Abstract
Sialadenoma papilliferum (SP) is a rare benign tumor of the salivary glands, and only three unequivocal cases of SP arising in the bronchus have been reported. We herein describe the histomorphologic and molecular features of four bronchial SP cases and discuss the differential diagnosis of this entity and the relationship with its clinicopathological mimics, in particular, glandular papilloma and mixed squamous cell and glandular papilloma (GP/MP). We encountered 2 male and 2 female patients with bronchial SP (mean 66.8 years old). All four tumors arose in the central bronchus and were characterized by a combination of surface exophytic endobronchial papillary proliferation and a submucosal multicystic component with complex architecture. The neoplastic epithelium consisted predominantly of non-ciliated stratified columnar cells with ciliated, squamous, and mucinous cells present focally. While 2 tumors (50%) harbored a BRAF V600E mutation by molecular and immunohistochemical analysis, similar to GP/MP, no KRAS, HRAS, AKT1 or PIK3CA mutations were detected in any of the cases. Two patients were treated with limited resection, while two patients underwent lobectomy based on the diagnosis of adenocarcinoma or possible squamous cell carcinoma in situ in the preoperative biopsy. All survived without recurrence or metastasis for 23 to 122 months after treatment. SP can develop in the central bronchus as the bronchial counterpart of the salivary gland tumor and should be considered in the differential diagnosis of endobronchial tumors. In addition, some histological resemblance and frequent BRAF V600E mutation raise the possibility of SP and GP/MP being on the same disease spectrum.
Keywords: BRAF V600E mutation, bronchial tumor, glandular papilloma, mixed squamous cell and glandular papilloma, sialadenoma papilliferum
INTRODUCTION
Tracheobronchial tumors are uncommon but include a variety of benign and malignant conditions derived from the surface epithelium or submucosal glands or caused by direct bronchial invasion or metastasis from other organs.1, 2 While the vast majority of tracheobronchial tumors in adults are malignant including squamous cell carcinoma and adenoid cystic carcinoma,1 benign neoplasms can rarely occur, and the differential diagnosis is crucial for patient management.
Salivary gland-type tumors infrequently arise from the bronchial glands of the bronchial wall. Most of them are adenoid cystic carcinoma and mucoepidermoid carcinoma, but other benign and malignant tumor types, including epithelial-myoepithelial carcinoma, pleomorphic adenoma, and sialadenoma papilliferum (SP), have also been rarely reported.2-7 Of those, SP is considered to originate in an excretory duct of the salivary gland and is histologically characterized by a combination of surface exophytic papillary proliferation and a deeper multicystic intraductal papillary epithelial component.8-11 It is a benign tumor, but can be erroneously diagnosed as malignancy because of its irregular and complex architecture.10 While SP almost exclusively affects the intraoral minor salivary glands, a small number of cases have been described in the major salivary glands and submucosal glands of the nasal cavity and bronchus.5-8, 12, 13 Importantly, however, not all reported cases of SPs arising in the bronchus can be confirmed based upon the histologic images in the reports and given the necessity of evaluating by the architectural and cellular components of both superficial and submucosal components in the diagnosis SP. Recently, BRAF V600E mutations were reported in 75%-100% of salivary gland SP cases;10, 14 thus the presence of BRAF V600E mutations can support the diagnosis of SP; however, the genetic alterations associated with bronchial SP have not been investigated.
Glandular papilloma and mixed glandular and squamous papilloma (GP/MP) are benign bronchial tumors arising from the surface mucosa.15 They share some histological characteristics with SP including an endobronchial papillary epithelial growth pattern.16 Interestingly, BRAF V600E mutations have also been reported in a considerable number of GP/MP cases.17-19 These histologic and molecular similarities raise the possibility of SP and GP/MP being on the same disease spectrum.
We herein report the clinical, pathologic, immunohistochemical and molecular features of four cases of SP arising in the bronchus and discuss its distinction from and relationship with GP/MP as well as potential diagnostic pitfalls.
MATERIALS AND METHODS
Patient Selection and Histologic Review
This study was approved by the Institutional Review Board of each institution (2018-0190). Five expert head and neck pathologists (M.N., M.U., I.O., Y.H. and T.N.) performed a retrospective search to identify institutional and consultation cases with an initial diagnosis of “SP” or “papillary-cystic tumor” of the bronchus, and retrieved a total of four cases. One case has been previously reported elsewhere.5 Histological diagnoses were confirmed by 5 expert pathologists (M.N., M.M.-K., M.U., I.O., and T.N.) in accordance with the 2017 WHO Classification of Head and Neck Tumours9 and related studies.10, 13, 20 In addition to routine haematoxylin and eosin, periodic acid-Schiff and mucicarmine staining, immunohistochemistry was performed using the following antibody clones with Bond 3 automated immunostainer (Leica Microsystems, Bannockburn, IL) in accordance with the manufacturer’s recommendations: p63 (4A4, 1:100, Dako, Carpinteria, CA), α-smooth muscle actin (1A4, 1:100; Dako, Carpinteria, CA), BRAF V600E (VE-1, 1:50; Spring Bioscience, Pleasanton, CA), and TTF-1 (8G6G3/1, 1:300; Dako).
