Abstract
The Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) consortium brings together researchers from around the world to try to identify the genetic underpinnings of brain structure and function, along with robust, generalizable effects of neurological and psychiatric disorders. The recently-formed ENIGMA Brain Injury working group includes 10 subgroups, based largely on injury mechanism and patient population. This introduction to the special issue summarizes the history, organization, and objectives of ENIGMA Brain Injury, and includes a discussion of strategies, challenges, opportunities and goals common across 6 of the subgroups under the umbrella of ENIGMA Brain Injury. The following articles in this special issue, including 6 articles from different subgroups, will detail the challenges and opportunities specific to each subgroup.
Introduction to ENIGMA
The Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA; enigma.usc.edu) consortium was formed in 2009 in an effort to increase power to detect associations between genetic variation and brain structure and function. ENIGMA has since expanded to examine alterations in brain structure and function across a number of disorders, with or without also including genetic data. The name ENIGMA, which Webster defines as “mysterious, puzzling, or difficult to understand or explain”, also invokes the endeavors of the British team at Bletchley Park to decode highly sophisticated war-time communications during World War II; similarly, ENIGMA brings investigators together to decode the complex and multifaceted factors that influence brain structure and function. At the time of its inception, the focus on candidate genes in imaging genetics led to a crisis of reproducibility, but less biased genome-wide association studies (GWAS) required tens or hundreds of thousands of participants to achieve significance. In 2014, ENIGMA was funded as an NIH Big Data to Knowledge (BD2K) Center of Excellence. ENIGMA has resulted in the largest-ever neuroimaging datasets of numerous disorders to date, including Major Depression, Schizophrenia, and Epilepsy. ENIGMA currently includes 30 disease working groups, 4 groups on healthy variation over the lifespan, and 9 groups focused on methods development. There are currently over 1400 investigators from 40 countries participating in ENIGMA activities (see Figure 1). ENIGMA has received funding through over 20 grants across the United States, the European Union, and Australia. For a recent review of broader ENIGMA activities, see (Thompson et al. 2020).
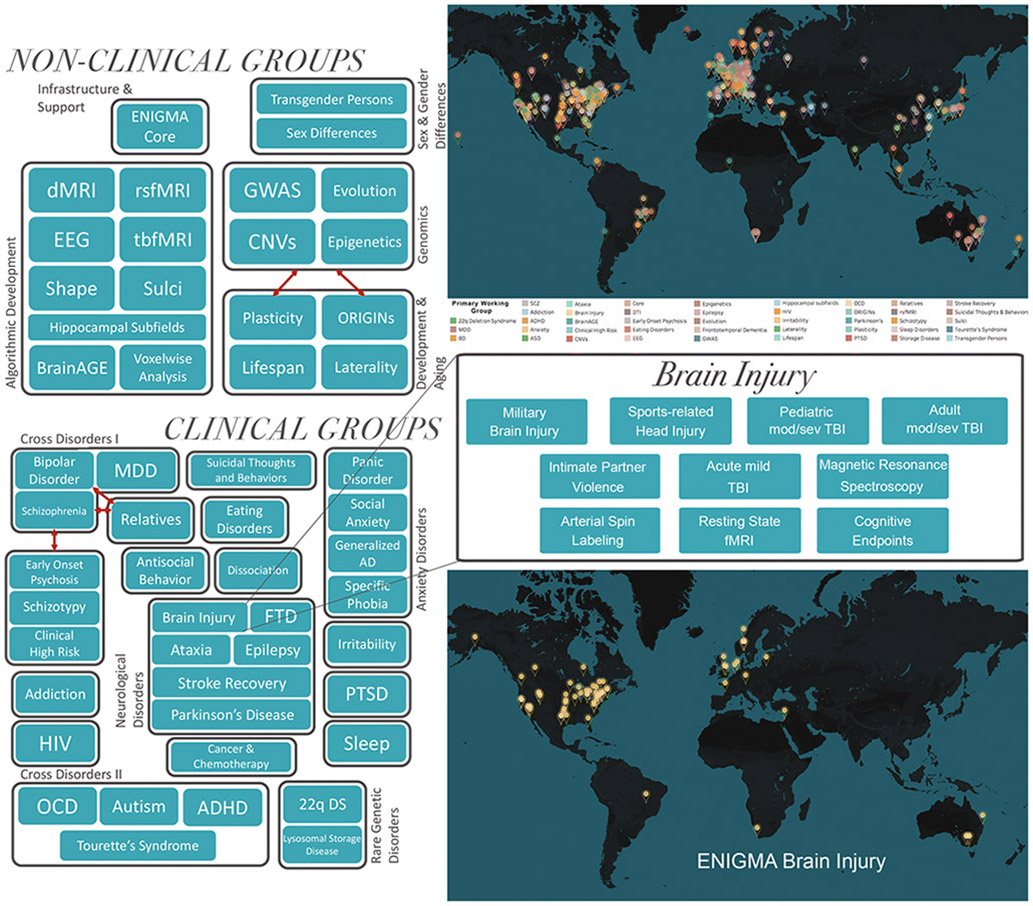
Figure 1.
