Abstract
Traumatic brain injury (TBI) is common among military personnel and the civilian population and is often followed by a heterogeneous array of clinical, cognitive, behavioral, mood, and neuroimaging changes. Unlike many neurological disorders that have a characteristic abnormal central neurologic area(s) of abnormality pathognomonic to the disorder, a sufficient head impact may cause focal, multifocal, diffuse or combination of injury to the brain. This inconsistent presentation makes it difficult to establish or validate biological and imaging markers that could help improve diagnostic and prognostic accuracy in this patient population. The purpose of this manuscript is to describe both the challenges and opportunities when conducting military-relevant TBI research and introduce the Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA) Military Brain Injury working group. ENIGMA is a worldwide consortium focused on improving replicability and analytical power through data sharing and collaboration. In this paper, we discuss challenges affecting efforts to aggregate data in this patient group. In addition, we highlight how “big data” approaches might be used to understand better the role that each of these variables might play in the imaging and functional phenotypes of TBI in Service member and Veteran populations, and how data may be used to examine important military specific issues such as return to duty, the late effects of combat-related injury, and alteration of the natural aging processes.
Introduction
Recent worldwide military conflicts have demonstrated a critical need for understanding the many clinical and societal impacts that follow traumatic brain injury (TBI) in Service members, Veterans, and their families. Patients with military-relevant TBI present with a number of unique features that can impact research approaches and clinical care, including different mechanisms of injury (e.g., blunt force or blast-related injury (which may be due to pure over-pressure or most often a combination of over-pressure and blunt force), the prevalence of comorbidities (e.g., posttraumatic stress disorder [PTSD], mood disorders, etc.), and diagnostic challenges specific to a combat environment. In fact, military service and deployment may have implications for brain structure and function, even in the absence of brain injury, that are yet to be fully understood (Butler et al., 2017). Given these unique group characteristics that will be discussed in detail below, research including this population can be complicated, or, at the very least, nuanced, as many of these variables potentially impact outcomes independently.
Importantly, recent reviews of the literature specific to this population (Mu et al., 2017; Salat et al., 2017; Wilde et al., 2015) conclude that neuroimaging should play a critical role in understanding military-related TBI, and may prove particularly useful in evaluating interventions relevant to military/Veteran populations. With the exception of sport-related concussion, the vast majority of TBI quantitative structural MRI studies include mixed patient samples across the full range of injury severity. These tend to be cross-sectional, and they vary in chronicity. Determining the imaging biomarkers that are predictive of the short- and long-term outcomes in TBI should be an emphasis in future research, as accurate identification of these markers will improve our ability to identify injury, properly diagnose (i.e., PTSD vs. TBI), guide treatments, predict recovery, and both develop and evaluate new therapeutic interventions.
Additional research, however, is required to understand the contribution of unique, military-specific features and their impact on function following TBI. Military-related TBI research will benefit from better coordination of data collection and analysis efforts across multiple sites and cohorts. This would facilitate a “big data” approach that is not fully realized in military-related TBI research at this time. Better coordination across the studies would allow researchers to accumulate data across these studies to address important clinical questions. As such, the purpose of this manuscript is to (1) describe the unique challenges of military-relevant brain injury, (2) discuss how data aggregation across multiple cohorts may help us to understand TBI in this patient group, (3) provide basic recommendations on data collection, usage, and analysis for those performing clinical research in this unique patient group, and (4) introduce the Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA) Military Brain Injury working group.
Recent discussions among the ENIGMA Military Brain Injury working group and with groups of collaborating scientists and clinicians from around the world have highlighted many challenges in working with Veterans and Service members. These discussions have clearly emphasized the need for more coordinated collaboration to address complicated diagnostic and prognostic issues that clinicians experience when treating these individuals. Moreover, although challenging, “big data” approaches that aggregate data from different sources (i.e., research cohorts) that results in large amounts of accumulated data with unique and overlapping clinical, cognitive, and imaging variables can provide powerful opportunities to address these challenges and to help understand how these variables interact with one another in military-related TBI.
Challenges and Opportunities
A number of challenges exist for studies of TBI in populations of Service members and Veterans. Specific consideration of diagnostic criteria, comparison groups, and comorbidities are particularly warranted. Studies also differ in the methods used in considering symptom validity concerns, as well as how TBI may interact with cognitive aging or other comorbidities. Moreover, unique challenges exist when combining legacy or prospective accrual data, including variability in scales used to collect injury information and regulatory and logistical issues within and between institutions.
TBI Diagnosis and Severity Classification:
Military-related TBI research uses different diagnostic and severity schemes, compared to civilian cohorts. For example, TBI in the United States Veterans Affairs/Department of Defense (VA/DoD) nosology (https://www.healthquality.va.gov/guidelines) is defined as a structural or physiological disruption of the brain due to external force(s). Disruption of brain structure or physiology usually manifests as a common set of symptoms surrounding the injury, including alteration of consciousness (AOC), loss of consciousness (LOC), post-traumatic amnesia (PTA), neurologic deficits, cognitive difficulties, and/or imaging findings. TBI severity in the United States DoD/VA is categorized along a continuum (mild to moderate to severe), using variable timings experienced at the time of injury for these clinical symptoms (AOC, LOC, and PTA; see Table 1 for specifics). Perhaps unique to DoD/VA criteria is that the Glasgow Coma Scale score (GCS; a measure often used in civilian literature to establish severity) is not required and is rarely used except in the case of trauma. Likewise, the presence of any trauma-related intracranial abnormalities on medical imaging warrants classification as moderate injury using the DoD/VA criteria, even though the injury can be diagnosed as mild TBI based on duration of AOC, LOC, and PTA. In civilian studies, this category can be known as “complicated mild”, but this designation does not exist in the DoD/VA criteria. Further, it is possible that findings might be seen in advanced research imaging, but not in conventional medical imaging, and it is entirely possible that a Veteran or Service member might not receive either type.
Table 1.
Diagnostic criteria for mild TBI for the Veterans Administration/Department of Defense Clinical Guidelines (Management of Concussion/mTBI Working Group, 2009) and the American College of Rehabilitation Medicine (Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine, 1993).
| Structural Imaging | Loss of consciousness (LOC) | Alteration of consciousness/mental state (AOC) | Post-traumatic amnesia (PTA) | Glasgow Coma Scale (GCS) | |
|---|---|---|---|---|---|
| VA/DoD | Normal | 0-30 min | ≤24 hours | ≤24 hours | Typically not available |
| ACRM | May be normal | 0-30 min | Any | ≤24 hours | 13-15 within 30 min of injury |
In addition, it is not unusual in Service member cohorts for diagnosis to occur after the patient has been stabilized medically for other more major life-threatening injuries (i.e., hemorrhagic, traumatic amputations, etc.) using retrospective self-report. Deployment-related active combat settings can delay assessment and validation of TBI symptoms at the point of injury. These factors can lead to some significant disparity between the time of diagnosis and time of injury. Although there may be practical reasons for these diagnostic differences and the delay in diagnosis, reliance on self-report and the use of different diagnostic and severity classification schemes may play a role in what is reported. Importantly, future research may need to take additional precautions when combining data or comparing findings across cohorts that might apply different TBI severity classifications, have variable intervals between injury and assessment, or use different standardized assessment tools when questioning patients about their injury history.
Variability in Injury Scales:
Assessing lifetime history of TBI is another critical element in understanding recovery trajectories, treatment effectiveness, and long-term health outcomes. However, reliable and valid assessment of lifetime TBI has been an ongoing challenge for clinicians and researchers. In many contemporary research studies, participants are prompted to recall details from potential TBI exposure events that occurred years earlier. These events can be subject to retrospective memory bias and loss of specific details regarding the duration of LOC, AOC, and PTA. TBIs, particularly those of mild severity, can go undocumented for long periods of times for a variety of reasons. For example, the individual may believe his or her injury does not necessitate medical treatment, or he or she may feel afraid that immediate reporting will lead to removal from active duty, or other more life threatening medical issues that require immediate attention (i.e., traumatic amputation, hemorrhaging, etc.) may take precedence over assessment for TBI without an obvious head trauma. As such, lifetime assessment without a medical record verification has been a criticism of TBI research, especially in this patient population.
Despite these challenges in assessment, structured and semi-structured instruments of lifetime TBI have been developed and many are considered the “gold standards” in TBI assessment. However, having many measures may in fact complicate the issues of assessment in military-relevant TBI and result in diagnostic challenges across studies (i.e., blast-related vs blast TBI) that may impact the comparability of findings. Regardless, these measures are useful and allow the interviewers to document exposure history. This may be accomplished by using unique or distinct questions or algorithms. Importantly, however, the validity of these measures is not necessarily based on veridical recounting of the TBI event, but rather the degree to which the measure is useful in determining whether negative outcomes stemmed from individual or lifetime TBI or exposure to possible TBI as measured by the instrument. In this section, we discuss some of the most widely used tools to assess an individual’s TBI history - some specific to military-related TBI, and others more general.
There are many measures that can be used to assess TBI history in Veteran and Service members. Many of these are summarized briefly in TABLE 2. Though each has various strengths, they are designed to collect the same types of information. Primarily, these instruments are used to identify possible events that could lead to a head injury, then the patient is queried for clinical evidence of AOC, LOC, and PTA for each event. Many of these measures are designed to capture information across multiple exposures so that the accumulated history of TBI exposure can be better characterized. In addition, some will ask questions about symptom or symptom development post-injury, but questions regarding post-injury symptom development goes beyond the diagnostic and severity determinations these measures are meant to capture and more importantly, are sometimes used as evidence of a TBI when they are not part of the diagnostic criteria (e.g. symptoms that develop in the days to weeks rather than immediately after injury).
TABLE 2:
Common TBI injury scales used to assess TBI severity and history in veterans and Service members. It should be noted that this table is not an exhaustive list and there are omissions. However, the point of this table is to show that there is a host of measures that have cropped up as “standardized” means of assessing TBI history and severity of specific TBI events in military cohorts.
| Measure | Strength | Weakness | Relevant References |
|---|---|---|---|
| Ohio State University TBI Identification Method (OSU TBI-ID) | Short administration time (3-5 minutes), administered to person or proxy, assesses the entire lifetime history, administered by a variety of staff with minimal training | Focuses patient on specific types of injuries, does not assess symptoms following TBI, relies primarily on self-report | (Dams-O’Connor et al., 2013), (Bogner and Corrigan, 2009) |
| Brain Injury Screening Questionnaire (BISQ) | Comprehensive to include symptoms following brain injury, short administration times, translated into multiple languages | Relies primarily on self-report | (Dams-O’Connor et al., 2014) |
| The Boston Assessment of TBI-Lifetime (BAT-L) | More open-ended questions, captures more detailed information about blast-related exposures, can be used to quantify features of the injury exposure | Relies primarily on self-report, requires additional training and clinical expertise to administer and grade correctly | (Fortier et al., 2014) |
| The VCU Retrospective Concussion Diagnostic Interview | Developed using a clinical algorithm and consensus diagnostic procedure, looks to distinguished between physical and psychological effects of TBI | Relies primarily on self-report, requires training to use follow-up questions in a consistent manner | (Walker et al., 2014) |
| The Minnesota Blast Exposure Screening Tool (MN-BEST) | Questions can be quantified, captures frequency, severity, and plausibility of blast-related TBI, assesses other neurologic signs, test-retest and interrater reliability can be assessed | Relies primarily on self-report, not really compared to other measures | (Nelson et al., 2011) |
| Quantification of Cumulative Blast Exposure (QCuBE) | Specific to deployment related injuries, gets additional information about the types of device used in the blast, whether armor was worn, distance from blast | Relies primarily on self-report, not really been compared to other measures | (Petrie et al., 2014) |
| Military Acute Concussion Evaluation (MACE) | Rapid assessment screening instrument, has follow-up of incident to include assessment of symptoms, can be quantified, meant to be administered close to the event, multiple versions available. | Relies primarily on self-report | (French et al., 2008) |
Of further note, many of these measures were developed in the context of studies where researchers have identified gaps in the available methods when interviewing military and veteran patient groups. Though this tendency to develop new measures is common in clinical research, it can prevent more direct comparison of findings across studies as it effectively leads to psychometrically and quantitatively distinct measures. Additionally, with rare exceptions, limited direct comparisons between various measures in the literature, makes it difficult to quantify similarities and differences between these measures. As most of these measures use similar questioning methods and even similar questions to elicit information on TBI exposure, these different measures should be comparable. However, there are minor modifications or differences that are used to generate additional metrics that could lead to unique groupings (i.e., severity staging). Thus, it could be important to consider the specific measure used when attempting to aggregate data across studies.
Blast over-pressure exposure and blast-related head injury:
One area of evolving discovery and research deals with blast over- pressure exposure, which is more common in military and Veteran cohorts but increasing in prevalence among civilians in conflict zones or in terrorist events such as the Boston Marathon bombing in 2013. Though Service members and Veterans can incur a TBI from a number of different causes, some researchers have reported that most deployed combat-related TBI in military and Veterans result from explosives or blast injury (Covey, 2006; Fox et al., 2005). However, since these studies were published, DVBIC consistently reports that the majority of military TBIs are diagnosed in the non-deployed setting (training, accidents, falls, sports-related, MVAs…etc). Combat diagnosed TBI include injury while being exposed to improvised explosive devices (IED), advanced artillery, mines, mortars, rockets, grenades, aerial bombing, and other explosives. Blast over-pressure exposure is associated with alterations in brain function (Robinson et al., 2019, 2017, 2015) and cognitive performance (Grande et al., 2018), independent of TBI status, which may be due to a number of biological or functional changes including (but not limited to) disrupted cerebral blood flow or impaired neurovascular coupling. In addition, blast over-pressure exposure may result in additional white matter abnormalities in APOE e4 carriers, especially in those within 10 meters of the blast (Sullivan et al., 2019). There is also mounting neuroimaging support for potentially discrete and distinguishable effects of biomechanical forces of blast-related TBI compared to other mechanisms of injury that are detected by DTI and volumetric techniques. However, some studies conflict with these findings, reporting no detectable effects of blast exposure, or show effects using functional magnetic resonance imaging (fMRI) but not with volumetry (Fischer et al., 2014; Newsome et al., 2015). These equivocal results highlight the complexity of this issue and future work is needed to further understand the unique aspects of blast-induced TBI, particularly the effects of repetitive blast exposure over the careers of military personnel.
However, it is important to realize that a blast-related head injury is further defined as a complex type of physical trauma resulting from direct or indirect exposure to an explosion. This definition goes on to categorize five injury types resulting from blast exposures: Primary: resulting from the high pressure, or overpressure created by explosions; Secondary: resulting from strong winds following the blast wave that propel fragments and debris against the body; Tertiary: resulting from strong blast winds and pressure gradients that can accelerate and cause blunt force injury; Quaternary: resulting from other explosive products (heat and light) and from exposure to toxic substances that can cause burns, blindness, and inhalation injuries; Quinary: the clinical consequences of post-detonation environmental contaminants including chemical, biological, and radiological substances (Blast Injury Research Coordinating Office (BIRCO), n.d.). Regardless, diagnosing a head injury in the individual would still require documentation of AOC, LOC, or PTA and there is still much research required to fully understand the amount of blast exposure required for injury.
Differences between Veteran cohorts across combat operations:
Though obvious differences in age exist between Veterans from different conflicts and wars (i.e., World War II, Korea, Vietnam, OIF/OEF, others), there are other variables that render direct comparisons complicated. For example, cold (Korean War) and heat (African and Middle East Operations) related injuries, exposure to toxins (i.e., Agent Orange, exposure to burn pits) and/or IEDs (OEF/OIF/OND), and the length and number of deployments vary for each of the conflicts (https://www.publichealth.va.gov/exposures/index.asp). Many of these factors have been examined independently and have shown deleterious effects on clinical and cognitive function separate from TBI (Barrett et al., 2001; Cedeño Laurent et al., 2018; Chao, 2017; Cooper et al., 2018; Gopinath et al., 2019; Martin et al., 2019; Veitch et al., 2013). Another potential difference over the course of military history has been in the innovations in trauma care that has led to improved mortality rates with each successive conflict (Ingalls et al., 2014; Maddry et al., 2018; Ramasamy et al., 2009). Consequently, morbidity in conflict has increased due to the many medical developments that make it possible for more seriously injured soldiers to survive combat trauma previously deemed not survivable (Kotwal et al., 2018). In the context of TBI, these differences between cohorts may lead to variance in symptom presentation and comorbidity risks. As larger cohorts become available by aggregating data, investigation of the individual and collective impacts of many of these unique variables could help us understand how they might interact with TBI to augment or worsen clinical and functional outcomes.
Comparison groups:
Selection of an adequate comparison group is imperative in TBI research, especially in military and Veteran cohorts (Levin et al., 2014). Some studies of deployment-related TBI have used civilians (Michael et al., 2015) or a combination of military personnel and civilians as controls (Woods et al., 2015). Most researchers prefer to enroll Service members or Veterans as their controls (Donald et al., 2014; Han et al., 2014; Hoge et al., 2008; Troyanskaya et al., 2015) to ensure that study groups match not only on basic demographics, but also military specific and potential deployment-related characteristics such as history of exposure to combat-related stressors, comorbidities, and even premorbid environments.
A review of the military and Veteran literature quickly illustrates the variability in control samples analyzed. Individuals who serve as controls may report having been unexposed (Troyanskaya et al., 2015) or exposed to blasts (Donald et al., 2014; Han et al., 2014). These participants may have sustained extracranial injuries or been uninjured, but do not report a history of, or exposure to TBI. Though some suggest that these different control groups may result in unique findings, a previous investigation showed that it is appropriate to combine uninjured and injured controls in one group, as long as the extracranial injuries are mild (Troyanskaya et al., 2016). However, studies have found transient brain alterations from blast exposure even in the absence of a TBI diagnosis (Bazarian et al., 2013; Carr et al., 2016; Taber et al., 2015; Tate et al., 2013), and the possibility of confounding (within study) or equivocal (between studies) results should be considered when using blast-exposed controls.
