Summary
Progressive loss of dopamine inputs in Parkinson’s disease leads to imbalances in coordinated signaling of dopamine and acetylcholine (ACh) in the striatum, which is thought to contribute to parkinsonian motor symptoms. As reciprocal interactions between dopamine inputs and cholinergic interneurons (ChIs) control striatal dopamine and ACh transmission, we examined how partial dopamine depletion in an early-stage mouse model for Parkinson’s disease altered nigral regulation of cholinergic activity. We found region specific alterations in how remaining dopamine inputs regulated cholinergic excitability that differed between the dorsomedial (DMS) and dorsolateral (DLS) striatum. Specifically, we found that dopamine depletion downregulated metabotropic glutamate receptors (mGluR1) on DLS ChIs at synapses where dopamine inputs co-release glutamate, abolishing the ability of dopamine inputs to drive burst firing. This loss underlied parkinsonian motor impairments as viral rescue of mGluR1 signaling in DLS ChIs was sufficient to restore circuit function and attenuate motor deficits in early-stage parkinsonian mice.
eTOC
Cai et al. find that partial degeneration of dopamine inputs in mice differentially alters how remaining SNc terminals regulate cholinergic interneurons via dopamine and glutamate transmission across the dorsomedial and dorsolateral striatum. Rescuing the dysfunction in glutamatergic excitation of cholinergic interneurons alleviated early stage Parkinsonian motor symptoms.
Introduction
Parkinson’s disease is a neurodegenerative disorder characterized by the progressive loss of dopamine neurons in the substantia nigra pars compacta (SNc) resulting in dopaminergic denervation of the dorsal striatum, a region critical for voluntary movements. Loss of striatal dopamine alters the strength of synaptic inputs to medium spiny neurons (MSNs) and their activity, which is thought to contribute to the impairment of movement control (Albin et al., 1989; DeLong, 1990; Gerfen and Surmeier, 2011; Kreitzer and Malenka, 2008). In addition to changes in MSN activity, dysfunction of striatal cholinergic interneurons (ChIs) has also been implicated in Parkinson’s disease (Ding et al., 2006; Kharkwal et al., 2016; McKinley et al., 2019; Tanimura et al., 2019; Ztaou et al., 2016). Although ChIs comprise only 1 - 2% of total striatal neurons, they form dense arborizations and robustly regulate both synaptic inputs to MSNs and their output through muscarinic and nicotinic acetylcholine (ACh) receptors (Mamaligas and Ford, 2016; Mamaligas et al., 2016; Nelson et al., 2014; Pakhotin and Bracci, 2007; Threlfell et al., 2012). In advanced stage Parkinson’s disease, the depletion of dopamine leads to an aberrant increase in striatal cholinergic activity, and this imbalance between dopamine and ACh has long been considered the main pathology leading to movement disability (Aosaki et al., 2010; Pisani et al., 2007). However, despite the onset of motor symptoms occurring when striatal dopamine is only partially decreased (Cheng et al., 2010; Pavese et al., 2011), less is known about the changes in dopamine and ACh balance in early stages of the disease and how this alters striatal output.
Dopaminergic and cholinergic signaling have multiple interactions within the striatum. While ChIs facilitate dopamine release via pre-synaptic nicotinic receptors (Cachope et al., 2012; Threlfell et al., 2012), dopamine neurons differentially regulate cholinergic activity across sub-regions of the dorsal striatum. Dopamine neurons primarily inhibit ChI firing via dopamine D2 receptors in the dorsomedial striatum (DMS), but generate burst firing in ChIs via glutamate co-release evoked mGluR1 activation in the dorsolateral striatum (DLS) (Cai and Ford, 2018; Chuhma et al., 2018). However, how this region-specific modulation of ChIs by SNc inputs is altered in early stage Parkinson’s disease and how it may contribute to motor deficits is not known. Here we find that following partial loss of dopamine inputs, dopamine-driven inhibition of cholinergic activity in the DMS is preserved due to reduced dopamine reuptake, but glutamate co-release evoked excitation in the DLS is lost due to a downregulation of mGluR1 receptors. Expressing mGluR1 in ChIs to restore mGluR signaling was sufficient to alleviate the circuit deficits and improve motor impairments in early stage parkinsonian mice.
Results
Low dose 6-OHDA evokes a partial reduction in striatal dopamine inputs
To mimic early stage Parkinsonism, we used a model in which striatal dopamine inputs were partially lesioned (Boix et al., 2015; Park et al., 2018). A low-dose of 6-hydroxydopamine (6-OHDA, 1 μg / μL) was unilaterally injected into the medial forebrain bundle (MFB) of male and female wild-type C57Bl6 mice (Figure 1A). Three to four weeks post-injection, there were fewer tyrosine hydroxylase (TH) positive dopamine inputs across the dorsal striatum of 6-OHDA-injected mice relative to sham vehicle-injected hemispheres (p < 0.001, ANOVA) (Figure 1B and 1C) and reduced TH immunoreactivity in the midbrain (p < 0.01, Mann-Whitney) (Figure 1D - 1E). To quantify the reduction of striatal dopamine release associated with the loss of dopamine inputs, a carbon fiber electrode was inserted into the dorsal striatum in coronal brain slices and fast-scan cyclic voltammetry (FSCV) was performed using a triangular waveform (−0.4 to +1.3 V at 400 V/s; 10 Hz) to measure evoked dopamine release following a single electrical stimulus. The peak extracellular concentration of dopamine ([DA]o) was decreased in the 6-OHDA injected hemisphere by ~ 57% compared to control contralateral hemisphere injected with saline (p < 0.01, Mann-Whitney) (Figure 1F). This reduction in striatal dopamine inputs led to parkinsonian motor deficits, including a decrease in contralateral paw use in the cylinder motor test (p < 0.001, Kruskal–Wallis) (Figure 1G).
Figure 1. Partial depletion of striatal dopamine following low-dose 6-OHDA induces mild motor impairment without altering ChI pacemaker firing.
(A) Schematic and timeline of 6-OHDA injection.
(B) Coronal striatal section of TH-immunoreactivity following low dose (1 μg/ μL) unilateral 6-OHDA injection.
(C) TH-immunoreactivity intensity of injected hemisphere relative to contralateral saline injected hemisphere. N = 4.
(D) Coronal midbrain section showing the loss of TH-immunoreactivity in the SNc following low dose (1 μg/ μL) unilateral 6-OHDA injection.
(E) TH-immunoreactivity intensity of injected hemisphere relative to contralateral saline injected hemisphere. N = 5.
(F) Peak striatal FSCV [DA]o from saline and low-dose 6-OHDA injected hemispheres. Dopamine evoked with a single electrical stimulus (1 ms). Left: illustration of the FSCV waveform. N = 6 for both groups.
(G) Contralateral paw use in cylinder behavioral test. N = 12 – 16.
(H) Cell attached recordings of DMS and DLS ChIs from control and low-dose 6-OHDA (1 μg / μL) groups.
(I) Quantification of cell attached baseline pacemaker firing rates. n = 8 - 16. N = 3 - 6. Summary data are mean ± SEM. ** = p < 0.01, *** = p < 0.001, n.s = p > 0.05.
To examine whether the partial loss of striatal dopamine inputs altered Chi firing, coronal striatal brain slices were prepared three to four weeks post unilateral 6-OHDA injection into the MFB (1 μg / μL) and cell-attached recordings were made from ChIs. ChIs were identified by their large somatic size and presence of tonic pacemaker firing (Cai and Ford, 2018). The baseline pacemaker firing rate of ChIs in the dorsomedial striatum (DMS) and the dorsolateral striatum (DLS) was similar between low-dose 6-OHDA treated animals and saline-injected sham controls (p > 0.05, Kruskal–Wallis) (Figure 1H - 1I). As significant loss of striatal dopamine decreases the frequency and regularity of ChI firing due to a downregulation of HCN and SK channels (Choi et al., 2020; McKinley et al., 2019), ChI firing rate was only reduced in the DLS from high dose (4μg / μL) 6-OHDA-injected animals (p < 0.05, Mann-Whitney) which exhibited a near complete loss of TH+ dopamine inputs to the striatum (p < 0.001, ANOVA) and further decreased use of the contralateral paw (p < 0.001, Kruskal–Wallis) (Supplemental Figure S1). Thus, while the partial loss of dopamine inputs was sufficient to induce a parkinsonian motor impairment, it did not alter the pacemaker firing of ChIs.
Regulation of cholinergic activity by SNc inputs is differentially altered across dorsal striatal regions in early Parkinsonian mice
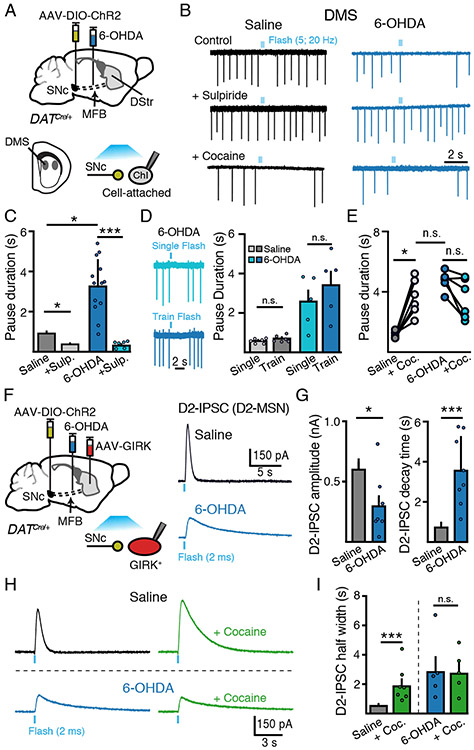
To examine how partial degeneration of dopamine inputs disrupted the regulation of ChI activity by remaining dopamine terminals, an adeno-associated virus (AAV) encoding double-floxed channelrhodopsin 2 (ChR2) was unilaterally injected into the midbrain of mice expressing Cre recombinase under the dopamine transporter promoter (DAT-Cre) (Figure 2A). In the dorsomedial striatum (DMS) the release of dopamine drives D2-receptor mediated pauses in ChI firing (Aosaki et al., 1994; Cai and Ford, 2018; Chuhma et al., 2014, 2018; Straub et al., 2014). Optogenetic stimulation of dopamine terminals using a train of stimuli to mimic SNc burst firing (5 pulses, 20 Hz, 2 ms per pulse) led to a pause in pacemaker firing in randomly selected DMS ChIs from saline-injected control animals, which was abolished by the D2-receptor antagonist sulpiride (500 nM) (p < 0.05, Kruskal-Wallis) (Figure 2B - 2C). Surprisingly, this pause in firing was enhanced in DMS ChIs from 6-OHDA-injected animals (p < 0.05, Kruskal-Wallis) (Figure 2B - 2C). Thus, despite the partial reduction in TH+ striatal fibers, activation of remaining dopamine inputs drove longer pauses in ChI firing. Similar results were found using only a single optogenetic flash (2 ms) to evoke dopamine release (Figure 2D), suggesting that the prolonged pause was not due to changes in the summation of dopamine or plasticity of release following 6-OHDA treatment.
Figure 2. SNc dopamine-mediated inhibition of DMS Chi excitability is enhanced following partial dopamine loss.
(A) Schematic of MFB 6-OHDA and SNc AAV-DIO-ChR2 injections into DAT-Cre mice and recordings of DMS ChIs three weeks following.
(B) Cell attached recordings of DMS ChIs from control and low-dose 6-OHDA (1 μg/ μL) groups following optogenetic stimulation of dopamine inputs (5 flashes, 2 ms, 20 Hz). Sulpiride (500 nM) sensitive dopamine evoked pauses in DMS ChIs are enhanced following low-dose 6-OHDA.
(C) Quantification of the duration of SNc-evoked pause in ChI firing from (B). n = 6 - 15, N = 3 - 6.
(D) Representative traces from the same DMS ChI illustrating the effect of a single optogenetic stimulus (2 ms flash) and a train of stimuli (5 flashes, 2 ms, 20 Hz) and quantification of the duration of SNc-evoked pause in ChI firing.
