Abstract
Objective
To evaluate pregnancy and neonatal outcomes, disease severity, and mother-to-child transmission of pregnant women with Chikungunya infection (CHIKV).
Design
Retrospective observational study.
Setting
Grenada.
Population
Women who gave birth during a Chikungunya outbreak between January 2014 and September 2015 were eligible.
Methods
This descriptive study investigated 731 mother-infant pairs who gave birth during a CHIKV outbreak. Women and infants underwent serological testing for CHIKV by ELISA.
Main outcome measures
Primary outcomes: composite pregnancy complication (abruption, vaginal bleeding, preterm labour/cervical incompetence, cesarean delivery for fetal distress/abruption/placental abnormality or delivery for fetal distress) and composite neonatal morbidity.
Results
Of 416 mother-infant pairs, 150 (36%) had CHIKV during pregnancy, 135 (33%) had never had CHIKV, and 131 (31%) had CHIKV outside of pregnancy. Mean duration of joint pain was shorter among women infected during pregnancy (μ = 898 days, σ = 277 days) compared with infections outside of pregnancy (μ = 1064 days, σ = 244 days) (P < 0.0001). Rates of pregnancy complications (RR = 0.76, P = 0.599), intrapartum complications (RR = 1.50, P = 0.633), and neonatal outcomes were otherwise similar. Possible mother-to-child transmission occurred in two (1.3%) mother-infant pairs and two of eight intrapartum infections (25%).
Conclusion
CHIKV infection during pregnancy may be protective against long-term joint pain sequelae that are often associated with acute CHIKV infection. Infection during pregnancy did not appear to pose a risk for pregnancy complications or neonatal health, but maternal infection just prior to delivery might have increased risk of mother-to-child transmission of CHIKV.
Keywords: Chikungunya, mother-to-child-transmission, neonatal outcomes, pregnancy, pregnancy outcomes, vertical transmission
Tweetable abstract
Chikungunya infection did not increase risk of pregnancy complications or adverse neonatal outcomes, unless infection was just prior to delivery.
Introduction
Chikungunya virus (CHIKV) is a mosquito-borne virus with recent epidemic global spread to Americas, Africa, Asia and Europe.1 The name originates from the Makonde language meaning ‘disease that bends up joints’, referencing its hallmark symptom of crippling joint pain2 which may last for months or even years.3,4 In 2013, CHIKV reemerged as a large scale-outbreak in the Americas and, thus far, has infected over 1 million individuals in this region.5 Grenada, located in the Caribbean, experienced a large outbreak of CHIKV from August through December of 2014, with conservative estimates that 60% of the island population was infected.6,7
Prior studies of CHIKV during pregnancy have found that maternal-to-child transmission (MTCT) occurs almost exclusively in the setting of intrapartum maternal viraemia.8-10 In cases of MTCT, neonatal sequelae are common, including neurocognitive delays or postnatal onset of microcephaly.10-12 However, little is known about the impact of CHIKV infection on other pregnancy-related outcomes.13-16 Multiple studies have identified no significant maternal complications;14,15 however, one study demonstrated an association with pre-eclampsia, haemorrhage and sepsis.16
Detection of maternal and neonatal IgG and IgM antibody testing against CHIKV can help determine timing and mode of transmission leading to neonatal infection. Neonatal IgG can be detected in the newborn from passive placental transmission or can indicate fetal infection. Neonatal IgG antibodies that are acquired transplacentally from the mother can be detected in the neonate for the first few weeks to months of life.17,18 Because IgM cannot be transported across the placenta, the presence of IgM antibodies in the newborn reflects either fetal or neonatal disease.19
In this study, we investigated maternal, pregnancy and neonatal outcomes via interviews among pregnant women with laboratory-confirmed CHIKV infection during the 2014 CHIKV outbreak on the tri-island state of Grenada, West Indies. The objectives were to determine whether infection during pregnancy affects pregnancy and neonatal outcomes, if infection during pregnancy impacts the course of the disease, and to estimate rates of MTCT by interviewing women and performing serological testing 2–3 years following the outbreak. Our primary outcomes included composite pregnancy complication (defined as placental abruption, vaginal bleeding, preterm labour/cervical incompetence, caesarean delivery for fetal distress/abruption/placental abnormality or delivery for fetal distress) and composite neonatal morbidity (defined as haematological complications, seizures, admission to the neonatal ICU, failure to thrive, skin complication, congenital abnormality, infection or CHIKV diagnosis).
Methods
Study overview
This retrospective observational study recruited women who were pregnant or who gave birth during the Grenadian CHIKV outbreak from August through December of 2014 in Grenada. To include mothers exposed in pregnancy to CHIKV and a comparative cohort of unexposed women, mothers who delivered infants born between January 2014 and September 2015 were considered eligible for study inclusion. Mother-child pairs were enrolled from November 2016 to March 2018. Research assistants collected demographic, socio-economic, pregnancy and birth information from mothers and administered CHIKV-specific surveys. Maternal and infant sera were collected at enrolment. Institutional review boards at Stanford University (IRB-34992) and St. George’s University (IRB # 16026) approved the study protocol. The study was funded by a National Institute of Health Fogarty International Center, grant #1R21TW010536-01.
