Abstract
Background and aims:
Gastrointestinal infections have been linked to changes in the composition and function of gut microbiome and development of inflammatory bowel diseases. We therefore sought to examine the relationship between gastroenteritis and risk of microscopic colitis (MC).
Methods:
We conducted a case-control study of all adult MC patients diagnosed between 1990–2016 in Sweden matched to up to 5 general population controls according to age, sex, calendar year, and county. Cases of MC were identified using SNOMED codes from the ESPRESSO study, a cohort of gastrointestinal pathology reports from all 28 pathology centers in Sweden. We used logistic regression modeling to estimate adjusted odds ratios (aORs) and 95% CIs.
Results:
Through December of 2016, we matched 13,468 MC cases to 64,479 controls. The prevalence of previous diagnosed gastrointestinal infection was 7.5% among MC patients which was significantly higher than in controls (3.0%, Pcomparison <0.001). After adjustment, gastroenteritis was associated with an increased risk of MC (aOR 2.63; 95%CI=2.42–2.85). Among specific pathogens, Clostridioides difficile (aOR 4.39, 95%CI=3.42–5.63), Norovirus (aOR 2.87, 95%CI=1.66–4.87) and Escherichia species (aOR 3.82, 95%CI=1.22–11.58) but not Salmonella species were associated with an increased risk of MC. The association between gastrointestinal infections and risk of MC was stronger for collagenous subtype (aOR 3.23, 95% CI 2.81–3.70) as compared to lymphocytic colitis (aOR = 2.51, 95% CI 2.28–2.76, Pheterogeneity = 0.005). The associations remained significant after adjustment for immune-mediated conditions and polypharmacy and when compared to unaffected siblings.
Conclusion:
In a nationwide study, we found that gastrointestinal infection, particularly Clostridioides difficile is associated with an increased risk of subsequent MC.
Keywords: Pathogens, Epidemiology, Collagenous Colitis, Lymphocytic Colitis
LAY SUMMARY:
Microscopic colitis is a common cause of diarrhea among older adults. In a nationwide study in Sweden, we found that gastrointestinal infections, particularly from Clostridioides difficile may be a risk factor for microscopic colitis.
INTRODUCTION:
Microscopic colitis (MC) is an inflammatory bowel disease (IBD) of the large intestine that primarily affects older adults. Histologically, the disease is further categorized into lymphocytic (dense lymphocytic infiltration, >20 lymphocytes /100 epithelial cells) and collagenous (expansion of collagen fiber, >15 microns with lymphocytic infiltration) colitis subtypes. The pathophysiology of MC is largely unknown but is thought to be related to loss of immune tolerance to environmental-induced perturbances in the aging microbiota1. Genetic associations with the HLA region have been detected for MC in early small candidate studies2, and later larger Immunochip analyses3, 4, which were consistently replicated in independent cohorts and biobanks5, 6. Increased risk of collagenous colitis (CC), but not lymphocytic colitis (LC), 3,4 was observed in relation to an extended haplotype (8.1) that includes several genes coding for MHC molecules with a critical role in immune response to microbial pathogens. This observation supports the hypothesis that enteric infection through its effect on innate immune system and the community structure and function of the gut microbiome may increase risk of MC. This is further supported by previous observations that gastrointestinal infections have been linked to development of classical IBD (reviewed in 7). Additionally, a number of studies have demonstrated new onset MC in patients with recurrent Clostridioides difficile or following fecal microbiota transplantation8–12. Lastly, a recent study from Denmark demonstrated an association between Campylobacter infections and risk of MC13. Despite these compelling lines of evidence, no prior study has systematically examined the association between other bacterial, parasites and viral gastrointestinal infections and risk of MC.
We therefore sought to examine the association between all gastrointestinal infections and risk of MC in a nationwide histopathology database in Sweden.
METHODS:
The study was approved by the Stockholm Ethics Review Board. Informed consent was waived since the study was strictly a register-based analysis14.
Study population:
Ascertainment of MC cases:
We have previously outlined our method for identifying MC cases in Sweden15–17. Briefly, we used ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden) cohort18, a cohort of gastrointestinal histology from all 28 pathology departments in Sweden from January of 1965 until April of 2017. Each pathology report in ESPRESSO includes data on personal identity number, date of biopsy, anatomic location, and morphology according to Systematized Nomenclature of Medicine (SNOMED) system (collagenous colitis: M40600 and lymphocytic colitis: M47170). Supplementary Table 1 includes a list of definitions for all variables used to define study population. For this study, we only included MC cases diagnosed from January 1, 1990 until December 31, 2016. This time window was selected based on our prior study demonstrating a significant increase in incidence starting in early 1990s16. Similar time period has been used in other epidemiologic studies of MC in the US19–21. We defined date of diagnosis of MC as the date of first colorectal biopsy demonstrating MC. In a prior study, when comparing to a clinical diagnosis of MC obtained from review of medical records, our method of identifying cases had a positive predictive value of 95% demonstrating the validity of our outcome definition22.
Selection of population controls:
We matched each MC case to up to 5 general population controls without a prior diagnosis of IBD or MC according to age, sex, calendar year, and county of residence at date of diagnosis. We used the Total Population Register in Sweden to identify controls using personal identity number, a unique number assigned to all residents in Sweden14.
Selection of sibling controls:
We identified siblings through the Multigeneration Register, a component of the Total Population Register that has collected information on family relationships from all registered residents in Sweden born since 1932 and alive on January 1, 196114.
