Abstract
Most children with ADHD have impaired working memory abilities. These working memory deficits predict impairments in activities of daily living (ADLs) for adults with ADHD. However, our understanding of the relation between pediatric ADHD and ADLs is limited. Thus, this study aimed to examine (1) the extent to which pediatric ADHD is associated with ADL difficulties; and if so (2) the extent to which these difficulties are related to their well-documented working memory difficulties and/or core ADHD inattentive and hyperactive/impulsive symptom domains. A well-characterized, clinically evaluated sample of 141 children ages 8–13 years (M=10.36, SD=1.46; 51 girls; 70% White/non-Hispanic) were administered a battery of well-validated working memory tests and assessed for ADHD symptoms (teacher-ratings) and ADL difficulties (parent-ratings); cross-informant reports were used to control for mono-informant bias. Children with ADHD exhibited medium magnitude difficulties with ADLs (d=0.61, p<.005, 38% impaired). Results of the bias-corrected, bootstrapped conditional effects model indicated that lower working memory predicted reduced performance of age-expected ADLs (β=.28) and greater ADHD inattentive (β=−0.40) and hyperactive/impulsive symptoms (β=−0.16). Greater inattentive, but not hyperactive/impulsive, symptoms predicted greater ADL difficulties (β=−0.36) even after controlling for working memory. Interestingly, working memory exerted a significant indirect effect on ADLs via inattentive (indirect effect: β=.15, effect ratio=.54) but not hyperactive/impulsive symptoms. These findings implicate ADHD inattentive symptoms as a potential mechanism underlying ADL difficulties for children with ADHD, both independently and via working memory’s role in regulating attention.
Keywords: ADHD, activities of daily living, ADL, working memory, executive function
Activities of daily living (ADLs) is a broad term used to describe life tasks fundamental for supporting self-care and self-maintenance (Lawton & Brody, 1969). Initial definitions of ADLs encompassed a wide range of functions including locomotion, traveling, dressing, toileting, and eating (Mlinac & Feng, 2016). In clinical practice, difficulties with ADLs are often a qualitative marker of declines in cognitive processes such as working memory, stemming from the association between ADLs and executive functioning (Thames et al., 2011; Vaughan & Giovanello, 2010; Woods et al., 2008). ADLs have historically been used as a classifier to assess disability status among older adults (Katz, 1983); however, they are becoming more commonly used in assessing functional impairment in children as well (Blank et al., 2008; Cook et al., 2018; Duncan & Bishop, 2015; James et al., 2014; Thaler et al., 2012). Furthermore, research on age-related changes in ADL abilities (Hayase, et al., 2004) emphasizes the utility of ADL definitions that encompass skills in accordance with developmental age. For example, basic/personal ADL tasks (e.g., dressing, personal hygiene) are more commonly executed in younger children whereas adolescents increasingly perform more complex ADLs independently (e.g., preparing meals, completing household chores; James et al., 2014; Summers et al., 2008). To date, ADLs have been extensively investigated in adults with neurodegenerative disorders and in children with chromosomal and/or neurological disorders (James et al., 2014; Vaughan & Giovanello, 2010; Woods et al., 2008); however, only more recently have ADLs been investigated in children with neurodevelopmental disorders (Cook et al., 2018; Duncan & Bishop, 2015; Thaler et al., 2012).
Working Memory and ADL Performance
Results from the adult and geriatric literature on associations between ADLs and measures of cognition suggest deficits in ADLs are associated with both mild and severe degrees of cognitive decline (Jekel et al., 2015; Ranhoff, 1997; Vaughan & Giovanello, 2010; Woods et al., 2008), with more severe impairments in cognitive functioning relating to increased impairments in ADL performance (Arling & Williams, 2003; Tomaszewski Farias et al., 2009). Working memory has been increasingly indicated as an important executive function implicated in functional outcomes such as ADLs (Vaughan & Giovanello, 2010). For example, evidence suggests that working memory is a significant predictor of ADL performance in adults (Vaughan & Giovanello, 2010) and that training working memory abilities may positively impact ADL performance in older adults (Cantarella et al., 2017). Working memory, one of the three core executive functions (Miyake et al., 2000), refers to the active, top-down manipulation of information held in short-term memory (Baddeley, 2007), and includes interrelated functions of the mid-lateral prefrontal cortex and interconnected networks that involve dual-processing, updating, and reordering (Nee et al., 2013; Wager & Smith, 2003).
ADLs and Neurodevelopmental Disorders
Though ADLs have been widely studied in aging populations (Martyr & Clare, 2012), the literature has only recently begun to examine ADLs in pediatric populations. This paucity of research is surprising given that (a) several neurodevelopmental disorders have been associated with impaired executive functioning, which in turn has been linked with ADL difficulties in adults (e.g., Barkley & Murphy, 2010); and (b) childhood reflects the developmental period during which foundational ADLs are explicitly taught and learned (Demetriou et al., 2018; Kingdon et al., 2016). Research investigating ADL impairment and executive functioning in child and/or adolescent populations is mostly limited to samples of children with chromosomal and/or neurological disorders. For example, impaired ADL performance is well-documented in children diagnosed with Down’s Syndrome, Cerebral Palsy, and Developmental Coordination Disorder (DCD); although their ADL impairments appear to be associated largely with fine and gross motor delays rather than impairments in working memory or related executive functions (Summers et al., 2008; Volman et al., 2006; Magalhães et al., 2011; Wang et al., 2012). In contrast, research investigating ADL skills in neurotypical children suggests that age is the most significant predictor of ADL performance (Rosenberg, 2015). Research examining the association between executive functions, particularly working memory, and impairments in ADLs in children with neurodevelopmental disorders is limited, with most studies examining this relation in children with autism spectrum disorder and intellectual disability (Duncan & Bishop, 2015; Gantschnig et al., 2011).
However, pediatric attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder with broad and replicated evidence linking it with executive function impairments, particularly in working memory (Biederman et al., 2004; Kofler, Irwin et al., 2018; Nigg et al., 2005). Cross-sectional evidence indicates cortical underdevelopment in working memory-related brain regions in children with ADHD (for review, see Rapport et al., 2013) while longitudinal evidence indicates 3–5 year delays in cortical maturation of these regions in pediatric ADHD samples (Shaw et al., 2007). Thus, the lack of understanding among the interplay of working memory and ADLs in pediatric ADHD is concerning particularly given that research has shown that executive functions such as working memory (Vaughan & Giovanello, 2010) significantly predict ADL performance in other populations. It is important to investigate ADLs in this population given the well-established link between executive functioning and ADLs (de Paula et al., 2015; Jekel et al., 2015; Ranhoff, 1997; Vaughan & Giovanello, 2010; Woods et al., 2008). Taken together, the relation between deficits in working memory and ADL performance in pediatric ADHD is an understudied and potentially important area of research for understanding the influence of this disorder on children’s day to day functioning. Thus, this study aims to contribute to the pediatric literature by examining the association between working memory and ADL performance in children with ADHD.
ADLs and ADHD
ADHD is a neurodevelopmental disorder affecting approximately 5% of school-age children (Polanczyk et al., 2007, 2014). To date, the literature on ADLs in pediatric ADHD has primarily examined children’s disruptive behaviors and the quality of parent-child interactions during the performance of ADLs (Theule et al., 2013; Whalen, et al., 2006), rather than these children’s skill at independently performing ADLs. Furthermore, there is a surprising paucity of studies examining the relations between working memory or related executive functions and ADL performance in pediatric ADHD. Thus, to our knowledge no studies have examined whether childhood ADHD is associated with ADL impairments and, if so, whether those impairments are also linked with these children’s well-documented working memory deficits. These gaps in the literature are important to address given that prior literature indicates direct relations between working memory/related cognitive deficits and impairments in ADLs among children with other neurodevelopmental disorders including autism spectrum disorder and intellectual disability (Duncan & Bishop, 2015; Gantschnig et al., 2011; Gilotty et al., 2002; Kottorp et al., 2003). In addition, measures of working memory and related executive functions predict ADL performance for adults with ADHD both cross-sectionally (Barkley & Murphy 2010; Stavro et al., 2011) and longitudinally (Barkley & Fischer, 2011). These findings suggest that working memory may be an underlying causal mechanism for difficulties in ADL performance among adults with ADHD. However, due to differing symptom presentations across disorders and developmental demands in adulthood versus childhood (Weiss & Hechtman, 1993), further investigations are needed to examine whether these relations exist in pediatric ADHD and, if so, the extent to which the disorder’s well-documented working memory deficits (e.g., Kofler, Irwin et al., 2018) negatively impact ADL performance for these children.
