Abstract
In a subgroup of 337 participants (mean age 64±9 years; 45% women) from the Systolic Blood Pressure Intervention Trial (SPRINT), where participants were randomly assigned to intensive treatment (target systolic blood pressure (SBP)<120 mmHg) versus standard treatment (<140 mmHg), we examined the effect of intensive BP lowering on indexes of aortic stiffness. Carotid-femoral pulse-wave velocity (cfPWV), a validated global measure of aortic stiffness, was measured by echo-guided Doppler at baseline and 18-month follow-up visit. Aortic elastance, distensibility, and compliance were measured by cardiac magnetic resonance imaging. During follow-up, the intensive treatment produced a mean between-group reduction in SBP of 12.7 mmHg (95% confidence interval (CI):11.1–14.3 mm Hg). During follow-up, intensive treatment significantly attenuated the increase in cfPWV compared to standard treatment [adjusted follow-up least square mean (LSM) =9.0 m/sec (95% CI: 8.7–9.3) versus 10.0 m/sec (9.6–10.3); p<0.001], an effect that persisted even after adjusting for mean arterial pressure. Intensive treatment also decreased the aortic elastance index [LSM=1.38 mm Hg/mL/m2 (95% CI: 1.34–1.41) vs 1.48 mm Hg/mL/m2 (95% CI: 1.44–1.51), p=0.002] compared to standard treatment. No significant between-group differences were observed for aortic distensibility and compliance. We conclude that intensive treatment significantly attenuated increases in cfPWV and aortic elastance index. Attenuation of increases in aortic stiffness may be one of the mechanisms contributing to the benefit of intensive BP treatment observed in SPRINT.
Keywords: Hypertension treatment, aortic stiffness, pulse wave velocity, aortic distensibility, blood pressure, aging, older adults
Graphical Abstract
Introduction
Aging is associated with reduced effectiveness of the aortic volume reservoir that dampens cardiac pulsation. Loss of elasticity causes increased systolic blood pressure (SBP), reduced diastolic flow and diastolic blood pressure (DBP), and widening of pulse pressure (PP), the cardinal clinical sign of increased aortic stiffness. Increased SBP greatly exacerbates the aging-related increase in aortic stiffness,1 resulting in increased left ventricle (LV) afterload and adverse ventricular-vascular coupling, LV hypertrophy, and impaired coronary artery perfusion.2 Increased aortic stiffness is common in systolic hypertension and may contribute to the development of heart failure (HF), chronic kidney disease (CKD), and cerebrovascular disease, and is an independent predictor of cardiovascular (CV) morbidity and mortality in hypertensive patients.3–6
Carotid-femoral arterial pulse-wave velocity (cfPWV) is a validated, reproducible, noninvasive global measure of aortic stiffness and is a strong, independent predictor of CV events and all-cause mortality.7,8 Assessment of cfPWV has recently been added as a class IIA recommendation to the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) guidelines for the management of hypertension, which recommend screening for large artery stiffening in asymptomatic hypertensive patients in order to detect asymptomatic organ damage as an intermediate stage in the continuum of vascular disease.9 We and others have shown that cardiovascular magnetic resonance imaging (CMRI) local measures of aortic stiffness are reproducible, correlate with adverse outcomes, and are responsive to treatment.4,5,10 For example, decreased aortic distensibility as assessed by CMRI predicted all-cause mortality and incident CV disease (CVD) in the Multi-Ethnic Study of Atherosclerosis (MESA).11
The Systolic Blood Pressure Intervention Trial (SPRINT) showed that intensive BP control to a target SBP<120 mmHg compared to a standard target of SBP< 140 mmHg reduced the risk of CV morbidity and mortality by 25%, total mortality by 27%, and acute decompensated HF by 36%.12,13 While these results have led to revisions of the American Heart Association / American College of Cardiology hypertension management guidelines,14 the mechanistic link between the intensive SBP reduction, vascular function, and reduction in CVD events are not well understood.
We recently showed that intensive treatment did not significantly improve LV hypertrophy, LV function, or myocardial fibrosis compared to standard treatment.15 This suggests that mediators other than these LV measures may contribute to the improvements in CVD events associated with intensive treatment. A recent secondary analysis of SPRINT showed that the intensive treatment was associated with improvements in estimated aortic stiffness at 12 months of follow-up and improved risk prediction for incident CVD events and mortality.16 However, that study only examined the effect of intensive BP treatment on aortic stiffness through statistical predictions of PWV calculated as a function of age and BP, a method known to have significant limitations.17 Besides, that analysis was post hoc with inherent limitations.
SPRINT–HEART is distinct from and significantly extends that prior work. It was a pre-planned, prospective, combined CMRI and echo-Doppler ancillary study to SPRINT designed to assess the effect of intensive BP reduction on robust, well-established measures of vascular function and to overcome the limitations of the prior studies. We measured PWV directly and multiple other independent arterial function measures and did so in a different cohort of the SPRINT population.
Methods
Study Population and Study measurements.
All data will be publicly available at the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC, https://biolincc.nhlbi.nih.gov/home/). Details of SPRINT’s design and primary outcomes have been published previously.12,13,18 SPRINT-HEART was a 337 participant ancillary study within the main SPRINT trial.15 All methods and inclusion and exclusion criteria were identical to SPRINT, except that participants with contraindications to CMRI were excluded.12,18 Participants were recruited from four clinics, and the CMRI exams were performed at Wake Forest School of Medicine.15 The study was approved by the Institutional Review Board, and written informed consent was obtained.
Examinations were performed in the morning after an overnight fast and before taking study medication. CMRI and Doppler-echo ultrasound examinations were performed within 7–10 days of the randomization visit and again at the 18-month follow-up visit. Since titration of BP medications was generally complete within 3–6 months of randomization, this strategy allowed an intervention exposure of at least 12–15 months prior to the follow-up CMRI and Doppler-echo exams.