(E) Lack of effect of cocaine (3 μM) on SNc-evoked pauses following 6-OHDA. n = 6. N = 2.
(F) Schematic of viral injections of MFB 6-OHDA, SNc AAV-DIO-ChR2 and striatal AAV-tdTomato-GIRK2 injection into DAT-Cre mice and recordings of GIRK2 mediated D2-IPSCs in D2-MSNs evoked by optogenetic stimulation of dopamine inputs three weeks following.
(G) Summary of amplitude and decay time of D2-IPSCs from D2-MSNs in control and low-dose 6-OHDA (1 μg/ μL) groups. n = 6 - 8, N = 4 - 8.
(H) Representative traces of D2-MSN D2-IPSCs illustrating the effect of cocaine (3 μM) on the duration of D2-IPSCs in saline and 6-OHDA injected animals.
(I) Quantification of D2-IPSC half-width time from (H). n = 5 - 9, N = 4.
Summary data are mean ± SEM. * = p < 0.05, ** = p < 0.01, *** = p < 0.001, n.s. = p > 0.05.
As loss of dopamine leads to decreased DAT expression and subsequent dopamine uptake (Garris et al., 1997; Uhl et al., 1994), we tested if altered uptake contributed to the extended inhibition of ChI firing by applying the DAT inhibitor, cocaine. While cocaine (3 μM) had no effect on the basal firing rate of ChIs (p > 0.05, Wilcoxon) (Supplemental Figure S2), DAT inhibition prolonged the pause evoked by photoactivation of dopamine terminals in control slices (p < 0.05, Wilcoxon), but had no effect on pause duration in low-dose 6-OHDA treated animals, regardless of initial pause duration (p > 0.05, Wilcoxon) (Figure 2B and 2E and Supplemental Figure S2). This suggests that loss of DAT-mediated uptake likely accounts for the increased dopamine evoked pause in firing following partial degeneration of dopamine inputs.
To determine how reduced DAT function alters dopamine transmission onto other striatal neurons, we also examined the effect of 6-OHDA on the kinetics of dopamine mediated synaptic responses in D2-receptor expressing medium spiny neurons (D2-MSNs), since the time course of synaptic D2-receptor activation in these cells is tightly regulated by DAT-mediated clearance (Marcott et al., 2014). Unilaterally injected 6-OHDA (1 μg / μL) animals were also injected with an AAV encoding G-protein activated inwardly rectifying potassium channel (GIRK2) (AAV-tdTomato-GIRK2) in the striatum (Figure 2F). Since GIRK channels couple to endogenous D2-receptors in D2-MSNs, the viral overexpression of GIRK2 channels provides an electrophysiological readout of D2-receptor activation in D2-MSNs (Marcott et al., 2014, 2018). Three to four weeks post injection, whole-cell recordings from D2-MSNs revealed that D2-IPSCs in D2-MSNs from low-dose 6-OHDA injected animals were reduced in amplitude (p < 0.05, Mann-Whitney) but had longer decay times compared to vehicle-injected sham controls (p < 0.001, Mann-Whitney) (Figure 2F - 2G), indicating decreased dopamine uptake following 6-OHDA treatment. Furthermore, similar to the effect on pauses in ChI firing, blocking dopamine reuptake with cocaine (3 μM) potentiated the duration of D2-IPSCs from saline-injected controls (p < 0.001, Mann-Whitney), but had no effect on D2-IPSCs recorded in slices from low-dose 6-OHDA treated animals (p > 0.05, Wilcoxon) (Figure 2H - 2I). Together these results indicate that partial loss of dopamine inputs leads to a decrease in both dopamine release and clearance, which in DMS ChIs, has the net effect of enhancing dopamine-mediated inhibition of firing.
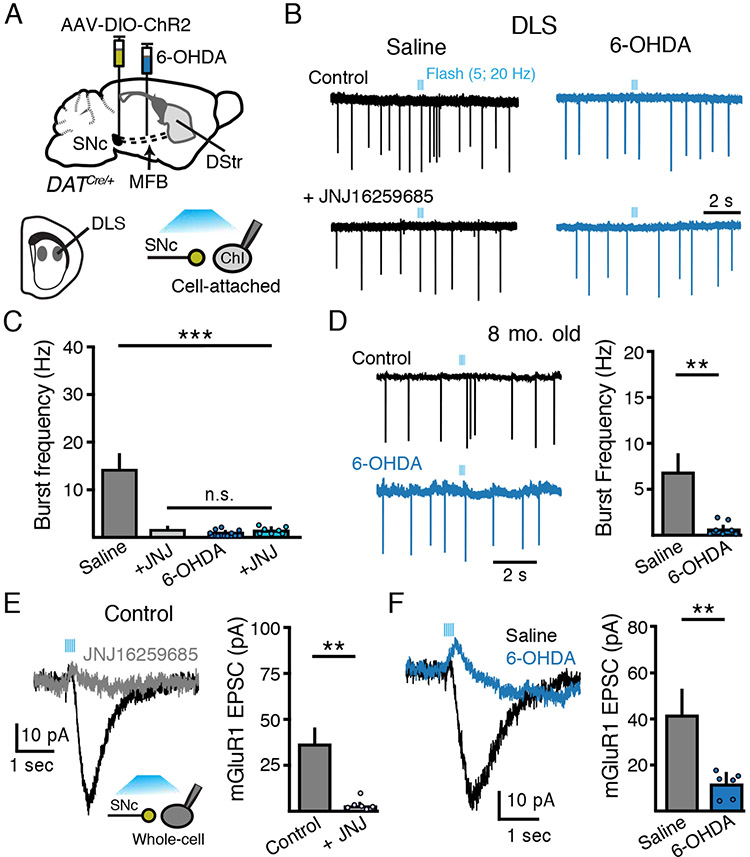
Midbrain dopamine inputs differentially regulate ChI firing across striatal regions (Cai and Ford, 2018; Chuhma et al., 2014, 2018; Straub et al., 2014). While dopamine release pauses the firing of ChIs in the DMS, stimulation of dopamine terminals evokes burst firing of DLS ChIs due to co-release of glutamate and subsequent activation of group 1 metabotropic glutamate receptors (mGluRs) (Berg et al., 2007; Cai and Ford, 2018; Chuhma et al., 2018). ChIs express both mGluR1 and mGluR5 group 1 receptors (Pisani et al., 2001; Tallaksen-Greene et al., 1998) but the excitation resulting from stimulation of midbrain inputs occurs primarily via mGluR1 (Chuhma et al., 2018). We found that photoactivation of DLS dopamine terminals led to a burst of action potentials in randomly selected DLS ChIs from saline-injected control animals, but had no effect on ChIs in slices from low-dose 6-OHDA treated animals (p < 0.001, Kruskal-Wallis) (Figure 3A - 3C). Application of the mGluR1 antagonist JNJ16259685 (20 μM; JNJ) abolished evoked burst firing in control slices (p < 0.001, Kruskal-Wallis), but had no effect in slices from 6-OHDA treated animals (p > 0.05, Kruskal-Wallis) (Figure 3A - 3C). We also repeated cell attached recordings in eight-month-old animals. Similar to young adult mice, photoactivation of DLS dopamine terminals also led to a burst of action potentials in old mice which was abolished following low-dose 6-OHDA treatment (p < 0.01, Mann-Whitney) (Figure 3D), indicating that similar loss of mGluR1-induced bursting occurs following 6-OHDA lesions in aged animals.
Figure 3. SNc glutamate corelease-evoked bursting of DLS ChIs is lost following partial dopamine loss.
(A) Schematic of MFB 6-OHDA and SNc AAV-DIO-ChR2 injections into DAT-Cre mice and recordings from DLS ChIs three weeks following.
(B) Cell attached recordings of DLS ChIs from control and low-dose 6-OHDA (1 μg/ μL) groups following optogenetic stimulation of dopamine inputs (5 flashes, 2 ms, 20 Hz). JNJ16259685 (20 μM) sensitive burst firing is abolished following low-dose 6-OHDA.
(C) Quantification of SNc-evoked ChI burst frequency in DLS ChIs firing from (B). n = 7 - 14, N = 2 - 4.
(D) Representative traces and quantification of DLS ChI burst firing following photoactivation of SNc inputs from saline or low-dose 6-OHDA treated 8-month-old mice. n = 6 - 7, N = 3.
(E) Representative trace and amplitude quantification of SNc-evoked (5 flashes, 2 ms, 20 Hz) JNJ16259685 (20 μM) sensitive mGluR EPSCs. n = 6, N = 2.
(F) Optogenetic stimulation evoked mGluR-mediated EPSCs from DLS ChIs are abolished following 6-OHDA. Right: summary of mGluR-mediated EPSCs in DLS ChIs from saline and 6-OHDA groups. n = 6 – 7, N = 3.
Summary data are mean ± SEM. ** = p < 0.01, n.s. = p > 0.05.
Group 1 type mGluRs are Gq-coupled and depolarize ChIs via the activation of an excitatory TRPc mediated inward current (Berg et al., 2007; Cai and Ford, 2018; Chuhma et al., 2018). To confirm that the loss of mGluR-evoked bursting was due to a loss of mGluR1 mediated currents, whole-cell recordings were made from DLS ChIs which were identified by their large somatic size and presence of a hyperpolarization activated inward current (Ih). Photoactivation of DLS dopamine terminals evoked a JNJ-sensitive mGluR1-mediated excitatory postsynaptic current (EPSC) in ChIs (p < 0.01, Wilcoxon) (Figure 3E), which was reduced in amplitude in slices from low-dose 6-OHDA treated animals compared to saline controls (p < 0.01, Mann-Whitney) (Figure 3F). Thus, partial degeneration of striatal dopamine terminals leads to a loss of glutamate co-release driven excitation and burst firing in DLS ChIs. The extent of TH+ fiber loss did not correlate with the extent of loss of evoked burst firing, as burst firing was abolished in all ChIs examined from low-dose 6-OHDA treated animals despite variable levels of TH+ fiber loss (Figure 1C and 3C). Because the expression of VGLUT2 confers neuroprotective properties to dopamine cells limiting their susceptibility to degeneration by parkinsonian neurotoxins (Steinkellner et al., 2018), dopamine neurons co-releasing glutamate are less vulnerable to degeneration. As the loss of burst firing did not correlate with striatal TH+ fiber degeneration, the results suggest that loss of glutamatergic excitation of ChIs may not be simply due to degeneration of SNc fibers but instead that midbrain evoked excitation of ChIs may be selectively vulnerable in parkinsonian mice.