Working Memory Impairments in ADHD: Associations with Functional Outcomes
Experimental and longitudinal evidence indicates that ADHD’s phenotypic behavioral presentation is likely driven, at least in part, by deficits in working memory (Rapport et al., 2009; Karalunas et al., 2017; Kofler et al., 2015). Working memory deficits in ADHD are well established, with meta-analytic evidence suggesting large effect sizes (d=2.01–2.15; Kasper et al., 2012). Recent literature suggests that a majority of children with ADHD display impairments in at least one executive function, with the largest proportion of children with ADHD exhibiting deficits in working memory abilities at a prevalence rate of 62% to 85% (Fosco et al., 2020; Karalunas et al., 2017; Kasper et al., 2012; Kofler et al., 2018; Sonuga-Barke et al., 2008).
In addition to the replicated evidence for a causal role of working memory deficits on core ADHD behavioral symptoms (e.g., Kofler et al., 2010), these deficits have also been associated experimentally and cross-sectionally with functional impairments across academic, organizational, peer, and family outcomes. The relation between working memory impairments and academic outcomes has been given considerable attention in the pediatric ADHD literature with studies establishing an association (Arnold et al., 2020; Friedman et al., 2016, 2017; Simone et al., 2018), as well as causal evidence (Kofler, Spiegel et al., 2018), between working memory and underachievement in reading and math. Working memory also predicts parent and teacher ratings of organizational difficulties in children with ADHD (Kofler, Sarver et al., 2018). In addition to academic outcomes, replicated evidence indicates that ADHD-related working memory impairments predict social problems (Bunford et al., 2014; Hoza, 2007; Kofler, Harmon et al., 2018). Thus, current literature suggests working memory is an important underlying mechanism both associated with and predictive of functional outcomes for ADHD. Yet, very limited studies have examined the relation between working memory and functional outcomes outside of the academic and peer domains, despite early evidence of working memory’s association with family functioning (Mares et al., 2007; Kofler, Sarver, et al., 2016). Towards addressing this gap in the ADHD literature, an examination of ADLs is important, as ADLs are diffuse in the home setting and consequently may provide meaningful insight into children with ADHD’s functioning at home.
Current Study
Taken together, there is well-documented evidence that (1) most children with ADHD have working memory deficits (Fosco et al., 2020; Kasper et al., 2012); (2) adults with ADHD exhibit difficulties performing ADLs (Barkley & Murphy 2010; Stavro et al., 2011), with these difficulties linked with their deficits in executive functions such as working memory (Barkley & Fischer, 2011); and (3) working memory deficits are associated with difficulties independently performing ADLs in adulthood (Vaughan & Giovanello, 2010). However, it is not known whether (1) children with ADHD show impairments in ADL performance, and (2) if so, if deficits in working memory are an underlying mechanism for this population. To address these omissions, the current study combined cross-informant ratings of ADHD symptoms and ADL performance with children’s performance on a battery of well-validated working memory tests (Alderson et al., 2013; Kofler, Irwin et al., 2018; Miyake et al., 2000; Rapport et al., 2009) to examine working memory’s association with ADLs in children with and without ADHD symptoms. It seemed reasonable to hypothesize that children with ADHD would exhibit difficulties in independently performing ADLs and that working memory abilities would predict these difficulties given replicated findings in adults with ADHD (Barkley & Murphy 2010; Barkley & Fischer, 2011; Stavro et al., 2011). We further hypothesized that working memory performance would be associated with ADLs both directly and indirectly via working memory’s impact on ADHD-related inattention and hyperactivity/impulsivity symptoms.
Method
Participants
The sample comprised 141 children ages 8–13 years (M=10.36, SD=1.46; 51 girls) from the Southeastern United States, recruited by or referred through community resources from 2015 to 2019 for participation in a larger study of the neurocognitive mechanisms underlying pediatric attention and behavioral problems. All parents and children gave informed consent/assent, and the Florida State University Institutional Review Board approved the study prior to and throughout data collection. Sample ethnicity was mixed with 99 Caucasian Non-Hispanic (70.2%), 17 Black/African American (12.1%), 12 Hispanic (8.5%), 12 multiracial children (8.5%), and 1 Asian (0.7%) child.
Group Assignment
All children and caregivers completed a detailed, semi-structured clinical interview using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS; Kaufman et al., 1997). K-SADS interviews were supplemented with parent and teacher rating scales from the Behavior Assessment System for Children (BASC-2/3; Reynolds & Kamphaus, 2004) and ADHD Rating Scale for DSM-4/5 (ADHD-RS-4/5; DuPaul et al., 2016).
Ninety-three children met all of the following criteria and were included in the ADHD group: (1) DSM-5 diagnosis of ADHD Combined (n = 65), Inattentive (n = 24), or Hyperactive/Impulsive Presentation (n = 4) by the CLC’s directing clinical psychologist based on K-SADS and differential diagnosis considering all available clinical information indicating onset, course, duration, and severity of ADHD symptoms consistent with the ADHD neurodevelopmental syndrome, (2) borderline/clinical elevations on at least one parent and one teacher ADHD subscale; and (3) current impairment based on parent report. Children with any current ADHD presentation specifiers were eligible given the instability of ADHD subtypes (Lahey et al., 2005; Valo & Tannock, 2010; Willcutt et al., 2012). To improve generalizability (Wilens et al., 2002), children with comorbidities were included. Comorbidities reflect clinical consensus best estimates and included oppositional defiant disorder (12.9%)1, depressive disorders (4.3%), and anxiety disorders (28.0%). Learning disabilities were suspected in 12.9% of the sample based on score(s) > 1.5 SD below age-norms on one or more KTEA-3 academic skills battery reading or math subtests.
The Non-ADHD group comprised 48 consecutive case-control referrals (22 girls) who did not meet ADHD criteria and included both neurotypical children and children with psychiatric disorders other than ADHD. Neurotypical children (62.5%) had normal developmental histories and nonclinical parent/teacher ratings, were recruited through community resources, and completed the same evaluation as clinically-referred cases. Clinically referred and evaluated children who did not meet ADHD criteria were also included in the Non-ADHD group. These Non-ADHD disorders were included to control for comorbidities in the ADHD group, and included best estimate diagnoses of anxiety (29.2%) and depressive (8.3%) disorders. Approximately 4.2% of the clinically-evaluated Non-ADHD cases screened positive for learning disorders based on score(s) > 1.5 SD below age-norms on one or more KTEA-3 academic skills battery reading or math subtests. Importantly, the ADHD and Non-ADHD groups did not differ compared to the ADHD group in the proportion of children diagnosed with anxiety (p = .89) and depression (p = .33). The ADHD group has a larger proportion of children with ODD as expected.
Children were excluded from the study if they presented with (a) gross neurological, sensory, or motor impairment, (b) history of a seizure disorder, psychosis, intellectual disability, or autism spectrum disorder, or (c) non-stimulant medications that could not be withheld for testing. Psychostimulants (Nprescribed=38) were withheld for a minimum of 24 hours prior to both research testing sessions.
Procedures
Children participated in two research sessions (3 hours each) following the baseline psychoeducational assessment. The working memory tasks were administered as part of a larger battery of executive and non-executive laboratory tasks. The tasks were counterbalanced within and across sessions to minimize order effects. Children were seated in a caster-wheel swivel chair approximately 0.66 meters from the computer monitor for all tasks. Performance was monitored at all times by the examiner, who was stationed just out of the child’s view to provide a structured setting while minimizing performance improvements associated with examiner demand characteristics (Gomez & Sanson, 1994). All children received brief (2–3 min) breaks after each task, and preset longer (10–15 min) breaks after every 2–3 tasks to minimize fatigue.
Working Memory Performance
Rapport working memory reordering tests.