More broadly, the issue of what control group to use remains an important area of interest. The use of different control groups could directly affect the findings in ENIGMA analyses that combine effect sizes across individual studies to determine how consistent a particular imaging finding is between sites. However, to date, only a limited number of studies use more than one control group and as a result, much of the concern remains speculative. Regardless, as a group, we acknowledge this as a potential limitation requiring additional investigation to truly understand how the selection of control participants might lead to different conclusions or findings. Moreover, the selection criteria for the control group(s) will depend in part on the specific research question being posed.
Comorbidities:
Research examining comorbid conditions following TBI is growing rapidly and though consideration of these conditions is essential particularly given a patient population such as military-related and veteran TBI patients who may have unique or unique combinations of comorbid conditions that require further attention. Studies designed to account for the effects of these co-occurring conditions are thus important as there is a relatively high occurrence of comorbidity that poses a challenge for clinicians to diagnose and treat combat related TBI. Overlapping symptoms of TBI and several concomitant mood disorders and including (but not limited to) poor concentration, anxiety, insomnia/sleep disturbances, and irritability add to this challenge (Sayer, 2012). (See also schematic of overlap in symptoms and neuroimaging findings in Figure 1.)
Figure 1.
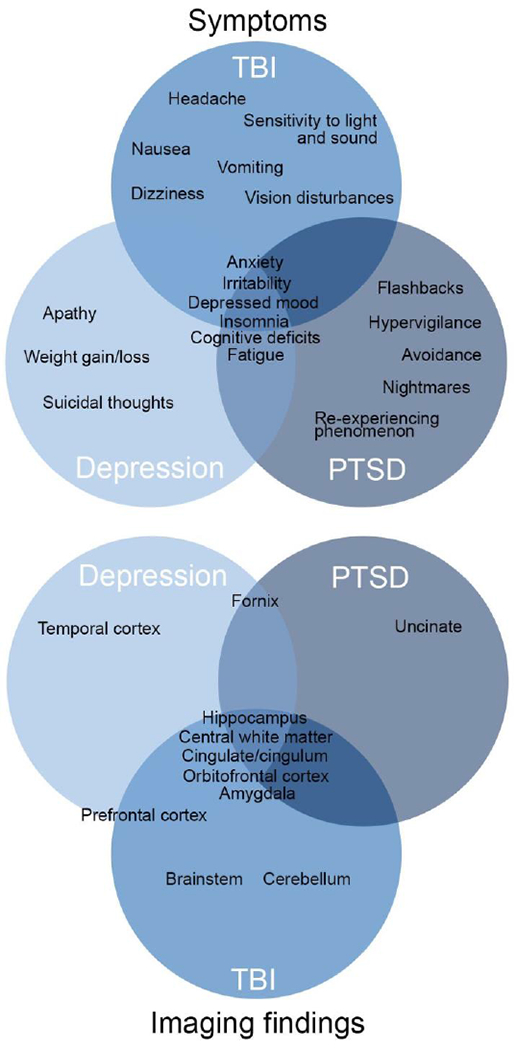
Overlap between TBI, PTSD, and Major Depression in symptoms and reported imaging differences. Imaging differences are shown across modalities, including structure, function, and connectivity.
One of the more obvious comorbidities that requires consideration in this patient group is post-traumatic stress disorder (PTSD). After experiencing sufficient life-threatening trauma (i.e., active combat), risk for concomitant psychological trauma is expected to increase (Marx et al., 2009; Schneiderman et al., 2008). In fact, a systematic review of the literature shows that the prevalence of PTSD comorbidity ranges from 33% in the general military population (Carlson et al., 2011) to over 89% of veterans with TBI (n = 22,053; (Taylor et al., 2012). Importantly, some studies have found that PTSD accounts for more variance in post-concussive symptom reporting than the presence or absence of LOC, the type of injury mechanism, or the characteristics of the blast exposure (Lippa et al., 2010). Historically, imaging studies of TBI have ignored psychiatric comorbidities in this patient group, but recent literature suggests a greater emphasis on accounting for their effects (Bazarian et al., 2013; Han et al., 2018, 2015; Levin et al., 2010; Lipton et al., 2012, 2009) and has shown poorer function in individuals who are comorbid for both TBI and PTSD (Kaup et al., 2019).The clinical picture can be further complicated as the co-occurrence of PTSD and/or major depressive disorder (MDD) with TBI can overlap and exacerbate symptoms by contributing to increased symptom severity, incomplete or delayed recovery, reduced quality of life, and increased risk of suicide (Barnes et al., 2012; Brenner et al., 2010; Hudak et al., 2012; Walter et al., 2012).
Though the most commonly occurring comorbid psychiatric condition in veterans is PTSD (72%), MDD (45%), anxiety (22%), and substance use disorder (20%) are also common. Other psychiatric difficulties may influence risk of injury and recovery from military-related TBI. For example, depression, suicidal behaviors, and substance use and abuse disorders are commonly experienced by Service members during and after deployment, and also frequently co-occur in the context of TBI (Hoge et al., 2008; Hoge and Castro, 2011, 2006). Overall, stress-related polytrauma and psychiatric factors may confound neuroimaging research in TBI in this patient population (Bazarian et al., 2013; Davenport et al., 2015; Isaac et al., 2015; Lopez-Larson et al., 2013; Matthews et al., 2011; Trotter et al., 2015; Ware et al., 2018).
Another important consideration is the recent transition from Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV to DSM-V diagnostic criterion for several mood disorders, especially PTSD. This transition has created unique harmonization challenges. It is possible to “translate” PTSD diagnostic criteria from DSM-V to DSM-IV, but the reverse process poses a challenge because of the introduction of new criteria to DSM-V that are absent in DSM-IV. For example, established methods for converting the Clinician Administered PTSD Scale-5 (CAPS-V) scores to CAPS-IV scores are available and straightforward to implement (Sun et al., 2019). Similarly, self-report instruments that use DSM-IV criteria are generally straightforward to harmonize with gold-standard clinician-administered instruments such as the CAPS, particularly if item-level data are available. However, the correlation of self-report measures with clinician-administered measures is imperfect (McDonald et al., 2009). In the absence of item-level data, the accuracy, sensitivity, and specificity of self-report measures in relation to gold-standard clinician-administered measures may depend on variables such as the clinical cut-off score that is applied, which can vary from study to study (McDonald et al., 2009). In addition, the definition of PTSD in the International Classification of Diseases (ICD)-11 differs in many respects from the diagnostic criteria in DSM-V. Though the ICD-11 criteria and DSM-V criteria perform equally well for identifying patients in distress, the overlap of PTSD diagnosis with ICD-11 and DSM-V is quite low, where ICD-11 identifies fewer cases than DSM-V (Hafstad et al., 2017). Thus, such challenges in identifying PTSD may result in an uneven diagnosis and treatment, depending on the part of the world and the instrument being applied.
As discussed in the introductory paper (Wilde et al., 2019), there are ENIGMA working groups dedicated to a number of disorders that can be comorbid with TBI in military populations, including MDD (Schmaal et al., 2016), PTSD (Dennis et al., 2019; Logue et al., 2017), anxiety (Groenewold et al., 2018), and addiction (Mackey et al., 2019). In fact, a recent study published by the PTSD ENIGMA-PGC Consortium working group demonstrated only limited evidence that PTSD moderates the effect of TBI on white matter integrity, based on the spatially circumscribed white matter disruption investigated in 3,047 individuals across several unique cohorts (Dennis et al., 2019). This finding highlights the value of examining data from several individual studies and illustrates the need for additional research in an area so pertinent to military and Veteran patient groups, as the biological mechanisms that may predispose individuals to poor outcomes to TBI may also predispose individuals to other psychiatric disorders. Thus, coordination with these other clinical working groups within the larger ENIGMA consortium will allow for additional well-powered, cross-disorder analyses that will help clarify whether psychiatric disturbances following a brain injury are similar to, or distinct from those experienced in the absence of physical brain injury.
Interactions between TBI and aging processes:
Prospective longitudinal studies of OEF/OIF/OND military Service members with histories of TBI show that neurobehavioral and pathological consequences of head-trauma are not static, but instead evolve slowly over time (Mac Donald et al., 2017; Stein et al., 2016). For example, five-year outcome studies of Service members who sustained a TBI in combat showed that, compared to military control participants, those with head injury histories demonstrated increased rates of disability, worsening severity of psychiatric and post-concussive symptoms, and neuroimaging evidence of greater white matter microstructural changes within individuals across time (Mac Donald et al., 2019, 2017). In addition, the Trotter et al. (2015) study demonstrated a significant blast exposure by age interaction, and was in a cross-sectional cohort of Veterans, with the older blast exposed Service members having worse diffusion scalar metrics (Trotter et al., 2015). Although the temporal course of the pathophysiological mechanisms associated with neurotrauma remains mostly unexamined, and thus poorly understood, the extant data shows that it involves a host of neurochemical, metabolic, inflammatory, and neurodegenerative processes (Greve and Zink, 2009). As our military Service members begin to enter advanced ages, we must consider how the negative long-term effects of head-trauma may compound age-related neurodegenerative processes.
Though not necessarily specific to Service Members and Veterans, TBI often represents a risk factor for the development of other complicating neurologic illnesses that can further complicate functional outcomes. More specifically, post-traumatic epilepsy or PTE is common following moderate and severe brain injury for civilian populations (between 4 and 50%; Verellen and Cavazos, 2014) and as such represents one of the most common types of focal epilepsy (~20%; Piccenna et al., 2017). Functionally, there is some significant overlap in the cognitive domains affected by epilepsy (independent of TBI) and most suspect that PTE worsens functional outcomes (Kolakowksy-Hayner et al., 2012). Importantly, there is very little information about PTE in Veteran and Service members and as such, this assumption remains unexamined. Currently, this work may be accomplished by smaller groups with more specific expertise in epilepsy until a more consistent approach is developed across a sufficient number of studies to warrant a big data approach.
Chronic neuropathologies of single and repetitive TBI are acknowledged risk factors for dementia, but concerns regarding the late-life effects of TBI have increased in recent years, partly due to broad media coverage of chronic traumatic encephalopathy (CTE) in athletes and in military personnel with repetitive head trauma (Smith et al., 2019, 2013). For these reasons, characterizing late-life effects of TBI in non-athlete civilians and Veterans remains very important to TBI survivors and their families. There is a growing body of epidemiological studies across both civilian and military samples which shows that TBI, even when mild, is an independent risk factor for dementia and neurodegenerative disorders in late life (see Gardner and Yaffe, 2015 for review). As recently demonstrated by (Barnes et al., 2018), there is a dose-response relationship between dementia risk and TBI severity, with a 3-fold increased risk in individuals with moderate or severe TBI, and a 2-fold increase in those with mild TBI. Moreover, individuals with TBI appear to have an earlier age of dementia onset, on average, relative to those with no history of head trauma, suggesting that TBI not only increases risk for dementia, but also accelerates disease course (Gardner et al., 2014; Gardner and Yaffe, 2014; Hayes et al., 2017). Importantly, when examining the relationship between TBI and dementia directly in prospective cohorts, there are mixed findings (Crane et al., 2016). In fact, TBI with LOC was related to more related to Lewy body accumulation and motor abnormalities (i.e., parkinsonism) and not dementia or AD. Thus, this remains a complicated area requiring additional research (Van Den Heuvel et al., 2007).
Many pathological features are common to both TBI and neurodegenerative disorders such as Alzheimer’s disease (AD) - including Aβ deposition, alpha-synuclein, TDP-43, neurite degeneration, synapse loss, and microgliosis (Uryu et al., 2007; Villapol, 2018). More recent theories suggest that prominent neurofibrillary and astrocytic tangles characteristic of CTE may play a role in those with repetitive head trauma. Interestingly, deep sulcal tau pathology has been observed in long-term TBI survivors of even single-event head injury (Johnson et al., 2012), and about 20% of cases without prior concussion also show this characteristic tau pathology (Bieniek et al., 2015; McKee et al., 2014; Stein et al., 2015). Beyond pathologic proteins, mounting evidence—particularly from the animal literature—strongly suggests that vascular damage vis-à-vis BBB breakdown may initially lead to neuroinflammatory cascades, blood flow dysfunction, white matter changes, and eventual neurodegeneration, particularly in those individuals who are already at increased risk for AD (Sundman et al., 2014).
To better understand TBI aging interactions, a set of validated biomarkers needs to be established for tracking progression over time. This might be more easily accomplished using aggregated data across studies using multi-modal assessment (e.g., neuropsychological and psychiatric evaluations), novel and emerging neuroimaging techniques (e.g., diffusion tensor imaging, arterial spin labeling), cerebrospinal fluid and plasma-based markers of neurodegeneration, pathologic proteins, and vascular dysfunction (inflammatory and endothelial markers). Examining these variables and the interactions between them will eventually allow us to better elucidate the myriad of polypathology underlying the long-term, chronic effects of TBI in older military and Veteran patients with a history of TBI.
Premorbid Considerations:
As with all TBI patient populations, there is a long list of premorbid factors that have been identified as factors that influence outcome after injury. Early life stress or adverse childhood experiences (ACEs) - broadly defined as negative experiences before age 18 - can have a wide range of physical and psychological effects. ACEs may include abuse, neglect, or household instability (Felitti et al., 2019). The CDC estimates that more than half of the population experiences at least one adverse event (CDC, 2016). Military brain injury studies often detail combat and deployment-related stressors, but detailed life histories are collected less often. ACEs can increase risk for a number of factors, that are themselves risk factors for poor outcome after injury, including cardiovascular disease, reduced cognitive capacity, substance abuse, and depression, highlighting the complex interplay among premorbid factors (Anda et al., 2002; Dong et al., 2004; Edwards et al., 2003; Nemeroff, 2016).
Premorbid psychiatric disorders or a familial history are also considered important risk factors in TBI outcomes. A premorbid history of psychiatric disorders (e.g., ADHD, or mood disorders) can increase the risk of both sustaining a brain injury and the risk of poor outcome after injury (Manners et al., 2016; Vassallo et al., 2007).
Premorbid IQ may also help explain some of the variance in outcomes following a TBI (Stewart-Willis et al., 2018). The idea of cognitive reserve is not a new idea and has been examined in a number of other neurologic conditions, especially in dementia research (Rodriguez et al., 2019). However, it has not been thoroughly examined in TBI patient groups. Preliminary studies that examined this in TBI demonstrate the utility of adjusting for premorbid IQ, though the need for replication and more nuanced investigation of premorbid IQ is required.
Additional research is clearly needed to examine interactions between early life stress, the chronic effects of early life stress, and brain injury. Inclusion of premorbid factors will help us to understand how these variables impact symptom presentation, symptom evolution or progression, and responses to treatment in the individual patient. Ultimately, knowledge gains in this burgeoning area of research will help TBI clinicians contextualize injury presentation in a much more productive manner. So, it will be important to consider different ways of assessing these factors in the individual patient in future prospective studies.
Other Risk Factors.
A number of other risk factors are beginning to garner additional research interest in this patient cohort which could independently affect or augment outcomes. Performanceenhancing drugs and supplements are used frequently by military personnel to improve alertness, physical performance, and focus, and these might require additional investigation (Ko et al., 2018). Use of supplements varies by sex (i.e., higher weight loss supplements in women and higher body-building supplements in men), by military rank (i.e., higher use in non-commissioned), and that increased combat exposure, mixed duty cycles, and longer hours are associated with higher use (Lui et al., 2019; van der Pols et al., 2017). More specifically, studies of Omega-3 and caffeine supplements in military personnel have shown improvements in cognitive function (Yarnell and Deuster, 2016) and resiliency in stressful situations (Hoffman et al., 2015), though some studies did not find any benefit (Coull et al., 2016; Dretsch et al., 2014). However, as pointed out in the review by Ko et al. (2018), published studies rarely report safety information regarding the use of supplements. Furthermore, researchers and clinicians have only begun to speculate how supplements might mediate or moderate function following TBI (Manchester et al., 2017). To date, there is only a paucity of studies examining these questions directly; however, in one animal TBI study, omega-3 supplemented rats showed faster recovery of body weight and improved cognitive function following multiple mild TBIs (Wang et al., 2013). Thus, examining the effects of supplements may be an important line of research that could be conducted in the context of “big data”, as aggregating data across studies will more likely yield the number of participants needed to examine the various supplements used.