Loss of glutamate co-transmission does not result from a decrease in presynaptic glutamate release
We proceeded to determine the mechanism underlying the loss of glutamate co-release mediated excitation in DLS ChIs. We first examined whether the effect was due to a presynaptic loss of glutamate co-release from SNc dopamine terminals. To initially estimate the extent that 6-OHDA reduced glutamatergic transmission from dopamine terminals, glutamate AMPA receptor mediated EPSCs were measured from neighboring MSNs in the DLS from control saline-injected animals and those treated with low-dose 6-OHDA (Figure 4A). As expected, a partial degeneration of SNc terminals reduced the amplitude of optogenetically evoked AMPA EPSCs in DLS MSNs (p < 0.01, Kruskal-Wallis), suggesting a presynaptic decrease in glutamate release (Figure 4A - 4B). To mimic the reduction in glutamate transmission induced by low-dose 6-OHDA, we directly perturbed presynaptic glutamate co-release, by generating mice in which the vesicular glutamate transporter (VGLUT2), which loads glutamate into synaptic vesicles in dopamine neurons (Dal Bo et al., 2004; Hnasko et al., 2010; Sulzer et al., 1998), was selectively knocked down in dopamine cells. DAT-Cre mice were crossed with VGLUT2-floxed mice, and the resulting VGLUT2 hemizygous conditional knockdown animals (VGLUT2 cKD; DATCre/+;VGLUT2flx/+) were injected with AAV-DIO-ChR2 in the midbrain (Figure 4A). Consistent with a 50-60% reduction in VGLUT2 expression in conditional knockdown mice (Moechars et al., 2006; Wallén-Mackenzie et al., 2010), the amplitude of optically evoked AMPA EPSCs was reduced in DLS MSNs from VGLUT2 cKD slices compared to those from control animals (p < 0.001, Kruskal-Wallis) (Figure 4A - 4B). Moreover, the amplitude of AMPA EPSCs from VGLUT2 cKD animals were similar to those recorded from low-dose 6-OHDA treated animals (p > 0.05, Kruskal-Wallis) (Figure 4A - 4B), suggesting that conditional knockdown reduced glutamate release from SNc terminals to similar levels as low-dose 6-OHDA treatment. By contrast, despite the reduction in VGLUT2 expression, optogenetic stimulation of dopamine terminals still evoked comparable bursts of action potentials in DLS ChIs as DAT-Cre controls (p > 0.05, Mann-Whitney) (Figure 4C - 4D). To further examine whether burst firing in DLS ChIs was affected by VGLUT2 knockdown we repeated experiments comparing control (DATCre/+) and VGLUT2 cKD (DATCre/+;VGLUT2flx/+) animals using a range of light intensities to determine if the threshold for burst firing was similar across groups. Decreasing the power of blue light similarly reduced both the frequency of evoked bursts (p > 0.05, Mann-Whitney) (Figure 4E) and the amplitude of evoked mGluR1 EPSCs (p > 0.05, Mann-Whitney) (Figure 4F) in DLS ChIs in both control and VGLUT2 cKD slices. This suggests that the threshold for burst firing was similar between control and VGLUT2 cKD groups. Similar results were obtained when control slices were incubated in bafilomycin A (500 nM), a blocker of vacuolar-type ATPase, to inhibit the voltage and proton gradient required by VGLUT2 so as to reduce the concentration of glutamate in synaptic vesicles (Harrison and Jahr, 2003; Zhou et al., 2000). While bafilomycin A reduced the amplitude of electrically evoked AMPA EPSCs in DLS MSNs (p < 0.05, Mann-Whitney) (Figure 4G), it did not impair optogenetically evoked burst firing from DLS ChIs (p > 0.05, Mann-Whitney) (Figure 4H). Furthermore, as in VGLUT2 cKD slices, decreasing the optogenetic light power decreased the frequency of bursts in slices incubated in bafilomycin A to a similar extent as in control slices (p > 0.05, Mann-Whitney) (Figure 4I). These results suggest that although presynaptic glutamate transmission from dopamine terminals is decreased by 6-OHDA treatment, this decrease is insufficient to eliminate mGluR1-mediated ChI bursting. Thus, a presynaptic decrease in glutamate release is unlikely to account for the loss of burst firing of DLS ChIs induced by low-dose 6-OHDA.
Figure 4. Presynaptic reduction in glutamate release does not mimic effect of dopamine terminal degeneration.
(A) Schematic of MFB saline or 6-OHDA and SNc AAV-DIO-ChR2 injections into either DAT-Cre mice or DAT-Cre;VGLUT2 cKD mice and recordings from MSNs three weeks following. Representative AMPA EPSCs from saline control, 6-OHDA or VGLUT2 cKD groups. Single flash (2 ms).
(B) Summary of AMPA-mediated EPSCs in DLS MSNs from saline, 6-OHDA groups and VGLUT2 cKD groups. n = 10 -15, N = 3.
(C) Optogenetic stimulation of dopamine inputs in VGLUT2 cKD mice. Train flash (5 flashes, 2 ms, 20 Hz)
(D) Summary of burst firing frequency in control and VGLUT2 cKD groups. n = 14 – 18, N = 2 - 3.
(E) Cell-attached recordings of SNc evoked bursting in DLS ChIs from saline control and VGLUT2 cKD groups. LED light power (5 flashes, 2 ms, 20 Hz) was varied from 0.5 to 2.0 mW/mm2. n = 6 - 8, N = 4.
(F) SNc evoked mGluR1 EPSCs from saline control and VGLUT2 cKD groups. LED light power (5 flashes, 2 ms, 20 Hz) was varied from 0.5 to 2.0 mW/mm2. n = 6, N = 2.
(G) Bafilomycin A (500 nM) reduces electrically evoked AMPA EPSCs from MSNs. Single electrical stimulus (0.5 ms). n = 8 -10, N = 3.
(H) Bafilomycin A (500 nM) does not affect optogenetically evoked bursts from DLS ChIs. Train flash (5 flashes at 20 Hz). n = 12 -18, N = 2 - 3.
(I) Cell-attached recordings of SNc evoked bursting in DLS ChIs from saline control and bafilomycin A treated slices. LED light power (5 flashes, 2 ms, 20 Hz) was varied from 0.5 to 2.0 mW/mm2. Right: quantification of the decrease in SNc-evoked burst firing frequency when LED light power was decreased from 2.0 to 0.5 mW/mm2. n = 5 - 8.
Summary data are mean ± SEM. * = p < 0.05, ** = p < 0.01, *** = p < 0.001, n.s. = p > 0.05
Partial loss of striatal dopamine inputs downregulates functional mGluR1s in ChIs
To determine if a post-synaptic loss of mGluR1-mediated signaling could instead account for the decrease in glutamate co-release driven excitation, whole-cell recordings were made from DLS ChIs while the mGluR agonist L-aspartate was exogenously applied by iontophoresis (200 mM; in the presence of AMPA and NMDA receptor antagonists) (Figure 5A). Application of L-aspartate (200 mM, 500 ms) evoked mGluR-mediated inward currents that were reduced in amplitude from low-dose 6-OHDA treated animals compared to saline-injected sham controls (p < 0.001, Mann-Whitney) (Figure 5A - 5B). Inward currents were eliminated in the presence of a mGluR1 antagonist (JNJ; 10 μM). Current-voltage analysis showed that the inward TRP currents (reversal potential ~ +10 mV) in ChIs from 6-OHDA treated animals also exhibited reduced conductance (Figure 5C). Similar to the effect of iontophoretic application of L-aspartate, bath application of the group 1 metabotropic glutamate receptor agonist DHPG (50 μM) also evoked smaller inward currents in DLS ChIs from low-dose 6-OHDA treated animals compared to saline-treated mice (p < 0.01, Mann-Whitney) (Figure 5D - 5E). As these results indicate a postsynaptic decrease in mGluR function in DLS ChIs, we also measured mGluR1 mRNA (Grm1) levels using single cell quantitative reverse transcriptase polymerase chain reaction following dopamine loss. Following the injection of AAV-DIO-tdTomato into ChAT-Cre mice, we aspirated the intracellular contents of fluorescently labeled DLS ChIs from saline control animals as well as animals 3 d and 21 d post low-dose 6-OHDA injection (Figure 5F). As expected, aspirated ChIs exhibited significant enrichment of Chat, a molecular marker of ChIs, over the transcription factor Ctip2 which is expressed in only MSNs in the striatum (Arlotta et al., 2008) (p < 0.0001, Mann-Whitney). Three days following 6-OHDA injection there was a trend towards decreased Grm1 levels (p = 0.06, Mann-Whitney) which was significantly lower than levels in DLS ChIs 21 d following 6-OHDA injection (p < 0.01, Mann-Whitney) (Figure 5F and Supplemental Figure S3). These data suggest 6-OHDA induces dynamic changes in GRM1 mRNA levels following degeneration of nigral inputs, which may contribute to the functional downregulation of mGluR1.
Figure 5. Down regulation of mGluR1 signaling in DLS ChIs following partial degeneration of dopamine inputs.
(A) Iontophoretic application of L-aspartate (200 μM; 500 ms) evoked mGluR-mediated inward currents in DLS ChIs are reduced following low-dose (1 μg/ μL) 6-OHDA.
(B) Summary L-aspartate evoked mGluR currents in DLS ChIs. n = 12 – 17, N = 3 - 4.
(C) Current-voltage relationship of L-aspartate evoked responses from saline and 6-OHDA DLS ChI groups. n = 8 - 9, N = 3.
(D) Bath application evoked DHPG (50 μM) inward currents from saline and 6-OHDA DLS ChI groups.
(E) Summary of DHPG evoked current amplitude. n = 6 – 10, N = 3.
(F) Relative amounts of Grm1 mRNA in DLS ChIs from 3d and 21d post 6-OHDA injected animals. Aspirated ChIs exhibited enriched levels of Chat over Ctip2 mRNA. n 16 - 44, N = 3 for both 3d and 21d groups.
(G) Representative 2-photon image of an Alexa594 (20 μM) and Cal520 (100 μM) filled DLS ChI. L-aspartate filled iontophoretic pipette and location of photometry measurement of Ca2+ response illustrated.
(H) L-aspartate evoked Cal520 Ca2+ fluorescence responses from saline and 6-OHDA DLS ChIs.
(I) Summary amplitude of Ca2+ responses. n = 7 - 8, N = 3 - 4.
(J) Substance P (1 μM) evoked inward currents from saline and 6-OHDA DLS ChIs.
(K) Summary of Substance P evoked current amplitude. n = 11 – 12, N = 3 - 4.
(L) CNO (20μM) evoked inward currents in hM3Dq-expressing DLS ChIs from saline and 6-OHDA DLS ChIs.
(M) Summary of amplitude of CNO inward currents. n = 19 – 20, N = 4.
Summary data are mean ± SEM. ** = p < 0.01, *** = p < 0.001, n.s. = p > 0.05.
Next, we tested whether the dysfunction in mGluR-mediated Chi excitation was due to the disruption of mGluR1 mediated signaling cascades. Group 1 metabotropic glutamate receptors are Gq-coupled receptors, which via the activation of phospholipase C (PLC), mobilize Ca2+ release from intracellular stores. We used two-photon Ca2+ spot photometry in DLS Chis filled with the Ca2+ indicator dye Cal520 (100 μM) to compare the amplitude of dendritic L-aspartate-evoked intracellular Ca2+ transients between 6-OHDA treated slices and saline controls (Figure 5G). This approach, which rapidly oscillates the position of two-photon laser beam position in a small circle (150 nm diameter) on a dendritic segment, continuously samples fluorescence intensity (2 KHz) allowing for high signal-to-noise ratio and temporal resolution of Ca2+ responses (Pressler and Strowbridge, 2019). An iontophoretic pipette containing L-aspartate (200 mM) was placed adjacent to a segment of dendrite ~50-100 μm from the soma (Figure 5G). The amplitude of the L-aspartate-evoked Ca2+ transients (expressed as ΔF/F) was reduced in Chis in slices taken from low-dose 6-OHDA treated animals compared to controls (p < 0.001, Mann-Whitney) (Figure 5H - 5I) indicating that, like the reduction in TRP-mediated currents, partial degeneration of dopamine inputs down-regulates mGluR1 signaling in DLS Chis.
We next examined whether the decrease in mGluR signaling also occurred for other Gq-coupled GPCRs in Chis. Chis express the Gq-coupled neurokinin 1 receptor and application of its agonist Substance P evokes an inward excitatory current similar to activation of mGluRs (Aosaki and Kawaguchi, 1996). However, unlike the effect of L-aspartate or DHPG (Figure 5B and 5E), bath application of Substance P (1 μM) evoked similar amplitude inward currents in DLS Chis in 6-OHDA treated and saline control slices (p > 0.05, Mann-Whitney) (Figure 5J - 5K). We also examined whether an exogenously expressed Gq-coupled receptor would be impacted by 6-OHDA. A chemogenetic approach was used to virally express the hM3D (Gq) DREADD receptor selectively in Chis using mice expressing Cre recombinase under the control of the choline acetyltransferase promoter (ChAT-Cre). Similar to the results using Substance P, bath application of the DREADD agonist CNO (20 μM) evoked similar amplitude inward currents in Chis from both saline and 6-OHDA treated animals (n = 19, p > 0.05, Mann-Whitney) (Figure 5L - 5M). Together these results suggest that partial loss of striatal dopamine inputs drives specific down-regulation of mGluR1 signaling in DLS ChIs, which accounts for the dysregulation of glutamate mediated excitatory transmission in parkinsonian mice.
