Abstract
Posttraumatic stress disorder (PTSD) is linked to both altered physiological functioning and poorer cardiovascular health outcomes, including an increased risk for cardiovascular disease and cardiovascular-related mortality. An important question is whether interventions for PTSD might ameliorate the risk for poorer health by improving cardiovascular physiological intermediaries. To begin to characterize the literature addressing this question, we conducted a systematic review of empirical studies examining the impact of PTSD interventions on cardiovascular physiological intermediaries, including blood pressure (BP), heart rate (HR), cardiac impedance, and subclinical atherosclerosis. Outcomes included both tonic (i.e., resting) cardiovascular functioning and cardiovascular reactivity (CVR). A total of 44 studies met the inclusion criteria. There was mixed evidence regarding whether PTSD treatment improved tonic cardiovascular functioning. There was stronger evidence that PTSD treatments reduced CVR to trauma-related stressors, particularly for higher-quality studies of cognitive behavioral interventions. No studies examined cardiac impedance or subclinical atherosclerosis. The studies had a high degree of heterogeneity in the populations sampled and interventions tested. Moreover, they generally included small sample sizes and lacked control conditions. Interventions for PTSD may improve cardiovascular physiological outcomes, particularly CVR to trauma cues, although additional methodologically rigorous studies are needed. We outline changes to future research that would improve the literature regarding this important question, including the more frequent use of control groups and larger sample sizes.
Posttraumatic stress disorder (PTSD) is a psychiatric disorder characterized by intrusive memories, negative changes in cognition and mood, avoidance, and hyperarousal symptoms that occur in individuals who have been exposed to a traumatic event (American Psychiatric Association [APA], 2013). It is a prevalent and costly illness (Kilpatrick et al., 2014) that results in poorer mental and physical health (Kessler, 2000). One of the major health risks associated with PTSD is cardiovascular disease (CVD; Edmondson & von Känel, 2017), which was recently identified as a critical public health priority by the U.S. Preventive Services Task Force (2018). Individuals with PTSD are more likely to develop CVD, experience cardiovascular events, and die from cardiovascular causes than those without PTSD (Boscarino, 2008; Edmondson & Cohen, 2013; Edmondson et al., 2013; Kubzansky et al., 2007; Vaccarino et al., 2013).
Individuals with PTSD may be at increased risk of poorer cardiovascular outcomes due to alterations in relevant physiology, particularly tonic (i.e., resting) cardiovascular functioning and cardiovascular reactivity (CVR). A hallmark feature of PTSD is elevated physiological arousal (APA, 2013). Meta-analytic studies have shown that individuals with PTSD have higher levels of tonic heart rate (HR) and blood pressure (BP; Buckley & Kaloupek, 2001; Pole, 2007) as well as lower levels of HR variability (HRV; Tan et al., 2011) compared to those without PTSD. Poorer tonic cardiovascular functioning is associated with a higher risk of CVD (Fox et al., 2007; Kannel, 1996; Thayer et al., 2010) and an increased risk of early mortality (Mensink & Hoffmeister, 1997; Prospective Studies Collaboration, 2002). Increased CVR to stressors, such as trauma-related stimuli (Kimble et al., 2014), may also link PTSD to poorer cardiovascular health. The frequency and intensity of CVR, particularly to trauma cues, can be increased by PTSD symptoms, which hold health relevance given links between CVR and an increased risk of later CVD and early mortality through atherosclerotic processes (Treiber et al., 2003). If interventions designed to reduce PTSD symptoms also improve cardiovascular physiological intermediaries, long-term health benefits for individuals with PTSD may also result. Figure 1 outlines this conceptual model.
Figure 1. Conceptual Model Visualizing the Hypothesized Role of Cardiovascular Physiological Dysregulation in Mediating the Observed Associations Between Posttraumatic Stress Disorder (PTSD), Altered Cardiovascular Functioning, and Poor Cardiovascular Health Outcomes.
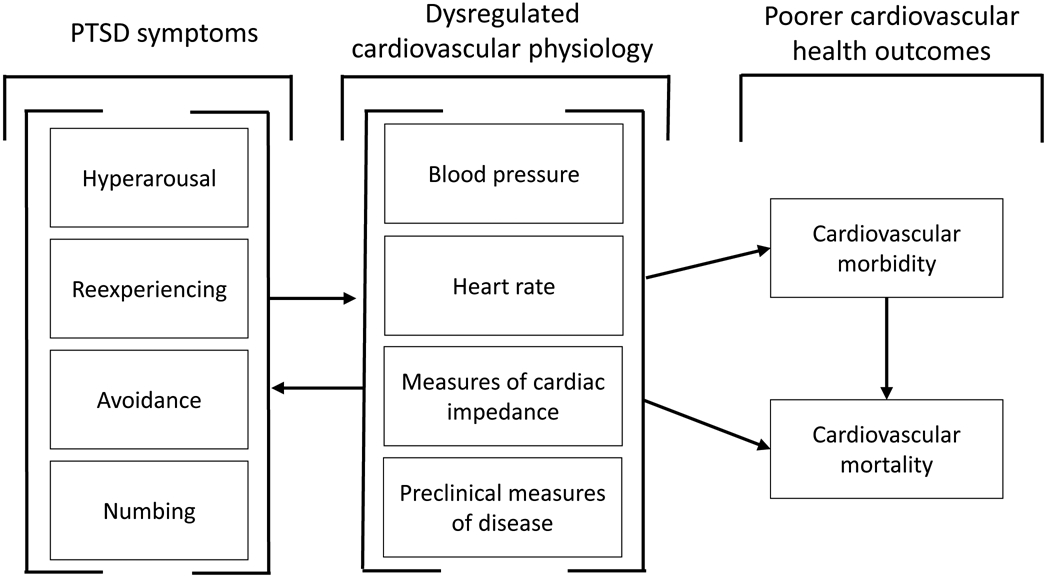
Note. Symptoms of PTSD could lead to dysregulated cardiovascular physiology via direct biologic pathways, such as increased activation of the sympathetic nervous system, or by altered health behaviors, such as increased use of substances or changes in eating patterns. In this model, it might be expected that PTSD treatment would also lead to the resolution of dysregulated cardiovascular physiology and improved cardiovascular health outcomes. Alternatively, variation in cardiovascular physiology before any traumatic stress exposure may be associated with a differential risk of developing PTSD; such a model would not predict that PTSD treatment would lead to altered cardiovascular physiology or improved health outcomes unless it did so via secondary or indirect effects, such as teaching generalizable skills for decreasing anxiety or improving sleep hygiene.