Deployment history offers several complex clinical and research confounds that also require consideration. The identification and classification of TBI in active-duty military personnel is largely complicated by factors related to the mechanism of injury. Deployment can increase the risk of TBI from disparate mechanisms of injury in military personnel as compared to civilians. It is therefore important to consider the historical timing and context of military deployment. While not known within the context of military-related TBI specifically, multiple deployments could worsen outcomes by increasing the psychological and physiological effects of polytrauma (i.e., two or more injuries in at least two areas of the body, Kroupa, 1990) due to acute and/or chronic stress in addition to increased risk for additional physical injury. Explosive blast is the most common mechanism of injury among deployed OEF/OIF/OND Veterans (Maas et al., 2010; Owens et al., 2008), yet the VA Polytrauma and Blast Related Injuries (PT/BRI) Quality Enhancement Research Initiative (QUERI) reported that this mechanism only accounts for a minority of TBI seen in Veterans who receive health care at the VA. This reflects the fact that TBI in older Veterans most likely resulted from blunt force trauma. Regardless, deployment, and perhaps especially combat deployment, are associated with single and repetitive exposure to acute and/or chronic stressors (Fear et al., 2010; Wittchen et al., 2012). However, neurotoxic effects of stress, chronic stress, and prolonged symptoms of PTSD are poorly understood in the context of military-relevant TBI (as discussed in Butler et al., 2017). It is not known whether stress exposure influences TBI risk (e.g., greater incidence with more severe stress exposure) or outcomes (e.g., worse recovery with more severe stress exposure). Polytrauma from exposure to stress may be more inherent to some mechanisms of military-relevant TBI than others. For instance, blast-related TBI is highly comorbid with post-traumatic stress disorder (PTSD; Bazarian et al., 2013), and may be associated with more deleterious long-term brain and cognitive outcomes (Disner et al., 2017; Nelson et al., 2019). Ultimately, deployment history, stress factors associated with combat, and number of exposures to combat and/or actual TBI require additional investigation in this patient population.
For Service members who were deployed, especially to active combat areas, TBI can occur along with significant physical injuries such as burns and fractures. These additional injuries complicate the diagnosis and potential outcomes in military-related TBI in several ways (e.g., Lange et al., 2014; Polusny et al., 2011). First, extra-cranial injuries requiring immediate medical interventions to prevent loss of life can obscure assessment of symptoms often used to diagnose TBI and may delay identification, diagnosis, and treatment of TBI. Treatment protocols also conflict for individuals with burn/hemorrhagic injuries and TBI and delays may exacerbate the dynamic acute and early post-injury biomechanical cascade of TBI in cases with severe physical polytrauma (e.g., excessive blood loss in relation to blood perfusion; neuroinflammation in response to systemic injury; e.g., Kaur et al., 1995). In addition, extensive polytrauma can confound retrospective recall of information pertaining to the time of injury, including in the presence of prolonged LOC, severe bodily injury requiring anesthesia or sedation, or exposure to severe acute physical and psychological stressors.
Recent investigations into methods used for evacuation of military personnel from the combat theater following significant life-threatening injury highlight additional risks to Service members with TBI. Ingalls et al. (2014) reviewed the first 10 years of critical care aeromedical transport during OIF/OEF, and found that rapid movement of critically injured casualties within hours of wounding was effective with minimal mortality during transport and for 30 days post-transport. Regardless, decreased partial pressure of oxygen, hypobaria, vibration, temperature changes, noise, decreased humidity, and gravitational forces are listed as potential stressors requiring additional investigation (AFI 48-307). In a replication of early research (Skovira et al., 2016), animal models (rat) of TBI and simulated aeromedical evacuation (cabin pressure equivalent to 8,000 ft, 12 hours, and vibration) revealed a complicated interaction between normobaric and hypobaric conditions, blast exposure, and inflammatory cytokines. Ultimately, the hypobaric blast exposed group had significantly more brain, lung, and other organ pathologies, including neuronal degeneration, scattered single cell apoptosis, and necrosis (Scultetus et al., 2019). Interestingly, the cytokine profile was more nuanced and did not appear to underlie the pathological changes observed in the hours following the flight simulation. This contrasts with the earlier study by Skovira et al. (2016), which demonstrated increased neuroinflammation in an animal model at a much longer post-simulated flight interval that was associated with poorer long-term cognitive function. In the Scultetus et al., 2016 swine model, brain perfusion was worsened in the simulated hypobaric conditions following TBI. It is clear that additional research needs to be conducted here, particularly in humans, but these findings may highlight additional risks and considerations when conducting research in military and Veteran groups.
Other factors that require further examination in military personnel and Veterans include the effects of genetics and sex. Current genetic research within military populations is very limited (Parnell et al., 2018), though the evidence is mounting that genetic variation may modulate both TBI recovery and outcomes (Williams et al., 1991). The polymorphism with the most support is the apolipoprotein E (APOE) ε4 allele. A meta-analysis suggests that the ε4 allele is associated with poorer outcomes after TBI (Haan et al., 2008), including a tenfold increased risk of dementia up to 30 years after TBI (Isoniemi et al., 2006). Additionally, boxers with the ε4 allele who had participated in many bouts were more likely to develop CTE than those who had only participated in a few fights (Jordan et al., 1997). Imaging and peripherally circulating proteomic biomarker correlations are currently being assessed in large longitudinal cohorts (e.g., CENC and TRACK-TBI). Sex is another factor that will require additional examination in this patient group and large samples may be required given the disparity between male and female enlistment rates. This fact certainly limits the generalizability of any findings back to the general population. Flowever, women appear to have worse outcomes across a number of variables (manifesting symptoms, surgical interventions, mortality) following TBI (Farace and Alves, 2000; Munivenkatappa et al., 2016). Preliminary results from our initial analyses using the ENIGMA approach seem to validate the need to examine the effects of sex, as there appears to be an interaction for diffusion measures (i.e., higher FA in males and lower FA in females). Though genetics and sex differences require examination across the TBI patient continuum, there may be genetic and sex differences unique to this patient cohort that could be examined with higher statistical power in the context of larger, aggregated data.
Finally, there may be other factors that need to be studied to fully understand how injury risk or injury mechanisms can be modified. For example, there is limited information about how much military armor, including uniforms and helmets, worn at the time of injury affects outcomes in military-related TBI, especially blast-related TBI. Also, more specific to blast-related TBI is the need to investigate whether the type of vehicle (e.g., armored, unarmored, enclosed, open, pedestrian), and the proximity of the vehicle to a blast influences injury risk (i.e., blast proximity and loading). Together, these literature gaps complicate clinical diagnosis and decision-making, and a better understanding could help shape future interventions aimed at improving neurobehavioral and social outcomes.
Symptom validity concerns:
As symptom reporting is inherently a subjective process, it is fraught with potential validity questions, including exaggerated symptom reporting and recall bias. As reviewed by (Silver, 2015), there are high levels of what are considered invalid symptom and neurocognitive test performance in those evaluated for the effects of an mTBI and/or PTSD, including military servicemen and women (Cooper et al., 2011; Spencer et al., 2017). Part of the problem with symptom reporting in those meeting criteria for having sustained an mTBI is that the symptoms are diverse, and none are specific to brain injury, as discussed in the Comorbidity section above (Hiploylee et al., 2017; Tator et al., 2016). All symptoms associated with mTBI also tend to occur with bodily injury not involving TBI (Lange et al., 2019), as well as a variety of medical, neurological, and neuropsychiatric conditions outside the realm of TBI (Donnell et al., 2012). Symptom validity testing (SVT) refers to specific metrics that are either embedded within the test, or part of a separate measure that assesses what is referred to as response bias (Larrabee, 2015). Some SVT measures also include findings related to unusual or atypical symptom reporting, though commonly, SVT measures will use a cut-score approach in defining valid versus invalid symptom reporting. Similarly, the term “performance validity testing” (PVT) has been suggested as a more accurate term when the focus is cognitive performance rather than symptom reporting.
Neuroimaging studies of military-related mTBI may need to use SVT measures to assess validity of symptom reporting and test performance. Some studies of neuroimaging correlates of symptoms, or performance on cognitive, motor, or sensory-perceptual functioning following mTBI exclude those who perform below a recommended SVT cut-point. However, as shown by Clark et al. (2016), a potentially superior approach may be to include those who score above chance, but below the cut-point, as the number of relevant factors associated with mTBI may influence embedded or separate measures SVT scores. Better understanding of neuroimaging correlates of SVT or PVT failure may provide novel insights into why validity failure rates are so high in those with mTBI in the military (Mooney et al., 2018).
Regulatory or Logistical Issues.
Those who have conducted research in military or Veteran populations may encounter regulatory or other logistical issues that are somewhat unique to this patient population and can appear more challenging to navigate. In the United States, Service member and Veteran health care are provided by different government-controlled groups or agencies. Though this makes logistical and budgetary sense, combined with the unique cultural aspects of each, investigating TBI using data from both these groups can create additional challenges, including regulatory (e.g., IRB governance), funding, and data harmonization issues. Many of these issues, especially as they relate to the United States military and VA systems, are discussed in detail in the VA-DoD Collaboration Guidebook for Healthcare Research (2013). Some of these issues are only briefly described here, to provide a well-rounded discussion of issues related to conducting military and Veteran research. Though many of these issues make VA/DoD research complicated, research in these settings has revolutionized medical care and treatment. As such, this type of work can be highly rewarding, and often leads to improved medical care for a broader range of patients following TBI.
One unique aspect of research in this patient population is the military and VA cultures that often require unique strategies to properly liaise and obtain permissions needed to conduct research. Understanding command structures, observing proper military etiquette, discerning differences between active and reserve personnel, training and operational units, credentialing requirements, resource utilization restrictions, occupational demands within military units, and acknowledging possibility of reassignment or deployment and significant changes in command etc. are critical to the success of research in military settings (http://www.ncdsv.org/images/VA-DOD_ResearchCollaborationGuidebook_2011.pdf). The VA also has a unique culture that requires consideration including different interpretations of management and regulatory guidelines and policies that exist across the VA system. To navigate many of these logistic and regulatory issues successfully, close direct interaction with relevant liaisons, specialty staff, and administrators who can guide researchers through these logistical challenges is often necessary. Thus, developing strong relationships of mutual respect and shared vision often facilitates research collaborations.
In the United States DoD/VA system, some challenges associated with data access can be mitigated using different legal documents, including complete/partial offsite research waivers, data use agreement (DUA), and/or cooperative research and development agreements (CRADA). These documents require legal staff to be involved, but can facilitate data sharing and collaboration between sites. However, changes in both VA and DoD have led to some simplification of data sharing between DoD and VA. For instance, the development of the DoD and VA Infrastructure for Clinical Intelligence (DaVINCI) platform facilitates longitudinal research with linked DoD and VA data. The transition of the DoD Trauma Registry to Defense Health Agency (DHA) also streamlines the data acquisition process by using a standard data sharing agreement application (DSAA) through the DHA.
VA and DoD research projects are also funded through a number of different peer reviewed mechanisms. DoD has both intramural and extramural funding opportunities, while the United States VA system only funds intramural investigations. Practically, this means that principal investigators for VA funds must have a VA appointment and be employed by the VA for a minimum of 5/8ths time. Principal investigators funded by DoD grants can be active duty military, civil service employees, academic, or other private industry personnel qualified to conduct the research. In addition, guidelines for expenditure limits, both overall and in certain categories, may differ between these funding agencies. While these factors may influence the kinds of research submitted to each agency, judicious use of these mechanisms can facilitate the ability to address research questions using diverse funding mechanisms.
Summary of Recommendations for Future Studies
Given the challenges outlined above, big data efforts may further our understanding of how these somewhat unique variables contribute to the clinical and functional outcomes in Service members and Veterans following TBI. However, there are challenges associated with using retrospective or archival data, and these limitations may be mitigated in future studies if prospective studies are designed with the idea that data acquired might be used in aggregated form. From the perspective of the ENIGMA Military Brain Injury Working Group, the following recommendations may be used to guide the acquisition of new data in military-relevant cohorts with TBI if investigators so choose. If these recommendations are followed in the design and implementation of new studies, data aggregation and the new knowledge that can come from big data approaches are likely to be greatly enhanced.
Imaging Clinical Guideline for TBI (DCoE).
Beginning in 2007 with the build-up in troop deployments in Afghanistan, the number of deployment and non deployment related TBIs sharply increased in US Service members. Soon after, the Department of Veterans Affairs allowed Veterans to claim TBI as a disability counting toward compensation. With these changes, there was a need for more objective verification of TBI (especially mTBI) and consistent documentation of TBI exposure. Ultimately, the Defense Centers of Excellence (DCoE) convened a panel of experts and issued a set of Clinical Recommendations entitled “Neuroimaging following mTBI in the non-deployed setting.” This set of clinical guidelines was intended to act as a set of standardized neuroimaging procedures (summarized in Table 3) that could be used by military physicians and radiologists to capture consistent imaging across military treatment facilities (MTF), VA medical centers, and community-based outreach clinics. These recommendations cover a number of imaging modalities including CT and PET/SPECT imaging. Further, although the standardization or harmonization recommendations that we are making here are more specific to MRI, these imaging modalities are clearly important in our understanding of TBI (see Raji and Henderson, 2018 and Byrnes, et al., 2014 for a review of these methods). As ENIGMA methods are developed to deal with these additional imaging types, efforts to standardize the acquisition of these imaging methods should be implemented. Nonetheless, establishing clinical guidelines using standardized methods for the assessment of TBI represents an important effort to improve comparability of clinical information across studies.
Table 3.
Current clinical guidelines for neuroimaging after TBI as proposed by the DCoE clinical radiology guidelines working group. CT=Computed Tomography, MRI=Magnetic Resonance Imaging, PET=Positron Emission Tomography, SPECT=Single-photon Emission Tomography, FDG=fluorodeoxyglucose, HMPAO=hexamethylpropyleneamineoxime, ECD=ethyl cysteinate dimer.
| Modality | Clinical Indications in mTBI | Acute (injury to 7 days post-injury) | Sub-Acute (8 to 89 days post injury) | Chronic (greater than 90 days post injury) |
|---|---|---|---|---|
| Goal of imaging | Identify surgical mass or lesions | Evaluate, enhance counseling, identify need for referral | Evaluate, enhance counseling, identify need for referral | |
| CT | Utility varies based on the length of time between injury and scanning | Modality of choice if clinical evaluation indicates need for imaging | Use only if MRI is contraindicated | Use only if MRI is contraindicated |
| MRI | Volumetric changes, microhemorrhage, diffuse axonal injury | If symptoms worsen after 72 hours | Modality of choice | Modality of choice |
| PET | 18 FDG-PET | No current clinical indication | If no structural abnormalities do not explain persistent symptoms, PET may be able to provide information. | If no structural abnormalities do not explain persistent symptoms, PET may be able to provide information. |
| SPECT | If no PET available, consider HMPAO or ECD SPECT | No current clinical indication | If no structural abnormalities do not explain persistent symptoms, SPECT may be able to provide information. | If no structural abnormalities do not explain persistent symptoms, SPECT may be able to provide information. |
Currently, standardization of imaging parameters is difficult, but not impossible. For example, the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study is well into its second decade of work and has been extremely successful in coordinating image data collection and analyses across multiple sites and neuroimaging vendors (Weiner et al., 2015). It is clear that this kind of coordinated rigor improves the quality of imaging data in research settings and makes it possible to ask more complicated clinical questions. Also of note are recent efforts by several large scale TBI studies (CENC, TRACK-TBI, CARE) to harmonize image acquisition parameters to generate images with minimal differences, particularly with respect to the results of planned analysis pipelines. Although these consortia focus on slightly different TBI cohorts, the attempt to minimize differences in the acquisition parameters, regardless of the cohort examined, will facilitate comparison of studies across sites that will allow us to address important clinical questions that may identify unique risk factors for each of different clinical cohorts. The fact that efforts are being coordinated between large studies is also encouraging and will create future opportunities to aggregate data that can benefit the investigation of many TBI patient groups.
Clinical and Cognitive CDE’s for Military Studies.
Limited guidelines exist related to Common Data Elements (CDEs) or measures that are recommended for mild TBI in military-relevant settings. Although combat-related TBI was not the focus of the initial version of the International (NIH) CDEs, the Department of Defense and the VA were prominent stakeholders, and the panels included several members with VA or other military-relevant affiliations (Thurmond et al., 2010; Whyte et al., 2010). In addition to TBI, psychological health was a focus of these early efforts, and working groups also addressed common data elements for PTSD (Kaloupek et al., 2010) and operational stress. As the CDE initiative evolved to specifically consider elements for use in cohorts of different severity, including mild TBI and concussion, new working groups again included several members with experience in combat-related TBI and did consider several domains and measures that had been used and would be applicable in this population (Hicks et al., 2013). However, the variables included in the second edition of the CDEs were restricted to supplemental CDEs, and measures designated for military studies include the Combat Exposure Scale (Keane et al., 1989), Military Acute Concussion Evaluation (McCrea, 2001; McCrory et al., 2017; Mucha et al., 2014), Veterans Rand 36-Item Health Survey (Kazis et al., 2004; “Traumatic Brain Injury,” 2018). Other measures commonly applied in this population, include the Neurobehavioral Symptom Inventory (King et al., 2012), Automated Neuropsychological Assessment Metrics (ANAM; Kabat et al., 2001), which is recommended for in-theater post-injury assessment of cognitive function (“Indications and Conditions for In-Theater Post-Injury Neurocognitive Assessment Tool (NCAT) Testing Clinical Recommendation,” 2013) and the Post-Traumatic Stress Disorder Checklist 5 (Weathers et al., 2013) are also included in the list of general measures.
The Consortium to Alleviate PTSD (CAP) has performed field testing of certain mandated core CDEs that relate to common comorbidities in Service members and Veterans (including PTSD, mood disorders, substance misuse) as well as symptoms that may be common across both TBI and PTSD (including sleep, headaches, etc. Ben Barnes et al., 2019). Initial testing of these measures suggests that they may be useful in data aggregation efforts, but the authors conclude that additional testing and experience are necessary. In addition to efforts targeting PTSD, the Military Research Suicide Consortium has also suggested items for assessment of suicide risk factors (Ringer et al., 2018), and that this is also a current area of interest for the NINDS Common Data Elements (https://www.commondataelements.ninds.nih.gov/Traumatic%20Brain%20injury).