The Rapport et al. (2009) computerized phonological and visuospatial working memory tests correctly classify children with versus without ADHD at similar rates as ADHD rating scales (Tarle et al., 2018), and predict hyperactivity (Rapport et al., 2009), inattention (Kofler et al., 2010), and impulsivity (Raiker et al., 2012). Reliability and validity evidence includes high internal consistency (α = .81–.97) and 1–3-week test-retest reliability (.76–.90; Kofler et al., 2019; Sarver et al., 2015), and expected relations with criterion working memory complex span (r = .69) and updating tasks (r = .61) (Wells et al., 2018). Internal consistency in the current sample was .84 (phonological) and .88 (visuospatial). Six trials per set size were administered in randomized/unpredictable order (3–6 stimuli/trial; 1 stimuli/second) as recommended (Kofler et al., 2016). Five practice trials were administered before each task (80% correct required). Task duration was approximately 5 (visuospatial) to 7 (phonological) minutes. Partial-credit unit scoring (i.e., stimuli correct per trial) was used to index overall working memory performance at each set size 3–6 as recommended (Conway et al., 2005).
The Rapport phonological working memory task assesses phonological working memory based on Baddeley’s (2007) model. Children were presented a series of jumbled numbers and a capital letter on a computer monitor. Each number and letter (4 cm height) appeared on the screen for 800 ms, followed by a 200 ms interstimulus interval. The letter never appeared in the first or last position of the sequence to minimize potential primacy and recency effects, and was counterbalanced across trials to appear an equal number of times in the other serial positions (i.e., position 2, 3, 4, or 5). Children were instructed to recall the numbers in order from smallest to largest, and to say the letter last (e.g., 4 H 6 2 is correctly recalled as 2 4 6 H).
The Rapport visuospatial working memory task assesses visuospatial working memory based on Baddeley’s (2007) model. Children were shown nine squares arranged in three offset vertical columns. A series of 2.5 cm diameter dots (3, 4, 5, or 6) were presented sequentially in one of the nine squares during each trial, such that no two dots appeared in the same square on a given trial. All but one dot presented within the squares was black—the exception being a red dot that was counterbalanced across trials to appear an equal number of times in each of the nine squares, but never presented as the first or last stimulus in the sequence to minimize potential primacy and recency effects. Each dot was displayed for 800 ms followed by a 200 ms interstimulus interval. Children reordered the dot locations (black dots in serial order, red dot last) and responded on a modified keyboard.
Working memory updating: Letter updating.
The Miyake et al. (2000) letter memory test was adapted for use with children and exemplifies working memory updating based on the Miyake et al. (2000) model. Working memory updating tasks involve the constant monitoring and rapid addition/deletion of working memory contents (Miyake & Friedman, 2012). Psychometric support for this version includes high internal consistency (α=.75), expected magnitude relations with other working memory tests (Kofler et al., 2018), and large magnitude ADHD/Non-ADHD between group differences (Fosco et al., 2020; Kofler et al., 2018). Internal consistency in the current sample was .75. In this computerized task, letters were presented on the screen one at a time, and children were instructed to keep track of the last three letters presented. To ensure the task required continuous updating, children were instructed to rehearse out loud the last three letters by mentally adding the most recent letter and dropping the fourth letter back and then saying the new string of three letters out loud (Miyake et al., 2000). The number of letters presented (4–8 stimuli presented/trial, 1200 ms presentation, 2400 ms ISI) was varied randomly across trials to ensure that successful performance required continuous updating until the end of each trial. A practice block was administered; children advanced to the test phase following three correct trials. Four blocks of three trials each were administered. Children responded via mouse click. The dependent variables were mean stimuli correct per trial recalled in the correct serial order during each of the four task blocks.
Independent variable: Working memory.
Task impurity was controlled by computing a Bartlett maximum likelihood component score (DiStefano et al., 2009), which combined the 4 phonological reordering (set sizes 3–6), 4 visuospatial reordering (set sizes 3–6), and 4 letter updating (blocks 1–4) variables (43.92% variance accounted; component loadings = .50 – .76). This factor-analytically derived component score provides an estimate of reliable, component-level variance attributable to domain-general working memory. This formative method for estimating working memory was selected because (a) such methods have been shown to provide higher construct stability relative to confirmatory/reflective approaches (Willoughby et al., 2016); and (b) estimating working memory at the construct-level rather than measure-level was expected to maximize associations with the study’s behavioral outcomes of interest via the removal of task-specific and error variance. Conceptually, this process isolates reliable variance across estimates of working memory by removing task-specific demands associated with nonworking memory processes, time-on-task effects via inclusion of four blocks per task, and non-construct variance attributable to other measured nonworking memory processes (e.g., short-term memory load). This working memory component score was used in all analyses below. Higher scores reflect better working memory abilities.
Activities of Daily Living (ADL)
The Behavioral Assessment System for Children (BASC-2/3; Reynolds & Kamphaus, 2015) Activities of Daily Living subscale was used in the current study (8–9 items; 4-point Likert scale), and contains both positively and negatively valanced items assessing caregiver perceptions of the frequency with which their child independently performs functional ADLs (e.g., brushing teeth, getting dressed, cleaning up after themselves, acting in a safe manner, making healthy food choices; Kamphaus & Reynolds, 2015). As an initial study, we selected a global, parent-report measure of ADLs because (a) adults with ADHD demonstrate difficulties with daily-living skills and ADLs (Barkley & Murphy, 2010; Barkley & Fischer, 2011), and (b) the concurrent validity of these scales has been demonstrated relative to global as well as specific indices of ADL difficulties. For example, the BASC-2/3 Activities of Daily Living subscale demonstrates the expected pattern of relations with measures of activities competence (r=.24 to .34; Achenbach & Rescorla, 2001) and measures of DSM-based disorders whose core features include ADL deficits (r=.54; Goldstein & Naglieri, 2010). Additional psychometric support for the Activities of Daily Living subscale includes high internal consistency (α=.77 to .80) and test-retest reliability (r = .86 to .90). Of note, the BASC teacher-report form does not have an ADL subscale; therefore, only parent-reported ADLs are assessed in the current study.
Dependent variable: Activities of daily living.
Raw scores from the BASC-2/3 Activities of Daily Living subscale were used to measure children’s skill at performing ADLs at home. Raw scores were selected a priori as recommended for research purposes (Achenbach, 1991). Age and sex were controlled in all analyses. Higher scores reflect better/more consistent performance of ADLs.
ADHD Symptoms
The ADHD Rating Scale (ADHD-RS-4/5; DuPaul et al., 2016) teacher forms were used to assess the frequency and severity of ADHD symptoms along with other childhood psychopathology based on DSM criteria in children and adolescents aged 5 to 17 (18 items; 4-point Likert scale). The ADHD-RS-4/5 teacher report form was selected a priori to minimize the potential impact of mono-instrument and mono-informant bias for models predicting ADLs from ADHD symptoms (i.e., illusory correlations occurring when comparing subscales from the same informant or same rating scale). The ADHD-RS-4/5 comprises two symptom subscales: Inattention (9 items) and Hyperactivity-Impulsivity (9 items). Psychometric support for the ADHD-RS-4/5 includes high internal consistency (α=0.94) and test-retest reliability (r= 0.79 to 0.85; DuPaul et al., 2016). Internal consistency in the current sample was .94 (inattentive items) and .95 (hyperactive/impulsive items).
Conditional effect variables: Inattention & Hyperactivity/impulsivity.
Raw scores from the ADHD-RS-4/5 were used to assess inattentive and hyperactive/impulsive behaviors. Raw scores for ADHD symptoms were selected a priori as recommended for research purposes (Achenbach, 1991). Age and sex were controlled in all analyses. Higher scores reflect greater quantity/severity of teacher-reported ADHD symptoms.
Global Intellectual Functioning (IQ) and Socioeconomic Status (SES)
All children were administered the Verbal Comprehension Index (VCI) of a Wechsler (2014) scale (WISC-V). Hollingshead (1975) SES was estimated based on caregiver(s)’ education and occupation.
Data Analysis Overview
The current study was the first to examine the extent to which (1) pediatric ADHD is associated with impaired performance of ADLs; and (2) ADL difficulties reflect the outcome of the interfering effects of ADHD inattentive and/or hyperactivity/impulsive behavioral symptoms, both independently and as an outcome of the well-documented effects of underlying working memory difficulties on producing elevated ADHD symptoms. Thus, our analytic plan was organized into two tiers. In the first analytic tier, we conducted data screening/cleaning and examined the extent to which ADHD is associated with difficulties in ADL performance as hypothesized (Table 1).