Mutation Analyses
To investigate the mutation status of key oncogenes, we performed polymerase chain reaction followed by Sanger sequencing.10 In brief, DNA from formalin-fixed paraffin-embedded tissue was extracted with a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). The tumor component of each unstained slide was carefully microdissected to increase tumor cellularity. Polymerase chain reaction products were purified using a QIAquick Spin Kit (Qiagen). Each purified product was directly sequenced using a forward primer with a BigDye Terminator v3.1 Cycle Sequencing Kit on an ABI 3730 instrument (Applied Biosystems Inc., Foster City, CA). Mutation analyses were carried out for 5 key oncogenes of the RAS-RAF-MEK-MAPK and PI3K-Akt pathways: BRAF (exon 15), AKT1 (exon 2), PIK3CA (exons 9 and 20), HRAS (exons 2 and 3) and KRAS (exons 2 and 3). The primer sequences are listed in the Supplementary Table S1 (Supplemental Digital Content 1).
RESULTS
The radiological, bronchoscopic, and histopathological features of each case are shown in Figs. 1-4.
FIGURE 1.
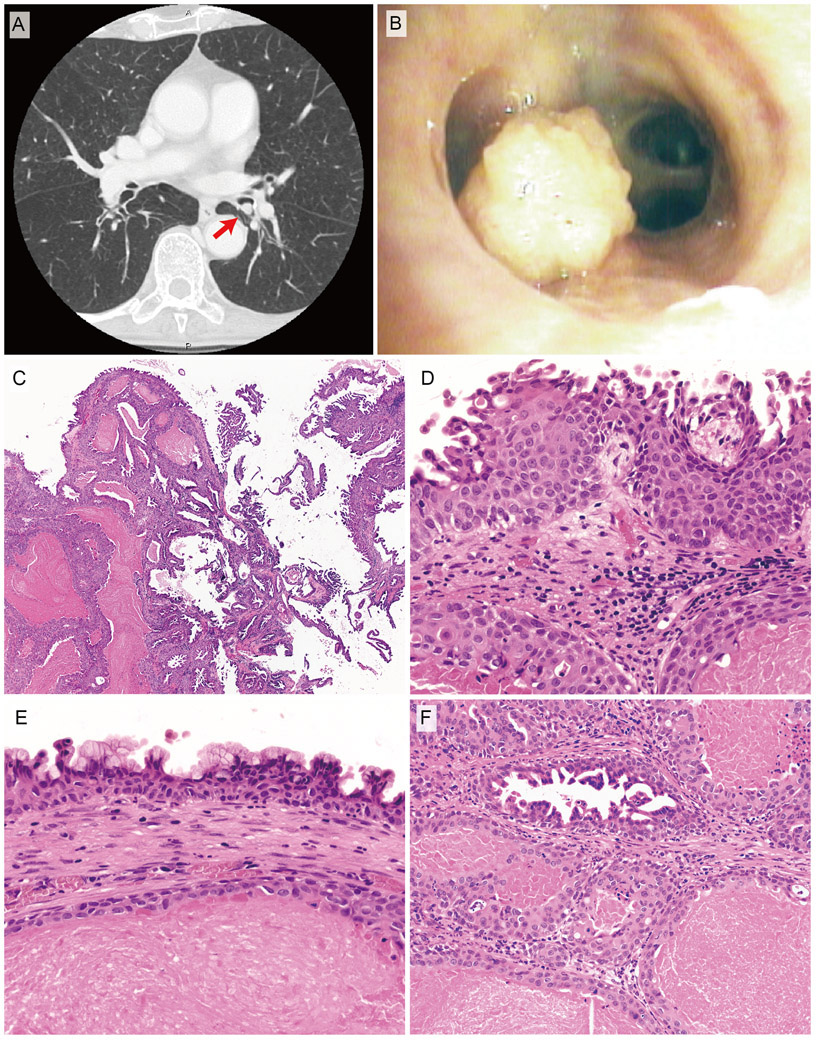
Radiologic, bronchoscopic, and histopathological features of bronchial sialadenoma papilliferum (case 1). Computed tomography reveals endobronchial consolidation mass attached to the bronchiolar wall (arrows) (A). Bronchoscopy shows a polypoid lesion with the papillary surface protruding into the bronchiolar lumen (B). C, Low-power view. The tumor consists of a combination of surface papillary proliferation (right portion) and a submucosal multicystic component (left portion). D, Surface squamous epithelium. E, Mucous cells in the surface epithelium. F, Submucosal multicystic component. The inner ductal epithelium cells display multiple intraluminal micropapillary configurations. Each lumen contains proteinaceous material.
Clinical Characteristics
The clinicopathologic characteristics of SP of the bronchus are summarized in Table 1. The patients were 2 males and 2 females, 52-77 years old (mean 66.8 years old). Tumors arose in the secondary to quaternary bronchus of the right (three cases) or left (one case) lung. The tumor diameter was 6-16mm (mean 9.5mm). Two patients presented with chest pain and the others were referred for abnormal chest X-ray findings. On chest computed tomography, the tumors were depicted as endobronchial polypoid lesions (Figs. 1A, 2A). Bronchoscopy revealed polypoid tumors arising in the bronchial walls (Figs. 1B, 2B). The preoperative biopsies of the study cohort rendered various diagnoses. The diagnosis of SP was made in one case (case 1). This case and another case with the diagnosis of atypical columnar papilloma (case 2) were treated with bronchoscopic resection. One patient had undergone lobectomy based on the preoperative diagnosis of adenocarcinoma (case 3). The biopsy of case 4 exhibited an atypical squamous proliferation from which the possibility of squamous cell carcinoma in situ could not be excluded. The surgeons opted sleeve lobectomy considering the tumor location and the possibility of malignancy. None of the 4 patients has experienced recurrence with a mean follow-up of 63.8 months (23-122 months).
