Abstract
Temporal lobe epilepsy (TLE) has been conceptualized as focal disease with a discrete neurobiological focus and can respond well to targeted resection or ablation. In contrast the neuro-cognitive deficits resulting from TLE can be widespread involving regions beyond the primary epileptic network. We hypothesize that this seemingly paradoxical findings can be explained by differences in connectivity between the primary epileptic region which is hyper-connected and its secondary influence on global connectome organization. This hypothesis is tested using regional and global graph theory metrics where we anticipate that regional mesial-temporal hyperconnectivity will be found and correlate with seizure frequency while global networks will be disorganized and be more closely associated with neuro-cognitive deficits. Resting state fMRI was used to examine temporal lobe regional connectivity and global functional connectivity from 102 TLE patients and 55 controls. Connectivity matrices were calculated for subcortical volumes and cortical parcellations. Graph theory metrics (global clustering coefficient (GCC), degree, closeness), were compared between groups and in relation to neuropsychological profiles and disease covariates using permutation testing and causal analysis. In TLE there was a decrease in GCC (p=0.0345) associated with a worse neuropsychological profile (p=0.0134). There was increased connectivity in the left hippocampus/amygdala (degree p=0.0103, closeness p=0.0104) and a decrease in connectivity in the right lateral temporal lobe (degree p=0.0186, closeness p=0.0122). A ratio between the hippocampus/amygdala and lateral temporal lobe—temporal lobe connectivity ratio (TLCR) revealed differences between TLE and controls for closeness (left p=0.00149, right p=0.0494) and for degree on left p=0.00169; with trend on right p=0.0567. Causal analysis suggested that “Epilepsy Activity” (seizure frequency, anti-seizure medications) was associated with increase in TLCR but not in GCC, while cognitive decline was associated with decreased GCC. These findings support the hypothesis that in TLE there is hyperconnectivity in the hippocampus/amygdala and hypoconnectivity in the lateral temporal lobe associated with “Epilepsy Activity.” While, global connectome disorganization was associated with worse neuropsychological phenotype.
Keywords: Temporal Lobe Epilepsy, Functional MRI, Functional Connectivity, Connectome, Graph Theory, Causal Analysis
INTRODUCTION:
Temporal lobe epilepsy (TLE) is the most common focal adult epilepsy[1]. Traditionally focal epilepsy was conceptualized as a discrete area referred to as the “epileptogenic zone [2]”, but emerging evidence from invasive stereoEEG [3–5], connectivity analysis using scalp EEG [6,7], as well as structural[8,9] and functional [10,11] MRI suggest that focal epilepsy may affects regions beyond the epileptogenic zone. Supporting the “network hypothesis” [12] of focal epilepsy is the neuropsychology literature examining cognitive deficits in TLE. As expected, it is common to find cognitive deficits attributable to the involved temporal lobe such as verbal memory in left mesio-temporal epilepsy [13], but maybe more surprising is that patients with focal epilepsy often have multi-domain cognitive deficits[14]. Together these data suggest broad network abnormalities in TLE, but these results need to be balanced against the causal evidence from TLE surgical outcome data. Clearly, well-selected patients not only become seizure free [15] but can have an improvement in cognitive deficits [16]. These results raise the question of which of the network abnormalities being highlighted in these studies are markers of the primary epileptic network that is both necessary and sufficient for seizure generation and which are secondary or reactive.
Previous investigations into global and regional connectivity in epilepsy include morphological connectivity based on structural MRI as well as connectivity based on functional MR. There are several pertinent results from these investigations which inform the current study. In particular there are several studies that examine the global connectivity measures as they related to clinical disease variables, neuropsychological profile, and type of epilepsy [17–20]. An example is Garcia-Ramos et al that finds a decreased modularity and a dissociation of the cortical and subcortical networks in juvenile myoclonic epilepsy [21]. For regional connectivity patterns in focal epilepsy, Larivière et al found increased connectivity within the “epileptogenic zone” independent of structural atrophy [10]. Other investigations in focal epilepsy have found decreased connectivity involving subcortical gray structures including the thalamus and nucelus basalis [22–24]. Using time-varying i.e. dynamic resting state connectivity, Yang et al demonstrated a disruption across the default-mode network that varied based on the type of epilepsy [25] as well as previous investigations with dynamic resting state connectivity that showed dynamic shifts in global network dysfunction associated with the epileptiform abnormalities [26]. Despite varying methodologies, the general findings from these and other studies suggest that in the interictal state there is an increased connectivity within the seizure generating network and a more general and dynamic dysfunction of the global connectome architecture. Here we use a relatively large cohort of TLE patients with comprehensive neuropsychological and clinical data to determine how the focal increased connectivity in the hippocampus/amygdala relates to global network dysfunction, epilepsy activity cognitive deficits.
To address these questions, we used the Epilepsy Connectome Project (ECP) database, which contains clinical, neuropsychological, and resting state functional MRI (rs-fMRI) data on patients with TLE (N=102) and controls (N=55). Our analysis used a series of global and nodal graph theory measures on rs-fMRI. Specifically, we hypothesized that there is a global breakdown of the typical modular network connectivity of the brain and that this corresponds to the neuropsychological profile, in line with previous investigations [17–20,27]. On a regional or nodal level we hypothesized that there are regions of increased connectivity [28] as measured by “degree” and a metric of centrality known as “closeness” specifically in the most likely seizure-generating zones of the amygdala and hippocampus. We hypothesized that the surrounding regions of the temporal lobe will be hypoconnected, corresponding to regions influenced by the primary seizure-generating region. In turn, these regional connectome abnormalities may affect the global functional connectome and explain the multi-domain cognitive deficits seen in some patients with TLE.
METHODS:
Participants:
Participants included 102 patients with TLE (seizure lateralization 23 right, 53 left, 7 bilateral, and 19 uncertain, Supplementary Table 1) and 55 healthy controls. Data collection occurred from March 2016 to December 2018 from the Medical College of Wisconsin and University of Wisconsin-Madison. The project was approved by the respective institutional review boards. All participants provided written informed consent.
TLE participants met the following inclusion criteria: age 18–60 years, estimated full-scale Intelligence Quotient ≥70, fluent in English, diagnosis of TLE based on at least two of 1) observed or described semiology consistent with TLE, 2) interictal electrographic evidence of TLE, 3) temporal lobe seizure captured on video EEG monitoring, or 4) mesial temporal sclerosis (MTS) or hippocampal atrophy seen on MRI. Exclusion criteria included 1) presence of a structural lesion on MRI other than MTS or hippocampal atrophy, and 2) suspected or confirmed active infectious/autoimmune/inflammatory etiology for TLE. Healthy controls were aged 18–60 years, fluent in English, with no structural brain lesion, central neurological disease or major psychiatric condition, or contraindication to MRI.
MRI Acquisition:
MRI was performed on 3T General Electric 750 scanners. T1 images were acquired with an MPRAGE sequence using TR/TE=604ms /2.516ms, TI=1060.0 ms, flip angle=8°, FOV=25.6 cm, and 0.8 mm isotropic voxels. rs-fMRI images were obtained with whole-brain 8-band simultaneous multi-slice (SMS) imaging, 72 slices, TR/TE=802 ms/33.5 ms, flip angle 50°, matrix = 104×104, FOV=20.8, and 2.0 mm isotropic voxel with a Nova 32-channel receive head coil. Participants were asked to fixate on a white cross at the center of a black screen during the scans.
MRI Data Processing:
Images were processed with the Human Connectome Project (HCP) processing pipelines[29], based on FreeSurfer [30] and FSL [31], including the non-linear registration tool FNIRT which is able to register brain regions with significant atrophy [32]. More details on the HCP processing can be found in Glasser et al [29]. Using the Connectome Workbench (1.1.1) [33], time-series data from four 5-min rs-fMRI scans acquired in a single session were concatenated. 360 time-series from cortical parcels defined by the Glasser parcellation [34] plus 19 FreeSurfer subcortical regions [35] were extracted. Further pre-processing were performed on rs-fMRI images with AFNI (Analysis of Functional Neuro-Images) [36], including motion regression with 12 parameters, regression-based removal of signal changes in the white matter, CSF, global signal, and band-pass filtering (0.01–0.1 Hz). There is uncertainty regarding motion correction method for multiband images[37], but we performed preprocessing pipelines recommended by the HCP, which included frame-wise registration to the single-band reference image to correct for head motion [38] and regressing 12 parameters (X/Y/Z, pitch/roll/yaw and temporal derivatives), as well as signals from white matter, cerebro-spinal fluid and global signal [39] was considered adequate. There were no differences in absolute or relative RMS motion between groups (P > 0.3).
Connectivity Matrices:
Connectivity matrices were generated from pairwise Pearson correlations between the 379 regions of interest (ROI) time series. Binary undirected adjacency matrices were created by first taking the absolute value of all correlation coefficients and then using a proportional density threshold from 0.35 to 0.65 using 0.05 steps creating a total of 7 binary adjacency matrices per participant. 0.65 was the threshold limit to maintain a fully connected graph for all participants. The threshold density was centered around 0.5 (to assume an uninformed prior) with 0.15 to either side creating the range of 0.35 to 0.65. No optimal method for determining edges has been determined in rs-fMRI connectivity. Binary edges with proportional thresholding were used instead of weighted edges as binary graph theory metrics have a longer history and weighted-equivalents are not available for all measures such as rich-club proportion. Additionally, proportional thresholding is at least one method to account for global differences in correlation matrices between individuals.