Table 1.
Descriptive Statistics.
| ADHD | Non-ADHD | |||||||
|---|---|---|---|---|---|---|---|---|
| Demographics | M | SD | M | SD | Cohen’s d | t | χ2 | p |
| N (Boys/Girls) | 93 (64/29) | 48 (26/22) | - | - | 2.94 | .09, n.s. | ||
| Ethnicity (A, MR, B, H, W) | (0, 6, 13, 5, 69) | (1, 6, 4, 7, 30) | - | - | 7.91 | .10, n.s. | ||
| Age | 10.30 | 1.48 | 10.46 | 1.45 | - | 0.54 | - | .55, n.s. |
| SES | 49.01 | 10.60 | 49.81 | 11.98 | - | 0.38 | - | .69, n.s. |
| VCI | 103.42 | 14.98 | 107.58 | 11.83 | 0.31 | 1.67 | - | .08, n.s. |
| Parent - Activities of Daily Living (T-scores) | 35.70 | 9.58 | 42.58 | 9.00 | 0.74 | 4.13 | - | <.001 |
| Working Memory Component Score (z-scores) | −0.31 | 0.97 | 0.60 | 0.77 | 1.17 | 6.33 | - | <.001 |
| Parent - Activities of Daily Living (raw scores) | 10.63 | 4.06 | 13.1 | 3.71 | 0.61 | 3.39 | - | <.005 |
| Parent - Activities of Daily Living Modified (raw scores) | 9.03 | 3.61 | 11.0 | 3.22 | 0.57 | 3.21 | - | .002 |
| Teacher - Attention Problems (raw scores) | 16.87 | 6.13 | 10.54 | 7.78 | 0.90 | −5.34 | - | <.001 |
| Teacher - Hyperactivity/Impulsivity (raw scores) | 12.59 | 8.80 | 6.06 | 7.09 | 0.80 | −4.37 | - | <.001 |
Note: Parent - Activities of Daily Living (Modified) reflects raw scores with the ‘organizes tasks’ item removed due to its similarity to a core ADHD inattentive symptom as described in the Method (footnote 2). A = Asian; B = Black/African American; MR = Multiracial; H = Hispanic; SES =Hollingshead SES total score; VCI=Wechsler Verbal Comprehension Index; W = White/Non-Hispanic
In Tier 2, we conducted conditional effects analyses using PROCESS (Model 4; Hayes, 2013) with 10,000 bias-corrected, bootstrapped samples to analyze the relations among working memory, ADHD symptoms, and ADLs (Preacher, Rucker, & Hayes, 2007). Bias-corrected, bootstrapped conditional effects modeling was preferred as it allows shared variance among predictors to be parsed according to theory and previous research. As such, this analysis was used to determine whether working memory performance predicted ADLs through two proposed conditional effects: ADHD inattentive and ADHD hyperactive/impulsive symptoms. Working memory was modeled to predict ADHD symptoms, rather than vice versa, based on prior theoretical work and experimental evidence that increasing working memory demands evokes inattentive and hyperactive behavior (Kofler et al., 2010; Rapport et al., 2009), whereas working memory deficits remain large when covarying attentive behavior during testing (Kofler et al., 2010). Inattention and hyperactivity/impulsivity were included as separate predictors based on evidence that they differentially predict relations between working memory and other ADHD-related impairments (e.g., Bunford et al., 2014; Kofler et al., 2018b). Additionally, ADHD symptoms were modeled as predictors of ADLs given conceptualizations of ADL difficulties as secondary features of ADHD. Importantly, the ADHD-RS-4/5 Inattention and Hyperactivity subscales were considered appropriate predictors as they do not contain any items that explicitly assess ADLs as indexed by the BASC-2/3 with one exception2 (Reynolds & Kamphaus, 2015). Of note, the cross-sectional design precludes testing of competing models regarding directionality of effects (i.e., reversing arrows does not distinguish plausible models; Thoemmes, 2015). Effects are statistically significant if their 95% CIs do not contain 0.0.
Results
Power Analysis
Large effects were predicted based on large relations between working memory and ADL performance (d = 0.87; Vaughan & Giovanello, 2010) and large relations between working memory and ADHD symptoms (d ≥ 2.0; Kasper et al., 2012). Our N of 141 exceeds the N = 34 required for bias-corrected bootstrapping to detect an effect of these expected magnitudes for α = .05 and power = .80 with one intermediate effect (Fritz & MacKinnon, 2007). Conservatively assuming partial mediation and approximately equal contributions of each mediator, N = 100 produced power = .94–.96 to detect the total indirect effect with two intermediate effects (Briggs, 2006). Power for detecting significance for each intermediate effect with N = 100 was .92–.94. Thus, the current study’s N of 141 is adequately powered to detect effects of the expected magnitude.
Tier 1: Preliminary Analyses and Group Differences
All independent and dependent variables were screened for univariate outliers, defined as values greater than 3 SD above or below the within-group mean. Seven data points (0.003% of data points) were identified as outliers and were corrected to the most extreme value 3 SD above or below the within-group mean. Task data from subsets of the current battery have been reported for subsets of the current sample to examine conceptually unrelated hypotheses in prior reports (please see Irwin et al., 2020). Data for the study’s primary outcome, ADLs, have not been previously reported. Inspection of Table 1 indicated that the ADHD group exhibited significantly higher ADHD symptoms (d = 0.80–0.90, p < .001) and lower working memory performance (d = 1.17, p < .001) as expected. Interestingly, children with ADHD also exhibited medium magnitude difficulties with ADLs as hypothesized (d = 0.61, p < .005), suggesting that approximately 38% of children with ADHD are perceived by their caregivers as demonstrating ADL impairments based on converting this effect size into the proportion of population non-overlap (Zakzanis, 2001). The ADHD and Non-ADHD groups did not differ on age, sex, or IQ (all p > .08). Age and sex were included as covariates in the conditional effects models. IQ was not included as a covariate based on compelling statistical, methodological, and conceptual rationale against covarying IQ when investigating cognitive processes in ADHD (Dennis et al., 2009). Intercorrelations among study variables are shown in Table 2.
Table 2.
Zero-order correlations.
| Variable | Age | VCI | Gender | WM | Parent - ADL | Teacher - AP | Teacher - HI |
|---|---|---|---|---|---|---|---|
| Age | 1 | -- | -- | -- | -- | -- | -- |
| VCI | −.03 | 1 | -- | .44** | .23** | −.30** | −.06 |
| Gender | .05 | −.09 | 1 | -- | -- | -- | -- |
| WM | .41** | .39** | .09 | 1 | .26** | −.37** | −.16 |
| Parent - ADL | −.21* | .23** | −.12 | .141 | 1 | −.37** | −.09 |
| Teacher - AP | −.12 | −.30** | .09 | −.38** | −.34** | 1 | .51** |
| Teacher - HI | −.19* | −.08 | .23** | −.20* | −.07 | .52** | 1 |
Note: Parent - ADL = Parent-rated Activities of Daily Living; Teacher - AP = Teacher-rated Attention Problems; Teacher - HI = Teacher-rated Hyperactivity/Impulsivity; WM = Working Memory Performance. Bottom triangle = Zero-order correlations; Top triangle = partial correlations (controlling for age and sex).