TABLE 1.
Clinicopathologic Characteristics of Sialadenoma Papilliferum Cases of the Bronchus
| Case no. | Preoperative biopsy diagnosis |
Initial diagnosis of resected specimen |
Age (years) |
Sex | Anatomical site | Size (mm) |
Symptom | Treatment | Outcome/ Follow-up (mo) |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sialadenoma papilliferum | Sialadenoma papilliferum | 77 | F | Left lung, B6-basal bifurcation | 8 | Abnormal chest x-ray | Bronchoscopic resection | NED (23) | |
| 2 | Columnar papilloma, atypical | Sialadenoma papilliferum | 52 | F | Right lung, B3b | 6 | Chest pain, abnormal x-ray | Bronchoscopic resection | NED (74) | |
| 3 | Adenocarcinoma | Carcinoma of salivary gland type* | 63 | M | Right lung, B6a | 16 | NA | Lobectomy | NED (122) | |
| 4 | Atypical squamous proliferation** | Sialadenoma papilliferum | 75 | M | Right lung, Bu, eparterial bronchus | 8 | Chest pain, abnormal x-ray | Sleeve lobectomy | NED (36) | 5 |
| Previous case 1 | Benign papillary lesion | Sialadenoma papilliferum | 53 | M | Right lung, B6 | 18 | Abnormal chest x-ray | Lobectomy | NED (8) | 6 |
| Previous case 2 | Not diagnostic | Sialadenoma papilliferum | 66 | M | Right lung, middle lobe | 13 | Abnormal chest CT | Lobectomy | NED (36) | 7 |
The diagnosis made on the resection specimen at the referring laboratory. The diagnosis of sialadenoma papilliferum was confirmed by the consultant pathologist.
Case 4 showed significant basal cell hyperplasia/immature squamous metaplasia underneath the surface columnar epithelium that may have been captured in the biopsy and interpreted as atypical squamous proliferation.
CT indicates computed tomography; NA, not available; NED, no evidence of disease.
FIGURE 2.
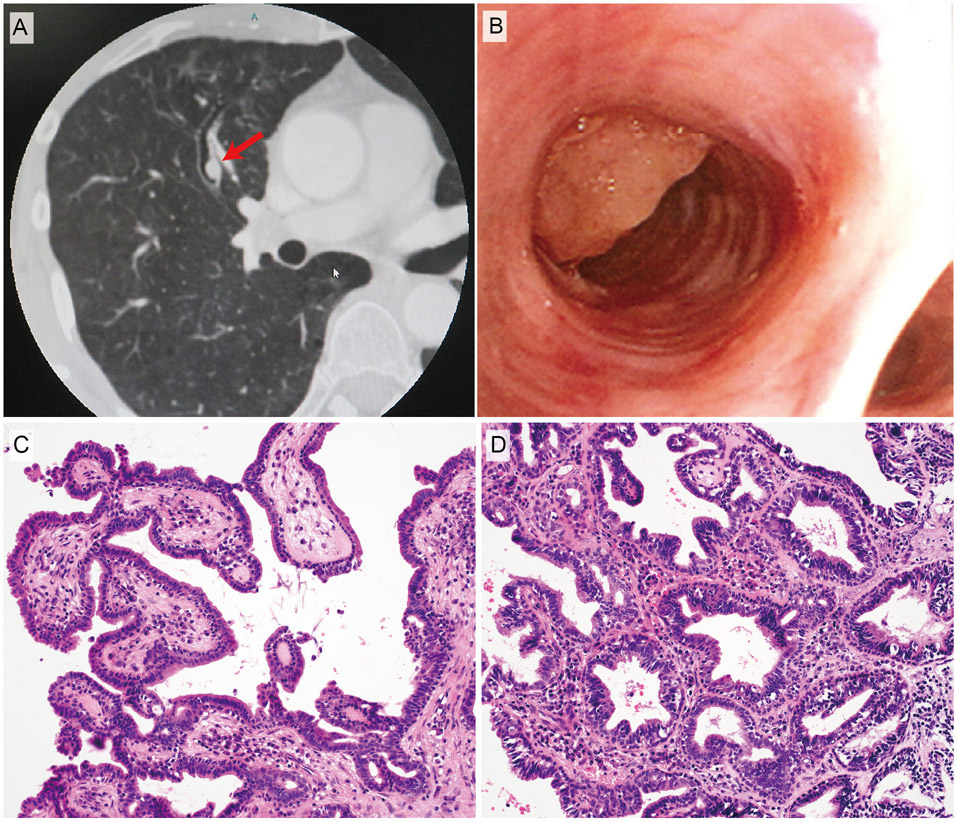
Radiologic, bronchoscopic, and histopathological features of case 2. A, Computed tomography image. B, Bronchoscopic findings. C, Exophytic papillary proliferation of columnar epithelium. D, Submucosal multicystic component with intraluminal papillary formations. The basal cells are cytologically bland and show an orderly linear arrangement.