Finally, the VA/DoD has established and updated Clinical Practice Guidelines (Management of Concussion/mTBI Working Group, 2009; “VA/DoD CLINICAL PRACTICE GUIDELINE FOR THE MANAGEMENT OF CONCUSSION-MILD TRAUMATIC BRAIN INJURY,” 2016), which address additional measures and domains to be queried in military-relevant populations. These include the assessment of headache, sleep quality, mood, and other conditions. Although these represent clinical guidelines rather than research recommendations, these tools may facilitate consistency for investigators using these data. Aligning future research efforts with at least a core subset of tools that are widely used in clinical practice may also facilitate large scale data aggregation. As research in this population expands, the need for additional consideration of data points that could be commonly collected across studies has become increasingly evident, and this is a goal of ENIGMA Brain Injury. Table 4 below summarizes measures that are currently recommended in each of these efforts.
Table 4.
Commonly recommended measures for research and clinical assessment of TBI and associated comorbidities in military-relevant populations. NINDS=National Institute of Neurologic Disorders and Stroke, CDE=Common Data Elements, VA=Veterans Affairs, DoD=Department of Defense, CPG=Clinical Practice Guidelines, CAP=Consortium to Alleviate PTSD.
| Domain | Measure | NINDS mild TBI CDE | VA/DoD CPG | CAP |
|---|---|---|---|---|
| Global Functioning or Outcome | ||||
| Short Form 36 Medical Outcome Study (SF-36 v2) | X | |||
| Neuropsychological Impairment or Cognition | ||||
| Automated Neuropsychological (ANAM) | X | X | ||
| Brief Visuospatial Memory Test – Revised (BVMT-R) | X | |||
| Color Word Interference Test | X | |||
| Controlled Oral Word Association Test | X | |||
| Grooved Pegboard Test | X | |||
| NIH Toolbox Cognitive Battery | X | |||
| Rey Auditory Verbal Learning Test (RAVLT) or California Verbal Learning Test – III (CVLT – III) | X | |||
| Symbol Digit Modalities Test (SDMT) | X | |||
| Trail Making Test (TMT) | X | |||
| Wechsler Adult Intelligence Scale (WAIS-IV) Digit Span, Letter-Number Sequencing subtests and Processing Speed Index | X | |||
| General Health Symptoms | ||||
| Veterans RAND 36- or 12-Item Short Form Health Survey | X | X | ||
| Post-concussive Symptoms | ||||
| Neurobehavioral Symptom Inventory (NSI) | X | X | ||
| Rivermead Postconcussive Symptom Inventory (RPQ) | X | |||
| Headaches | ||||
| Headache Impact Test (HIT-6) | X | |||
| Sleep | ||||
| Pittsburgh Sleep Quality Index (PSQI) | X | |||
| Epworth Sleepiness Scale (ESS) Adult Scale | X | X | ||
| Insomnia Severity Index (ISI) | X | X | ||
| Snoring Tired Observed Pressure Body Mass Index Age Neck and Gender (STOP-BANG) | X | |||
| PROMIS Sleep Disturbance and Sleep-Related Impairment short forms | X | |||
| Dizziness | ||||
| Dizziness Handicap Inventory | X | |||
| Interpersonal/occupational functioning | ||||
| Brief Inventory of Psychosocial Functioning | X | |||
| PTSD | ||||
| Posttraumatic Stress Disorder Checklist – 5 (PCL-5) or, PTSD Checklist – Civilian/Military/ Stressor Specific (PCL-C/M/S) | X | X | X | |
| Clinician-Administered PTSD Scale (CAPS) | X | X | ||
| Depression /Mood Disorder | ||||
| Beck Depression Inventory – 2 (BDI-2) | X | |||
| Center for Epidemiological Studies Depression Scale (CES-D) | X | |||
| Patient Health Questionnaire (9 Item) (PHQ-9) | X | X | X | |
| Self-Injurious Thoughts and Behaviors Interview | X | |||
| Anxiety | ||||
| Generalized Anxiety Disorder (GAD-7) (sports-related concussion CDEs only) | X | X | X | |
| Other Psychiatric and Psychological Status | ||||
| Brief Symptom Inventory – 18 Item (BSI-18) | X | |||
| NIH Toolbox Emotion Battery | X | |||
| Substance Use | ||||
| Alcohol Use Disorders Identification Test: Self Report Version (AUDIT) | X | X | ||
| Alcohol, Smoking, and Substance Use Involvement Screening Test (ASSIST) | X | |||
| Quick Drinking Screen self-report version | X | |||
| Fagerstrom Test for Nicotine Dependence and Smokeless Tobacco | X | |||
| Quality of Life | ||||
| Quality of Life in Neurological Disorders (Neuro-QOL) | X | |||
| TBI-QOL | X | |||
| Quality of Life after Brain Injury (QOLIBRI) | X | |||
| Satisfaction with Life Scale (SWLS) | X | |||
| Symptom/Performance Validity | ||||
| Medical Symptom Validity Test (MSVT) | X | |||
| Test of Memory Malingering (TOMM) | X | |||
| Victoria Symptom Validity Test | X | |||
| Word Memory Test | X | |||
| Combat Exposure | ||||
| Combat Exposure Scale (CES) | X | |||
| Deployment Risk and Resilience Inventory 2 (DRRI-2) Combat Experiences | X | |||
Fluid biomarkers:
One approach to further characterize imaging correlates of the multiple and often co-existing pathologic processes of a condition as complex and heterogeneous as TBI (e.g., distinguish microvascularfrom axonal shearing injury or demonstrate its colocalization), is to partner advanced imaging with blood and biofluid biomarkers in acute, subacute, chronic, and remote TBI. TBI biomarker discovery projects that analyze candidate biomarker panels from large cohorts that encompass diverse TBI mechanisms, partnered with robust clinical, imaging and biofluid datasets, and statistical modeling, may advance the field to actuate TBI diagnostic multi-modal biomarker panels. Currently, there are no accepted clinical guidelines that incorporate the assessment fluid biomarkers after a TBI that can aid recovery and prevent further complications. There is a critical need to identify such biomarkers so that management of such injuries and post-concussive symptoms can be addressed with objective treatments. Recent reviews (e.g., Kim et al., 2018) address several categories of these biomarkers, such as those involved in blood brain barrier (BBB) dysfunction, cerebral blood flow dysfunction, traumatic axonal injury, neuroinflammatory response, and genetic variation in relation to the type of TBI (severe, moderate or mild). More specifically, disruptions of the BBB can lead to increased CSF/serum albumin ratio in patients with severe TBI. Although data are limited, such changes have not been reported in mild or moderate TBI (Bowman et al., 2012; Pisani et al., 2012; Tibbling et al., 1977). BBB disruption due to TBI can also lead to an upregulation of S100B, which is one of the few astrocyte-specific CNS proteins that are currently used clinically to detect mTBI if CT cannot be performed (Marchi et al., 2013, 2003). Another promising marker is plasma-soluble prion protein (PrP), which is increased in the CNS after mTBI in athletes when compared to healthy young adults and non-concussed athletes (Pham et al., 2015). More research is required to clarify the role of PrP as a biomarker in mTBI.
The first blood-based TBI biomarkers to receive FDA approval in acute TBI are plasma glial fibrillary acidic protein (GFAP) and Ubiquitin C-terminal hydrolase-L1 (UCH-L1), when measured in the first 12 hours after a TBI (Papa et al., 2016; Posti et al., 2016). Both neurofilament light chain (NfL) and tau in CSF (Gatson et al., 2014; Neselius et al., 2012) and blood (Gill et al., 2017) are associated with acute axonal injury; tau demonstrates less CNS specificity than NfL in polytrauma cases, likely because of peripheral sources of tau. In a military population, Gill et al. (Gill et al., 2018a) showed similar results, correlating increased acute plasma levels of tau, GFAP, and UCH-L1 and CT abnormalities in a cohort of 277 military Service members with suspected mTBI with an area under the curve (AUC) of 0.77 for increased GFAP in those with mTBI and imaging findings. Additional association between GFAP and abnormal imaging findings are also observed in other more recent large TBI cohorts (Yue et al, 2020). Studies of plasma tau in acute sports concussion collected within the first 6 hours after injury suggest that higher levels may be prognostic biomarkers of prolonged recovery (Blumbergs et al., 1994; Czeiter et al., 2020). Amyloid species, including amyloid precursor protein (APP), amyloid beta 40 (Aβ40), and amyloid beta 42 (Aβ42), accumulate as early as 2-3 hours after TBI with axonal injury (Smith et al., 2003). Studies in acute severe TBI have shown neuroinflammatory responses (IL-1β, IL-6, IL-8, IL-10, TNF-α)(Blennow et al., 2011), but no similar changes in military personnel with blast exposures, suggesting that these are less sensitive as diagnostic TBI biomarkers (Neselius et al., 2012). Candidate prognostic biomarkers of early TBI outcomes (3-6 months) include S100B, tau and phosphorylated tau (p-tau), NfL, αII-spectrin breakdown product (SNTF), and neuroinflammatory markers (IL-6, IL-8, IL-10 and TNF-α). The most promising and sensitive biomarkers to date are plasma measures of tau, NF-L, and GFAP in predicting poor outcomes after TBI (Blennow et al., 2011). There are few studies of TBI biomarkers in the chronic stage, ≥ 6 months after injury, but early reports of altered levels identify plasma and exosomal NfL, phosphorylated tau (p-tau) GFAP, and IL-6 as candidate prognostic biomarkers (Gill et al., 2018b; Kenney et al., 2018; Olivera et al., 2015; Rubenstein et al., 2017; Stern et al., 2016) with Nfl, followed by tau and inflammatory biomarkers currently the most promising prognostic biomarkers (Guedes et al., 2020; Peltz et al, 2020; Shahim et al., 2020; Shahim et al, 2020).
Future studies will be enriched by including several of these fluid biomarker assessments as they are developed and implemented. Even if the expertise and funds are not available for analyses of these types of data, it would be our strong recommendation that samples be acquired and stored for future analyses if at all possible. This area of research has significant potential and there have been important clinical gains in improving diagnostic and prognostic accuracy using fluid biomarkers.
Premorbid Variable Assessments.
Assessing clinically-relevant factors that occur prior to military service is another important area of research that might be accomplished using big data analytic methods. As noted in the challenges section, early life stress, and personal and familial psychiatric history are also potential factors influencing clinical and functional outcomes across a wide range of patient groups. As these premorbid factors influence functional and clinical outcomes, our recommendation for new studies is to include premorbid assessments that capture this type of social, psychological, and familial information. Capturing these data will allow us to further characterize these risk factors in those who experience TBI.
The recent research and clinical interest in these early life experiences has led to the development of many standardized instruments that could be used in this space to optimize consistent data collection on a number of these early life events. Though there is some debate about the validity of retrospective assessments (prospective collection appears to be reliable), a number of studies show how these variables can shape imaging, health, psychological, and cognitive outcomes (Grainger et al., 2019; Heany et al., 2018; Khosravani et al., 2019; Meinert et al., 2019; Olson et al., 2019; Tozzi et al., 2019). As such, including assessments such as the Childhood Trauma Questionnaire (CTQ) or the Adverse Childhood Events Scale (ACES) could improve our ability to characterize how these events shape TBI symptom presentation, prognosis, and recovery.
Other Recommendations.
Examining data collected across studies and conducted by different investigators poses additional challenges that warrant further discussion. First, datasets collected by individual groups are heavily nuanced. Thus another challenge is understanding the organization, the intent, and the limitations each dataset might present when aggregating data or asking a clinical question. Further, critical to the ENIGMA process (even beyond our working group) is the inclusion of investigators from each cohort that have intimate knowledge of the data collection. In this way, these kinds of issues will be known to the entire working group. For example, inclusion of investigators involved in the data collection can provide critical information about missing data. Missing data is the bane of all clinical research and can impact findings in unique ways when examined or when there is an effort to aggregate data into larger datasets for analyses. Data can be missing for a number of reasons and these reasons will impact the quality of the data in unique ways which are best understood by the investigators collecting the data. Thus by including experts involved in the collection of the data, there is a set of expert checks and balances needed to ensure that the data are used and interpreted correctly.
Summary of Recommendations
Our recent experience using big data to investigate the effects of TBI on imaging in military and veteran populations has been remarkably successful despite challenges inherent in this endeavor. This experience has resulted in the production of this manuscript and motivates the recommendations for future TBI studies. We recognize that a set of specific recommendations might place additional burden on groups of investigators, but a core set of imaging, biomarker, clinical, and cognitive measures is likely to greatly improve the efficiency, utility, and power of big data approaches. This type of harmonization effort is critical and would maximize and extend the use of data collected using public research funds. For the past decade, researchers have been first encouraged, then required, to submit data to repositories established by federal and other international agencies to maximize data use via sharing. However, using data from these repositories is challenging given the variability in data collection.
To improve the success of these endeavors to share data in the future, our recommendations include primary/core, supplementary, and comprehensive suggestions. This type of approach could ensure that a basic, standardized data set (primary/core) would exist across studies. This could benefit the larger TBI research and clinical community, while also meeting the individual needs of the specific studies designed to answer important more specific clinical questions.
One general suggestion to ensure successful completion of research in military and veteran cohorts is that researchers involve military-specific experts and stakeholders. Given the unique access requirements, and logistic, cultural, and funding requirements, it is critical to liaise with military or research experts with successful experience in conducting research in these participant groups. Preferably, new studies would be designed a priori in collaboration with military liaisons to ensure that proper consideration is given to these unique elements of this kind of research. This would allow investigators to anticipate issues and avoid unnecessary delays and complications.
Beyond this general recommendation, we make the following more specific recommendations regarding CDEs. The following contains both the recommendations and a brief rationale for each.
Demographic and Clinical.
Primary/core recommendations are to report a consistent set of demographic and clinical information for each research cohort. In our opinion, the minimal set of measures include age, sex, education, branch of service, TBI exposure history, and key premorbid features (prior to military service/injury).
Future studies should consider the use of one of the standardized TBI history assessments described above. Though we would prefer future studies to agree on a single measure (OSU-TBI ID for example), we recognize that this will be extremely difficult and may not be practical for many reasons. However, as these measures can be subtly different, we would encourage investigators to know how their chosen instrument performs with regard to other more frequently used measures, such as the OSU or BAT-L. Publishing this information would be extremely useful when trying to aggregate data from different studies and could create a more consistent set of clinical groupings for analyses across studies. Regardless, the core features of any assessment need to include accurate identification of injury (i.e., was there a TBI?), timing of injury (i.e., time since injury), and quantification of prior TBI exposure (i.e., number of TBIs over a lifetime, including childhood, predeployment and post-deployment) across study groups.
Our core demographic recommendations would be to use the NINDS demographic form, recording and reporting data as contained in the document (F1535_Demographics.doc at https://www.commondataelements.ninds.nih.gov/Traumatic%20Brain%20Injury).
- Additional core demographic and clinical recommendations for military and Veteran groups would include, but are not limited to:
- Branch of service (i.e., Army, Navy, Marines, Air Force, Coast Guard, other)
- Rank (Enlisted and Officer levels for US Service members)
- Job category (i.e., Military Occupational Specialty (MOS) Code for US Military; see https://asvabpracticetestonline.com/army-mos-codes/ for example codes)
- Combat exposure using a standardized scale
- Combat Exposure Scale (primary/core)
- Deployment Risk and Resilience Inventory-2 (DRRI-2; supplemental)
Time since injury (in days, if possible), number of injuries, and intervals between injuries (in days, if possible)
Headache assessment (HIT-6; primary/core)
- Standardized measure that captures the lifetime TBI exposure history (OSU-TBI ID as preferred measure, acknowledging that significant variability exists in the literature). Note that more research is required here to determine how comparable these measures are and what means are available to integrate information from different measures.
- If using a unique measure, including additional information as to how the measure performs relative to other more established measures.
As time and resources allow, supplementary demographic and clinical variables that research groups might consider collecting and reporting include:
Premorbid psychiatric diagnostic history (i.e., ADHD, depression, PTSD)
DRRI-2 (beyond assessing combat exposure, it assesses pre-deployment stresses, childhood function, work environment, training information, familial support and stressors, and post-deployment stressors)
Current and chronic pain assessment
Early life stress
Adverse childhood events
Recruitment methods (e.g., from clinic or community) to be able to explain any differences in the sample characteristics of TBI and control groups across studies
Imaging.
Currently, the core imaging recommendations follow the DCoE recommendations outlined above. To improve standardization, we would encourage investigators to reach out to the larger consortium studies (e.g., CENC/LIMBIC, CARE, VA TRACTS) to request more specific parameter recommendations for their particular scanner equipment so that additional coordination of acquisitions can be accomplished. These groups have unique experience collecting data across sites and variable scanner platforms that can be used to improve overall acquisition consistency. It is our experience that many groups are willing to share this type of information to improve future coordinated research.
The following core sequence recommendations are provided for prospective data collection for groups acquiring MRI data, regardless of their intention to analyze the data. Any unanalyzed data could be uploaded to repositories for other investigators to examine. Thus, the core imaging recommendations follow NINDS (https://www.commondataelements.ninds.nih.gov/Traumatic%20Brain%20Injury) and DCoE (https://dvbic.dcoe.mil/material/neuroimaging-following-mild-tbi-non-deployed-setting-reference-card) guidelines and include:
3D T1-weighted Images (1 mm isotropic voxel acquisition minimum, for volumetric, cortical thickness, and anatomical shape analyses)
3D T2-weighted Images (matching T1 resolution if possible)
Susceptibility weighted imaging (SWI; microlesion assessment), or quantitative susceptibility mapping (QSM)
3D fluid attenuated inversion recovery (FLAIR; for hyperintensity assessment)
Diffusion tensor imaging (minimum 64-direction, single shell; white matter integrity and microstructural assessment)
Resting state functional imaging (for network connectivity assessment)
As time, expertise, and funds allow, supplementary imaging sequences that could be acquired include:
MR spectroscopy (metabolite assessment; please see paper in this issue for specific recommendations related to MRS in TBI)
Arterial spin labeling (perfusion assessment)
Multi-shell diffusion imaging
Though not an emphasis in ENIGMA or in this manuscript (due to the lack of standardized processing routines at this time), additional recommendations can be seen in the DCoE documents for computed tomography (CT) and nuclear medicine (PET/SPECT) imaging that should be considered by clinicians as these methods clearly have promising clinical applications
EEG monitoring might also be included, especially if post-traumatic epilepsy is suspected.