There are a number of efficacious treatments for PTSD. The U.S. Veterans Affairs and Department of Defense (VA/DoD; 2017) designated trauma-focused psychotherapies—particularly those with a primary exposure and/or cognitive restructuring component—as first-line treatments, with pharmacological approaches or other evidence-based individual psychotherapy treatment recommended in cases where such treatments are not available or individuals seeking treatment are not interested in such approaches. A number of additional treatments have also been used to treat PTSD symptoms (e.g., HRV biofeedback; Reyes, 2014). Despite the availability of effective treatments for PTSD and established cardiovascular health risks for individuals with PTSD, few studies have examined whether PTSD interventions improve CVD outcomes (National Heart, Lung, and Blood Institute, 2018; see Burg et al. 2017 for an example). It is possible that when PTSD treatments reduce posttraumatic symptoms, they also result in concomitant improvement in cardiovascular physiological intermediaries. If this were the case, it could reduce the risk for CVD or early death among individuals with PTSD. In this systematic review, we summarize the empirical evidence examining whether interventions designed to treat PTSD improve cardiovascular physiological intermediaries in the form of lower levels of tonic HR and BP, higher HRV, and reduced CVR.
Method
The study selection process was based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (i.e., PRISMA) guidelines (Moher et al., 2009) and was preregistered in the PROSPERO database (CRD42019131029. The MEDLINE, PubMED, and PsychINFO search databases were used, and search terms included several variations of “PTSD” combined with measures of cardiovascular physiology. Full search terms and search strings are available online (https://osf.io/pvewy/). The searches were current to November 6, 2019, and resulted in 792 unique abstracts for review. To meet study inclusion criteria, articles needed to (a) be a peer-reviewed empirical journal article, excluding systematic reviews, case studies, and other similar article types; (b) include human participants; (c) not include participants under 18 years of age; (d) include an intervention that targeted PTSD symptoms; (e) include at least one posttreatment physiological outcome variable; and (f) include a sample that was selected for PTSD symptomology.
The results of the article selection and exclusion process are outlined in Figure 2. The first author reviewed all abstracts, and the last author double-coded a randomly selected subsample (19.1%, n = 151). The double-coded subsample resulted in 100% agreement regarding inclusion and exclusion criteria and 99.3% agreement on the specific reason for exclusion. Of the original 792 abstracts, 705 abstracts were excluded after the initial review. Of the remaining 87 articles, 43 were excluded after a full review. This resulted in a final sample of 44 articles for inclusion and study data extraction. The first author coded all studies, with coding independently checked by a second author. All disagreements were reconciled through discussion and included a third author as needed.
Figure 2. Flowchart Outlining Article Exclusion and Inclusion for the Final Data Extraction and Synthesis.
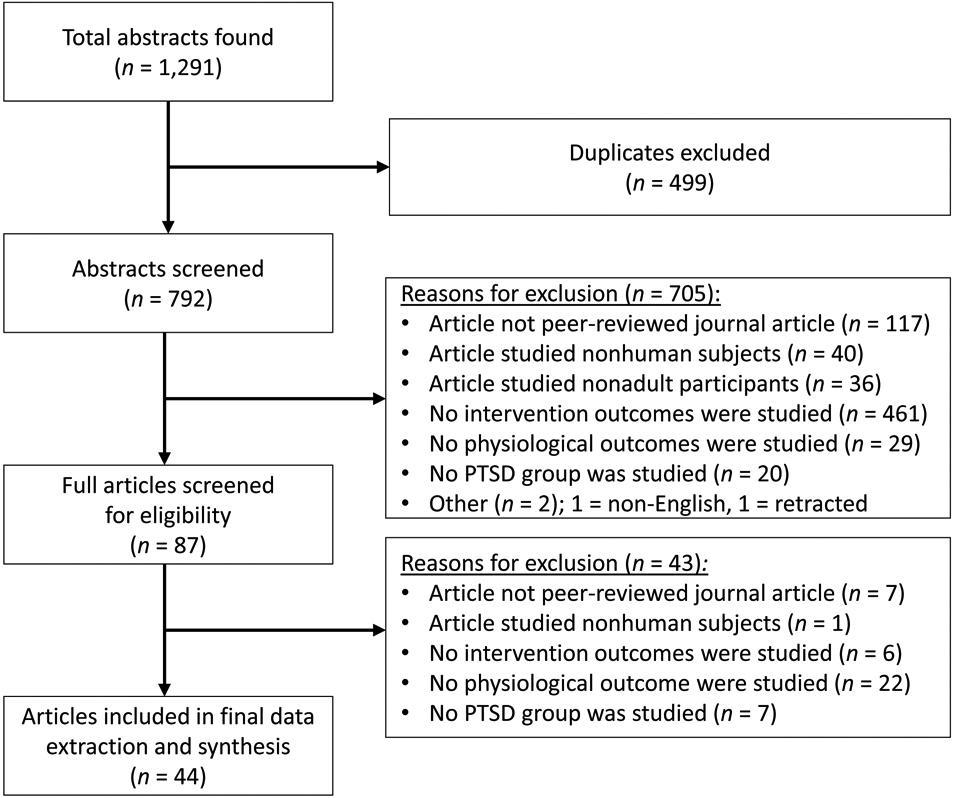
Note. Reasons for exclusions were recorded in order from the top to bottom category. PTSD = posttraumatic stress disorder.
Study characteristics included demographic information, intervention type, treatment length, and the population sampled. Participants selected for non-PTSD comparison groups were not included in these data. The results for studies that did not include a control group described the change in the outcomes over the course of treatment, whereas studies with control groups described differences between control and treatment conditions. Outcomes reported in the included studies reflected the measurement occasion most recently following treatment. We coded study quality using a previously published system (McCann et al., 2014), which we adapted slightly for the present review. Four of the eight criteria were broadened to allow coding for all studies included in the review (e.g., medication trials), and one criterion was added to assess whether the study included a control group. Studies received 1 point for each of the nine quality items making up the 10-point scale (range: 0–9). All items are included in Supplemental Table 2.
Results
Study Demographic Characteristics
In total, 44 articles met the inclusion criteria, with a mean publication year of 2010 (SD = 7). The 1,766 total participants across the 44 studies were an average of 41.7 years old, 65.1% were male, and 30.5% were reported as a minority of ethnicity and/or race. The mean sample size was 40.1 participants (SD = 50.0). There were 19 community samples (43.2%), 16 veteran or active duty military (36.4%), and eight samples (18.2%) that included participants who had experienced a specific type of traumatic event (e.g., sexual assault, motor vehicle accident). Treatments averaged 8.9 weeks (SD = 10.5) in length. Twelve studies (27.2%) primarily tested psychopharmacological interventions, 24 studies (54.5%) primarily tested psychosocial interventions, and 8 (18.2%) studies primarily tested other (e.g., nonpharmacologic, nonpsychosocial) interventions. Of the 41 studies that reported PTSD symptom outcomes, 38 (92.7%) reported a significant decrease in PTSD symptoms. Full data are summarized in Supplementary Table 1.
Outcome Measures
Tonic cardiovascular functioning outcomes included resting BP, HR, and HRV. Measures of HRV included low- and high-frequency domains, standard deviations of beat-to-beat intervals, root mean squares of the successive differences, and respiratory sinus-arrhythmia. Outcomes related to CVR included HR and BP reactivity to general and trauma-specific stressors. No studies examined measures of cardiac impedance (e.g., preejection period, cardiac output) or subclinical atherosclerosis.