CMRI local aortic stiffness measures
CMRI scans were performed as previously described 4,5,10 on a 1.5 Tesla Avanto scanner (Siemens Medical Solutions, Erlangen, Germany) with a phased array chest coil. For assessing aortic distensibility, a series of sagittal and axial images of the aorta were obtained in standard and oblique planes. The purpose of obtaining these planes was to ensure a circular aortic lumen throughout the cardiac cycle to minimize partial volume effects during image acquisition. Cardiac cycle-dependent changes in the aortic lumen were assessed according to previously published techniques with interleaved, velocity-encoded, phase-contrast (PC), gradient-echo images acquired perpendicular to the course of the proximal ascending thoracic aorta approximately 4 cm above the aortic valve.4,5 We utilized 8 mm thick slices with 256×256 matrices, a field of view of 28–32 cm (yielding voxel sizes of 0.94×0.94×8 mm), a flip angle of 40 degrees, a repetition time of 11 msec, and an echo-time of 3.5 msec. A non-ferromagnetic brachial BP cuff was applied to record BP during the PC-CMR image acquisition, and systolic and diastolic BP were measured in triplicate and then averaged. 4,5,10 Using custom software, the cross-sectional area of the vessel lumen was planimetered during end-systole and end-diastole.
As previously described, aortic distensibility by CMRI was calculated by the following formula.4,5,10 Aortic distensibility=Area of aorta at end-systole(mm2) - area aorta at end-diastole(mm2) / brachial PP(mmHg) × area aorta at end-diastole(mm2) (Figure 1). Brachial PP was measured noninvasively with a non-ferromagnetic arm BP and recorded at the time of the phase-contrast acquisition.4,5,10 Aortic compliance can be defined as the change in arterial blood volume within an aortic segment caused by a given change in arterial BP. Practically, the arterial blood volume was defined as the aortic cross‐sectional area multiplied by the slice thickness. As the slice thickness was constant for a patient examination, we consider only the aortic cross‐sectional area. We define compliance as the change in the cross‐sectional area during a cardiac cycle corresponding to a given change in arterial BP.4,5,19 Aortic elastance index was measured as the ratio of end-systolic pressure to LV stroke volume index (mmHg/mL/m2), in which end-systolic pressure was estimated by multiplying the brachial arterial systolic BP by 0.85. This metric to assess end-systolic pressure has been shown to correlate well with that obtained invasively (r=0.98, p<0.0001).20,21 Aortic stiffness was also assessed as the ratio of brachial artery PP (PP; measured by a CMR-compatible sphygmomanometer) relative to the LV stroke volume indexed to body surface area (SVi).20,21 PP/SV has been shown to correlate well with other measures of arterial stiffness (r=0.98, p<0.001).22
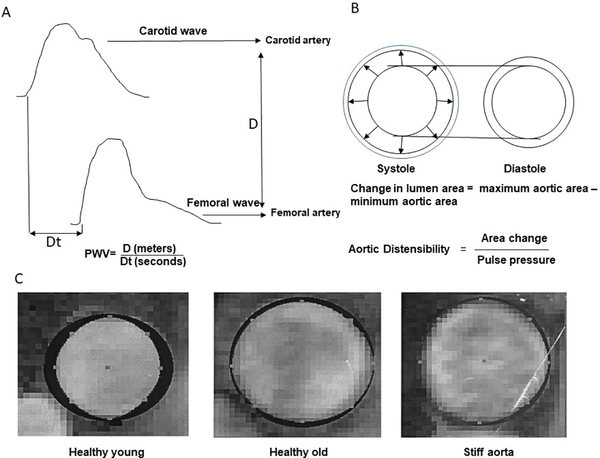
Figure 1.
A explains pulse wave velocity. B shows the aortic distensibility C. Phase-contrast gradient-echo cardiography images of the ascending thoracic aorta from a participant with healthy controls, healthy aging and patients with stiff aorta. The blackened silhouettes on the images of the aorta represent the difference in aortic area between end and end systole. Cardiac cycle-dependent change in aortic area decreased with advancing age and, importantly, were most reduced in older participants with stiff aorta (Part of the image reproduced with permission from reference 4 )
PWV=pulse wave velocity, D=distance, DT=time
Global aortic stiffness measure: Carotid Femoral Pulse wave velocity
As previously described, cfPWV was measured by a two-dimensional echo-guided Doppler.23 Standard longitudinal B-mode images of the left common carotid artery were recorded with the subject in the supine position using a commercially available ultrasound instrument (Sequoia, Accuson, Inc) fitted with a high-frequency (10-MHz) linear probe and using vascular software and optimized settings. The wave Doppler flow was identified simultaneously with ECG. The process was repeated on the left femoral artery in the groin. The measurement of cfPWV is made by dividing the distance (from the carotid point to the femoral point) by the so-called transit time (the time of travel of the foot of the wave over the distance). The foot of the wave is defined at the end of the diastole when the steep rise of the waveform begins. To find the transit time, the time from the R wave of the QRS to the foot of the waveform was measured using digital calipers. PWV was defined as the distance between the carotid and femoral arteries divided by the transit time calculated by Doppler (Figure 1).22 Arm-cuff BPs were recorded in triplicate and averaged. The cfPWV and MRI measures were done on the same day among most of the participants.
Statistical Methods
SPRINT-HEART was designed to detect hypothesized effects of intensive BP control on CV structure and function, the details of which have been previously described.15 Linear mixed models with participant-specific and clinic site-specific random effects were used to compare longitudinal trajectories for systolic BP between the treatment groups. Change in measures of aortic stiffness and other outcome measures were compared between the treatment groups using linear models, including adjustments for the baseline value of each measure, age, and sex. Prespecified subgroups for the overall trial included age (<75 versus. ≥75 years), sex, race/ethnicity (Black versus. non-Black), baseline systolic BP tertiles (≤132, >132 to <145, and ≥145 mmHg), history of CVD, CKD, and orthostatic hypotension at baseline. Interactions between treatment effects and subgroups were assessed with a likelihood ratio test. To account for multiple comparisons, we utilized the step-down procedure of Holm.24
We estimated the incidence of the primary composite CVD outcome using Kaplan-Meier techniques. We used Cox proportional hazards regression to evaluate the association between the change in the cfPWV measure between baseline and the follow-up visit and incident CVD events. For analyses of CVD events, we excluded participants who experienced a CVD event before the 18-month follow-up visit (8 events excluded). All analyses were performed using SAS v9.4 (Cary, NC) and The R Statistical Computing Environment.
