Abstract
The steady rise in opioid users and abusers has uncovered multiple detrimental health consequences of perturbed opioid receptor signaling, thereby creating the need to better understand the biology of these systems. Among endogenous opioid networks, μ-receptors have received special attention due to their unprecedented biological complexity and broad implications in homeostatic functions. Here, we review the origin, molecular biology and physiology of endogenous opioids with a special focus on μ-opioid receptor networks within the endocrine system. Moreover, we summarize the current evidence supporting an involvement of the latter in regulating distinct endocrine functions. Finally, we combine these insights to present an integrated perspective on μ-opioid receptor biology and provide an outlook on future studies and unresolved questions in this field.
Keywords: opioids, oprm1, μ-receptors, beta endorphin, hormones, evolution, GPCR
1. Medical use of opioids: an unusual story
The terms “opiates” and “opioids” are often used synonymously but in fact refer to distinct molecular classes: the former comprises natural occurring alkaloids derived from Papaver somniferum (see Glossary) such as morphine, codeine or thebaine, whereas the latter reflects an umbrella term further including peptidergic, synthetic as well as semisynthetic opioid receptor ligands (Figure 1) [1]. In contrast to many other drugs used in modern medicine, the pharmacological implementation of opiates did not arise as a consequence from elucidating the physiology of the underlying system. Instead, opiates have been used for thousands of years, while the molecular basis for their action was only discovered in the late 20th century. Simultaneously, the ongoing opioid crisis (Box 1) has raised awareness for the multiple detrimental health consequences related to perturbed opioid signaling, thereby creating an urgent need to better understand both the physiology as well as pathology of these systems. Among unfavorable opioid-induced side effects, the endocrine system is particularly severely affected by these substances [2], suggesting that opioid receptors hold critical functions in endocrine homeostasis per se. While the link between a biological system best-known for its involvement in nociception and endocrine hormones may not be intuitive, we herein relay evolutionary principles such as life history theory to opioid biology, thereby providing a possible explanation for the broad involvement of these networks in whole-body homeostasis. This theoretical framework may aid in better understanding the trade-offs arising from opioid receptor activation as well as generating novel perspectives on diseases that could be amenable to manipulation of this system.
Figure 1: Chemical structure of opioids.
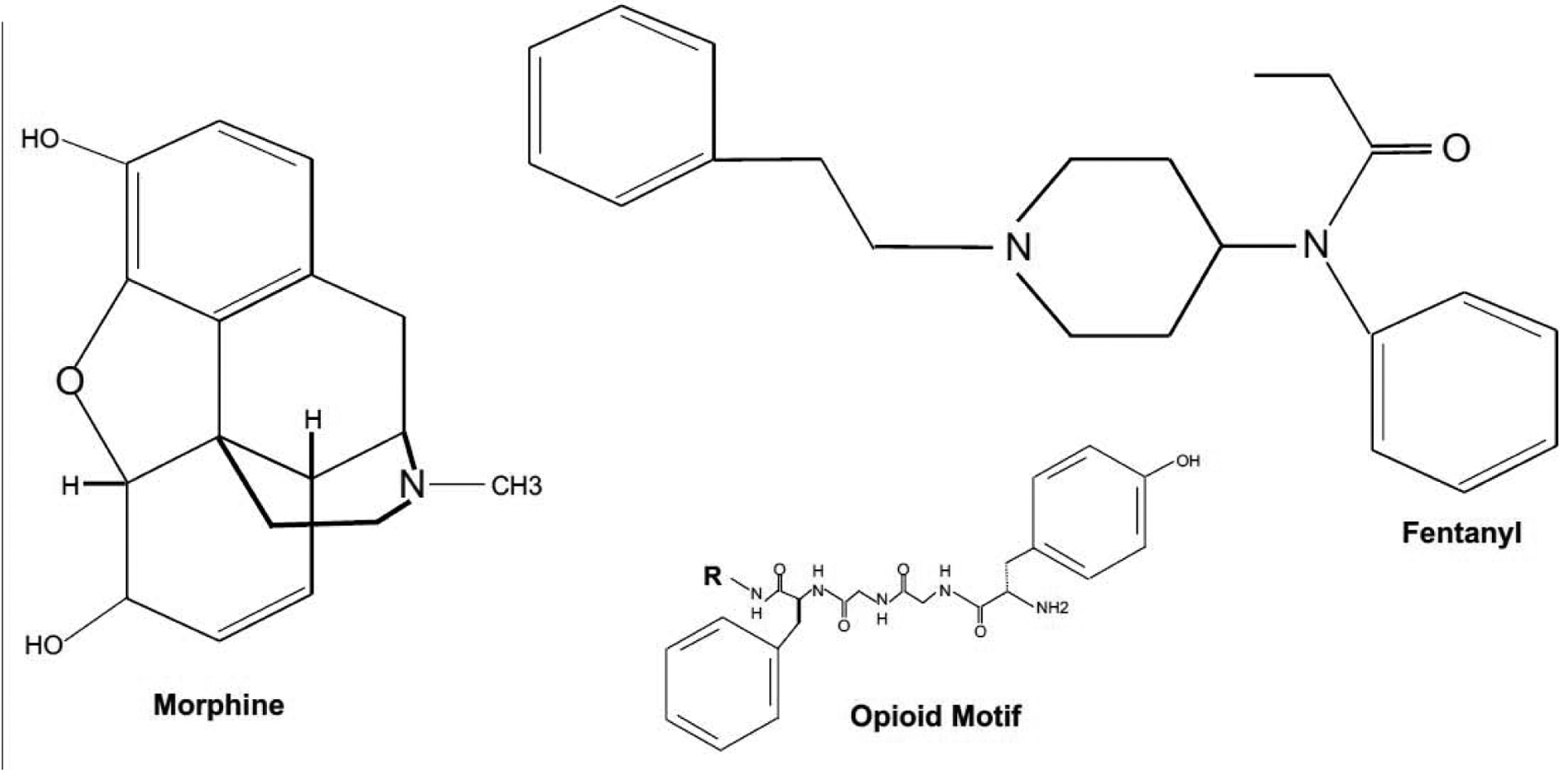
Alkaloids derived from Papaver Somniferum including morphine or codeine share a common carbon-scaffold and preferentially bind to μ-opioid receptors. Semi-synthetic opioids such as Fentanyl bind and activate these receptors even more potently. Endogenous opioids all share a core amino acid pentasequence coined the “opioid motif” consisting of Tyr-Gly-Gly-Phe-Met or Leu. Enkephalins are exclusively built from these 5 amino acids, whereas all other opioids peptides exhibit various additional residues. The core opioid tetrasequence with “R” indicating the respective residue (Met/Leu-XXX) is shown above.
Box 1: Trajectories of the opioid crisis.
In 1980, a one-paragraph letter postulating a low risk for the development of addiction following opioid treatment was published in the New England Journal of Medicine [3]. The authors’ claim was based on a retrospective data evaluation revealing that few hospitalized patients (4 out of 11882) developed relevant addictive disease subsequent to an opioid prescription. Although no further evidence supporting this hypothesis was presented, the same article has been widely cited (> 600 times) in the scientific literature as evidence that opioid use confers negligible risks for addiction [4].
Today, the United States and Canada (and to a lesser extent, other developed countries of the world) are facing an opioid crisis characterized by increasing numbers of overdose-related deaths from both legally as well as illegally obtained opioids [5–7]. In 2018 alone, 46 802 Americans succumbed to a lethal opioid overdose, corresponding to an average of 128 opioid-related deaths per day [8]. Although the above cited article may not be held responsible for the current crisis, it is indeed representative of the changing perspective on opioids that arose around this time. While these substances were traditionally used to treat acute pain related to injury or terminal illness, an increasing awareness for alleviating chronic pain conditions advocated by both scientific publications as well as the World Health Organization became apparent in the 1980s. The subsequent development and introduction of semisynthetic opioids such as Oxycodone as well as aggressive marketing strategies (most prominently illustrated by the case of OxyContin) further fueled increasing opioid prescriptions across the US [9]. Additionally, studies indicating insufficient pain control in a large percentage of patients with chronic illnesses, as well as novel remuneration structures in the health care system rewarding patient satisfaction (whereby pain control took a great share of the overall score) also contributed to this evolution [9, 10]. Finally, an increasing number of semisynthetic opioids became widely available, thus increasing the accessibility, while lowering the cost of the individual compound. Of note, these developments did not remain unnoticed and prescribed opioid doses leveled off by 2010 in the US [11]. Conversely, the number of deaths related to illicit opioid use continue to rise, suggesting that the distribution and availability of these substances among the population remains high [6]. Taken together, the opioid crisis imposes a substantial socioeconomic burden with an estimated annual cost of almost 80 billion dollars in the US [12].
2. Biology of endogenous opioids
In the middle of the 20th century, researchers proposed the existence of specific receptors facilitating the analgesic effects of morphine and other opioids, simultaneously raising the question which endogenous ligands might potentially interact with these structures [13]. The discovery of naloxone-reversible analgesia arising from electrical stimulation of certain brain regions corroborated the assumed existence of endogenous opioid ligands acting through receptors, which are shared with exogenous opiates such as morphine [14]. Shortly thereafter, endogenous opioid peptides were discovered, followed by the cloning of the three main opioid receptor classes, namely μ-(MOR), κ-(KOR) and δ-(DOR) opioid receptors [15]. The nomenclature of these proteins was based on the first activating ligands to be identified (mu for morphine, kappa for ketocyclazocin) or the initial tissue of discovery (delta for Vas deferens) [16]. Later, the existence of a fourth receptor class coined nociception/orphanin FQ receptor (encoded by OPRL1; opioid-receptor like 1) was confirmed, although the latter differs from other opioid receptors in exhibiting poor binding affinity for cognate opioid peptides [17].