Pharmacological Interventions
Twelve studies focused on a pharmacological intervention: six studies reported on selective serotonin reuptake inhibitors (SSRIs), three studies on the α-1 adrenergic receptor antagonist prazosin, two studies on the nonselective β-adrenoreceptor antagonist propranolol, and one study that reported on both propranolol and the glucocorticoid/progesterone antagonist mifepristone. Five studies examined tonic cardiovascular functioning outcomes. Two studies that tested SSRI effects on HRV found contrasting results: HRV increased after treatment in one study (Cohen et al., 2000) and decreased in the other (Ramaswamy et al., 2015). Two studies that tested the effect of prazosin found no changes in supine and standing BP (Raskind et al., 2007; Raskind et al., 2013), whereas a third study found a significant decrease in BP (Raskind et al., 2018). Seven studies examined CVR outcomes: Four studies reported on SSRIs and three on propranolol or mifepristone. Two single-arm treatment studies of SSRIs (Tucker et al., 2000, 2004) found significant decreases in HR and BP reactivity to trauma-related stressors, whereas two other studies, including the only RCT of SSRIs, did not find significant changes in CVR to trauma-related (Tucker et al., 2003) or general stressors (Vermetten et al., 2006). Two studies of propranolol (Brunet et al., 2008, 2014)—one placebo-controlled study and one single-arm treatment study—found that administration prior to trauma recall reduced CVR. A third study included three separate placebo-controlled studies (one of propranolol and two of mifepristone) attempting to affect traumatic memory reconsolidation; all three placebo-controlled studies found no significant changes in CVR to trauma cues (Wood et al., 2015). These studies provided limited evidence that pharmacological interventions improved tonic cardiovascular physiology, with mixed evidence of reduced CVR to trauma cues.
Psychosocial Interventions
There were 24 studies that focused on psychosocial interventions. Of these, 15 studies reported on cognitive behavioral therapies (CBTs), six studies on eye movement desensitization and reprocessing (EMDR), two studies on multiple psychosocial interventions, and one study on autogenic training, a relaxation technique focused on statements related to somatic sensations.
Ten studies assessed changes in tonic cardiovascular functioning. Six of these studies examined the effect of CBT on tonic cardiovascular functioning. Two studies (Bourassa, Stevens, et al., 2020; Lindauer et al., 2006) found some evidence of lower tonic levels after treatment, and three studies found no effect (Blanchard et al., 2002; Griffin et al., 2012; Rabe et al., 2006;). The results of one study demonstrated that CBT increased HRV during REM sleep in treatment responders (n = 5; Nishith et al., 2003). Another study showed no difference in HR or HRV for individuals engaged in mindfulness meditation or paced breathing compared to those in an active control condition (Wahbeh et al., 2016). The authors of another study found that a combination of prolonged exposure therapy, EMDR, and exercise reduced tonic BP (Voorendonk et al., 2019). A study of EMDR found improved tonic HRV but not tonic HR in a single-arm treatment study (Sack et al., 2007). Finally, the results of a single-arm treatment study demonstrated that autogenic training improved tonic HRV (Mitani et al., 2006).
There were 20 studies that examined the effect of psychosocial interventions on CVR. Eleven of the 12 studies that tested some implementation of CBT found reduced CVR to trauma-related stressors (Blanchard et al., 2002; Dunne et al., 2012; Fecteau & Nicki, 1999; Griffin et al., 2012; Rabe et al., 2006; Lindhauer et al., 2006), including all six studies that utilized a primary exposure component (Bourassa, Stevens, et al., 2020; Loucks et al., 2019; Maples-Keller et al., 2019; Tuerk et al., 2018; Wangelin & Tuerk, 2015; Wells et al., 2015). The effects of CBT on CVR with regard to general stressors were more mixed—two studies found some evidence for reduced CVR (Hinton et al., 2009; Schubert et al. 2019), whereas two others did not (Rabe et al., 2006; Blanchard et al., 2002). Five of the six studies that examined the effect of EMDR on CVR found that EMDR reduced CVR to trauma-related stressors (Aubert-Khalfa et al., 2008; Montgomery & Ayllon, 1994; Rogers et al., 1999; Sack et al., 2008; Wahbeh et al., 2016), and one study found that EMDR attenuated CVR to a general stressor (Renfrey & Spates, 1994). Of note, none of the studies of EMDR included a control group.
These studies provide mixed evidence that psychosocial interventions improve tonic cardiovascular functioning. The 20 studies that examined CVR provided stronger evidence that psychosocial interventions reduced CVR to trauma cues. However, there was little evidence of reduced CVR to general stressors.
Other Interventions
Eight studies included nonpharmacological or nonpsychosocial interventions. Four studies examined HRV biofeedback, two studies assessed acoustic neurofeedback, and one study each looked at electroconvulsive therapy and transcranial magnetic stimulation. Seven of these studies assessed changes in tonic HRV. All four studies of HRV biofeedback found increased tonic HRV (Ginsberg et al., 2010; Reyes, 2014; Schuman & Killian, 2019; Zucker et al., 2009). The two studies of acoustic neurofeedback (Tegeler et al., 2016, 2017) also found improvements in tonic HRV. A retrospective study of maintenance electroconvulsive therapy demonstrated improved tonic HRV after 1 year of treatment (Ahmadi et al., 2018). Only one study assessed changes in CVR, and the authors found that transcranial magnetic stimulation decreased HR reactivity to a trauma script compared to sham treatment (Isserles et al., 2013). All told, these studies provide evidence of improved tonic HRV, particularly for HRV biofeedback. However, caution is warranted, as the studies generally had small sample sizes (i.e., less than 40 participants each).
Study Quality
The average study quality was 4.0 points out of 9 possible points. Twelve studies (27.2%) received two points or less, 24 studies (54.5%) received between 3 and 6 points, and eight studies received 7 points or more (18.2%). When assessing differences in quality by type of intervention, pharmacological (M quality score = 4.8) and psychosocial treatments (M quality score = 4.3) were of significantly higher quality than other types of treatment studies (M quality score = 2.3), t(19) = 2.85, p = .011 and t(31) = 2.15, p = .040 for pharmacological and psychosocial treatments, respectively.
Conclusions From Higher Quality Studies
The eight higher-quality studies (i.e., a quality score of 7 or higher) demonstrated mixed evidence for PTSD treatment effects on tonic cardiovascular outcomes. The results of two studies showed that treatment decreased tonic cardiovascular functioning (Bourassa, Stevens, et al., 2020; Raskind et al., 2018;), whereas the findings from the two other studies did not (Raskind et al., 2013; Wabeh et al. 2016). Of note, the two studies that found significant effects were the two largest studies. The effects were moderately sized, d = −0.44 for Bourassa, Stevens, et al., 2020 (N = 104, two conditions) and ds = −0.47 and −0.33 for Raskind et al., 2018 (N = 304, two conditions) and would be difficult to detect in studies with smaller numbers of participants in each condition. The results for treatment effects on CVR to trauma stressors were clearer. The four studies that included some form of a CBT all found significantly reduced CVR to trauma-related stressors following treatment (Blanchard et al., 2002; Bourassa, Stevens, et al., 2020; Tuerk et al., 2018; Wells et al., 2015). However, one treatment study of SSRIs did not demonstrate an effect on CVR (Tucker et al., 2003).