Results
Participant characteristics
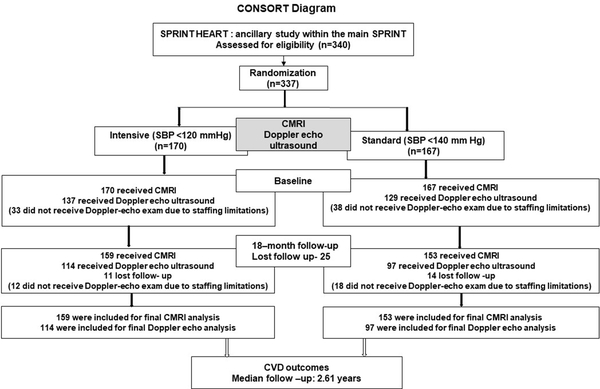
A total of 337 SPRINT participants (mean age=64.3±8.9 years (Standard Deviation, SD), 45% female) completed the baseline CMRI exam, with 170 and 167 randomized to intensive treatment and standard treatment, respectively (Figure 2). The 2D Doppler-echo exam was performed on 266 SPRINT participants at baseline; non-completion of the Doppler-echo exam was due primarily to staffing limitations. There were no significant baseline differences in characteristics between the treatment groups (Table 1).15 There were modest differences in some characteristics compared to the rest of the SPRINT cohort (Supplemental Table S1).15
Figure 2.
CONSORT diagram of SPRINT HEART
SPRINT=Systolic Blood Pressure Intervention Trial, SBP= systolic blood pressure, CMRI= cardiovascular magnetic resonance imaging; CVD=cardiovascular disease
Table 1.
Baseline characteristics of SPRINT- HEART participants by treatment group
| Characteristics | Intensive Treatment | Standard Treatment |
|---|---|---|
| N=170 | N=167 | |
| Age (Years), mean±SD | 64.1±8.3 | 64.5±9.4 |
| 75 years or older, No. (%) | 20(11.8) | 27(16.2) |
| Female sex, No. (%) | 82(48.2) | 68(40.7) |
| Race / Ethnicity, No. (%) | ||
| White | 98(57.6) | 85(50.9) |
| Black | 67(39.4) | 78(46.7) |
| Hispanic | 3(1.8) | 2(1.2) |
| Other | 2(1.2) | 2(1.2) |
| Smoking status, No. (%) | ||
| Current smoker | 31(18.2) | 34(20.4) |
| Former smoker | 59(34.7) | 61(36.5) |
| Never smoker | 80(47.1) | 72(43.1) |
| Body Mass Index (kg/m2), mean±SD | 30.5±5.9 | 30.7±6.1 |
| Systolic Blood Pressure (mmHg), mean±SD | 140.8±16.5 | 141.5±17.0 |
| Diastolic Blood Pressure (mmHg), mean±SD | 81.3±12.6 | 80.5±13.4 |
| Systolic Blood Pressure, No. (%)* | ||
| ≤132 mm Hg | 59(34.7) | 47(28.1) |
| 133 to <145 mm Hg | 47(27.6) | 59(35.3) |
| ≥145 mm Hg | 64(37.6) | 61(36.5) |
| History of CVD, No. (%) | 22(12.9) | 25(15.0) |
| 10-year Framingham CVD risk≥15%, No. (%) | 118(69.4) | 116(69.5) |
| No. of antihypertensive agents, mean±SD | 1.7±1.1 | 1.6±1.2 |
| Not using antihypertensive agents, No. (%) | 25(14.7) | 36(21.6) |
| Use of statins, No. (%) | 59(34.7) | 44(26.3) |
| Use of aspirin, No. (%) | 84(49.4) | 66(39.5) |
| Serum creatinine (mg/dl), median (IQR) | 1.0 [0.8 to 1.2] | 1.0 [0.8 to 1.2] |
| eGFR (ml/min/1.73 m2), mean±SD | 75.5±19.6 | 75.6±19.1 |
| eGFR<60 ml/min/1.73 m2, No. (%) | 35(20.6) | 35(21.0) |
| Urine albumin to creatinine ratio (mg/g), median (IQR) | 8.1[4.9 to 14.8] | 7.8 [5.3 to 15.8] |
| Glucose (mg/dl), mean±SD | 98.7±11.4 | 100.0±12.6 |
| Triglycerides (mg/dl), median (IQR) | 109.0 [81.0 to 147.8] | 104.0 [73.0 to 150.5] |
| Total cholesterol (mg/dl), mean±SD | 192.4±40.0 | 200.0±42.4 |
| HDL cholesterol (mg/dl), mean±SD | 51.9±14.6 | 51.9±14.0 |
| Pulse Wave Velocity(m/sec) | 9.04±0.17 | 9.26±0.16 |
| Aortic Elastance Index(mm Hg/mL/m2) | 1.45±0.02 | 1.47±0.03 |
| Aortic Distensibility(10−3 / mm Hg) | 1.39±0.07 | 1.24±0.05 |
| Aortic Compliance(mm/mmHg) | 1.25±0.06 | 1.09±0.04 |
| Aortic Area Change(mm2) | 61.6±2.42 | 55.6±2.07 |
| Pulse Pressure/Stroke Volume(mmHg/mL) | 0.72±0.02 | 0.72±0.02 |
CVD indicates cardiovascular disease, eGFR estimated glomerular filtration rate, HDL high-density lipoprotein, IQR Interquartile Range, SD Standard Deviation.