Ascertainment of Exposure Information:
The Swedish Patient Register includes individual-level data on hospital discharges on a nationwide level since 1987 (and countywide since 1964)14. The register was expanded in 2001 to also include specialized outpatient care. Information on enteric infections (bacterial, parasitic, viral and not otherwise defined) prior to study entry (among cases or matched controls) were collected from the register using international classification of disease (ICD)-codes (Supplementary Table 2). The accuracy of ICD coding for ascertainment of diagnoses for the inpatient component of Swedish Patient Register has been previously validated with a positive predictive value of 85–95%23.
Other covariates:
Demographic information on age, sex, death or emigration were collected from the Swedish Total Population Register14. Additionally, we obtained data on education level as a measure of socioeconomic status from the LISA (longitudinal integrated database for health insurance and labor market studies). This database compiles and annually updates administrative information from the labor market and educational and social sectors from 1990 onward on all individuals 16 years or older registered as residents in Sweden24.
We used the Swedish Prescribed Drug Register to obtain data on medication use25. Briefly, this Register was started on July 1, 2005 when information on all dispended medications including date of redemption, amount of drug, and dosage were directly transferred from pharmacy to the registry. Consistent with our prior analyses, we defined active treatment for MC as use of budesonide (ATC code: A07EA06). This was based on prior published randomized controlled trials26–28 and American Gastroenterological Association (AGA) and the European guidelines29, 30 showing that budesonide is effective in treatment of MC and is the recommended first line treatment. In this study, we defined active treatment as any use of budesonide within the first year of diagnosis of MC.
Lastly, information on other medical conditions including type 1 diabetes and thyroid disease were collected from the Swedish Patient Register while data on diagnosis of celiac disease were collected from the ESPRESSO study (Supplementary Table 1). We defined history of immune-mediated conditions as having at least one encounter for celiac disease, type 1 diabetes, psoriasis, systemic lupus, sarcoidosis, rheumatoid arthritis, thyroid disease, primary biliary cirrhosis, autoimmune hepatitis, and primary sclerosing cholangitis (Supplementary Table 3).
Statistical analysis:
We conducted a case-control study of all incident MC patients diagnosed in Sweden from January 1, 1990 until December 1, 2016 matched to up to 5 population controls according to age, sex, calendar year, and county of residence at date of diagnosis. We excluded patients with history of IBD prior to study entry or those with a gastrointestinal infection within 6 months of diagnosis for cases and index date for controls to minimize the bias related to miscoding of gastrointestinal infections in MC cases with delayed diagnosis (Figure 1). We modeled gastrointestinal infections as any infection, bacterial, parasitic, viral and others. We also examined the following specific bacterial and viral infections: Salmonella, Escherichia, Clostridioides difficile, and Norovirus. We used logistic regression modeling to estimate odds ratio and 95%CI while adjusting for age, sex, county, calendar year, education (≤ 9, 10–12, > 12 years), and other immune-mediated conditions. Since MC is a rare outcome, we assumed that odds ratios are good approximations of relative risk estimates. Additionally, we examined the associations according to histologic subtypes of collagenous and lymphocytic colitis and used log likelihood ratio test to compare model fit between models assuming similar effect and models allowing for differential association according to histologic subtypes31.
Figure 1:
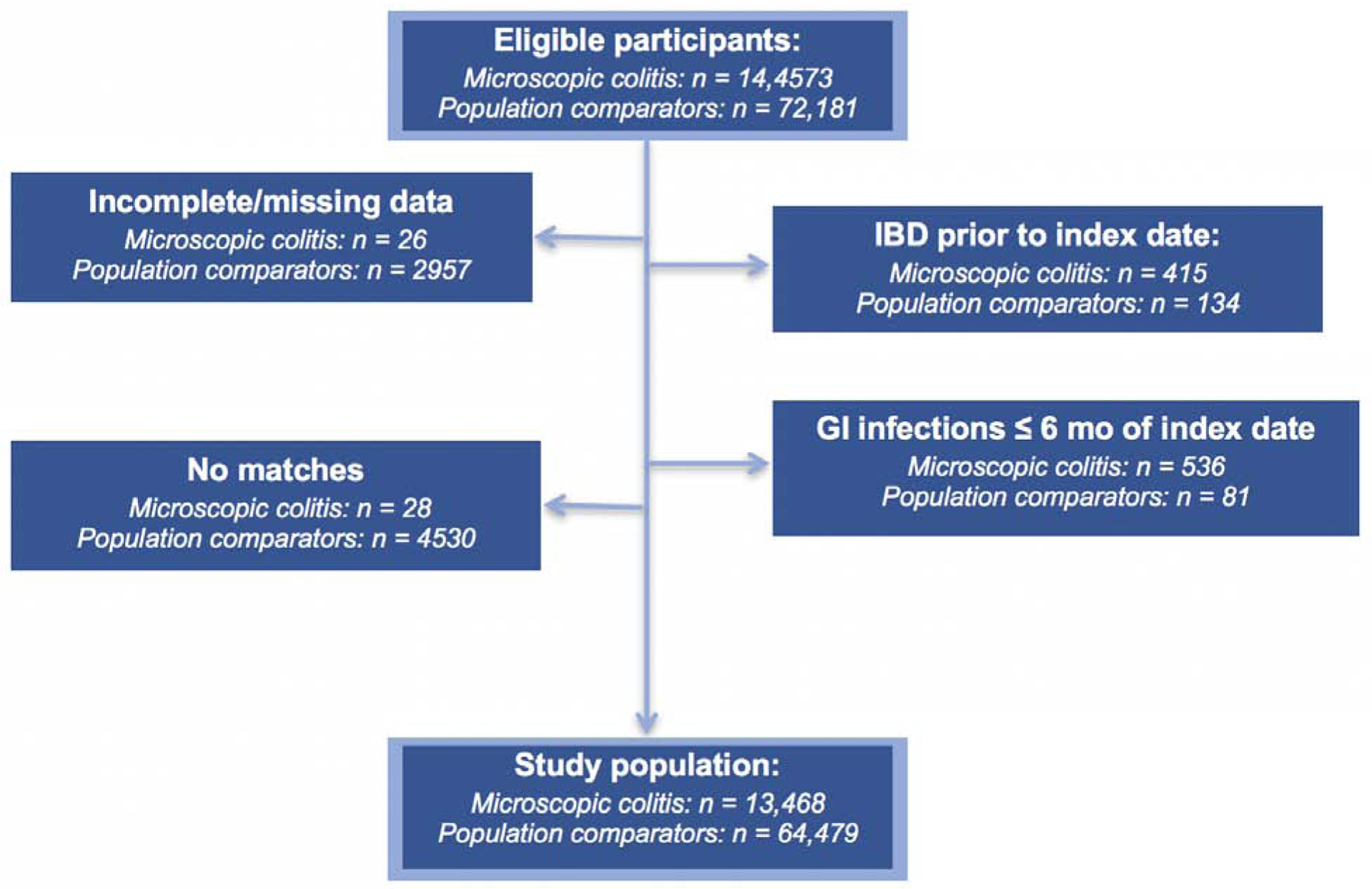
Flow chart of eligible participants in the study