Enrolment criteria
We defined our study group as women who were pregnant or gave birth around the time of the CHIKV outbreak in Grenada (August–December 2014), in which at least 60% of the population is estimated to have been infected with the virus.6,7 Women were eligible if they gave birth to a liveborn child between January 2014 and September 2015, a time period that coincided with potential CHIKV exposure during gestation. Live birth information was obtained from all 34 medical stations and five health centres in the tri-island state of Grenada, Carriacou and Petite Martinique, so gestational losses were eliminated. An enumeration list of all births in Grenada between 2014 and 2015 was created from birth records and the telephone contact of the mother extracted. Mothers who responded to a telephone call and showed interest in participating in the study were interviewed in person when their infant was 2 years of age (24–26 months). Maternal and neonatal data were obtained from mothers’ antenatal clinic cards and maternal self-report. Mother-child pairs were excluded if the woman was not a permanent resident of the study catchment area, did not give birth during the pre-specified dates or if the infant no longer was in the woman’s care at the time of study enrolment (i.e. neonatal death or stillbirth).
Laboratory testing
Women and their offspring (aged 2 years) underwent serological testing for CHIKV by enzyme-linked immunosorbent assay (ELISA) (InBios CHIKjj IgG kit).20 The serum was tested locally and results were disseminated to health centres and mothers. For any neonate who tested positive for CHIKV IgG, the presence of CHIKV IgM was assessed by ELISA (InBios CHIKjj IgM kit) in maternal and/or neonatal serum to investigate timing of CHIKV exposure.
CHIKV IgG antibodies detected in child serum originated from a fetal (in utero) infection or postnatal infection (infection occurring after birth). By waiting to test children over 23 months of age (upon completion of maternal IgG antibody degradation in infant circulation), we eliminated the possibility of detecting maternally sourced IgG in our infant cohort.21,22 In addition, given the absence of CHIKV circulation reported after the official end of the epidemic in Grenada, infants were less likely to have been infected postnatally. Those from the study group who tested positive for CHIKV IgG antibodies were considered to be likely infected while in utero.
Exposure definitions
We defined exposed mothers as those who tested positive for IgG CHIKV antibodies at time of enrollment and who recalled onset of typical symptomatic CHIKV infection during pregnancy (joint pain in combination with muscle pain, rash or fever, as presented in 85% of IgM- or polymerase chain reaction (PCR)-positive CHIKV cases during the CHIKV outbreak in Grenada7). Because CHIKV infection typically presents with acute and distinctly unique clinical characteristics, we relied on self-reported symptom onset, assuming a low proportion of asymptomatic infections (< 10%) in Grenada given the 60% attack rate.6,7 To support this, an inverse correlation was noted between proportions of seropositive subjects and asymptomatic infections.23,24
We compared our exposed group with one of two groups depending on the study question. To assess the maternal and perinatal impacts of CHIKV, we contrasted the pregnancy and neonatal outcomes of our exposed group to pregnant women without CHIKV infection (unexposed group: negative serum IgG antibody testing for CHIKV). To assess characteristics of CHIKV infection onset during pregnancy versus outside of pregnancy, we contrasted our exposed group to our second comparator group, which consisted of women who tested positive for CHIKV IgG antibodies and reported CHIKV infection outside of pregnancy (see Box 1).
Box.
Overview of participants and exposure definitions by exposed group and control groups 1 and 2
| Exposed group: Maternal CHIKV during pregnancy |
Control group 1: No history of maternal CHIKV |
Control group 2: Maternal CHIKV outside of pregnancy |
|---|---|---|
| Pregnant during CHIKV outbreak | Pregnant during CHIKV outbreak | Pregnant during CHIKV outbreak |
| and | and | and |
| Reported history of CHIKV during pregnancy (joint pain and fever, rash or muscle pain | CHIKV IgG-negative | Reported history of CHIKV outside of pregnancy (joint pain and fever, rash or muscle pain) |
| and | and | |
| CHIKV IgG positive | CHIKV IgG-positive |
Children who tested negative for CHIKV IgG were considered not to have been exposed to CHIKV. All children who tested positive for CHIKV IgG antibodies had CHIKV IgM testing performed. CHIKV IgM antibodies often persist for at least 6 months after infection and can persist up to 2 years.25,26 Children born to mothers with CHIKV during pregnancy (exposed group) who tested positive for IgG antibodies were considered to have in utero CHIKV infection. Children born to mothers with no evidence of CHIKV during pregnancy (unexposed or infected outside of pregnancy groups) were considered to have been infected postnatally.
Core outcomes and definitions
We collected data on pregnancy and neonatal outcomes per maternal report, postnatal health records and antenatal clinic cards. Pregnancy-associated illness included: diabetes or hypertensive disorders (such as GDM, pre-eclampsia, gestational hypertension). We defined ‘pregnancy complication’ as presence of any of the following: placental abruption, vaginal bleeding, preterm labour/cervical incompetence, caesarean delivery for fetal distress/abruption/placental abnormality and delivery for fetal distress. We defined ‘intrapartum complication’ as pregnancy complications that impacted the intrapartum period, including delivery for fetal distress, abruption, shoulder dystocia, cervical insufficiency, abruption and meconium-stained amniotic fluid. We intentionally overlapped the categories of ‘pregnancy complication’ and ‘intrapartum complication’ because of the inextricable overlap between many intrapartum and antenatal pregnancy complications. Persistence of maternal joint pain in days was calculated based on self-reported joint pain at 1 month prior to, 1 week prior to, and on the date of the enrolment.