Graph Theory Metrics:
Metrics were calculated for each participant at each density threshold. The mean across density thresholds was calculated for each metric on a per participant basis and used for analysis. Metrics were calculated with ‘igraph 1.25’ [40] except rich club proportion, which was calculated using ‘brainGraph 2.7.3’ [41].
Degree:
The degree of node i is the number of edges i makes with all other nodes. Degree is the simplest measure of node connectivity.
Nodal Clustering Coefficient (NCC):
The nodal clustering coefficient of node i Is the number of closed triangles (three vertices with three edges) of which i is a member divided by the number of open (three vertices with two edges) and closed triangles. That is, if a node is connected to two other nodes how likely is it that those nodes are connected with each other.
Closeness Centrality[42]:
Closeness Centrality is a measure of how closely connected node i is with the rest of the graph, and is calculated
Where d(i,j) is the shortest path between node i and node j.
Rich Club Proportion (RCP).
RCP is defined as the proportion of the graph nodes within the rich-club core using the random walk methodology described by Ma and Mondragón [43].
Global Clustering Coefficient (GCC):
GCC is the mean of all nodal clustering coefficients for a given graph.
Regional Nodal Metrics:
The temporal lobes were each divided into three regions: lateral, medial, and amygdala/hippocampal. The first two networks were defined by the HCP description of medial and lateral temporal networks[34] and are surface based. Each of these networks contains 8 nodes. The amygdala/hippocampal region is volumetric and defined by FreeSurfer segmentation with 2 nodes. The mean of the nodes within each region was used for analysis. A ratio of the graph theory metrics from the ipsilateral amygdala/hippocampus region to the lateral temporal region was generated and termed the temporal lobe connectivity ratio (TLCR).
Neuropsychological Testing:
Neuropsychological evaluation included 16 tests covering intelligence, language, visuospatial processing, memory, executive functions, and psychomotor speed (Figure 1).
Figure 1:
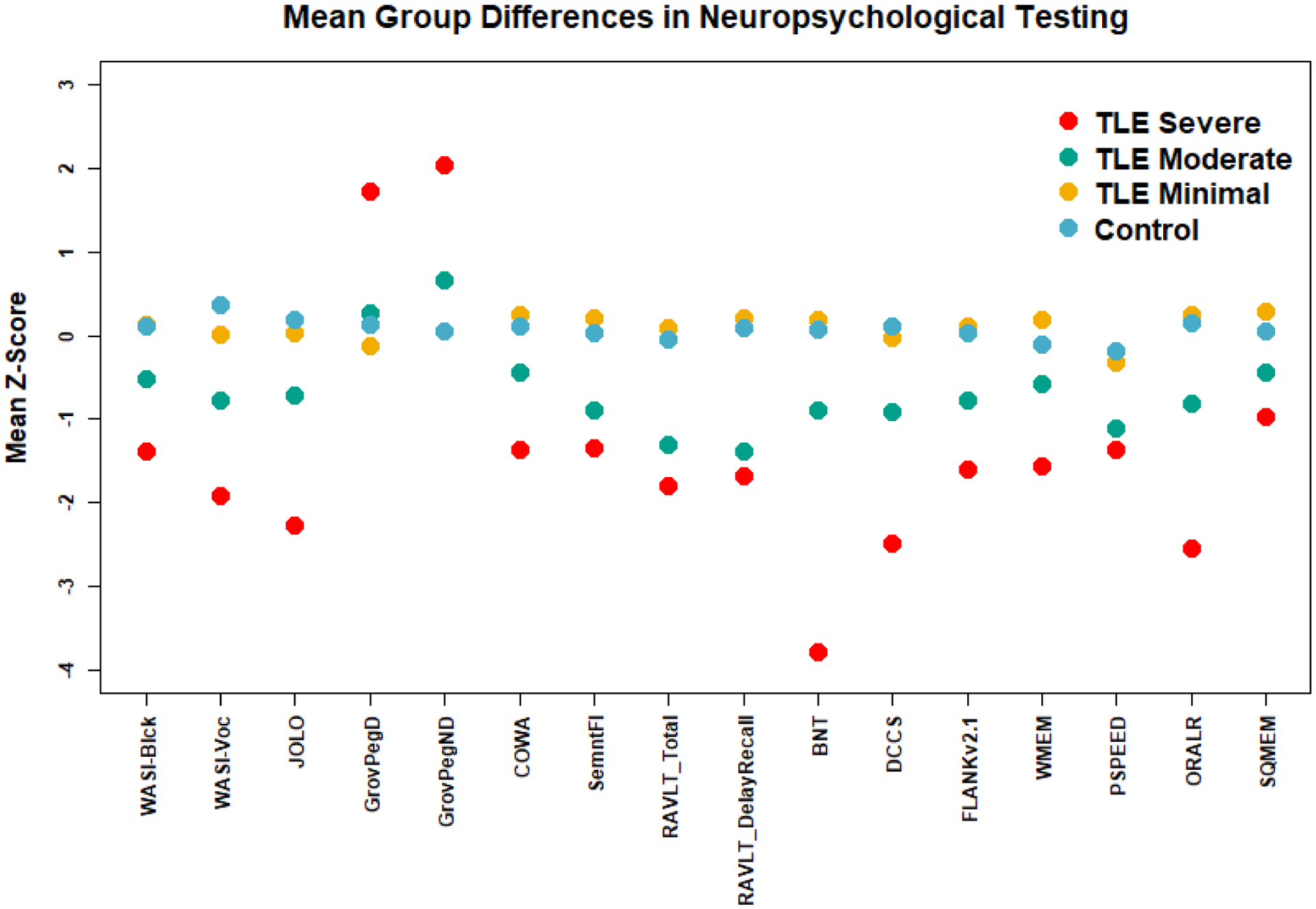
Difference in neuropsychological tests between controls and groups defined by k-means clustering analysis of neuropsychological testing on the TLE subjects. TLE groups are labeled ‘Minimal’, ‘Moderate’, and ‘Severe’ based on degree of cognitive impairment. Abbreviations for tests, WASI-Blck: Wechsler Abbreviated Scale of Intelligence Block Design; WASI-Voc: Wechsler Abbreviated Scale of Intelligence Block Design; JOLO: Judgement of Line Orientation; GrovPegD and GrovPegND: groved pegboard dominant and non-dominant; COWA: controlled oral word association test, SemnFl: semantic fluency; RAVLT_Total and RAVLT_DelayedRecall: Rey auditory verbal learning test total words and delayed recall; BNT: Boston naming test. Measures from the NIH Toolbox Cognitive Batter include: DCCS: dimensional change card sort task; FLANKv2.1: flanker inhibitory control and attention test version 2.1; WMEM: list sorting working memory; PSPEED: pattern comparison processing speed; ORALR: oral reading recognition; SQMEM: picture sequence memory test. Scores were z-scored using mean and standard error from controls. Of note elevated scores from the grove pegged tests indicate increased time of task completion with higher scores consistent with impairment.
Clustering into Neuropsychological Phenotypes:
The 16 age-adjusted z-scored neuropsychological metrics were used for K-means cluster analysis. Gap statistic was used to determine optimal number of clusters[44] with 1000 bootstraps to estimate error. Group stability was assessed using 1000 trials of bootstrapping. Clustering analysis was performed with ‘cluster’ package 2.1.0 [45] and optimal cluster number with ‘NBClust’ package 3.0 [46]. This clustering approach differs from one used previously to identify cognitive subgroups within this TLE population [47], but represents a more unsupervised approach as previously clinician-determined assignments of tests to a limited number of cognitive domains were applied with the cognitive domains then subjected to clustering, whereas the current approach makes no such a priori assumptions or assignments and leaves the cognitive tests free to covary into discrete groups without bias.
Statistical Procedure:
All comparison of means between two or several groups was performed using two-tailed permutation testing (‘perm’ 1.0.0 [48]). P-values were adjusted for multiple comparison using the methodology of Benjamini [49] with false discovery rate (FDR) of <0.05.
Causal Inference:
Causal inference in statistics is a methodology developed by Dr. Pearl [50] and utilizes investigator proposed diagrams to visualize potential casual relationships between the putative model variables. Associated with the diagram are a series of structural equations. If enough of the variables are measureable and meet requirements such as the “Back-Door Criteria” some of the casual relationships of the model may be testable by examining conditional statistical dependencies. Examples of this techniques and are found in http://www.dagitt.net. Similar methodologies which do not utilize causal diagrams, but have similar underlying mathematical premises are described by Dr. Rubin et al and include propensity score matching and instrumental variables [51]. To this end a causal diagram was created to illustrate the proposed relationships between the connectivity metrics and TLE clinical/neuropsychological factors. The conditional independencies predicted by the model were tested to confirm or disconfirm underlying model assumptions. The causal model was used to explore the specific hypothesis that regional hyperconnectivity and the surrounding hypoconnectivity (described as the temporal lobe connectivity ratio-TLCR) is correlated with ‘Epilepsy Activity’. To examine this hypothesis, the conditional dependencies between seizure frequency, anti-seizure medications, and TLCR were assessed using global connectivity metrics and neuropsychology testing as outcome variables. WASI-Vocabulary and WASI-Block design were selected for left and right hemispheric dominant measures respectively. This procedure was performed independently for both right- and left-lateralized TLE. Variable dependencies and conditional independence were calculated using Pearson partial correlation coefficients and correlation coefficients, with a p<0.05, (with no correction) used to determine if a significant dependency was present between variables. ‘Dagitty 0.2–2’ [52] was used for causal analysis, ‘ppcor 1.1’ [53] was used to calculate partial correlation coefficients.
