Abstract
Surface-induced thrombosis is a frequent, critical issue for blood-contacting medical devices that poses a serious threat to patient safety and device functionality. Antithrombotic material design strategies including the immobilization of anticoagulants, alterations in surface chemistries and morphology, and the release of antithrombotic compounds have made great strides in the field with the ultimate goal of circumventing the need for systemic anticoagulation, but have yet to achieve the same hemocompatibility as the native endothelium. Given that the endothelium achieves this state through the use of many mechanisms of action, there is a rising trend in combining these established design strategies for improved antithrombotic actions. Here, we describe this emerging paradigm, highlighting the apparent advantages of multiple antithrombotic mechanisms of action and discussing the demonstrated potential of this new direction.
Graphical Abstract
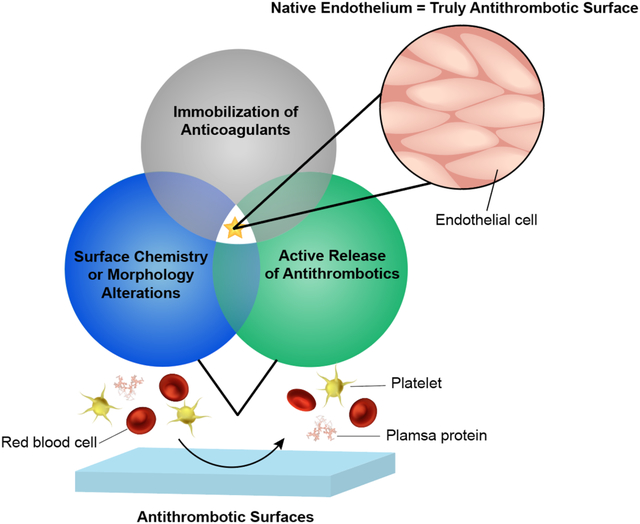
This minireview introduces the emerging trend of combining antithrombotic surface design strategies for improved hemocompatibility.
1. Introduction
Millions of blood-contacting medical devices (BCMD) are used every year in the form of catheters, stents, heart valves, vascular grafts, etc. However, even with the aid of systemic anticoagulation, thrombosis remains one of the most common complications and causes of failure for these devices.1 Despite over 50 years of research and development in improving blood-material interactions, the only truly hemocompatible surface remains the endothelium. To achieve and maintain this state, the endothelium employs a number of antithrombotic mechanisms to supplement the specialized physiology of endothelial cells.2 Preventing coagulation via similar mechanisms used by the endothelium can improve the safety and efficacy of blood-contacting surfaces. While much progress has been made, the administration of anticoagulants, such as heparin, with BCMD use is still necessary to attempt to prevent thrombotic complications.3 However, anticoagulant therapies are associated with a number of adverse effects, some life-threatening,3, 4 and therefore, the development of surfaces that eliminate the need for their use is extremely attractive.
Contact with foreign surfaces such as BCMD disrupts blood homeostasis and leads to thrombus formation via the contact activation/intrinsic pathway of coagulation. There are several excellent reviews that describe this complex pathway in depth;5–7 very briefly, the process starts with plasma protein adsorption, leading to platelet adhesion and activation, further cell deposition and fibrin polymerization, and finally results in thrombus formation (Fig. 1). Plasma proteins rapidly adsorb to BCMD surfaces, triggering a number of complex reactions regulating thrombosis. Adsorbed fibrinogen and von Willebrand factor (vWF) mediate platelet adhesion through interaction with GPIIb/IIIa platelet