For neonates, composite neonatal morbidity comprised haematological complications, seizures, admission to the neonatal ICU, failure to thrive, skin complication, congenital abnormality, infection or CHIKV diagnosis. Composite severe neonatal morbidity comprised seizures, admission to neonatal ICU or respiratory distress.
Statistical analysis
To study how infection during pregnancy affects maternal and neonatal outcomes, we used bivariate analyses to compare the listed core outcomes for mothers with and without CHIKV during pregnancy. In these, P-values were determined using Student’s t-test or the Mann–Whitney U-test for continuous variables and the Chi-square test and Fisher’s exact test for categorical variables. Additionally, we assessed independent predictors of composite outcomes among children of mothers with or without CHIKV infection during pregnancy in multivariable zero-inflated beta-binomial regression analyses, while controlling for potential confounders (gestational age at delivery, mode of delivery and maternal age).
To explore how pregnancy affects the course of CHIKV infection, we constructed life tables and plotted persistence of joint pain since acute infection using Kaplan–Meier plots stratified by maternal CHIKV during pregnancy and maternal CHIKV outside of pregnancy (unexposed group 2). Time since acute infection in days was calculated as the difference between self-reported onset of symptoms and time at enrolment. Differences in persistent joint pain between pregnant and non-pregnant women were compared using the Log-rank test. As the immune milieu varies physiologically along the pregnancy, from a Th1 to a Th2 milieu,27,28 and chronic chikungunya arthritis partially stems from a Th1 bias3,29, we further stratified maternal CHIKV during pregnancy by trimester of pregnancy at onset of infection. To determine the influence of the timing of infection, we used a Cox proportional hazard model with non-pregnant women as the reference group. As age is another driver of CHIKV arthritis,3 we adjusted for age. All analyses were performed using R statistical language (version 1.1.383, 2017, Boston, MA, USA). P-values were adjusted using a Bonferroni correction (alpha of 5%) to allow for multiple comparisons. Missing data were excluded from the analysis (Table S5).
Results
Characteristics of participants
We enrolled 731 mother-infant pairs from November 2016 to March 2018, corresponding to 21% of total births in Grenada from 2014 to 2015. Of these, 42 were excluded due to missing maternal IgG data, 88 due to absence of symptoms compatible with acute symptomatic CHIKV infection (joint pain together with fever, body ache, muscle/bone pain or rash), and 185 because they did not answer the question CHIKV was present during pregnancy (Figures 1 and 2). After applying exclusion criteria, 416 mother-infant pairs were eligible. Of these, 150 (36%) mothers experienced CHIKV infection during pregnancy, 135 (33%) had no history or laboratory evidence of CHIKV infection, and 131 (31%) experienced CHIKV outside of pregnancy. Demographic and socio-economic characteristics of mothers were similar between the three groups (Table 1).
Figure 1.
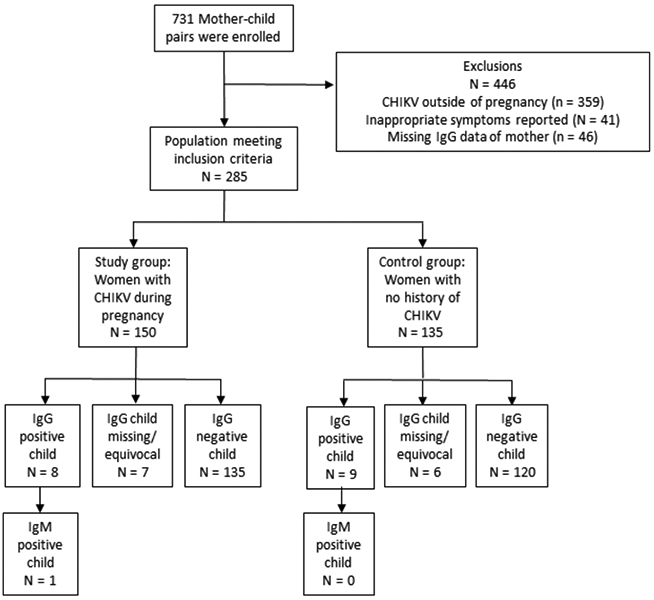
Enrolment and exposure categories for maternal chikungunya infection during pregnancy compared with no history of chikungunya infection. Compatible symptoms were joint pain and one of the following: fever, generalised body ache, muscle pain, bone pain or rash.
Figure 2.