Organization of the ENIGMA consortium and the ENIGMA Brain Injury working group. Map in top right shows current sites across ENIGMA, map in bottom right shows current sites in the ENIGMA Brain Injury working group. TBI=traumatic brain injury, dMRI=diffusion magnetic resonance spectroscopy, EEG=electroencephalography, rsfMRI=resting state functional MRI, tbfMRI=task based fMRI, GWAS=genome-wide association study, CNV=copy number variation, MDD=major depressive disorder, AD=anxiety disorder, PTSD=post-traumatic stress disorder, FTD=frontotemporal dementia, HIV=human immunodeficiency virus, OCD=obsessive compulsive disorder, ADHD=attention-deficit/hyperactivity disorder, 22q DS=22q11.2 deletion syndrome. Adapted from Thompson et al., 2020.
Formation of ENIGMA Brain Injury
In the fall of 2016, the ENIGMA Brain Injury group was formed. From its inception, it was clear that the complexity of brain injury would necessitate specialized groups that could more readily address unique features of the varying cohorts. Within the first year, multiple subgroups were identified, including Pediatric Moderate/Severe Traumatic Brain Injury (TBI) (msTBI) (Dennis et al. 2020), Military-Relevant TBI (Tate et al. 2020), and Sport-Related Head Injury (Koerte et al. 2020). Soon after, groups for Adult msTBI (Olsen et al. 2020) and Acute Emergency Department (Civilian) Mild TBI were formed, followed by a group focusing on Intimate Partner Violence (Esopenko et al. 2020). The newest groups to be formed are focused on emerging imaging methods that may have particular relevance in TBI, namely Magnetic Resonance Spectroscopy (MRS)(Bartnik-Olson et al. 2020), Arterial Spin Labeling (ASL), resting state fMRI (rsfMRI), and Cognitive Endpoints (see Figure 1). Additional groups will likely be added in the future to address other aspects of methodological and imaging development as well as other TBI-relevant patient populations. The focus of this special issue is on the ENIGMA-Brain Injury working group’s efforts to facilitate research in TBI and concussion.
Goals and Benefits
The overarching goal of the ENIGMA effort is to create a collaborative framework where investigators can work together to address questions and objectives that require large amounts of data and to promote replication of preliminary findings through the use of multiple and independent samples. Collaboration enables investigators to overcome common obstacles which often limit sample sizes in this area of research, including the expense of acquiring neuroimaging data and limited sample sizes. The intent of ENIGMA is to accelerate the pace of investigation through harnessing the enormous intellectual resources and computational power that exists across the globe, not only with regard to a particular disease entity, but also through interfacing with others possessing technical expertise in imaging, genetics, computational science, or with expertise in conditions that may be comorbid (e.g., TBI and PTSD) or may modify disease outcome (e.g., developmental issues). Given many unique clinical and functional features of TBI (i.e., spatial and functional heterogeneity of injury, common comorbidities, etc), the ENIGMA model poses many attractive solutions to addressing important clinical questions.
Immediate goals of the ENIGMA Brain Injury working group are to conduct analyses using multiple datasets to find robust effects of brain injury across samples, using mega-analysis (direct pooling of data points from different sources) when possible to answer questions that require larger samples. Meta-analysis (use of effect sizes from the existing studies or data to obtain an overall effect) can function as replication analyses, as effects that are only present in a minority of cohorts, or small cohorts that are not likely to survive multiple comparisons corrections in the overall analysis. With an increase in statistical power, we can more definitively address major questions in the field, such as the existence and nature of sex-related differences after TBI, how different comparison group impact results (such as contact vs. non-contact controls in sports), how comorbid disorders interact with TBI to affect the brain (such as PTSD or depression), and how differences in injury mechanisms may manifest in the brain. Additionally, large sample sizes allow us to employ machine learning approaches to identify patient subgroups based on demographic, clinical, and imaging variables, potentially with implications for prognostication and tailored treatment. ENIGMA working groups are committed to publishing both positive and negative results, as transparency is critical for advancing science and avoiding the “file-drawer” problem (Duncan et al. 2018). In addition to an increase in statistical power, this collaboration leads to an increase in intellectual power by leveraging the collective expertise of a large network of scientists. Each researcher brings their own perspective, training background, experience, and interests, leading to a rich array of possible projects.
Beyond the immediate goals, ENIGMA Brain Injury is meant to be hypothesis-generating for future studies. Although large meta-analyses have increased power, this approach is not appropriate for all questions, so we consider it to be complementary to more in-depth individual cohort studies. We hope that the findings that result from our efforts raise hypotheses that individual sites can interrogate in more depth within their cohorts. Our results will hopefully serve as preliminary data to support future individual grant submissions by members of ENIGMA Brain Injury. Although the current ENIGMA Brain Injury activities will center largely on retrospective data analysis (with the exception of the Intimate Partner Violence group, Esopenko et al. 2020), this framework will lay the foundation for future collaboration between teams. We hope that the exchange of ideas, methodology, protocols, data, and analytic tools will lead to further attempts to harmonize prospective data collection and will synergize the development of new analytic pipelines and techniques.
Approach
ENIGMA approaches team science in a unique way that differs both conceptually and practically from other consortium efforts. We recognize the hesitation that researchers may feel in joining group science efforts as well as the logistical hurdles that can accompany data sharing, and we make every effort to “meet groups where they are” so that researchers both feel comfortable and invested. First, although there are advantages of an approach that favors centralized data storage and analysis from the standpoint of quality control, this approach has several notable disadvantages, particularly in a global forum. Regulatory mandates may prohibit or limit the transfer or sharing of some forms of health information and data, including neuroimaging and samples with genetic information. Additionally, centralized models may create logistical challenges for the institution where data reside, including issues related to the recurring personnel and infrastructure costs of storing and transferring large amounts of data. Centralized models also often create a situation where some investigators have more access to the data and resources than others, which may limit enthusiasm for contributing data. Centralized models may also lack incentives for sharing since funding is often awarded to a primary site and publication credit may favor investigators at the primary sites. The ENIGMA approach circumvents each of these issues in an innovative approach.