All analyses were performed with R version 4.0.0 (Vienna, Austria).
RESULTS:
Demographics:
There were 102 TLE and 55 controls. Cofactors evaluated included, Age: TLE cohort mean 39.9 years, standard deviation (SD) 11.6, range 19 to 59; Controls mean 31.6 years, SD 10.0, range 18 to 60, p-value=<0.001; Sex: TLE 62 female (60.1%), Controls 27 female (49.1%), p-value=0.18 (uncorrected). More demographic and epilepsy covariates for the TLE subjects are found in Supplementary Table 1. Hippocampal atrophy using adjusted (for age and intra-cranial volume) hippocampal volumes and a threshold (z ≤ −2), 11 (11%) of the TLE group had hippocampal atrophy, 5 (5%) had unilateral left hippocampal atrophy, 3 (3%) had unilateral right hippocampal atrophy, and 3 (3%) had bilateral hippocampal atrophy. To ensure that the hippocampal connectivity for closeness and degree was not an epiphenomenon related to ipsilateral hippocampal volume, a correlation test was performed with uncorrected p-values to identify this possible confounder. For left hippocampal volume there no significant correlation with degree (r=−0.08, p=0.32) or closeness (r=−0.08, p=0.31). Similarly, for right hippocampal volume there no significant correlation with degree (r=0.05, p=0.55) or closeness (r=0.05, p=0.52).
Given the group mean differences in age between TLE and control. Age was evaluated as a potential confounder and was found to be uncorrelated with regional metrics, reported with uncorrected p-values (degree p=0.868, closeness p=0.581, NCC p=0.0823) or global metrics (GCC p=0.799, RCP p=0.0848).
Neuropsychological Clusters:
The optimal number of clusters was determined to be three with a gap statistic of 0.403. Figure 1 shows the mean score for each cluster and controls on the 16 neuropsychological tests. The TLE ‘Minimal’ group is similar in cognitive profile to the controls. The ‘Moderate’ had specific deficits in semantic fluency (animal naming), verbal memory and visual-spatial ability. The ‘Severe’ group had widespread cognitive deficits.
Global Graph Theory Metrics: Rich Club Proportion and Global Clustering Coefficient
Group Comparison: TLE versus Controls
The GCC was significantly lower in TLE than in controls (p=0.0345). Mean RCP was higher across all sparsity levels but did not reach statistical significance (p=0.106), (Figure 2). P-values used FDR correction for 2-comparisons.
Figure 2:
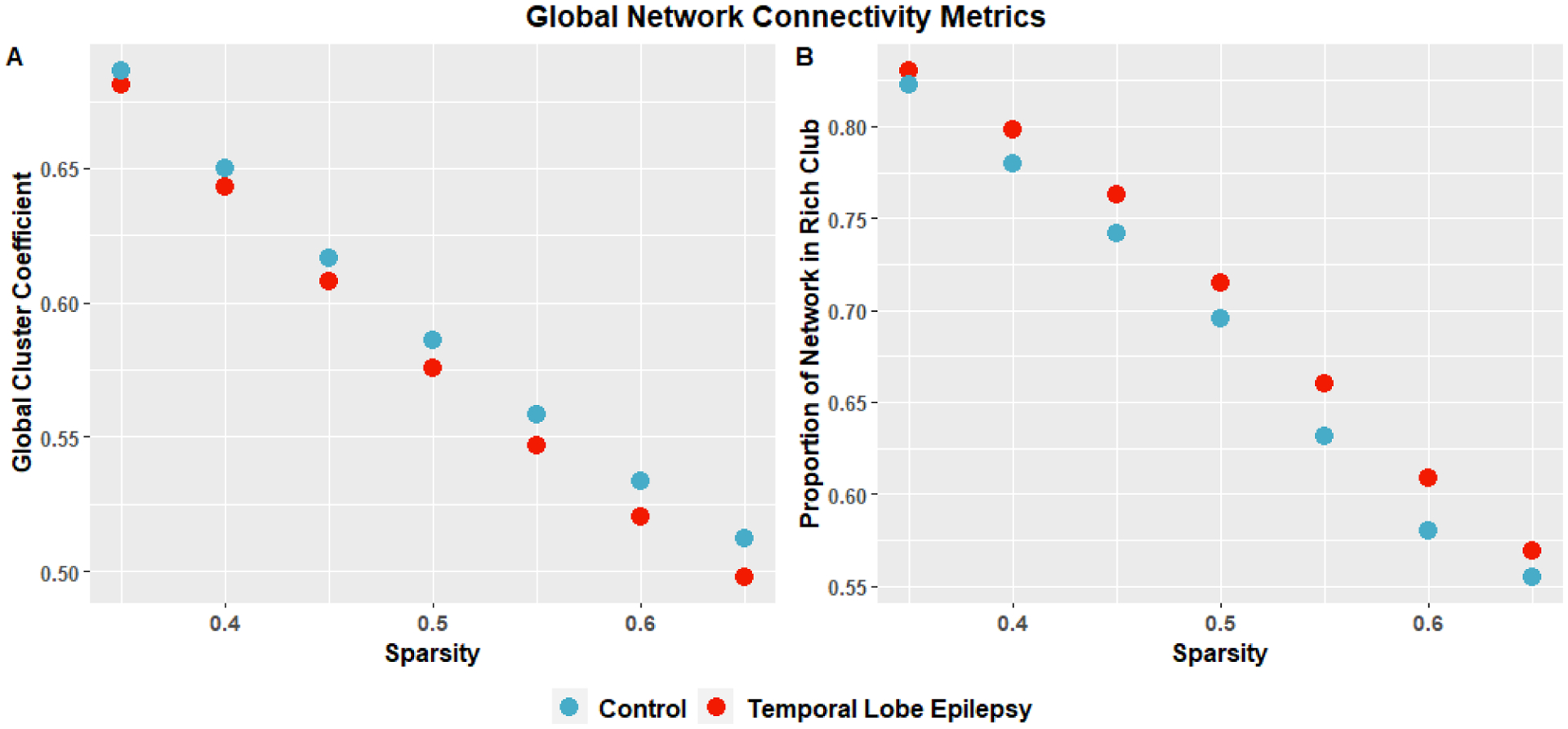
Comparison of global connectivity metrics: global clustering coefficient, and Rich Club Proportion across different proportional threshold connectivity matrices from 0.35 to 0.65. Using permutation testing with correction for FDR for the mean across sparsity levels were significant with Global Cluster Coefficient: p=0.0345, TLE: mean=0.582, standard deviation=0.0275; Controls: mean=0.592, standard deviation=0.0289. Rich Club Proportion: p=0.106, TLE: mean=0.706, standard deviation=0.0740; Control: mean=0.687, standard deviation=0.0740.
Correlation with Seizure Frequency
Linear regression between global metrics and overall seizure frequency showed no significant results were found (GCC p=0.844, RCP p=0.952). P-values used FDR correction for 2-comparisons.
Comparison between Neuropsychological Clusters
There are significant differences in mean GCC (p=0.0134) and RCP (p=0.0500) between the TLE neuropsychological clusters ‘Minimal’, ‘Moderate’, and ‘Severe’ and controls (Figure 3) using 4-sample permutation testing. GCC tended to a decrease from minimum to severe while the mean RCP tended to increase with neuropsychological severity. P-values used FDR correction for 2-comparisons.