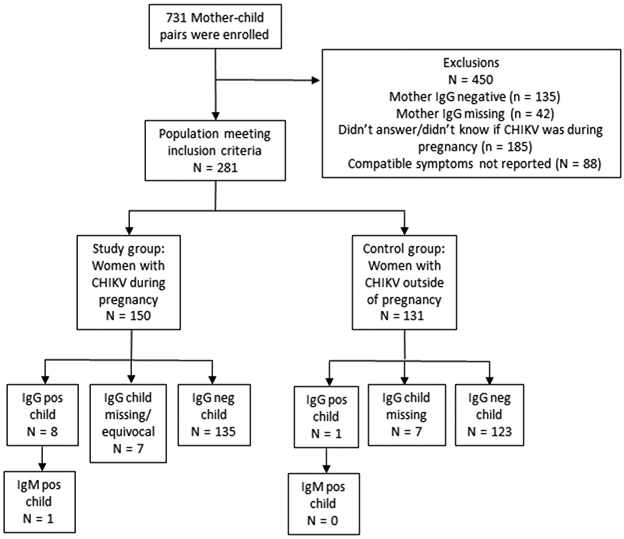
Enrolment and exposure categories for maternal chikungunya infection during pregnancy compared with chikungunya infection outside of pregnancy. Compatible symptoms were joint pain and one of the following: fever, generalised body ache, muscle painC, bone pain or rash.
Table 1.
Demographic characteristics of women by exposed and control groups 1 and 2
| Characteristic | Exposed group CHIKV infection during pregnancy (n = 150) |
Control group 1 No history of CHIKV infection (n = 135) |
Control group 2 History of CHIKV infection outside of pregnancy (n = 131) |
|---|---|---|---|
| Age (year) (mean ± SD) | 30.9 ± 6.0 | 30.2 ± 6.0 | 30.9 ± 6.3 |
| Race/ethnicity | |||
| African descent | 126 (84) | 115 (85) | 114 (87) |
| Asian descent | 7 (5) | 4 (3) | 6 (5) |
| Other | 16 (11) | 8 (6) | 10 (8) |
| Refused/Don’t know | 1 (1) | 8 (6) | 1 (1) |
| Education | |||
| Primary school | 15 (10) | 16 (13) | 14 (11) |
| Secondary school | 89 (60) | 64 (47) | 66 (50) |
| Bachelor’s degree | 17 (11) | 8 (6) | 11 (8) |
| Graduate/Professional degree | 4 (3) | 8 (6) | 2 (2) |
| Other | 24 (16) | 31 (23) | 38 (29) |
| Refused/Don’t know | 1 (1) | 8 (6) | 0 (0) |
| Occupation | |||
| Student | 6 (4) | 1 (1) | 3 (2) |
| Housewife | 8 (5) | 3 (2) | 4 (3) |
| Business/office worker | 48 (32) | 36 (28) | 43 (33) |
| Manual worker | 6 (4) | 11 (9) | 12 (9) |
| Unemployed | 28 (19) | 39 (31) | 29 (22) |
| Other | 54 (36) | 38 (30) | 40 (32) |
| Married/common-law/lives with partner | 79 (53) | 79 (63) | 74 (58) |
| Previously divorced or separated | 1 (5) | 8 (6) | 10 (8) |
| Mosquito precautions | |||
| Use of repellent/spray | 126 (85) | 99 (77) | 114 (87) |
| Use of mosquito coil/net | 95 (64) | 74 (58) | 90 (69) |
| Collect water on roof | 46 (31) | 38 (29) | 38 (330) |
| Store water in black tanks | 71 (47) | 65 (51) | 65 (50) |
| Prior pregnancies | |||
| Nulliparous | 39 (26) | 31 (23) | 26 (20) |
| Multiparous | 110 (73) | 93 (69) | 105 (80) |
| Missing | 1 (1) | 11 (8) | 0 (0) |
Values are frequency (%) or frequency ± standard deviation (SD).
Pregnancy outcomes
We found no difference in adverse pregnancy outcomes between women with CHIKV during pregnancy and women with no history of CHIKV (Table S1). Similar proportions of complications and fetal distress during the pregnancy were present for both groups.
Neonatal outcomes
We observed similar neonatal outcomes across groups (Table S2). After performing multivariable regression analysis controlling for potential confounding factors (gestational age at delivery, mode of delivery and maternal age), no differences in neonatal outcomes were observed between the groups (Table S2).
Congenital structural anomalies were rare, with a small but insignificant difference observed between groups: three children (2%) had congenital anomalies in pregnancies complicated by CHIKV infection compared with none (0%) in pregnancies without CHIKV (P = 0.273). In the three children with congenital anomalies (tracheomalacia, congenital hydrocoele, club foot), maternal CHIKV infection occurred in the third trimester.
Possible mother-to-child-transmission of CHIKV
Among 150 mothers with CHIKV infection during pregnancy, eight (5%) of their children tested positive for CHIKV IgG antibodies compared with nine (7%) children of mothers with no clinical history or laboratory evidence of CHIKV during pregnancy and one (1%) infant of a mother with CHIKV outside of pregnancy (P = 0.053). Six of these eight children were born after the outbreak ended, indicating they may have been exposed to CHIKV in utero (as circulating CHIKV was very limited during this time); however, postnatal infection cannot be ruled out. In terms of timing of infection, two mothers of these children reported CHIKV in the first trimester, two in the second trimester, and four in the third trimester. All the children were born at term, one via caesarean delivery for placental abruption and maternal haemorrhage.