First, to accommodate data sharing issues, contribution of raw data is not required for participation; though processing support (at several levels) is available for groups that request it. Basic requirements for participation are the contribution of raw or summary magnetic resonance imaging (MRI) data (e.g., T1-weighted imaging) and simple clinical and demographic information. Additional imaging sequences, such as diffusion MRI (dMRI), task-based and resting-state fMRI (tbfMRI and rsfMRI), magnetic resonance spectroscopy (MRS), and arterial spin labeling (ASL), and more detailed clinical and cognitive information allow for broader participation, but are not required. Sites will vary in both imaging acquisition parameters, but follow common, validated processing and analytic steps as part of the ENIGMA framework. Processing guidelines and scripts for subcortical volume, cortical measures, and diffusion MRI measures can be found on the ENIGMA website (http://enigma.ini.usc.edu/protocols/), and ENIGMA working groups are engaged in developing and adapting pipelines for additional imaging modalities. There are numerous challenges in combining and harmonizing distinct datasets, discussed in more detail in the Limitations and Challenges section below. Big data analytics is a rapidly advancing field, enabling more sophisticated modeling, but these approaches are suboptimal if the input data are not equivalent across sites.
The ENIGMA platform is intended to be flexible and expandable (see Figure 2). ENIGMA has been successful in other areas of research in part because of the flexibility with data sharing: sharing raw data is never required, but a central site is available to provide computing support or analysis training, if necessary. Investigators may choose to process and analyze their data locally, sending only summary statistics to the lead investigator for an analysis that has been proposed, thus maintaining the maximum amount of control over their data. Sites that have a stronger emphasis on clinical expertise may opt to send raw imaging data to a central site for a given investigation if they do not have the computational or personnel resources to process their own data locally. In all cases, analyses are opt in, meaning that participation in one project does not assume participation in all projects. The result is massively distributed computing, as processing is spread across many sites and investigators with different expertise. All working group members are encouraged to submit secondary proposals to the group for projects they wish to lead. In this respect, ENIGMA is a framework for collaboration, flexible to the interests and restrictions of each participating member. While the initial analyses can be completed on existing datasets, the infrastructure is established for coordinating aspects of study protocols in new projects which have not yet collected data. We encourage the participation of researchers at all levels, and aim to support early career researchers through an expanded network of collaborators and access to larger amounts of data than is commonly available.
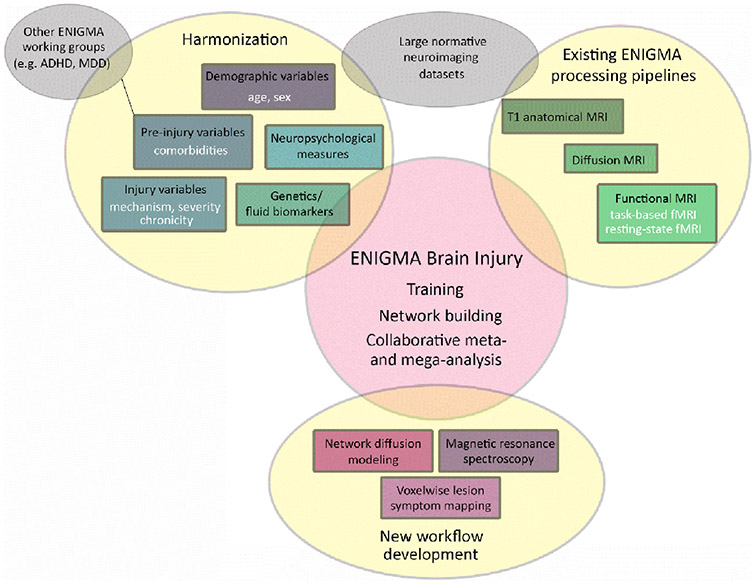
Figure 2.
Schematic showing the approach and goals of the ENIGMA Brain Injury working group.