Symptom, Mood, and Cognitive Measures.
For years, NIH has had a set of CDE recommendations for TBI assessment. However, the recommendations are broad and include multiple behavioral domains. Our recent efforts to aggregate data across several military and Veteran cohorts has been difficult because there still remains considerable variability in the battery of tests administered with little overlap in the specific measures administered even when investigators follow CDE requirements. Nonetheless, the following recommendations closely follow those of other established guidelines (see Table 4). Note that whenever possible, the response level items could be shared in repository form for these measures.
Our core symptom recommendations would be:
- Neurobehavioral Symptom Inventory (NSI; used broadly in VA data; primary/core)
- Note that inclusion of item-level responses may improve understanding of individual symptoms
Rivermead Post Concussion Symptoms Questionnaire (primary/core) as an alternative to the NSI
- Other recommendation for military settings
- Patient Global Impression of Change (PGIC) Scale (DVBIC recommendation)
Due to the incidence of comorbid sleep issues, dizziness and pain in this population, the following supplementary symptom measures to assess these in more detail are also recommended.
Pittsburgh Sleep Quality Inventory (PSQI)
Epworth Sleepiness Scale (ESS) Adult Scale
Pain
Headache Impact Test (HIT-6)
Dizziness Handicap Inventory
Our core and supplementary mood assessment recommendations would be:
- PTSD
- PTSD CheckList (PCL-5) (core)
- Clinician Administered PTSD Scale (CAPS), where possible
- Depression and suicidal ideation
- Center for Epidemiologic Studies-Depression (primary/core) or Patient Health Questionnaire-9 (PHQ-9) (core)
- Self-injurious Thoughts and Behaviors Interview (supplemental)
- Anxiety
- Generalized Anxiety Disorder-7 (GAD-7; core)
- Alcohol and Substance Use Disorders
- Alcohol Use Disorders Identification Test (AUDIT, core)
- Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST; supplementary)
Our core cognitive measure recommendations would be:
- Premorbid Function
- Test of Premorbid Function (TOPF; core)
- WRAT-4 Reading Subtest (alternative)
- Computerized Battery: Computerized batteries often sample different cognitive domains. Though the psychometric properties are not well-known (especially in TBI), these measures offer additional standardization and accuracy (i.e., response times) that are not easy to match when using more traditional paper and pencil measures.
- Automated Neuropsychological Assessment Metrics (ANAM)
- NIH-Toolbox Cognitive Battery
- Attention
- Trail-Making Test (core)
- Wechsler Adult Intelligence Scale (WAIS) Symbol Digits subtest (core)
- Continuous Performance Test (CPT) (supplemental)
- Delis-Kaplan Executive Function Scale (D-KEFS) Trails Test (alternative to TMT)
- Language
- D-KEFS Verbal Fluency Test (core)
- Controlled Oral Word Fluency (supplemental or alternative)
- Memory
- California Verbal Learning Test-III (CVLT-III) OR Hopkins Verbal Learning Test-Revised (HVLT-R; for serial testing) (core)
- Benton Visual Memory Test-Revised (BVMT-R; supplemental)
- Executive
- Stroop Color-Word (core)
- D-KEFS Color-Word Interference (alternative)
- Processing Speed
- WAIS-IV Symbol Search and Coding (core)
- Computerized Test of Information Processing (CTIP) (supplemental)
- Motor
- Grooved Pegboard Test (core)
Symptom/Performance Validity core and supplemental recommendations include:
Test of Memory Malingering (TOMM; core)
Word Memory Test (WMT) or Medical Symptom Validity Test (MSVT), or Victoria Symptom Validity Test (VSVT) (supplemental)
Having made these core and supplemental recommendations, we recognize that there may be limitations in neuropsychological assessments with regard to TBI assessment (especially mild TBI). Many neuropsychological measures used to assess TBI were developed independent of TBI and the testing materials that were based on theoretical frameworks that are almost a century old (Miller and Barr, 2017). Currently, assessment also relies heavily on measures that are labor intensive, expensive, and are gross measures of the cognitive domains they claim to examine (Miller and Barr, 2017; Rabin et al., 2016). Though current versions of these tests reflect modest modifications and updated normative data, there has been minimal effort to incorporate technology into neuropsychological assessment and as a result there may be limited sensitivity and the specificity of these measurements when investigating the effects of TBI. Current neuropsychological assessment methods continue to be lab-based, administered 1:1 which may not reflect real-world neurocognitive and neurobehavioral challenges experienced by a brain-injured examinee (Kessels, 2019; Ziemnik and Suchy, 2019). For these reasons, supplementary suggestions for assessment would include new measures from various disciplines that might improve the sensitivity and specificity of findings in TBI:
Dual task paradigms
Eye tracking paradigms
Balance testing (e.g., Computerized Dynamic Posturography (CDP); the emphasis here would be to use measures that are not based only on clinical observation)
Virtual reality testing environments
Biomarkers.
The interest in fluid biomarkers is growing and sampling these biomarkers in new cohorts offers a significant opportunity to understand how these measures interact with, or help to predict, the clinical, imaging, and cognitive measures following TBI. We recognize that processing storing and analyzing biospecimens requires additional expertise and funding that may not be available to every study. However, collection of blood and bio-samples and storage of these specimens for future analyses remain a core recommendation whenever feasible.
Our core biomarker recommendations would be:
- Collect and store serial biosamples from each participant in a uniform manner, including collection, local processing and storage. The current TBI biorepositories were developed based on the NINDS Parkinson’s Disease Biomarker Program and include routine collection of whole blood (for DNA extraction from buffy coat), plasma, serum, RNA and saliva at multiple preset time points..
- Recommendations for drawing and storing biosamples can be found here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4824671/ or in consultation with biospecimen biorepositories currently collecting and curating specimens (e.g. CNRM, CENC, TRACK-TBI, Center-TBI)
- If capable of analyzing, report the following:
- FDA approved TBI biomarkers
- GFAP
- UCHL-1
- Other
- NF-L
- Tau and p-tau
- Amyloid β peptides
- IL-1β, IL-6, IL-8, IL-10
- TNF-α
Supplementary biomarker analyses of biosamples could include:
- Genetic/Epigenetic data
- APOE genotype
- miRNA expression
- DNA methylation
- GWAS (from blood or saliva samples)
- More novel technology considerations for biomarker analyses
- Single molecule array (Simoa; Bogoslovsky et al., 2019)
- P-tau isoforms (e.g. cis/trans or tau phosphorylation site)
- CNS-derived exosomal cargo (proteomics, lipidomics, miRNA)
Again, we recognize the fact that more specific recommendations can be burdensome for diverse research groups. However, harmonization at the level of acquisition maximizes and extends the use of data collected with public research funds and has great potential for additional advances in personalized medicine including targeted treatments for patients who have sustained a TBI during military service.
Conclusion
Neuroimaging and genetics studies in Veterans/military-relevant populations with exposure to concussive events and TBI have been rapidly increasing over the past two decades. Big data approaches in these populations are associated with numerous challenges, including those inherent in heterogeneous definitions of TBI and assessment of injury in theatre, inconsistent use of measures in data collection, and frequencies of certain comorbidities. However, significant opportunities exist for both retrospective analysis and design of prospective studies in this area of TBI research. The ENIGMA Military Brain Injury working group will address many of these issues through collaborative analyses. The ENIGMA Military Brain Injury working group will address many of these issues through collaborative meta- and mega-analyses.
Acknowledgements
VA R&D SPIRE AWARD to MA. K99NS096116 to ELD. VA RR&D IK2RX002922-01A1 to SGD. Dutch Ministry of Defence. R01AG058822 to JPH. R01NS100973 and DoD contract W81XWH-18-1-0413 to AI. VA Rehabilitation Research and Development Service I01RX003443, I01RX003442; VA Health Services Research and Development Service Research Career Scientist Award IK6HX002608 to MJP. This work was supported (or supported in part) by Merit Review Award Number I01CX001820 from the United States (U.S.) Department of Veterans Affairs Clinical Sciences R&D (CSRD) Service. VA CSR&D 1IK2CX001772-01 to DRS. Defense and Veterans Brain Injury Centers, the U.S. Army Medical Research and Materiel Command (USAMRMC; W81XWH-13-2-0025) and the Chronic Effects of Neurotrauma Consortium (CENC; PT108802-SC104835) to DFT. UL1TR002649 to BAT. U54EB020403 to PMT. Department of Defense, Chronic Effects of Neurotrauma Consortium (CENC) Award W81XWH-13-2-0095, 5 I01 RX002174 to EAW.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest
Dr. Adamson is on the scientific advisory board for TruGenomics, a start-up developing a biomarker for PTSD. Dr. Zafonte received royalties from 1) Oakstone for an educational CD- Physical Medicine and Rehabilitation a Comprehensive Review; 2) Demos publishing for serving as co-editor of the text Brain Injury Medicine. Dr. Zafonte also serves on the Scientific Advisory Board of Myomo, Oxeia Biopharma, Biodirection and ElMINDA. He also evaluates patients in the MGH Brain and Body-TRUST Program which is funded by the NFL Players Association. The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, Department of Veterans Affairs, or the U.S. Government.
References
- Anda RF, Whitfield CL, Felitti VJ, Chapman D, Edwards VJ, Dube SR, Williamson DF, 2002. Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatr. Serv 53, 1001–1009. [DOI] [PubMed] [Google Scholar]
- Attilio PJ, Flora M, Kamnaksh A, Bradshaw DJ, Agoston D, Mueller GP, 2017. The Effects of Blast Exposure on Protein Deimination in the Brain. Oxid. Med. Cell. Longev 2017, 8398072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Byers AL, Gardner RC, Seal KH, Boscardin WJ, Yaffe K, 2018. Association of Mild Traumatic Brain Injury With and Without Loss of Consciousness With Dementia in US Military Veterans. JAMA Neurol. 75, 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SM, Walter KH, Chard KM, 2012. Does a history of mild traumatic brain injury increase suicide risk in veterans with PTSD? Rehabilitation Psychology, 10.1037/a0027007 [DOI] [PubMed] [Google Scholar]
- Barrett DH, Morris RD, Akhtar FZ, Michalek JE, 2001. Serum dioxin and cognitive functioning among veterans of Operation Ranch Hand. Neurotoxicology 22, 491–502. [DOI] [PubMed] [Google Scholar]
- Bartnik-Olson B, Alger J, Babikian T, Harris AD, Holshouser B, Kirov II, Maudsley AA, Thompson PM, Dennis EL, Tate DF, Wilde EA, Lin A, 2019. The Clinical Utility of Magnetic Resonance Spectroscopy in Traumatic Brain Injury: Recommendations from the ENIGMA MRS Working Group. Brain Imaging Behav Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, Donnelly K, Peterson DR, Warner GC, Zhu T, Zhong J, 2013. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during Operations Enduring Freedom and Iraqi Freedom. J. Head Trauma Rehabil 28, 1–12. [DOI] [PubMed] [Google Scholar]
- Ben Barnes J, Presseau C, Jordan AH, Kline NK, Young-McCaughan S, Keane TM, Peterson AL, Litz BT, the Consortium to Alleviate PTSD, 2019. Common Data Elements in the Assessment of Military-Related PTSD Research Applied in the Consortium to Alleviate PTSD. Mil. Med 184, e218–e226. [DOI] [PubMed] [Google Scholar]
- Bieniek KF, Ross OA, Cormier KA, Walton RL, Soto-Ortolaza A, Johnston AE, DeSaro P, Boylan KB, Graff-Radford NR, Wszolek ZK, Rademakers R, Boeve BF, McKee AC, Dickson DW, 2015. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 130, 877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blast Injury Research Coordinating Office (BIRCO), n.d. Blast Injury Research Coordinating Office (BIRCO) [WWW Document], URL https://blastinjuryresearch.amedd.army.mil/index.cfm/blast_injury_101 (accessed 11.8.19).