Categories reflect tertiles of systolic blood pressure in the overall trial population at baseline.
Achieved BP
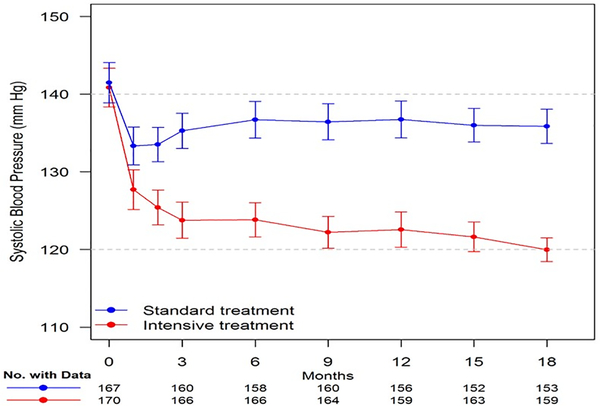
Figure 3 displays SBP by treatment group through the 18-month follow-up study visit. The least-square mean (LSM) SBPs during follow-up were 123.2 mmHg and 135.8 mmHg in the intensive treatment and standard treatment groups. The between-group difference in SBP was 12.7 mmHg (95% confidence interval (CI):11.1–14.3 mm Hg). Most (11.4 mmHg, 90%) of this between-group difference was achieved by the 3 months of follow-up visit, thereby allowing >15 months exposure to observe changes in the aortic stiffness measures. We also compared the SBP and DBP at the time of baseline and 18-month follow-up Doppler and CMRI exams. As shown previously,15, this did not significantly change the separation in BP between the 2 groups.
Figure 3.
Systolic Blood Pressure (SBP) for participants in SPRINT-HEART through the-18 month follow-up visit. Points denote mean SBP, 95% confidence intervals.
Aortic stiffness measures
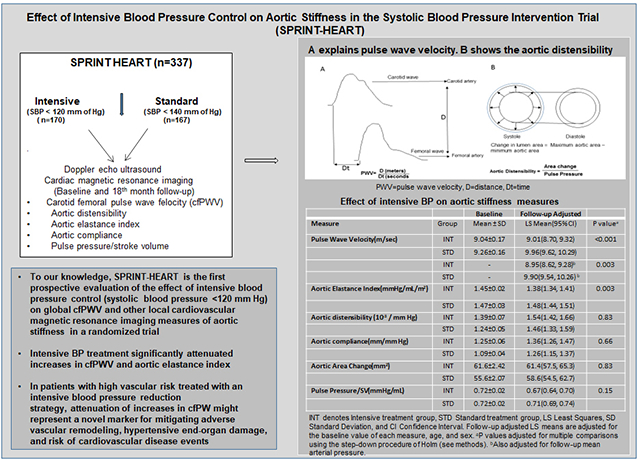
Intensive treatment attenuated the increase in cfPWV observed with standard treatment [adjusted follow-up LSM, 9.0 m/sec, 95%(CI), 8.7 to 9.3 versus 10.0 m/sec, 95% CI, 9.6 to 10.3, for intensive and standard treatment respectively p<0.001] (Table 2). This effect on cfPWV was generally unchanged upon adjusting for mean arterial pressure, [adjusted follow-up LSM = 8.95 (95%CI: 8.62–9.28) versus 9.90 (95% CI: 9.54–10.26) for intensive and standard treatment respectively, p=0.003]. The aortic elastance index was significantly decreased with intensive treatment (adjusted follow-up LSM = 1.38 mm Hg/mL/m2 (95% CI: 1.34–1.41) versus 1.48 (95% CI: 1.44 to 1.51), p=0.002). There were no significant between-group differences for the aortic stiffness index, stroke volume, or the other aortic stiffness measures we examined.
TABLE 2.
Effect of intensive blood pressure control on aortic stiffness measures
| Baseline | Follow-up | Difference | Follow-up Adjusted | |||
|---|---|---|---|---|---|---|
| Measure | Group | Mean ± SD | Mean ± SD | Mean ± SD | LS Mean(95% CI) | p value* |
| Pulse Wave Velocity(m/sec) | INT | 9.04±0.17 | 8.92±0.16 | −0.10±0.17 | 9.01(8.70, 9.32) | <0.001 |
| STD | 9.26±0.16 | 10.01±0.24 | 0.76±0.20 | 9.96(9.62, 10.29) | ||
| INT | - | - | - | 8.95(8.62, 9.28)† | 0.003 | |
| STD | - | - | - | 9.90(9.54, 10.26)† | ||
| Aortic Elastance Index(mmHg/mL/m2) | INT | 1.45±0.02 | 1.37±0.03 | −0.09±0.02 | 1.38(1.34, 1.41) | 0.003 |
| STD | 1.47±0.03 | 1.47±0.03 | 0.00±0.02 | 1.48(1.44, 1.51) | ||
| Aortic distensibility (10−3 / mm Hg) | INT | 1.39±0.07 | 1.57±0.08 | 0.19±0.07 | 1.54(1.42, 1.66) | 0.83 |
| STD | 1.24±0.05 | 1.43±0.07 | 0.17±0.06 | 1.46(1.33, 1.59) | ||
| Aortic compliance(mm/mm Hg) | INT | 1.25±0.06 | 1.41±0.07 | 0.16±0.06 | 1.36(1.26, 1.47) | 0.66 |
| STD | 1.09±0.04 | 1.24±0.06 | 0.14±0.05 | 1.26(1.15, 1.37) | ||
| Aortic Area Change(mm2) | INT | 61.6±2.42 | 62.8±2.52 | 1.73±2.39 | 61.4(57.5, 65.3) | 0.83 |
| STD | 55.6±2.07 | 58.0±2.22 | 1.92±2.27 | 58.6(54.5, 62.7) | ||
| Stroke Volume [SV](mL) | INT | 76.3±1.16 | 75.8±1.19 | 0.9±0.63 | 77.1(75.8, 78.3) | 0.12 |
| STD | 78.2±1.30 | 76.4±1.32 | −1.69±0.69 | 74.9(73.6, 76.2) | ||
| Pulse Pressure (mmHg) | INT | 53.0±1.03 | 48.7±1.07 | −3.95±1.07 | 49.2(47.5, 50.9) | 0.28 |
| STD | 54.1±1.10 | 51.4±1.13 | −2.26±1.1 | 51.6(49.8, 53.3) | ||
| Pulse Pressure/SV(mmHg/mL) | INT | 0.72±0.02 | 0.67±0.02 | −0.06±0.02 | 0.67(0.64, 0.70) | 0.15 |
| STD | 0.72±0.02 | 0.70±0.02 | −0.01±0.02 | 0.71(0.69, 0.74) |
INT denotes Intensive treatment group, STD Standard treatment group, LS Least Squares, SD Standard Deviation, and CI Confidence Interval. Follow-up adjusted LS means are adjusted for the baseline value of each measure, age, and sex.