Of further note, there have been a number of recent consortia efforts in TBI and concussion neuroimaging. Most of these are multi-site studies with varying degrees of harmonization in study protocol. A number have been focused on brain injury in Military Service Members, including the Chronic Effects of Neurotrauma Consortium (CENC, Walker et al. 2016) and the Long-term Impact of Military-relevant brain Injury Consortium (LIMBIC-CENC), the Study of Brain Aging in Vietnam War Veterans (DoD ADNI, Weiner et al. 2014), the Vietnam-Era Twin Study (VETSA, Kremen et al. 2013), and the Injury and Traumatic Stress (INTRuST, Lepage et al. 2018) study. Others have been focused on sports-related head impacts, including the NCAA-DoD Grand Alliance Concussion Assessment, Research, and Education (CARE, Broglio et al. 2017) consortium and the Big Ten-Ivy League Traumatic Brain Injury Research Collaboration (Putukian et al. 2019). Lastly, Translating Research and Clinical Knowledge in TBI (TRACK-TBI, Yue et al. 2013) and Collaborative European NeuroTrauma Effectiveness Research in TBI (CENTER-TBI, Maas et al. 2015) are multi-site studies recruiting from emergency departments (EDs), covering a wide range of injury types and severities. These large studies will significantly advance our understanding of factors that influence outcome after TBI, but the large cost of collecting such large samples limits participation of all interested investigators and requires dedicated funding opportunities. Some ENIGMA working groups are examining ways to work together to converge data collection methods in studies that are just being designed or launched. However, historically, the main differences between these consortia and ENIGMA Brain Injury is the use of prospective vs. existing retrospective data harmonization and the degree of data centralization at a specific site. Each approach has benefits and drawbacks, and we believe there is a place for both in the field of TBI research. Studies that are prospectively harmonized obviously generate data that are more equivalent and simplify the harmonization steps, but they require large amounts of funding, planning, and coordination across sites. While the ENIGMA model requires more effort to produce comparable data, using legacy datasets represents a cost-effective way to gain further insight from completed projects. Harmonization can occur at multiple points during data processing, allowing multiple datasets to be used in a unified approach. With the flexibility in data sharing, the ENIGMA model engages a larger group of researchers – data sharing regulations differ tremendously across sites and across countries and clearly it is not possible to join a prospectively harmonized multi-site study after it has begun.
Successes in Other ENIGMA Groups
Among ENIGMA working groups, the Brain Injury group is relatively young, allowing it to benefit from the experiences of more established working groups. We highlight three working groups here that have some comorbidity with TBI. The Major Depressive Disorder (MDD) was one of the first disease groups to be formed in 2014. To date, they have published a large number of papers across a variety of modalities examining both broad disease effects and more specific symptoms (Frodl et al. 2017; Kelly et al. 2017; Rentería et al. 2017; Schmaal et al. 2016, 2017; Tozzi et al. 2019). Additionally, this group has led the creation of related focus groups, such as the Suicidal Thoughts and Behaviors (STB) working group. While the MDD group was supported by the initial NIH BD2K Center of Excellence grant along with 6 other psychiatric working groups, this initial phase of funding has been completed. One group that has been successful in receiving grant support is the Post-Traumatic Stress Disorder (PTSD) working group. The PTSD working group has considerable overlap in membership with the Military Brain Injury subgroup and has also published papers on subcortical volume (Logue et al. 2017) and white matter microstructure (Dennis et al. 2019). The ENIGMA Addiction working group has similarly received grant support, and recently published a paper of 3,240 individuals examining multiple substances. They found that alcohol abuse was associated with the most substantial alterations in cortical measures (Mackey et al. 2019). Depression, PTSD, and substance use disorders (SUDs) are all potentially comorbid with TBI, as either pre-injury and/or outcome so interfacing with these groups will support important cross-disorder analyses.
Potential
ENIGMA has the potential to address many of the challenges listed above. There is tremendous heterogeneity in TBI and outcome is likely influenced by a large range of demographic and clinical variables. When variability is high, large samples are necessary to detect reliable effects. Through the increased sample size ENIGMA facilitates, there is greater power to detect abnormalities that are consistent across patients, and also to perhaps identify subgroups with distinct clinical prognoses. As discussed in more detail in the following papers of this issue written by the leaders of each subgroup, there are a large number of potentially confounding variables when researching TBI. For example, with regard to the complex intersection of TBI and psychiatric disorders, TBI has been cited as both a risk factor for subsequent development of post-injury psychopathology or developmental disorder (e.g., ADHD), but a history of pre-existing psychopathology may also increase the risk of sustaining a head injury. Therefore, comorbid disorders must be carefully considered, as mentioned above. Moreover, some comorbid disorders are more prevalent in certain subgroups (e.g., ADHD in children, PTSD in Military Service Members and Intimate Partner Violence), while others are generally comorbid with TBI of any population or severity (e.g., MDD). Larger sample sizes made possible by ENIGMA allow consideration of these confounds, and investigators will collaborate with existing ENIGMA working groups dedicated to these potentially comorbid disorders. Through collaboration with these groups, we endeavor to identify neural phenotypes that are distinct and also identify common features that exist across disorders.
A central aim of the ENIGMA Brain Injury group is identifying factors that affect outcome. Some of these may be variables that cannot be modified, such as gender/sex, age, or genetic variability, but could warrant more targeted treatment. Others might be modifiable, and amenable to treatment or intervention. There may be subgroups of individuals within the larger patient group that manifest different patterns of dysfunction. The use of “big data” may thus allow us to more accurately predict future recovery or neurodegeneration. Another central aim of the ENIGMA Brain Injury group is to develop new image processing workflows that are appropriate for brain-injured populations or specifically aimed at characterizing injury-related pathology. With individual lesion maps, we can optimize existing image processing pipelines and directly examine associations between lesion location and functional disruption. We aim to work with others to develop pipelines for automated detection of white matter hyperintensities. Additionally, there are a number of approaches that have been well studied in individual cohorts, including multimodal approaches like connectomics, which we plan to extend for use across multiple cohorts.