Association Between Physiological Outcomes and PTSD Symptom Improvement
Twelve studies examined the association between changes in physiological outcomes and PTSD symptom change. Four of these studies found evidence supporting an association between changes in tonic HRV and PTSD symptoms (Ginsberg et al., 2010; Wahbeh et al., 2016; Zucker et al., 2009). Three studies did not find evidence that changes in tonic HR were correlated with PTSD symptom change (Bourassa, Stevens, et al., 2020; Lindhauer et al., 2006; Sack et al., 2008). Five studies found evidence that changes in CVR to trauma cues were correlated with PTSD symptom change (Blanchard et al., 2002; Isserles et al., 2013; Lindhauer et al., 2006; Rabe et al., 2006; Tuerk et al., 2018), whereas three studies did not (Bourassa, Stevens, et al., 2020; Sack et al., 2008; Tucker et al., 2000).
Assessing the Evidence of Bias
Several possible indicators of bias were observed in the studies reviewed. First, study sample sizes were small relative to current expectations for sample size (Chow et al., 2017). Of note, there was evidence that sample sizes increased by publication date such that a more recent publication date was correlated with a larger sample size, r = .33, p = .031. Concerns regarding sample sizes were exacerbated by the fact that only three studies included any type of power analysis. This may reflect that cardiovascular physiology was an intermediary or secondary outcome. Regarding missing data, only nine studies (20.5%) reported using an intent-to-treat-methodology: Half of these studies excluded noncompleters and/or used listwise deletion. Finally, at least one physiological outcome was likely available for study, but changes in that outcome, typically tonic HR, were not tested and/or reported in 68.2% of studies.
Discussion
To our knowledge, this was the first systematic review of the literature examining PTSD intervention effects on cardiovascular physiology. Overall, the studies demonstrated mixed results regarding whether treatment reduced tonic BP and HR across all interventions, with more evidence that PTSD treatment increased tonic HRV. There was stronger evidence that PTSD treatment reduced CVR to trauma cues, particularly treatments using a CBT approach.
There were significant limitations to the reviewed literature. First, studies were heterogeneous with regard to the types of PTSD interventions tested and the populations sampled. This was partially by design—the preregistered inclusionary criteria were permissive of any intervention designed to treat PTSD. However, this heterogeneity makes comparing interventions more difficult. It is possible that different interventions are associated with different outcomes. For example, pharmacological interventions may affect physiological intermediaries via pathways independent of their efficacy for PTSD symptoms (Shores et al., 2001). Teasing apart such differences would require a larger literature base. The heterogeneity in studies also made data synthesis methods (e.g., meta-analysis) problematic, although such methods may be warranted when additional studies are published.
There were also methodological limitations to the reviewed studies. Only eight of the 44 included studies (18.2%) received 7 points or more out of nine coded categories, and most studies (63.6%) had 30 or fewer participants. Small sample sizes can result in both low power to detect effects and increased instability of effect sizes. For example, the one study that did not demonstrate that CBT significantly reduced CVR reactivity to trauma cues had a small sample size (N = 20; Fecteau & Nicki, 1999). With equal groups, the study was only powered at roughly 43% to detect a large effect size, dropping to only 20% for a medium effect. When additional studies are published, a meta-analysis that synthesizes the results of smaller studies may help alleviate these concerns.
Most of the studies reviewed (59.1%) did not include any type of control condition, making it difficult to know whether changes in cardiovascular outcomes in these single-arm treatment studies were due to PTSD treatment or alternative explanations, such as habituation to study tasks or regression to the mean. Three studies by Tucker and colleagues (2000, 2003, 2004) highlight this concern. Two single-arm treatment studies (Tucker et al., 2000, 2004) found decreased CVR to a trauma-related stressor after treatment with an SSRI; however, an RCT that tested SSRIs did not find reduced CVR when participants taking SSRIs were compared to control participants (Tucker et al., 2003).
Understanding the effects of PTSD treatment on cardiovascular functioning is important from both clinical and theoretical perspectives. Determining whether PTSD treatments affect cardiovascular physiological intermediaries is an essential step to understanding whether such treatments can ameliorate observed risks for poorer cardiovascular health among individuals with PTSD. Reductions in tonic cardiovascular functioning or CVR could net long-term improvement in cardiovascular health, as each predicts the incidence and progression of CVD and early mortality (Mensink & Hoffmeister, 1997; Prospective Studies Collaboration, 2002; Treiber et al., 2003). Evidence that PTSD treatments improve cardiovascular physiology intermediaries would make a powerful case for the importance of PTSD treatment to stakeholders, including hospital administrators, insurance companies, healthcare providers, and individuals experiencing symptoms of PTSD.
There are several pathways through which PTSD symptomology may impact cardiovascular physiology. Models that focus on psychological mechanisms of fear generalization and the role of avoidance in preventing fear extinction (e.g., Craske et al., 2008; Sripada et al., 2013) would predict that interventions that interrupt these processes should decrease CVR to trauma-related stressors (e.g., reminders of the traumatic event). This might help explain the relatively stronger evidence for the effect of CBT treatments that included elements of exposure on CVR to trauma-related stressors. Such therapies explicitly create physiological reactions to feared stimuli through imaginal and in vivo exposures, with the goal of reducing future affective and physiological reactivity to trauma cues.
Alternatively, models of PTSD pathophysiology that focus on neuromodulatory neurotransmitter signaling pathways (Geracioti et al., 2001) would make different predictions. For example, there is evidence that PTSD can change both the release and postsynaptic reactivity to noradrenaline (Nylocks et al., 2015), and these changes may occur both in the central nervous system, where they contribute to the core symptoms of PTSD, and in the peripheral nervous system, which affects cardiovascular physiology. It is less clear whether these models would predict that PTSD treatments that ameliorate subjective symptoms of PTSD would also normalize altered cardiovascular intermediaries, as there is currently limited evidence as to whether such hypothesized underlying changes in neuromodulatory neurotransmitter signaling are altered by different options for PTSD treatment. Studies such as those reviewed herein would provide initial evidence as to whether PTSD treatment might normalize altered physiology observed among individuals with PTSD. Of note, there are additional aspects of peripheral physiology implicated in PTSD that are not covered by the present review, including the renin–angiotensin (Nylocks et al., 2015), endocrine (Deslauriers et al., 2017), and immune systems (Shorter & Fink, 2017). These systems could have further implications for health risks beyond those included in the present review.