Similar to the three main opioid receptor classes, endogenous opioid peptides are generated from three precursors proteins: preopiomelanocortin, preprodynorphin and preproenkephalin, encoded by POMC, PDYN and PENK, respectively [18, 19]. These precursor proteins undergo post-translational processing (e.g. cleavage by proprotein convertases) to generate a variety of hormones and mature opioid peptides [20]. The latter share a consensus amino acid pentasequence known as the “opioid motif” (Tyr-Gly-Gly-Phe-Met/Leu) [21] (Figure 2). The existence of an additional (fourth) class of endogenous opioid ligands termed endomorphins has also been proposed [22]. Yet, the encoding gene remains unidentified, thus leaving the possibility that these peptides are either exclusively generated via post-translation mechanisms or may not exist after all [23]. Of note, the characteristics of opioid biology with multiple ligands in the presence of relatively few receptors (convergence) stands in sharp contrast to most other mediators in the CNS, where few ligands typically bind to a plethora of receptors (divergence). Additionally, many functions are shared between different opioid receptors, implying that sufficient engagement of opioid-induced biological programs might be of high priority to organisms (see below).
Figure 2: Endogenous opioid peptides are generated from precursor proteins.
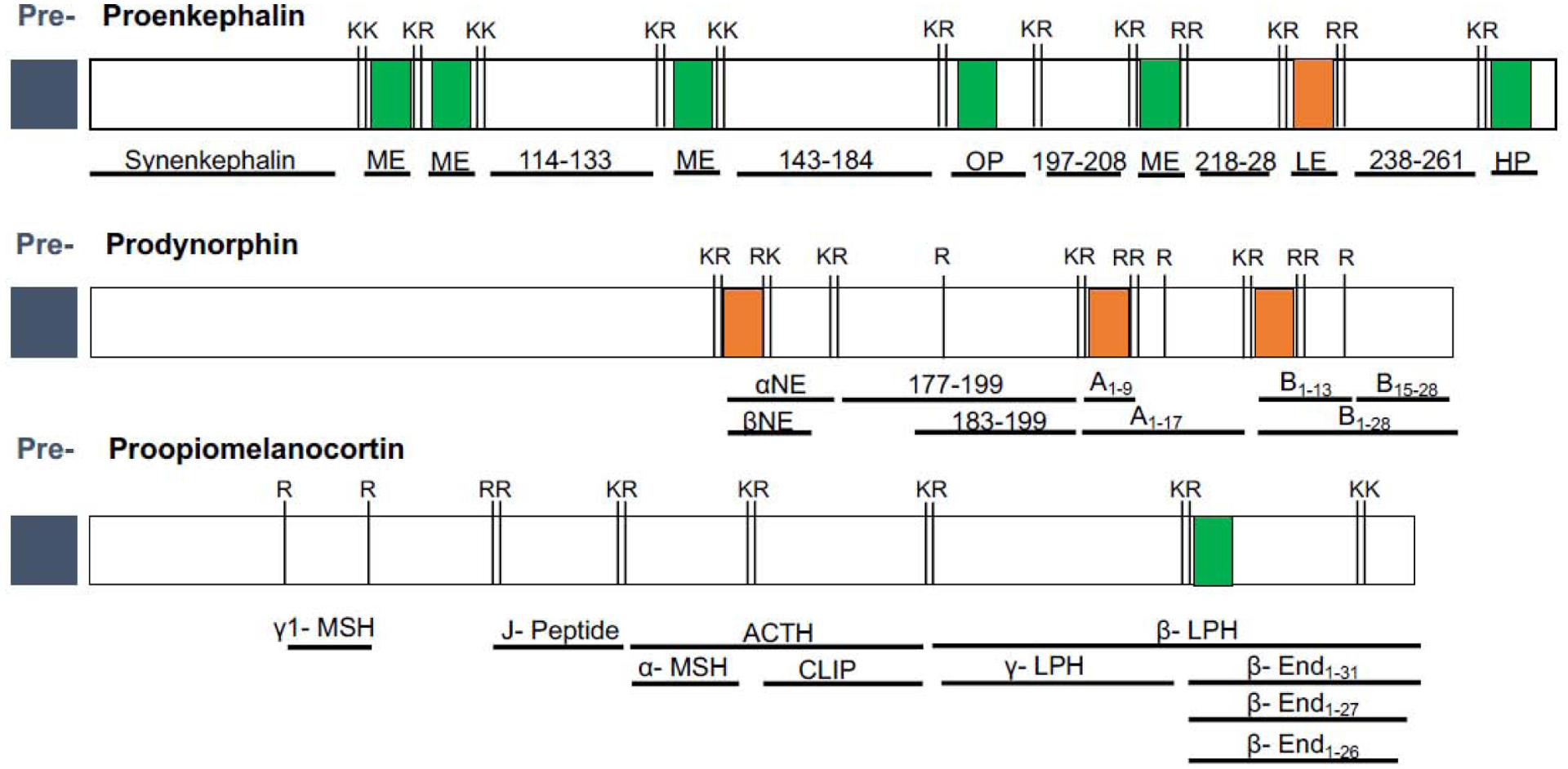
Schematic illustration of the three major opioid peptide precursor proteins (conserved between mice and humans with subtle differences; the former shown above): preproenkephalin (PENK), preprodynorphin (PDYN) and preproopiomelanocortin (POMC). Major cleavage sites consisting of Lysine (K) and Arginine (R) residues are indicated. The two core opioid motifs are highlighted in green (Tyr-Gly-Gly-Phe-Met) and orange (Tyr-Gly-Gly-Phe-Leu), respectively. Post-translational processing of each precursor generates a variety of different hormones and peptides, some of which have not been named (referred to as numbers in the figure). Lower case numbers reflect different isoforms of the same peptide that vary in their length and thus, biological activity. Note that cleavage of POMC also generates non-opioid mediators such as ACTH or MSH (Adapted from [42]).
ME= Met-enkephalin, OP=octapeptide, LE= Leu-enkephalin, HP=heptapeptide, αNE= alpha neoendorphin, βNE= beta neoendorphin, Ax-y= dynorphin A variants, Bx-y= dynorphin B variants, γ1-/α- MSH= gamma1/alpha melanocyte stimulating hormone, J-Peptide= joining peptide, CLIP= corticotropin-like intermediate lobe peptide; β-/γ-LPH = beta/gamma Lipotropin; β- Endx-y=beta endorphin variants
The long-held belief of distinct receptor selectivity of endogenous opioids has recently been challenged by studies demonstrating wide and potent interactions between different endogenous opioids and opioid receptor classes. Nonetheless, all peptides exhibit varying affinities and potencies to activate individual opioid receptors with the POMC-derivative β-endorphin representing the most potent endogenous agonist for μ-receptors [24]. Given that most desired (analgesia, sedation, anxiolysis, cough suppression), as well as undesired (constipation, respiratory depression, addiction, tolerance, nausea, endocrine perturbations) effects in opioid pharmacology are facilitated by μ-receptors, these signaling networks warrant special attention.
3. OPRM1: from genetics to signaling
G-protein coupled receptors (GPCRs) constitute the largest group of cell membrane proteins expressed across human tissue. GPCRs are evolutionarily related and share conserved genetic as well as structural patterns [25]. As part of the latter family, μ-opioid receptors typically exhibit 7 transmembrane (TM) domains. Together with an intracellular C-terminal tip, these domains are involved into the coupling to small G-proteins and thus, intracellular signaling responses. Transmembrane domains also constitute the ligand binding pocket, whereas N-terminal regions modulate the interaction between ligands and the receptor (Box 2) [26]. In humans and mice, μ-receptors are encoded by a single gene (OPRM1 and Oprm1) consisting of at least 20 and 26 exons, respectively (Figure 3) [27]. Of note, μ-opioid receptors share 94% sequence homology at the protein level between the two species [28]. Phylogenetic studies have mapped the appearance of the quartet of opioid receptors to the origin of jawed vertebrates early in vertebrate evolution approximately 450 million years ago, whereas opioid-like systems have been described in even more primitive invertebrate species (see below) [29].
Box 2: Intracellular signaling of μ-opioid receptors.
MORs belong to the GPCR class A rhodopsin family and typically couple to Gi/o proteins. As such, MORs mainly harness two distinct intracellular signaling pathways, one of which relies on G-protein signaling, the other being facilitated by β-arrestin proteins. Activation of the G-protein dependent branch typically yields a reduction in intracellular cAMP levels through the inhibition of adenylatcyclases with subsequent effects on protein kinases and/or ion channels, ultimately culminating in dampened neuronal excitability and/or cellular responses. On the other hand, ligand binding to μ-receptors may also trigger intracellular phosphorylation events, thereby promoting the recruitment of β-arrestin proteins, which subsequently bind to the receptor, attenuate further G-protein signaling and may foster internalization of the protein. However, β-arrestin binding also redirects the cellular response to alternative pathways such as mitogen activated protein kinase signaling [37]. Overall, β-arrestin-dependent pathways are believed to account for the majority of undesired consequences of long-term opioid use including the development of tolerance as indicated by the absence of the latter in β-arrestin-2 knock-out mice [38]. These observations led researchers to hypothesize that synthesizing “biased” opioid receptor ligands favoring G-protein over β-arrestin-dependent signaling might be sufficient to overcome many obstacles of current opioid pharmacology, a promise that has thus far not been convincingly fulfilled. Crucially, endogenous opioids themselves are biased agonists with different peptides preferentially activating one of the two intracellular signaling branches [24]. Further complexity to the μ-receptor system is added by the fact, that alternatively spliced C-terminal variants differ in their inherent bias for promoting G-protein vs. β-arrestin-dependent signaling [39, 40]. Thus, μ-opioid receptor signaling bias is created at both the ligand, as well as the receptor level.