Reported neonatal disease from possible MTCT of CHIKV infection occurred in two (1.3%) mother-infant pairs and two of eight intrapartum infections (25%). In both MTCT cases, mothers reported symptom onset within a few days of delivery. In the first case, a 29-year-old multiparous female reported CHIKV 2 days prior to delivery and delivered vaginally at term. She reported that her child developed CHIKV infection within 2 weeks of delivery. No further details on neonatal symptoms were available. Maternal and infant serum 2 years after delivery was IgG- and IgM-positive for the mother and IgG-positive and IgM-negative for the infant. In the second case, a 29-year-old multiparous female at 36 weeks’ gestation reported symptoms the day before delivery. She delivered vaginally without complications and her infant was reported to have CHIKV infection after delivery. No further details of neonatal symptoms were available. Serum testing performed 2 years after delivery revealed IgG antibodies in the mother and infant and equivocal IgM antibodies in the infant.
Maternal symptomatology of CHIKV
To determine whether CHIKV infection was more severe or long-term sequelae more prevalent among women with infection during pregnancy, we compared symptoms for women with CHIKV during and outside of pregnancy (Table 2). Although the case definition of reported CHIKV symptoms was required (joint pain with fever, muscle pain or rash) in all participants with CHIKV infection, women in their first or third trimesters of pregnancy reported more muscle pain compared with those with CHIKV outside of pregnancy (44 and 45% versus 17%, P = 0.001 and P = 0.002, respectively). The remainder of symptoms were similar, with the exception of persistent joint pain. Mean duration of joint pain was shorter among women infected during pregnancy (μ = 898 days, σ = 277 days) than among women infected outside of pregnancy (μ = 1064 days, σ = 244 days) (P < 0.0001) (Figure S1). When compared with women with CHIKV outside of pregnancy, women infected during pregnancy reported shorter duration of joint pain overall, with small differences by trimester of infection: third trimester (adjusted hazard ratio: 41.5, 95% CI 12.6–136.6), second trimester (adjusted hazards ratio: 30.0, 95% CI 12.4–72.6) and first trimester (adjusted hazards ratio: 5.5, 95% CI 2.9–10.5) (Tables S3 and S4).
Table 2.
Symptoms of CHIKV during pregnancy versus outside of pregnancy
| Symptom | CHIKV infection during pregnancy (n = 150) by trimester of infection |
CHIKV infection outside of pregnancy (n = 131) |
P-value | ||
|---|---|---|---|---|---|
| 1st (n = 52) | 2nd (n = 47) | 3rd (n = 42) | |||
| Joint pain* | 52 (100) | 47 (100) | 42 (100) | 131 (100) | 1 |
| Fever* | 39 (75) | 32 (68) | 32 (76) | 98 (75) | 0.795 |
| Muscle pain* | 23 (44) | 15 (32) | 19 (45) | 22 (17) | <0.001 |
| Rash* | 28 (54) | 30 (64) | 17 (41) | 64 (49) | 0.147 |
| Chills | 16 (31) | 10 (21) | 12 (29) | 25 (19) | 0.296 |
| General body ache | 20 (39) | 18 (38) | 13 (31) | 75 (57) | 0.005 |
| Bone pain | 5 (10) | 5 (11) | 5 (12) | 4 (3) | 0.057 |
| Headache | 25 (48) | 18 (38) | 18 (43) | 57 (44) | 0.809 |
| Retro-orbital pain | 12 (23) | 4 (9) | 3 (7) | 10 (8) | 0.029 |
| Vomiting | 8 (15) | 7 (15) | 6 (14) | 4 (3) | 0.003 |
| Mean duration of joint pain (SD) (days) | 935 (223) | 931 (85) | 816 (141) | 1064 (244) | <0.0001 |
Bold values indicated P < 0.05
SD, standard deviation.
Values are frequency (%) or mean (± SD).
We required that participants fulfill the case definition of CHIKV infection; this required all participants to report joint pain along with one of the following symptoms: fever, muscle pain and rash. Bonferroni-adjusted P-value = 0.005 cutoff adjusting alpha of 5% for n = 9 tests. Pairwise Chi-square test found insignificant inter-group differences at P-value threshold of 0.005.
Discussion
Main findings
In this mother-child cohort study, we did not identify any difference in adverse pregnancy outcomes or neonatal outcomes between women with CHIKV infection during pregnancy compared to women without CHIKV infection. Rare in utero MTCT of CHIKV in the intrapartum period was suspected in this study and may have led to two cases of neonatal CHIKV infection after delivery. CHIKV infection during pregnancy was associated with differing symptomatology compared with infection outside of pregnancy, with women who experienced CHIKV during pregnancy reporting more muscle and bone pain, but of a shorter duration.