It is also possible that PTSD treatment may not affect cardiovascular health—treatment could provide individuals with skills that enable them to address some or all of their PTSD symptoms without improving their underlying cardiovascular physiology. Alternatively, the increased risk of CVD observed among individuals with PTSD may reflect a preexisting vulnerability to developing PTSD due to higher levels of physiological reactivity and may not be reversible as a result. Finally, PTSD is linked to poorer health through lifestyle correlates, such as health behaviors and changes in social support (Bergman et al., 2019; Bourassa, Smolenski, et al., 2020; Vujanovic et al., 2013). Such correlates may account for some or all of the risk for poor cardiovascular health among individuals with PTSD rather than alterations to cardiovascular physiology. These alternative explanations highlight the theoretical importance of studies that investigate the effect of PTSD treatment on cardiovascular physiological outcomes, which could provide insight into the physiological predictors and outcomes for PTSD.
There are three methodological changes that would immediately benefit the literature: larger sample sizes, the use of control conditions, and the inclusion of cardiovascular physiology as a primary outcome in PTSD treatment studies. Larger samples would allow for more accurate estimates of effects as well as tests of the moderators and mediators of such effects. Second, the use of control groups is particularly important. The types of cardiovascular assessment required for the studies included in the present review likely result in habituation to both baseline assessments of cardiovascular physiology and the tasks used to test CVR over time. Third, PTSD treatment studies should use measures of cardiovascular physiology as primary outcomes to complement reduced PTSD symptomology. This would support research aims related to both the practical goal of identifying ways to reduce cardiovascular morbidity and mortality for individuals who have experienced traumatic stress, as well as theoretical questions about the mechanisms by which treatments modulate the underlying pathophysiology of PTSD.
We did not identify any studies that assessed the change in clinical indicators of cardiovascular disease or atherosclerosis (e.g., intima-media thickness) following PTSD treatment. This absence in the current literature is striking. Measures of disease progression would be a valuable addition and provide another link to clinical endpoints. Similarly, it was noted in a 2013 review (Edmondson & Cohen, 2013) that no studies had examined whether PTSD treatment reduced the risk for cardiovascular disease, and, to our knowledge, only one other study has done so since that time (Burg et al., 2017). Although it is challenging to conduct prospective studies of cardiovascular health endpoints due to the long periods of observation and large samples required, longitudinal epidemiological datasets or electronic medical record reviews might provide opportunities to address this question in the short term (e.g., Burg et al., 2017). In the long term, future studies of PTSD treatment could include measures of cardiovascular risk and CVD outcomes, particularly those when researchers are planning to conduct long-term follow-up.
Studies of cardiovascular physiology would benefit from data on cardiovascular physiology collected using ecologically valid methods. Changes in ambulatory measures would better support the efficacy of using PTSD treatments to improve cardiovascular health, particularly given the utility of ambulatory measures of cardiovascular physiology in predicting the risk of early mortality (Dolan et al., 2005). Finally, the heterogeneity of treatments in the present review suggests the need for increased attention to how psychiatric medications and psychosocial treatments operate together to impact cardiovascular health. Many studies of psychosocial treatments included individuals who were using pharmacologic interventions and vice versa. Testing whether multiple interventions moderate PTSD treatment outcomes could provide evidence as to whether such studies generalize to community treatment, where multiple treatment modalities are common.
The results of this systematic review point to limited evidence that PTSD treatments improve tonic BP and HR levels, with mixed evidence of improved tonic HRV. There is stronger evidence that PTSD treatment reduces CVR to trauma cues, particularly for psychosocial interventions that use a cognitive behavioral approach. The characteristics of the studies included in this review highlight the need for future studies that include larger sample sizes and control groups. In addition, the absence of studies examining CVD progression and clinical outcomes presents opportunities for future study. To the extent that PTSD treatment improves cardiovascular physiology, such effects could result in important health, quality of life, and financial benefits for both individuals with PTSD and the health care systems that treat them.
Supplementary Material
Acknowledgments
This work was supported in part by a National Institute on Aging (NIA) Training Grant (T32-AG000029) for the first author and the Department of Veterans Affairs (VA) Northwest Network MIRECC and Career Development Award (IK2CX001774) from the U.S. Department of Veterans Affairs, Clinical Science Research and Development Service granted to the second author. The funding sources had no input in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The views expressed are those of the authors and do not reflect the official policy of the Department of Veterans Affairs or the U.S. Government.
Footnotes
Preregistration: PROSPERO database CRD42019131029.
Open Practices Statement
The preregistration for this study, including primary questions of interest, search strings, and study inclusion criteria, can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019131029.
References
- Ahmadi N, Moss L, Hauser P, Nemeroff C, & Atre-Vaidya N (2018). Clinical outcome of maintenance electroconvulsive therapy in comorbid posttraumatic stress disorder and major depressive disorder. Journal of Psychiatric Research, 105, 132–136. 10.1016/j.jpsychires.2018.08.023 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Author. [Google Scholar]
- Aubert-Khalfa S, Roques J, & Blin O (2008). Evidence of a decrease in heart rate and skin conductance responses in PTSD patients after a single EMDR session. Journal of EMDR Practice and Research, 2(1), 51–56. 10.1891/1933-3196.2.1.51 [DOI] [Google Scholar]
- Bergman HE, Chan PK, Cooper AA, Shirley E, Goto T, Fine T, Cohen GH, Sampson L, Ganocy S, Tamburrino M, Liberzon I, Calabrese J, Galea S, & Liberzon I (2019). Examining the relationship between PTSD symptomatology and cigarette smoking among Ohio Army National Guard soldiers. Military Behavioral Health, 7(1), 46–56. 10.1080/21635781.2018.1556139 [DOI] [Google Scholar]