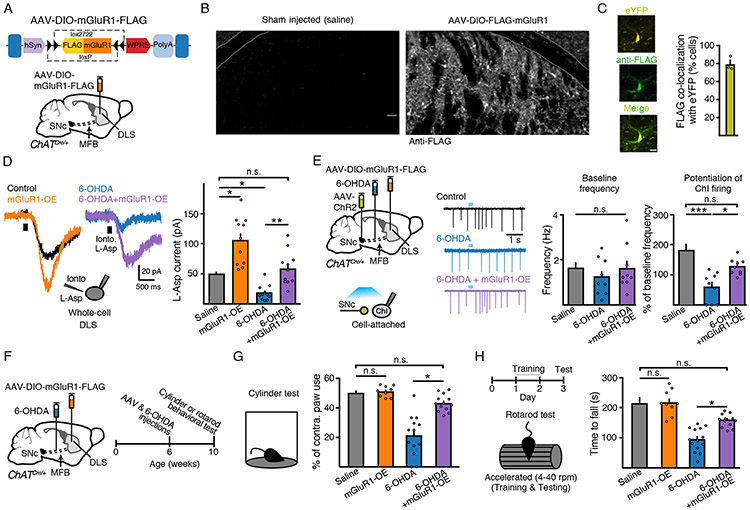
Overexpression of mGluR1 in DLS ChIs restores glutamate co-release mediated transmission and rescues parkinsonian motor impairment
If downregulation of functional mGluR1 levels is responsible for the dysregulation of ChI excitability in the DLS in parkinsonian mice, limiting this reduction may ameliorate parkinsonian motor deficits. To test this, we designed an AAV encoding double-floxed mGluR1 (Figure 6A). Three weeks following injection of AAV-DIO-mGluR1-FLAG into ChAT-Cre mice, widespread FLAG immunoreactivity could be detected in ChIs of the striatum (Figure 6B). To determine the proportion of ChIs that expressed the construct, in separate ChAT-Cre mice we co-injected AAV-DIO-eYFP and AAV-DIO-mGluR1-FLAG. Out of 233 eYFP+ putative ChIs, 179 co-expressed FLAG (79.3 ± 4.5 %) (Figure 6C). The overall density of FLAG+ putative ChIs in the DLS was 43 cells / mm2, which based on previous estimates of ChI density in the DLS (Cai and Ford, 2018) suggests that ~79% of putative ChIs expressed the transgene. In slices from AAV-DIO-mGluR1-FLAG injected animals, iontophoretic application of L-aspartate (200 mM; 500 ms) evoked larger amplitude inward currents in ChIs than in vehicle-injected control hemispheres (p < 0.05, Kruskal-Wallis) (Figure 6D), indicating that overexpressed mGluR1s were functional and that increasing the expression of these receptors could evoke larger amplitude postsynaptic responses. Importantly, viral overexpression of mGluR1 potentiated the amplitude of L-aspartate-evoked currents in DLS ChIs from low-dose 6-OHDA treated mice (p < 0.01, Kruskal-Wallis) and restored the amplitude of currents similar to those seen in saline-injected sham control mice (p > 0.05, Kruskal-Wallis) (Figure 6D). To verify that the overexpression of mGluR1s could rescue the circuit deficits in parkinsonian mice, low-dose 6-OHDA treated ChAT-cre mice were injected with AAV-DIO-mGluR1-FLAG into the striatum and AAV-ChR2 into the midbrain (Figure 6E). While optogenetic stimulation of midbrain inputs failed to evoke action potential bursts in DLS ChIs from 6-OHDA treated mice, bursting was restored in 6-OHDA treated animals overexpressing mGluR1 (p < 0.05, Kruskal-Wallis) (Figure 6E). Overexpression of mGluR1 did not alter the input resistance (p > 0.05, Kruskal-Wallis) or basal firing rate of ChIs (p > 0.05, Kruskal-Wallis), nor did it alter dopamine D2-receptor mediated transmission onto adjacent D2-MSNs (p > 0.05, Mann-Whitney) (Figure 6E and Supplemental Figure S4). Taken together, these results suggest that decreased functional mGluR1 expression in DLS ChIs is a major factor limiting the regulation of their excitability in parkinsonian mice and that preventing this decrease via the overexpression of mGluR1 rescues this dysfunction.
Figure 6. Overexpression of mGluR1s rescues parkinsonian circuit and motor deficits.
(A) Schematic of design and injection of AAV-DIO-mGluR1-FLAG in ChAT-Cre mice.
(B) Immunohistochemical images illustrating anti-FLAG immunoreactivity from saline and AAV-DIO-mGluR1-FLAG injected hemispheres in ChAT-Cre mice.
(C) eYFP and FLAG co-localization IHC images and quantification following AAV-DIO-eYFP and AAV-DIO-mGluR1-FLAG co-injection into the DLS of ChAT-Cre mice. Scale bar = 25 μm. N = 3.
(D) L-aspartate (200 nM, 500 ms) evoked mGluR mediated inward currents. mGluR overexpression rescues 6-OHDA-induced loss of current amplitude. n = 11 - 12, N = 2 - 3.
(E) Schematic of MFB saline or 6-OHDA, midbrain AAV-ChR2 and striatal AAV-DIO-mGluR1-FLAG injections into ChAT-Cre mice and recordings from ChIs three weeks following. Representative traces and quantification of baseline firing and ChR2 evoked DLS ChI bursting and rescue in 6-OHDA + mGluR1 overexpressing mice. n = 9 - 10, N = 3.
(F) Schematic and timeline of injections and cylinder or rotarod behavioral tests.
(G) Contralateral paw use in cylinder behavioral test. N = 9 – 14.
(H) Latency to fall on test day (day 3) on rotarod (4 - 40 rpm). N = 6 - 14.
Summary data are mean ± SEM. ** = p < 0.01, *** = p < 0.001, n.s. = p > 0.05
Next, the effect of expressing mGluR1 in DLS ChIs was examined on motor performance in parkinsonian mice. We first used the cylinder test in partial unilaterally lesioned animals to assess forelimb symmetry movement (Boix et al., 2015; Park et al., 2018). As seen before (Figure 1D), unilateral low-dose 6-OHDA treated mice had reduced forelimb use on the contralateral side (p < 0.001, Kruskal-Wallis) (Figure 6G). Overexpression of mGluR1 in DLS ChIs, following AAV-DIO-mGluR1-FLAG injection into the DLS of ChAT-Cre mice, had no effect on contralateral paw use in control saline-injected animals but significantly improved the ability of 6-OHDA unilaterally treated animals to use their contralateral paws (p < 0.05, Kruskal-Wallis) (Figure 6G). Thus, virally expressing mGluR1 in DLS ChIs was sufficient to alleviate parkinsonian limb use asymmetry. Next, we used the rotarod test to further assay parkinsonian motor deficits (Figure 6H). Bilateral partially lesioned ChAT-Cre mice were trained on rotarod with an acceleration protocol (4-40 rpm) for two days before being tested on day three using this same acceleration protocol (Figure 6H). Overexpression of mGluR1 in DLS ChIs in vehicle-injected sham control animals had no effect, but significantly improved the ability of 6-OHDA treated mice to stay on the rotarod (p < 0.001, Kruskal-Wallis) (Figure 6H). Taken together, these results suggest that selectively expressing mGluR1 in DLS ChIs alleviates motor deficits in low-dose 6-OHDA-induced early stage parkinsonian mice.
Loss of mGluR1 receptors limits SNc-evoked excitation
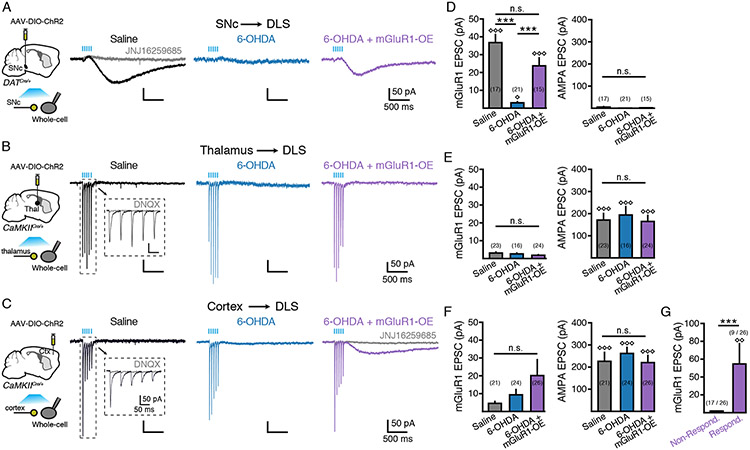
The current data indicate that loss of mGluR1s affects SNc-driven ChI excitability. However, it is not clear whether other glutamatergic inputs also activate mGluR1s and if loss of mGluR1 following dopaminergic degeneration limits the excitatory drive of these inputs via this receptor. ChIs receive fast excitatory glutamatergic input from the sensorimotor cortex and the parafascicular nucleus of the thalamus (Buendia et al., 2019; Ding et al., 2008, 2010; Doig et al., 2014; Kosillo et al., 2016; Mamaligas et al., 2019; Matsumoto et al., 2001). To determine if glutamate release from cortical and thalamic inputs also activate mGluR1s, AAV-DIO-ChR2 was injected into the SNc of DAT-Cre mice, or the sensorimotor cortex or the parafascicular thalamus of CaMKII-Cre mice. Three weeks post injection, a train of light pulses (5 pulses, 20 Hz, 2 ms per pulse) was used to evoke glutamate release from each input in slices while recording from DLS ChIs (Figure 7A - 7C). As noted previously (Chuhma et al., 2018), photoactivation of midbrain inputs from control animals evoked only small amplitude AMPA EPSCs onto DLS ChIs (Figure 7A). While these AMPA EPSCs were difficult to detect above the baseline noise (p > 0.05, Mann-Whitney versus 2 x S.D. baseline noise), stimulation of midbrain inputs reliably evoked JNJ-sensitive mGluR1 EPSCs (p < 0.001 versus 2 x S.D. baseline noise, Mann-Whitney) (Figure 7B, 7D). In contrast, photoactivation of either thalamic or cortical terminals evoked only AMPA EPSCs, which were blocked by the antagonist DNQX (10 μM) (p < 0.001, Mann-Whitney), without evoking measurable mGluR1 EPSCs (p > 0.05, Mann-Whitney) (Figure 7B, 7C, 7E, 7F). Thus, under control conditions while midbrain inputs activate mGluR1s on DLS ChIs, cortical and thalamic inputs do not. We did observe that stimulation of cortical, but not thalamic, inputs evoked an unidentified late-slow EPSC in a subset of DLS ChIs (7 out of 21 cells) that had significantly slower kinetics than mGluR1 EPSCs (p < 0.001, Mann-Whitney) (Supplemental Figure S5). This late-slow EPSC was not blocked by the mGluR1 antagonist JNJ16259685 (10 μM), confirming that it was not mediated by mGluR1 receptors. As only a subset of DLS ChI exhibited a late-slow EPSC, the amplitude overall was not significantly different than baseline (p > 0.05, Mann-Whitney) (Figure 7F).
Figure 7. Parkinsonian motor impairments result from loss of SNc-mediated mGluR1 signaling.
(A) Representative traces from DLS ChIs from saline (left), 6-OHDA (middle) and 6-OHDA + mGluR1 overexpressing (right) groups following photostimulation (5 flashes, 2 ms, 20 Hz) of midbrain inputs.
(B) Representative traces from DLS ChIs from saline (left), 6-OHDA (middle) and 6-OHDA + mGluR1 overexpressing (right) groups following photostimulation (5 flashes, 2 ms, 20 Hz) of parafascicular thalamic inputs.
(C) Representative traces from DLS ChIs from saline (left), 6-OHDA (middle) and 6-OHDA + mGluR1 overexpressing (right) groups following photostimulation (5 flashes, 2 ms, 20 Hz) of sensorimotor cortical inputs.