Figure 3:
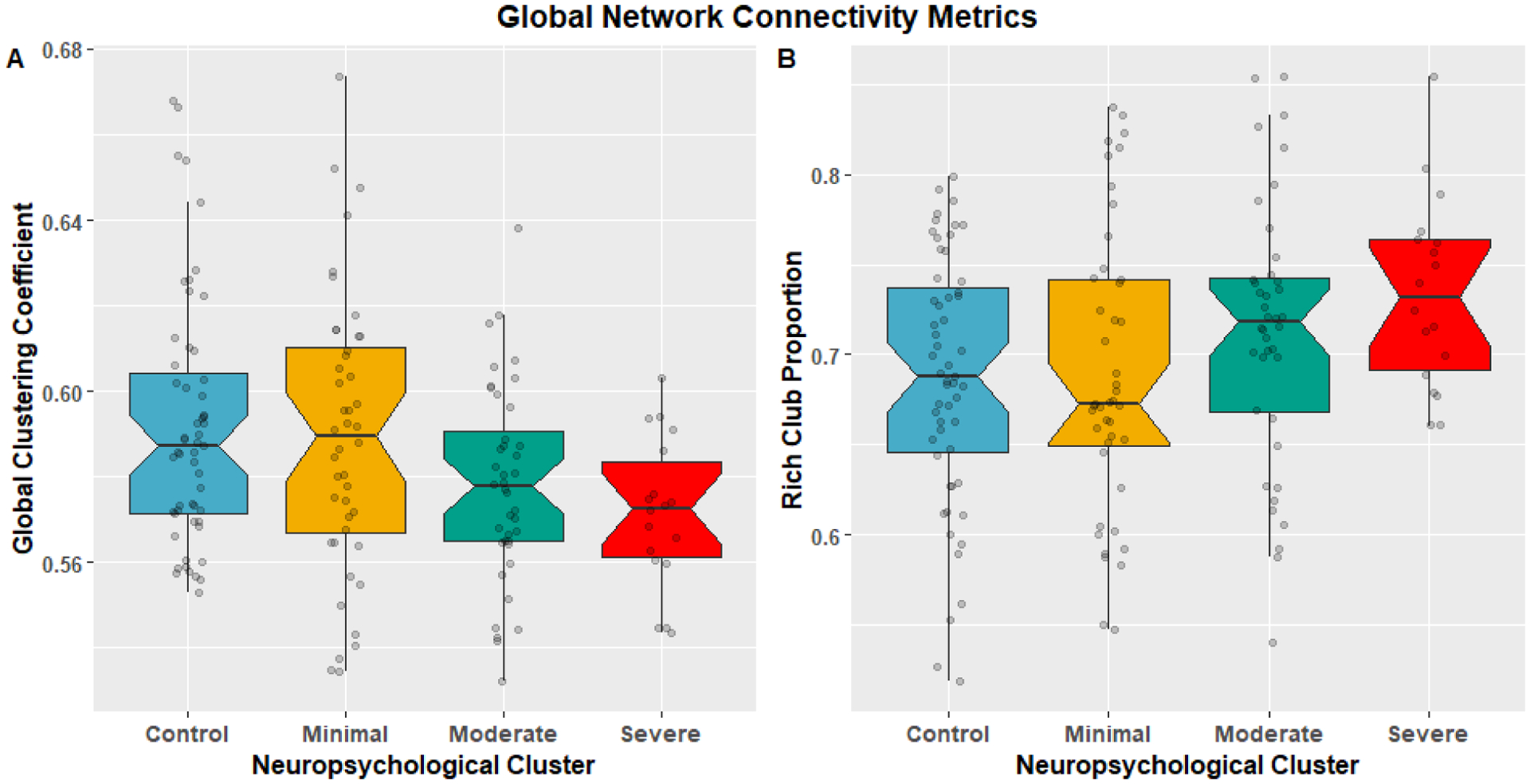
Notched boxplots comparing the distributions of global GT metrics global clustering coefficient (A), and Proportion of Rich Club (B) with superimposed subject scatter plot between TLE neuropsychological clusters. Using permutation testing with correction for FDR for the mean across sparsity levels were significant with Global Cluster Coefficient: p=0.0134; Control: mean=0.5918, standard deviation=0.0289; Minimal: mean=0.589 standard deviation=0.0325; Moderate: mean=0.578, standard deviation=0.0231; Severe: mean=0.572, standard deviation=0.0175. Rich Club Proportion: p=0.05; Control: mean=0.687, standard deviation=0.0694; Minimal: mean=0.690, standard deviation=0.0786, Moderate: mean=0.712, standard deviation=0.0734; Severe: mean=0.734, standard deviation=0.0526.
Nodal Metrics: Nodal Clustering Coefficient, Degree, and Closeness
Mean Regional Nodal Metric, Group Comparison TLE versus Control:
Figure 4. There were significant increases in degree (p=0.0103) and closeness (p=0.0104) in the left amygdala/hippocampus and decreases in degree (p=0.0186) and closeness (p=0.0232) in the right lateral temporal lobe for TLE. For NCC there was a decrease in TLE for the left lateral temporal (p=0.0363), right amygdala/hippocampus (p=0.0141), right medial temporal (p=0.00930), and right lateral temporal (p=0.0122) nodes. P-values used FDR correction for 12-comparisons.
Figure 4:
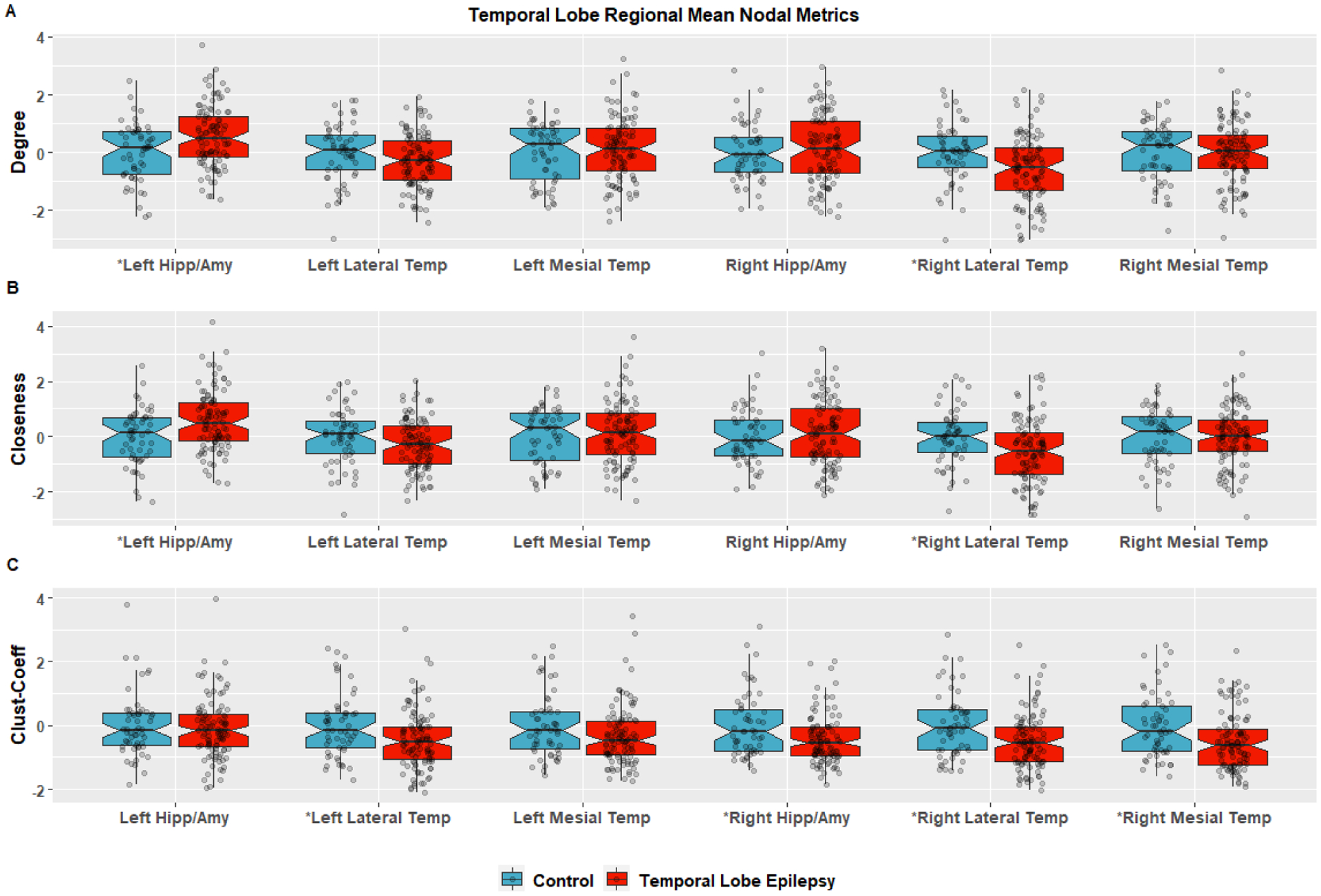
Regional graph theory metrics are the mean of the nodal metrics for that region specified by the Glasser et al parcellation/network scheme[34] for the Lateral Temporal the Mesial Temporal Lobes. These are regions based on surface vertices, which are limited in their ability to map connectivity for subcortical regions so the amygdala and hippocampal region is volumetric and based of freesurfer segmentation. The mean regional Degree is in plot (A), Closeness is (B) and the regional Clustering Coefficient is in plot (c). The data is scaled by the control mean and standard deviation for ease of visual comparison. Group comparisons were performed with two-sided permutation testing FDR correction and those with an *were significant for (A) Degree for left hippocampus/amygdala (p=0.0103), right lateral temporal (p=0.0186) and for Closeness (B) left amygdala/hippocampus (p=0.0104) and Right lateral temporal lobe (p=0.0122). Other regions did not have a statistical difference for closeness or degree. For regional Clustering Coefficient (C) there was a decrease in TLE versus control for the left lateral temporal (p=0.0363), right amygdala/hippocampus (p=0.0141), right medial temporal (p=0.00930), and right lateral temporal (p=0.0122).
Temporal Lobe Connectivity Ratio (TLCR):
From the group comparison, a trend was observed for a decrease in connectivity (degree, closeness) in the lateral temporal lobe and an increase in the amygdala/hippocampus. To quantify this observation a ratio was calculated for each participant (amygdala/hippocampus divided by lateral temporal) for both closeness and degree. This ratio was termed the “temporal lobe connectivity ratio” (TLCR). The TLCR was checked with a Shapiro test for deviation from normality with no significant results (Degree: left p=0.7557, right p=0.093; Closeness: left p=0.755, right p=0.166) and then z-scored using controls mean and SD.
TLCR, TLE versus Control:
The left and right z-scored TLCR (zTLCR) were compared between TLE and controls for both degree and closeness. For degree the Left zTLCR was (TLE: mean=0.587, SD=1.02, p-value=0.00169), for degree Right zTLCR (TLE: mean=0.434, SD=1.5, p-value=0.0567). For closeness the Left zTLCR (TLE: mean=0.45, SD=0.97, p-value=0.00149); Right zTLCR (TLE: mean=0.452, SD=1.52, p-value=0.0494). In summary, there was a significant group difference in right and left zTLCR for closeness; for degree zTLCR was significant on the left and showed a trend on the right. P-values used FDR correction for 4-comparisons.
zTLCR, Epilepsy Laterality:
The left zTLCR was significantly different between groups with 3-sample permutation testing (controls, left TLE, right TLE) for both degree (p=0.0321) and closeness (p=0.0456). Right zTLCR shows a trend towards a difference for both degree (p=0.0720) and closeness (p=0.0630). Figure 5. P-values used FDR correction for 2-comparisons.