A number of exploratory and sensitivity analyses were done. First, we conducted latency analyses examining the association between gastrointestinal infections and risk of MC according to different exposure time window (0.5 – < 1, 1- < 3, 3-< 5, ≥ 5 years). Second, we additionally adjusted our models for number of encounters (3 years to 6 months prior to index date) and use of medications commonly associated with MC including non-steroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors (PPIs), serotonin reuptake inhibitors (SSRIs), and statins to account for health seeking behaviors among those diagnosed with MC as well as residual confounding related to polypharmacy. Third, we explored the associations according to strata defined by sex, age of diagnosis (< 50 versus ≥ 50 years), calendar period (1990–2006, 2007– 2011, and 2012–2016), and history of an immune-mediated disease. Fourth, we limited our cases of MC to those receiving treatment with budesonide. Lastly, we limited our definition of enteric infections to those that resulted in dispensed antibiotics (ATC codes are listed in Supplementary Table 1) within 2 weeks of diagnosis and examined their associations with risk of MC. All statistical analyses were carried out using R statistical software (version 3.5.2, R Foundation for Statistical Computing, Vienna, Austria). A p-value <0.05 was considered statistically significant.
RESULTS:
We matched 13,468 MC cases (4295 CC and 9173 LC) to 64,479 controls (Table 1). The mean age at MC diagnosis was 60 years (SD=17) with women accounting for more than 70% of participants. Compared to population controls, patients with MC were more likely to have been born in a Nordic country (94% vs 85%, respectively) and have an earlier diagnosis of celiac disease (3.4% vs. 0.1%, respectively) and immune-mediated condition (23.7% vs 11.6%, respectively). There was no difference in socioeconomic status as defined by number of years of education between MC and population controls (Table 1).
Table 1:
Characteristics of cases and controls at index data*
| Population controls N=64,479 |
Microscopic colitis N=13,468 |
Collagenous colitis N=4295 |
Lymphocytic colitis N=9173 |
|
|---|---|---|---|---|
| Sex, female | 46,525 [72.2] | 9697 [72.0] | 3310 [77.1] | 6387 [69.6] |
| Age at start of follow up | ||||
| Mean [SD] years | 59.6 [16.7] | 60.2 [16.8] | 63.13 [15.0] | 58.79 [17.4] |
| Median [IQR] years | 63.0 [50.0–72.0] | 63.0 [51.0–73.0] | 65.0 [55.0–75.0] | 62.0 [49.0–72.0] |
| < 50 years | 15,945 [24.7] | 3230 [24.0] | 750 [17.5] | 2480 [27.0] |
| ≥ 50 years | 48,534 [75.3] | 10,238 [76.0] | 3545 [82.5] | 6693 [73.0] |
| Country of birth | ||||
| Nordic | 57,170 [88.7] | 12,642 [93.9] | 4114 [95.8] | 8528 [93.0] |
| Other | 7309 [11.3] | 826 [6.1] | 181 [4.2] | 645 [7.0] |
| Age at diagnosis | ||||
| Mean [SD] years | N.A. | 60.2 [16.8] | 63.13 [15.0] | 58.79 [17.4] |
| Years of diagnosis | ||||
| 1990 – 2006 | 21,420 [33.2] | 4442 [33.0] | 1444 [33.6] | 2998 [32.7] |
| 2007 – 2011 | 21,645 [33.6] | 4528 [33.6] | 1468 [34.2] | 3060 [33.4] |
| 2012 – 2016 | 21,414 [33.2] | 4498 [33.4] | 1383 [32.2] | 3115 [34.0] |
| History of gastroenteritis | 1920 [3.0] | 1030 [7.6] | 358 [8.3] | 672 [7.3] |
| Time from gastroenteritis to index date | ||||
| Mean [SD] years | 16.2 [12.4] | 14.3 [11.7] | 13.7 [11.9] | 14.6 [11.6] |
| Median [IQR] years | 13.4 [4.7–26.3] | 11.0 [4.0–23.1] | 9.7 [3.5–22.0] | 11.7 [4.4–23.9] |
| 0.5 < 1 years | 79 [0.1] | 56 [0.4] | 22 [0.5] | 34 [0.4] |
| 1 < 3 years | 223 [0.4] | 138 [1.0] | 52 [1.2] | 86 [0.9] |
| 3 < 5 years | 199 [0.3] | 111 [0.8] | 44 [1.0] | 67 [0.7] |
| > = 5 years | 1419 [2.2] | 725 [5.4] | 240 [5.6] | 485 [5.3] |
| Education¶ | ||||
| ≤ 9 years | 17,957 [27.9] | 3526 [26.2] | 1290 [30.0] | 2236 [24.4] |
| 10–12 years | 25,785 [40.0] | 5486 [40.7] | 1771 [41.2] | 3715 [40.5] |
| ≥ 13 years | 18,490 [28.7] | 4080 [30.3] | 1122 [26.1] | 2958 [32.3] |
| Missing | 2247 [3.5] | 376 [2.8] | 112 [2.6] | 264 [2.9] |
| Comorbidities | ||||
| Celiac disease | 45 [0.1] | 462 [3.4] | 156 [3.6] | 306 [3.3] |
| Immune-mediated diseases ∆ | 7,507 [11.6] | 3196 [23.7] | 1223 [28.5] | 1973 [21.5] |
Abbreviations: Not applicable (NA), standard deviation (SD) and interquartile range (IQR).