P values adjusted for multiple comparisons using the step-down procedure of Holm (see methods).
Also adjusted for follow-up mean arterial pressure.
Effects of the intensive BP reduction on aortic stiffness measures among the prespecified subgroups
The effects of the intensive treatment on cfPWV were generally consistent across prespecified subgroups, recognizing such analyses are typically underpowered. The lone exception was a more considerable difference in cfPWV between intensive and standard treatment in participants with a prior history of CVD (Table 3, interaction p = 0.03). There was no other strong evidence of heterogeneity for the other measures of aortic stiffness across the prespecified subgroups. (Supplemental Tables S2–S5).
TABLE 3.
Effect of intensive blood pressure control on pulse wave velocity within subgroups
| No. | Baseline | Follow-up | Difference | Follow-up Adjusted | Interaction | ||
|---|---|---|---|---|---|---|---|
| Subgroup | Group | B/FU | Mean ± SD | Mean ± SD | Mean ± SD | LS Mean(95% CI) | p value* |
| Age | 0.65 | ||||||
| <75 years | INT | 122/103 | 8.82±1.78 | 8.84±1.61 | −0.03±1.66 | 9.00(8.66, 9.33) | |
| STD | 107/80 | 9.06±1.68 | 9.69±2.33 | 0.66±1.96 | 9.75(9.38, 10.12) | ||
| 75 years or older | INT | 15/11 | 10.91±2.96 | 9.70±2.52 | −0.73±2.62 | 8.96(7.94, 9.97) | |
| STD | 22/17 | 10.19±2.29 | 11.52±2.01 | 1.21±1.98 | 10.84(10.02, 11.67) | ||
| Sex | 1.00 | ||||||
| Male | INT | 71/62 | 8.93±1.90 | 8.79±1.74 | −0.13±1.80 | 8.93(8.50, 9.36) | |
| STD | 73/55 | 9.20±1.64 | 9.69±2.21 | 0.48±1.95 | 9.64(9.19, 10.09) | ||
| Female | INT | 66/52 | 9.17±2.18 | 9.07±1.71 | −0.07±1.75 | 9.07(8.60, 9.53) | |
| STD | 56/42 | 9.33±2.08 | 10.43±2.53 | 1.13±1.95 | 10.33(9.81, 10.85) | ||
| Race / Ethnicity | 1.00 | ||||||
| Non-Black | INT | 83/73 | 9.02±1.96 | 8.91±1.59 | −0.12±1.85 | 8.97(8.58, 9.37) | |
| STD | 69/54 | 9.28±1.98 | 9.94±2.38 | 0.71±1.98 | 9.88(9.42, 10.34) | ||
| Black | INT | 54/41 | 9.09±2.16 | 8.93±1.94 | −0.05±1.64 | 9.04(8.5, 9.57) | |
| STD | 60/43 | 9.24± 1.68 | 10.10±2.39 | 0.82±1.97 | 10.01(9.5, 10.53) | ||
| Chronic Kidney Disease | 1.00 | ||||||
| No | INT | 106/89 | 8.89±1.88 | 8.76±1.72 | −0.13±1.80 | 8.91(8.55, 9.27) | |
| STD | 99/74 | 9.19±1.76 | 10.01±2.39 | 0.86±1.99 | 10.00(9.61, 10.39) | ||
| Yes | INT | 31/25 | 9.58±2.44 | 9.49±1.64 | 0.02±1.70 | 9.28(8.61, 9.96) | |
| STD | 30/23 | 9.48±2.09 | 10.01±2.37 | 0.43±1.89 | 9.74(9.04, 10.45) | ||
| History of CVD | 0.03 | ||||||
| No | INT | 120/102 | 8.99±1.90 | 9.01±1.76 | −0.02±1.75 | 9.08(8.75, 9.41) | |
| STD | 109/83 | 9.07±1.67 | 9.70±2.14 | 0.67±1.82 | 9.76(9.40, 10.12) | ||
| Yes | INT | 17/12 | 9.41±2.83 | 8.16±1.17 | −0.78±1.85 | 8.27(7.31, 9.22) | |
| STD | 20/14 | 10.26±2.40 | 11.85±2.90 | 1.30±2.69 | 11.04(10.14, 11.94) | ||
| Systolic Blood Pressure | 1.00 | ||||||
| ≤132 mmHg | INT | 48/39 | 8.87±2.05 | 8.71±1.50 | 0.09±1.52 | 9.04(8.49, 9.59) | |
| STD | 33/25 | 8.99±1.64 | 9.84±1.89 | 0.91±1.59 | 9.96(9.28, 10.64) | ||
| >132 to <145 mmHg | INT | 40/34 | 9.14±2.39 | 9.03±1.98 | −0.04±1.79 | 9.07(8.48, 9.65) | |
| STD | 48/39 | 9.19±2.06 | 10.22±2.61 | 0.92±1.78 | 10.12(9.57, 10.66) | ||
| ≥145 mmHg | INT | 49/41 | 9.14±1.71 | 9.02±1.71 | −0.32±1.98 | 8.90(8.37, 9.42) | |
| STD | 48/33 | 9.51±1.73 | 9.89±2.46 | 0.46±2.40 | 9.71(9.12, 10.30) | ||
| Orthostatic Hypotension | 1.00 | ||||||
| No | INT | 131/108 | 9.02±2.03 | 8.95±1.73 | −0.03±1.70 | 9.05(8.73, 9.38) | |
| STD | 123/93 | 9.27±1.85 | 10.03±2.36 | 0.75±1.98 | 9.94(9.60, 10.29) | ||
| Yes | INT | 6/6 | 9.70±2.24 | 8.31±1.64 | −1.38±2.62 | 7.97(6.60, 9.35) | |
| STD | 6/4 | 9.00±1.67 | 9.52±2.86 | 0.88±1.83 | 9.82(8.13, 11.50) |
INT denotes Intensive treatment group, STD Standard treatment group, LS Least Squares, SD Standard Deviation, CI Confidence Interval, and CVD Cardiovascular Disease. Follow-up adjusted LS means are adjusted for the baseline value of each measure, age, and sex.