(D) Quantification of mGluR1 and AMPA EPSCs evoked by stimulation of midbrain inputs from (A). n = 15 - 21. N = 3 - 5.
(E) Quantification of mGluR1 and AMPA EPSCs evoked by stimulation of thalamic inputs from (B). n = 16 - 24. N = 2 - 3.
(F) Quantification of mGluR1 and AMPA EPSCs evoked by stimulation of cortical inputs from (B). n = 21 - 26. N = 3 - 4.
(G) Amplitude of mGluR1 EPSCs following cortical stimulation in 6-OHDA + mGluR1 overexpressing responding and non-responding DLS ChIs.
Summary data are mean ± SEM. *** = p < 0.001, n.s. = p > 0.05. Diamonds represent p < 0.01 or < 0.001 versus 2 x S.D of baseline.
Comparing synaptic responses from saline control mice to those from low dose 6-OHDA injected animals again showed that partial dopamine degeneration reduced the amplitude of midbrain-evoked mGluR1 EPSCs (p < 0.001 vs saline control, Kruskal-Wallis) (Figure 7A - 7D). The amplitude of AMPA EPSCs was similar from both thalamic and cortical inputs in low dose 6-OHDA treated animals as saline controls (p>0.05, Kruskal-Wallis) (Figure 7B, 7C, 7E, 7F), confirming that dopamine lesions do not alter the strength or short-term plasticity of AMPA-mediated glutamatergic synaptic events from either cortical or thalamic inputs to ChIs (Buendia et al., 2019). Expressing mGluR1 by co-injecting AAV-DIO-FLAG-mGluR1 and AAV-mCherry-Cre into the DLS of low-dose 6-OHDA treated mice restored the amplitude of SNc-evoked mGluR1 EPSCs in DLS ChIs to levels similar to sham-lesioned controls (p > 0.05 versus saline control, Kruskal-Wallis) (Figure 7A, 7D). Conversely, viral overexpression of mGluR1 had no effect on thalamic EPSCs (p > 0.05, Kruskal-Wallis) (Figure 7B, 7E) but did however generate mGluR1 EPSCs following photostimulation of cortical inputs in 6-OHDA treated mice in a subset of ChIs (Figure 7C, 7F, 7G). While the overall amplitude of cortically-evoked mGluR1 EPSCs was not significantly different than baseline (p > 0.05, Mann-Whitney), separating responders from non-responders revealed mGluR1 EPSCs in a minority of cells following viral expression of mGluR1 in 6-OHDA treated mice (p < 0.05, Mann-Whitney). The kinetics of these JNJ-sensitive mGluR1 EPSCs were similar to mGluR1 EPSCs evoked by midbrain inputs (Supplemental Figure S5). Together these results indicate that the loss of mGluR1s following dopamine terminal degeneration primarily limits the excitatory influence of midbrain inputs, and that viral overexpression of mGluR1 restores SNc-evoked mGluR1-mediated excitation of DLS ChIs but also allows for activation of these receptors by cortical inputs in some cells.
Discussion
The interaction between dopamine and ACh in the dorsal striatum is central to motor and cognitive functions (Aosaki et al., 2010; Di Chiara et al., 1994). Dopamine neurons differentially modulate cholinergic activity across sub-regions of the dorsal striatum through dopamine and glutamate co-release (Cai and Ford, 2018; Chuhma et al., 2018). Past work has shown that the actions of dopamine and ACh differ across the medial and lateral striatum due to differences in both the extent of co-release and the strength of post-synaptic receptor signaling. Midbrain dopamine neurons projecting to the striatum originate from topographically organized populations of SNc and VTA neurons that differ in their gene expression, transmitter profiles and in the motivational signals they encode (Cai and Ford, 2018; Chuhma et al., 2014, 2014; Haber et al., 2000; Lerner et al., 2015; Poulin et al., 2014). As DMS projecting SNc dopamine neurons do not express VGLUT2, only DLS dopamine inputs drive Chi burst firing through glutamate co-release, despite mGluR1 receptors being expressed across Chis in the dorsal striatum (Berg et al., 2007; Cai and Ford, 2018; Chuhma et al., 2018). The strength of dopamine inputs onto Chis also varies across regions. While similar amounts of dopamine are released in both regions (Chuhma et al., 2018), dopamine evokes only brief pauses in DLS Chis due to weaker D2-receptor signaling in these cells, which is quickly overwhelmed by glutamate co-release mGluR1-mediated bursting (Cai and Ford, 2018). Here we show in an early stage Parkinson’s disease mouse model, that while dopamine-mediated inhibition of Chis is preserved in the DMS due to a reduction of dopamine re-uptake, SNc-driven excitation of Chis by glutamate co-release is diminished in the DLS due to a decrease in postsynaptic mGluR1 expression. Rescuing the loss of mGluR1 signaling in dorsolateral Chis was sufficient to alleviate several parkinsonian motor deficits. This work reveals the vulnerability of SNc glutamatergic modulation of striatal cholinergic activity in Parkinson’s disease and the importance of this modulation for motor performance.
ChIs exhibit pacemaker activity (Bennett and Wilson, 1999) and are driven to fire bursts by excitatory inputs from the thalamus and motor cortex (Ding et al., 2010; Doig et al., 2014; Mamaligas et al., 2019; Matsumoto et al., 2001) as well as SNc dopamine neurons through glutamate co-release (Cai and Ford, 2018; Chuhma et al., 2018). While cortical and thalamic inputs are known to excite Chis through AMPA and NMDA receptors (Ding et al., 2010; Mamaligas et al., 2019), it is not clear the extent to which these inputs may also regulate burst firing via mGluRs. Comparing sensorimotor, parafascicular thalamic and nigral inputs revealed that during brief trains of stimuli, mGluR1 receptors are activated primarily by SNc dopamine inputs. Fast glutamatergic synapses are surrounded by glutamate transporter enriched astrocytic processes that help confine glutamate to the synaptic cleft and limit spillover. This arrangement may not exist for nigral inputs, which are likely opposed at a further distance from postsynaptic sites (Descarries et al., 1996). Thus, glutamate released from SNc terminals may more easily reach mGluR1 receptors on Chis than when released from cortical and thalamic terminals. The selective loss of SNc-evoked mGluR1 mediated excitation of Chis following dopamine terminal degeneration suggests that selective loss of this input may most strongly contribute to the associated motor impairment in Parkinsonian animals.
The loss of SNc-evoked ChI bursting was unlikely due simply to a decrease in glutamate release. While 6-OHDA induced degeneration of SNc terminals did lead to a decrease in glutamate co-release from dopamine terminals, mimicking this change by reducing presynaptic VGLUT2 expression or activity, failed to alter midbrain-evoked DLS ChI bursting. Varying the LED stimulus power further confirmed that the threshold for burst firing was similar between control and VGLUT2 cKD groups, suggesting that the lack of effect of decreasing glutamate release on burst firing was not due to use of a supramaximal optogenetic stimulus. The high affinity of mGluRs for glutamate (Niswender and Conn, 2010; Traynelis et al., 2010) likely enables robust receptor activation despite the reduced glutamate levels. Instead electrophysiology and calcium imaging experiments revealed a postsynaptic loss of mGluR signaling. The decrease in mGluR1 mediated excitation was not due to loss of downstream signaling, as other Gq-coupled receptor excitatory currents were unaffected by 6-OHDA, suggesting instead a decrease in functional mGluR1 receptor levels. This was supported by single-cell qPCR analysis which showed dynamic changes in mGluR mRNA levels in ChIs from 6-OHDA treated animals. Future work will be required to determine how transcriptional or translational changes potentially alter mGluR1 expression on ChIs. Degradation or post-translational modification may also contribute to the decreased functional expression following loss of dopamine, as altered expression of scaffolding molecules such as Homer1, which regulates mGluR surface trafficking (Brakeman et al., 1997; Ciruela et al., 2000; Roche et al., 1999) is decreased following dopamine depletion (Kurz et al., 2010; Kuwajima et al., 2007).
In Parkinson’s patients, dopaminergic denervation initially emerges in the putamen (DLS). We found that an early consequence of dopamine degeneration was the downregulation of mGluR1 signaling and a loss of SNc-mediated excitation of DLS ChIs. Past studies have found reduced striatal mGluR1 levels across the striatum in Parkinson’s patients as well as in animal models for Parkinson’s disease (Kumar et al., 2014; Kuwajima et al., 2007; Yamasaki et al., 2016). However, as mGluR1 is broadly expressed in the striatum, the locus of this change has been unclear. Interestingly, group 1 mGluR signaling in ChIs has previously been reported to be unaffected in PINK1 and Parkin knockout mice (Martella et al., 2009). This work however was done in young animals at ages where striatal dopamine levels and motor activity has yet to be impacted. Human PET imaging studies have shown that there is approximately a 50% decrease in dopamine terminals in the putamen at the onset of Parkinson’s disease (Cheng et al., 2010; Pavese et al., 2011) which is similar to the degree of striatal dopamine terminal loss and evoked dopamine release that we observed in low-dose 6-OHDA treated animals. In light of the present findings, this suggests that the loss of dopamine may initiate the decrease in mGluR1 in ChIs and that mGluR dysfunction may only become apparent near the onset of symptom development.
Selective viral expression of mGluR1 in ChIs in parkinsonian mice was sufficient to restore SNc-mediated excitatory drive and alleviated several motor deficits in these parkinsonian mice. One beneficial effect of this rescue may be that bursts of ChIs potentiate dopamine release from the remaining SNc axons in the DLS. Synchronous firing of ChIs evokes dopamine release by activating presynaptic nicotinic receptors independent of dopamine neuron firing (Cachope et al., 2012; Threlfell et al., 2012). Therefore, phasic activation of ChIs by dopamine neurons may drive additional release of dopamine in the DLS. In addition, as ChIs inhibit cortical and thalamic inputs via presynaptic muscarinic receptors (Ding et al., 2010; Pakhotin and Bracci, 2007), rescue of SNc-driven ChI bursts may also attenuate the altered excitatory drive (Picconi et al., 2004; Villalba and Smith, 2011) and imbalance to direct and indirect pathways that arise following dopamine depletion (Day et al., 2006; Parker et al., 2016; Tanimura et al., 2019). We did observe that in a subset of ChIs, overexpression of mGluR1 led to the de novo appearance of mGluR1 EPSCs following stimulation of cortical inputs. Increased mGluR1 bursting as a result of cortical inputs may also help ameliorate motor deficits.
It has long been proposed that striatal depletion of dopamine in Parkinson’s disease leads to an excess of ACh (Barbeau, 1962; Ding et al., 2006; Ztaou et al., 2016), with the imbalance being central to the underlying motor deficits (Aosaki et al., 2010; Pisani et al., 2007). However, recent work has found that DLS ChIs exhibit reduced excitability and spontaneous firing in a progressive mouse model for Parkinson’s disease (McKinley et al., 2019). As the present work has identified a loss of SNc-driven excitation in Parkinsonian mice, the rescue of which can alleviate several motor deficits, these results together suggest a potential decrease in ChI activity following loss of dopamine. Targeting ChIs to rescue the dysfunction in their firing may be a strategy for alleviating cholinergic imbalances that arise in Parkinson’s disease. Due to the broad neuronal expression of group 1 mGluRs, rather than pharmacological strategies, gene-therapy approaches that could target mGluR1 may be a synapse-specific approach for therapeutic development.
Star Methods
Lead Contact
Further information and requests for resources and reagents should be direct to and will be fulfilled by the Lead Contact, Christopher Ford (christopher.ford@cuanschutz.edu)
Materials Availability
Plasmids generated have been uploaded to Addgene (plasmid #166227).
Data and Code Availability
All data and codes generated during this study are available upon request.