Figure 5:
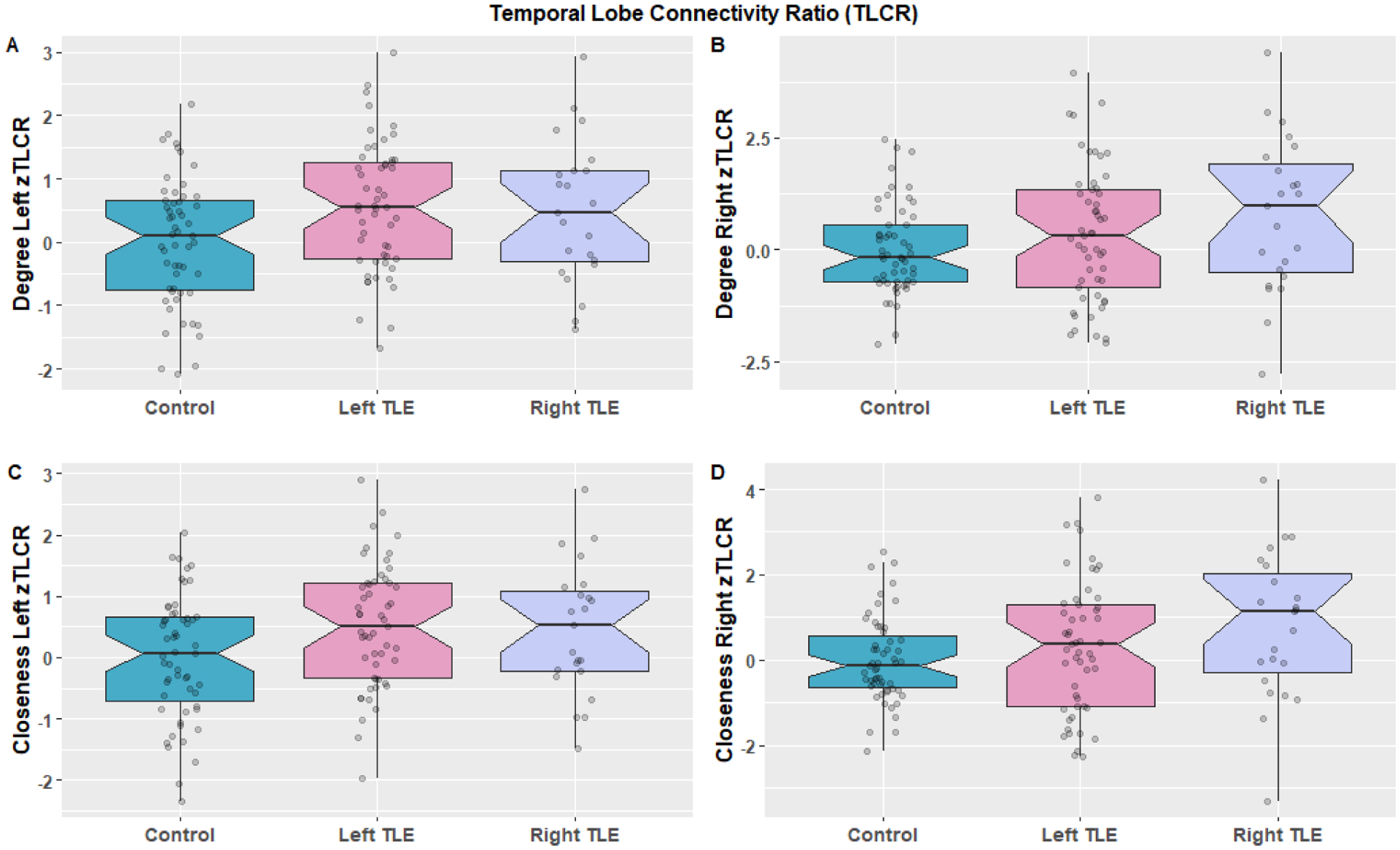
Comparison of the left and right temporal lobe connectivity ratio defined as the amygdala/hippocampus regional connectivity divided by the ipsilateral lateral temporal lobe connectivity which was then z-scored based on mean and standard deviation of controls. This was calculated for both Degree (A,B) and Closeness (C,D) and groups were compared between controls left lateralized epilepsy and right lateralized epilepsy using permutation testing with FDR correction. (A) Degree Left zTLCR (p=0.0321; Left TLE: mean=0.559, standard deviation=1.02; Right TLE: mean=0.479, standard deviation=1.12), (B) Degree Right zTLCR (p=0.0720; Left TLE: mean=0.341, standard deviation=1.49; Right TLE: mean=0.765, standard deviation =1.71). (C) Closeness Left zTLCR (p=0.0465; Left TLE: mean=0.515, standard deviation =0.993; Right TLE: mean=0.460, standard deviation =1.06); Closeness Right zTLCR (p=0.0630; Left TLE: mean=0.316, standard deviation=1.54; Right TLE: mean=0.806 standard deviation=1.71).
zTLCR in Neuropsychological Clusters:
Right and left zTLCR for both degree and closeness were calculated for the TLE neuropsychological clusters ‘Minimal’, ‘Moderate’, ‘Severe’ and controls. There was no significant differences found for 4-sample permutation testing between the 4 groups: Degree Left zTLCR (p=0.991), Right zTLCR (p=0.930); Closeness Left zTLCR (p=0.997), Right zTLCR (p=0.887). P-values used FDR correction for 5-comparisons.
zTLCR correlation with epilepsy activity.
TLE participants were divided into those with unifocal right or left TLE. A linear model was fit for overall seizure prevalence (mean seizure frequency for a month, including all seizure types) for left TLE and left zTLCR and for right TLE relative to right zTLCR for both degree and closeness. There was a significant correlation for right zTLCR for both degree (p=0.00433) and closeness (p=0.00621), with a positive coefficient for both indicating that an increase in zTLCR is associated with more frequent seizures. There was not a significant correlation for in left zTLCR for either degree (p=0.359) or closeness (p=0.255). However, left degree zTLCR was significantly correlated with number of anti-seizure medications (#ASM), (p=0.0381). This relationship is examined further in the following section. P-values used FDR correction for 6-comparisons.
Causal Inference:
A causal diagram representing the relationship between the focal and global connectivity measures and other epilepsy related variables is proposed (Figure 6). Variables L1–L3 represent latent variables. L1 is “Epilepsy Activity”, which represents the time-dependent instantaneous risk of a seizure. It is dependent on the underlying “Epilepsy Severity” (L2), the unobserved environmental and genetic variables (U1) and #ASM (E1). U1 has components that are endogenous (e.g. hormonal cycles, and circadian rhythms) and exogenous (e.g. sleep deprivation, environmental stress, alcohol use). L2 “Epilepsy Severity” is considered static for this model but in reality may change slowly over time and reflects the underlying propensity for seizure generation. “Epilepsy Severity” directly influences the L1 and is primarily determined by the underlying etiology of the epilepsy (U2) which may be related to genetic and/or acquired underlying factors such as variability in ion channel morphology and expression, and structural brain pathology. There is controversy[54] over whether the ‘Epilepsy Activity’ (L1) can in turn lead to a progressive worsening of the underlying ‘Epilepsy Severity’ (L2), but given the cross-sectional nature of cohort used here this relationship is assumed to be unidirectional. L3 is conceptually similar to general cognitive capacity with domain-specific abilities measured via neuro-psychological testing M3. L3 is dependent on underlying genetic and environmental factors (U3) such as underlying general intelligence (“g”) and education. The M variables are measureable and the following are used for analysis, M1-Seizure Frequency: overall seizure frequency per month including all seizure types, M2: the zTLCR (right and left) calculated from degree, M3: a measure of global disruption of functional connectivity and quantified as the global clustering coefficient. M4: WASI Vocabulary (WASI-V) for left TLE, WASI Block Design (WASI-B) for right TLE. E1 is a measureable exposure and quantified as the number of anti-seizure medications (#ASM) at the time of imaging.
Figure 6:
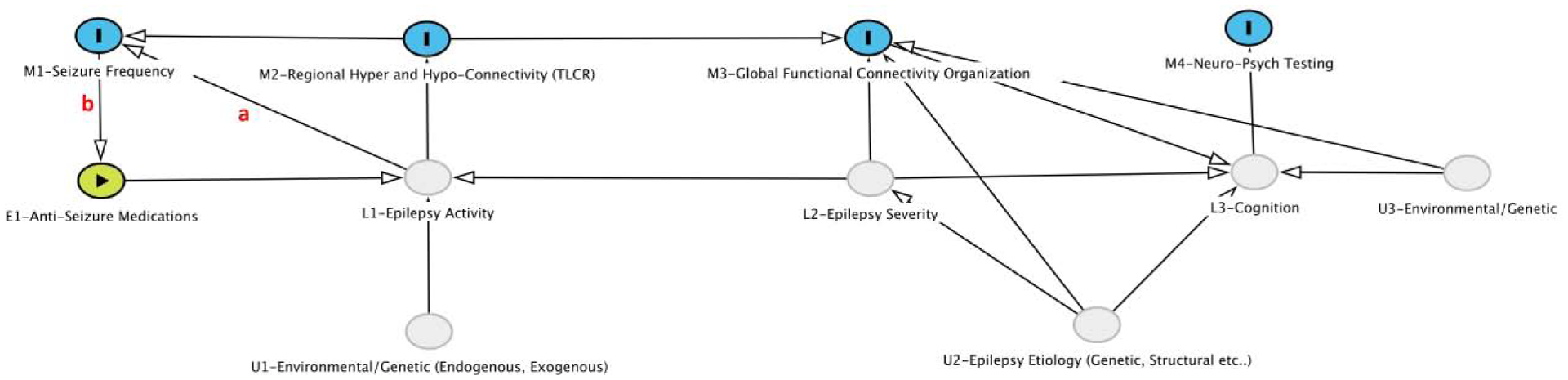
Causal diagram of relationships between latent variables (L1–L3), measured variables (M1–M4), and unobserved variables (U1–U3) and a measureable exposure (E1) in TLE. Arrows represent direction of effect. The red a and b are specific effects that are explored in more detail in the causal analysis. Further description of the variables and analysis is found the “Result” section.