P values adjusted for multiple comparisons using the step-down procedure of Holm (see methods).
Cardiovascular Events
Although the sample size of this ancillary study provides very limited power to examine CVD events, the results in SPRINT-HEART were consistent with the overall trial.13 Compared with standard treatment, intensive treatment had a lower incidence of the primary composite CVD endpoint (9 versus 18 events, Hazard Ratio = 0.48; 95% CI, 0.22–1.06) and all-cause mortality (3 versus 8 deaths, Hazard Ratio = 0.32; 95% CI, 0.08–1.22). Adjusting for baseline cfPWV, larger decreases in cfPWV were associated with decreased risk of a CVD event subsequent to the 18-month study visit (HR per 1 SD decrease=0.46; 95% CI: 0.27 to 0.79, p=0.005), although this analysis is only based on 10 CVD events (follow up cfPWV data were available in only 211 participants).
Discussion
In SPRINT-HEART, a prospective, ancillary study to SPRINT, combined CMRI, and Doppler-echo imaging was performed to examine the effects of intensive treatment (target SBP<120 mm Hg) compared with standard treatment (target SBP<140 mmHg) on measures of aortic stiffness. Intensive BP treatment significantly attenuated increases in cfPWV and aortic elastance index. There were no significant between-group differences in other measures of aortic stiffness. Although based on a modest number of events, larger increases in cfPWV at 18 months of follow-up were strongly and significantly associated with increased risk of subsequent CVD events. These data suggest the possibility that improved aortic stiffness may have contributed, in part, to the CV benefit observed in SPRINT from intensive treatment.
Studies utilizing noninvasive methodologies to measure cfPWV have identified the importance of this measure of arterial stiffness as a CV risk factor–often independent of BP.25,26 However, there have been surprisingly few reports from randomized trials that have examined the effect of intensive BP treatment on PWV. In contrast to the present study, prior trials examined BP levels much higher than those tested in SPRINT, primarily compared different antihypertensive drug classes (no control group), and were unable to examine relationships of PWV with events.27–29 However, the mechanistic links between BP lowering, cfPWV attenuation, and improved CV outcomes are not well understood, particularly at lower BP ranges as tested in SPRINT.
To our knowledge, SPRINT-HEART is the first study to evaluate the effect of intensive treatment of SBP on global cfPWV and other local CMRI measures of aortic stiffness in a randomized trial. Our results are consistent with a previous SPRINT analysis, which showed that intensive treatment was associated with improvements in ePWV at 12 months of follow-up. In our data, we found a modest correlation between cfPWV and ePWV (Spearman’s rank correlation = 0.37). This modest correlation likely explains why the analysis of ePWV indicated decreases in PWV on average with intensive treatment, with generally stable levels in participants randomized to standard treatment.16 In contrast, our results suggest that intensive treatment attenuates age and hypertension-related increases in cfPWV associated with higher BP targets over time.
The present study significantly expands upon prior reports in multiple ways. It is unique that it is the only study that has examined the relationship between change in both global cfPWV and local other CMRI measures as assessed by validated methods within the context of a randomized trial focused on intensive BP (<120/80 mmHg) reduction. SPRINT recruited a high vascular risk population with hypertension and existing CVD, CKD, or an elevated estimated CVD risk based on age and other risk factors. Besides, SPRINT had a large proportion of older participants (28% ≥75 years) who have previously been under-represented in most hypertension trials. In a subset of SPRINT participants, baseline cfPWV assessed by the SphygmoCor® CPV device was significantly higher than in prior studies of normotensive persons, further supporting the high-risk nature of the SPRINT population.30 The present study also found that intensive BP reduction had a favorable effect on the aortic elastance index. This highlights the effect of the increased pulsatile load caused by aging or hypertension on the pressure-volume loop and further supports the importance of intensive BP reduction among older adults.31 Taken together, our results show that in this hypertensive population with high CVD risk, intensive BP treatment attenuates age and hypertension-related increases in cfPWV associated with higher BP targets over time.