Figure 3: The genetic complexity of μ-receptors.
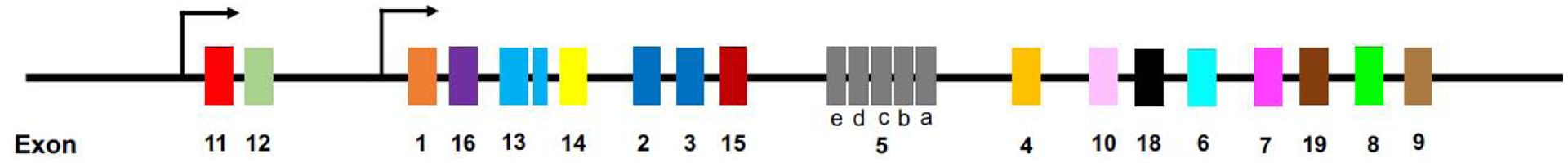
Although only a single μ-receptor encoding gene exists in humans and rodents (the latter shown above), alternative splicing of the OPRM1 pre-mRNA gives rise to a plethora of receptor variants that exhibit distinct biochemical properties. All 7TM variants contain a consensus sequence built from exons 1, 2 and 3, whereas 6 TM variants replace exon 1 with exon 11. Note that the exon nomenclature does not reflect their chromosomal locus but rather their timepoint of discovery.
Over the past two decades, several scientific discoveries have reshaped our understanding of μ-receptor physiology by revealing much greater complexity of these networks than once anticipated. OPRM1 pre-mRNA undergoes extensive alternative splicing to generate a plethora of receptor variants or isoforms with distinct biochemical properties. These can be divided into three main classes: 1.) 7-transmembrane C-terminal variants generated by 3’ splicing, all of which contain a consensus sequence built from exons 1, 2 and 3 but exhibit varying intracellular C-terminal amino acid tails; 2.) 6TM variants generated by 5’ splicing that replace exon 1 with exon 11, thereby yielding N-terminally truncated receptors; and 3.) single TM variants, generated by exon skipping or insertion, presumably acting as molecular chaperons for other opioid receptors [27, 30]. Of note, most clinically used μ-receptor agonists require 7TM variants to elicit analgesic effects as demonstrated by the absence of morphine-induced analgesia in Exon 1 knock-out animals devoid of these variants [31]. On the other hand, 6TM μ-receptor variants are responsible for the analgesic effects of a novel class of opioids such as 3-iodobenzoyl-6β-naltrexamide (IBNtxA), a naltrexone-derivative producing potent analgesia with less side-effects [32–34]. To date, it remains unclear if endogenous opioid peptides can interact with 6TM μ-receptor variants [35]. Of note, overexpression of 6TM variants alone in cell lines in vitro did not confer binding of any available radio-labeled opioid ligand [36], raising questions regarding the molecular mechanisms underlying the functions of 6TM variants.
Although the existence of multiple μ-receptor variants has been recognized for years, an appreciation of potential interactions between individual isoforms at the cellular and/or tissue level has only recently emerged. By integrating human RNA sequencing data sets, GPRC sequences and structures as well as proteomics, genetic approaches and pharmacological in vitro experiments, Marti-Solano et al. revealed conserved patterns across GPRC classes producing similar functional consequences [41]. More specifically, this study demonstrated that N-terminal modifications (resulting from 5’ alternative splicing) of such receptors typically alter ligand binding or efficacy, whereas C-terminal changes impact G-protein coupling, signaling and trafficking, which is in line with μ-opioid receptor physiology as outlined above. Most importantly, these authors reported that the combinatorial expression of different isoforms of the same receptor gene has critical consequences for net signaling outcomes (Figure 4). For example, co-expression of the canonical and alternative isoforms of cannabinoid receptor 1 (CNR1) had pronounced impact on intracellular cAMP levels upon forskolin exposure.
Figure 4: Expression profiles of GPCR variants determine net signaling outcomes.
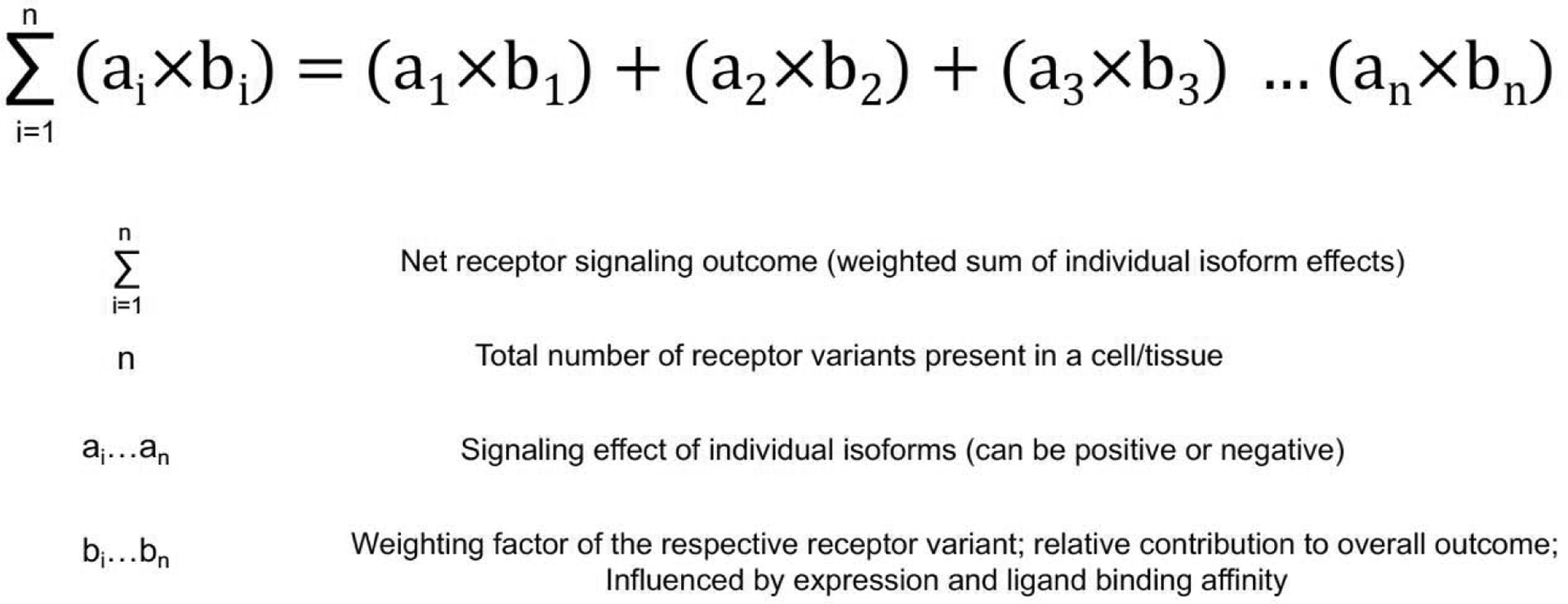
Simplified mathematical modelling of signaling responses arising from the combinatorial expression of different GPCR variants in a given cell or tissue.
Taken together, the combinatorial expression of different GPCR isoforms diversifies the cellular response to a given ligand. Hence, predicting physiological and/or pharmacological effects of receptor ligands requires a more holistic approach that has thus far received little attention, which also holds true for μ-opioid receptors.
4. Endocrine functions of μ-opioid receptor networks
Besides the CNS, the highest expression of μ-opioid receptors has been described in endocrine organs such as the testis or the adrenal gland (see human protein atlas, GTEx dataset or [42]) with an accumulating body of evidence supporting the existence of peripheral opioid networks functioning locally within such tissues. Taken together with the observation that chronic opioid use has been linked to a variety of endocrine diseases such as hypogonadism and infertility, osteoporosis, adrenal insufficiency, as well as diabetes, an involvement of μ-opioid receptor networks in endocrine homeostasis appears likely [2, 43]. Indeed, a large body of preclinical and clinical evidence has implicated μ-receptors and their ligands in regulating endocrine systems. Although the implementation of the Cre-LoxP-system has allowed scientists to explore organ-specific functions of selected genes and both global as well as conditional μ-opioid receptor knock-out mice have been generated [44, 45], surprisingly little effort has been invested into exploring the causal involvement of these networks in endocrine health and disease. The following sections will summarize the currently available evidence for such functions of μ-receptors with a special focus on peripheral effects on endocrine tissues.
4.1. Central and peripheral effects on reproduction
In both men and women, opioid receptor activation yields a reduction of gonadotropin releasing hormone (GnRH) release paralleled by a decrease in circulating sex hormone levels [46]. Indeed, hypogonadism is commonly observed among opioid users with varying prevalence depending on the substance used, duration of the treatment as well as the route of administration. In male patients receiving opioid therapy for non-cancer pain, 20–85% exhibit hypogonadism depending on the testosterone threshold used to define the condition [47]. As for most data reported in such patient collectives, these findings are likely blurred by confounders such as pain, concomitant medications or underlying diseases. Nevertheless, a plethora of experimental evidence supports a direct involvement of opioid receptors in regulating reproductive functions.