This model was used to determine if the influence of L1 (Epilepsy Activity) on M1 (Seizure Frequency) is primarily mediated by M2 (zTLCR). Specifically, is the arrow a between L1 and M1 necessary (is there influence on L1 on M1 outside of M2)? To address this question, we first need to examine the influence of ‘Seizure Frequency’ on the #ASM (b) as the relationship is potentially fraught with complex dependencies as seizures lead to increasing ASM, which in turn are expected to suppress epilepsy activity and seizure frequency. We examine the model independently for right and left TLE using the ipsilateral degree TLCR.
Right Temporal Lobe Epilepsy:
In the absence of both a and b pathways, the following conditional independence would apply: M1⊥E1|M2—that is Seizure Frequency (M1) is independent of #ASM (E1) conditional on TLCR (M2). This, was the case with a p=0.350, r=0.210. Of note without conditioning, M1 is dependent on M2 (p=0.004, r=0.572). Based on this result for right TLE b can be ignored. Further conditional independencies that would apply only in the absence of a include M3⊥M1|M2 (p=0.788, r=−0.0610) and M4⊥M1|M2 (p=0.623, r=0.111). That is global clustering coefficient (M3) is independent of seizure frequency (M1) conditional on zTLCR (M2) and WASI-B (M4) is independent of seizure frequency (M1) conditional on zTLCR (M2). Without conditioning, the Global Clustering Coefficient (M3) is dependent on zTLCR M2 (p=0.004, r=−0.582), but not seizure frequency (M1) (p=0.079, r=−0.373) showing that the relationship between M2 and M3 is stronger than between M1 and M3. While neither correlation coefficient is significant between WASI-B and zTLCR (p=0.265, r=−0.242) or WASI-B and seizure frequency (p=0.819, r=−0.0504) the overall magnitude of the correlation is higher with zTLCR. These data suggest that the dependency of “Seizure Frequency” on “Epilepsy Activity” goes through an intermediary of zTLCR and the a pathway can be ignored.
Left Temporal Lobe Epilepsy:
In contrast to right TLE, in left TLE M1⊥E1|M2 is not true—in other words seizure frequency (M1) remains dependent on the number of #ASM conditional on zTLCR (p=0.0381, r=0.288), this means the b pathway is present i.e. seizure frequency is positively correlated with #ASM (p=0.0226, r=0.313)—essentially both seizure frequency and anti-seizure medication need to be considered when using clinical data to assess the underlying “Epilepsy Activity”. Therefore, in assessing conditional independences the #ASM is relevant and the conditional independencies become: M3⊥E1|(M1, M2) and M4⊥E1|(M1, M2). So global clustering coefficient (M3) is independent of #ASM (E1) conditional on both seizure frequency (M1) and ipsilateral zTLCR (M2) with a p=0.332 and r=−0.136. Without conditioning, the Global Clustering Coefficient is significantly correlated with both zTLCR (p=<0.001 r=−0.597) and #ASM (p=0.0205 r=−0.318).
The verbal intelligence test (WASI-V) was used as M4 for left TLE and a similar pattern emerges as occurred in right TLE, M4⊥E1|(M1, M2), (p=0.102, r=−0.231), so WASI-V is conditionally independent of #ASM, but without conditioning is significantly correlated with #ASM (p=0.0286 r=−0.301). This result shows again that a pathway can be ignored in left TLE as well as it was for right TLE—that “Epilepsy Activity” is best measured through zTLCR in this conceptual causal framework.
DISCUSSION:
Using rs-fMRI based functional connectivity analysis on 102 TLE participants and 55 controls we demonstrated a statistical difference in the global graph theory measure of GCC. When comparing between the TLE neuropsychological phenotypes there was a significant difference between group means for both RCP and GCC, with a declining GCC and increasing RCP associated with worse cognitive profile (Figure 2). These findings suggest a breakdown of the typical macro-scale inter-regional network connectivity in TLE associated with multi-domain cognitive dysfunction. On nodal analysis there were regions of both increased connectivity (amygdala/hippocampus) and areas of decreased connectivity (lateral temporal lobes). The global measures were more closely related to neuropsychological deficits while the regional metrics were more closely aligned with average number of seizures, this second finding was explored further with causal analysis and it was found that the ratio of amygdala/hippocampus to lateral temporal lobe (TLCR) has a putative causal relationship to “Epilepsy Activity” conceptualized as the time-varying probability of a seizure. TABLE 1 is a summary of major findings from the study with brief explanations.
TABLE 1:
Summary of Graph Theory Results
| Global Clustering Coefficient | |||||
| TLE-Minimal Cognitive | TLE-Moderate Cognitive | TLE-Severe Cognitive | |||
| Controls | Deficits | Deficits | Deficits | ||
| Mean | 0.592 | 0.589 | 0.578 | 0.572 | p=0.0134 |
| Decreased global clustering coefficient is associated with worsening cognitive deficits in TLE. This finding suggests that the normal clustering of neural activity within tightly connected networks is decreased in TLE and related to cognitive deficits. | |||||
| Rich Club Proportion | |||||
| TLE-Minimal Cognitive | TLE-Moderate Cognitive | TLE-Severe Cognitive | |||
| Controls | Deficits | Deficits | Deficits | ||
| Mean | 0.687 | 0.690 | 0.712 | 0.734 | p=0.0500 |
| Similar to Global Clustering Coefficient, the Rich Club Proportion reflects how brain regions organize into subnetworks; specifically in this case the metric is the proportion of overall regions contained in the rich club core. Essentially this finding of increased Rich Club Proportion in TLE means that the traditional “Rich Club” of highly connected regions, which serve as efficient hubs for interaction of brain networks, is diminished. The “Rich Club” core in TLE has more regions, which are overall less connected making for less overall efficient organization. | |||||
| Degree | |||
| Left Temporal Connectivity Ratio (mesial temporal divided by lateral temporal) | |||
| Left Lateralized TLE | Right Lateralized TLE | ||
| Mean | 0.559 | 0.479 | p=0.0321 |
| Right Temporal Connectivity Ratio | |||
| Left Lateralized TLE | Right Lateralized TLE | ||
| Mean | 0.341 | 0.765 | p=0.0720 |
| Degree is the simplest measure of how many connections a region has. The above results show that the mesio-temporal structures are highly connected with other regions, while the lateral temporal lobes are less connected. These findings also correlate with seizure frequency and laterality of the epilepsy. | |||
| Closeness Centrality | |||
| Left Temporal Connectivity Ratio | |||
| Left Lateralized TLE | Right Lateralized TLE | ||
| Mean | 0.515 | 0.46 | p=0.0465 |
| Right Temporal Connectivity Ratio | |||
| Left Lateralized TLE | Right Lateralized TLE | ||
| Mean | 0.316 | 0.806 | p=0.0630 |
| Closeness Centrality measures how many steps it would take to be connected with any other brain region, so it is marker of how connected a node is to the rest of the brain. This finding is similar to Degree it that shows that the mesial-temporal structures are hyperconnected to the rest of brain and the opposite occurs in lateral temporal lobes. Again, this finding correlates with seizure frequency and laterality. | |||
Neuropsychological Clusters:
K-means clustering demonstrated three stable neuropsychological clusters with a minimally affected group, which has a cognitive profile similar to controls and similar global GT metrics; a moderately affected group, which has deficits in memory, fluency, and visual-spatial tests, and severely affected group with widespread cognitive deficits and lower GCC and higher RCP. These results are largely concordant to the evolving literature of neuropsychological phenotypes in TLE [14]. As noted, our clustering approach differed from one used previously with this dataset to identify cognitive subgroups [47], but even with the completely unsupervised approach used here the same three phenotype groups were identified pointing to the robustness of the findings. Somewhat paradoxical are the often widespread cognitive deficits in TLE despite being a seemingly focal disease process, which is an argument in favor of the network hypothesis of focal epilepsy.
Global Graph Theory Measures:
Similar to prior studies there was a breakdown of the typical macroscale connectivity pattern in TLE. This disruption can be quantified in several ways, including a decrease in GCC [17], decrease in small worldness [18], or increase in RCP [19]. The common theme amongst these various studies is that nodes that would typically be clustered together in a network are instead less organized with seemingly more random connections. Similar results have been obtained with structural connectivity showing decreased GCC and increased efficiency[20]. It is unclear if this process causes the cognitive deficits associated with TLE or is a compensatory mechanism. In the causal analysis proposed in this study (Figure 6), we suggest that the disruption in global connectome organization is a means by which focal epilepsy causes multi-domain cognitive dysfunction and therefore there should be a closer relationship between these global graph theory metrics and the cognitive profile versus the measure of the “Epilepsy Activity”, which was the case.