Limitations and Challenges
One of the key challenges of multi-site efforts is adequate data harmonization. Large sample sizes will not overcome uncharacterized heterogeneity between datasets, and there is a risk of a “garbage in, garbage out” outcome if appropriate harmonization and quality control steps are not taken. TBI manifests in different forms across severity, acuity, and age at injury, highlighting the importance of defining the patient population. Harmonization crosses multiple domains, including imaging, neuropsychological assessment, clinical outcomes, and blood biomarkers. Combining imaging data is first challenged by different naming conventions and data organization, which can be helped by using BIDS (Brain Imaging Data Structure) standards (Gorgolewski et al. 2016). As ENIGMA mainly works with data that have already been collected, harmonization is completed post hoc as a data processing step. For new data collection, we have the opportunity to harmonize aspects of different of protocols. Of note, for structural imaging, T1-weighted MRI is more straightforward. While protocols do differ across manufacturers, a voxel-size of 1 mm3 is standard, making volume calculations less variable. For diffusion MRI (dMRI), there is considerable variability in angular resolution, diffusion weighting, and voxel size. Even two scanners from the same manufacturer running the same protocols will yield slightly different average diffusivity measures, making it critical that dMRI analyses are meta-analyses, not mega-analyses, unless harmonization like ComBat or similar algorithms are applied (Cetin-Karayumak et al. 2019; Cetin Karayumak et al. 2019; Johnson et al. 2007). One benefit of this variability, however, is that it increases the generalizability of results. We can have more confidence in effects that are detected at both 12 direction dMRI and 128 direction dMRI. Additionally, there are current efforts in the ENIGMA consortium to develop harmonized methods for functional MRI and resting-state fMRI (Adhikari et al. 2018; Adhikari et al. 2018; Veer et al. 2019). To address this challenge, the ENIGMA Brain Injury group will experiment with various harmonization approaches mentioned above, taking advantage of the multi-site projects already included that have more formal harmonization procedures as part of the study protocol. This work will yield further insight into factors that impact the within sequence imaging heterogeneity.
An additional challenge lies in harmonizing cognitive and clinical measures. Although the introduction of International Common Data Elements for TBI has facilitated use of recommended measures within a variety of outcome domains, considerable variability still exists as appropriate measures differ between populations of interest (e.g., athletes vs military), age range (measures developed and normed for young children differ from those used in older children or adults), acuity (symptoms and outcome for acute vary from those in chronic phases of recovery) and severity (outcome domains and measures most relevant for concussion differ from those used in more severe TBI). Although neuropsychological testing or other outcome assessment is common in many studies, the specific assessments used necessarily vary widely. Several options exist for harmonizing these data or developing common comparable cognitive endpoints that could be used to further the research. The most conservative approach involves identification of the most commonly used scales across studies and focus analyses around domains and cohorts where common data was collected. Another approach would be to convert scores within a given outcome domain to standardized T-scores based on population means and standard deviations. This allows for more variability in the specific measures that can be included, but care must be applied in ensuring that the cognitive constructs are indeed consistent. A third approach involves calculating a cognitive composition score created by assigning weighted scores based on the degree to which an individual test score deviates from normative expectations (increased deviation, increased weight). This method is expected to improve sensitivity by creating finer gradations across patients and increase the ceiling for improved detection even in mTBI (Silverberg et al. 2017). Finally, in addition to working together to harmonize existing outcome data and to address novel imaging analytic pipelines, the development, testing, and optimization of innovative cognitive and neurobehavioral outcome measures that are specific to the assessment of mTBI, concussion, and repetitive head hits may be a goal of the working groups, where appropriate.
Though not discussed directly in this special issue, the recently established Cognitive Endpoints group has begun piloting additional statistical methods that are intended to quantify the disparity in cognitive measures administered between cohorts. Once the disparity is known, the goal would be to apply a set of flexible statistical methods designed to minimize the disparity (i.e., item response theory, machine learning) in measures administered to produce a set of co-calibrated and validated measures that can be used in analyses with the harmonized imaging data. Following these steps (see Figure 3), seemingly disparate cognitive data acquired independently across cohorts can move from a low value state with regards to big data approaches toward a more useful high value set of variables that can examined in an aggregated manner. Data curated and processed in this manner will be especially critical when defining the relevant brain-behavior relationships or identifying any unique behavioral phenotypes that exist across or within TBI cohorts. In addition, this promising effort could be applied more broadly to other ENIGMA working data to allow for the exploration of behavioral/functional relationships between imaging findings and these common cognitive endpoints.
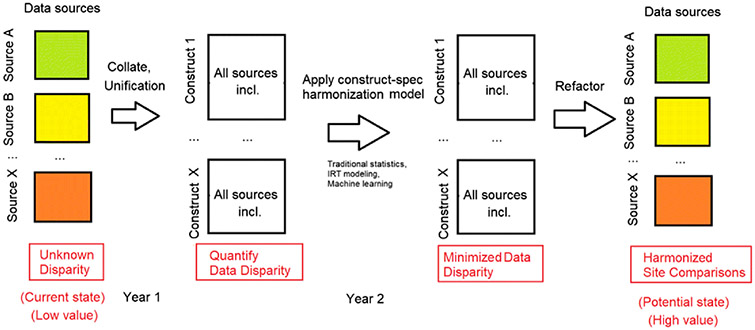
Figure 3.
This illustration shows several steps in producing common cognitive endpoints using data from different sources/cohorts. The first column shows a theoretical set of data from a variety of sources with unknown disparity, for which the value of the data in aggregated form is unknown. When data is collated from these various sources, the disparity can be quantified and constructs emerge. Based on the degree of disparity, different statistical methods for harmonizing the constructs can be applied to minimize disparity. Finally, these constructs can be refactored into data that would allow investigators to perform comparisons across these datasets, thereby improving the value and extending the usability of the data in big data analyses.