Administration of naloxone to male rats provoked an increase in luteinizing hormone (LH) levels, which was reversed by co-administration of morphine [48]. Similarly, chronic treatment of male rabbits with naloxone for 14 days resulted in increased circulating LH and testosterone levels [49]. Likewise, opioid receptor blockade in men evoked increased LH pulse frequency and enhanced sex hormone secretion [50, 51]. Similar observations have been made in females, where acute morphine administration yielded a suppression of LH levels [52]. Together, these findings led researchers to hypothesize that opioids might be involved in facilitating the negative feedback inhibition of GnRH release by sex hormones. Indeed, circulating β-endorphin levels rise during the follicular phase of the menstrual cycle in healthy women and increase even further in the luteal phase, thus paralleling the peak of progesterone-mediated feedback inhibition of GnRH release [53]. The crucial involvement of β-endorphin in this process is further highlighted by studies performed in rhesus monkeys [54]. Here, ovarectomized animals were supplemented with either estradiol or estradiol/progesterone and β-endorphin levels were measured in hypophyseal portal blood. Whereas the former treatment had only mild effects on β-endorphin levels, the latter provoked a strong increase, reminiscent of circulating β-endorphin kinetics found in humans as noted above. Consistently, the suppression of LH pulse frequency induced by continuous dihydrotestosterone infusion was reversed by naloxone administration in healthy women, further supporting the concept that opioid receptors play a role in sex hormone-mediated feedback inhibition [55].
Of note, most of these studies do not allow to preclude the possibility that opioid-receptors other than μ-receptors might be involved in facilitating the reported effects. However, the preferential binding of both exogenous (morphine, naloxone), as well as endogenous opioid receptor ligands (β-endorphin) investigated in these studies [56], corroborates the hypothesis that these receptors are crucially involved in governing hypothalamic GnRH release. Of note, β-endorphin-deficient mice (devoid of the principle endogenous μ-receptor ligand) apparently breed normally [57], suggesting that functional redundancy within the endogenous opioid system allows for compensatory μ-receptor activation or engagement of these receptors is dispensable to reproduction after all. Conversely, an increased endogenous opioid tone apparently perturbs fertility as noted above.
More recently, peripheral opioid receptor networks have been implicated in contributing to the regulation of reproductive functions. In women undergoing in vitro fertilization (IVF), β-endorphin concentrations in follicular fluid predicted the number of retrieved metaphase II oocytes, thus pointing towards an involvement of endogenous opioids and μ-receptors in oocyte maturation [58]. Indeed, the presence of MOR on oocytes has been confirmed [59]. Subsequent studies revealed that follicular fluid β-endorphin content associates with pregnancy outcomes and live birth rates in women with polycystic ovary syndrome and diminished ovarian reserve undergoing IVF, further underpinning a potential role of opioid peptides in oocyte maturation [60]. Additionally, β-endorphin levels in follicular fluid of healthy young women were found to be 10–40 times higher than those in plasma, suggesting local synthesis and/or clearance mechanisms [61]. Of note, opioid receptors are also expressed by male germ cells [62]. Similar to reports in women, β-endorphin concentrations in seminal plasma exceeded those in systemic plasma by a factor >10 [63]. Intriguingly, in vitro exposure to morphine increased the number of immotile sperm cells [64], mirroring the clinical picture of impaired semen quality in heroin abusers [65]. Finally, β-endorphin and met-enkephalin have been detected in the uterine fluid of women with subsequent studies revealing local POMC expression in primary endometrial tissue [66, 67]. Consistently, endometrial OPRM1 mRNA and protein expression reach a maximum at the time of ovulation, further supporting a physiological function of μ-receptor networks in the uterine cavity [68]. It is worth mentioning that other opioid receptor classes (i.e. DOR and KOR) have been detected in peripheral reproductive tissues as well [59]. In view of the rather high binding affinity of β-endorphin to κ- and δ- receptors (see above), an involvement of these receptors in facilitating some of the peptide’s peripheral effects is likely.
In summary, preclinical and clinical evidence implicates a critical involvement of μ-opioid receptors in regulating the hypothalamic pituitary gonadal axis, effects which are mainly of inhibitory nature. On the other hand, the existence of peripheral opioid networks in reproductive tissues has been discovered only recently and remains to be further explored.
4.2. Regulation of glucose homeostasis
In contrast to recent discoveries of opioid receptor networks in the gonads, peripheral effects of opioid peptides on the endocrine pancreas have long been appreciated. Almost three decades ago, β-endorphin was found to inhibit insulin release from isolated pancreatic beta cells [69]. Likewise, β-endorphin infusion strongly decreased pancreatic insulin secretion in healthy volunteers, while stimulating glucagon release, effects which were found to be cAMP-dependent [70]. In contrast, ambiguous results were reported by other authors with differential effects of β-endorphin on insulin kinetics depending on glucose concentrations and whole-body metabolism [71–73].
The arguably most compelling evidence for an involvement of μ-opioid receptor networks in modulating pancreatic beta cell function stems from global μ-receptor knock-out mice (MOR−/−) [74], which were generated by targeting exon 1 of the Oprm1 locus. This approach renders mice devoid of all 7TM variants, while leaving 6TM μ-receptor variants intact. On a regular chow diet, these animals exhibited exaggerated weight gain compared to wildtype littermates, which was explained by an apparent increase in adipose tissue fat mass. Intriguingly, these effects were observed as early as 5 days after birth. Phenotypic characterization of these mice revealed enhanced glucose tolerance characterized by pronounced hyperinsulinemia. Most interestingly, MOR−/− animals displayed a prominently increased pancreas weight with enhanced beta cell mass and heightened insulin content. Consistently, pancreatic beta cells isolated from knockout mice secreted more insulin upon tolbumatide exposure than cells derived from wildtype controls. Taken together, these data suggest that 1.) μ-receptors hold crucial inhibitory functions in modulating insulin release 2.) pancreatic beta cell proliferation is partly governed by μ-receptors and 3.) a loss of opioid-tone (activity) results in an anabolic state characterized by hyperinsulinemia and weight gain. In view of the global nature of the receptor knockout and the lack of information on food intake provided by the authors, contributions of central effects of μ-receptors to the phenotype cannot be excluded. Indeed, μ-receptors also modulate insulin release via mechanisms in the CNS. Intracerebroventricular administration of the μ-agonist DAMGO blunted glucose-stimulated insulin release in mice in an alpha2-adrenoreceptor dependent fashion, suggesting additional indirect effects via the sympathetic nervous system [75]. On the other hand, an independent μ-opioid receptor knock-out strain did not exhibit overt metabolic abnormalities when fed ad libitum. Yet, these mice displayed resistance against high fat diet-induced obesity [76], which stands in sharp contrast to findings from other MOR−/− mice reported above. Importantly, this strain was generated by targeting exons 2 and 3 of the Oprm1 locus, thus yielding a predicted loss of all 7TM and 6TM, while not affecting single TM μ-receptor variants [77]. Taken together, 6TM and 7TM μ-receptor variants may hold distinct (and even opposing) physiological functions, which highlights the necessity to consider the genetic complexity of μ-receptors when interpreting experimental data.
Finally, endogenous opioids are heavily involved in reward circuits, regulating food intake and hedonistic behavior with μ-receptor agonists generally promoting feeding irrespective of satiety, both in vertebrate, as well as invertebrate species [78, 79]. Consistently, opioid receptor blockade by a naltrexone-bupropion sustained release formulation elicited weight loss in obese individuals with type II diabetes, which was paralleled by improvements in glycemic control [80]. Similar observations have been made in overweight individuals with polycystic ovary syndrome [81]. Of note, the pharmacological utility of naltrexone is compromised by its side effects with a large percentage of patients experiencing nausea and/or vomiting [80].
4.3. Modulation of the stress response
Given the apparent homeostatic regulation of glucose metabolism by μ-receptors, one may wonder whether these networks are also involved in other immediate responses requiring glycemic adaptations such as the stress (fight and flight) response. Indeed, significant expression of μ-receptors has been reported in the adrenal gland ([82] and GTEx dataset). However, which functions these receptors fulfill in this tissue remains largely unclear with some in vitro studies reporting inhibitory, while others demonstrating stimulatory effects on catecholamine and glucocorticoid secretion, respectively [83–85]. Yet, in patients with hypothalamic-pituitary disconnection, naloxone administration increased cortisol, but not andrenocorticotropic hormone (ACTH) levels, supporting the idea of direct effects of opioids on the adrenal glands [86]. Likewise, β-endorphin infusion suppressed circulating catecholamine levels in hypertensive and healthy subjects, respectively, further supporting inhibitory actions of μ-receptors on adrenal gland responses [87].
Centrally, μ-receptors generally appear to inhibit hypothalamic-pituitary-adrenal (HPA) axis activity. Male heroin addicts on methadone maintenance therapy exhibited reduced circulating ACTH and cortisol levels both basally, as well as after metyrapone administration [88]. Consistently, β-endorphin infusion reduced circulating cortisol levels in healthy volunteers [89]. Moreover, a single nucleotide polymorphism (SNP) in the OPRM1 gene (A118G) proposed to confer enhanced ligand binding to the receptor has been linked to blunted ACTH responses in humans undergoing metyrapone testing [90]. In line with these observations, long-term opioid treatment may evoke hypoadrenalism [91], further underpinning the inhibitory effects of opioid receptor activation on HPA axis activity.
Of note, SNPs within the OPRM1 locus eliciting functional consequences reminiscent of those found in humans have also been described in non-human primates [92, 93], demonstrating how common evolutionary pressures may have produced similar phenotypes across species. Furthermore, these findings could imply a shared genetic response to environmental challenges mediated by opioid receptor networks [92].