Experimental Model and Subject Details
Experimental models
All experiments were approved by and performed in agreement with the guidelines of the Institutional Animal Care and Use Committee (IACUC) at University of Colorado School of Medicine. 10 week-old mice used in experiments were: DAT-IRES-Cre heterozygote mice (Slc6a3IRES-Cre, Jackson Laboratory stock #006660), ChAT-IRES-Cre heterozygote mice (Chattm2(cre)Lowl/J, Jackson Laboratory stock #006410), VGLUT2 conditional knockdown (Slc17a6+/fl ; Slc6a3+/IRESCre Jackson Laboratory stock #006660 x #007583), CaMKII-Cre heterozygote mice (generated from WT and homozygous B6.Cg-Tg(Camk2a-cre)T29-1Stl/J, Jackson Laboratory stock# 005359), and wild-type C57BL/6J mice (Jackson Laboratory stock #000664). Both male and female mice were used for all experiments.
Stereotaxic injection
Male or female mice were injected at the age of 6-7 weeks. Mice were anesthetized with 1~2% isoflurane and mounted in a stereotaxic frame (Kopf Instruments). Mice were injected with a Nanoject III (Drummond Scientific). All coordinates were relative to bregma. For MFB injections, 1 μL (1 μg / μL or 4 μg / μL) of 6-hydroxydopamine was injected into either one or both hemispheres with the following coordinates (relative to Bregma): AP −1.2 mm, ML −1.3 mm (± 1.3 mm for bilateral injections), DV −4.75 mm. Sham vehicle-injected controls were injected using the same coordinates with control saline (0.9%, 1 μl). To express channelrhodopsin in the SNc, 500 nL of AAV5.EF1a.DIO.hChR2 (H134R)-EYFP was injected at the following coordinates: AP −2.3 mm, ML −1.0 mm, DV −4.7 mm. To express GIRK2 in dorsal striatum, 400 nL of AAV9.hSyn.tdTomato.T2A.mGIRK2-1 was injected at the following coordinates: AP +0.9 mm, ML −1.9 mm, DV −2.8 mm. For experiments requiring expression of Gq-coupled DREADDs in DLS, 400 nL of AAV5-hSyn-DIO-hM3D-mCherry was injected at the following coordinates: AP + 0.7 mm, ML −2.25 mm, DV −2.65 mm. To express ChR2 in the sensorimotorcortex 500 nL of AAV5.EF1a.DIO.hChR2 (H134R)-EYFP was injected at the following coordinates: AP +1.1 mm, ML +/− 1.45 mm, DV −1.2 mm. To express ChR2 in the parafascicular thalamus 500 nL of AAV5.EF1a.DIO.hChR2 (H134R)-EYFP was injected at the following coordinates: AP −1.65 mm, ML +/− 0.85 mm, DV −3.4 mm. To overexpress mGluR1 in the DLS of ChAT-Cre mice, 600 nL of AAVDJ-DIO-mGluR1-FLAG was injected with the following coordinates: AP + 0.7 mm, ML −2.25 mm (± 2.25 mm for bilateral injections), DV −2.65 mm. In cases where eYFP was co-expressed in ChIs, 300 nL of AAVDJ-DIO-mGluR1-FLAG and 300 nL of AAV-EF1a-DIO-eYFP were injected in the same coordinates. To overexpress mGluR1 in the DLS of DAT-cre or CaMKII-Cre mice, 400 nL of AAVDJ-DIO-mGluR1-FLAG was co-injected with 400nL of AAV5-EF1a-mCherry-Cre with the following coordinates: AP + 0.7 mm, ML −2.25 mm (± 2.25 mm for bilateral injections), DV −2.65 mm. Sham vehicle-injected controls were injected using the same coordinates with control saline (600 nL). Mice were allowed to recover for at least 3 weeks prior to testing. For behavioral experiments in Figure 6, the control group was injected with saline in the MFB and DLS, the mGluR1-OE group was injected with saline in the MFB and AAV-DIO-FLAG-mGluR1 in the DLS, the 6-OHDA group was injected with 6-OHDA in the MFB and saline in the DLS, and the 6-OHDA + mGluR1-OE group was injected with 6-OHDA in the MFB and AAV-DIO-FLAG-mGluR1 in the DLS.
Mouse model of PD
Either 1μg or 4 μg 6-OHDA was dissolved in 1 μL sterile saline and injected into MFB as described above. Sham control mice were injected with 1 μL sterile saline using the same coordinates. 30 minutes prior to 6-OHDA injection, mice were weighed and given desipramine at 25 mg/kg to inhibit noradrenaline / 5HT uptake and pargyline at 5 mg/kg to reduce extrasynaptic breakdown of 6-OHDA. After surgery, mice were provided with hydro gel (ClearH2O) and i.p. injected with saline daily for 7 days.
Method Details
Slice preparation
Mice were anesthetized with isoflurane and then perfused with ice-cold cutting solution containing (in mM): 75 NaCl, 2.5 KCl, 6 MgCl2, 0.1 CaCl2, 1.2 NaH2PO4, 25 NaHCO3, 2.5 D-glucose, 50 sucrose and bubbled with 95% O2 and 5% CO2. Coronal slices (240 μm) containing the dorsal striatum were cut in the same cutting solution. Slices were then incubated for 1 h at 32°C in artificial cerebrospinal fluid (aCSF) containing (in mM): 126 NaCl, 2.5 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 21.4 NaHCO3, 11.1 D-glucose and 10 μM MK-801, and bubbled with 95% O2 and 5% CO2. After incubation, slices were transferred into a recording chamber and constantly perfused with aCSF (33 ± 2°C) at a rate of 2 mL/min. Neurons were visualized using a BX51WI microscope (Olympus) with an infrared LED (Thorlabs). Fluorescent neurons were visualized with custom-made green or blue LEDs.
Electrophysiology
All recordings were made in the dorsal striatum using Axopatch 200B amplifiers (Molecular Devices). Patch pipettes were made using a pipette puller (Narishige, PC-10). Pipettes for cell-attached recordings from ChIs contained aCSF. Pipettes (~2MΩ) for whole-cell recordings from ChIs contained (in mM): 135 CsCl, 10 HEPES(K), 0.1 CaCl2, 2 MgCl2, 0.1 EGTA, 0.1 mg/mL GTP, 1 mg/mL ATP, and 1.5 mg/mL phosphocreatine (pH 7.35, 275 mOsm). Pipettes (~2MΩ) for whole-cell recordings from MSNs contained (in mM): 115 K-methylsulphate, 20 NaCl, 1.5 MgCl2, 10 HEPES(K), 10 BAPTA-tetrapotassium, 0.1 mg/mL GTP, 1 mg/mL ATP, and 1.5 mg/mL phosphocreatine (pH 7.35, 275 mOsm). All recordings were acquired with Axograph X (Axograph Scientific) at 10 kHz and filtered to 2 kHz. The amplitude of cell attached action currents have been normalized for presentation. For whole-cell recordings, cells were held at −60 mV. All putative ChIs were identified or confirmed by the presence of H-current with a hyperpolarization protocol (−30 mV, 5s).
Optogenetic stimulation of ChR2-expressing inputs was achieved with wide-field illumination using a blue LED (2 ms, 470 nm). LED 470 nm light power was measured at the plane of the back aperture of the objective. Unless otherwise stated, LED power was set to ~1.5 mW/mm2. As stated, either a single flash (2 ms) or a train of 5 stimuli (2 ms) at 20 Hz was used to evoke dopamine and glutamate release. Iontophoresis of aspartic acid was performed using an iontophoresis generator (Dagan). Iontophoretic pipettes were pulled using a horizontal puller (Sutter Instrument). Pipettes containing L-aspartic acid (200 mM) was positioned 15-20 μm from the recorded cell. Aspartic acid was then focally delivered to the cell using a negative current (−180 nA, +15 nA backing current to prevent leakage). Unless otherwise stated DNQX (10 μM) and picrotoxin (100 μM) were included in the aCSF to block AMPA and GABAA receptors and slices were pretreated with MK-801 (10 μM) to block NMDA receptors.
For experiments comparing midbrain, cortical and thalamic inputs to ChIs, the amplitude of mGluR EPSCs was determined by measuring the peak inward current within a window between 450 - 900 ms following the onset of the optogenetic stimulation. In cases where JNJ was applied to inhibit mGluR1 EPSCs, the amplitude of late-slow EPSCs was measured by subtracting the current in the presence of JNJ.
Calcium imaging
2-photon calcium imaging was performed using a home-built 2-photon laser scanning microscopy system with a BX51WI microscope (Olympus). A Ti:Sapphire laser (Chameleon Ultra I; Coherent) was tuned to emit pulsed excitation at 810 nm, and scanned using a pair of X-Y galvanometer mirrors (6215, Cambridge Technology). The fluorescence was collected through a water-immersion objective (60X, Olympus), a dichroic mirror (T700LPXXR, Chroma) and filters (ET680sp and ET525/50m-2P, Chroma), and was detected using a GaAsP photomultiplier tube (PMT, H10770PA-40, Hamamatsu). A current preamplifier (SR570, Stanford Research Systems) was used to convert the output to voltage, which was then digitized by a data acquisition device (PCI-6110, National Instruments). For calcium imaging, ChIs were filled with Cal-520 (100 μM) and Alexa 594 (20 μM, for visualization of morphology). The internal solution for whole-cell recordings from ChIs contained (in mM): 115 K-methylsulphate, 20 NaCl, 1.5 MgCl2, 10 HEPES(K), 0.1 mg/mL GTP, 1 mg/mL ATP and 1.5 mg/mL phosphocreatine. Fluorescent changes of calcium indicator, Cal-520, were measured using 2-photon calcium photometry with a custom software (Toronado; https://github.com/StrowbridgeLab/Toronado-Laser-Scanning) as previously described (Pressler and Strowbridge, 2019). Briefly, the laser spot repeatedly scanned a circular path (157 nm diameter) around the region of interest. The PMT signal was converted by the same preamplifier (SR570, Stanford Research Systems; sensitivity 100 nA / V), but further filtered to 500 Hz with the gain increased two-fold (FLA-01, Cygnus Technologies). Then the signal was acquired using a data acquisition device (ITC-18, HEKA Instruments) and recorded using Axograph X (Axograph Scientific). Aspartic acid was focally applied by iontophoresis. An iontophoretic electrode was filled with L-aspartic acid (200 mM) and the fluorescent indicator sulforhodamine 101 (500 μM). To help visualize the pipette tip, the pipette was dipped in PBS containing BSA-conjugated Alexa 594 (0.06 %) for 60 seconds. Pipettes containing aspartic acid were placed 15-20 μm from the region of interest. L-aspartic acid was ejected using a negative current (−180 nA, +15 nA backing current to prevent leakage).
FSCV
Carbon fiber electrodes (34 – 700, Goodfellow) were encased with a glass pipette with an exposed diameter of 7 μm and length of 50 – 100 μm. The tip of fiber was placed in the DStr 30 – 70 μm below the surface of the slice. While holding the carbon fiber at −0.4 V, triangular waveforms (−0.4 to 1.3 V versus Ag/AgCl at 400 V/s) were applied to the fiber at 10 Hz. Background subtracted cyclic voltammogram currents were obtained by subtracting the average of 10 voltammograms obtained prior to stimulation from each voltammogram obtained after stimulation. Peak [DA]o was determined from the peak oxidation potential. The carbon fiber was calibrated to known concentrations of dopamine after experiments. DHβE (1 μM) was included in the recording solution for FSCV experiments.