- Blennow K, Jonsson M, Andreasen N, Rosengren L, Wallin A, Hellström PA, Zetterberg H, 2011. No neurochemical evidence of brain injury after blast overpressure by repeated explosions or firing heavy weapons. Acta Neurol. Scand 123, 245–251. [DOI] [PubMed] [Google Scholar]
- Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ, 1994. Stalning af amyloid percursor protein to study axonal damage in mild head Injury. Lancet 344, 1055–1056. [DOI] [PubMed] [Google Scholar]
- Bogner J, Corrigan JD, 2009. Reliability and predictive validity of the Ohio State University TBI identification method with prisoners. J. Head Trauma Rehabil 24, 279–291. [DOI] [PubMed] [Google Scholar]
- Bowman GL, Kaye JA, Quinn JF, 2012. Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr. Gerontol. Geriatr. Res 2012, 184042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner LA, Ivins BJ, Schwab K, Warden D, Nelson LA, Jaffee M, Terrio H, 2010. Traumatic brain injury, posttraumatic stress disorder, and postconcussive symptom reporting among troops returning from iraq. J. Head Trauma Rehabil 25, 307–312. [DOI] [PubMed] [Google Scholar]
- Butler O, Adolf J, Gleich T, Willmund G, Zimmermann P, Lindenberger U, Gallinat J, Kühn S, 2017. Military deployment correlates with smaller prefrontal gray matter volume and psychological symptoms in a subclinical population. Transl. Psychiatry 7, e1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes KR, Wilson CM, Brabazon F, von Leden R, Jurgen JS, Oakes TR, Selwyn RG 2014. FDG-PET imaging in mild traumatic brain injury: a critical review. Front Neuroenergetics. 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson KF, Kehle SM, Meis LA, Greer N, Macdonald R, Rutks I, Sayer NA, Dobscha SK, Wilt TJ, 2011. Prevalence, assessment, and treatment of mild traumatic brain injury and posttraumatic stress disorder: a systematic review of the evidence. J. Head Trauma Rehabil 26, 103–115. [DOI] [PubMed] [Google Scholar]
- Carr W, Stone JR, Walilko T, Young LA, Snook TL, Paggi ME, Tsao JW, Jankosky CJ, Parish RV, Ahlers ST, 2016. Repeated Low-Level Blast Exposure: A Descriptive Human Subjects Study. Mil. Med 181,28–39. [DOI] [PubMed] [Google Scholar]
- Cedeño Laurent JG, Williams A, Oulhote Y, Zanobetti A, Allen JG, Spengler JD, 2018. Reduced cognitive function during a heat wave among residents of non-air-conditioned buildings: An observational study of young adults in the summer of 2016. PLoS Med. 15, e1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, 2017. Evidence of Objective Memory Impairments in Deployed Gulf War Veterans With Subjective Memory Complaints. Mil. Med 182, e1625–e1631. [DOI] [PubMed] [Google Scholar]
- Chen H, Constantini S, Chen Y, 2015. A Two-Model Approach to Investigate the Mechanisms Underlying Blast-Induced Traumatic Brain Injury. Brain Neurotrauma. 10.1201/b18126-21 [DOI] [PubMed] [Google Scholar]
- Chen Y, Huang W, 2011. Non-impact, blast-induced mild TBI and PTSD: concepts and caveats. Brain Inj. 25, 641–650. [DOI] [PubMed] [Google Scholar]
- Clark AL, Sorg SF, Schiehser DM, Bigler ED, Bondi MW, Jacobson MW, Jak AJ, Delano-Wood L, 2016. White Matter Associations With Performance Validity Testing in Veterans With Mild Traumatic Brain Injury: The Utility of Biomarkers in Complicated Assessment. J. Head Trauma Rehabil 31, 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DB, Curtiss G, Armistead-Jehle P, Belanger HG, Tate DF, Reid M, Bowles AO, Velez CS, Kennedy JE, Vanderploeg RD, 2018. Neuropsychological Performance and Subjective Symptom Reporting in Military Service Members With a History of Multiple Concussions: Comparison With a Single Concussion, Posttraumatic Stress Disorder, and Orthopedic Trauma. J. Head Trauma Rehabil 33, 81–90. [DOI] [PubMed] [Google Scholar]
- Cooper DB, Nelson L, Armistead-Jehle P, Bowles AO, 2011. Utility of the mild brain injury atypical symptoms scale as a screening measure for symptom over-reporting in operation enduring freedom/operation iraqi freedom service members with post-concussive complaints. Arch. Clin. Neuropsychol 26, 718–727. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Bogner J, 2007. Screening and identification of TBI. J. Head Trauma Rehabil [DOI] [PubMed] [Google Scholar]
- Coull N, Chrismas B, Watson P, Horsfall R, Taylor L, 2016. Tyrosine Ingestion and Its Effects on Cognitive and Physical Performance in the Heat. Med. Sci. Sports Exerc 48, 277–286. [DOI] [PubMed] [Google Scholar]
- Covey DC, 2006. Combat orthopaedics: a view from the trenches. J. Am. Acad. Orthop. Surg 14, S10–7. [DOI] [PubMed] [Google Scholar]
- Czeiter E, Amrein K, Gravesteijn Benjamin Y, Lecky F, Menon DK, Mondello S, Newcombe VFJ, Richter S, Steyerberg EW, Vande Vyvere T, Verheyden J, Xu H, Yang Z, Maas AIR, Wang KKW, Büki A, CENTER-TBI Participants and Investigators. 2020. Blood biomarkers on admission in acute traumatic brain injury: Relations to severity, CT findings and care path in the CENTER-TBI study. EBioMedicine 2020. June;56:102785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dams-O’Connor K, Spielman L, Singh A, Gordon WA, Lingsma HF, Maas AIR, Manley GT, Mukherjee P, Okonkwo DO, Puccio AM, Schnyer DM, Valadka AB, Yue JK, Yuh EL, TRACK-TBI Investigators, 2013. The impact of previous traumatic brain injury on health and functioning: a TRACK-TBI study. J. Neurotrauma 30, 2014–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dams-O’Connor K, Cantor JB, Brown M, Dijkers MP, Spielman LA, Gordon WA, 2014. Screening for traumatic brain injury: findings and public health implications. J. Head Trauma Rehabil 29, 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport ND, Lim KO, Sponheim SR, 2015. White matter abnormalities associated with military PTSD in the context of blast TBI. Hum. Brain Mapp 36, 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Caeyenberghs K, Asarnow RF, Babikian T, Bartnik-Olson B, Bigler ED, Figaji A, Hodges CB, Levin H, Lindsey H, Livny-Ezer A, Max J, Newsome M, Olsen A, Ryan NP, Suskauer SJ, Thomopoulos S, Ware AL, Watson CG, Wheeler AL, Zielinski BA, Thompson PM, Tate DF, Wilde EA, 2019. Brain Imaging in Young Brain-Injured Patients: A Coordinated Effort Towards Individualized Predictors from the ENIGMA Pediatric msTBI Group. Brain Imaging Behav Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Disner SG, Fani N, Salminen LE, Logue M, Clarke EK, Haswell CC, Averill CL, Baugh LA, Bomyea J, Bruce SE, Cha J, Choi K, Davenport ND, Densmore M, du Plessis S, Forster GL, Frijling JL, Gönenc A, Gruber S, Grupe DW, Guenette JP, Hayes J, Hofmann D, Ipser J, Jovanovic T, Kelly S, Kennis M, Kinzel P, Koch SBJ, Koerte I, Koopowitz S, Korgaonkar M, Krystal J, Lebois LAM, Li G, Magnotta VA, Manthey A, May GJ, Menefee DS, Nawijn L, Nelson SM, Neufeld RWJ, Nitschke JB, O’Doherty D, Peverill M, Ressler K, Roos A, Sheridan MA, Sierk A, Simmons A, Simons RM, Simons JS, Stevens J, Suarez-Jimenez B, Sullivan DR, Théberge J, Tran JK, van den Heuvel L, van der Werff SJA, van Rooij SJH, van Zuiden M, Velez C, Verfaellie M, Vermeiren RR, Wade BSC, Wager T, Walter H, Winternitz S, Wolff J, York G, Zhu Y, Zhu X, Abdallah CG, Bryant R, Daniels JK, Davidson RJ, Fercho KA, Franz C, Geuze E, Gordon EM, Kaufman ML, Kremen W, Lagopoulos J, Lanius RA, Lyons MJ, McCauley SR, McGlinchey R, McLaughlin KA, Milberg W, Neria Y, Olff M, Seedat S, Shenton M, Sponheim SR, Stein DJ, Stein MB, Straube T, Tate DF, van der Wee NJA, Veltman DJ, Wang L, Wilde EA, Thompson PM, Kochunov P, Jahanshad N, Morey RA, 2019. Altered White Matter Microstructural Organization in Post-Traumatic Stress Disorder across 3,049 Adults: Results from the PGC-ENIGMA PTSD Consortium. bioRxiv. 10.1101/677153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG, Kramer MD, Nelson NW, Lipinski AJ, Christensen JM, Polusny MA, Sponheim SR, 2017. Predictors of postdeployment functioning in combat-exposed US military veterans. Clin. Psychol. Sci 5, 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald CLM, Mac Donald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, Werner NJ, Zonies D, Oh J, Fang R, Brody DL, 2014. Prospectively Assessed Clinical Outcomes in Concussive Blast vs Nonblast Traumatic Brain Injury Among Evacuated US Military Personnel. JAMA Neurology. 10.1001/jamaneurol.2014.1114 [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF, 2004. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation 110, 1761–1766. [DOI] [PubMed] [Google Scholar]
- Donnell AJ, Kim MS, Silva MA, Vanderploeg RD, 2012. Incidence of postconcussion symptoms in psychiatric diagnostic groups, mild traumatic brain injury, and comorbid conditions. Clin. Neuropsychol 26, 1092–1101. [DOI] [PubMed] [Google Scholar]
- Dretsch MN, Johnston D, Bradley RS, MacRae H, Deuster PA, Harris WS, 2014. Effects of omega-3 fatty acid supplementation on neurocognitive functioning and mood in deployed U.S. soldiers: a pilot study. Mil. Med 179, 396–403. [DOI] [PubMed] [Google Scholar]
- Edwards VJ, Holden GW, Felitti VJ, Anda RF, 2003. Relationship Between Multiple Forms of Childhood Maltreatment and Adult Mental Health in Community Respondents: Results From the Adverse Childhood Experiences Study. American Journal of Psychiatry. 10.1176/appi.ajp.160.8.1453 [DOI] [PubMed] [Google Scholar]
- Esopenko C, Meyer J, Wilde EA, Marshall A, Tate DF, Lin A, Koerte IK, Werner KB, Dennis EL, Ware AL, de Souza NL, Menefee DS, Dams-O’Connor K, Stein DJ, Hillary FG, 2019. Harmonization of Measures to Assess IPV-Related Head Trauma: Recommendations from the ENIGMA IPV Working Group. Brain Imaging Behav Under Review. [Google Scholar]
- Farace E, Alves WM, 2000. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J. Neurosurg 93, 539–545. [DOI] [PubMed] [Google Scholar]
- Fear NT, Jones M, Murphy D, Hull L, Iversen AC, Coker B, Machell L, Sundin J, Woodhead C, Jones N, Greenberg N, Landau S, Dandeker C, Rona RJ, Hotopf M, Wessely S, 2010. What are the consequences of deployment to Iraq and Afghanistan on the mental health of the UK armed forces? A cohort study. Lancet 375, 1783–1797. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, 2019. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults: The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med 56, 774–786. [DOI] [PubMed] [Google Scholar]
- Fischer BL, Parsons M, Durgerian S, Reece C, Mourany L, Lowe MJ, Beall EB, Koenig KA, Jones SE, Newsome MR, Scheibel RS, Wilde EA, Troyanskaya M, Merkley TL, Walker M, Levin HS, Rao SM, 2014. Neural activation during response inhibition differentiates blast from mechanical causes of mild to moderate traumatic brain injury. J. Neurotrauma 31, 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Amick MM, Grande L, McGlynn S, Kenna A, Morra L, Clark A, Milberg WP, McGlinchey RE, 2014. The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) semistructured interview: evidence of research utility and validity. J. Head Trauma Rehabil 29, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Gillespie DL, O’Donnell SD, Rasmussen TE, Goff JM, Johnson CA, Galgon RE, Sarac TP, Rich NM, 2005. Contemporary management of wartime vascular trauma. J. Vase. Surg 41,638–644. [DOI] [PubMed] [Google Scholar]
- Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K, 2014. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 71, 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Yaffe K, 2015. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell. Neurosci 66, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Yaffe K, 2014. Traumatic brain injury may increase risk of young onset dementia. Ann. Neurol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatson JW, Barillas J, Hynan LS, Diaz-Arrastia R, Wolf SE, Minei JP, 2014. Detection of neurofilament-H in serum as a diagnostic tool to predict injury severity in patients who have suffered mild traumatic brain injury. J. Neurosurg 121, 1232–1238. [DOI] [PubMed] [Google Scholar]
- Gill J, Latour L, Diaz-Arrastia R, Motamedi V, Turtzo C, Shahim P, Mondello S, DeVoto C, Veras E, Hanlon D, Song L, Jeromin A, 2018a. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology 91, e1385–e1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J, Merchant-Borna K, Jeromin A, Livingston W, Bazarian J, 2017. Acute plasma tau relates to prolonged return to play after concussion. Neurology 88, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J, Mustapic M, Diaz-Arrastia R, Lange R, Gulyani S, Diehl T, Motamedi V, Osier N, Stern RA, Kapogiannis D, 2018b. Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj. 32, 1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath KS, Sakoglu U, Crosson BA, Haley RW, 2019. Exploring brain mechanisms underlying Gulf War Illness with group ICA based analysis of fMRI resting state networks. Neurosci. Lett 701, 136–141. [DOI] [PubMed] [Google Scholar]
- Grainger SA, Crawford JD, Kochan NA, Mather KA, Chander RJ, Draper B, Brodaty H, Sachdev PS, Henry JD, 2019. An investigation into early-life stress and cognitive function in older age. Int. Psychogeriatr 1–5. [DOI] [PubMed] [Google Scholar]
- Grande LJ, Robinson ME, Radigan LJ, Levin LK, Fortier CB, Milberg WP, McGlinchey RE, 2018. Verbal Memory Deficits in OEF/OIF/OND Veterans Exposed to Blasts at Close Range. J. Int. Neuropsychol. Soc 24, 466–475. [DOI] [PubMed] [Google Scholar]
- Greve MW, Zink BJ, 2009. Pathophysiology of traumatic brain injury. Mt. Sinai J. Med 76, 97–104. [DOI] [PubMed] [Google Scholar]
- Guedes VA, Kenney K, Shahim P, Qu BX, Lai C, Devoto C, Walker WC, Nolen T, Diaz-Arrastia R, Gill JM; CENC Multisite Observational Study Investigators. Exosomal neurofilament light: A prognostic biomarker for remote symptoms after mild traumatic brain injury? Neurology 2020. June 9;94(23):e2412–e2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MN, Aiello AE, West NA, Jagust WJ, 2008. C-reactive protein and rate of dementia in carriers and non carriers of Apolipoprotein APOE4 genotype. Neurobiol. Aging 29, 1774–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafstad GS, Thoresen S, Wentzel-Larsen T, Maercker A, Dyb G, 2017. PTSD or not PTSD? Comparing the proposed ICD-11 and the DSM-5 PTSD criteria among young survivors of the 2011 Norway attacks and their parents. Psychol. Med 47, 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Chapman SB, Krawczyk DC, 2015. Altered Amygdala Connectivity in Individuals with Chronic Traumatic Brain Injury and Comorbid Depressive Symptoms. Front. Neurol 6, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Mac Donald CL, Johnson AM, Barnes Y, Wierzechowski L, Zonies D, Oh J, Flaherty S, Fang R, Raichle ME, Brody DL, 2014. Disrupted modular organization of resting-state cortical functional connectivity in U.S. military personnel following concussive “mild” blast-related traumatic brain injury. Neuroimage 84, 76–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Martinez D, Chapman SB, Krawczyk DC, 2018. Neural correlates of reduced depressive symptoms following cognitive training for chronic traumatic brain injury. Hum. Brain Mapp 39, 2955–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Logue MW, Sadeh N, Spielberg JM, Verfaellie M, Hayes SM, Reagan A, Salat DH, Wolf EJ, McGlinchey RE, Milberg WP, Stone A, Schichman SA, Miller MW, 2017. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain 140, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heany SJ, Groenewold NA, Uhlmann A, Dalvie S, Stein DJ, Brooks SJ, 2018. The neural correlates of Childhood Trauma Questionnaire scores in adults: A meta-analysis and review of functional magnetic resonance imaging studies. Dev. Psychopathol 30, 1475–1485. [DOI] [PubMed] [Google Scholar]
- Hicks R, Giacino J, Harrison-Felix C, Manley G, Valadka A, Wilde EA, 2013. Progress in developing common data elements for traumatic brain injury research: version two--the end of the beginning. J. Neurotrauma 30, 1852–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiploylee C, Dufort PA, Davis HS, Wennberg RA, Tartaglia MC, Mikulis D, Hazrati L-N, Tator CH, 2017. Longitudinal Study of Postconcussion Syndrome: Not Everyone Recovers. J. Neurotrauma 34, 1511–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JR, Stout JR, Harris RC, Moran DS, 2015. β-Alanine supplementation and military performance. Amino Acids 47, 2463–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, 2011. Blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, 2006. Post-traumatic stress disorder in UK and US forces deployed to Iraq. Lancet. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA, 2008. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med 358, 453–463. [DOI] [PubMed] [Google Scholar]
- Hudak AM, Hynan LS, Harper CR, Diaz-Arrastia R, 2012. Association of depressive symptoms with functional outcome after traumatic brain injury. J. Head Trauma Rehabil 27, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indications and Conditions for In-Theater Post-Injury Neurocognitive Assessment Tool (NCAT) Testing Clinical Recommendation [WWW Document], 2013. DVBIC. URL https://dvbic.dcoe.mil/material/indications-and-conditions-theater-post-injury-neurocognitive-assessment-tool-ncat-testing-clinical-recommendation (accessed 12.12.19). [Google Scholar]
- Ingalls N, Zonies D, Bailey JA, Martin KD, Iddins BO, Carlton PK, Hanseman D, Branson R, Dorlac W, Johannigman J, 2014. A review of the first 10 years of critical care aeromedical transport during operation iraqi freedom and operation enduring freedom: the importance of evacuation timing. JAMA Surg. 149, 807–813. [DOI] [PubMed] [Google Scholar]