Vascular stiffening develops from a complex interaction between dynamic changes involving structural and cellular elements of the vessel wall.3,8 BP plays a significant role in determining vessel wall structure, with remodeling compensating for wall stress changes. Arterial stiffening is not merely an adaptive response of blood vessels to distending pressures; instead, when accelerated, arterial stiffening is an underlying pathophysiological cause of the increase in pressure.1,3,8 In addition, oxidative stress, and inflammation aggravate both micro-and macrovascular function, including rarefaction/remodeling and endothelial dysfunction, thus further worsening hypertension, vascular remodeling arterial stiffness.32–34 Noninvasive measurement of cfPWV may assess the overall effects of these complex, dynamic processes on arterial stiffness, changes with effective treatment, and relation to clinical events. Attenuation of cfPWV may reflect the reduction of arterial wall damage and decreased transmission of pulsatile forces towards the microvasculature, thereby protecting capillaries of high flow and low resistance organs brain and kidneys.8
Despite significant attenuation of the increase in cfPWV with intensive BP reduction, there were no significant between-group differences in other measures of aortic stiffness such as aortic distensibility or compliance. This may be because these measures differ fundamentally in several ways. PWV is a global measure of overall aortic stiffness measured between the carotid and femoral arteries and excludes the ascending aorta where aortic distensibility and compliance, which are more local measures, were measured.8 In addition, aortic distensibility and compliance are dependent upon intra-arterial pressure (central aortic pressure) and lumen cross-sectional area (or diameter), in contrast to cPWV, which depends on the speed at which the arterial pulse propagates. In addition, distensibility depends on local BP, which may differ substantially from brachial cuff values due to pulse amplification as it travels from the heart to the periphery.8 Combined hypertension and aging can induce structural changes in the arterial wall as evidenced by an early decline in proximal aortic elasticity, increased smooth muscle stiffness, replacement fibrosis, and calcium deposition, which indeed affect the intrinsic properties of the vessel wall; however, these changes are expressed heterogeneously throughout the arterial system, and this may affect local stiffness measures differently than global measures.8,34,35
Our study has several strengths, including a racially diverse population, a large number of patients >75 years old, random treatment assignment, adjudication of clinical events, use of modern imaging techniques, and centralized, blinded image analyses. To our knowledge, this is the largest study using combined CMRI and Doppler-echo to assess multiple measures of arterial stiffness embedded in a large hypertension clinical trial of intensive SBP reduction and with strong benefit on clinical events. Our study has potential limitations. Due to practical and ethical limitations, we used well-validated, reproducible measures of aortic stiffness rather than invasive measures from catheterization. To estimate aortic PP for calculating aortic distensibility, we used a noninvasive brachial cuff PP measurement instead of a direct assessment of the aortic PP via a catheter. While practical for serial measurements in older adults,36 this methodology does not consider amplification of PP that may occur from the aorta to the brachial artery. For this reason, our calculation of aortic distensibility should be considered an approximation. Till now, all the available noninvasive methods and devices suffer the calibration error in the estimation of central aortic PP. Future studies that measure distensibility could incorporate new noninvasive techniques that more accurately assess aortic PP. Finally, most, if not all, of the arterial stiffness indices displayed some degree of BP dependency on various forms of presser tasks. Thus, in human studies, one cannot be certain about the relative contributions of BP reduction alone versus concomitant beneficial changes in arterial wall biology; however, that does not change the fact that stiffness improved.
Perspectives
Intensive treatment significantly attenuated increases in cfPWV and aortic elastance index, suggesting that improvements in aortic stiffness may be one of the mechanisms contributing to the CV benefits of intensive BP treatment observed in SPRINT. In patients with high vascular risk treated with an intensive BP reduction strategy, attenuation of increases in cfPW might represent a novel marker for mitigating adverse vascular remodeling, hypertensive end-organ damage, and risk of CVD events.
Supplementary Material
Novelty and Significance.
What Is New?
To our knowledge, SPRINT (Systolic Blood Pressure Intervention Trial)-HEART is the first prospective evaluation of the effect of intensive blood pressure control (systolic blood pressure <120 mm Hg) on global carotid femoral pulse wave velocity (cfPWV) and other local cardiovascular magnetic resonance imaging measures of aortic stiffness in a randomized trial.
Intensive BP treatment significantly attenuated increases in cfPWV and aortic elastance index.
Although based on a modest number of events, larger increases in cfPWV at 18 months of follow-up were strongly and significantly associated with increased risk of subsequent cardiovascular disease events.
What Is Relevant?
In patients with high vascular risk treated with an intensive blood pressure reduction strategy, attenuation of increases in cfPW might represent a novel marker for mitigating adverse vascular remodeling, hypertensive end-organ damage, and risk of cardiovascular disease events.
Summary
Intensive treatment significantly attenuated increases in cfPWV and aortic elastance index, suggesting that improvements in aortic stiffness may be one of the mechanisms contributing to the CV benefits of intensive BP treatment observed in SPRINT.
Acknowledgments
Sources of Funding
The SPRINT-HEART Ancillary study was supported by R01HL107257 as well as R01AG18917, R01AG045551, P30-AG21331, and the Kermit G. Phillips II Chair in Cardiovascular Medicine at Wake Forest School of Medicine. SPRINT was by National Institutes of Health (NIH) under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported partly through the Department of Veterans Affairs and contribution of study medications from Takeda Pharmaceuticals International, Inc. For a full of contributors to SPRINT, please see: https://www.sprinttrial.org/public/dspScience.cfm.
Disclosures
Dr. Kitzman: Consultant for Bayer, Corvia Medical, Boehringer-Ingelheim, Astra Zeneca, and Novartis, grant funding from Novartis, Bayer, and Astra Zeneca, and stock ownership in Gilead Sciences.
Dr. Upadhya: research funding from Novarits and Corvia
Footnotes
All other authors: No disclosures to report.