Categories based on compulsory school, high school, and college.
includes psoriasis, thyroid disease, rheumatoid arthritis, systemic lupus erythematosus, sarcoidosis, vasculitis, autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis, and celiac disease.
The prevalence of previous diagnosed gastrointestinal infection was 7.5% among patients with MC which was significantly higher than in population controls 3.0% (Pcomparison < 0.001). Specifically, compared to population controls, the OR of MC after adjusting for matching factors for any gastrointestinal infection was 2.72 (95%CI=2.51–2.94) (Figure 2). The estimate was similar when additionally adjusting for education and immune-mediated diseases (aOR= 2.63, 95%CI=2.42–2.85). The increased risk was consistent when the analyses were limited to infections from bacteria (aOR=2.85, 95%CI=2.45–3.30), parasites (aOR=2.46, 95%CI=1.32–4.41) and virus (aOR=2.58, 95%CI=1.95–3.38). Among specific pathogens, we saw an increased risk of MC with Clostridioides difficile (aOR=4.39, 95%CI=3.42–5.63), Escherichia species (aOR 3.82, 95%CI=1.22–11.58), and Norovirus (aOR=2.87, 95%CI=1.66–4.87) but not Salmonella. We observed an association with both histologic subtypes of CC and LC, albeit the magnitude of association was stronger with CC subtype (Supplementary Table 5). Specifically, compared to population controls, any gastrointestinal infection was associated with an aOR of 3.12 (95%CI=2.70–3.59) for CC and 2.42 (95%CI=2.19–2.68) for LC. Formal statistical comparison of these estimates did reach significance (Pheterogeneity = 0.005). Similar to our primary analysis, we observed an association between Clostridioides difficile infection and risk of both CC (aOR=4.07, 95%CI=2.70–6.12) and LC (aOR=4.56, 95%CI=3.33–6.25).
Figure 2:
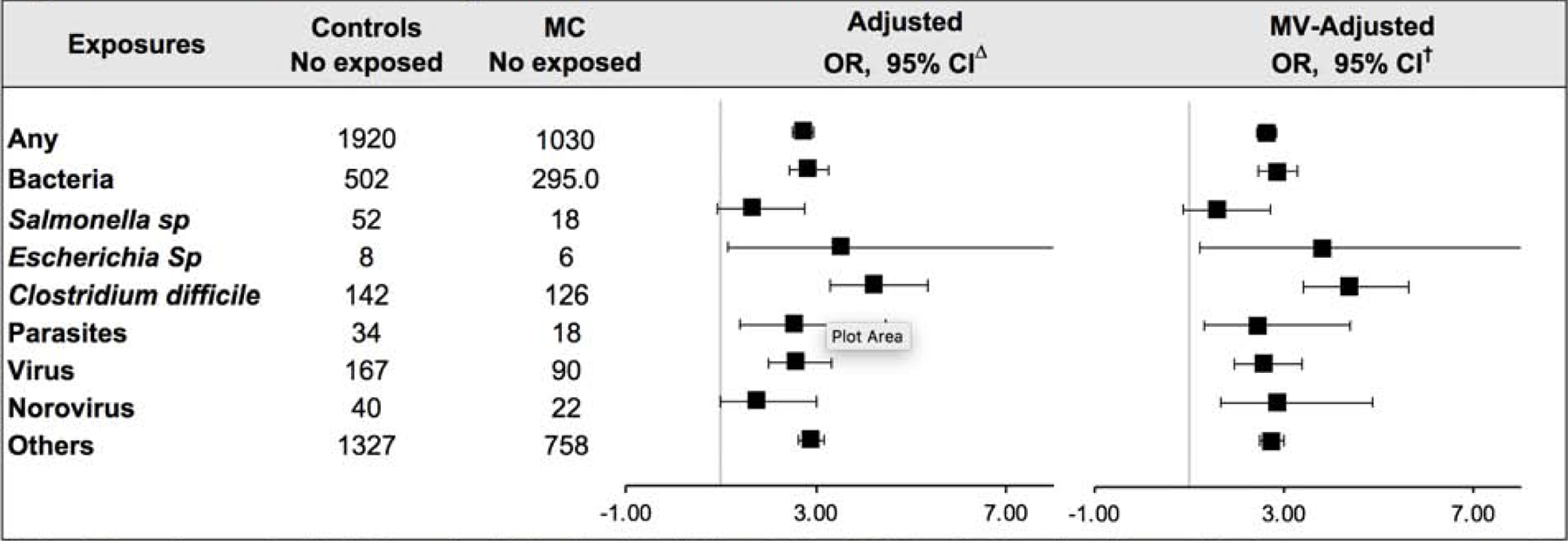
Association between gastrointestinal infection and risk of MC
*Abbreviations: microscopic colitis (MC), number (No.) multivariable (MV), and odds ratio (OR). ∆ Models adjusted for matching factors: age, sex, county, and calendar year. † Models were additionally adjusted for education, and comorbidities according to categories listed in Table 1.