Pathologic Characteristics
The histopathologic characteristics of the current cases are summarized in Table 2. The four cases showed similar histologic features. The tumors arose from the wall of the central bronchi, where submucosal gland and cartilage tissue were present (Figs. 3A, 4A). The tumors consisted of surface exophytic papillary proliferations as well as a submucosal multicystic component (Figs. 1C, 3A, 4A). On the tumor surface, the papillae were accompanied by a fibrovascular core with occasional irregular micropapillary epithelial tufts (Figs. 2C, 3B, 4B). Although most of the lining epithelium consisted of non-ciliated stratified columnar cells (Figs. 3B, 4B), ciliated columnar (Fig. 4C), squamous (Fig. 1D) or mucinous types of cells (Fig. 1E) were also focally present in some cases (Table 2). The ciliated columnar cells were cytologically bland and were present in a segment of the lining epithelium, and the transition from non-ciliated epithelium was abrupt suggestive of entrapped non-neoplastic cells (Fig. 4C). Case 4 showed significant basal cell hyperplasia/immature squamous metaplasia with a monotonous appearance and mitotic activity (Fig. 4B). The fibrovascular stroma of the papillae was accompanied by a moderate degree of chronic inflammatory infiltrates consisting primarily of lymphocytes.
TABLE 2.
Histologic and Molecular Characteristics of Sialadenoma Papilliferum Cases of the Bronchus
| Histology |
Epithelium |
Nuclear atypia | Immunohistochemistry |
Gene mutation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no. |
Exophytic papillary component |
Multicystic component |
Non-ciliated columnar |
Ciliated | Squamous | Mucinous | TTF-1 | BRAF V600E | Ki-67 | ||
| 1 | (+) | (+) | (+) | (+) | (+) | (+) | Mild to moderate | (−) | weakly (+) | 5% | BRAF V600E |
| 2 | (+) | (+) | (+) | (−) | (−) | (+) | Mild | (−) | ND | 1% | (−) |
| 3 | (+) | (+) | (+) | (−) | (−) | (+) | Moderate | (−) | ND | 3% | (−) |
| 4 | (+) | (+) | (+) | (+) | (+) | (+) | Mild to moderate | (−) | (+) | 3% | BRAF V600E |
ND indicates no data.
FIGURE 3.
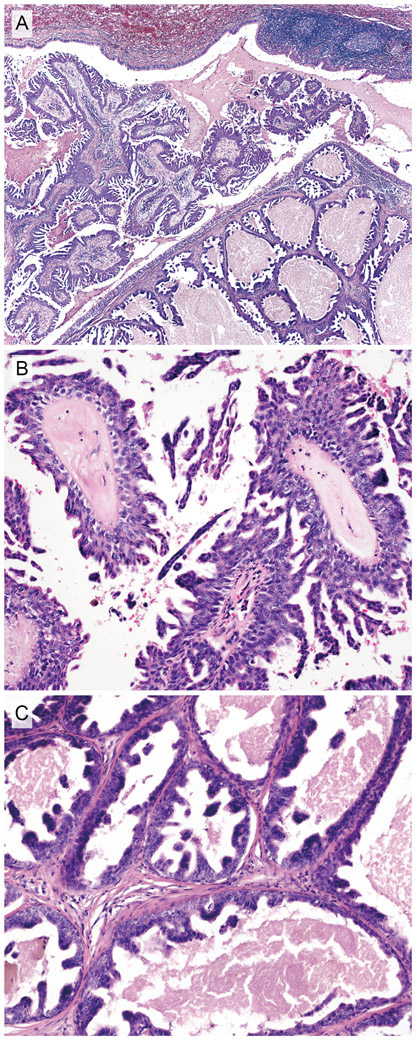
Histopathological features of case 3. A, Low-power view. Endobronchial exophytic papillary fronds (left portion) and submucosal multicystic structures (right portion). B, Exophytic papillary component. Irregular micropapillary epithelial tufts are present on the surface. C, Submucosal multicystic component. Inner ductal epithelium forms multiple irregular papillary projections. Each lumen contains proteinaceous material.
FIGURE 4.
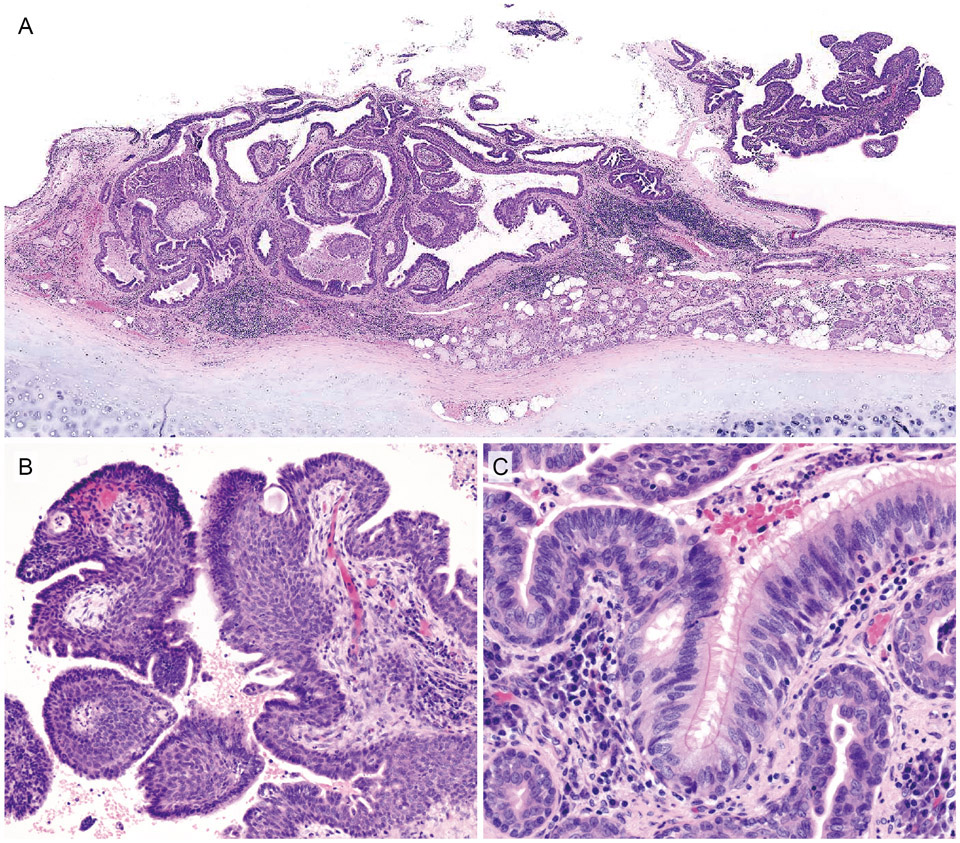
Histopathological features of case 4. A, Low-power view. The tumor is situated adjacent to the bronchial gland in the central bronchus. The tumor consists of a combination of partly detached surface papillary proliferation and a submucosal multicystic component. B, Exophytic papillary proliferation accompanied by a fibrovascular core. Significant basal cell hyperplasia/immature squamous metaplasia underneath the surface columnar epithelium is noted. C, On the exophytic component, the surface stratified columnar epithelium abruptly changed from the non-ciliated type to the ciliated type, resembling normal respiratory epithelium. Note the moderate chronic inflammatory cell infiltrates in the subepithelial stroma.