= significant at .05 level
= significant at .01 level
= p < .10
Tier 2: Conditional Effects Models
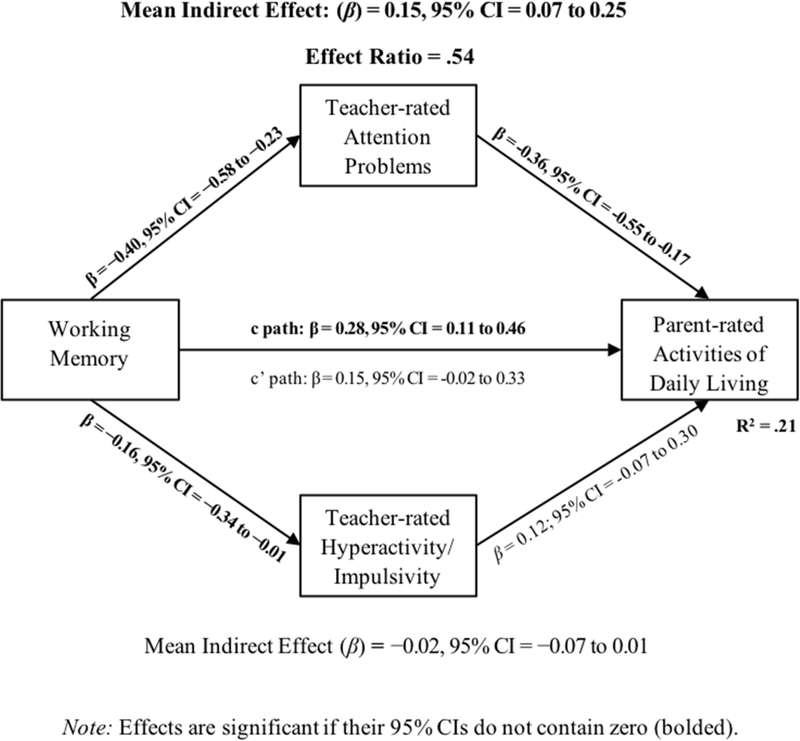
As shown in Figure 1, results of the bias-corrected, conditional effects model indicated that children with lower working memory abilities had more difficulty consistently performing age-expected ADLs (β = .28, 95% CI = 0.11 to 0.46). Reduced working memory abilities also predicted greater inattentive (β = −0.40, 95% CI = −0.58 to −0.23) and hyperactive/impulsive symptoms (β = −0.16, 95% CI = −0.34 to −0.01). In addition, greater inattentive symptoms, but not hyperactive/impulsive symptoms, predicted more difficulty completing ADLs (β = −0.36, 95% CI = −0.55 to −0.17) even after controlling for working memory.
Figure 1.
Cross-informant conditional effects models. Significant pathways are bolded. Age and sex were covaried but are not shown for visual clarity.
Conditional effects of working memory on ADLs through cross-informant inattentive symptoms were significant (indirect effect: β = .15, 95% CI = 0.07 to 0.25, ER = .54). The direct effect of working memory on parent-reported ADLs was no longer significant after accounting for working memory’s effects on ADLs via the inattentive symptom pathway (95% CI includes 0.0), indicating that working memory’s impact on ADL performance was carried, in large part, via working memory’s role in regulating/maintaining attention. No conditional effects via the hyperactive/impulsive symptoms pathway were observed (95% CI includes 0.0).
Sensitivity Analyses
Finally, we conducted sensitivity analyses to probe the extent to which the results were influenced by our a priori decision to include age and sex as covariates. This involved repeating the Tier 2 analyses, this time without the statistical control for age and sex. Results were highly consistent with those reported above, with one notable exception: Without control for age and sex, there was no evidence for a direct effect of working memory on ADL performance (β = .14, 95% CI = −0.03 to 0.31 in the model without age/sex controlled vs. β = .28, 95% CI = 0.11 to 0.46 in the primary Tier 2 analyses reported above), highlighting the importance of controlling for developmental changes in these constructs. The indirect effect of working memory on ADLs via the attention problems pathway remained significant (β = .15, 95% CI = 0.07 to 0.27, ER = 1.0), and all other findings were consistent with those reported above, including direct effects of inattention on ADLs when controlling for working memory (β = −.40, 95% CI= −0.60 to −0.21) and no evidence for direct or indirect effects via the hyperactivity/impulsivity symptoms pathway (all 95% CIs include zero).
Discussion
The current study used a combination of cross-informant ratings of ADHD symptoms and ADL performance in conjunction with children’s performance on a battery of validated working memory tests (e.g., Fosco et al., 2020) to examine the extent to which ADL difficulties in pediatric ADHD reflect a behavioral outcome of underlying working memory difficulties, both directly and indirectly via working memory’s association with elevated ADHD symptoms. Additional strengths of the study include the use of a multi-method, multi-informant, and multi-task design and the inclusion of a clinically-evaluated and carefully-phenotyped sample of children with and without ADHD. Results from the current study indicated that a sizable minority of children with ADHD exhibit difficulties in their skill at consistently performing age-expected ADLs (d=0.61; 38% impaired). These difficulties appear to be related, to a significant extent, to their inattentive symptoms, both independently and as an outcome of their well-documented working memory difficulties. These findings were consistent with previous evidence indicating that deficits in ADLs are associated with both mild and severe degrees of cognitive decline in geriatric and adult-ADHD samples (Barkley & Murphy 2010; Barkley & Murphy, 2011; Jekel et al., 2015; Ranhoff, 1997; Stavro et al., 2011) and that working memory is a significant predictor of ADL performance (Cloutier et al., 2017; Vaughan & Giovanello, 2010). These results extend previous findings by documenting the association between working memory and ADLs in children with ADHD, and suggest that working memory’s relation with ADL difficulties may be attributable, in large part, to working memory’s impact on inattention symptoms which in turn impact ADL performance.
Interestingly, ADHD inattentive symptoms independently predicted ADLs, even when controlling for working memory, whereas ADHD hyperactive/impulsive symptoms were not significantly associated with ADLs even at the bivariate level (Table 2). These findings were consistent with prior literature indicating that children with other neurodevelopmental disorders, such as autism spectrum disorder and intellectual disorder, exhibit difficulties with ADLs (Gilotty et al., 2002; Kottorp et al., 2003) and that measures of executive functioning predict ADL impairments in adults with ADHD both cross-sectionally (Barkley & Murphy 2010; Stavro et al., 2011) and longitudinally (Barkley & Fischer, 2011). These results extend previous findings by demonstrating that ADL difficulties in pediatric ADHD appear to be due, in part, to interference effects from inattentive symptoms. In other words, these results suggest that children with ADHD exhibit difficulties in ADLs in part due to their difficulties attending to the task at hand, rather than impairments resulting from their elevated gross motor movement/physical activity. These findings contrast with evidence indicating that ADL difficulties in children with chromosomal and/or neurological disorders are associated with fine and gross motor difficulties rather than cognitive functioning (Summers et al., 2008; Volman et al., 2006; Magalhães et al., 2011; Wang et al., 2012), and highlight the potential for different developmental pathways to lead to the same/similar difficulties at the phenotypic/behavioral endpoint across different syndromes. For children with ADHD, our results suggest that ADL difficulties are more closely associated with cognitive impairments (working memory) and interfering behaviors (inattention), rather than motor-related functions such as overactivity or impulsivity.
Previous literature indicates that the phenotypic expression of ADHD-related inattentive and hyperactive/impulsive behaviors may be driven, in part, by deficits in working memory (Barkley, 1997; Rapport et al., 2009; Karalunas et al., 2017; Kasper et al., 2012; Kofler et al., 2015), and that deficits in working memory in children with ADHD are associated with functional outcomes at school (Friedman et al., 2017, 2018; Kofler, Sarver et al., 2018; Simone et al., 2018) and with peers (Hoza, 2007; Kofler, Harmon et al., 2018). The present study tested the hypothesis that working memory would predict ADHD symptoms, which would in turn predict ADLs. We found partial support for this prediction, with indirect effects of working memory on ADLs through parent-reported inattentive but not hyperactive/impulsive symptoms. Thus, it appears that working memory may be implicated in ADL performance to the extent that underdeveloped working memory contributes to the expression of ADHD-related inattentive symptoms as they are perceived by teachers. That is, difficulties with ADLs in children with ADHD appear to reflect, in large part, the behavioral expression of underlying working memory deficits that result in difficulty attending to external stimuli for developmentally expected durations (e.g., Kofler et al., 2010; Rapport et al., 2009). This conclusion is consistent with findings in the adult and aging literature indicating that ADL impairments are driven in large part by cognitive deficits (Jekel et al., 2015; Ranhoff, 1997; Vaughan & Giovanello, 2010; Woods et al., 2008) and extend previous findings by demonstrating this association in children with ADHD. An interesting implication of our findings is that they suggest that children with ADHD may not have ADL deficits per se, but rather that working memory ‘slips’ may produce interfering behaviors (e.g., inattentive symptoms) that in turn prevent these children from being able to consistently demonstrate their ADL skills at age-expected levels. In other words, children with ADHD may have learned to complete ADLs at developmentally expected levels (i.e., they possess the requisite knowledge and skills), but have difficulty consistently doing so due to interfering neurocognitive and behavioral factors. This hypothesis is of course speculative because the current study used a global measure of ADL skills, but is generally consistent with prior literature indicating that social problems in ADHD reflect difficulty consistently performing learned social skills rather than a lack of social knowledge or skills acquisition (Aduen et al., 2018), and extends previous findings by suggesting that differentiating ADL knowledge from performance difficulties may be a promising avenue for further research in this area.