- Isaac L, Main KL, Soman S, Gotlib IH, Furst AJ, Kinoshita LM, Fairchild JK, Yesavage JA, Ashford JW, Bayley PJ, Adamson MM, 2015. The impact of depression on Veterans with PTSD and traumatic brain injury: a diffusion tensor imaging study. Biol. Psychol 105, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoniemi H, Tenovuo O, Portin R, Himanen L, Kairisto V, 2006. Outcome of traumatic brain injury after three decades—relationship to ApoE genotype. J. Neurotrauma 23, 1600–1608. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH, 2012. Widespread ⊺ and amyloid-β pathology many years after a single traumatic brain injury in humans. Brain Pathol. 22, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan AD, Relkin NR, Ravdin LD, Jacobs AR, 1997. Apolipoprotein E e4 Associated With Chronic Traumatic Brain Injury in Boxing. JAMA. [PubMed] [Google Scholar]
- Kabat MH, Kane RL, Jefferson AL, DiPino RK, 2001. Construct validity of selected Automated Neuropsychological Assessment Metrics (ANAM) battery measures. Clin. Neuropsychol 15, 498–507. [DOI] [PubMed] [Google Scholar]
- Kaloupek DG, Chard KM, Freed MC, Peterson AL, Riggs DS, Stein MB, Tuma F, 2010. Common data elements for posttraumatic stress disorder research. Arch. Phys. Med. Rehabil 91, 1684–1691. [DOI] [PubMed] [Google Scholar]
- Kaup AR, Toomey R, Bangen KJ, Delano-Wood L, Yaffe K, Panizzon MS, Lyons MJ, Franz CE, Kremen WS, 2019. Interactive Effect of Traumatic Brain Injury and Psychiatric Symptoms on Cognition among Late Middle-Aged Men: Findings from the Vietnam Era Twin Study of Aging. J. Neurotrauma 36, 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C, Singh J, Lim MK, Ng BL, Yap EPH, Ling EA, 1995. The response of neurons and microglia to blast injury in the rat brain. Neuropathology and Applied Neurobiology. 10.1111/j.1365-2990.1995.tb01073.x [DOI] [PubMed] [Google Scholar]
- Kazis LE, Miller DR, Clark JA, Skinner KM, Lee A, Ren XS, Spiro A 3rd, Rogers WH, Ware JE Jr, 2004. Improving the response choices on the veterans SF-36 health survey role functioning scales: results from the Veterans Health Study. J. Ambul. Care Manage 27, 263–280. [DOI] [PubMed] [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora CA, 1989. Clinical evaluation of a measure to assess combat exposure. Psychological Assessment: A Journal of Consulting and Clinical Psychology 1,53. [Google Scholar]
- Kenney K, Qu B-X, Lai C, Devoto C, Motamedi V, Walker WC, Levin HS, Nolen T, Wilde EEA, Diaz-Arrastia R, Gill J, CENC Multisite Observational Study Investigators, 2018. Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury. Brain Inj. 32, 1276–1284. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, 2019. Improving precision in neuropsychological assessment: Bridging the gap between classic paper-and-pencil tests and paradigms from cognitive neuroscience. Clin. Neuropsychol 33, 357–368. [DOI] [PubMed] [Google Scholar]
- Khosravani V, Samimi Ardestani SM, Sharifi Bastan F, Mohammadzadeh A, Amirinezhad A, 2019. Childhood maltreatment, cognitive emotion regulation strategies, and alcohol craving and dependence in alcohol-dependent males: Direct and indirect pathways. Child Abuse Negl. 98, 104197. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Tsao JW, Stanfill AG, 2018. The current state of biomarkers of mild traumatic brain injury. JCI Insight 3. 10.1172/jci.insight.97105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King PR, Donnelly KT, Donnelly JP, Dunnam M, Warner G, Kittleson CJ, Bradshaw CB, Alt M, Meier ST, 2012. Psychometric study of the Neurobehavioral Symptom Inventory. J. Rehabil. Res. Dev 49, 879–888. [DOI] [PubMed] [Google Scholar]
- Koerte IK, Dennis EL, Bazarian JJ, Bigler ED, Buckley T, Choe M, Esopenko C, Gill J, Giza CC, Hodges CB, Irimia A, Kenney K, Levin H, Lin A, Lindsey HM, Max J, Mayer AR, Merchant-Borna K, Mills B, Porfido T, Tartaglia C, Ware AL, Zeineh M, Thompson PM, Tate DF, Wilde EA, Baron D, 2019. Neuroimaging of Sport-Related Brain Injury: Challenges and Recommendations from the ENIGMA Sports-Related Brain Injury group. Brain Imaging Behav Under Review. [Google Scholar]
- Ko H, Hunter KE, Scott AM, Ayson M, Willson ML, 2018. A systematic review of performance-enhancing pharmacologicals and biotechnologies in the Army. J. R. Army Med. Corps 164, 197–206. [DOI] [PubMed] [Google Scholar]
- Kolakowksy-Hayner SA, Wright J, Englander J, Duong T, Ladley-O’Brien S 2013. Impact of late post-traumatic seizures on physcial health and functioning for individuals with brain injury within the community. Brain Inj. 27(5):578–86. [DOI] [PubMed] [Google Scholar]
- Kotwal RS, Staudt AM, Mazuchowski EL, Gurney JM, Shackelford SA, Butler FK, Stockinger ZT, Holcomb JB, Nessen SC, Mann-Salinas EA, 2018. A US military Role 2 forward surgical team database study of combat mortality in Afghanistan. J. Trauma Acute Care Surg 85, 603–612. [DOI] [PubMed] [Google Scholar]
- Kroupa J, 1990. [Definition of “polytrauma” and “polytraumatism”]. Acta Chir. Orthop. Traumatol. Cech 57, 347–360. [PubMed] [Google Scholar]
- Lange RT, Brickell TA, Kennedy JE, Bailie JM, Sills C, Asmussen S, Amador R, Dilay A, Ivins B, French LM, 2014. Factors influencing postconcussion and posttraumatic stress symptom reporting following military-related concurrent polytrauma and traumatic brain injury. Arch. Clin. Neuropsychol 29, 329–347. [DOI] [PubMed] [Google Scholar]
- Lange RT, Lippa SM, French LM, Bailie JM, Gartner RL, Driscoll AE, Wright MM, Sullivan JK, Varbedian NV, Barnhart EA, Holzinger JB, Schaper AL, Reese MA, Brandler BJ, Camelo-Lopez V, Brickell TA, 2019. Long-term neurobehavioural symptom reporting following mild, moderate, severe, and penetrating traumatic brain injury in U.S. military service members. Neuropsychol. Rehabil. 1–24. [DOI] [PubMed] [Google Scholar]
- Larrabee GJ, 2015. The multiple validities of neuropsychological assessment. Am. Psychol 70, 779–788. [DOI] [PubMed] [Google Scholar]
- Levin HS, Shum D, Chan RCK, 2014. Understanding Traumatic Brain Injury: Current Research and Future Directions. Oxford University Press. [Google Scholar]
- Levin HS, Wilde E, Troyanskaya M, Petersen NJ, Scheibel R, Newsome M, Radaideh M, Wu T, Yallampalli R, Chu Z, Li X, 2010. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J. Neurotrauma 27, 683–694. [DOI] [PubMed] [Google Scholar]
- Ling G, Bandak F, Armonda R, Grant G, Ecklund J, 2009. Explosive blast neurotrauma. J. Neurotrauma 26, 815–825. [DOI] [PubMed] [Google Scholar]
- Lippa SM, Pastorek NJ, Benge JF, Thornton GM, 2010. Postconcussive symptoms after blast and nonblast-related mild traumatic brain injuries in Afghanistan and Iraq war veterans. J. Int. Neuropsychol. Soc 16, 856–866. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Gulko E, Zimmerman ME, Friedman BW, Kim M, Gellella E, Gold T, Shifteh K, Ardekani BA, Branch CA, 2009. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology 252, 816–824. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Kim N, Park YK, Hulkower MB, Gardin TM, Shifteh K, Kim M, Zimmerman ME, Lipton RB, Branch CA, 2012. Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging Behav. 6, 329–342. [DOI] [PubMed] [Google Scholar]
- Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, Densmore M, Haswell CC, Ipser J, Koch SB, 2017. Smaller hippocampal volume in posttraumatic stress disorder: a multi-site ENIGMA-PGC study. Biol. Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson M, King JB, McGlade E, Bueler E, Stoeckel A, Epstein DJ, Yurgelun-Todd D, 2013. Enlarged thalamic volumes and increased fractional anisotropy in the thalamic radiations in veterans with suicide behaviors. Front. Psychiatry 4, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui C-W, Waller M, Bell A, van der Pols JC, 2019. Retrospective self-reported dietary supplement use by Australian military personnel during deployment to Iraq and Afghanistan: results from the Middle East Area of Operations Health Study. Appl. Physiol. Nutr. Metab 44, 674–680. [DOI] [PubMed] [Google Scholar]
- Maas AI, Harrison-Felix CL, Menon D, Adelson PD, Balkin T, Bullock R, Engel DC, Gordon W, Orman JL, Lew HL, Robertson C, Temkin N, Valadka A, Verfaellie M, Wainwright M, Wright DW, Schwab K, 2010. Common data elements for traumatic brain injury: recommendations from the interagency working group on demographics and clinical assessment. Arch. Phys. Med. Rehabil 91, 1641–1649. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Barber J, Patterson J, Johnson AM, Dikmen S, Fann JR, Temkin N, 2019. Association between 5-year clinical outcome in patients with nonmedically evacuated mild blast traumatic brain injury and clinical measures collected within 7 days postinjury in combat. JAMA network open 2, e186676–e186676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, Werner NJ, Adam OR, Rivet DJ, Flaherty SF, Oh JS, Zonies D, Fang R, Brody DL, 2017. Outcome Trends after US Military Concussive Traumatic Brain Injury. J. Neurotrauma 34, 2206–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, Allen NB, Alia-Klein N, Batalla A, Blaine S, Brooks S, Caparelli E, Chye YY, Cousijn J, Dagher A, Desrivieres S, Feldstein-Ewing S, Foxe JJ, Goldstein RZ, Goudriaan AE, Heitzeg MM, Hester R, Hutchison K, Korucuoglu O, Li C-SR, London E, Lorenzetti V, Luijten M, Martin-Santos R, May A, Momenan R, Morales A, Paulus MP, Pearlson G, Rousseau M-E, Salmeron BJ, Schluter R, Schmaal L, Schumann G, Sjoerds Z, Stein DJ, Stein EA, Sinha R, Solowij N, Taped S, Uhlmann A, Veltman D, van Holst R, Whittle S, Wright MJ, Yücel M, Zhang S, Yurgelun-Todd D, Hibar DP, Jahanshad N, Evans A, Thompson PM, Glahn DC, Conrod P, Garavan H, ENIGMA Addiction Working Group, 2019. Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects. Am. J. Psychiatry 176, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddry JK, Perez CA, Mora AG, Lear JD, Saveli SC, Bebarta VS, 2018. Impact of prehospital medical evacuation (MEDEVAC) transport time on combat mortality in patients with non-compressible torso injury and traumatic amputations: a retrospective study. Mil Med Res 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchester J, Eshel I, and Marion DW. 2017. The benefits and risks of energy drinks in young adults and military service members. Mil Med 182(7), e1726–e1733. [DOI] [PubMed] [Google Scholar]
- Management of Concussion/mTBI Working Group, 2009. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Journal of Rehabilitation Research & Development 46. [Google Scholar]
- Manners JL, Forsten RD, Kotwal RS, Elbin RJ, Collins MW, Kontos AP, 2016. Role of Pre-Morbid Factors and Exposure to Blast Mild Traumatic Brain Injury on Post-Traumatic Stress in United States Military Personnel. J. Neurotrauma 33, 1796–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Bazarian JJ, Puvenna V, Janigro M, Ghosh C, Zhong J, Zhu T, Blackman E, Stewart D, Ellis J, Butler R, Janigro D, 2013. Consequences of repeated blood-brain barrier disruption in football players. PLoS One 8, e56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Rasmussen P, Kapural M, Fazio V, Kight K, Mayberg MR, Kanner A, Ayumar B, Albensi B, Cavaglia M, Janigro D, 2003. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor. Neurol. Neurosci 21, 109–121. [PMC free article] [PubMed] [Google Scholar]
- Martin K, McLeod E, Périard J, Rattray B, Keegan R, Pyne DB, 2019. The Impact of Environmental Stress on Cognitive Performance: A Systematic Review. Hum. Factors 18720819839817. [DOI] [PubMed] [Google Scholar]
- Marx BP, Brailey K, Proctor SP, Macdonald HZ, Graefe AC, Amoroso P, Heeren T, Vasterling JJ, 2009. Association of time since deployment, combat intensity, and posttraumatic stress symptoms with neuropsychological outcomes following Iraq war deployment. Arch. Gen. Psychiatry 66, 996–1004. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Strigo IA, Simmons AN, O’Connell RM, Reinhardt LE, Moseley SA, 2011. A multimodal imaging study in U.S. veterans of Operations Iraqi and Enduring Freedom with and without major depression after blast-related concussion. Neuroimage 54 Suppl 1, S69–75. [DOI] [PubMed] [Google Scholar]
- McCrea M, 2001. Standardized Mental Status Testing on the Sideline After Sport-Related Concussion. J. Athl. Train 36, 274–279. [PMC free article] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, Cantu RC, Cassidy D, Echemendia RJ, Castellani RJ, Davis GA, Ellenbogen R, Emery C, Engebretsen L, Feddermann-Demont N, Giza CC, Guskiewicz KM, Herring S, Iverson GL, Johnston KM, Kissick J, Kutcher J, Leddy JJ, Maddocks D, Makdissi M, Manley GT, McCrea M, Meehan WP, Nagahiro S, Patricios J, Putukian M, Schneider KJ, Sills A, Tator CH, Turner M, Vos PE, 2017. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med 51, 838–847. [DOI] [PubMed] [Google Scholar]
- McDonald SD, Beckham JC, Morey RA, Calhoun PS, 2009. The validity and diagnostic efficiency of the Davidson Trauma Scale in military veterans who have served since September 11th, 2001. J. Anxiety Disord. 23, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Daneshvar DH, Alvarez VE, Stein TD, 2014. The neuropathology of sport. Acta Neuropathol. 127, 29–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert S, Repple J, Nenadic I, Krug A, Jansen A, Grotegerd D, Förster K, Enneking V, Dohm K, Schmitt S, Stein F, Brosch K, Meller T, Redlich R, Böhnlein J, Sindermann L, Goltermann J, Leehr EJ, Opel N, Aldermann L, Reuter A, Schubotz RI, Hahn T, Kircher T, Dannlowski U, 2019. Reduced fractional anisotropy in depressed patients due to childhood maltreatment rather than diagnosis. Neuropsychopharmacology 44, 2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael AP, Stout J, Roskos PT, Bolzenius J, Gfeller J, Mogul D, Bucholz R, 2015. Evaluation of Cortical Thickness after Traumatic Brain Injury in Military Veterans. J. Neurotrauma 32, 1751–1758. [DOI] [PubMed] [Google Scholar]
- Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine, 1993. Definition of mild traumatic brain injury.
- Miller JB, Barr WB, 2017. The Technology Crisis in Neuropsychology. Arch. Clin. Neuropsychol 32, 541–554. [DOI] [PubMed] [Google Scholar]
- Mooney SR, Stafford J, Seats E, 2018. Medical Evaluation Board Involvement, Non-Credible Cognitive Testing, and Emotional Response Bias in Concussed Service Members. Mil. Med 183, e546–e554. [DOI] [PubMed] [Google Scholar]
- Mucha A, Collins MW, Elbin RJ, Furman JM, Troutman-Enseki C, DeWolf RM, Marchetti G, Kontos AP, 2014. A Brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am. J. Sports Med 42, 2479–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munivenkatappa A, Agrawal A, Shukla DP, Kumaraswamy D, Devi BI, 2016. Traumatic brain injury: Does gender influence outcomes? Int. J. Crit. Illn. Inj. Sci 6, 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu W, Catenaccio E, Lipton ML, 2017. Neuroimaging in Blast-Related Mild Traumatic Brain Injury. J. Head Trauma Rehabil. 32, 55–69. [DOI] [PubMed] [Google Scholar]
- Nelson NW, Disner SG, Anderson CR, Doane BM, McGuire K, Lamberty GJ, Hoelzle J, Sponheim SR, 2019. Blast concussion and posttraumatic stress as predictors of postcombat neuropsychological functioning in OEF/OIF/OND veterans. Neuropsychology. 10.1037/neu0000594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson NW, Hoelzle JB, McGuire KA, Ferrier-Auerbach AG, Charlesworth MJ, Sponheim SR, 2011. Neuropsychological evaluation of blast-related concussion: illustrating the challenges and complexities through OEF/OIF case studies. Brain Inj. 25, 511–525. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, 2016. Paradise Lost: The Neurobiological and Clinical Consequences of Child Abuse and Neglect. Neuron 89, 892–909. [DOI] [PubMed] [Google Scholar]
- Neselius S, Brisby H, Theodorsson A, Blennow K, Zetterberg H, Marcusson J, 2012. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS One 7, e33606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome MR, Durgerian S, Mourany L, Scheibel RS, Lowe MJ, Beall EB, Koenig KA, Parsons M, Troyanskaya M, Reece C, Wilde E, Fischer BL, Jones SE, Agarwal R, Levin HS, Rao SM,2015. Disruption of caudate working memory activation in chronic blast-related traumatic brain injury. Neuroimage Clin 8, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Lejbman N, Jeromin A, French LM, Kim H-S, Cashion A, Mysliwiec V, Diaz-Arrastia R, Gill J, 2015. Peripheral Total Tau in Military Personnel Who Sustain Traumatic Brain Injuries During Deployment. JAMA Neurol. 72, 1109–1116. [DOI] [PubMed] [Google Scholar]
- Olsen A, Babikian T, Bigler E, Caeyenberghs K, Conde V, Dams-O’Connor K, Dobryakova E , Grafman J, Håberg AK, Heggland I, Hellstrøm T, Hodges CB, Irimia A, Jha RM, Johnson PK, Levin H, Li LM, Lindsey HM, Livny A, Løvstad M, Medaglia J, Menon D, Mondello S, Monti MM, Newcombe VFJ, Petroni A, Ponsford J, Sharp D, Spitz G, Westlye LT, Thompson PM, Dennis EL, Tate DF, Wilde EA, Hillary FG, 2019. Toward a Global and Open Science for Imaging Brain Trauma: the ENIGMA Adult msTBI Working Group. Brain Imaging Behav Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EA, Overbey TA, Ostrand CG, Pizzagalli DA, Rauch SL, Rosso IM, 2019. Childhood maltreatment experiences are associated with altered diffusion in occipito-temporal white matter pathways. Brain Behav. e01485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens BD, Kragh JF Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB, 2008. Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. J. Trauma 64, 295–299. [DOI] [PubMed] [Google Scholar]
- Papa L, Brophy GM, Welch RD, Lewis LM, Braga CF, Tan CN, Ameli NJ, Lopez MA, Haeussler CA, Giordano DIM, Others, 2016. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 73, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell N, Rye K, Greenberg N, 2018. Health and well-being management in the military: a systematic review of genetic studies. J. R. Army Med. Corps 164, 302–308. [DOI] [PubMed] [Google Scholar]