The deeper portion of the tumor appeared multicystic composed of dilated ducts filled with proteinaceous or necrotic material (Figs. 1F, 3C). The inner ductal epithelial cells formed multiple intraluminal micropapillary tufts without a fibrovascular core (Figs. 1F, 2D, 3C). The ductal epithelial proliferations were largely similar to those of the surface components, but devoid of ciliated and squamous cells. A few mucous cells, which were inconspicuous in routine HE sections but were highlighted by mucicarmine stain, were also seen. The tumor cell nuclei showed mild to moderate nuclear atypia with rare mitotic figures (Fig. 4C). The basal cells present in the surface and ductal epithelium were cytologically bland and showed an orderly linear arrangement (Fig. 1F, 2D, 3C, 4C).
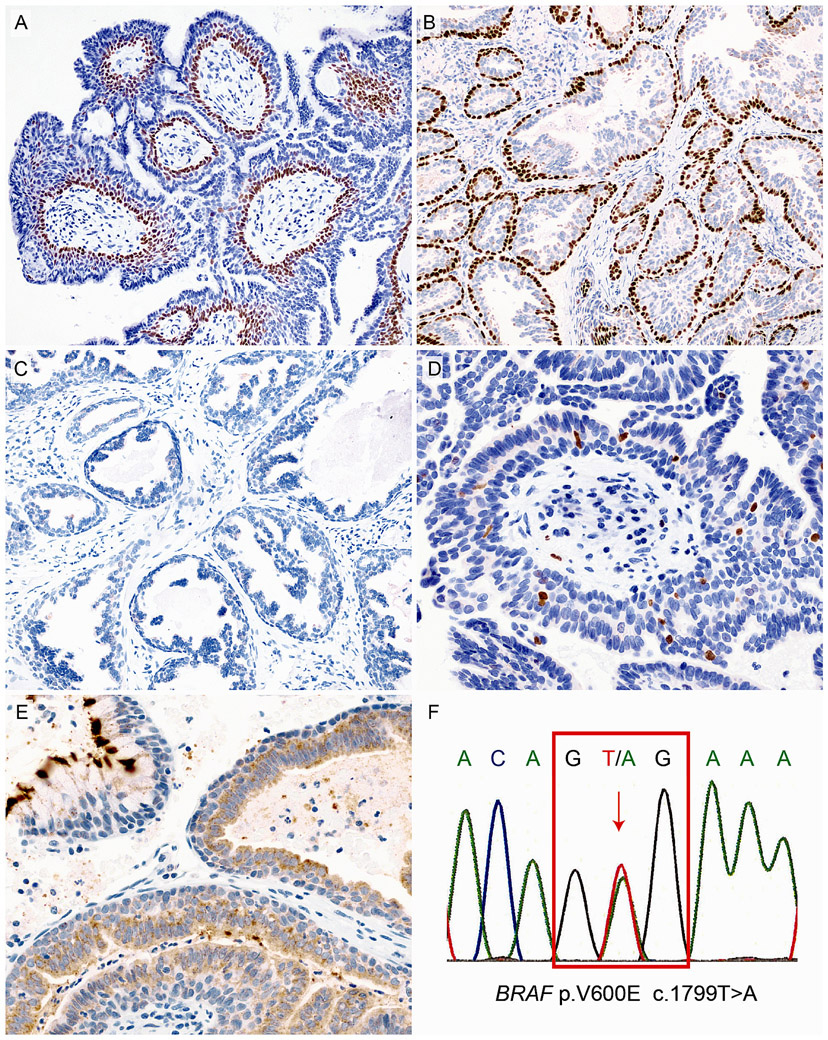
Immunohistochemically, the basally located cells of both the exophytic and multicystic components expressed p63 (Figs. 5A, B) but not α-smooth muscle actin, suggesting that the cells were basal cells rather than myoepithelial cells. Neither columnar epithelial cells nor basal cells in each component showed TTF-1 expression (Fig. 5C). The Ki-67 labeling index ranged from 1% to 5% (Fig. 5D). Two cases were positive for BRAF V600E-specific immunohistochemistry (Fig. 5E). While non-ciliated columnar, squamous, and mucous cells were immunoreactive for BRAF V600E, rimming basal cells were not. The surface cilia of ciliated columnar cells exhibited strong cross-reactivity to BRAF V600E antibody, but cytoplasmic expression was not observed (Fig. 5E).21
FIGURE 5.