Limitations
The current study has several strengths, including a relatively large and clinically evaluated sample of children and the multi-method, multi-informant, and multi-task design. Despite these methodological refinements and the carefully phenotyped sample, the following limitations must be considered when interpreting results. First, despite psychometric support for the use of the parent BASC Activities of Daily Living subscale, it provides an overall assessment of ADL performance rather than a fine-grained assessment of specific ADLs. As an initial study, this seemed the prudent course; however, the current findings cannot speak to whether ADHD may be associated with impairments in specific ADL skills. Thus, future studies are needed to replicate and extend these findings using more fine-grained ADL assessments (e.g., behavioral observations). Next, the current study used performance from validated working memory tests but did not include other executive and non-executive neurocognitive functions that may be related to ADHD symptoms and ADLs (Rosenburg, 2015; Thaler et al., 2012; Vaughan & Giovanello, 2010). Interestingly, the current literature base suggests that specific rather than domain-general cognitive domains may differentially influence an individual’s ability to perform ADLs (e.g., Razani et al., 2007; Royall et al., 2007), and prior research has shown than individuals’ level of ADL performance differs even within samples of individuals with intellectual disability (Lifshitz et al., 2008). In the current sample, IQ was not included as a covariate based on compelling statistical, methodological, and conceptual rationale against covarying IQ when investigating cognitive processes in ADHD (Dennis et al., 2009). In other words, covarying IQ would preclude conclusions regarding ADHD as a neurodevelopmental disorder by fundamentally changing our grouping variable, and remove significant variance associated with the outcomes of interest (Dennis et al., 2009). However, given the well documented heterogeneity of executive functioning among children with ADHD (Castellanos & Tannock, 2002; Kofler et al., 2019), it will be important for future work to consider the unique contributions of each executive function in addition to working memory as they relate to children’s performance of ADLs, as well as differentiate between skills deficits and performance deficits as it applies to ADLs (Aduen et al., 2018; Kofler, Harmon et al., 2018).
In that context, another strength of this study was the inclusion of a large sample of children with ADHD, given that the disorder’s neurocognitive heterogeneity was expected to provide a wider range of scores on the working memory tests and as such a greater likelihood of uncovering associations among children’s working memory abilities and ADLs. Nonetheless, the inclusion of children without ADHD may reduce specificity of the findings to ADHD, just as the oversampling of children with ADHD may reduce generalizability to the broader population of children. Similarly, co-occurring conditions are common in ADHD (Wilens et al., 2002) and therefore inclusion of children with these comorbidities was important to maximize external validity and generalizability of the larger study. We attempted to balance internal and external validity threats by recruiting a Non-ADHD group matched for the number of these Non-ADHD disorders. However, controlling for the number of other disorders does not perfectly equate the groups and thus future work is needed to examine more ‘pure’ (non-comorbid) ADHD samples.
Lastly, working memory was used as a predictor of ADLs given the preponderance of evidence indicating that working memory deficits may underlie ADHD’s phenotypic behavioral presentation for many if not most children with ADHD (Kofler et al., 2010; Rapport et al., 2009). Nonetheless, this conceptualization differs from models in the neurology literature, where illnesses such as Alzheimer’s, Parkinson’s, and HIV are acquired illnesses that predict declines in working memory, which then in turn predict declines in ADL performance (Jekel et al., 2015; Ranhoff, 1997; Woods et al., 2008). In contrast, neurodevelopmental disorders such as ADHD are not acquired illnesses; rather, delayed/altered development of working memory has been hypothesized to give rise to observable ADHD symptoms (e.g., Kofler et al., 2010; Rapport et al., 2009), which in turn interfere with ADL performance. In this context, we were unable to examine competing models regarding directionality due to the cross-sectional nature of our data (Thoemmes, 2015). Thus, experimental and longitudinal studies are needed to test for causality and directionality of the effects reported herein.
Clinical and Research Implications
Taken together, the current results indicate that a sizable minority of children with ADHD have difficulty completing their ADLs consistently, and suggest that these difficulties may be related, in large part, to their inattentive symptoms – both independently and via their underlying working memory difficulties. In contrast, ADHD hyperactive/impulsive symptoms do not appear to confer risk for difficulties with ADLs. Thus, these findings highlight an additional domain of functional impairment for children with ADHD (Aduen et al., 2018; Bunford et al., 2014; Hoza, 2007; Kofler, Harmon et al., 2018; Simone et al., 2018), while suggesting that directly treating ADL difficulties may not be the most effective strategy for helping these children perform their ADLs more consistently. That is, based on the current results we would speculate that ADL difficulties in ADHD may not reflect specific skill deficits, but rather the influence of interfering behaviors (i.e., inattentive symptoms) and neurocognitive vulnerabilities (i.e., working memory deficits). Of course, this hypothesis remains speculative because the current study was not an intervention study, but is consistent with recent evidence that the inefficacy of interventions targeting specific skill-sets (e.g., social skills training) in children with ADHD may be due to the fact that these children possess the age-expected skills but instead have difficulty implementing these skills consistently due to working memory and/or attention difficulties (Aduen et al., 2018; Kofler, Harmon et al., 2018).
Acknowledgements:
This work was supported in part by NIH grants (R34 MH102499, R01 MH115048, PI: Kofler). The sponsor had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to report.
As recommended in the K-SADS, oppositional defiant disorder was diagnosed clinically only with evidence of multi-informant/multi-setting symptoms. ODD comorbidity is 39.8% in the ADHD group based on parent-reported symptom counts.
The BASC ADL subscale item “organizes chores or other tasks well.” is highly similar to the DSM-5 ADHD symptom “often has difficulty organizing tasks or activities.” Exploratory analyses indicated that the pattern and interpretation of results was unchanged with this item removed from children’s BASC ADL scores.
