Than One Kind of Extinction Learning. Front Syst Neurosci 13, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akers KG et al. (2014) Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344 (6184), 598–602. [DOI] [PubMed] [Google Scholar]
- 82.Thompson BM et al. (2010) Activation of the infralimbic cortex in a fear context enhances extinction learning. Learn Mem 17 (11), 591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quirk GJ et al. (2003) Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23 (25), 8800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kiritoshi T and Neugebauer V (2018) Pathway-Specific Alterations of Cortico-Amygdala Transmission in an Arthritis Pain Model. ACS Chem Neurosci 9 (9), 2252–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ji G and Neugebauer V (2009) Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol 102 (4), 2253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sadler KE et al. (2017) Divergent functions of the left and right central amygdala in visceral nociception. Pain 158 (4), 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morris RG (2003) Long-term potentiation and memory. Philos Trans R Soc Lond B Biol Sci 358 (1432), 643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zorumski CF et al. (2016) Ketamine: NMDA Receptors and Beyond. J Neurosci 36 (44), 11158–11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McNicol ED et al. (2014) A systematic review and meta-analysis of ketamine for the prevention of persistent post-surgical pain. Acta Anaesthesiol Scand 58 (10), 1199–213. [DOI] [PubMed] [Google Scholar]
- 90.Xu Y et al. (2016) Effects of Low-Dose and Very Low-Dose Ketamine among Patients with Major Depression: a Systematic Review and Meta-Analysis. Int J Neuropsychopharmacol 19 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Costa-Mattioli M et al. (2009) Translational control of long-lasting synaptic plasticity and memory. Neuron 61 (1), 10–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferrari LF et al. (2013) Peripheral administration of translation inhibitors reverses increased hyperalgesia in a model of chronic pain in the rat. J Pain 14 (7), 731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Géranton SM et al. (2009) A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J Neurosci 29 (47), 15017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Megat S and Price TJ (2018) Therapeutic opportunities for pain medicines via targeting of specific translation signaling mechanisms. Neurobiol Pain 4, 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uttam S et al. (2018) eIF4E-Dependent Translational Control: A Central Mechanism for Regulation of Pain Plasticity. Front Genet 9, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moy JK et al. (2017) The MNK-eIF4E Signaling Axis Contributes to Injury-Induced Nociceptive Plasticity and the Development of Chronic Pain. J Neurosci 37 (31), 7481–7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kwon M et al. (2017) Inhibition of Mammalian Target of Rapamycin (mTOR) Signaling in the Insular Cortex Alleviates Neuropathic Pain after Peripheral Nerve Injury. Front Mol Neurosci 10, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shem K et al. (2018) Adverse cognitive effect of gabapentin in individuals with spinal cord injury: preliminary findings. Spinal Cord Ser Cases 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kamboj SK et al. (2005) The effects of immediate-release morphine on cognitive functioning in patients receiving chronic opioid therapy in palliative care. Pain 117 (3), 388–95. [DOI] [PubMed] [Google Scholar]
- 100.Lee JLC et al. (2017) An Update on Memory Reconsolidation Updating. Trends Cogn Sci 21 (7), 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zeidan F et al. (2012) Mindfulness meditation-related pain relief: evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett 520 (2), 165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kerns RD et al. (2011) Psychological treatment of chronic pain. Annu Rev Clin Psychol 7, 411–34. [DOI] [PubMed] [Google Scholar]
- 103.Adamczyk AK et al. (2020) The dynamics of pain reappraisal: the joint contribution of cognitive change and mental load. Cogn Affect Behav Neurosci 20 (2), 276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lazaridou A et al. (2017) Effects of Cognitive-Behavioral Therapy (CBT) on Brain Connectivity Supporting Catastrophizing in Fibromyalgia. Clin J Pain 33 (3), 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seminowicz DA et al. (2013) Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain 14 (12), 1573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feliu-Soler A et al. (2018) Current status of acceptance and commitment therapy for chronic pain: a narrative review. J Pain Res 11, 2145–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dindo L et al. (2018) Acceptance and Commitment Therapy for Prevention of Chronic Postsurgical Pain and Opioid Use in At-Risk Veterans: A Pilot Randomized Controlled Study. J Pain 19 (10), 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Husain M and Mehta MA (2011) Cognitive enhancement by drugs in health and disease. Trends Cogn Sci 15 (1), 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Millecamps M et al. (2007) D-cycloserine reduces neuropathic pain behavior through limbic NMDA-mediated circuitry. Pain 132 (1–2), 108–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schnitzer TJ et al. (2016) A randomized placebo-controlled pilot study of the efficacy and safety of D-cycloserine in people with chronic back pain. Mol Pain 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anacker C and Hen R (2017) Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci 18 (6), 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Malberg JE et al. (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20 (24), 9104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Papakostas GI et al. (2019) A phase 2, double-blind, placebo-controlled study of NSI-189 phosphate, a neurogenic compound, among outpatients with major depressive disorder. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Büchel C et al. (2014) Placebo analgesia: a predictive coding perspective. Neuron 81 (6), 1223–1239. [DOI] [PubMed] [Google Scholar]
- 115.Nesse RM and Schulkin J (2019) An evolutionary medicine perspective on pain and its disorders. Philos Trans R Soc Lond B Biol Sci 374 (1785), 20190288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tabor A and Burr C (2019) Bayesian Learning Models of Pain: A Call to Action. Current Opinion in Behavioral Sciences 26, 54–61. [Google Scholar]
- 117.Baliki MN and Apkarian AV (2015) Nociception, Pain, Negative Moods, and Behavior Selection. Neuron 87 (3), 474–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hebben N et al. (1985) Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H.M. Behav Neurosci 99 (6), 1031–9. [DOI] [PubMed] [Google Scholar]
- 119.Descarte R et al. (1664) L’homme et un Traitté de la ormation du Foetus du Mesme Autheur. Charles Angot. [Google Scholar]
- 120.Craig AD (2003) A new view of pain as a homeostatic emotion. Trends Neurosci 26 (6), 303–7. [DOI] [PubMed] [Google Scholar]