In exploratory analyses, we examined the association between gastrointestinal infection and MC according to different exposure time window. Although, the relative risk of MC appeared to be highest within 6–12 months (aOR=3.42, 95%CI=2.35–4.94), it remained elevated even 3 years after a gastrointestinal infection (aOR=2.63, 95%CI=2.04–3.36). We also explored the association between gastrointestinal infection and MC according to strata defined by sex, calendar year, and concomitant autoimmune disease observed no evidence for effect modification (All Pintereaction > 0.08) (Table 2). We did observe a stronger association between gastrointestinal infection and risk of MC with older age of diagnosis ≥ 50 years (Pinteraction = 0.038). Specifically, among participants with age at index date ≥ 50 years, the aOR of MC was 2.76 (95% CI 2.50– 3.04). The corresponding aOR participants with age index date < 50 years was 2.31 (95% CI 1.98–2.68).
Table 2:
Association between any gastrointestinal infection and risk of MC according to selected strata*
| Strata | Controls Number exposed | MC Number exposed | MV-adjusted OR, 95% CI | Pinteraction |
|---|---|---|---|---|
| Sex | ||||
| Males | 462 | 250 | 2.61 [2.21–3.08] | 0.940 |
| Females | 1458 | 780 | 2.63 [2.39–2.89] | |
| Age at index data | ||||
| < 50 years | 659 | 308 | 2.31 [1.98–2.68] | 0.038 |
| ≥ 50 years | 1261 | 722 | 2.76 [2.50–3.04] | |
| Years since exposed | ||||
| < 1 | 79 | 55 | 3.42 [2.35–4.94] | 0.222 |
| 1<=3 | 223 | 137 | 2.99 [2.38–3.74] | |
| 3<=5 | 199 | 111 | 2.63 [2.04–3.36] | |
| > = 5 | 1419 | 725 | 2.53 [2.30–2.78] | |
| Calendar year | ||||
| 1990 – 2006 | 498 | 285 | 2.80 [2.39–3.27] | 0.086 |
| 2007 – 2011 | 629 | 369 | 2.85 [2.48–3.27] | |
| 2012 – 2016 | 793 | 376 | 2.35 [2.05–2.67] | |
| Calendar year | ||||
| No | 1609 | 722 | 2.53 [2.31–2.78] | 0.599 |
| Yes | 311 | 308 | 2.68 [2.24–3.21] |
Abbreviations: collagenous colitis (CC), confidence interval (CI), lymphocytic colitis (LC), microscopic colitis (MC), number (No.), odds ratio (OR), Models were adjusted for age, sex, calendar year, county, education, and comorbidities according to categories listed in Table 1.
In sensitivity analyses, we considered the possibility that the observed associations may in part be related to differential health seeking behaviors between MC cases and controls and therefore additionally adjusted our models for the number of health-related encounters within the last 3 years of index date and observed similar associations. Specifically, compared to population controls, gastrointestinal infection was associated with aORs of 1.92 (95%CI = 1.76–2.09) for MC, 2.16(95%CI=1.85–2.52) for CC and 1.81(95%CI=1.63–2.01) for LC. We also considered the possibility that differential health seeking behavior (i.e. patients with MC are more likely to have been prescribed medications) may have explained our findings. Therefore, we limited our study population to 2007, one and a half year after introduction of the Swedish Prescribed Register and further adjusted our models for medications known to be associated with risk of MC including NSAIDs, PPI, SSRI, and statins as well as number of health-related encounters within 6 months to 3 years of index date. Compared to population controls, gastrointestinal infection was associated with an aOR of 1.52 (95% CI 1.28 −1.81) for MC. Similar to our primary analysis, Clostridioides difficile infection was particularly associated with an increased risk of MC (aOR=2.15, 95%CI=1.38–3.33). We also considered the possibility that our finding may be related to antibiotic exposure and therefore excluded participants who received antibiotics with diagnosis of gastrointestinal infection and observed an aOR for MC of 1.52 (95%CI=1.26–1.83) with any infection. Similarly, when we limited cases of gastrointestinal infections to those followed by antibiotics therapy, the association was slightly strengthened, aORs = 1.74 (95%CI=1.15–2.61) with any infection.
Lastly, we explored the possibility that shared genetics or early life factors may have accounted for our observed associations and therefore conducted an additional sensitivity analysis examining the relationship between gastrointestinal infections and risk of MC using unaffected siblings as comparators (Table 3, Supplementary Tables 4 and 6). The aORs of MC were 1.79, (95%CI 1.56–2.07) with any infection, 1.75, (95% CI 1.34–2.28) with bacterial infection, 3.04 (95%CI 1.11– 8.29) with parasitic infection, and 1.84 (95%CI 1.12 – 3.03) with viral infection, when compared to their unaffected siblings.