References
- Achenbach TM (1991). Manual for the Teacher’s Report Form and 1991 profile. Univ Vermont/Department Psychiatry. [Google Scholar]
- Alderson RM, Hudec KL, Patros CH, & Kasper LJ (2013). Working memory deficits in adults with attention-deficit/hyperactivity disorder (ADHD): An examination of central executive and storage/rehearsal processes. Journal of abnormal psychology, 122(2), 532. [DOI] [PubMed] [Google Scholar]
- Arling G, & Williams AR (2003). Cognitive impairment and resource use of nursing home residents: a structural equation model. Medical Care, 802–812. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Hodgkins P, Kahle J, Madhoo M, & Kewley G (2020). Long-term outcomes of ADHD: academic achievement and performance. Journal of Attention Disorders, 24(1), 73–85. [DOI] [PubMed] [Google Scholar]
- Aduen PA, Day TN, Kofler MJ, Harmon SL, Wells EL, & Sarver DE (2018). Social problems in ADHD: Is it a skills acquisition or performance problem?. Journal of Psychopathology and Behavioral Assessment, 40(3), 440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A (2007). Working memory, thought, and action. Oxford Press. [Google Scholar]
- Barkley RA, & Fischer M (2011). Predicting impairment in major life activities and occupational functioning in hyperactive children as adults: Self-reported executive function (EF) deficits versus EF tests. Developmental Neuropsychology, 36(2), 137–161. [DOI] [PubMed] [Google Scholar]
- Barkley RA, & Murphy KR (2010). Impairment in occupational functioning and adult ADHD: the predictive utility of executive function (EF) ratings versus EF tests. Archives of Clinical Neuropsychology, 25(3), 157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, … & Faraone SV (2004). Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. Journal of Consulting and Clinical Psychology, 72(5), 757. [DOI] [PubMed] [Google Scholar]
- Blank R, von Kries R, Hesse S, & von Voss H (2008). Conductive education for children with cerebral palsy: effects on hand motor functions relevant to activities of daily living. Archives of Physical Medicine and Rehabilitation, 89(2), 251–259. [DOI] [PubMed] [Google Scholar]
- Bunford N, Brandt NE, Golden C, Dykstra JB, Suhr JA, Owens JS (2014). ADHD symptoms mediate the association between deficits in executive functioning and social impairment in children. Journal of Abnormal Child Psychology, 43, 133–147. [DOI] [PubMed] [Google Scholar]
- Cantarella A, Borella E, Carretti B, Kliegel M, & de Beni R (2017). Benefits in tasks related to everyday life competences after a working memory training in older adults. International Journal of Geriatric Psychiatry, 32(1), 86–93. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, & Tannock R (2002). Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience, 3(8), 617–628. [DOI] [PubMed] [Google Scholar]
- Cloutier S, Chertkow H, Kergoat MJ, Gauthier S, & Belleville S (2017). Natural history of the decline in instrumental activities of daily living prior to dementia in persons with mild cognitive impairment. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 13(7), P1136. [Google Scholar]
- Conners CK, Pitkanen J, & Rzepa SR (2011). Conners 3rd Edition (Conners 3; Conners 2008). Encyclopedia of Clinical Neuropsychology. New York, NY: Springer, 675–678. [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, & Engle RW (2005). Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review, 12(5), 769–786. [DOI] [PubMed] [Google Scholar]
- Cook NE, Braaten EB, & Surman CB (2018). Clinical and functional correlates of processing speed in pediatric Attention-Deficit/Hyperactivity Disorder: A systematic review and meta-analysis. Child Neuropsychology, 24(5), 598–616. [DOI] [PubMed] [Google Scholar]
- Demetriou EA, Lampit A, Quintana DS, Naismith SL, Song YJC, Pye JE, … & Guastella AJ. (2018). Autism spectrum disorders: a meta-analysis of executive function. Molecular Psychiatry, 23(5), 1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula JJ, Diniz BS, Bicalho MA, Albuquerque MR, Nicolato R, de Moraes EN, … & Malloy-Diniz LF. (2015). Specific cognitive functions and depressive symptoms as predictors of activities of daily living in older adults with heterogeneous cognitive backgrounds. Frontiers in Aging Neuroscience, 7, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano C, Zhu M & Mîndrilă D (2009). Understanding and using factor scores: Considerations for the applied researcher. Practical Assessment, Research & Evaluation, 14, 1–11. [Google Scholar]
- Duncan AW, & Bishop SL (2015). Understanding the gap between cognitive abilities and daily living skills in adolescents with autism spectrum disorders with average intelligence. Autism, 19(1), 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, & Reid R (2016). ADHD rating scale-5 for children and adolescents: Checklists, norms, and clinical interpretation. New York: Guilford Press. [Google Scholar]
- Friedman LM, Rapport MD, Raiker JS, Orban SA, & Eckrich SJ (2017). Reading comprehension in boys with ADHD: The mediating roles of working memory and orthographic conversion. Journal of Abnormal Child Psychology, 45(2), 273–287. [DOI] [PubMed] [Google Scholar]
- Friedman LM, Rapport MD, Orban SA, Eckrich SJ, & Calub CA (2018). Applied problem solving in children with ADHD: The mediating roles of working memory and mathematical calculation. Journal of Abnormal Child Psychology, 46(3), 491–504. [DOI] [PubMed] [Google Scholar]
- Fosco WD, Kofler MJ, Groves NB, Chan ES, & Raiker JS (2020). Which ‘Working’Components of Working Memory aren’t Working in Youth with ADHD?. Journal of Abnormal Child Psychology, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantschnig BE, Page J, Nilsson I, & Fisher AG (2013). Detecting differences in activities of daily living between children with and without mild disabilities. American Journal of Occupational Therapy, 67(3), 319–327. [DOI] [PubMed] [Google Scholar]
- Gilotty L, Kenworthy L, Sirian L, Black DO, & Wagner AE (2002). Adaptive skills and executive function in autism spectrum disorders. Child Neuropsychology, 8(4), 241–248. [DOI] [PubMed] [Google Scholar]
- Goldstein S, & Naglieri JA (2010). Autism spectrum rating scales (ASRS) product overview. Multi-Health Syst Inc. [Google Scholar]
- Gomez R, & Sanson A (1994). Effects of experimenter and mother presence on the attentional performance and activity of hyperactive boys. Journal of Abnormal Child Psychology, 22, 517–529. [DOI] [PubMed] [Google Scholar]
- Hayase D, Mosenteen D, Thimmaiah D, Zemke S, Atler K, & Fisher AG (2004). Age‐related changes in activities of daily living ability. Australian Occupational Therapy Journal, 51(4), 192–198. [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press. [Google Scholar]
- Hollingshead AB (1975). Four factor index of social status. Yale: New Haven, CT. [Google Scholar]
- Hoza B (2007). Peer functioning in children with ADHD. Journal of Pediatric Psychology, 32(6), 655–663. [DOI] [PubMed] [Google Scholar]
- James S, Ziviani J, & Boyd R (2014). A systematic review of activities of daily living measures for children and adolescents with cerebral palsy. Developmental Medicine & Child Neurology, 56(3), 233–244. [DOI] [PubMed] [Google Scholar]
- Jekel K, Damian M, Wattmo C, Hausner L, Bullock R, Connelly PJ, … & Kramberger MG (2015). Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimer’s Research & Therapy, 7(1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LJ, Alderson RM, & Hudec KL (2012). Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clinical Psychology Review, 32(7), 605–617. [DOI] [PubMed] [Google Scholar]
- Katz S (1983). Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. Journal of the American Geriatrics Society, 31(12), 721–727. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, … Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Karalunas SL, Gustafsson HC, Dieckmann NF, Tipsord J, Mitchell SH, & Nigg JT (2017). Heterogeneity in development of aspects of working memory predicts longitudinal attention deficit hyperactivity disorder symptom change. Journal of Abnormal Psychology, 126(6), 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon D, Cardoso C, & McGrath JJ (2016). Research Review: Executive function deficits in fetal alcohol spectrum disorders and attention‐deficit/hyperactivity disorder–a meta‐analysis. Journal of Child Psychology and Psychiatry, 57(2), 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Harmon SL, Aduen PA, Day TN, Austin KE, Spiegel JA, … & Sarver DE. (2018). Neurocognitive and behavioral predictors of social problems in ADHD: A Bayesian framework. Neuropsychology, 32(3), 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Irwin LN, Soto EF, Groves NB, Harmon SL, & Sarver DE (2019). Executive functioning heterogeneity in pediatric ADHD. Journal of Abnormal Child Psychology, 47(2), 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, & Raiker JS (2010). ADHD and working memory: The impact of central executive deficits and exceeding storage/rehearsal capacity on observed inattentive behavior. Journal of Abnormal Child Psychology, 38, 149–161. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Sarver DE, Harmon SL, Moltisanti A, Aduen PA, Soto EF, & Ferretti N (2017). Working memory and organizational skills problems in ADHD. Journal of Child Psychology and Psychiatry, 59(1), 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Sarver DE, & Wells EL (2015). Working memory and increased activity level (hyperactivity) in ADHD: Experimental evidence for a functional relation. Journal of Attention Disorders, 1087054715608439. [DOI] [PubMed] [Google Scholar]
- Kottorp A, Bernspång B, & Fisher AG (2003). Activities of daily living in persons with intellectual disability: Strengths and limitations in specific motor and process skills. Australian Occupational Therapy Journal, 50(4), 195–204. [Google Scholar]