- Blanchard EB, Hickling EJ, Buckley TC, Taylor AE, Vollmer A, & Loos WR (1996). Psychophysiology of posttraumatic stress disorder related to motor vehicle accidents: Replication and extension. Journal of Consulting and Clinical Psychology, 64(4), 742–751. 10.1037//0022-006x.64.4.742 [DOI] [PubMed] [Google Scholar]
- Bourassa KJ, Smolenski DJ, Edwards-Stewart A, Campbell SB, Reger GM, & Norr AM (2020). The impact of prolonged exposure therapy on social support and PTSD symptoms. Journal of Affective Disorders, 260, 410–417. 10.1016/j.jad.2019.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa KJ, Stevens ES, Katz AC, Rothbaum BO, Reger GM, & Norr AM (2020). The impact of exposure therapy on resting heart rate and heart rate reactivity among active-duty soldiers with Posttraumatic Stress Disorder. Psychosomatic Medicine, 82(1), 108–114. 10.1097/PSY.0000000000000758 [DOI] [PubMed] [Google Scholar]
- Boscarino JA (2008). A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: Implications for surveillance and prevention. Psychosomatic Medicine, 70(6), 668–676. 10.1097/PSY.0b013e31817bccaf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, & Pitman RK (2008). Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. Journal of Psychiatric Research, 42(6), 503–506. 10.1016/j.jpsychires.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Brunet A, Thomas É, Saumier D, Ashbaugh AR, Azzoug A, Pitman RK, Orr SP, & Tremblay J (2014). Trauma reactivation plus propranolol is associated with durably low physiological responding during subsequent script-driven traumatic imagery. The Canadian Journal of Psychiatry, 59(4), 228–232. 10.1177/070674371405900408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TC, & Kaloupek DG (2001). A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosomatic Medicine, 63(4), 585–594. 10.1097/00006842-200107000-00011 [DOI] [PubMed] [Google Scholar]
- Burg MM, Brandt C, Buta E, Schwartz J, Bathulapalli H, Dziura J, Edmonson DE, & Haskell S (2017). Risk for incident hypertension associated with PTSD in military veterans, and the effect of PTSD treatment. Psychosomatic Medicine, 79(2), 181–188. 10.1097/PSY.0000000000000376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow SC, Shao J, Wang H, & Lokhnygina Y (2017). Sample size calculations in clinical research (3rd ed.). Chapman and Hall/CRC. 10.1201/9781315183084 [DOI] [Google Scholar]
- Cohen H, Kotler MD, Matar M, & Kaplan Z (2000). Normalization of heart rate variability in post-traumatic stress disorder patients following fluoxetine treatment: Preliminary results. Israeli Medical Association Journal, 2(4), 296–301. https://www.ncbi.nlm.nih.gov/pubmed/10804906 [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, & Baker A (2008). Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy, 46(1), 5–27. 10.1016/j.brat.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Deslauriers J, Powell SB, & Risbrough VB (2017). Immune signaling mechanisms of PTSD risk and symptom development: Insights from animal models. Current Opinion in Behavioral Sciences, 14, 123–132. 10.1016/j.cobeha.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Hond ED, McCormack P, Staessen JA, & O’Brien E (2005). Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: The Dublin Outcome Study. Hypertension, 46(1), 156–161. 10.1161/01.HYP.0000170138.56903.7a [DOI] [PubMed] [Google Scholar]
- Dunne RL, Kenardy J, & Sterling M (2012). A randomized controlled trial of cognitive-behavioral therapy for the treatment of PTSD in the context of chronic whiplash. The Clinical Journal of Pain, 28(9), 755–765. 10.1097/AJP.0b013e318243e16b [DOI] [PubMed] [Google Scholar]
- Edmondson D, & Cohen BE (2013). Posttraumatic stress disorder and cardiovascular disease. Progress in Cardiovascular Diseases, 55(6), 548–556. 10.1016/j.pcad.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Kronish IM, Shaffer JA, Falzon L, & Burg MM (2013). Posttraumatic stress disorder and risk for coronary heart disease: A meta-analytic review. American Heart Journal, 166(5), 806–814. 10.1016/j.ahj.2013.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, & von Känel R (2017). Post-traumatic stress disorder and cardiovascular disease. The Lancet Psychiatry, 4(4), 320–329. 10.1016/S2215-0366(16)30377-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau G, & Nicki R (1999). Cognitive behavioural treatment of post-traumatic stress disorder after motor vehicle accident. Behavioural and Cognitive Psychotherapy, 27(3), 201–214. https://doi.org/0D9B53137FB04E8974D7BAF39ECDF2E0 [Google Scholar]
- Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Sendon JLL, Steg PG, Tardif J, Tavazzi L, Tendera M, & the Heart Rate Working Group. (2007). Resting heart rate in cardiovascular disease. Journal of the American College of Cardiology, 50(9), 823–830. 10.1016/j.jacc.2007.04.079 [DOI] [PubMed] [Google Scholar]
- Geracioti TD Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE, & Kasckow JW (2001). CSF norepinephrine concentrations in posttraumatic stress disorder. American Journal of Psychiatry, 158(8), 1227–1230. 10.1176/appi.ajp.158.8.1227 [DOI] [PubMed] [Google Scholar]
- Ginsberg JP, Berry ME, & Powell DA (2010). Cardiac coherence and posttraumatic stress disorder in combat veterans. Alternative Therapies in Health & Medicine, 16(4). https://search.proquest.com/docview/670770930 [PubMed] [Google Scholar]
- Griffin MG, Resick PA, & Galovski TE (2012). Does physiologic response to loud tones change following cognitive–behavioral treatment for posttraumatic stress disorder? Journal of Traumatic Stress, 25(1), 25–32. 10.1002/jts.21667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton DE, Hofmann SG, Pollack MH, & Otto MW (2009). Mechanisms of efficacy of CBT for Cambodian refugees with PTSD: Improvement in emotion regulation and orthostatic blood pressure response. CNS Neuroscience & Therapeutics, 15(3), 255–263. 10.1111/j.1755-5949.2009.00100.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB (1996). Blood pressure as a cardiovascular risk factor: Prevention and treatment. JAMA, 275(20), 1571–1576. 10.1001/jama.1996.03530440051036 [DOI] [PubMed] [Google Scholar]
- Kessler RC (2000). Posttraumatic stress disorder: The burden to the individual and to society. The Journal of Clinical Psychiatry, 61(Suppl 5), 4–14. https://pubmed.ncbi.nlm.nih.gov/10761674/ [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, & Friedman MJ (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM‐IV and DSM‐5 criteria. Journal of Traumatic Stress, 26(5), 537–547. 10.1002/jts.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble M, Boxwala M, Bean W, Maletsky K, Halper J, Spollen K, & Fleming K (2014). The impact of hypervigilance: Evidence for a forward feedback loop. Journal of Anxiety Disorders, 28(2), 241–245. 10.1016/j.janxdis.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Spiro A, Vokonas PS, & Sparrow D (2007). Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Archives of General Psychiatry, 64(1), 109–116. 10.1001/archpsyc.64.1.109 [DOI] [PubMed] [Google Scholar]