Table 3:
Association between gastrointestinal infection and risk of MC using unaffected sibling as comparators*
| Exposures | Controls Number exposed | MC Number exposed | MV-adjusted OR, 95% CI |
|---|---|---|---|
| Any | 538 | 543 | 1.79 [1.56–2.07] |
| Bacteria | 138 | 141 | 1.75 [1.34–2.28] |
| Salmonella sp | 15 | 9 | 1.24 [0.51–3.00] |
| Escherichia sp | 5 | 2 | 0.59 [0.11–3.25] |
| Clostridioides difficile | 35 | 53 | 2.55 [1.58–4.10] |
| Parasites | 8 | 13 | 3.04 [1.11–8.29] |
| Virus | 43 | 44 | 1.84 [1.12–3.03] |
| Norovirus | 11 | 9 | 2.32 [0.83–6.47] |
| Others | 379 | 400 | 1.88 [1.59–2.23] |
DISCUSSION:
In a nationwide case-control study of over 13,000 patients with MC, we found that gastrointestinal infection was associated with a nearly 3-fold increased risk of MC, and this excess risk persisted beyond 3 years after the infection. The increased risk of MC appeared to be strongest following infection with Clostridioides difficile and with older age of diagnosis and CC histologic subtype. Our primary finding of an increased association between gastrointestinal infection and risk of MC remained consistent across multiple sensitivity and exploratory analyses.
One recent study by Nielson and colleagues from Denmark examined the association between positive stool culture for Campylobacter concisus, Campylobacter jejuni, non-typhoidal Salmonella or negative stool culture and risk of MC13. The study found an association with C. concisus (HR=9.3, 95%CI=4.1 to 20.1) and negative stool culture (HR=5.6, 95%CI=4.6–7.2) but not Salmonella species (HR=1.2, 95%CI=0.2–11.1). These findings are at least in part consistent with our observation that no or negative bacterial culture but not Salmonella species are associated with increased risk of MC. Nevertheless, our study that included nationwide coverage of MC cases and comprehensively assessed the relationship with all gastrointestinal infections (including viral and parasites) significantly expand on these prior findings. Our finding that Clostridioides difficile infection is particularly associated with an increased risk of MC is consistent with prior epidemiologic studies of Clostridioides difficile infection with classical IBD (reviewed in 7). Importantly, we have previously confirmed this relationship using a similar study population and study design in Swedish Patient Register32. Lastly, a number of case series have reported new onset MC following recurrent C.difficile infection or fecal microbiota transplantation8–12.
The pathophysiology of MC remains largely unknown. Emerging evidence suggests that similar to classical IBD, the disease occurs as a result of an aberrant immune response to the luminal microenvironment in a genetically susceptible host. First, genetic association studies have shown that variants within HLA genes are associated with risk of MC2, 4–6, similar to other chronic diseases with aberrant immune response to the gut microenvironment (i.e. celiac disease33 and classical IBD34). Second, human translational studies have demonstrated complete histologic remission with fecal diversion (i.e. creation of an ileostomy bag) in patients with refractory disease, and recurrence of histologic inflammation after re-establishing the fecal stream35–37, underscoring the critical role of gut microbiota in the pathogenesis of MC. Moreover, recent work from our group has demonstrated that compared to patients with functional diarrhea (i.e. chronic diarrhea without underlying inflammation), gut microbiota in active MC is characterized by significant dysbiosis 38. Lastly, medications such as PPIs and NSAIDs previously shown to be associated with risk of MC are known to have significant impact on the composition of the gut microbiota39–43. Therefore, it’s plausible that gastrointestinal infections increase risk of MC broadly through their effect on the gut microbiome composition and function. Additionally, our findings that Clostridioides difficile infection, concomitant antibiotics therapy, and older age are more strongly associated with risk of MC is likely related to the characteristically profound and persistent pro-inflammatory changes in the gut microbiota observed in these subgroups. However, we note that our observed associations were independent of antibiotics use. Although, it’s unclear how microbiome changes can trigger an immune response, genetic association studies have suggested a possible link between genes coding for MHC molecules3,4,5, 6, which are involved in recognition of extracellular pathogens by innate immunity, and risk of MC. This raises the possibility that gastrointestinal infections either directly or through alteration of the gut microenvironment may lead to T-cell activation through MHC molecules. This proposed mechanism is in part supported by our observation of a stronger association between enteric infection and CC, which appears to be the only subtype possibly linked to genes encoding MHC molecules3,4.
Our study has several strengths. This is the first nationwide cohort study that comprehensively examines the association between gastrointestinal infections and risk of MC in cohorts that collected data prospectively in routine medical care. Therefore, our study is less likely to be susceptible to selection and recall biases that are inherent to many observational studies. We identified cases of MC using a validated method demonstrating a positive predictive value of 95%. Lastly, we used multiple comparator groups (i.e. siblings) and definitions (i.e. active treatment) and performed latency analysis to demonstrate that our results were robust and not due to misclassification of exposure related to delayed diagnosis of MC.
We highlight several limitations. We used ICD coding to ascertain information on gastrointestinal infection, which may be susceptible to underreporting or inaccurate representation of the type of infection, introducing exposure misclassification. However, since such misclassification is likely non-differential, it would have likely biased our results toward null. Additionally, although the sensitivity of capturing all gastrointestinal infection in patient register may be only modest, in a recent validation study in Denmark, the positive predictive value was 82% (95%CI 73–88%) for definitive gastroenteritis and 92% (95%CI 85–96%) for definitive or probable gastroenteritis44. We also did not have specific information on the results of stool culture and therefore were limited in examining the associations according to more specific pathogens. However, we note that majority of stool studies in routine clinical practice are negative due to difficulties in culturing pathogens45. Lastly, we did not have information on lifestyle factors that may have confounded our observed associations and could not assess whether the associations were consistent according to other racial and ethnic groups.