Immunohistochemical and molecular features of bronchial sialadenoma papilliferum. p63-positive basal cells are evident in the exophytic papillary epithelium (A) and multicystic component (B) (Case 4 and case 1, respectively). No TTF-1-positive cells are found in the tumor (C, Case 3). Ki-67-positive cells are scattered, and the labeling index is <1% (D, Case 4). Positive immunoreactivity for BRAF V600E is observed in the inner ductal epithelial cells. Cross-reactivity of the cilia is noted (E, case 4). Sanger sequencing of case 1 showing a BRAF V600E mutation (F).
Molecular Features
Mutational analysis revealed BRAF V600E mutations in 2 of 4 cases (50%) corresponding to the results of BRAF V600E immunohistochemistry (Fig. 5F). Conversely, no AKT1, PIK3CA, HRAS or KRAS mutations were detected. No significant histological differences were found between tumors in relation to the mutation status.
DISCUSSION
We investigated the histologic and molecular features of four SP cases in the bronchus. SP is an extremely rare tumor even in the salivary gland. In addition to one case included in the current cohort,5 only two convincing cases have previously been reported in the bronchus (Table 1).6, 7 Likely due to its extreme rarity, SP is not currently included in the “salivary gland-type tumors” section of the 4th edition of WHO Classification of Tumours of the Lung, Pleura, Thymus, and Heart.22 Furthermore, genetic alterations in bronchial SP have not been reported.
The most common tracheobronchial tumor is squamous cell carcinoma and adenoid cystic carcinoma.1 Although these tumors may have similar clinical and radiological presentations to SP, the histomorphology differs markedly among these lesions. The main differential diagnosis of bronchial SP is GP/MP, but there is some confusion regarding the diagnostic categorization between these two entities. The superficial portion of both SP and GP/MP exhibits papillary growth of columnar epithelium accompanied by a basal cell layer,19 while the histological features of these two entities differ in several aspects. First, the submucosal multicystic component, which consists of dilated ducts with intraductal micropapillary epithelial tufts, is essential for a diagnosis of SP but is not a feature of GP/MP. The presence of a rimming of bland basal cells in the ducts of bronchial SP, which are positive for p63 and negative for α-smooth muscle actin, is comparable to that of SP of salivary gland origin and suggests its excretory duct derivation.10, 11 Second, the tumor location differs slightly between SP and GP/MP. While GP/MP can occur in both central and peripheral bronchi,16 all SPs in the previously reported and current cases arose in the central bronchi.5-7 Given that peripheral bronchi lack submucosal glandular tissue, SP may be limited in its development to the central bronchi. Third, the composition of the epithelium in SP differs from that in GP/MP. GP/MP is considered to originate from the bronchial epithelium; thus, it is plausible to consider that the epithelial tumor components of this entity are mostly made up of a mixture of ciliated columnar cells and mucous cells.16, 19, 23 In contrast, in the current cases of SP, the surface papillary epithelium was primarily composed of non-ciliated columnar cells with ciliated, mucous, and squamous cells present focally. In addition, the ciliated cells identified in the current SP cases were cytologically bland, well-circumscribed with an abrupt transition to the non-ciliated cells and negative for BRAF V600E cytoplasmic expression even in BRAF-mutated cases; thus, they likely represent non-neoplastic cells entrapped in the tumor proliferation.
A neoplastic lesion in the peripheral lung parenchyma with papillary architecture composed of ciliated columnar, mucous, and basal cells was initially designated as ciliated muconodular papillary tumor (CMPT).24 The term “bronchiolar adenoma” was later proposed to extend the disease spectrum of CMPT.25 Recently, based on the histologic similarity and frequent BRAF V600E mutations, (at least peripheral) GP/MP was suggested to be a part of the bronchiolar adenoma spectrum.17 The reported prevalence of BRAF V600E mutations in bronchiolar adenoma and GP/MP was 38%-40% and 57%, respectively.19, 25-27 Now we report BRAF V600E mutations in 2 of the 4 SP cases. Considering the bronchial gland development, where a bud from respiratory epithelium invaginates, elongates, and finally forms the bronchial gland,28 SP might fall within the same disease spectrum as GP/MP and bronchiolar adenoma. However, SP is suspected to originate from an excretory duct of the bronchial gland present just below an orifice to the surface epithelium, as the tumor is composed of both surface epithelial proliferation and a submucosal multicystic component.10, 11 While bronchial SP is considered analogous to salivary SP, these may not necessarily be identical entities, as the covering/surface epithelium differs between the salivary glands and bronchial gland (squamous epithelium and ciliated stratified columnar epithelium, respectively).
Of note, genetic alterations reported in CMPT or bronchiolar adenoma include EGFR, KRAS, HRAS, ALK and AKT1, in addition to BRAF.26, 27, 29, 30 In the current study, however, none of the tumors harbored KRAS, HRAS, AKT1 or PIK3CA mutations. The mutation frequency in the present cohort was lower than that in the salivary gland counterpart, but the precise rate is difficult to estimate because of the small number of cases analyzed. With wider recognition of the disease and the accumulation of more cases, further studies will be required in order to delineate the genetic characteristics of bronchial SP precisely.