Immunohistochemistry
Mice were anesthetized with isoflurane and transcardially perfused with 4% paraformaldehyde in PBS containing (in mM): 137 NaCl, 1.5 KH2PO4, 8 NaH2PO4, and 2.7 KCl (pH = 7.4). Brains were post-fixed in 4% PFA for 2 hours and then dehydrated with 30% sucrose in PBS at 4°C for 24h. Serial coronal slices (30 μm in thickness) containing the dorsal striatum or midbrain were obtained using a cryostat. For tyrosine hydroxylase staining, sections were mounted on slides and blocked in 5% normal donkey serum in PBS-T (with 0.3% Triton X-100 for permeabilization) for 1 hour at room temperature. Slides were washed in PBS and then incubated with 1:200 rabbit anti-TH antibody (AB152, Millipore) in PBS overnight at 4°C. Slides were then washed in PBS and incubated in 1:500 donkey anti-rabbit Alexa Fluor 488 (A21206, Life Technologies) for 1 hour at room temperature and washed afterwards. For immunofluorescence staining of DYKDDDDK tag (FLAG tag), 30 μm sections containing the dorsal striatum were mounted on slides and blocked in 5% normal donkey serum in PBS-T (with 0.3% Triton X-100 for permeabilization) for 1 hours at room temperature. Slides were then washed in PBS and incubated with 1:200 rabbit anti-DDDDK tag (Binds to FLAG® tag sequence) antibody (ab1162, Abcam) in PBS for overnight at 4°C. Slides were washed in PBS and incubated in 1:500 donkey anti-rabbit Alexa Fluor 594 (ab150076, Abcam) for 1 hours at room temperature and washed. Fluorescent images were obtained using a slide scanner (VS120, Olympus). Image processing were performed in Fiji (ImageJ).
Single-cell reverse transcriptase quantitative PCR
ChIs in acute striatum slices, were aspirated and expelled into PCR tubes containing lysis buffer and RNAse inhibitor, spun briefly, then snap-frozen on dry ice and stored at −80 C until further processing. cDNA was synthesized from single cells using Takara SMART-Seq HT kit according to the manufacturer’s protocol. qPCR was then performed in triplicate to assess relative expression levels of Grm1. Analysis was performed with Bio-Rad CFX 384 Real-Time PCR System and using PerfeCTa ToughMix Low Rox reaction mix and the following IDT Primetime probes: B-Actin (mmPT 39a.2214843.g), Chat (mmPT 58.31626536), Ctip2 (Bcl11b) (mmPT 58.31107142), Grm1 (mmPT 58.8395588). dCT values were obtained by normalizing Ct values for Grm1, Chat and Ctip2 against Ct values for B-actin. Relative mRNA levels were determined by normalizing Grm1 values from 6-OHDA treated animals against saline controls.
AAV generation
To generate cre-dependent mGluR1-Myc/DDK (Origene, MR211136) expressing AAVs, a pAAV hSyn DIO mGluR1-Myc/DDK plasmid was created by arranging the following elements downstream of the left-ITR of AAV2: synapsin promoter, loxP2272, loxP, inverted mGluR1-Myc/DDK, loxP 2272, loxP, WPRE, bhGH polyA and right ITR. AAVs were produced as previously described (Aoto et al., 2013). Briefly, pAAV hSyn DIO mGluR1-Myc/DDK plasmid was co-transfected with pHelper and pRC DJ into HEK-293T cells (ATCC) via the calcium phosphate transfection method. 72 hours post transfection, cells were harvested, lysed and loaded onto an iodixanol gradient. The lysate was centrifuged at 400,000 xg for 2 hours and the 40% iodixanol fraction was collected and concentrated in a 100,000 MWCO Amicon tube filter (Millipore). The titer was 7 x 1012 GC / ml.
Motor Behavior
Behavioral tests were performed 3 weeks following injections. A cylinder test was performed to evaluate forelimb use symmetry. Individual mice were put in a clean plastic cylinder (10.5 cm diameter; 14.5 cm height). Mice were not habituated to the cylinder prior to the test. Video data of full-body movement in the cylinder was acquired (3 minutes) and scored post-hoc. Wall touches with fully extended digits were counted for the contralateral and ipsilateral paws. The percent contralateral paw use was calculated as a ratio of total paw use (both paws). To assess motor coordination of mice with bilateral injections of 6-OHDA, a rotarod test was performed using a rotarod. Mice were habituated to the testing room for 40 minutes, then tested under an acceleration protocol of 4 rpm to 40 rpm. The latency to fall was recorded. Mice were trained for 2 days and tested on third day. Each mouse underwent 3 trials of a maximum of 5 minutes on each day with a separation of 10 minutes between each trial.
Chemicals
Picrotoxin and MK-801 were from Abcam. Sulpiride, JNJ16259685, Clozapine N-oxide, Bafilomycin A1, (RS)-3,5-DHPG and Substance P were obtained from Tocris Bioscience. K-methylsulfate was obtained from Acros Organic. All the other chemicals were from Sigma-Aldrich.
Quantification and Statistical Analysis
All data are shown as mean ± SEM. Statistical tests used for comparisons were parametric t test, non-parametric Mann-Whitney test, Wilcoxon matched-pairs signed rank test, and Kruskal-Wallis ANOVA test. The statistical significance was considered as p < 0.05 (*), p < 0.01 (**), p < 0.001 (***) and p < 0.001 (****). ‘N’ signifies number of animals and ‘n’ signifies number of cells, which can be found in the figure legends.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-TH | Millipore | Cat# AB152 |
| Donkey anti-rabbit Alexa Fluor 488 | Life Technologies | Cat# A21206 |
| Rabbit anti-DDDDK tag | Abcam | Cat# ab1162 |
| Donkey anti-rabbit Alexa Fluor 594 | Abcam | Cat# ab150076 |
| Bacterial and Virus Strains | ||
| AAV5.EF1a.DIO.hChR2 (H134R)-EYFP | pAAV-EF1a-double floxed-hChR2(H134R)-EYFP-WPRE-HGHpA was a gift from Karl Deisseroth | Addgene plasmid: 20298 |
| AAV9.hSyn.tdTomato.T2A.mGIRK2-1 | Marcott et al., 2014 | Penn Vector Core: V3992MI-R |
| AAV5-hSyn-DIO-hM3D-mCherry | Krashes et al., 2011 | Addgene plasmid: 44361 |
| AAV5-EF1a-mCherry-Cre | UNC Vector Core | AV6144B |
| AAVDJ-DIO-mGluR1-myc-FLAG | This manuscript | Addgene plasmid: 166227 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Alexa Fluor 594 | Thermo Fisher | Cat# A10436 |
| Cal520 | ATT Bioquest | Cat# 21141 |
| 6-hydroxydopamine hydrobromide | Tocris | Cat# 2547 |
| Desipramine hydrochloride | Tocris | Cat# 3067 |
| Pargyline hydrochloride | Sigma | Cat# P8013 |
| DNQX | Tocris | Cat# 0189 |
| Picrotoxin | Tocris | Cat# 1128 |
| MK-801 | Tocris | Cat# 0924 |
| JNJ16259685 | Tocris | Cat# 2333 |
| L-aspartate | Tocris | Cat# 0214 |
| Sulforhodamine 101 | Sigma | Cat# S7635 |
| Sulpiride | Tocris | Cat# 0895 |
| Clozapine N-oxide | Tocris | Cat# 4936 |
| (RS)-3,5-DHPG | Tocris | Cat# 0342 |
| Substance P | Tocris | Cat# 1156 |
| Bafilomycin A1 | Tocris | Cat# 1334 |
| BAPTA | Acros Organic | Cat# 123311000 |
| Critical Commercial Assays | ||
| PerfeCTa ToughMix Low Rox | Quantabio | Cat# 95112 |
| SMART-Seq HT kit | Takara | Cat# 634455 |
| Experimental Models: Cell Lines | ||
| HEK-239T cells | ATCC | Cat# 11268 |
| Experimental Models: Organisms/Strains | ||
| Mouse / DAT-IRES-Cre | Jackson Labs | JAX: 006660 |
| Mouse / ChAT-IRES-Cre | Jackson Labs | JAX: 006410 |
| Mouse / Floxed VGLUT2 | Jackson Labs | JAX: 007583 |
| Mouse / CaMKII-Cre | Jackson Labs | JAX: 005359 |
| Mouse / C57Bl6 | Jackson Labs | JAX: 000664 |
| Oligonucleotides | ||
| B-actin | IDT | mmPT: 39a.2214843.g |
| Chat | IDT | mmPT: 58.31626536 |
| Ctip2 | IDT | mmPT: 58.31107142 |
| Grm1 | IDT | mmPT: 58.8395588 |
| Recombinant DNA | ||
| mGluR1-Myc/DDK | Origene | Cat# MR211136 |
| Software and Algorithms | ||
| Axograph X | Axograph Scientific | RRID SCR_014284 https://axograph.com |
| Prism | GraphPad Software | RRID SCR_002798 https://www.graphpad.com/scientific-software/prism |
| Toronado | Pressler & Strowbridge, 2019 | https://github.com/StrowbridgeLab/Toronado-Laser-Scanning |
Highlight Bullets (85 characters including spaces max).
Partial DA loss alters SNc signaling to ACh interneurons (ChIs) across the striatum
DA inhibition of DMS ChIs is preserved due to reduced dopamine reuptake
Glutamate co-release excitation of DLS ChIs is lost due to downregulation of mGluR1
ChI mGluR1 overexpression improved motor deficits in early stage parkinsonian mice
Acknowledgments:
This work was funded by NIH grants R01-DA35821 (CPF), R01-NS95809 (CPF) and R01-MH116901 (JA). We thank Ben Strowbridge for providing Toronado code for 2-photon imaging, Robert Edwards for providing VGLUT2 floxed mice, the University of Colorado Anschutz Behavioral Core for assistance with behavioral experiments, and the NIDA drug supply program for providing cocaine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: The authors declare no competing financial interests.