- Lindauer RT, van Meijel EP, Jalink M, Olff M, Carlier IV, & Gersons BP (2006). Heart rate responsivity to script-driven imagery in posttraumatic stress disorder: Specificity of response and effects of psychotherapy. Psychosomatic Medicine, 68(1), 33–40. 10.1097/01.psy.0000188566.35902.e7 [DOI] [PubMed] [Google Scholar]
- Loucks L, Yasinski C, Norrholm SD, Maples-Keller J, Post L, Zwiebach L, Fiorillo D, Goodin M, Janovic T, & Rothbaum BO (2019). You can do that?!: Feasibility of virtual reality exposure therapy in the treatment of PTSD due to military sexual trauma. Journal of Anxiety Disorders, 61, 55–63. 10.1016/j.janxdis.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, & Zangen A (2013). Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder: A pilot study. Brain Stimulation, 6(3), 377–383. 10.1016/j.brs.2012.07.008 [DOI] [PubMed] [Google Scholar]
- Maples-Keller JL, Rauch SA, Jovanovic T, Yasinski CW, Goodnight JM, Sherrill A, Black K, Michopoulos V, Dunlop BW, Rothbaum BO, & Norrholm SD (2019). Changes in trauma-potentiated startle, skin conductance, and heart rate within prolonged exposure therapy for PTSD in high and low treatment responders. Journal of Anxiety Disorders, 68, 102147. 10.1016/j.janxdis.2019.102147 [DOI] [PubMed] [Google Scholar]
- McCann RA, Armstrong CM, Skopp NA, Edwards-Stewart A, Smolenski DJ, June JD, Metzger-Abumkong M, & Reger GM (2014). Virtual reality exposure therapy for the treatment of anxiety disorders: An evaluation of research quality. Journal of Anxiety Disorders, 28(6), 625–631. 10.1016/j.janxdis.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Mensink GBM, & Hoffmeister H (1997). The relationship between resting heart rate and all-cause, cardiovascular, and cancer mortality. European Heart Journal, 18(9), 1404–1410. 10.1093/oxfordjournals.eurheartj.a015465 [DOI] [PubMed] [Google Scholar]
- Mitani S, Fujita M, Sakamoto S, & Shirakawa T (2006). Effect of autogenic training on cardiac autonomic nervous activity in high-risk fire service workers for posttraumatic stress disorder. Journal of Psychosomatic Research, 60(5), 439–444. 10.1016/j.jpsychores.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. 10.1136/bmj.b2535 [DOI] [PubMed] [Google Scholar]
- Montgomery RW, & Ayllon T (1994). Eye movement desensitization across subjects: Subjective and physiological measures of treatment efficacy. Journal of Behavior Therapy and Experimental Psychiatry, 25, 217–230. 10.1016/0005-7916(94)90022-1 [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute. (2018). NHLBI Working Group: The cardiovascular consequences of post-traumatic stress disorder: Workshop recommendations. U.S. Department of Health & Human Services. [Google Scholar]
- Nishith P, Duntley SP, Domitrovich PP, Uhles ML, Cook BJ, & Stein PK (2003). Effect of cognitive behavioral therapy on heart rate variability during REM sleep in female rape victims with PTSD. Journal of Traumatic Stress, 16(3), 247–250. 10.1023/A:1023791906879 [DOI] [PubMed] [Google Scholar]
- Nylocks KM, Michopoulos V, Rothbaum AO, Almli L, Gillespie CF, Wingo A, Habib L, Gamwell KL, Marvar PJ, Bradley B & Ressler KJ, (2015). An angiotensin‐converting enzyme (ACE) polymorphism may mitigate the effects of angiotensin‐pathway medications on posttraumatic stress symptoms. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 168(4), 307–315. 10.1002/ajmg.b.32313 [DOI] [PubMed] [Google Scholar]
- Pole N (2007). The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin, 133(5), 725–746. 10.1037/0033-2909.133.5.725 [DOI] [PubMed] [Google Scholar]
- Prospective Studies Collaboration. (2002). Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. The Lancet, 360(9349), 1903–1913. 10.1016/S0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- Rabe S, Dörfel D, Zöllner T, Maercker A, & Karl A (2006). Cardiovascular correlates of motor vehicle accident-related posttraumatic stress disorder and its successful treatment. Applied Psychophysiology and Biofeedback, 31(4), 315–330. 10.1007/s10484-006-9027-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Selvaraj V, Driscoll D, Madabushi JS, Bhatia SC, & Yeragani V (2015). Effects of escitalopram on autonomic function in posttraumatic stress disorder among veterans of operations Enduring Freedom and Iraqi Freedom (OEF/OIF). Innovations in Clinical Neuroscience, 12(5–6), 13–19. https://pubmed.ncbi.nlm.nih.gov/26155373/ [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Chow B, Harris C, Davis-Karim A, Holmes HA, Hart KL, McFall M, Mellman TA, Reist C, Romesser J, Rosenheck R, Shih M, Stein MB, Swift R, Gleason T, Lu Y, & Huang GD (2018). Trial of prazosin for post-traumatic stress disorder in military veterans. New England Journal of Medicine, 378(6), 507–517. 10.1056/NEJMoa1507598 [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, Shoefer J, O’Connell J, Taylor F, Gross C, Rohde K & McFall ME (2007). A parallel group placebo-controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biological Psychiatry, 61(8), 928–934. 10.1016/j.biopsych.2006.06.032 [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, Homas D, Hill J, Daniels C, Calohan J, Millard SP Rohde K, O’Connell J, Pritzl D, Fieszli K, Petrie EC, Gross C, Mayer CL, Freed MC, Engel C, Perkind ER (2013). A trial of prazosin for combat trauma PTSD with nightmares in active duty soldiers returned from Iraq and Afghanistan. American Journal of Psychiatry, 170(9), 1003–1010. 10.1176/appi.ajp.2013.12081133 [DOI] [PubMed] [Google Scholar]
- Renfrey G, & Spates CR (1994). Eye movement desensitization: A partial dismantling study. Journal of Behavior Therapy and Experimental Psychiatry, 25(3), 231–239. 10.1016/0005-7916(94)90023-X [DOI] [PubMed] [Google Scholar]
- Reyes FJ (2014). Implementing heart rate variability biofeedback groups for veterans with posttraumatic stress disorder. Biofeedback, 42(4), 137–142. 10.5298/1081-5937-42.4.02 [DOI] [Google Scholar]
- Rogers S, Silver SM, Goss J, Obenchain J, Willis A, & Whitney RL (1999). A single session, group study of exposure and eye movement desensitization and reprocessing in treating posttraumatic stress disorder among Vietnam War veterans. Journal of Anxiety Disorders, 13(1–2), 119–130. 10.1016/S0887-6185(98)00043-7 [DOI] [PubMed] [Google Scholar]
- Sack M, Hofmann A, Wizelman L, & Lempa W (2008). Psychophysiological changes during EMDR and treatment outcome. Journal of EMDR Practice and Research, 2(4), 239–246. 10.1891/1933-3196.2.4.239 [DOI] [Google Scholar]
- Sack M, Lempa W, & Lamprecht F (2007). Assessment of psychophysiological stress reactions during a traumatic reminder in patients treated with EMDR. Journal of EMDR Practice and Research, 1(1), 15–23. 10.1891/1933-3196.1.1.15 [DOI] [Google Scholar]