- Lawton MP, & Brody EM (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist, 9(3), 179–186. [PubMed] [Google Scholar]
- Lifshitz H, Merrick J, & Morad M (2008). Health status and ADL functioning of older persons with intellectual disability: Community residence versus residential care centers. Research in Developmental Disabilities, 29(4), 301–315. [DOI] [PubMed] [Google Scholar]
- Magalhães LC, Cardoso AA, & Missiuna C (2011). Activities and participation in children with developmental coordination disorder: A systematic review. Research in Developmental Disabilities, 32(4), 1309–1316. [DOI] [PubMed] [Google Scholar]
- Mares D, McLuckie A, Schwartz M, & Saini M (2007). Executive function impairments in children with attention-deficit hyperactivity disorder: Do they differ between school and home environments?. The Canadian Journal of Psychiatry, 52(8), 527–534. [DOI] [PubMed] [Google Scholar]
- Martyr A, & Clare L (2012). Executive function and activities of daily living in Alzheimer’s disease: a correlational meta-analysis. Dementia and geriatric cognitive disorders, 33(2–3), 189–203. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current directions in psychological science, 21(1), 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlinac ME, & Feng MC (2016). Assessment of activities of daily living, self-care, and independence. Archives of Clinical Neuropsychology, 31(6), 506–516. [DOI] [PubMed] [Google Scholar]
- Nee DE, & Brown JW (2013). Dissociable frontal–striatal and frontal–parietal networks involved in updating hierarchical contexts in working memory. Cerebral Cortex, 23(9), 2146–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, & Sonuga-Barke EJ (2005). Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes?. Biological Psychiatry, 57(11), 1224–1230. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, De Lima MS, Horta BL, Biederman J, & Rohde LA (2007). The worldwide prevalence of ADHD: a systematic review and metaregression analysis. American Journal of Psychiatry, 164(6), 942–948. [DOI] [PubMed] [Google Scholar]
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, & Rohde LA (2014). ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. International Journal of Epidemiology, 43(2), 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, & Hayes AF (2007). Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research, 42, 185–227. [DOI] [PubMed] [Google Scholar]
- Raiker JS, Rapport MD, Kofler MJ, & Sarver DE (2012). Objectively-measured impulsivity and ADHD: Testing competing predictions from the working memory and behavioral inhibition models of ADHD. Journal of Abnormal Child Psychology, 40, 699–713. [DOI] [PubMed] [Google Scholar]
- Razani J, Casas R, Wong JT, Lu P, Alessi C, & Josephson K (2007). Relationship between executive functioning and activities of daily living in patients with relatively mild dementia. Applied Neuropsychology, 14(3), 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhoff AH (1997). Activities of daily living, cognitive impairment and other psychological symptoms among elderly recipients of home help. Health & Social Care in the Community, 5(3), 147–152. [Google Scholar]
- Rapport MD, Bolden J, Kofler MJ, Sarver DE, Raiker JS, & Alderson RM (2009). Hyperactivity in boys with ADHD: A ubiquitous core symptom or manifestation of working memory deficits? Journal of Abnormal Child Psychology, 37, 521–534. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Orban SA, Kofler MJ, & Friedman LM (2013). Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clinical Psychology Review, 33(8), 1237–1252. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, & Kamphaus RW (2015). BASC-3: Behavior assessment system for children. [DOI] [PubMed]
- Rosenberg L (2015). The associations between executive functions’ capacities, performance process skills, and dimensions of participation in activities of daily life among children of elementary school age. Applied Neuropsychology: Child, 4(3), 148–156. [DOI] [PubMed] [Google Scholar]
- Royall DR, Lauterbach EC, Kaufer D, Malloy P, Coburn KL, & Black KJ (2007). The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. The Journal of Neuropsychiatry and Clinical Neurosciences, 19(3), 249–265. [DOI] [PubMed] [Google Scholar]
- Tarle SJ, Alderson RM, Patros CHG, Lea SE, Hudec KL, & Arrington EF (2017). Attention-deficit/hyperactivity disorder and phonological working memory: Methodological variability affects clinical and experimental performance metrics. Neuropsychology, 31(4), 383–394. [DOI] [PubMed] [Google Scholar]
- Thaler NS, Bello DT, & Etcoff LM (2012). WISC-IV profiles are associated with differences in symptomatology and outcome in children with ADHD. Journal of Attention Disorders, 17(4), 291–301. [DOI] [PubMed] [Google Scholar]
- Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, … & Hinkin CH (2011). Medication and finance management among HIV-infected adults: the impact of age and cognition. Journal of Clinical and Experimental Neuropsychology, 33(2), 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theule J, Wiener J, Tannock R, & Jenkins JM (2013). Parenting stress in families of children with ADHD: A meta-analysis. Journal of Emotional and Behavioral Disorders, 21(1), 3–17. [Google Scholar]
- Thoemmes F (2015). Reversing arrows in mediation models does not distinguish plausible models. Basic and Applied Social Psychology, 37, 226–234. [Google Scholar]
- Tomaszewski Farias S, Cahn-Weiner DA, Harvey DJ, Reed BR, Mungas D, Kramer JH, & Chui H (2009). Longitudinal changes in memory and executive functioning are associated with longitudinal change in instrumental activities of daily living in older adults. The Clinical Neuropsychologist, 23(3), 446–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver DE, Rapport MD, Kofler MJ, Raiker JS, & Friedman LM (2015). Hyperactivity in ADHD: Impairing deficit or compensatory behavior? Journal of Abnormal Child Psychology, 43, 1219–1232. [DOI] [PubMed] [Google Scholar]
- Simone AN, Marks DJ, Bédard AC, & Halperin JM (2018). Low working memory rather than ADHD symptoms predicts poor academic achievement in school-aged children. Journal of Abnormal Child Psychology, 46(2), 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro GM, Ettenhofer ML, & Nigg JT (2007). Executive functions and adaptive functioning in young adult attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society, 13(2), 324–334. [DOI] [PubMed] [Google Scholar]
- Summers J, Larkin D, & Dewey D (2008). Activities of daily living in children with developmental coordination disorder: dressing, personal hygiene, and eating skills. Human movement science, 27(2), 215–229. [DOI] [PubMed] [Google Scholar]
- Vaughan L, & Giovanello K (2010). Executive function in daily life: Age-related influences of executive processes on instrumental activities of daily living. Psychology and Aging, 25(2), 343–355. [DOI] [PubMed] [Google Scholar]
- Valo S, & Tannock R (2010). Diagnostic instability of DSM–IV ADHD subtypes: Effects of informant source, instrumentation, and methods for combining symptom reports. Journal of Clinical Child & Adolescent Psychology, 39, 749–760. [DOI] [PubMed] [Google Scholar]
- Volman MJ, Visser JJ, & Lensvelt-Mulders GJ (2007). Functional status in 5 to 7-year-old children with Down syndrome in relation to motor ability and performance mental ability. Disability and Rehabilitation, 29(1), 25–31. [DOI] [PubMed] [Google Scholar]
- Wager TD, & Smith EE (2003). Neuroimaging studies of working memory. Cognitive, Affective, & Behavioral Neuroscience, 3, 255–274. [DOI] [PubMed] [Google Scholar]
- Wang HY, Chen CC, & Hsiao SF (2012). Relationships between respiratory muscle strength and daily living function in children with cerebral palsy. Research in Developmental Disabilities, 33(4), 1176–1182. [DOI] [PubMed] [Google Scholar]
- Wells EL, Kofler MJ, Soto EF, Schaefer H, & Sarver DE (2018). Assessing working memory in children with ADHD: Minor administration and scoring changes may improve digit span backward’s construct validity. Research in Developmental Disabilities, 72, 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children-Fourth Edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (2014). Wechsler Intelligence Scale for Children-Fourth/Fifth Edition. San Antonio: Pearson. [Google Scholar]
- Weiss G, & Hechtman LT (1993). Hyperactive children grown up: ADHD in children, adolescents, and adults. Guilford Press. [Google Scholar]
- Whalen CK, Henker B, Jamner LD, Ishikawa SS, Floro JN, Swindle R, … & Johnston JA. (2006). Toward mapping daily challenges of living with ADHD: Maternal and child perspectives using electronic diaries. Journal of Abnormal Child Psychology, 34(1), 111–126. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Brown S, Tanguay S, Monuteaux MC, Blake C, & Spencer TJ (2002). Psychiatric comorbidity and functioning in clinically referred preschool children and school-age youths with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry, 41, 262–268. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, … & Lahey BB (2012). Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. Journal of Abnormal Psychology, 121(4), 991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby MT, Blair CB, & The Family Life Project Investigators. (2016). Measuring executive function in early childhood: A case for formative measurement. Psychological Assessment, 28(3), 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS Grant I (2008). HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology, 22: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK (2001). Statistics to tell the truth, the whole truth, and nothing but the truth: formulae, illustrative numerical examples, and heuristic interpretation of effect size analyses for neuropsychological researchers. Archives of clinical neuropsychology, 16, 653–667. [PubMed] [Google Scholar]