Regional Connectivity:
The regional connectivity showed an increase in connectivity in the hippocampus and amygdala and a decrease in the lateral temporal lobe best quantified by the temporal lobe connectivity ratio (TLCR), a ratio between the ipsilateral amygdala/hippocampus and lateral temporal lobe region. Likely most of these patients have epilepsy originating from the hippocampus and/or amygdala given that is the most common form of TLE, around 89% [55]—suggesting the region of hyperconnectivity is likely the seizure generating region. The surrounding regions of hypoconnectivity are potentially secondarily involved or a compensatory response to hippocampal/amydalar hyperconnectivity, though more detailed invasive EEG studies and surgical outcomes are needed to refine and prove this hypothesis. Contrary to the global metrics the regional connectivity did not correlate well with cognitive deficits, but they did with “Epilepsy Activity”.
A recent investigation with concordant findings, is an fMRI study on 30 surgical TLE patients and 57 controls. They found a reduction in the functional connectivity “difference distance” in the temporoinsular and prefrontal networks, these findings are consistent with our finding of increased connectivity in relationship to the putative epileptogenic region. Of note they performed morphological analysis and found that connectivity changes were independent of atrophy [10]. Another study by Lee et al using the method of intrinsic connectivity contrast showed that the epileptogenic zone in TLE was associated with increase connectivity on the hemisphere ipsilateral to the seizure focus [56]. This finding is again consistent with our general conclusion of increased connectivity related to the seizure focus, but we did not look at hemispheric differences partly as the ECP study cohort is not as well lateralized, lacking surgical outcome and invasive EEG. A further study looked at 12 TLE subjects and age matched controls and found increased connectivity with the TLE network defined by 8 nodes [57]. This increased connectivity decreased as a function of disease duration. This first finding is in line with our results. The second finding is of interest as we found an increased correlation of connectivity with “epilepsy activity”. These findings might seem to be a contradiction, but potentially the explanation could be found in the hypoconnectivity in the lateral temporal lobe. With disease progression or epilepsy activity there is not only an increase of connectivity in the primary epileptogenic region, but also a decrease in the connectivity of associated regions (lateral temporal lobe). In summary, our results build upon previous investigations by demonstrating increased connectivity within the mesial-temporal structures and decreased connectivity in lateral temporal lobe in a large non-surgical cohort of TLE patients.
Causal Analysis:
The causal analysis method proposed by Pearl et al[50] is used to explore a theoretical model for macro-scale functional connectivity in TLE and associated clinical consequences. We found that “Epilepsy Activity” (Figure 6, L1) is associated with the regional zTLCR (Figure 6, M2), but that careful accounting of both the #ASM and seizure frequency needs to be considered when assessing the underlying disease activity. Interestingly there were differences in this relationship between right and left TLE. Complicating any analysis of epilepsy activity is the unreliability of patient-reported seizure frequency[58]. Nonetheless, the conditional dependencies dictated by the proposed causal model were met. This model is meant only as a theoretical construct, the unobserved variables (U1–3) need to be better defined and quantified to create more directly testable relationships. Similarly rs-fMRI is only one methodology to quantify connectivity and different techniques (e.g. MEG/HD-EEG) and different scales (intracranial micro-electrodes) are needed to confirm the proposed relationships. Of particular interest is if Epilepsy Activity (L1) is not only affected by Epilepsy Severity (L2), but can in turn worsen the severity of epilepsy—the Gowerian concept of “seizures begetting seizures”. This is long-standing debate in the epilepsy research and given the cross-sectional nature of this study not testable [59] here, but could be explored with longitudinal data.
Limitations/Future Directions:
Only 34% of patients had ictal EEG monitoring and no patient had surgical outcome data to confirm accuracy of lateralization, though even with ictal monitoring and surgical outcome, epilepsy lateralization and localization remain imperfect[60]. It is also unclear how many of these patients had neocortical TLE. Our hypothesis would be an inversion of zTLCR in neocortical TLE, but given their likely rarity (probably on the order of ~11% [55]) within this cohort and the lack of intracranial monitoring this hypothesis cannot be tested and these patients are likely a source of noise for the mesial TLE. Only 11% of this TLE cohort had hippocampal atrophy making this a relatively benign group of TLE compared to the typical TLE surgical group. This study cohort is thus different from other datasets and potentially representing a TLE cohort more reflective of TLE in the community, but with the major drawback of lack of localization, lateralization, and outcome data available in a surgical cohort. Retesting the TLCR in a surgical cohort and including a longitudinal analysis is warranted to confirm the stability of the results and examine the potentially progressive element of TLE as well to determine if zTLCR is clinically useful.
Supplementary Material
Highlights:
Global Clustering Coefficients are decreased in TLE.
Decrease in Global Clustering Coefficient and increase in Rich Club Proportion were associated with worse neuropsychological profile.
In TLE there is increased regional connectivity in left hippocampus/amygdala; decreased connectivity in right lateral temporal lobe.
A ratio of connectivity of hippocampus/amygdala divided by lateral temporal lobe (TLCR) is increased on both the right and left in TLE.
Higher TLCR is associated with increased “Epilepsy Activity”.
Acknowledgments:
The authors would like to thank all the participants and their families. Additionally, the authors would like to thank Megan Rozman, Taylor McMillan, Elizabeth Awe, Courtney Forseth, Peter Kraegel, Cole Cook, Charlene Rivera-Bonet, Anna Freiberg, Neelima Tellapragada, Dace Almane for recruitment and data collection, MRI technologists for their assistance in scanning and other support staff.
Funding: This study was supported by grant number U01NS093650 (Epilepsy Connectome Project) from the National Institutes of Health. Funding for healthy control participants’ data acquisition was provided in part by the Department of Radiology at the University of Wisconsin - Madison. Funding support for Dr. Struck also comes from R01NS111022.
Abbreviations:
- TLE
Temporal Lobe Epilepsy
- MTS
Mesiotemporal sclerosis
- #ASM
Number of Anti-Seizure Medications
- TLCR
Temporal Lobe Connectivity Ratio
- zTLCR
Z-scored Temporal Lobe Connectivity Ratio
- rs-fMRI
Resting state functional MRI
- LOOCV
Leave one out cross validation
- SD
Standard Deviation
- GTC
Generalized Tonic Clonic Seizure
- GCC
Global Clustering Coefficient
- NCC
Nodal Clustering Coefficient
- RCP
Rich Club Proportion
- ROI
Region of Interest
- ⊥
Statistically Independent
- |
Conditional Probability
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The imaging data will be released publically through the human connectome project. The date of data release is pending.
The project was approved by the Medical College of Wisconsin and University of Wisconsin-Madison institutional review boards. All participants provided written informed consent.
This study is not a clinical trial and does not using material reproduced from other sources.
Disclosures: None of the authors have any conflict of interest to disclose.