Finally, chronic opioid use has also been associated with the development of another common endocrine disease, namely osteoporosis. However, the underlying mechanisms remain incompletely understood (Box 3).
Box 3: Opioids and bone health.
Chronic opioid use has been associated with impaired bone quality and an increased fracture risk [94]. Mechanistically, opioid-induced hypogonadism likely plays a prominent role in this pathology, while an enhanced susceptibility to falls secondary to dizziness may also contribute. Additionally, direct effects of opioids on bone have been proposed [95]. Yet, experimental evidence supporting this hypothesis is scarce. A widely cited publication demonstrating morphine-induced inhibition of osteocalcin production used excessively high doses (mM range) that are unlikely to occur neither physiologically, nor pathologically [96]. Moreover, the cell line used to study these effects (MG-63) was derived from sarcoma tissue and poorly resembles primary osteoblast characteristics [97]. Finally, the detection of opioid receptors in bone cells has thus far relied on semi-quantitative techniques and suboptimal primer design with most studies failing to detect relevant expression levels of any opioid receptor subclass in bone [98]. Of note, global dynorphin knock-out mice (Dyn−/−) displayed enhanced bone mass, although this effect was found to be mediated via the CNS, rather than peripherally [98].
5. An integrated perspective on μ-opioid receptor networks
The evolutionary advantage conferred by the implementation of endogenous opioid networks is highlighted by several observations. First, opioid-like systems are not confined to higher developed organisms but also found in more primitive invertebrate species such as Molluscs, Annelids or Arthropods [99]. Second, the structure of opioid receptors is highly conserved across vertebrate species despite originating early in their evolution (see above). Third, opioid receptor networks are involved in the regulation of central physiological functions such as reproduction or feeding behavior and fourth, the high homology between different opioid receptor subclasses as well as their redundant activation by different endogenous opioids indicates that ensuring sufficient engagement of these networks is of high priority to organisms.
From an evolutionary perspective, reproductive fitness reflects the main driver of natural selection. Thus, a genetic repertoire conferring optimal adaptation to a given environment (resulting in maximized reproductive fitness) will be selected for. Consistently, traits mediating such adaptive responses are of particular importance to organisms [100].
In Caenorhabiditis elegans, endogenously expressed nlp-24 (neuropeptide-like protein 24)-derived peptides were found to exert agonistic activity at an opioid-like receptor (nrp-17), which could also be activated by prototypical pharmacological opioid receptor agonists such as morphine [101]. Engagement of this system promoted locomotion and feeding behavior in starved animals, both of which aid in nutrient uptake under challenging conditions, suggesting that opioids mediate survival responses. In vertebrates, the versatile effects arising from μ-opioid receptor activation might seem unrelated to such functions. Yet, the net organismal outcome elicited by these programs may be summarized as a state of energy preservation, also referred to as maintenance. The latter reflects an orchestrated response to constraints imposed by unfavorable environmental conditions (e.g. nutrient scarcity). Collectively, maintenance programs are characterized by an accentuation of catabolic pathways at the expense of anabolism (i.e. growth and reproduction), corresponding to a prioritization of resource allocation through trade-offs [102].
Indeed, prolonged μ-opioid receptor engagement, either invoked exo- or endogenously, yields a cessation of multiple programs that are energetically costly: reproduction (inhibition of GnRH release) [52], macromolecule synthesis und nutrient storage (reduction of insulin secretion) [74], tissue renewal (decreased bone formation) [94], cardio-respiration (attenuated cardiac function, hypotension and respiratory depression) [1], digestion (decreased gastrointestinal motility) [34], fight and flight response (reduced HPA axis activity) [91] as well as the pro-inflammatory response (immunosuppression) [103]. These adaptations are crucial in face of challenging environmental conditions due to the inherently finite nature of organismal resources.
The most impressive examples for these annotations are hibernating animals, who temporarily suppress a broad range of metabolic functions (including most anabolic programs) in order to survive in unfavorable environments [104]. Intriguingly, intracerebroventricular administration of naloxone to hibernating Syrian hamsters provoked an arousal response, suggesting that endogenous opioids might be involved in facilitating maintenance programs [105]. Indeed, subsequent studies revealed an increase in β-endorphin immunoreactivity in the arcuate nucleus of these animals after hibernation onset and anti-β-endorphin antibodies elicited arousal, reminiscent of the effects of naloxone noted above. Conversely, intracerebral administration of the selective μ-receptor agonist DAMGO invoked a sharp decrease in body temperature, a characteristic adaptation occurring in hibernating species when engaging such programs [106].
Taken together, these findings suggest a role of central μ-receptors and their principle endogenous ligand β-endorphin in facilitating adaptations to challenging environments, which are crucial to survive. Pertinent to this, the strongest triggers for endogenous β-endorphin release thus far reported comprise painful stimuli, exhausting exercise, fasting or stressful tasks, all of which indicate challenging environments and are similarly found in invertebrate species [99, 107–109]. In mammals, the common molecular origin of ACTH and β-endorphin (i.e. POMC) should be considered when interpreting such findings since elevated β-endorphin levels under these conditions could partly arise from increased HPA axis activity.
Although humans do not hibernate, the adaptations occurring in response to environmental challenges are similar to other mammals: prolonged stress, excessive exercise and/or caloric mismatch yield hypogonadism (as exemplified by the clinical entity of hypothalamic amenorrhea) as does cold exposure, infection or any other evolutionary relevant environmental stressor [110–113]. Similarly, bone formation is typically decreased in such individuals, thus conferring a risk for the development of osteoporosis and fractures [113]. Pertinent to an involvement of central opioid receptors in facilitating such responses in humans, patients with hypothalamic amenorrhea have successfully been treated with opioid antagonists (i.e. naltrexone) to restore gonadal-axis activity and even induce pregnancy [114].
Thus, temporal increases in endogenous opioid tone likely confer a significant survival advantage to organisms through facilitating a variety of systemic adaptations, which are conserved across species and tailored to demands arising in challenging environments. On the other hand, a persistently increased opioid tone may eventually become maladaptive and contribute to disease since the engagement of this system occurs at the expense of certain other aspects of physiology (trade-off) (Figure 5). The relative contributions of central vs. peripheral μ-opioid receptor networks to these phenomena remain unknown.
Figure 5: Perturbed μ-opioid receptor activity contributes to disease.
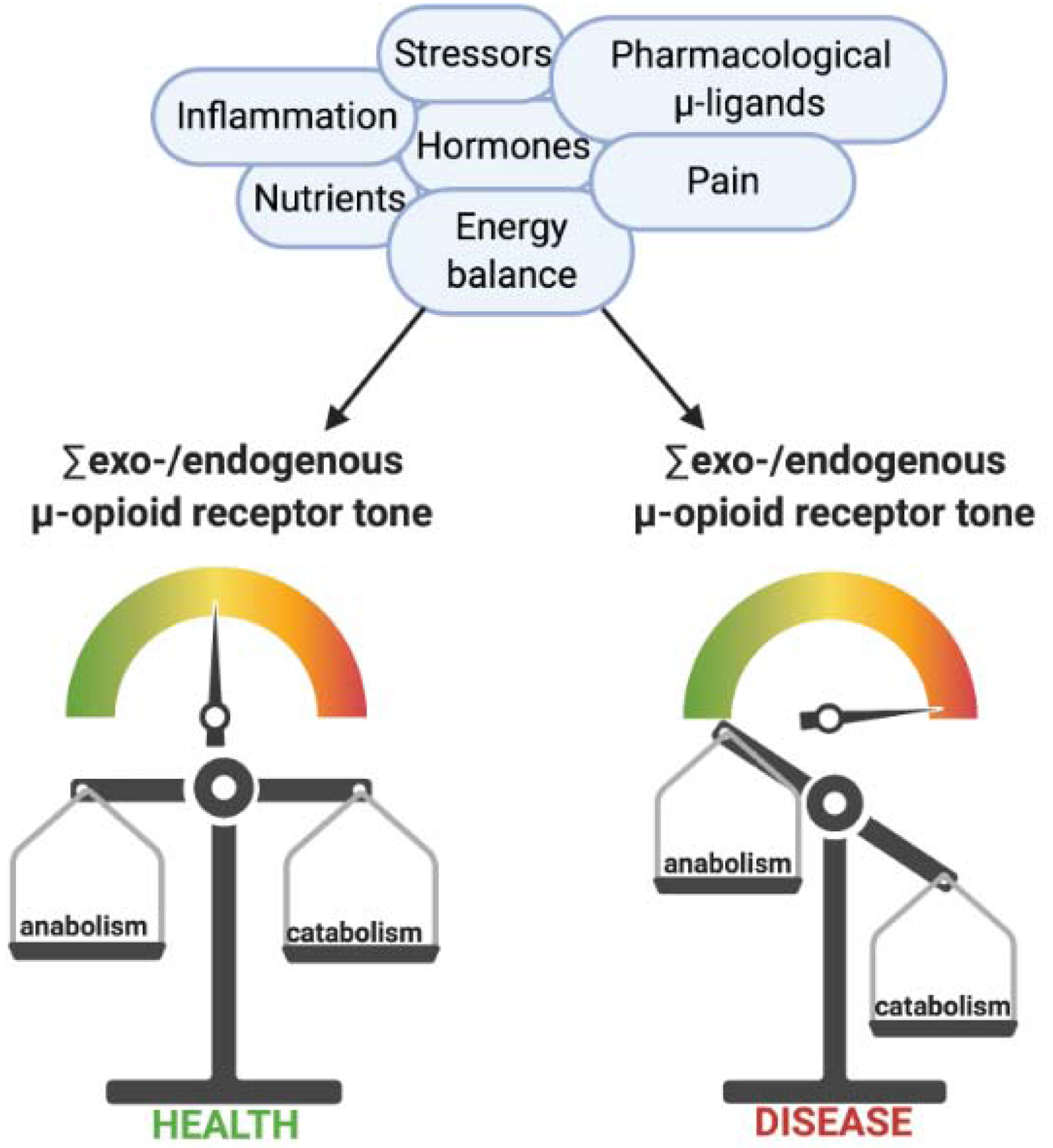
Engagement of μ-opioid receptors (sum activity) emanates from both exogenous (pharmacological), as well as endogenous sources (endogenous opioid peptides), the latter being shaped by a plethora of factors as exemplified above. Overall, biological responses prompted by μ-receptor activation occur at the expense of energetically costly (anabolic) programs such as reproduction, the immune response or bone formation. While such changes may be temporally beneficial in the face of challenging environments, their persistence (e.g. due to long-term opioid treatment) culminates in disease.