Although SP is considered a benign tumor, there were several histomorphologic features that might prompt confusion with a malignancy. The complex architecture of the epithelial tufts resembles the filigree pattern of micropapillary adenocarcinoma of the lung.31 In addition, the tumor cells exhibited mildly to moderately atypical nuclei with rare mitoses in both the previously reported and current cases.10 Indeed, two of our cases were classified as adenocarcinoma and possible squamous cell carcinoma in situ at the initial biopsy leading to pulmonary lobectomy. To avoid the misinterpretation of bronchial SP as malignant or pre-malignant lesions, it is important to recognize that the tumor is located in the central bronchus, proliferates with a combination of a surface exophytic endobronchial papillary configuration and a submucosal multicystic component, and has evidence of an intraductal nature, as confirmed by the presence of p63-positive basal cell rimming.
Malignant transformation of SP in the salivary glands has been described in some case reports;32-34 however, such reports did not show unequivocal evidence to support the diagnosis of SP or malignancy.13 Interestingly, in a recent report, adenocarcinoma arising in “mixed squamous and glandular papilloma” of the lung was diagnosed based on the presence of anaplasia and invasion beyond the bronchial cartilage;35 however, the provided figures only show the tumor exhibiting a multicystic intraductal papillary growth without foci of invasion raising the possibility of SP. The follow-up data for the case was not included in the report.35 In addition, none of the patients in our cohort experienced recurrence or metastases during a follow-up of over two years. Thus, we believe that bronchial SP primarily demonstrates a benign clinical course, although the possibility of low-grade malignancy cannot be completely excluded.
In conclusion, SP can arise in the bronchus as the bronchial counterpart of the salivary gland tumor and frequently harbors a BRAF V600E mutation. Given its complex architecture, bronchial SP may be misinterpreted as malignancy, especially in a small biopsy and treated as such; thus, it is important to consider SP in the differential diagnosis of endobronchial tumors. Given the histological resemblance and common genetic alterations, SP might fall within the same disease spectrum as GP/MP and bronchiolar adenoma.
Supplementary Material
Supplemental Digital Content 1. Supplementary Table 1:
PCR Primers Used for Sanger Sequencing pdf
ACKNOWLEDGEMENTS
MN, MMK, MU, IO, and NT contributed to the conception and pathological evaluation. HH and TN performed the genetic analysis. MN, MMK and TN drafted the manuscript. All authors contributed to the scientific discussion and final approval of the manuscript.
The authors thank Yoshinari Yamamoto, Mayumi Yokotsuka and Hitomi Yokota, Tokyo Medical University, for their technical assistance.
Footnotes
Conflicts of Interest and Source of Funding:
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Park CM, Goo JM, Lee HJ, et al. Tumors in the tracheobronchial tree: CT and FDG PET features. Radiographics. 2009;29:55–71. [DOI] [PubMed] [Google Scholar]
- 2.Stevic R, Milenkovic B. Tracheobronchial tumors. J Thorac Dis. 2016;8:3401–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resio BJ, Chiu AS, Hoag J, et al. Primary Salivary Type Lung Cancers in the National Cancer Database. Ann Thorac Surg. 2018;105:1633–1639. [DOI] [PubMed] [Google Scholar]
- 4.Falk N, Weissferdt A, Kalhor N, et al. Primary Pulmonary Salivary Gland-type Tumors: A Review and Update. Adv Anat Pathol. 2016;23:13–23. [DOI] [PubMed] [Google Scholar]
- 5.Honda Y, Shiraishi K, Nomori H, et al. Sialadenoma papilliferum of the bronchus: rare tracheobronchial tumor of salivary gland type. Pathol Int. 2009;59:572–576. [DOI] [PubMed] [Google Scholar]
- 6.Bobos M, Hytiroglou P, Karkavelas G, et al. Sialadenoma papilliferum of bronchus. Virchows Arch. 2003;443:695–699. [DOI] [PubMed] [Google Scholar]
- 7.Campisi A, Dell'Amore A, Bertolaccini L, et al. Sialadenoma papilliferum of the bronchus: a rare tumour of salivary gland origin. Adv Respir Med. 2020;88:267–270. [DOI] [PubMed] [Google Scholar]
- 8.Abrams AM, Finck FM. Sialadenoma papilliferum. A previously unreported salivary gland tumor. Cancer. 1969;24:1057–1063. [DOI] [PubMed] [Google Scholar]
- 9.Foschini MP, Bell D, Katabi N. Sialadenoma papilliferum. In: El-Naggar AK, Chan JKC, Grandis JR, et al. , eds. WHO Classification of Head and Neck Tumours. Lyon: International Agency for Research on Cancer; 2017:192. [Google Scholar]
- 10.Nakaguro M, Urano M, Ogawa I, et al. Histopathological evaluation of minor salivary gland papillary-cystic tumours: focus on genetic alterations in sialadenoma papilliferum and intraductal papillary mucinous neoplasm. Histopathology. 2020;76:411–422. [DOI] [PubMed] [Google Scholar]
- 11.Maiorano E, Favia G, Ricco R. Sialadenoma papilliferum: an immunohistochemical study of five cases. J Oral Pathol Med. 1996;25:336–342. [DOI] [PubMed] [Google Scholar]