The use of fluid biomarkers and genetics also brings challenges. As with imaging, important considerations surround differences in the collection, processing, and analysis of biofluid samples that will necessarily vary by site. An optimal approach includes the use of standardized procedures on the actual samples using a limited number of “batches”, but meta-analyses are also possible with these data (Berger et al. 2012; Manley et al. 2010).
While advances in technology have supported the growing movement towards data sharing and open science, there are important legal, ethical, and regulatory issues with global data sharing (Palk et al. 2019). All of these considerations are aimed at protecting participant privacy and controlling data use. Anonymization is often required, although what is considered “anonymized” differs (Sariyar et al. 2015), and this can include both meta-data in a file header and physical features from MR images (Milchenko and Marcus 2013). While some institutions and countries allow for data sharing to be considered under a “broad consent”, others require explicit statements regarding future potential uses of individual data. The recently enacted General Data Protection Regulation (GDPR) imposed restrictions on sharing personal data within and outside the European Union and requires explicit consent from an individual to share data outside of a few lawful purposes (Voigt and Von dem Bussche 2017). Material Transfer Agreements (MTAs) and Data Use Agreements (DUAs) are required by some institutions, and may be applied more stringently in certain populations (e.g., U.S. Veterans and Active Duty Service Members, children, etc.). A Uniform Biological Material Transfer Agreement (UBMTA) can expedite transfer between participating universities (Carr et al. 2017). Sharing genomic data brings additional concerns, as release of this information could have broad legal, medical, and other consequences for the individual. The NIH mandates that genomic data be submitted to dbGaP (database of Genotypes and Phenotypes) after identifying information is removed (names, dates, locations)(Paltoo et al. 2014). Controlled access to genomic data is then granted to researchers for a specific project. Sharing summary level data within ENIGMA, as opposed to raw imaging data, addresses many of these concerns, although some institutions restrict this level of sharing as well. In these cases, scripts for site-level statistical analysis can be shared with summary statistics returned to the primary site. COINSTAC (Collaborative Informatics and Neuroimaging Suite Toolkit for Anonymous Computation) is web-based framework for executing harmonized processing and analysis across multiple sites that allows the data to stay local, negating the need for explicit sharing consent or MTA/DUAs (Plis et al. 2016). Encouragingly, other consortia have reported that harmonization was more of a challenge than permissions (Budin-Ljøsne et al. 2014). The activities of ENIGMA over the last decade have shown that while there are numerous hurdles to data-sharing and international collaboration, in most cases there are solutions that satisfy the ethical and legal requirements while facilitating group science (Thompson et al. 2020). ENIGMA is active in efforts to enhance understanding of regulations in several regions of the world around data sharing, and to formulate solutions to facilitate global collaboration.
Progress and Deliverables
We will continuously assess our progress to ensure that ENIGMA Brain Injury is moving the field forward and supporting the researchers involved. Our immediate goals are to establish and expand our network of interested researchers along with potential new datasets to include, and identify funding mechanisms to support the effort. Intermediate goals include developing and testing new pipelines for processing neuroimaging data that consider structural deformations, improve classification of neuropathology such as white matter hyperintensities, allow for longitudinal modeling of change after injury, and are stable and useful across sites. Continuous goals include supporting the advancement of junior investigators through the opportunity to propose and lead new analyses and to provide a forum for researchers to discuss controversies and open questions in the field. In the long-term, we hope that this effort will yield hypotheses that researchers can interrogate in greater depth in their individual cohorts and lead to new collaborations among participants as they plan future studies with a goal of improving comparability in a mutually beneficial manner. The deliverables for the ENIGMA Brain Injury group include publications, grants, new pipeline development, establishment of best practices for combining data and for processing TBI neuroimaging data (particularly with regard to lesions), and datasets that have been curated and harmonized. The ENIGMA Brain Injury group has quarterly conference calls along with regular in-person meetings coinciding with relevant conferences to inform collaborators of progress and make plans moving forward. Each subgroup has monthly or bi-monthly conference calls to discuss specific analyses.
Conclusions
The ENIGMA Brain Injury group aims to bring together researchers from around the world with the shared goal of furthering our understanding of the impact of brain injury and factors that may influence outcome. Building off of the framework of the extremely productive broader ENIGMA consortium, we are optimistic that this effort will yield new information and will help answer open questions in the field. We also expect our research to introduce new questions and hypotheses that individual cohorts can investigate in greater detail and will hopefully inspire new data collection. In contrast to other efforts, raw data are not centralized, and contributing sites maintain ownership and control of their data, with all analyses being opt-in. All members are welcome to submit secondary proposals, benefitting from an expanded collaborative network and larger sample size. Researchers interested in joining or learning more about the ENIGMA Brain Injury group are encouraged to read the companion articles in this special issue and contact the authors.
Acknowledgements
We acknowledge funding sources including K99 NS096116 to Dr. Dennis and PT12051 and I01 RX002174 (CENC) to Drs. Wilde, Tate, and Dennis. The authors wish to acknowledge the leadership of Dr. Paul Thompson (ENIGMA PI) as well as the leadership of Drs. Frank Hillary, Alexander Olsen, Inga Koerte, David Baron, Alexander Lin, Brenda Bartnik-Olson, Karen Caeyenberghs, Carrie Esopenko, and Neda Jahanshad, as well as ENIGMA support personnel and all working group members and contributors. We wish to acknowledge the contribution of Eamonn Kennedy in the conceptualization and creation of the figures. We also wish to thank all participants who have contributed to this research. Finally, we extend our gratitude to Drs. Erin D. Bigler and Martha E. Shenton for their thoughtful review of this manuscript and their enduring mentorship and support.