In non-pregnant populations, long-term sequelae are common following CHIKV infection, including a 40% prevalence of chronic inflammatory rheumatism.30 Similarly, we found that a high proportion of women with CHIKV in pregnancy reported continued joint pain. Persistent joint pain in this population constitutes a novel finding in the field of chikungunya chronic arthritis, which deserves further investigation. Two years after acute infection, it was unexpectedly high in the younger population of parturient women, both close to the upper limit of prevalence reported with the Asian lineage of CHIKV and to the average prevalence reported with the East-Central-African diverged CHIKV lineage, the most virulent CHIKV genotype with respect to joint pain.31 We hypothesise that this high prevalence of persistent joint pain in pregnant women with CHIKV infection may be influenced by the Th1 to Th2 cytokine bias that normally occurs during the second half of gestation,27 CHIKV chronic arthritis being mainly driven by interleukin-6,32 a Th2 pro-inflammatory cytokine prevailing at the end of pregnancy.28
Maternal to child transmission of CHIKV has been reported previously8-10 and is most common during the intrapartum period, as was suspected to have occurred in this study.
Strengths and limitations
Strengths of our study include the use of a large mother-child cohort to study rare maternal outcomes and persistent symptoms during pregnancy during an epidemic period. We performed IgG and IgM antibody testing on maternal and child sera; given that IgG testing for CHIKV has little known cross-reactivity,33 specificity was likely high.
Our study is limited by the reliance on self-report rather than medical records, potentially resulting in recall bias. This includes maternal report of their child being diagnosed with CHIKV infection after delivery and maternal self-report of CHIKV symptoms. Moreover, we lacked the ability to confirm maternal timing of CHIKV infection by assessing for viraemia. We improved the specificity of CHIKV infection onset by using a case definition based on symptomatology for CHIKV infection. We postulate that timing of reported CHIKV infection was specific in our study, as CHIKV infection features easily recognisable symptoms, and our case definition of CHIKV infection features an 85% serological overlap with CHIKV IgM antibodies.7 Although CHIKV antibodies are thought to be lifelong and enrolment 2 years after the outbreak may not pose a problem for classifying maternal exposure, transplacental antibodies in the infants disappear within 2 years.22 As our focus is on pregnancy outcomes, this limitation should not impact our results. However, importantly, in this study we were unable to confirm mother-to-fetal transmission because we only tested for the presence of absence of antibodies in children more than 23 months after delivery and did not directly test umbilical cord blood of the newborn to detect maternal-to-fetal transmission. Therefore, postnatal infection could not be ruled out.
Because our sample collection occurred after the pregnancy, we had to rely on symptoms as indicators of infection and to determine timing of infection. Asymptomatic CHIKV infections occur; however, our data here can only examine the impact of symptomatic infections in pregnant women. Further limitations include a lack of generalisability, as our study was performed in Grenada. In this retrospective study, we relied on a convenience sample of mothers who were interested in participating in our study, which may have introduced bias. Additionally, we did not have access to hospital data for other maternal outcomes but instead relied on maternal report, which was prone to a memory (recall) bias towards underreporting. It is important to note that CHIKV continued to circulate in the country until 2017; however, because transmission was very limited after the outbreak period, IgG-positive infants are believed to have been primarily infected through MTCT.
Interpretation
Consistent with published literature,14 CHIKV infection during pregnancy in our cohort was not associated with an increased risk for our studied pregnancy outcomes. In a study of 658 women infected with CHIKV during pregnancy in Réunion, France, there was no increased rate of caesarean delivery, haemorrhage, congenital anomalies or preterm birth.14 Similarly, our study demonstrated comparable proportions of pregnancy complications in pregnant women with and without CHIKV infection. However, acute infection near time of delivery may confer an increased risk of not only MTCT but also pregnancy complications. For example, Gérardin et al.8 found increased fetal decelerations and caesarean deliveries among 61 women with viraemia at term, suggesting a potentially increased risk of fetal distress in affected pregnancies. Similar results were found during the 2005–2006 CHIKV outbreak across Reunion Island; 678 women had acute infections throughout pregnancy and 22 were infected intrapartum.8
Although variation exists,8,10,14,16,34 we detected similar proportions of suspected neonatal in utero CHIKV exposure and symptomatic neonatal infection to Gérardin et al.8 In their study of over 700 women with CHIKV during pregnancy, 10% of neonates had CHIKV exposure in utero, compared with 5% in our cohort. MTCT leading to symptomatic neonatal infection occurred in 2.5% (19/739) of neonates compared with 1.3% (2/150) in our cohort.8 Other studies have documented rates between 0%16,34 and 48%9 for symptomatic neonatal CHIKV infection from MTCT. Multiple studies report that symptomatic neonatal infection from MTCT is greatest with maternal infection near delivery,8,10,14 consistent with our findings. In a meta-analysis of 46 women with intrapartum symptomatic CHIKV infection, Contopoulos-Ioannidis et al.10 report a 50% risk of symptomatic neonatal disease. In the current and prior studies, CHIKV infection during pregnancy does not appear to be associated with congenital anomalies.9,14,34
In both our study and previously published literature,8,10,14 CHIKV infection does not appear convey a significant pregnancy-associated risk unless acute maternal infection occurs near delivery. In contrast, Zika virus results in adverse pregnancy outcomes in all trimesters,35,36 with congenital anomalies most common in first trimester infections.35 This is reassuring for pregnant women at risk for CHIKV infection and their providers, as the likelihood of acute infection during delivery is less than at any point during pregnancy.