6. Concluding remarks and future perspectives
The ongoing opioid crisis has reignited the scientific interest in opioids and specifically, μ-receptors. Although a considerable body of evidence supports an involvement of these signaling networks in endocrine homeostasis, much of our knowledge on μ-receptors is derived from older studies that were unable to address critical questions due to the lack of adequate molecular techniques in former times, thereby yielding an underestimation of the respective biological complexity. Indeed, recent advances in μ-opioid receptor research have highlighted the necessity to consider differential effects elicited by truncated and full-length receptor variants, although the physiological (including endocrine) functions of the former remain poorly understood.
The theoretical framework presented in this manuscript suggests that manipulation of opioid receptor signaling may reflect a widespread, conserved organismal effector mechanism in response to constraints imposed by challenging environments. The resulting trade-offs arise from the prioritization of organismal resource allocation and may aid in understanding many of the unfavorable health consequences elicited by prolonged opioid therapy. Vice versa, opioid receptors could perhaps be pharmacologically targeted in a much broader context than presently appreciated. However, this transition needs to be preceded by a significant body of research to unravel the basic homeostatic functions of endogenous opioid receptor networks beyond pain perception, both in- and outside the CNS.
Now, it is up to scientists to harness the available tools to elucidate the plethora of outstanding questions, all of which will aid in better understanding the intricate complexity of these networks.
Outstanding questions.
Do endogenous opioids interact with 6TM μ-receptor variants?
Does the selective 6TM variant agonist IBNtxA invoke similar endocrine side-effects as “classical” opioids?
What is the relative contribution of G-protein vs. β-arrestin-dependent signaling to endocrine side effects elicited by opioid therapy?
Do peripheral tissues significantly contribute to circulating β-endorphin levels?
Do combinatorial expression profiles of μ-receptors differ between endocrine organs? Does this allow the creation of tissue-specific ligands?
Are central and peripheral opioid receptor networks mechanistically interconnected or do they function autonomously?
Which factors regulate the production of opioid peptides in the periphery?
Is the increase in circulating β-endorphin under stressful conditions blunted in animals with hypophyseal-specific POMC deletion?
Can μ-receptor ligands be used in vitro to improve oocyte quality?
Does the microbiome contribute to shaping the endogenous opioid tone of the host? If so, how is this accomplished?
Highlights.
The endogenous opioid system holds crucial functions in endocrine homeostasis
Opioid peptides are not only produced in the CNS, but also in peripheral organs such as the gonads
Alternative splicing mechanisms give rise to a plethora of μ-opioid receptor variants with distinct biochemical properties
The combinatorial expression of different μ-receptor variants determines signaling responses in a given cell or tissue
Opioid networks fulfill conserved functions across species
The net organismal outcome of μ-receptor activation reflects a state of energy preservation (maintenance), which is a key adaptative response of organisms to survive in challenging environments
Persistent opioid receptor engagement (either elicited exo- or endogenously) occurs at the expense of distinct physiological programs (e.g. reproduction), thus eventually invoking disease
Acknowledgments
The authors would like to thank Paul Heidler for his thoughtful comments and suggestions. Graphical illustrations were partly created using biorender.com. This work was funded by the Deutsche Forschungsgemeinschaft (DFG) to LCH (HO 1875/24-1 and HO 1875/26-1), TDR (RA 2151/4-1). Further funding was provided by the German Academic Scholarship Foundation and Mildred Scheel Nachwuchszentrum Dresden (MSNZ) to NJ. YXP received grants from the National Institute on Drug Abuse of the National Institutes of Health, DA042888 and DA007242 and the Mayday Foundation.
Glossary
- Papaver somniferum
also known as „opium poppy”; species of flowering plant; contains several alkaloids, some of which potently bind to opioid receptors (collectively referred to as opiates) including morphine and codeine
- life history theory
theoretical framework seeking to explain how organisms allocate their resources into three main biological programs (growth, reproduction, maintenance) in order to maximize reproductive success; investment strategy is dictated by the quality of the environmental conditions encountered
- naloxone
synthetic, unselective, competitive opioid antagonist; highest binding affinity for μ-opioid receptors
- alternative splicing
regulated process occurring after gene transcription; enables the production of multiple proteins from a single genetic sequence; relies on the creation of different exon combinations in the mature mRNA
- DAMGO
[D-Ala2, N-MePhe4, Gly-ol]-enkephalin; synthetic enkephalin-derivative; exhibits high affinity and specificity for μ-opioid receptors, which contrasts the preferential binding of endogenous enkephalins to δ-opioid receptors
- forskolin
geranylgeranyl-pyrophosphate derivative naturally occurring in plants (Plectranthus barbatus); experimentally used as a potent adenylate cyclase stimulator; provokes an intracellular cAMP increase
- Cre-LoxP-system
site-specific recombinase technology; mostly used to insert or delete specific DNA sequences, thus yielding “knock-in” and “knock-out” phenotypes, respectively; consist of the enzyme Cre (causing recombinase) and loxP sites (locus of x-over, P1), the latter flanking the genetic sequence of interest
- hypogonadism
insufficient gonadal function characterized by reduced circulating sex hormone levels and associated clinical symptoms; may be accompanied by infertility
- polycystic ovary syndrome
also known as PCOS; common endocrinopathy among women in reproductive age; characterized by biochemical and/or clinical hyperandrogenemia, menstrual cycle abnormalities and polycystic morphology of the ovaries; presence of two out of three listed symptoms justifies a PCOS diagnosis (Rotterdam criteria)
- tolbutamide
first-generation potassium channel blocker; yields a depolarization of pancreatic beta cell membranes, thereby provoking opening of voltage-gated calcium channels and subsequent insulin release
- ad libitum
“as desired”, animals have free access to food
- metyrapone
reversible CYP11B1 (11-beta-hydroxylase) inhibitor; blocks adrenal cortisol synthesis; yields an increase in circulating 11-deoxycortisol, paralleled by enhanced corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) secretion due to a loss of negative feedback inhibition; used in endocrine practice to exclude tertiary adrenal insufficiency/evaluate hypothalamic-pituitary-adrenal (HPA) axis functionality
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have received honoraria, unrestricted educational grants and research funding to the individual or the institution from Alexion (LCH), Amgen (LCH, TDR), Roche (TDR), Shire (LCH, TDR) and UCB (LCH, TDR). YXP is a Co-Scientific founder of Sparian Biosciences. The remaining authors have no potential conflicts of interest to declare.