References
- Albin RL, Young AB, and Penney JB (1989). The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375. [DOI] [PubMed] [Google Scholar]
- Aosaki T, and Kawaguchi Y (1996). Actions of substance P on rat neostriatal neurons in vitro. J. Neurosci. Off. J. Soc. Neurosci 16, 5141–5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T, Graybiel AM, and Kimura M (1994). Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science 265, 412–415. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Miura M, Suzuki T, Nishimura K, and Masuda M (2010). Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr. Gerontol. Int 10 Suppl 1, S148–157. [DOI] [PubMed] [Google Scholar]
- Aoto J, Martinelli DC, Malenka RC, Tabuchi K, and Südhof TC (2013). Presynaptic Neurexin-3 Alternative Splicing trans-Synaptically Controls Postsynaptic AMPA Receptor Trafficking. Cell 154, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, and Macklis JD (2008). Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J. Neurosci. Off. J. Soc. Neurosci 28, 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau A (1962). The Pathogenesis of Parkinson’s Disease: A New Hypothesis. Can. Med. Assoc. J 87, 802–807. [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, and Wilson CJ (1999). Spontaneous activity of neostriatal cholinergic interneurons in vitro. J. Neurosci 19, 5586–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AP, Sen N, and Bayliss DA (2007). TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons. J. Neurosci. Off. J. Soc. Neurosci 27, 8845–8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boix J, Padel T, and Paul G (2015). A partial lesion model of Parkinson’s disease in mice – Characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behav. Brain Res 284, 196–206. [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, and Worley PF (1997). Homer: a protein that selectively binds metabotropic glutamate receptors. Nature 386, 284–288. [DOI] [PubMed] [Google Scholar]
- Buendia J. de J.A., Tiroshi L, Chiu W-H, and Goldberg JA (2019). Selective remodeling of glutamatergic transmission to striatal cholinergic interneurons after dopamine depletion. Eur. J. Neurosci 49, 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang H-L, Morales M, Lovinger DM, and Cheer JF (2012). Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, and Ford CP (2018). Dopamine Cells Differentially Regulate Striatal Cholinergic Transmission across Regions through Corelease of Dopamine and Glutamate. Cell Rep. 25, 3148–3157.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H-C, Ulane CM, and Burke RE (2010). Clinical Progression in Parkinson’s Disease and the Neurobiology of Axons. Ann. Neurol 67, 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Ma TC, Ding Y, Cheung T, Joshi N, Sulzer D, Mosharov EV, and Kang UJ (2020). Alterations in the intrinsic properties of striatal cholinergic interneurons after dopamine lesion and chronic L-DOPA. ELife 9, e56920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Mingote S, Moore H, and Rayport S (2014). Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron 81, 901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Mingote S, Yetnikoff L, Kalmbach A, Ma T, Ztaou S, Sienna A-C, Tepler S, Poulin J-F, Ansorge M, et al. (2018). Dopamine neuron glutamate cotransmission evokes a delayed excitation in lateral dorsal striatal cholinergic interneurons. ELife 7, e39786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Soloviev MM, Chan W-Y, and McIlhinney RAJ (2000). Homer-1c/Vesl-1L Modulates the Cell Surface Targeting of Metabotropic Glutamate Receptor Type 1α: Evidence for an Anchoring Function. Mol. Cell. Neurosci 15, 36–50. [DOI] [PubMed] [Google Scholar]
- Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, and Trudeau L-E (2004). Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J. Neurochem 88, 1398–1405. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, et al. (2006). Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci 9, 251–259. [DOI] [PubMed] [Google Scholar]
- DeLong MR (1990). Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285. [DOI] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Bosler O, and Doucet G (1996). Dual character, asynaptic and synaptic, of the dopamine innervation in adult rat neostriatum: a quantitative autoradiographic and immunocytochemical analysis. J. Comp. Neurol 375, 167–186. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, and Consolo S (1994). Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 17, 228–233. [DOI] [PubMed] [Google Scholar]
- Ding J, Guzman JN, Tkatch T, Chen S, Goldberg JA, Ebert PJ, Levitt P, Wilson CJ, Hamm HE, and Surmeier DJ (2006). RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat. Neurosci 9, 832–842. [DOI] [PubMed] [Google Scholar]
- Ding J, Peterson JD, and Surmeier DJ (2008). Corticostriatal and Thalamostriatal Synapses Have Distinctive Properties. J. Neurosci 28, 6483–6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, and Surmeier DJ (2010). Thalamic Gating of Corticostriatal Signaling by Cholinergic Interneurons. Neuron 67, 294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig NM, Magill PJ, Apicella P, Bolam JP, and Sharott A (2014). Cortical and thalamic excitation mediate the multiphasic responses of striatal cholinergic interneurons to motivationally salient stimuli. J. Neurosci. Off. J. Soc. Neurosci 34, 3101–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Walker QD, and Wightman RM (1997). Dopamine release and uptake rates both decrease in the partially denervated striatum in proportion to the loss of dopamine terminals. Brain Res. 753, 225–234. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, and Surmeier DJ (2011). Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci 34, 441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, and McFarland NR (2000). Striatonigrostriatal Pathways in Primates Form an Ascending Spiral from the Shell to the Dorsolateral Striatum. J. Neurosci 20, 2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J, and Jahr CE (2003). Receptor occupancy limits synaptic depression at climbing fiber synapses. J. Neurosci. Off. J. Soc. Neurosci 23, 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, and Edwards RH (2010). Vesicular Glutamate Transport Promotes Dopamine Storage and Glutamate Corelease In Vivo. Neuron 65, 643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkwal G, Brami-Cherrier K, Lizardi-Ortiz JE, Nelson AB, Ramos M, Del Barrio D, Sulzer D, Kreitzer AC, and Borrelli E (2016). Parkinsonism Driven by Antipsychotics Originates from Dopaminergic Control of Striatal Cholinergic Interneurons. Neuron 91, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosillo P, Zhang Y-F, Threlfell S, and Cragg SJ (2016). Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cereb. Cortex N. Y. N 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, and Lowell BB (2011). Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest 121, 1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, and Malenka RC (2008). Striatal plasticity and basal ganglia circuit function. Neuron 60, 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar JD, Arango V, Kassir S, Majo V, Prabhakaran J, Simpson N, Bakalian M, Underwood M, and Mann J (2014). Alterations of mGluR1 in Parkinson’s disease and suicide depression: An autoradiography study with [18F]MK1312. J. Nucl. Med 55, 1830–1830. [Google Scholar]
- Kurz A, Double KL, Lastres-Becker I, Tozzi A, Tantucci M, Bockhart V, Bonin M, Garcia-Arencibia M, Nuber S, Schlaudraff F, et al. (2010). A53T-Alpha-Synuclein Overexpression Impairs Dopamine Signaling and Striatal Synaptic Plasticity in Old Mice. PLoS ONE 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M, Dehoff MH, Furuichi T, Worley PF, Hall RA, and Smith Y (2007). Localization and Expression of Group I Metabotropic Glutamate Receptors in the Mouse Striatum, Globus Pallidus, and Subthalamic Nucleus: Regulatory Effects of MPTP Treatment and Constitutive Homer Deletion. J. Neurosci 27, 6249–6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, Crow AK, Malenka RC, Luo L, Tomer R, et al. (2015). Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits. Cell 162, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamaligas AA, and Ford CP (2016). Spontaneous Synaptic Activation of Muscarinic Receptors by Striatal Cholinergic Neuron Firing. Neuron 91, 574–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamaligas AA, Cai Y, and Ford CP (2016). Nicotinic and opioid receptor regulation of striatal dopamine D2-receptor mediated transmission. Sci. Rep 6, 37834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamaligas AA, Barcomb K, and Ford CP (2019). Cholinergic Transmission at Muscarinic Synapses in the Striatum Is Driven Equally by Cortical and Thalamic Inputs. Cell Rep. 28, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcott PF, Mamaligas AA, and Ford CP (2014). Phasic Dopamine Release Drives Rapid Activation of Striatal D2-Receptors. Neuron 84, 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcott PF, Gong S, Donthamsetti P, Grinnell SG, Nelson MN, Newman AH, Birnbaumer L, Martemyanov KA, Javitch JA, and Ford CP (2018). Regional Heterogeneity of D2-Receptor Signaling in the Dorsal Striatum and Nucleus Accumbens. Neuron 98, 575–587.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella G, Platania P, Vita D, Sciamanna G, Cuomo D, Tassone A, Tscherter A, Kitada T, Bonsi P, Shen J, et al. (2009). Enhanced sensitivity to group II mGlu receptor activation at corticostriatal synapses in mice lacking the familial parkinsonism-linked genes PINK1 or Parkin. Exp. Neurol 215, 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N, Minamimoto T, Graybiel AM, and Kimura M (2001). Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J. Neurophysiol 85, 960–976. [DOI] [PubMed] [Google Scholar]
- McKinley JW, Shi Z, Kawikova I, Hur M, Bamford IJ, Sudarsana Devi SP, Vahedipour A, Darvas M, and Bamford NS (2019). Dopamine Deficiency Reduces Striatal Cholinergic Interneuron Function in Models of Parkinson’s Disease. Neuron 103, 1056–1072.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, Buist A, Cik M, Spek P. van der, Kass S, et al. (2006). Vesicular Glutamate Transporter VGLUT2 Expression Levels Control Quantal Size and Neuropathic Pain. J. Neurosci 26, 12055–12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Hammack N, Yang CF, Shah NM, Seal RP, and Kreitzer AC (2014). Striatal Cholinergic Interneurons Drive GABA Release from Dopamine Terminals. Neuron 82, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, and Conn PJ (2010). Metabotropic Glutamate Receptors:Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol 50, 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhotin P, and Bracci E (2007). Cholinergic Interneurons Control the Excitatory Input to the Striatum. J. Neurosci 27, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SE, Song K-I, Kim H, Chung S, and Youn I (2018). Graded 6-OHDA-induced dopamine depletion in the nigrostriatal pathway evokes progressive pathological neuronal activities in the subthalamic nucleus of a hemi-parkinsonian mouse. Behav. Brain Res 344, 42–47. [DOI] [PubMed] [Google Scholar]
- Parker PRL, Lalive AL, and Kreitzer AC (2016). Pathway-Specific Remodeling of Thalamostriatal Synapses in Parkinsonian Mice. Neuron 89, 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese N, Rivero-Bosch M, Lewis SJ, Whone AL, and Brooks DJ (2011). Progression of monoaminergic dysfunction in Parkinson’s disease: A longitudinal 18F-dopa PET study. NeuroImage 56, 1463–1468. [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Rossi S, Bernardi G, and Calabresi P (2004). Therapeutic doses of l-dopa reverse hypersensitivity of corticostriatal D2-dopamine receptors and glutamatergic overactivity in experimental parkinsonism. Brain 127, 1661–1669. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Centonze D, Bernardi G, and Calabresi P (2001). Functional coexpression of excitatory mGluR1 and mGluR5 on striatal cholinergic interneurons. Neuropharmacology 40, 460–463. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J, and Surmeier DJ (2007). Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 30, 545–553. [DOI] [PubMed] [Google Scholar]
- Poulin J-F, Zou J, Drouin-Ouellet J, Kim K-YA, Cicchetti F, and Awatramani RB (2014). Defining Midbrain Dopaminergic Neuron Diversity by Single-Cell Gene Expression Profiling. Cell Rep. 9, 930–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler RT, and Strowbridge BW (2019). Functional Specialization of Interneuron Dendrites: Identification of Action Potential Initiation Zone in Axonless Olfactory Bulb Granule Cells. J. Neurosci 39, 9674–9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Tu JC, Petralia RS, Xiao B, Wenthold RJ, and Worley PF (1999). Homer 1b Regulates the Trafficking of Group I Metabotropic Glutamate Receptors. J. Biol. Chem 274, 25953–25957. [DOI] [PubMed] [Google Scholar]
- Steinkellner T, Zell V, Farino ZJ, Sonders MS, Villeneuve M, Freyberg RJ, Przedborski S, Lu W, Freyberg Z, and Hnasko TS (2018). Role for VGLUT2 in selective vulnerability of midbrain dopamine neurons. J. Clin. Invest 128, 774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Tritsch NX, Hagan NA, Gu C, and Sabatini BL (2014). Multiphasic modulation of cholinergic interneurons by nigrostriatal afferents. J. Neurosci 34, 8557–8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, and Rayport S (1998). Dopamine neurons make glutamatergic synapses in vitro. J. Neurosci. Off. J. Soc. Neurosci 18, 4588–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallaksen-Greene SJ, Kaatz KW, Romano C, and Albin RL (1998). Localization of mGluR1a-like immunoreactivity and mGluR5-like immunoreactivity in identified populations of striatal neurons. Brain Res. 780, 210–217. [DOI] [PubMed] [Google Scholar]
- Tanimura A, Du Y, Kondapalli J, Wokosin DL, and Surmeier DJ (2019). Cholinergic Interneurons Amplify Thalamostriatal Excitation of Striatal Indirect Pathway Neurons in Parkinson’s Disease Models. Neuron 101, 444–458.e6. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, and Cragg SJ (2012). Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, and Dingledine R (2010). Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev 62, 405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Walther D, Mash D, Faucheux B, and Javoy-Agid F (1994). Dopamine transporter messenger RNA in Parkinson’s disease and control substantia nigra neurons. Ann. Neurol 35, 494–498. [DOI] [PubMed] [Google Scholar]
- Villalba RM, and Smith Y (2011). Differential Structural Plasticity of Corticostriatal and Thalamostriatal Axo-Spinous Synapses in MPTP-Treated Parkinsonian Monkeys. J. Comp. Neurol 519, 989–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén-Mackenzie Å, Wootz H, and Englund H (2010). Genetic inactivation of the vesicular glutamate transporter 2 (VGLUT2) in the mouse: What have we learnt about functional glutamatergic neurotransmission? Ups. J. Med. Sci 115, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, Fujinaga M, Kawamura K, Furutsuka K, Nengaki N, Shimoda Y, Shiomi S, Takei M, Hashimoto H, Yui J, et al. (2016). Dynamic Changes in Striatal mGluR1 But Not mGluR5 during Pathological Progression of Parkinson’s Disease in Human Alpha-Synuclein A53T Transgenic Rats: A Multi-PET Imaging Study. J. Neurosci. Off. J. Soc. Neurosci 36, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Petersen CC, and Nicoll RA (2000). Effects of reduced vesicular filling on synaptic transmission in rat hippocampal neurones. J. Physiol 525 Pt 1, 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ztaou S, Maurice N, Camon J, Guiraudie-Capraz G, Kerkerian-Le Goff L, Beurrier C, Liberge M, and Amalric M (2016). Involvement of Striatal Cholinergic Interneurons and M1 and M4 Muscarinic Receptors in Motor Symptoms of Parkinson’s Disease. J. Neurosci. Off. J. Soc. Neurosci 36, 9161–9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and codes generated during this study are available upon request.