Conclusion
In our cohort, CHIKV infection did not appear to increase pregnancy complications for women who gave birth to a liveborn child and who were symptomatically infected during pregnancy than for women who were uninfected. Further, the risk of neonatal complications from in utero CHIKV exposure was not increased. Overall, this study is consistent with published literature that fetal and neonatal impact is minimal if maternal infection does not occur near delivery. Long-term CHIKV symptoms are not increased in infections acquired in pregnancy compared with the general population. Although CHIKV may be symptomatic in affected mothers, pregnant women and their providers can be reassured that pregnancy outcomes are generally favourable and typically only have significant consequences if infection occurs within days of delivery.
Acknowledgments
Funding
This study was funded by NIH Fogarty International Center, grant #1R21TW010536-01 (PIs Waechter and LaBeaud, with the help of Patrick Gérardin).
Footnotes
Disclosure of interests
MEF is a consultant for the World Health Organization and ADL received grant funding from the NIH for a R21 during the study period. No other authors have any disclosures. The authors report no conflicts of interest. Completed disclosure of interest forms are available to view online as supporting information.
Details of ethics approval
The institutional review boards at Stanford University (IRB# 37739, approved 31 May 2016, and renewed 20 January 2020) and St. George’s University (IRB # 16026, approved 1 June 2016) approved the study protocol. There is no clinical trial registration as the study does not meet criteria for a clinical trial.
Data availability
Individual participant data collected during the trial will be available and shared after de-identification. The data will be available immediately following publication. A link will be made available for all data sharing.
References
- 1.Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med 2015;372:1231–9. [DOI] [PubMed] [Google Scholar]
- 2.Robinson M An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. I. Clinical features. Trans R Soc Trop Med Hyg 1955;49:28–32. [DOI] [PubMed] [Google Scholar]
- 3.Zaid A, Gérardin P, Taylor A, Mostafavi H, Malvy D, Mahalingam S. Chikungunya arthritis: implications of acute and chronic inflammation mechanisms on disease management. Arthritis Rheumatol 2018;70:484–95. [DOI] [PubMed] [Google Scholar]
- 4.Heath CJ, Lowther J, Noël TP, Mark-George I, Boothroyd DB, Mitchell G, et al. The identification of risk factors for chronic chikungunya arthralgia in Grenada, West Indies: a cross-sectional cohort study. Open Forum Infect Dis 2018;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Number of reported cases of chikungunya fever in the Americas, by country or territory, 2013–2014, Cumulative cases Epidemiological Week/EW 52. Pan Am Heal Organ. 2014. [www.paho.org/hq/index.php?option=com_topics]. Accessed 29 December 2014. [Google Scholar]
- 6.Forde MS, Martin F, Mitchell G, Bidaisee S. Public health response and lessons learned from the 2014 chikungunya epidemic in Grenada. Rev Panam Salud Publica 2017;41:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macpherson C, Noël T, Fields P, Jungkind D, Yearwood K, Simmons M, et al. Clinical and serological insights from the Asian lineage chikungunya outbreak in Grenada, 2014: an observational study. Am J Trop Med Hyg 2016;95:890–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gérardin P, Barau G, Michault A, Bintner M, Randrianaivo H, Choker G, et al. Multidisciplinary prospective study of mother-to-child chikungunya virus infections′ on the Island of La Reunion. PLoS Med 2008;5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres JR, Falleiros-Arlant LH, Dueñas L, Pleitez-Navarrete J, Salgado DM, Del CJB. Congenital and perinatal complications of chikungunya fever: a Latin American experience. Int J Infect Dis 2016;51:85–8. [DOI] [PubMed] [Google Scholar]
- 10.Contopoulos-Ioannidis AD, Newman-Lindsay S, Chow C, LaBeaud AD. Mother-to-child transmission of chikungunya virus: a systematic review and meta-analysis. PLoS Negl Trop Dis 2018;12:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gérardin P, Sampériz S, Ramful D, Boumahni B, Bintner M, Alessandri JL, et al. Neurocognitive outcome of children exposed to perinatal mother-to-child chikungunya virus infection: the CHIMERE cohort study on Reunion Island. PLoS Negl Trop Dis 2014;8:e2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos R, Viana R, Brainer-Lima A, FloreÂncio T, Carvalho MD, van der Linden V, et al. Perinatal chikungunya virus-associated encephalitis leading to postnatal-onset microcephaly and optic atrophy. Pediatr Infect Dis J 2018;37:94–5. [DOI] [PubMed] [Google Scholar]
- 13.Dotters-Katz SK, Grace MR, Strauss RA, Chescheir N, Kuller JA. Chikungunya fever: obstetric considerations on an emerging virus. Obstet Gynecol Surv 2015;70:453–7. [DOI] [PubMed] [Google Scholar]