References
- 1.Pathan H and Williams J (2012) Basic opioid pharmacology: an update. Br J Pain 6 (1), 11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fountas A et al. (2020) Opioid-induced endocrinopathies. Lancet Diabetes Endocrinol 8 (1), 68–80. [DOI] [PubMed] [Google Scholar]
- 3.Porter J and Jick H (1980) Addiction rare in patients treated with narcotics. N Engl J Med 302 (2), 123. [DOI] [PubMed] [Google Scholar]
- 4.Leung PTM et al. (2017) A 1980 Letter on the Risk of Opioid Addiction. N Engl J Med 376 (22), 2194–2195. [DOI] [PubMed] [Google Scholar]
- 5.Rosner B et al. (2019) Opioid prescription patterns in Germany and the global opioid epidemic: Systematic review of available evidence. PLoS One 14 (8), e0221153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudd RA et al. (2016) Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep 65 (50–51), 1445–1452. [DOI] [PubMed] [Google Scholar]
- 7.Martins SS et al. (2015) Worldwide Prevalence and Trends in Unintentional Drug Overdose: A Systematic Review of the Literature. Am J Public Health 105 (11), 2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedegaard H et al. (2020) Drug Overdose Deaths in the United States, 1999–2018. NCHS Data Brief (356), 1–8. [PubMed] [Google Scholar]
- 9.Rummans TA et al. (2018) How Good Intentions Contributed to Bad Outcomes: The Opioid Crisis. Mayo Clin Proc 93 (3), 344–350. [DOI] [PubMed] [Google Scholar]
- 10.Cleeland CS et al. (1994) Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 330 (9), 592–6. [DOI] [PubMed] [Google Scholar]
- 11.Bedson J et al. (2016) Trends in long-term opioid prescribing in primary care patients with musculoskeletal conditions: an observational database study. Pain 157 (7), 1525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florence C et al. (2020) The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol Depend, 108350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portoghese PS (1965) A new concept on the mode of interaction of narcotic analgesics with receptors. J Med Chem 8 (5), 609–16. [DOI] [PubMed] [Google Scholar]
- 14.Adams JE (1976) Naloxone reversal of analgesia produced by brain stimulation in the human. Pain 2 (2), 161–6. [PubMed] [Google Scholar]
- 15.Pasternak GW (2018) Mu Opioid Pharmacology: 40 Years to the Promised Land. Adv Pharmacol 82, 261–291. [DOI] [PubMed] [Google Scholar]
- 16.Law PY et al. (2013) Opioid receptors: toward separation of analgesic from undesirable effects. Trends Biochem Sci 38 (6), 275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butour JL et al. (1997) Recognition and activation of the opioid receptor-like ORL 1 receptor by nociceptin, nociceptin analogs and opioids. Eur J Pharmacol 321 (1), 97–103. [DOI] [PubMed] [Google Scholar]
- 18.Kakidani H et al. (1982) Cloning and sequence analysis of cDNA for porcine beta-neoendorphin/dynorphin precursor. Nature 298 (5871), 245–9. [DOI] [PubMed] [Google Scholar]
- 19.Noda M et al. (1982) Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature 295 (5846), 202–6. [DOI] [PubMed] [Google Scholar]
- 20.Emery MA and Akil H (2020) Endogenous Opioids at the Intersection of Opioid Addiction, Pain, and Depression: The Search for a Precision Medicine Approach. Annu Rev Neurosci 43, 355–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akil H et al. (1998) Endogenous opioids: overview and current issues. Drug Alcohol Depend 51 (1–2), 127–40. [DOI] [PubMed] [Google Scholar]
- 22.Zadina JE et al. (1997) A potent and selective endogenous agonist for the mu-opiate receptor. Nature 386 (6624), 499–502. [DOI] [PubMed] [Google Scholar]
- 23.Terskiy A et al. (2007) Search of the human proteome for endomorphin-1 and endomorphin-2 precursor proteins. Life Sci 81 (23–24), 1593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes I et al. (2020) Biased signaling by endogenous opioid peptides. Proc Natl Acad Sci U S A 117 (21), 11820–11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser AS et al. (2018) Pharmacogenomics of GPCR Drug Targets. Cell 172 (1–2), 41–54.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Convertino M et al. (2015) Differential Regulation of 6- and 7-Transmembrane Helix Variants of μ-Opioid Receptor in Response to Morphine Stimulation. PLoS One 10 (11), e0142826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasternak GW et al. (2020) Emerging Insights into Mu Opioid Pharmacology. Handb Exp Pharmacol 258, 89–125. [DOI] [PubMed] [Google Scholar]
- 28.Pándy-Szekeres G et al. (2018) GPCRdb in 2018: adding GPCR structure models and ligands. Nucleic Acids Res 46 (D1), D440–D446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dreborg S et al. (2008) Evolution of vertebrate opioid receptors. Proc Natl Acad Sci U S A 105 (40), 15487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu A et al. (2018) Sex Associated Differential Expressions of the Alternatively Spliced Variants mRNA of OPRM1 in Brain Regions of C57BL/6 Mouse. Cell Physiol Biochem 50 (4), 1441–1459. [DOI] [PubMed] [Google Scholar]
- 31.Schuller AG et al. (1999) Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci 2 (2), 151–6. [DOI] [PubMed] [Google Scholar]
- 32.Lu Z et al. (2018) Truncated μ-Opioid Receptors With 6 Transmembrane Domains Are Essential for Opioid Analgesia. Anesth Analg 126 (3), 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Z et al. (2015) Mediation of opioid analgesia by a truncated 6-transmembrane GPCR. J Clin Invest 125 (7), 2626–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majumdar S et al. (2011) Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci U S A 108 (49), 19778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefano GB et al. (1995) Presence of the mu3 opiate receptor in endothelial cells. Coupling to nitric oxide production and vasodilation. J Biol Chem 270 (51), 30290–3. [DOI] [PubMed] [Google Scholar]
- 36.Zhang T et al. (2020) A Truncated Six Transmembrane Splice Variant MOR-1G Enhances Expression of the Full-Length Seven Transmembrane. Mol Pharmacol 98 (4), 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuitavi J et al. (2020) The Life Cycle of the Mu-Opioid Receptor. Trends Biochem Sci. [DOI] [PubMed] [Google Scholar]
- 38.Raehal KM et al. (2005) Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314 (3), 1195–201. [DOI] [PubMed] [Google Scholar]
- 39.Narayan A et al. (2020) Mu Opioids Induce Biased Signaling at the Full-Length Seven Transmembrane C-Terminal Splice Variants of the mu Opioid Receptor Gene, Oprm1. Cell Mol Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J et al. (2017) Alternatively spliced mu opioid receptor C termini impact the diverse actions of morphine. J Clin Invest 127 (4), 1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marti-Solano M et al. (2020) Combinatorial expression of GPCR isoforms affects signalling and drug responses. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fricker LD et al. (2020) Five Decades of Research on Opioid Peptides: Current Knowledge and Unanswered Questions. Mol Pharmacol 98 (2), 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gautam S et al. (2015) Longitudinal Analysis of Opioid Analgesic Dose and Diabetes Quality of Care Measures. Pain Med 16 (11), 2134–41. [DOI] [PubMed] [Google Scholar]
- 44.Weibel R et al. (2013) Mu opioid receptors on primary afferent nav1.8 neurons contribute to opiate-induced analgesia: insight from conditional knockout mice. PLoS One 8 (9), e74706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun J et al. (2020) μ-Opioid receptors in primary sensory neurons are involved in supraspinal opioid analgesia. Brain Res 1729, 146623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seeber B et al. (2019) Opioids and reproduction. Vitam Horm 111, 247–279. [DOI] [PubMed] [Google Scholar]
- 47.Coluzzi F et al. (2018) Testosterone deficiency in non-cancer opioid-treated patients. J Endocrinol Invest 41 (12), 1377–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cicero TJ et al. (1979) Endogenous opioids participate in the regulation of the hypothalamus-pituitary-luteinizing hormone axis and testosterone’s negative feedback control of luteinizing hormone. Endocrinology 104 (5), 1286–91. [DOI] [PubMed] [Google Scholar]
- 49.Pedrón N et al. (1996) Effect of naloxone on serum testosterone in adult male rabbits. Arch Androl 37 (1), 15–8. [DOI] [PubMed] [Google Scholar]
- 50.Kletter GB et al. (1992) Acute effects of testosterone infusion and naloxone on luteinizing hormone secretion in normal men. J Clin Endocrinol Metab 75 (5), 1215–9. [DOI] [PubMed] [Google Scholar]
- 51.Tenhola H et al. (2012) Effect of opioid antagonists on sex hormone secretion. J Endocrinol Invest 35 (2), 227–30. [DOI] [PubMed] [Google Scholar]
- 52.Hemmings R et al. (1982) Effect of morphine on the hypothalamic-pituitary axis in postmenopausal women. Fertil Steril 37 (3), 389–91. [DOI] [PubMed] [Google Scholar]
- 53.Ferrer J et al. (1997) Plasma levels of beta-endorphin during the menstrual cycle. Gynecol Endocrinol 11 (2), 75–82. [DOI] [PubMed] [Google Scholar]
- 54.Wehrenberg WB et al. (1982) beta-Endorphin in hypophyseal portal blood: variations throughout the menstrual cycle. Endocrinology 111 (3), 879–81. [DOI] [PubMed] [Google Scholar]
- 55.Vermesh M et al. (1987) Endogenous opioids modulate the inhibitory effects of androgen on the hypothalamic-pituitary axis of normal women. J Clin Endocrinol Metab 65 (6), 1183–6. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez-Nunez V et al. (2013) In vivo regulation of the μ opioid receptor: role of the endogenous opioid agents. Mol Med 19, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubinstein M et al. (1996) Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci U S A 93 (9), 3995–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaschke N et al. (2018) Beta endorphin in serum and follicular fluid of PCOS- and non-PCOS women. Arch Gynecol Obstet 298 (1), 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agirregoitia E et al. (2012) Expression and localization of opioid receptors during the maturation of human oocytes. Reprod Biomed Online 24 (5), 550–7. [DOI] [PubMed] [Google Scholar]