- Peltz CB, Kenney K, Gill J, Diaz-Arrastia R, Gardner RC, Yaffe K. Blood biomarkers of traumatic brain injury and cognitive impairment in older veterans. Neurology 2020. September 1;95(9):e1126–e1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie EC, Cross DJ, Yarnykh VL, Richards T, Martin NM, Pagulayan K, Hoff D, Hart K, Mayer C, Tarabochia M, Raskind MA, Minoshima S, Peskind ER, 2014. Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J. Neurotrauma 31,425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham N, Akonasu H, Shishkin R, Taghibiglou C, 2015. Plasma soluble prion protein, a potential biomarker for sport-related concussions: a pilot study. PLoS One 10, e0117286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccenna L, Shears G, O’Brien TJ 2017. Management of post-traumatic epilepsy: an evidence review over the last 5 years and future directions. Epilepsia Open. 2(2): 123–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani V, Stefani A, Pierantozzi M, Natoli S, Stanzione P, Franciotta D, Pisani A, 2012. Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson’s disease. J. Neuroinflammation 9, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polusny MA, Kehle SM, Nelson NW, Erbes CR, Arbisi PA, Thuras P, 2011. Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in national guard soldiers deployed to Iraq. Arch. Gen. Psychiatry 68, 79–89. [DOI] [PubMed] [Google Scholar]
- Posti JP, Takala RSK, Runtti H, Newcombe VF, Outtrim J, Katila AJ, Frantzén J, Ala-Seppälä H, Coles JP, Hossain MI, Kyllönen A, Maanpää H-R, Tallus J, Hutchinson PJ, van Gils M, Menon DK, Tenovuo O, 2016. The Levels of Glial Fibrillary Acidic Protein and Ubiquitin C-Terminal Hydrolase-L1 During the First Week After a Traumatic Brain Injury: Correlations With Clinical and Imaging Findings. Neurosurgery 79, 456–464. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Paolillo E, Barr WB, 2016. Stability in Test-Usage Practices of Clinical Neuropsychologists in the United States and Canada Over a 10-Year Period: A Follow-Up Survey of INS and NAN Members. Arch. Clin. Neuropsychol 31, 206–230. [DOI] [PubMed] [Google Scholar]
- Raji CA, Henderson TA 2018. PET and single-photon emission computed tomography in brain concussion. Neuroimaging Clin N Am. 28(1): 67–82. [DOI] [PubMed] [Google Scholar]
- Ramasamy A, Hill AM, Clasper JC, 2009. Improvised explosive devices: pathophysiology, injury profiles and current medical management. J. R. Army Med. Corps 155, 265–272. [DOI] [PubMed] [Google Scholar]
- Ringer FB, Soberay KA, Rogers ML, Hagan CR, Chu C, Schneider M, Podlogar MC, Witte T, Holm-Denoma J, Plant EA, Gutierrez PM, Joiner TE, 2018. Initial validation of brief measures of suicide risk factors: Common data elements used by the Military Suicide Research Consortium. Psychol. Assess 30, 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ME, Clark DC, Milberg WP, McGlinchey RE, Salat DH, 2017. Characterization of Differences in Functional Connectivity Associated with Close-Range Blast Exposure. J. Neurotrauma 34, S53–S61. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Lindemer ER, Fonda JR, Milberg WP, McGlinchey RE, Salat DH, 2015. Close-range blast exposure is associated with altered functional connectivity in Veterans independent of concussion symptoms at time of exposure. Hum. Brain Mapp 36, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ME, McKee AC, Salat DH, Rasmusson AM, Radigan LJ, Catana C, Milberg WP, McGlinchey RE, 2019. Positron emission tomography of tau in Iraq and Afghanistan Veterans with blast neurotrauma. Neuroimage Clin 21, 101651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein R, Chang B, Yue JK, Chiu A, Winkler EA, Puccio AM, Diaz-Arrastia R, Yuh EL, Mukherjee P, Valadka AB, Gordon WA, Okonkwo DO, Davies P, Agarwal S, Lin F, Sarkis G, Yadikar H, Yang Z, Manley GT, Wang KKW, Cooper SR, Dams-O’Connor K, Borrasso AJ, Inoue T, Maas AIR, Menon DK, Schnyer DM, Vassar MJ, 2017. Comparing Plasma Phospho Tau, Total Tau, and Phospho Tau–Total Tau Ratio as Acute and Chronic Traumatic Brain Injury Biomarkers. JAMA Neurol. 74, 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Robinson ME, Miller DR, Clark DC, McGlinchey RE, 2017. Neuroimaging of deployment-associated traumatic brain injury (TBI) with a focus on mild TBI (mTBI) since 2009. Brain Inj. 31, 1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer NA, 2012. Traumatic brain injury and its neuropsychiatric sequelae in war veterans. Annu. Rev. Med 63, 405–419. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, van Erp TGM, Sämann PG, Frodl T, Jahanshad N, Loehrer E, Tiemeier H, Hofman A, Niessen WJ, Vernooij MW, Ikram MA, Wittfeld K, Grabe HJ, Block A, Hegenscheid K, Völzke H, Hoehn D, Czisch M, Lagopoulos J, Hatton SN, Hickie IB, Goya-Maldonado R, Krämer B, Gruber O, Couvy-Duchesne B, Rentería ME, Strike LT, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie NA, Wright MJ, Hall GB, MacQueen GM, Frey EM, Carballedo A, van Velzen LS, van Tol MJ, van der Wee NJ, Veer IM, Walter H, Schnell K, Schramm E, Normann C, Schoepf D, Konrad C, Zurowski B, Nickson T, McIntosh AM, Papmeyer M, Whalley HC, Sussmann JE, Godlewska BR, Cowen PJ, Fischer FH, Rose M, Penninx BWJH, Thompson PM, Hibar DP, 2016. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatry 21, 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman AI, Braver ER, Kang HK, 2008. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am. J. Epidemiol 167, 1446–1452. [DOI] [PubMed] [Google Scholar]
- Scultetus AH, Haque A, Chun SJ, Hazzard B, Mahon RT, Harssema MJ, Auker CR, Moon-Massat P, Malone DL, McCarron RM, 2016. Brain hypoxia is exacerbated in hypobaria during aeromedical evacuation in swine with traumatic brain injury. J. Trauma Acute Care Surg 81, 101–107. [DOI] [PubMed] [Google Scholar]
- Scultetus AH, Jefferson MA, Haque A, Ho LTVT, Hazzard B, Saha BK, Chun SJ, Auker CR, Moon-Massat PF, McCarron RM, Malone DL, 2019. Hypobaria during long-range flight resulted in significantly increased histopathological evidence of lung and brain damage in a swine model. J. Trauma Acute Care Surg 86, 116–122. [DOI] [PubMed] [Google Scholar]
- Shahim P, Politis A, van der Merwe A, Moore B, Chou YY, Pham DL, Butman JA, Diaz-Arrastia R, Gill JM, Brody DL, Zetterberg H, Blennow K, Chan L. Neurofilament light as a biomarker in traumatic brain injury. Neurology. 2020. August 11;95(6):e610–e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahim P, Politis A, van der Merwe A, Moore B, Ekanayake V, Lippa SM, Chou YY, Pham DL, Butman JA, Diaz-Arrastia R, Zetterberg H, Blennow K, Gill JM, Brody DL, Chan L. Time course and diagnostic utility of NfL, tau, GFAP, and UCH-L1 in subacute and chronic TBI. Neurology 2020. August 11 ;95(6):e623–e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver JM, 2015. Invalid symptom reporting and performance: What are we missing? NeuroRehabilitation 36, 463–469. [DOI] [PubMed] [Google Scholar]
- Skovira JW, Kabadi SV, Wu J, Zhao Z, DuBose J, Rosenthal R, Fiskum G, Faden AI, 2016. Simulated Aeromedical Evacuation Exacerbates Experimental Brain Injury. J. Neurotrauma 33, 1292–1302. [DOI] [PubMed] [Google Scholar]
- Smith DH, Chen X-H, Iwata A, Graham DI, 2003. Amyloid beta accumulation in axons after traumatic brain injury in humans. J. Neurosurg 98, 1072–1077. [DOI] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, Stewart W, 2013. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol 9, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, Trojanowski JQ, Stewart W, 2019. Chronic traumatic encephalopathy—confusion and controversies. Nat. Rev. Neurol 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa MAG, De Gasperi R, Paulino AJ, Pricop PE, Shaughness MC, Maudlin-Jeronimo E, Hall AA, Janssen WGM, Yuk FJ, Dorr NP, Dickstein DL, McCarron RM, Chavko M, Hof PR, Ahlers ST, Elder GA, 2013. Blast overpressure induces shear-related injuries in the brain of rats exposed to a mild traumatic brain injury. Acta Neuropathol Commun 1, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RJ, Waldron-Perrine B, Drag LL, Pangilinan PH, Axelrod BN, Bieliauskas LA, 2017. Neuropsychological test validity in Veterans presenting with subjective complaints of “very severe” cognitive symptoms following mild traumatic brain injury. Brain Inj. 31, 32–38. [DOI] [PubMed] [Google Scholar]
- Stein MB, Ursano RJ, Campbell-Sills L, Colpe LJ, Fullerton CS, Heeringa SG, Nock MK, Sampson NA, Schoenbaum M, Sun X, Jain S, Kessler RC, 2016. Prognostic Indicators of Persistent Post-Concussive Symptoms after Deployment-Related Mild Traumatic Brain Injury: A Prospective Longitudinal Study in U.S. Army Soldiers. J. Neurotrauma 33, 2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TD, Alvarez VE, McKee AC, 2015. Concussion in Chronic Traumatic Encephalopathy. Curr. Pain Headache Rep 19, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Tripodis Y, Baugh CM, Fritts NG, Martin BM, Chaisson C, Cantu RC, Joyce JA, Shah S, Ikezu T, Zhang J, Gercel-Taylor C, Taylor DD, 2016. Preliminary Study of Plasma Exosomal Tau as a Potential Biomarker for Chronic Traumatic Encephalopathy. J. Alzheimers. Dis 51, 1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Willis JJ, Heyanka D, Proctor-Weber Z, England H, Bruhns M, 2018. Premorbid IQ Predicts Postconcussive Symptoms in OEF/OIF/OND Veterans with mTBI. Arch. Clin. Neuropsychol 33, 206–215. [DOI] [PubMed] [Google Scholar]
- Sullivan DR, Logue MW, Wolf EJ, Hayes JP, Salat DH, Fortier CB, Fonda JR, McGlinchey RE, Milberg WP, Miller MW, 2019. Close-Range Blast Exposure Is Associated with Altered White Matter Integrity in Apolipoprotein ε4 Carriers. J. Neurotrauma. 10.1089/neu.2019.6489 [DOI] [PubMed] [Google Scholar]
- Sundman MH, Hall EE, Chen N-K, 2014. Examining the relationship between head trauma and neurodegenerative disease: A review of epidemiology, pathology and neuroimaging techniques. J. Alzheimers Dis. Parkinsonism 4. 10.4172/2161-0460.1000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Phillips RD, Mulready HL, Zablonski ST, Turner JA, Turner MD, McClymond K, Nieuwsma JA, Morey RA, 2019. Resting-state brain fluctuation and functional connectivity dissociate moral injury from posttraumatic stress disorder. Depress. Anxiety 36, 442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber KH, Hurley RA, Haswell CC, Rowland JA, Hurt SD, Lamar CD, Morey RA, 2015. White matter compromise in veterans exposed to primary blast forces. J. Head Trauma Rehabil 30, E15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate CM, Wang KKW, Eonta S, Zhang Y, Carr W, Tortella FC, Hayes RL, Kamimori GH, 2013. Serum brain biomarker level, neurocognitive performance, and self-reported symptom changes in soldiers repeatedly exposed to low-level blast: a breacher pilot study. J. Neurotrauma 30, 1620–1630. [DOI] [PubMed] [Google Scholar]
- Tator CH, Davis HS, Dufort PA, Tartaglia MC, Davis KD, Ebraheem A, Hiploylee C, 2016. Postconcussion syndrome: demographics and predictors in 221 patients. J. Neurosurg 125, 1206–1216. [DOI] [PubMed] [Google Scholar]
- Taylor BC, Hagel EM, Carlson KF, Cifu DX, Cutting A, Bidelspach DE, Sayer NA, 2012. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med. Care 50, 342–346. [DOI] [PubMed] [Google Scholar]
- Thurmond VA, Hicks R, Gleason T, Miller AC, Szuflita N, Orman J, Schwab K, 2010. Advancing integrated research in psychological health and traumatic brain injury: common data elements. Arch. Phys. Med. Rehabil 91, 1633–1636. [DOI] [PubMed] [Google Scholar]
- Tibbling G, Link H, Ohman S, 1977. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand. J. Clin. Lab. Invest 37, 385–390. [DOI] [PubMed] [Google Scholar]
- Tozzi L, Garczarek L, Janowitz D, Stein DJ, Wittfeld K, Dobrowolny H, Lagopoulos J, Hatton SN, Hickie IB, Carballedo A, Brooks SJ, Vuletic D, Uhlmann A, Veer IM, Walter H, Bülow R, Völzke H, Klinger-König J, Schnell K, Schoepf D, Grotegerd D, Opel N, Dannlowski U, Kugel H, Schramm E, Konrad C, Kircher T, Jüksel D, Nenadič I, Krug A, Hahn T, Steinsträter O, Redlich R, Zaremba D, Zurowski B, Fu CHY, Dima D, Cole J, Grabe HJ, Connolly CG, Yang TT, Ho TC, LeWinn KZ, Li M, Groenewold NA, Salminen LE, Walter M, Simmons AN, van Erp TGM, Jahanshad N, Baune BT, van der Wee NJA, van Tol M-J, Penninx BWJH, Hibar DP, Thompson PM, Veltman DJ, Schmaal L, Frodl T, “for the ENIGMA-MDD Consortium,” 2019. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol. Med 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traumatic Brain Injury [WWW Document], 2018. URL https://www.commondataelements.ninds.nih.gov/Traumatic%20Brain%20injury (accessed 11.20.19).
- Trotter BB, Robinson ME, Milberg WP, McGlinchey RE, Salat DH, 2015. Military blast exposure, ageing and white matter integrity. Brain 138, 2278–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanskaya M, Pastorek NJ, Scheibel RS, Petersen NJ, McCulloch K, Wilde EA, Henson HK, Levin HS, 2015. Combat exposure, PTSD symptoms, and cognition following blast-related traumatic brain injury in OEF/OIF/OND service members and Veterans. Mil. Med 180, 285–289. [DOI] [PubMed] [Google Scholar]
- Troyanskaya M, Pastorek NJ, Scheibel RS, Petersen NJ, Walder A, Henson HK, Levin HS, 2016. Choosing appropriate comparison group participants in studies of veterans: Characteristics of orthopedically injured and uninjured Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn veterans. J. Clin. Exp. Neuropsychol 38, 811–819. [DOI] [PubMed] [Google Scholar]
- Uryu K, Chen X-H, Martinez D, Browne KD, Johnson VE, Graham DI, Lee VM-Y, Trojanowski JQ, Smith DH, 2007. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp. Neurol 208, 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VA/DoD CLINICAL PRACTICE GUIDELINE FOR THE MANAGEMENT OF CONCUSSION-MILD TRAUMATIC BRAIN INJURY [WWW Document], 2016. URL https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGClinicianSummary50821816.pdf (accessed 12.12.19).
- van der Pols JC, Kanesarajah J, Bell A, Lui C-W, 2017. Current dietary supplement use of Australian military veterans of Middle East operations. Public Health Nutr. 20, 3156–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo JL, Proctor-Weber Z, Lebowitz BK, Curtiss G, Vanderploeg RD, 2007. Psychiatric risk factors for traumatic brain injury. Brain Inj. 21, 567–573. [DOI] [PubMed] [Google Scholar]
- Veitch DP, Friedl KE, Weiner MW, 2013. Military risk factors for cognitive decline, dementia and Alzheimer’s disease. Curr. Alzheimer Res 10, 907–930. [DOI] [PubMed] [Google Scholar]
- Verellen RM, Cavazos JE 2014. Post-traumatic epilepsy: an overview. Therapy, 7(5): 527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S, 2018. Neuropathology of Traumatic Brain Injury and Its Role in the Development of Alzheimer’s Disease. Amyloidosis [Working Title], 10.5772/intechopen.81945 [DOI] [Google Scholar]
- Walter KH, Barnes SM, Chard KM, 2012. The influence of comorbid MDD on outcome after residential treatment for veterans with PTSD and a history of TBI. Journal of Traumatic Stress. 10.1002/jts.21722 [DOI] [PubMed] [Google Scholar]
- Wang T, Van KC, Gavitt BJ, Grayson JK, Lu Y-C, Lyeth BG, Pichakron KO, 2013. Effect of fish oil supplementation in a rat model of multiple mild traumatic brain injuries. Restor. Neurol. Neurosci 31,647–659. [DOI] [PubMed] [Google Scholar]
- Ware AL, Wilde EA, Newsome MR, Moretti P, Abildskov T, Vogt GS, McCauley SR, Hanten G, Hunter JV, Chu ZD, Levin HS, 2018. A preliminary investigation of corpus callosum subregion white matter vulnerability and relation to chronic outcome in boxers. Brain Imaging Behav. 10.1007/s11682-018-0018-7 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP, 2013. The ptsd checklist for dsm-5 (pcl-5). Scale available from the National Center for PTSD at www.ptsd.va.gov. [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Cedarbaum J, Donohue MC, Green RC, Harvey D, Jack CR Jr, Jagust W, Morris JC, Petersen RC, Saykin AJ, Shaw L, Thompson PM, Toga AW, Trojanowski JQ, Alzheimer’s Disease Neuroimaging Initiative, 2015. Impact of the Alzheimer’s Disease Neuroimaging Initiative, 2004 to 2014. Alzheimers. Dement 11, 865–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte J, Vasterling J, Manley GT, 2010. Common data elements for research on traumatic brain injury and psychological health: current status and future development. Arch. Phys. Med. Rehabil 91, 1692–1696. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Bouix S, Tate DF, Lin AP, Newsome MR, Taylor BA, Stone JR, Montier J, Gandy SE, Biekman B, Shenton ME, York G, 2015. Advanced neuroimaging applied to veterans and service personnel with traumatic brain injury: state of the art and potential benefits. Brain Imaging Behav. 9, 367–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Dennis EL, Tate DF, 2019. The ENIGMA Brain Injury Working Group: Approach, Challenges, and Potential Benefits. Brain Imaging Behav Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DB, Annegers JF, Kokmen E, O’Brien PC, Kurland LT, 1991. Brain injury and neurologic sequelae: a cohort study of dementia, parkinsonism, and amyotrophic lateral sclerosis. Neurology 41, 1554–1557. [DOI] [PubMed] [Google Scholar]
- Wittchen H-U, Schönfeld S, Kirschbaum C, Thurau C, Trautmann S, Steudte S, Klotsche J, Höfler M, Hauffa R, Zimmermann P, 2012. Traumatic experiences and posttraumatic stress disorder in soldiers following deployment abroad: how big is the hidden problem? Dtsch. Arztebl. Int 109, 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DL, Yund EW, Wyma JM, Ruff R, Herron TJ, 2015. Measuring executive function in control subjects and TBI patients with question completion time (QCT). Front. Hum. Neurosci 9, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell AM, Deuster PA, 2016. Caffeine and Performance. J. Spec. Oper. Med 16, 64–70. [DOI] [PubMed] [Google Scholar]
- Yue JK, Upadhyayula PS, Avalos LN, Deng H, Wang KKW. 2020. The Role of Blood Biomarkers for Magnetic Resonance Imaging Diagnosis of Traumatic Brain Injury. Medicina (Kaunas) 2020. February 22;56(2):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemnik RE, Suchy Y, 2019. Ecological validity of performance-based measures of executive functions: Is face validity necessary for prediction of daily functioning? Psychol. Assess 31, 1307–1318. [DOI] [PubMed] [Google Scholar]