In conclusion, in a nationwide case-control study we demonstrate that previous diagnosed gastrointestinal infection is associated with an increased risk of MC. The associations were particularly stronger for Clostridioides difficile infection and older age of diagnosis and CC histologic subtype but appeared to be independent of shared genetics and early life factors. Our findings further highlight the role of perturbances in the gut microenvironment in the pathogenesis of MC. Future investigations on the potential mechanism by which gastrointestinal infection increases risk of MC, particularly in genetically susceptible hosts are warranted.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT:
Gastrointestinal infections have been linked to changes in the composition and function of gut microbiome and development of inflammatory bowel diseases. However, the relationship between gastrointestinal infections and risk of microscopic colitis has not been fully investigated.
NEW FINDINGS:
In a nationwide study, we found that gastrointestinal infection, particularly Clostridioides difficile is associated with an increased risk of microscopic colitis. The associations were stronger with collagenous colitis subtype as compared to lymphocytic colitis.
LIMITATIONS:
Information on gastrointestinal infections were extracted from diagnostic codes and stool culture information was not available. Additionally, the vast majority of participants in the study were white, possibly limiting the generalizability of the findings.
IMPACT:
Understanding the underlying mechanism of these associations may provide significant insights into the pathogenesis of microscopic colitis.
Funding:
This project is funded by the Karolinska Institutet (Ludvigsson) and by Stockholm county council (Ludvigsson). Dr. Khalili is supported by American College of Gastroenterology, a Senior Research Award from Crohn’s and Colitis Foundation and the Beker Foundation. Dr Olén is supported by grants from the Swedish medical society, Karolinska Institutet foundations, and the Strategic Research Area Epidemiology program at Karolinska Institutet while working on this project. Financial support is also provided through the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet (ALF).
None of the funding organizations has had any role in the design and conduct of the study; in the collection, management, and analysis of the data; or in the preparation, review, and approval of the manuscript.
Abbreviations used in this article:
- aOR
Adjusted odds ratio
- CI
confidence interval
- ESPRESSO
Epidemiology Strengthened by histoPathology Reports in Sweden
- HR
hazard ratio
- IBD
inflammatory bowel disease
- ICD
international classification of disease
- MC
microscopic colitis
- SNOMED
Systematized Nomenclature of Medicine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Details of ethics approval: This study was approved by the Regional Ethics Committee, Stockholm, Sweden (Protocol no 2014/1287-31/4).
Conflict of Interest:
Dr Khalili receives grant funding from Takeda and Pfizer. He has received consulting fees from Abbvie and Takeda. Dr Axelrad receives grant funding from BioFire Diagnostics. He has received consulting fees from Janssen and BioFire Diagnostics. Dr Ludvigsson coordinates a study on behalf of the Swedish IBD quality register (SWIBREG). That study has received funding from Janssen corporation. Karolinska Institutet has received investigator-initiated study grants and fees for lectures and consulting performed by Dr Olén for Janssen, Pfizer, Ferring, and Takeda.
Data sharing statement: No additional data available due to Swedish regulation.
REFERENCES:
- 1.Miehlke S, Verhaegh B, Tontini GE, et al. Microscopic colitis: pathophysiology and clinical management. Lancet Gastroenterol Hepatol 2019;4:305–314. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Banares F, Esteve M, Farre C, et al. Predisposing HLA-DQ2 and HLA-DQ8 haplotypes of coeliac disease and associated enteropathy in microscopic colitis. Eur J Gastroenterol Hepatol 2005;17:1333–8. [DOI] [PubMed] [Google Scholar]
- 3.Westerlind H, Bonfiglio F, Mellander MR, et al. HLA Associations Distinguish Collagenous From Lymphocytic Colitis. Am J Gastroenterol 2016;111:1211–3. [DOI] [PubMed] [Google Scholar]
- 4.Westerlind H, Mellander MR, Bresso F, et al. Dense genotyping of immune-related loci identifies HLA variants associated with increased risk of collagenous colitis. Gut 2017;66:421–428. [DOI] [PubMed] [Google Scholar]
- 5.Green HD, Beaumont RN, Thomas A, et al. Genome-wide association study of microscopic colitis in the UK Biobank confirms immune-related pathogenesis. J Crohns Colitis 2019. [DOI] [PMC free article] [PubMed]
- 6.Stahl E, Roda G, Dobbyn A, et al. Collagenous Colitis Is Associated with Hla Signature and Shares Genetic Risks with Other Immune-Mediated Diseases. Gastroenterology 2020. [DOI] [PMC free article] [PubMed]
- 7.Axelrad JE, Cadwell KH, Colombel JF, et al. Systematic review: gastrointestinal infection and incident inflammatory bowel disease. Aliment Pharmacol Ther 2020;51:1222–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agrawal M, Aroniadis OC, Brandt LJ, et al. The Long-term Efficacy and Safety of Fecal Microbiota Transplant for Recurrent, Severe, and Complicated Clostridium difficile Infection in 146 Elderly Individuals. J Clin Gastroenterol 2016;50:403–7. [DOI] [PubMed] [Google Scholar]
- 9.Fasullo MJ, Al-Azzawi Y, Abergel J. Microscopic Colitis After Fecal Microbiota Transplant. ACG Case Rep J 2017;4:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erim T, Alazmi WM, O’Loughlin CJ, et al. Collagenous colitis associated with Clostridium difficile: a cause effect? Dig Dis Sci 2003;48:1374–5. [DOI] [PubMed] [Google Scholar]
- 11.Byrne MF, McVey G, Royston D, et al. Association of Clostridium difficile infection with collagenous colitis. J Clin Gastroenterol 2003;36:285. [DOI] [PubMed] [Google Scholar]