- 60.Zhang C et al. (2020) β-Edorphin predict pregnancy outcome of PCOS and DOR women after IVF-ET. Arch Gynecol Obstet. [DOI] [PubMed] [Google Scholar]
- 61.Petraglia F et al. (1985) Beta-endorphin and met-enkephalin in peritoneal and ovarian follicular fluids of fertile and postmenopausal women. Fertil Steril 44 (5), 615–21. [DOI] [PubMed] [Google Scholar]
- 62.Estomba H et al. (2016) Expression and Localization of Opioid Receptors in Male Germ Cells and the Implication for Mouse Spermatogenesis. PLoS One 11 (3), e0152162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davidson A et al. (1989) Presence of immunoreactive beta-endorphin and calcitonin in human seminal plasma, and their relation to sperm physiology. Fertil Steril 51 (5), 878–80. [DOI] [PubMed] [Google Scholar]
- 64.Agirregoitia E et al. (2006) Expression and localization of delta-, kappa-, and mu-opioid receptors in human spermatozoa and implications for sperm motility. J Clin Endocrinol Metab 91 (12), 4969–75. [DOI] [PubMed] [Google Scholar]
- 65.Ragni G et al. (1985) Semen evaluation in heroin and methadone addicts. Acta Eur Fertil 16 (4), 245–9. [PubMed] [Google Scholar]
- 66.Petraglia F et al. (1986) Endogenous opioid peptides in uterine fluid. Fertil Steril 46 (2), 247–51. [DOI] [PubMed] [Google Scholar]
- 67.Wahlström T et al. (1985) Immunoreactive beta-endorphin is demonstrable in the secretory but not in the proliferative endometrium. Life Sci 36 (10), 987–90. [DOI] [PubMed] [Google Scholar]
- 68.Totorikaguena L et al. (2017) Mu opioid receptor in the human endometrium: dynamics of its expression and localization during the menstrual cycle. Fertil Steril 107 (4), 1070–1077.e1. [DOI] [PubMed] [Google Scholar]
- 69.Schleicher RL (1989) Beta-endorphin inhibits insulin secretion from isolated pancreatic islets. Endocrinology 124 (3), 1254–8. [DOI] [PubMed] [Google Scholar]
- 70.Giugliano D et al. (1989) Beta-endorphin and islet hormone release in humans: evidence for interference with cAMP. Am J Physiol 257 (3 Pt 1), E361–6. [DOI] [PubMed] [Google Scholar]
- 71.Khawaja XZ and Green IC (1991) Dual action of beta-endorphin on insulin release in genetically obese and lean mice. Peptides 12 (2), 227–33. [DOI] [PubMed] [Google Scholar]
- 72.Giugliano D et al. (1988) Beta-endorphin-induced inhibition and stimulation of insulin secretion in normal humans is glucose dependent. Diabetes 37 (9), 1265–70. [DOI] [PubMed] [Google Scholar]
- 73.Giugliano D et al. (1988) Altered metabolic and hormonal responses to epinephrine and beta-endorphin in human obesity. J Clin Endocrinol Metab 67 (2), 238–44. [DOI] [PubMed] [Google Scholar]
- 74.Wen T et al. (2009) The MOR-1 opioid receptor regulates glucose homeostasis by modulating insulin secretion. Mol Endocrinol 23 (5), 671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tudurí E et al. (2016) Acute stimulation of brain mu opioid receptors inhibits glucose-stimulated insulin secretion via sympathetic innervation. Neuropharmacology 110 (Pt A), 322–332. [DOI] [PubMed] [Google Scholar]
- 76.Tabarin A et al. (2005) Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a “thrifty gene”. Diabetes 54 (12), 3510–6. [DOI] [PubMed] [Google Scholar]
- 77.Matthes HW et al. (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383 (6603), 819–23. [DOI] [PubMed] [Google Scholar]
- 78.MacDonald AF et al. (2003) Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol 285 (5), R999–R1004. [DOI] [PubMed] [Google Scholar]
- 79.Ndubuka C et al. (1986) Opiates stimulate food consumption in the land snail Helix aspersa. NIDA Res Monogr 75, 493–6. [PubMed] [Google Scholar]
- 80.Hollander P et al. (2013) Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 36 (12), 4022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fruzzetti F et al. (2002) Effect of long-term naltrexone treatment on endocrine profile, clinical features, and insulin sensitivity in obese women with polycystic ovary syndrome. Fertil Steril 77 (5), 936–44. [DOI] [PubMed] [Google Scholar]
- 82.Peng J et al. (2012) Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend 124 (3), 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keating DJ et al. (2004) Opioid receptor stimulation suppresses the adrenal medulla hypoxic response in sheep by actions on Ca(2+) and K(+) channels. J Physiol 555 (Pt 2), 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kapas S et al. (1995) Action of opioid peptides on the rat adrenal cortex: stimulation of steroid secretion through a specific mu opioid receptor. J Endocrinol 144 (3), 503–10. [DOI] [PubMed] [Google Scholar]
- 85.Twitchell WA and Rane SG (1993) Opioid peptide modulation of Ca(2+)-dependent K+ and voltage-activated Ca2+ currents in bovine adrenal chromaffin cells. Neuron 10 (4), 701–9. [DOI] [PubMed] [Google Scholar]
- 86.Coiro V et al. (2011) Stimulatory effect of naloxone on plasma cortisol in human: Possible direct stimulatory action at the adrenal cortex. Regul Pept 166 (1–3), 1–2. [DOI] [PubMed] [Google Scholar]
- 87.Cozzolino D et al. (2005) Acute pressor and hormonal effects of beta-endorphin at high doses in healthy and hypertensive subjects: role of opioid receptor agonism. J Clin Endocrinol Metab 90 (9), 5167–74. [DOI] [PubMed] [Google Scholar]
- 88.Vescovi PP et al. (1990) Metyrapone effects on beta-endorphin, ACTH and cortisol levels after chronic opiate receptor stimulation in man. Neuropeptides 15 (3), 129–32. [DOI] [PubMed] [Google Scholar]
- 89.Taylor T et al. (1983) beta-endorphin suppresses adrenocorticotropin and cortisol levels in normal human subjects. J Clin Endocrinol Metab 57 (3), 592–6. [DOI] [PubMed] [Google Scholar]
- 90.Ducat E et al. (2013) Mu-opioid receptor A118G polymorphism in healthy volunteers affects hypothalamic-pituitary-adrenal axis adrenocorticotropic hormone stress response to metyrapone. Addict Biol 18 (2), 325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abs R et al. (2000) Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab 85 (6), 2215–22. [DOI] [PubMed] [Google Scholar]
- 92.Sweeney CG et al. (2017) Convergent Balancing Selection on the Mu-Opioid Receptor in Primates. Mol Biol Evol 34 (7), 1629–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwandt ML et al. (2011) OPRM1 gene variation influences hypothalamic-pituitary-adrenal axis function in response to a variety of stressors in rhesus macaques. Psychoneuroendocrinology 36 (9), 1303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gotthardt F et al. (2017) Bone mineral density and its determinants in men with opioid dependence. J Bone Miner Metab 35 (1), 99–107. [DOI] [PubMed] [Google Scholar]
- 95.Coluzzi F et al. (2015) The unsolved case of “bone-impairing analgesics”: the endocrine effects of opioids on bone metabolism. Ther Clin Risk Manag 11, 515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pérez-Castrillón JL et al. (2000) Expression of opioid receptors in osteoblast-like MG-63 cells, and effects of different opioid agonists on alkaline phosphatase and osteocalcin secretion by these cells. Neuroendocrinology 72 (3), 187–94. [DOI] [PubMed] [Google Scholar]
- 97.Pautke C et al. (2004) Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res 24 (6), 3743–8. [PubMed] [Google Scholar]
- 98.Baldock PA et al. (2012) The endogenous opioid dynorphin is required for normal bone homeostasis in mice. Neuropeptides 46 (6), 383–94. [DOI] [PubMed] [Google Scholar]
- 99.Harrison LM et al. (1994) The opiate system in invertebrates. Peptides 15 (7), 1309–29. [DOI] [PubMed] [Google Scholar]
- 100.Stearns S and Medzhitov R, Evolutionary Medicine, Sinauer Associates, Oxford University Press, 2016, p. 306. [Google Scholar]
- 101.Cheong MC et al. (2015) An opioid-like system regulating feeding behavior in C. elegans. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang A et al. (2019) An evolutionary perspective on immunometabolism. Science 363 (6423). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Philippe D et al. (2003) Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest 111 (9), 1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dave KR et al. (2012) Neuroprotection: lessons from hibernators. Comp Biochem Physiol B Biochem Mol Biol 162 (1–3), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tamura Y et al. (2005) Phase-specific central regulatory systems of hibernation in Syrian hamsters. Brain Res 1045 (1–2), 88–96. [DOI] [PubMed] [Google Scholar]
- 106.Tamura Y et al. (2012) Regulatory mechanism of body temperature in the central nervous system during the maintenance phase of hibernation in Syrian hamsters: involvement of β-endorphin. Brain Res 1448, 63–70. [DOI] [PubMed] [Google Scholar]
- 107.Rasmussen NA and Farr LA (2009) Beta-endorphin response to an acute pain stimulus. J Neurosci Methods 177 (2), 285–8. [DOI] [PubMed] [Google Scholar]
- 108.Harte JL et al. (1995) The effects of running and meditation on beta-endorphin, corticotropin-releasing hormone and cortisol in plasma, and on mood. Biol Psychol 40 (3), 251–65. [DOI] [PubMed] [Google Scholar]
- 109.Komaki G et al. (1990) Plasma beta-endorphin during fasting in man. Horm Res 33 (6), 239–43. [DOI] [PubMed] [Google Scholar]
- 110.Parua S et al. (1998) Effect of cold exposure on testicular delta 5–3 beta and 17 beta hydroxysteroid dehydrogenase activities and plasma levels of testosterone in toad (Bufo melanostictus) in breeding and hibernating season: duration-dependent response. Andrologia 30 (2), 105–8. [DOI] [PubMed] [Google Scholar]
- 111.Christeff N et al. (1988) Elevated oestrogen and reduced testosterone levels in the serum of male septic shock patients. J Steroid Biochem 29 (4), 435–40. [DOI] [PubMed] [Google Scholar]
- 112.Kanik KS et al. (2000) Adrenocorticotropin, glucocorticoid, and androgen secretion in patients with new onset synovitis/rheumatoid arthritis: relations with indices of inflammation. J Clin Endocrinol Metab 85 (4), 1461–6. [DOI] [PubMed] [Google Scholar]
- 113.Kandemir N et al. (2018) Bone Parameters in Anorexia Nervosa and Athletic Amenorrhea: Comparison of Two Hypothalamic Amenorrhea States. J Clin Endocrinol Metab 103 (6), 2392–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wildt L et al. (1993) Opiate antagonist treatment of ovarian failure. Hum Reprod 8 Suppl 2, 168–74. [DOI] [PubMed] [Google Scholar]