References
- 1.Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension. 2014;64:210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure-flow and pressure-volume relations in humans. Hypertension. 2010;56: 563–570 [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 2003;107:139–46. [DOI] [PubMed] [Google Scholar]
- 4.Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle dependent changes in aortic area and aortic distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001; 38:796–802. [DOI] [PubMed] [Google Scholar]
- 5.Rerkpattanapipat P, Hundley WG, Link KM, Brubaker PH, Hamilton CA, Darty SN, Morgan TM, Kitzman DW. Relation of aortic distensibility determined by magnetic resonance imaging in patients > or =60 years of age to systolic heart failure and exercise capacity. Am J Cardiol. 2002; 90:1221–5. [DOI] [PubMed] [Google Scholar]
- 6.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001; 37:1236–41. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann ED, Parker JR, Hopkins KD, Taylor MG, Gosling RG. Validation and reproducibility of pressure-corrected aortic distensibility measurements using pulse-wave-velocity Doppler ultrasound. J Biomed Eng. 1993; 15:221–8. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006; 27:2588–605. [DOI] [PubMed] [Google Scholar]
- 9.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013; 34:2159–219. [DOI] [PubMed] [Google Scholar]
- 10.Kitzman DW, Brubaker P, Morgan T. Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomised clinical trial. JAMA. 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redheuil A, Wu CO, Kachenoura N, Ohyama Y, Yan RT, Bertoni AG, Hundley GW, Duprez DA, Jacobs DR Jr, Daniels LB, Darwin C, Sibley C, Bluemke DA, Lima JA. Proximal aortic distensibility is an independent predictor of all-cause mortality and incident CV events: the MESA study. J Am Coll Cardiol. 2014; 64:2619–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upadhya B, Rocco M, Lewis CE, Oparil S, Lovato LC, Cushman WC, Bates JT, Bello NA, Aurigemma G, Fine LJ, et al. SPRINT Research Group. Effect of intensive blood pressure treatment on heart failure events in the systolic blood pressure reduction intervention trial. Circ Heart Fail 2017;10:e003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/ NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324 [DOI] [PubMed] [Google Scholar]
- 15.Upadhya B, Rocco MV, Pajewski NM, Morgan T, Blackshear J, Hundley WG, Oparil S, Soliman EZ, Cohen DL, Hamilton CA et al. SPRINT Research Group. Effect of Intensive Blood Pressure Reduction on Left Ventricular Mass, Structure, Function, and Fibrosis in the SPRINT-HEART. Hypertension. 2019. July: HYPERTENSIONAHA11913073.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlachopoulos C, Terentes-Printzios D, Laurent S, Nilsson PM, Protogerou AD, Aznaouridis K, Xaplanteris P, Koutagiar I, Tomiyama H, Yamashina A, Sfikakis PP, Tousoulis D. Association of Estimated Pulse Wave Velocity With Survival: A Secondary Analysis of SPRINT. JAMA Netw Open. 2019;2:e1912831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz JE, Feig PU, Izzo JL Jr. Pulse Wave Velocities Derived From Cuff Ambulatory Pulse Wave Analysis. Hypertension. 2019; 74:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr, Johnson KC, Killeen AA, et al. SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014;11:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Rourke MF, Staessen JA. Clinical applications of arterial stiffness; definitions and references values. Am J Hypertens. 2002; 15:426–44. [DOI] [PubMed] [Google Scholar]
- 20.Vasu S, Morgan TM, Kitzman DW, Bertoni A, Stacey RB, Hamilton C, Chiles C, Thohan V, Hundley WG. Abnormal stress-related measures of arterial stiffness in middle-aged and elderly men and women with impaired fasting glucose at risk for a first episode of symptomatic heart failure. J Am Heart Assoc. 2015; 14; 4:e000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992; 86:513–521. [DOI] [PubMed] [Google Scholar]
- 22.Rerkpattanapipat P, D’Agostino RB Jr, Link KM, Shahar E, Lima JA, Bluemke DA, Sinha S, Herrington DM, Hundley WG. Location of arterial stiffening differs in those with impaired fasting glucose versus diabetes: implications for left ventricular hypertrophy from the Multi-Ethnic Study of Atherosclerosis. Diabetes. 2009; 58:946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upadhya B, Hundley WG, Brubaker PH, Morgan TM, Stewart KP, Kitzman DW. Effect of Spironolactone on Exercise Tolerance and Arterial Function in Older Adults with Heart Failure with Preserved Ejection Fraction. J Am Geriatr Soc. 2017;65:2374–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holm S A simple sequentially rejective multiple test procedure. Scandanavian Journal of Statistics. 1979. Vol 6: 65–70. [Google Scholar]
- 25.Matsuoka O, Otsuka K, Murakami S, Hotta N, Yamanaka G, Kubo Y, Yamanaka T, Shinagawa M, Nunoda S, Nishimura Y, et al. Arterial stiffness independently predicts cardiovascular events in an elderly community -- Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) study. Biomed Pharmacother 2005;59 Suppl 1:S40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A; Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005;111:3384–90. [DOI] [PubMed] [Google Scholar]
- 27.Benetos A, Adamopoulos C, Bureau J-M, Temmar M, Labat C, Bean K, Thomas F, Pannier B, Asmar R, Zureik M, Safar M, Guize L: Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 2002; 105:1202–1207 [DOI] [PubMed] [Google Scholar]
- 28.Ichihara A, Hayashi M, Koura Y, Tada Y, Hirota N, Saruta T. Long-term effects of intensive blood-pressure lowering on arterial wall stiffness in hypertensive patients. Am J Hypertens. 2003:959–65. [DOI] [PubMed] [Google Scholar]
- 29.Asmar R Effect of antihypertensive agents on arterial stiffness as evaluated by pulse wave velocity: clinical implications. Am J Cardiovasc Drugs 2001; 1:387–97 [DOI] [PubMed] [Google Scholar]
- 30.Supiano MA, Lovato L, Ambrosius WT, Bates J, Beddhu S, Drawz P, Dwyer JP, Hamburg NM, Kitzman D, Lash J et al. Pulse wave velocity and central aortic pressure in systolic blood pressure intervention trial participants. PLoS One. 2018;13: e0203305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. SPRINT Research Group. JAMA. 2016; 315:2673–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003; 290:2945–51. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension. 2004; 44:112–6. [DOI] [PubMed] [Google Scholar]
- 34.Redheuil A, Yu WC, Wu CO, Mousseaux E, de Cesare A, Yan R, Kachenoura N, Bluemke D, Lima JA. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010; 55:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007; 50: 1–13. [DOI] [PubMed] [Google Scholar]
- 36.Stratos C, Stefanadis C, Kallikazaros I, Boudoulas H, Toutouzas P. Ascending aorta distensibility abnormalities in hypertensive patients and response to nifedipine administration. Am J Med. 1992; 93:505–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.