- 12.Khan MA, Brunt EM, Longo WE, et al. Persistent Clostridium difficile colitis: a possible etiology for the development of collagenous colitis. Dig Dis Sci 2000;45:998–1001. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen HL, Dalager-Pedersen M, Nielsen H. High risk of microscopic colitis after Campylobacter concisus infection: population-based cohort study. Gut 2020. [DOI] [PubMed]
- 14.Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125–36. [DOI] [PubMed] [Google Scholar]
- 15.Khalili H, Burke KE, Roelstraete B, et al. Microscopic Colitis and Risk of Inflammatory Bowel Disease in a Nationwide Cohort Study. Gastroenterology 2020;158:1574–1583 e2. [DOI] [PubMed] [Google Scholar]
- 16.Bergman D, Clements MS, Khalili H, et al. A nationwide cohort study of the incidence of microscopic colitis in Sweden. Aliment Pharmacol Ther 2019;49:1395–1400. [DOI] [PubMed] [Google Scholar]
- 17.Khalili H, Bergman D, Roelstraete B, et al. Mortality of Patients With Microscopic Colitis in Sweden. Clin Gastroenterol Hepatol 2019. [DOI] [PubMed]
- 18.Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol 2019;11:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke KE, Ananthakrishnan AN, Lochhead P, et al. Identification of Menopausal and Reproductive Risk Factors for Microscopic Colitis-Results From the Nurses’ Health Study. Gastroenterology 2018;155:1764–1775 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu PH, Burke KE, Ananthakrishnan AN, et al. Obesity and Weight Gain Since Early Adulthood Are Associated With a Lower Risk of Microscopic Colitis. Clin Gastroenterol Hepatol 2018. [DOI] [PMC free article] [PubMed]
- 21.Liu PH, Lebwohl B, Burke KE, et al. Dietary Gluten Intake and Risk of Microscopic Colitis Among US Women without Celiac Disease: A Prospective Cohort Study. Am J Gastroenterol 2019;114:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svensson M, Bergman D, Olen O, et al. Validating microscopic colitis (MC) in Swedish pathology registers. Scand J Gastroenterol 2018;53:1469–1475. [DOI] [PubMed] [Google Scholar]
- 23.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludvigsson JF, Svedberg P, Olen O, et al. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol 2019;34:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16:726–35. [DOI] [PubMed] [Google Scholar]
- 26.Miehlke S, Aust D, Mihaly E, et al. Efficacy and Safety of Budesonide, vs Mesalazine or Placebo, as Induction Therapy for Lymphocytic Colitis. Gastroenterology 2018;155:1795–1804 e3. [DOI] [PubMed] [Google Scholar]
- 27.Miehlke S, Madisch A, Kupcinskas L, et al. Budesonide is more effective than mesalamine or placebo in short-term treatment of collagenous colitis. Gastroenterology 2014;146:1222–30 e1–2. [DOI] [PubMed] [Google Scholar]
- 28.Miehlke S, Madisch A, Karimi D, et al. Budesonide is effective in treating lymphocytic colitis: a randomized double-blind placebo-controlled study. Gastroenterology 2009;136:2092–100. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen GC, Smalley WE, Vege SS, et al. American Gastroenterological Association Institute Guideline on the Medical Management of Microscopic Colitis. Gastroenterology 2016;150:242–6; quiz e17–8. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Banares F, Casanova MJ, Arguedas Y, et al. Current concepts on microscopic colitis: evidence-based statements and recommendations of the Spanish Microscopic Colitis Group. Aliment Pharmacol Ther 2016;43:400–26. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med 2016;35:782–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Axelrad JE, Olen O, Askling J, et al. Gastrointestinal Infection Increases Odds of Inflammatory Bowel Disease in a Nationwide Case-Control Study. Clin Gastroenterol Hepatol 2019;17:1311–1322 e7. [DOI] [PubMed] [Google Scholar]
- 33.van Heel DA, Franke L, Hunt KA, et al. A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat Genet 2007;39:827–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goyette P, Boucher G, Mallon D, et al. High-density mapping of the MHC identifies a shared role for HLA-DRB1*01:03 in inflammatory bowel diseases and heterozygous advantage in ulcerative colitis. Nat Genet 2015;47:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daferera N, Kumawat AK, Hultgren-Hornquist E, et al. Fecal stream diversion and mucosal cytokine levels in collagenous colitis: A case report. World J Gastroenterol 2015;21:6065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veress B, Lofberg R, Bergman L. Microscopic colitis syndrome. Gut 1995;36:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarnerot G, Bohr J, Tysk C, et al. Faecal stream diversion in patients with collagenous colitis. Gut 1996;38:154–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan DM, Cao Y, Miller K, et al. Microscopic Colitis is Characterized by Intestinal Dysbiosis. Clin Gastroenterol Hepatol 2019. [DOI] [PubMed]
- 39.Imhann F, Vich Vila A, Bonder MJ, et al. The influence of proton pump inhibitors and other commonly used medication on the gut microbiota. Gut Microbes 2017;8:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016;352:565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freedberg DE, Toussaint NC, Chen SP, et al. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology 2015;149:883–5 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masclee GM, Coloma PM, Kuipers EJ, et al. Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal anti-inflammatory drugs. Am J Gastroenterol 2015;110:749–59. [DOI] [PubMed] [Google Scholar]
- 44.Skyum F, Andersen V, Chen M, et al. Infectious gastroenteritis and the need for strict contact precaution procedures in adults presenting to the emergency department: a Danish register-based study. J Hosp Infect 2018;98:391–397. [DOI] [PubMed] [Google Scholar]
- 45.Axelrad JE, Freedberg DE, Whittier S, et al. Impact of Gastrointestinal Panel Implementation on Health Care Utilization and Outcomes. J Clin Microbiol 2019;57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