- 14.Fritel X, Rollot O, Gerardin P, Gaüzére BA, Bideault J, Lagarde L, et al. Chikungunya virus infection during pregnancy, Réunion, France, 2006. Emerg Infect Dis 2010;16:418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Gupta N. Short-term pregnancy outcomes in patients chikungunya infection: an observational study. J Fam Med Prim Care 2019;8:985–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escobar M, Nieto AJ, Loaiza-osorio S, Barona JS, Rosso F. Pregnant women hospitalized with chikungunya virus infection, Colombia, 2015. Emerg Infect Dis 2017;23:1777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilks SC, Nimmanitya S, Nisalak A, Burke D. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg 1988;38:411–9. [DOI] [PubMed] [Google Scholar]
- 18.Hartter H, Oyedele O, Dietz K, Kreis S, Hoffman J, Muller C. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr Infect Dis J 2000;19:635–41. [DOI] [PubMed] [Google Scholar]
- 19.Malek A Role of IgG antibodies in association with placental function and immunologic diseases in human pregnancy. Expert Rev Clin Immunol 2013;9:235–49. [DOI] [PubMed] [Google Scholar]
- 20.Fritel X, Catteau C, Calliez F, Brodel A, Vaillant JY, Ansquin H. Chikungunya outbreak, pregnancy outcome and perinatal mortality: observational study about 40,000 pregnancies and deliveries on Reunion island during 2004–2006. In Proceedings of the 13th international congress on infectious diseases, 2008 June 19–22, Kuala Lumpur. Int J Infect Dis 2008;12 (Suppl 1):e328. [Google Scholar]
- 21.Watanaveeradej V, Endy TP, Simasathien S, Kerdpanich A, Polprasert N. Transplacental chikungunya virus antibody. Emerg Infect Dis 2006;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramful D, Sampériz S, Fritel X, Michault A, Jaffar-Bandjee M, Rollot O. et al. Antibody kinetics in infants exposed to chikungunya virus infection during pregnancy reveals absence of congenital infection. J Infect Dis 2014;209:1726–30. [DOI] [PubMed] [Google Scholar]
- 23.Bustos Carrillo F, Gordon A, Harris E. Reply to Gérardin. Clin Infect Dis 2019;68:172–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bustos Carrillo F, Collado D, Sanchez N, Ojeda S, Lopez Mercado B, Burger-Calderon R, et al. Epidemiological evidence for lineage-specific differences in the risk of inapparent chikungunya virus infection. J Virol 2019;93:pii: e01622–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grivard P, Le Roux K, Laurent P, Fianu A, Perrau J, Gigan J, et al. Molecular and serological diagnosis of chikungunya virus infection. Pathol Biol 2007;55:490–4. [DOI] [PubMed] [Google Scholar]
- 26.Malvy D, Ezzedine K, Mamani-matsuda M, Autran B, Tolou H, Receveur MC, et al. Destructive arthritis in a patient with chikungunya virus infection with persistent specific IgM antibodies. BMC Infect Dis 2009;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shigeru Saito S, Akitoshi Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol 2010;63:601–10. [DOI] [PubMed] [Google Scholar]
- 28.Aris A, Lambert F, Bessette P, Moutquin JM. Maternal circulating interferon-γ and interleukin-6 as biomarkers of Th1/Th2 immune status throughout pregnancy. J Obstet Gynecol Res 2008;34:7–11. [DOI] [PubMed] [Google Scholar]
- 29.Dupuis-Maguiraga L, Noret M, Brun S, Le Grand R, Gras G, Roques P. Chikungunya disease: infection-associated markers from the acute to the chronic phase of arbovirus-induced arthralgia. PLoS Negl Trop Dis 2012;6:e1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez-Morales AJ, Cardona-Ospina JA, Fernanda Urbano-Garzón S, Sebastian H-Z. Prevalence of post-chikungunya infection chronic inflammatory arthritis: a systematic review and meta-analysis. Arthritis Care Res 2016;68:1849–58. [DOI] [PubMed] [Google Scholar]
- 31.Paixão ES, Rodrigues LC, Costa MCN, Itaparica M, Barreto F, Gérardin P, et al. Chikungunya chronic disease: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg 2018;112:301–16. [DOI] [PubMed] [Google Scholar]
- 32.Chow A, Her Z, Ong KS, Chen J, Dimatatac F, Kwek DJC, et al. Persistent arthralgia induced by chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis 2011;203:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prince HE, Seaton BL, Matud J, Batterman H. Chikungunya virus RNA and antibody testing at a National Reference Laboratory since the emergence of chikungunya virus in the Americas. Clin Vaccine Immunol 2014;22:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laoprasopwattana K, Suntharasaj T, Petmanee P, Suddeaugrai O, Geater A. Chikungunya and dengue virus infections during pregnancy: seroprevalence, seroincidence and maternal – fetal transmission, southern Thailand, 2009–2010. Epidemiol Infect 2016;144:381–8. [DOI] [PubMed] [Google Scholar]
- 35.Petersen L, Jamieson D, Powers A, Honein M. Zika virus. N Engl J Med 2016;374:1552–63. [DOI] [PubMed] [Google Scholar]
- 36.Brasil P, Pereira J, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 2016;375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data collected during the trial will be available and shared after de-identification. The data will be available immediately following publication. A link will be made available for all data sharing.