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References:
- [1].Téllez-Zenteno JF, Hernández-Ronquillo L. A Review of the Epidemiology of Temporal Lobe Epilepsy. Epilepsy Research and Treatment 2011;2012:e630853. 10.1155/2012/630853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Luders H Textbook of Epilepsy Surgery. London: CRC Press; 2008. [Google Scholar]
- [3].Mao J-W, Ye X-L, Li Y-H, Liang P-J, Xu J-W, Zhang P-M. Dynamic Network Connectivity Analysis to Identify Epileptogenic Zones Based on Stereo-Electroencephalography. Front Comput Neurosci 2016;10. 10.3389/fncom.2016.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li Y-H, Ye X-L, Liu Q-Q, Mao J-W, Liang P-J, Xu J-W, et al. Localization of epileptogenic zone based on graph analysis of stereo-EEG. Epilepsy Res 2016;128:149–57. 10.1016/j.eplepsyres.2016.10.021. [DOI] [PubMed] [Google Scholar]
- [5].Goodale SE, González HFJ, Johnson GW, Gupta K, Rodriguez WJ, Shults R, et al. Resting-State SEEG May Help Localize Epileptogenic Brain Regions. Neurosurgery 2020;86:792–801. 10.1093/neuros/nyz351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Storti SF, Galazzo IB, Khan S, Manganotti P, Menegaz G. Exploring the Epileptic Brain Network Using Time-Variant Effective Connectivity and Graph Theory. IEEE J Biomed Health Inform 2017;21:1411–21. 10.1109/JBHI.2016.2607802. [DOI] [PubMed] [Google Scholar]
- [7].van Mierlo P, Carrette E, Hallez H, Vonck K, Van Roost D, Boon P, et al. Accurate epileptogenic focus localization through time-variant functional connectivity analysis of intracranial electroencephalographic signals. Neuroimage 2011;56:1122–33. 10.1016/j.neuroimage.2011.02.009. [DOI] [PubMed] [Google Scholar]
- [8].Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage 2008;40:728–37. 10.1016/j.neuroimage.2007.12.031. [DOI] [PubMed] [Google Scholar]
- [9].Bernhardt BC, Bernasconi N, Concha L, Bernasconi A. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology 2010;74:1776–84. 10.1212/WNL.0b013e3181e0f80a. [DOI] [PubMed] [Google Scholar]
- [10].Larivière S, Weng Y, de Wael RV, Royer J, Frauscher B, Wang Z, et al. Functional connectome contractions in temporal lobe epilepsy: Microstructural underpinnings and predictors of surgical outcome. Epilepsia 2020;n/a. 10.1111/epi.16540. [DOI] [PubMed] [Google Scholar]
- [11].Morgan VL, Chang C, Englot DJ, Rogers BP. Temporal lobe epilepsy alters spatio-temporal dynamics of the hippocampal functional network. Neuroimage Clin 2020;26:102254. 10.1016/j.nicl.2020.102254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kramer MA, Cash SS. Epilepsy as a disorder of cortical network organization. Neuroscientist 2012;18:360–72. 10.1177/1073858411422754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Saling MM. Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain 2009;132:570–82. 10.1093/brain/awp012. [DOI] [PubMed] [Google Scholar]
- [14].Reyes A, Kaestner E, Ferguson L, Jones JE, Seidenberg M, Barr WB, et al. Cognitive phenotypes in temporal lobe epilepsy utilizing data- and clinically driven approaches: Moving toward a new taxonomy. Epilepsia 2020. 10.1111/epi.16528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness, Efficiency of Surgery for Temporal Lobe Epilepsy Study G. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–8. 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- [16].Baxendale S, Thompson PJ, Duncan JS. Improvements in memory function following anterior temporal lobe resection for epilepsy. Neurology 2008;71:1319–25. 10.1212/01.wnl.0000319699.04265.fd. [DOI] [PubMed] [Google Scholar]
- [17].Mazrooyisebdani M, Nair VA, Garcia-Ramos C, Mohanty R, Meyerand E, Hermann B, et al. Graph Theory Analysis of Functional Connectivity Combined with Machine Learning Approaches Demonstrates Widespread Network Differences and Predicts Clinical Variables in Temporal Lobe Epilepsy. Brain Connectivity 2020. 10.1089/brain.2019.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, et al. Altered Functional Connectivity and Small-World in Mesial Temporal Lobe Epilepsy. PLOS ONE 2010;5:e8525. 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lopes MA, Richardson MP, Abela E, Rummel C, Schindler K, Goodfellow M, et al. An optimal strategy for epilepsy surgery: Disruption of the rich-club? PLOS Computational Biology 2017;13:e1005637. 10.1371/journal.pcbi.1005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Garcia-Ramos C, Song J, Hermann BP, Prabhakaran V. Low functional robustness in mesial temporal lobe epilepsy. Epilepsy Res 2016;123:20–8. 10.1016/j.eplepsyres.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Garcia-Ramos C, Dabbs K, Lin JJ, Jones JE, Stafstrom CE, Hsu DA, et al. Progressive dissociation of cortical and subcortical network development in children with new-onset juvenile myoclonic epilepsy. Epilepsia 2018;59:2086–95. 10.1111/epi.14560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].González HFJ, Chakravorti S, Goodale SE, Gupta K, Claassen DO, Dawant B, et al. Thalamic arousal network disturbances in temporal lobe epilepsy and improvement after surgery. J Neurol Neurosurg Psychiatry 2019;90:1109–16. 10.1136/jnnp-2019-320748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].González HFJ, Narasimhan S, Johnson GW, Wills KE, Haas KF, Konrad PE, et al. Role of the Nucleus Basalis as a Key Network Node in Temporal Lobe Epilepsy. Neurology 2021. 10.1212/WNL.0000000000011523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wills KE, González HFJ, Johnson GW, Haas KF, Morgan VL, Narasimhan S, et al. People with mesial temporal lobe epilepsy have altered thalamo-occipital brain networks. Epilepsy Behav 2020:107645. 10.1016/j.yebeh.2020.107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yang S, Zhang Z, Chen H, Meng Y, Li J, Li Z, et al. Temporal variability profiling of the default mode across epilepsy subtypes. Epilepsia 2021;62:61–73. 10.1111/epi.16759. [DOI] [PubMed] [Google Scholar]
- [26].Liao W, Zhang Z, Mantini D, Xu Q, Ji G-J, Zhang H, et al. Dynamical intrinsic functional architecture of the brain during absence seizures. Brain Struct Funct 2014;219:2001–15. 10.1007/s00429-013-0619-2. [DOI] [PubMed] [Google Scholar]
- [27].Song J, Nair VA, Gaggl W, Prabhakaran V. Disrupted Brain Functional Organization in Epilepsy Revealed by Graph Theory Analysis. Brain Connect 2015;5:276–83. 10.1089/brain.2014.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Englot DJ, Konrad PE, Morgan VL. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia 2016;57:1546–57. 10.1111/epi.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Glasser M, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 2013;80:105–24. 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9:179–94. 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- [31].Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage 2012;62:782–90. 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- [32].Andersson JLR, Jenkinson M, Smith S. High resolution nonlinear registration with simultaneous modelling of intensities. BioRxiv 2019:646802. 10.1101/646802. [DOI] [Google Scholar]
- [33].Marcus DS, Harms MP, Snyder AZ, Jenkinson M, Wilson JA, Glasser MF, et al. Human Connectome Project informatics: quality control, database services, and data visualization. Neuroimage 2013;80:202–19. 10.1016/j.neuroimage.2013.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Glasser M, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. A multi-modal parcellation of human cerebral cortex. Nature 2016;536:171–8. 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–55. [DOI] [PubMed] [Google Scholar]
- [36].Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–73. 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- [37].Hoinkiss DC, Erhard P, Breutigam N-J, von Samson-Himmelstjerna F, Günther M, Porter DA. Prospective motion correction in functional MRI using simultaneous multislice imaging and multislice-to-volume image registration. Neuroimage 2019;200:159–73. 10.1016/j.neuroimage.2019.06.042. [DOI] [PubMed] [Google Scholar]
- [38].Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the Human Connectome Project. Neuroimage 2013;80:144–68. 10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59:2142–54. 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Csardi G, Nepusz T. The igraph software package for complex network research 2019:9.
- [41].Watson C brainGraph. 2020.
- [42].Freeman LC. Centrality in social networks conceptual clarification. Social Networks 1978;1:215–39. 10.1016/0378-8733(78)90021-7. [DOI] [Google Scholar]
- [43].Ma A, Mondragón RJ. Rich-Cores in Networks. PLOS ONE 2015;10:e0119678. 10.1371/journal.pone.0119678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J Royal Statistical Soc B 2001;63:411–23. 10.1111/1467-9868.00293. [DOI] [Google Scholar]
- [45].Maechler M, Strufy A, Hubert M, Hornik K. cluster: Cluster Analysis Basics and Extensions. R package version 2.1.0 2019. [Google Scholar]
- [46].Charrad M, Ghazzali N, Boiteau V, Niknafs A. NbClust: An R Package for Determining the Relevant Number of Clusters in a Data Set. Journal of Statistical Software 2014;61:1–36. 10.18637/jss.v061.i06. [DOI] [Google Scholar]
- [47].Hermann B, Conant LL, Cook CJ, Hwang G, Garcia-Ramos C, Dabbs K, et al. Network, clinical and sociodemographic features of cognitive phenotypes in temporal lobe epilepsy. Neuroimage Clin 2020;27. 10.1016/j.nicl.2020.102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fay MP, Shaw PA. Exact and Asymptotic Weighted Logrank Tests for Interval Censored Data: The interval R Package. Journal of Statistical Software 2010;36:1–34. 10.18637/jss.v036.i02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Benjamini YHW. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Soceity 1995;57:289–300. [Google Scholar]
- [50].Pearl J, Glymour M, Jewell NP. Causal inference in statistics : a primer. West Sussex, England: Wiley,; 2016. [Google Scholar]
- [51].Imbens GW, Rubin DB. Causal Inference for Statistics, Social, and Biomedical Sciences: An Introduction. 1st edition. New York: Cambridge University Press; 2015. [Google Scholar]
- [52].Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package “dagitty.” Int J Epidemiol 2016;45:1887–94. 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- [53].Kim S ppcor: An R Package for a Fast Calculation to Semi-partial Correlation Coefficients. Commun Stat Appl Methods 2015;22:665–74. 10.5351/CSAM.2015.22.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cole AJ. Is epilepsy a progressive disease? The neurobiological consequences of epilepsy. Epilepsia 2000;41 Suppl 2:S13–22. 10.1111/j.1528-1157.2000.tb01520.x. [DOI] [PubMed] [Google Scholar]
- [55].Schramm J, Kral T, Grunwald T, Blümcke I. Surgical treatment for neocortical temporal lobe epilepsy: clinical and surgical aspects and seizure outcome. Journal of Neurosurgery 2001;94:33–42. 10.3171/jns.2001.94.1.0033. [DOI] [PubMed] [Google Scholar]
- [56].Lee HW, Arora J, Papademetris X, Tokoglu F, Negishi M, Scheinost D, et al. Altered functional connectivity in seizure onset zones revealed by fMRI intrinsic connectivity. Neurology 2014;83:2269–77. 10.1212/WNL.0000000000001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Morgan VL, Abou-Khalil B, Rogers BP. Evolution of functional connectivity of brain networks and their dynamic interaction in temporal lobe epilepsy. Brain Connect 2015;5:35–44. 10.1089/brain.2014.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chen H, Koubeissi M. Seizure Occurrences: Patient Report, Scalp EEG, and RNS Electrocorticography Findings. J Clin Neurophysiol 2020. 10.1097/WNP.0000000000000684. [DOI] [PubMed] [Google Scholar]
- [59].Sutula TP, Hagen J, Pitkänen A. Do epileptic seizures damage the brain? Curr Opin Neurol 2003;16:189–95. 10.1097/01.wco.0000063770.15877.bc. [DOI] [PubMed] [Google Scholar]
- [60].Smart O, Rolston JD, Epstein CM, Gross RE. Hippocampal seizure-onset laterality can change over long timescales: A same-patient observation over 500days. Epilepsy & Behavior Case Reports 2013;1:56–61. 10.1016/j.ebcr.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