- 12.Gera M, Tan A. Sialadenoma papilliferum arising in the nasal cavity: immunohistochemical analysis. Pathology. 2017;49:94–95. [DOI] [PubMed] [Google Scholar]
- 13.Fowler CB, Damm DD. Sialadenoma Papilliferum: Analysis of Seven New Cases and Review of the Literature. Head Neck Pathol. 2018;12:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh MS, Bishop JA, Wang YP, et al. Salivary Sialadenoma Papilliferum Consists of Two Morphologically, Immunophenotypically, and Genetically Distinct Subtypes. Head Neck Pathol. 2020;14:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flieder DB, Nicholson AG, Travis WD, et al. Papillomas. In: Travis WD, Brambilla E, Burke AP, et al. , eds. WHO classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer; 2015:106–109. [Google Scholar]
- 16.Flieder DB, Koss MN, Nicholson A, et al. Solitary pulmonary papillomas in adults: a clinicopathologic and in situ hybridization study of 14 cases combined with 27 cases in the literature. Am J Surg Pathol. 1998;22:1328–1342. [DOI] [PubMed] [Google Scholar]
- 17.Huang YL, Chang YL, Chen KC, et al. Mixed squamous cell and glandular papilloma of the lung: A case report of a novel mutation in the BRAF gene and coexistent HPV infection, possible relationship to ciliated muconodular papillary tumor. Pathol Int. 2019;69:104–109. [DOI] [PubMed] [Google Scholar]
- 18.Kitawaki Y, Fujishima F, Taniuchi S, et al. Coexistence of glandular papilloma and sclerosing pneumocytoma in the bronchiole. Pathol Int. 2018;68:425–430. [DOI] [PubMed] [Google Scholar]
- 19.Lin DL, Xing XM, Ran WW, et al. Pulmonary peripheral glandular papilloma and mixed squamous cell and glandular papilloma frequently harbour the BRAF V600E mutation. Histopathology. 2020;76:997–1004. [DOI] [PubMed] [Google Scholar]
- 20.Brannon RB, Sciubba JJ, Giulani M. Ductal papillomas of salivary gland origin: A report of 19 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:68–77. [DOI] [PubMed] [Google Scholar]
- 21.Jones RT, Abedalthagafi MS, Brahmandam M, et al. Cross-reactivity of the BRAF VE1 antibody with epitopes in axonemal dyneins leads to staining of cilia. Mod Pathol. 2015;28:596–606. [DOI] [PubMed] [Google Scholar]
- 22.WHO classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer; 2015. [Google Scholar]
- 23.Aida S, Ohara I, Shimazaki H, et al. Solitary peripheral ciliated glandular papillomas of the lung: a report of 3 cases. Am J Surg Pathol. 2008;32:1489–1494. [DOI] [PubMed] [Google Scholar]
- 24.Kamata T, Yoshida A, Kosuge T, et al. Ciliated muconodular papillary tumors of the lung: a clinicopathologic analysis of 10 cases. Am J Surg Pathol. 2015;39:753–760. [DOI] [PubMed] [Google Scholar]
- 25.Chang JC, Montecalvo J, Borsu L, et al. Bronchiolar Adenoma: Expansion of the Concept of Ciliated Muconodular Papillary Tumors With Proposal for Revised Terminology Based on Morphologic, Immunophenotypic, and Genomic Analysis of 25 Cases. Am J Surg Pathol. 2018;42:1010–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamata T, Sunami K, Yoshida A, et al. Frequent BRAF or EGFR Mutations in Ciliated Muconodular Papillary Tumors of the Lung. J Thorac Oncol. 2016;11:261–265. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Aesif SW, Kipp BR, et al. Ciliated Muconodular Papillary Tumors of the Lung Can Occur in Western Patients and Show Mutations in BRAF and AKT1. Am J Surg Pathol. 2016;40:1631–1636. [DOI] [PubMed] [Google Scholar]
- 28.May A, Tucker A. Understanding the development of the respiratory glands. Dev Dyn. 2015;244:525–539. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi R, Higuchi K, Sudo M, et al. A case of anaplastic lymphoma kinase (ALK)-positive ciliated muconodular papillary tumor (CMPT) of the lung. Pathol Int. 2017;67:99–104. [DOI] [PubMed] [Google Scholar]
- 30.Jin Y, Shen X, Shen L, et al. Ciliated muconodular papillary tumor of the lung harboring ALK gene rearrangement: Case report and review of the literature. Pathol Int. 2017;67:171–175. [DOI] [PubMed] [Google Scholar]
- 31.Emoto K, Eguchi T, Tan KS, et al. Expansion of the Concept of Micropapillary Adenocarcinoma to Include a Newly Recognized Filigree Pattern as Well as the Classical Pattern Based on 1468 Stage I Lung Adenocarcinomas. J Thorac Oncol. 2019;14:1948–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ide F, Kikuchi K, Kusama K, et al. Sialadenoma papilliferum with potentially malignant features. J Clin Pathol. 2010;63:362–364. [DOI] [PubMed] [Google Scholar]
- 33.Shimoda M, Kameyama K, Morinaga S, et al. Malignant transformation of sialadenoma papilliferum of the palate: a case report. Virchows Arch. 2004;445:641–646. [DOI] [PubMed] [Google Scholar]
- 34.Solomon MP, Rosen Y, Alfonso A. Intraoral papillary squamous cell tumor of the soft palate with features of sialadenoma papilliferum? malignant sialadenoma papilliferum. Cancer. 1978;42:1859–1869. [DOI] [PubMed] [Google Scholar]
- 35.An AR, Park SY, Kim JH, et al. Adenocarcinoma-Papillary Cystic Pattern Arising in a Mixed Squamous and Glandular Papilloma of the Lung. Int J Surg Pathol. 2020;28:658–662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Supplementary Table 1:
PCR Primers Used for Sanger Sequencing pdf