- Schubert CF, Schreckenbach M, Kirmeier T, Gall-Kleebach DJ, Wollweber B, Buell DR, Uhr M, Rosner R, & Schmidt U (2019). PTSD psychotherapy improves blood pressure but leaves HPA axis feedback sensitivity stable and unaffected: First evidence from a pre–post-treatment study. Psychoneuroendocrinology, 100, 254–263. 10.1016/j.psyneuen.2018.10.013 [DOI] [PubMed] [Google Scholar]
- Schuman DL, & Killian MO (2019). Pilot study of a single-session heart rate variability biofeedback intervention on veterans’ posttraumatic stress symptoms. Applied Psychophysiology and Biofeedback, 44(1), 9–20. 10.1007/s10484-018-9415-3 [DOI] [PubMed] [Google Scholar]
- Shores MM, Pascualy M, Lewis NL, Flatness D, & Veith RC (2001). Short-term sertraline treatment suppresses sympathetic nervous system activity in healthy human subjects. Psychoneuroendocrinology, 26(4), 433–439. 10.1016/S0306-4530(01)00002-6 [DOI] [PubMed] [Google Scholar]
- Shorter E, & Fink M (2010). Endocrine psychiatry: Solving the riddle of melancholia. Oxford University Press. [Google Scholar]
- Sripada RK, Garfinkel SN, & Liberzon I (2013). Avoidant symptoms in PTSD predict fear circuit activation during multimodal fear extinction. Frontiers in Human Neuroscience, 7, 672. 10.3389/fnhum.2013.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G, Dao TK, Farmer L, Sutherland RJ, & Gevirtz R (2011). Heart rate variability (HRV) and posttraumatic stress disorder (PTSD): A pilot study. Applied Psychophysiology and Biofeedback, 36(1), 27–35. 10.1007/s10484-010-9141-y [DOI] [PubMed] [Google Scholar]
- Tegeler CH, Cook JF, Tegeler CL, Hirsch JR, Shaltout HA, Simpson SL, Fidali BC, Gerdes L, & Lee SW (2016). Clinical, hemispheric, and autonomic changes associated with the use of closed-loop, allostatic neurotechnology by a case series of individuals with self-reported symptoms of post-traumatic stress. BMC Psychiatry, 17(1), 141. 10.1186/s12888-017-1299-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeler CL, Gerdes L, Shaltout HA, Cook JF, Simpson SL, Lee SW, & Tegeler CH (2017). Successful use of closed-loop allostatic neurotechnology for post-traumatic stress symptoms in military personnel: Self-reported and autonomic improvements. Military Medical Research, 4(1), 38. 10.1186/s40779-017-0147-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, & Brosschot JF (2010). The relationship of autonomic imbalance, heart rate variability, and cardiovascular disease risk factors. International Journal of Cardiology, 141(2), 122–131. 10.1016/j.ijcard.2009.09.543 [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, & Taylor T (2003). Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine, 65(1), 46–62. https://doi.org/0033-3174/03/6501-0046 [DOI] [PubMed] [Google Scholar]
- Tucker P, Beebe KL, Burgin C, Wyatt DB, Parker DE, Masters BK, & Nawar O (2004). Paroxetine treatment of depression with posttraumatic stress disorder: Effects on autonomic reactivity and cortisol secretion. Journal of Clinical Psychopharmacology, 24(2), 131–140. https://www.ncbi.nlm.nih.gov/pubmed/15206659 [DOI] [PubMed] [Google Scholar]
- Tucker P, Potter-Kimball R, Wyatt DB, Parker DE, Burgin C, Jones DE, & Masters BK (2003). Can physiologic assessment and side effects tease out differences in PTSD trials? A double-blind comparison of citalopram, sertraline, and placebo. Psychopharmacological Bulletin, 37(3), 135–149. https://www.ncbi.nlm.nih.gov/pubmed/14608246 [PubMed] [Google Scholar]
- Tucker P, Smith KL, Marx B, Jones D, Miranda R Jr, & Lensgraf J (2000). Fluvoxamine reduces physiologic reactivity to trauma scripts in posttraumatic stress disorder. Journal of Clinical Psychopharmacology, 20(3), 367–372. http://www.ncbi.nlm.nih.gov/pubmed/10831026 [DOI] [PubMed] [Google Scholar]
- Tuerk PW, Wangelin BC, Powers MB, Smits JA, Acierno R, Myers US, Orr SP, Foa EB, & Hamner MB (2018). Augmenting treatment efficiency in exposure therapy for PTSD: A randomized double-blind placebo-controlled trial of yohimbine HCl. Cognitive Behaviour Therapy, 47(5), 351–371. 10.1080/16506073.2018.1432679 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs & Department of Defense. (2017). VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
- U.S. Preventive Services Task Force. (2018) Eighth annual report to Congress on high-priority evidence gaps for clinical preventive services. https://www.uspreventiveservicestaskforce.org/Page/Name/eighth-annual-report-to-congress-on-high-priority-evidence-gaps-for-clinical-preventive-services [PubMed]
- Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, Votaw JR, Forsberg CW, & Bremner JD (2013). Post-traumatic stress disorder and incidence of coronary heart disease: A twin study. Journal of the American College of Cardiology, 62(11), 970–978. 10.1016/j.jacc.2013.04.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Schmahl C, De Kloet C, Southwick SM, Charney DS, & Bremner JD (2006). Alterations in stress reactivity after long‐term treatment with paroxetine in women with posttraumatic stress disorder. Annals of the New York Academy of Sciences, 1071(1), 184–202. 10.1196/annals.1364.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorendonk EM, Sanches SA, De Jongh A, & Van Minnen A (2019). Improvements in cardiorespiratory fitness are not significantly associated with post-traumatic stress disorder symptom reduction in intensive treatment. European Journal of Psychotraumatology, 10(1), 1654783. 10.1080/20008198.2019.1654783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic AA, Farris SG, Harte CB, Smits JA, & Zvolensky MJ (2013). Smoking status and exercise in relation to PTSD symptoms: A test among trauma-exposed adults. Mental Health and Physical Activity, 6(2), 132–138. 10.1016/j.mhpa.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Goodrich E, Goy E, & Oken BS (2016). Mechanistic pathways of mindfulness meditation in combat veterans with posttraumatic stress disorder. Journal of Clinical Psychology, 72(4), 365–383. 10.1002/jclp.22255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangelin BC, & Tuerk PW (2015). Taking the pulse of prolonged exposure therapy: Physiological reactivity to trauma imagery as an objective measure of treatment response. Depression and Anxiety, 32(12), 927–934. 10.1002/da.22449 [DOI] [PubMed] [Google Scholar]
- Wells A, Walton D, Lovell K, & Proctor D (2015). Metacognitive therapy versus prolonged exposure in adults with chronic post-traumatic stress disorder: A parallel randomized controlled trial. Cognitive Therapy and Research, 39(1), 70–80. 10.1007/s10608-014-9636-6 [DOI] [Google Scholar]
- Wood NE, Rosasco ML, Suris AM, Spring JD, Marin MF, Lasko NB, Goetz JM, Fischer AM, Orr SP, & Pitman RK (2015). Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: Three negative psychophysiological studies. Psychiatry Research, 225(1–2), 31–39. 10.1016/j.psychres.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Zucker TL, Samuelson KW, Muench F, Greenberg MA, & Gevirtz RN (2009). The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: A pilot study. Applied Psychophysiology and Biofeedback, 34(2), 135–143. 10.1007/s10484-009-9085-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