Footnotes
Conflicts of interest
The authors report no potential conflicts of interest related to this project.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Adhikari BM, Jahanshad N, Shukla D, Glahn DC, Blangero J, Reynolds RC, et al. (2018). Heritability estimates on resting state fMRI data using ENIGMA analysis pipeline. Pacific Symposium on Biocomputing. Pacific Symposium on Biocomputing, 23, 307–318. [PMC free article] [PubMed] [Google Scholar]
- Adhikari BM, Jahanshad N, Shukla D, Turner J, Grotegerd D, Dannlowski U, et al. (2018). A resting state fMRI analysis pipeline for pooling inference across diverse cohorts: an ENIGMA rs-fMRI protocol. Brain imaging and behavior. doi: 10.1007/s11682-018-9941-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik-Olson B, Alger J, Babikian T, Harris AD, Holshouser B, Kirov II, et al. (2020). The Clinical Utility of Magnetic Resonance Spectroscopy in Traumatic Brain Injury: Recommendations from the ENIGMA MRS Working Group. Brain imaging and behavior, In Press (Special Issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RP, Beers SR, Papa L, Bell M, & Pediatric TBI CDE Biospecimens and Biomarkers Workgroup. (2012). Common data elements for pediatric traumatic brain injury: recommendations from the biospecimens and biomarkers workgroup. Journal of neurotrauma, 29(4), 672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio SP, McCrea M, McAllister T, Harezlak J, Katz B, Hack D, et al. (2017). A National study on the effects of concussion in collegiate athletes and US military service academy members: the NCAA--DoD concussion assessment, research and education (CARE) consortium structure and methods. Sports medicine, 47(7), 1437–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budin-Ljøsne I, Isaeva J, Knoppers BM, Tassé AM, Shen H-Y, McCarthy MI, et al. (2014). Data sharing in large research consortia: experiences and recommendations from ENGAGE. European journal of human genetics: EJHG, 22(3), 317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr N, Shin I, & Maier S (2017, March 1). Material Transfer and Data Use Agreements. Journal of Clinical Research Best Practices. https://papers.ssrn.com/abstract=3195015. Accessed 11 September 2019 [Google Scholar]
- Cetin Karayumak S, Bouix S, Ning L, James A, Crow T, Shenton M, et al. (2019). Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters. NeuroImage, 184, 180–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin-Karayumak S, Di Biase MA, Chunga N, Reid B, Somes N, Lyall AE, et al. (2019). White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Molecular psychiatry. doi: 10.1038/s41380-019-0509-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Caeyenberghs K, Asarnow RF, Babikian T, Bartnik-Olson B, Bigler ED, et al. (2020). Brain Imaging in Young Brain-Injured Patients: A Coordinated Effort Towards Individualized Predictors from the ENIGMA Pediatric msTBI Group. Brain imaging and behavior, In Press (Special Issue). [Google Scholar]
- Dennis EL, Disner SG, Fani N, Salminen LE, Logue M, Clarke EK, et al. (2019, June 20). Altered White Matter Microstructural Organization in Post-Traumatic Stress Disorder across 3,049 Adults: Results from the PGC-ENIGMA PTSD Consortium. bioRxiv. doi: 10.1101/677153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan D, Barisano G, Cabeen R, Sepehrband F, Garner R, Braimah A, et al. (2018). Analytic Tools for Post-traumatic Epileptogenesis Biomarker Search in Multimodal Dataset of an Animal Model and Human Patients. Frontiers in neuroinformatics, 12, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esopenko C, Meyer J, Wilde EA, Marshall A, Tate DF, Lin A, et al. (2020). Harmonization of Measures to Assess IPV-Related Head Trauma: Recommendations from the ENIGMA IPV Working Group. Brain imaging and behavior, In Press (Special Issue). [Google Scholar]
- Frodl T, Janowitz D, Schmaal L, Tozzi L, Dobrowolny H, Stein DJ, et al. (2017). Childhood adversity impacts on brain subcortical structures relevant to depression. Journal of psychiatric research, 86, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski KJ, Auer T, Calhoun VD, Craddock RC, Das S, Duff EP, et al. (2016). The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Scientific data, 3, 160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, & Rabinovic A (2007). Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics, 8(1), 118–127. [DOI] [PubMed] [Google Scholar]
- Kelly S, van Velzen L, Veltman D, Thompson P, Jahanshad N, Schmaal L, & Group, Group. (2017). 941. White Matter Microstructural Differences in Major Depression: Meta-Analytic Findings from Enigma-MDD DTI. Biological psychiatry, 81(10), S381. [Google Scholar]
- Koerte IK, Dennis EL, Bazarian JJ, Bigler ED, Buckley T, Choe M, et al. (2020). Neuroimaging of Sport-Related Brain Injury: Challenges and Recommendations from the ENIGMA Sports-Related Brain Injury group. Brain imaging and behavior, In Press (Special Issue). [Google Scholar]
- Kremen WS, Franz CE, & Lyons MJ (2013). VETSA: the Vietnam Era Twin Study of Aging. Twin research and human genetics: the official journal of the International Society for Twin Studies, 16(1), 399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage C, de Pierrefeu A, Koerte IK, Coleman MJ, Pasternak O, Grant G, et al. (2018). White matter abnormalities in mild traumatic brain injury with and without post-traumatic stress disorder: a subject-specific diffusion tensor imaging study. Brain imaging and behavior, 12(3), 870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. (2017). Smaller hippocampal volume in posttraumatic stress disorder: a multi-site ENIGMA-PGC study. Biological psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AIR, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, et al. (2015). Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery, 76(1), 67–80. [DOI] [PubMed] [Google Scholar]
- Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, et al. (2019). Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects. The American journal of psychiatry, 176(2), 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GT, Diaz-Arrastia R, Brophy M, Engel D, Goodman C, Gwinn K, et al. (2010). Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Archives of physical medicine and rehabilitation, 91(11), 1667–1672. [DOI] [PubMed] [Google Scholar]
- Milchenko M, & Marcus D (2013). Obscuring surface anatomy in volumetric imaging data. Neuroinformatics, 11(1), 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A, Babikian T, Bigler E, Caeyenberghs K, Conde V, Dams-O’Connor K, et al. (2020). Toward a Global and Open Science for Imaging Brain Trauma: the ENIGMA Adult msTBI Working Group. Brain imaging and behavior, In Press (Special Issue). [Google Scholar]
- Palk A, Illes J, Thompson PM, & Stein DJ (2019). Ethical Issues in Global Imaging Genetics Collaborations. NeuroImage, In Review. [DOI] [PubMed] [Google Scholar]
- Paltoo DN, Rodriguez LL, Feolo M, Gillanders E, Ramos EM, Rutter JL, et al. (2014). Data use under the NIH GWAS data sharing policy and future directions. Nature genetics, 46(9), 934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plis SM, Sarwate AD, Wood D, Dieringer C, Landis D, Reed C, et al. (2016). COINSTAC: A Privacy Enabled Model and Prototype for Leveraging and Processing Decentralized Brain Imaging Data. Frontiers in Neuroscience. doi: 10.3389/fnins.2016.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putukian M, D’Alonzo BA, Campbell-McGovern CS, & Wiebe DJ (2019). The Ivy League-Big Ten Epidemiology of Concussion Study: A Report on Methods and First Findings. The American journal of sports medicine, 47(5), 1236–1247. [DOI] [PubMed] [Google Scholar]
- Rentería ME, Schmaal L, Hibar DP, Couvy-Duchesne B, Strike LT, Mills NT, et al. (2017). Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Translational psychiatry, 7(5), e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariyar M, Schluender I, Smee C, & Suhr S (2015). Sharing and Reuse of Sensitive Data and Samples: Supporting Researchers in Identifying Ethical and Legal Requirements. Biopreservation and biobanking, 13(4), 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular psychiatry, 22(6), 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, van Erp TGM, Sämann PG, Frodl T, Jahanshad N, et al. (2016). Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Molecular psychiatry, 21(6), 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg ND, Crane PK, Dams-O’Connor K, Holdnack J, Ivins BJ, Lange RT, et al. (2017). Developing a Cognition Endpoint for Traumatic Brain Injury Clinical Trials. Journal of neurotrauma, 34(2), 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate DF, Dennis EL, Adams JT, Adamson MM, Belanger HG, Bigler ED, et al. (2020). Coordinating Global Multi-Site Studies of Military TBI: Potential, Challenges, and Harmonization Guidelines from the ENIGMA Military Brain Injury Group. Brain imaging and behavior, In Press (Special Issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P, Jahanshad N, Ching CRK, Salminen L, Thomopoulos SI, Bright J, et al. (2020). ENIGMA and Global Neuroscience: A Decade of Large-Scale Studies of the Brain in Health and Disease across more than 40 Countries. Translational Psychiatry, 10(1), 1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi L, Garczarek L, Janowitz D, Stein DJ, Wittfeld K, Dobrowolny H, et al. (2019). Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychological medicine, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer I, Waller L, Lett T, Erk S, & Walter H (2019). ENIGMA task-based fMRI: A workgroup studying the genetic basis of task-evoked brain activity. Presented at the Organization for Human Brain Mapping. [Google Scholar]
- Voigt P, & Von dem Bussche A (2017). The eu general data protection regulation (gdpr). A Practical Guide, 1st Ed., Cham: Springer International Publishing. https://link.springer.com/content/pdf/10.1007/978-3-319-57959-7.pdf [Google Scholar]
- Walker WC, Carne W, Franke LM, Nolen T, Dikmen SD, Cifu DX, et al. (2016). The Chronic Effects of Neurotrauma Consortium (CENC) multi-centre observational study: Description of study and characteristics of early participants. Brain injury: [BI], 30(12), 1469–1480. [DOI] [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Hayes J, Neylan T, Grafman J, Aisen PS, et al. (2014). Effects of traumatic brain injury and posttraumatic stress disorder on Alzheimer’s disease in veterans, using the Alzheimer's Disease Neuroimaging Initiative. Alzheimer’s & dementia: the journal of the Alzheimer's Association, 10(3 Suppl), S226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue JK, Vassar MJ, Lingsma HF, Cooper SR, Okonkwo DO, Valadka AB, et al. (2013). Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. Journal of neurotrauma, 30(22), 1831–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]