receptors, transforming platelets to a procoagulant state.1, 8 In this state, platelets can bind to factors Va (FVa) and Xa (FXa), forming a prothrombinase complex capable of converting prothrombin to thrombin.8 Thrombin polymerizes fibrinogen to fibrin, which further stabilizes the blood clot. Circulating leukocytes adhere to adsorbed fibrinogen and release platelet activating factor, interleukins, and tumor necrosis factor, further promoting thrombosis.1 Contact system components factor XII (FXII) and prekallikrein (PK) initiate the coagulation cascade, ultimately leading to fibrin formation. In addition, activated prekallikrein (PKa) generates kallikrein, triggering complement activation that further propagates platelet activation and tissue factor expression on leukocytes.9
Figure 1:
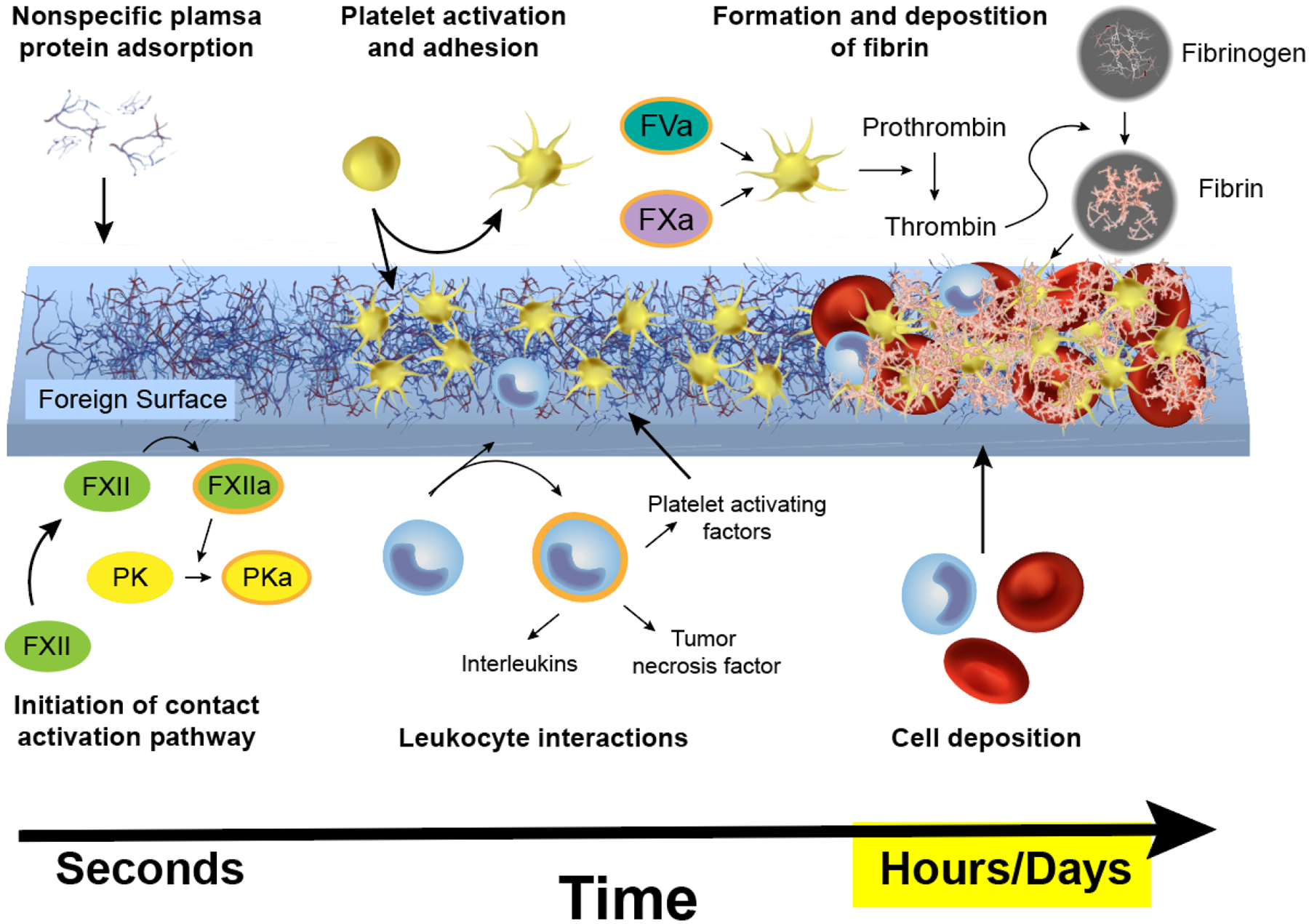
Contact activation results in thrombi on foreign surfaces. Factor XII (FXII); Activated factor XII (FXIIa); Prekallikrein (PK); Activated factor V (FVa); Activated factor X (FXa).
The endothelium prevents coagulation by inhibiting or interfering with many parts of the cascade (Fig. 2). Endothelial cells express antithrombotic proteins such as tissue factor pathway inhibitor (TFPI), thrombomodulin, endothelial protein C receptor (EPCR), and heparin-like molecules on their surfaces.2 TFPI inhibits the formation of two coagulation factors: it prevents the formation of tissue factor-factor VIIa complexes and, therefore, also the activation of factor X to FXa.10 Thrombomodulin likewise has multiple actions. It binds thrombin, preventing its conversion of fibrinogen into fibrin, and the thrombomodulin-thrombin complex additionally promotes the activation of protein C (another antithrombotic protein) either by itself or more efficiently when it is bound to EPCR.11 Heparin and heparin-like molecules enhance antithrombin’s ability to bind thrombin, again, preventing the polymerization of fibrinogen into fibrin.12 In addition to the specialized and responsive surface of endothelial cells, the endothelium actively releases antithrombotic agents to mitigate thrombosis. When released, nitric oxide (NO) and prostacyclin (PG12) have antiplatelet activity, temporarily inhibiting their ability to activate and aggregate.13 Tissue-Plasminogen Activator (t-PA), also produced in and released by endothelial cells, is thrombolytic instead of preventative of thrombosis.14 It activates plasminogen, which, in turn, activates plasmin. Plasmin is able to degrade fibrin and dissolve thrombi. Through the production, expression, and release of these many antithrombotic factors, the endothelium can successfully control thrombosis on its surface. The antithrombotic mechanisms of the endothelium have been incorporated into material designs with varying frequency and success. However, a comparison study of these components has yet to be conducted; therefore, a determination of each factor’s effectiveness and importance has yet to be made.
Figure 2:
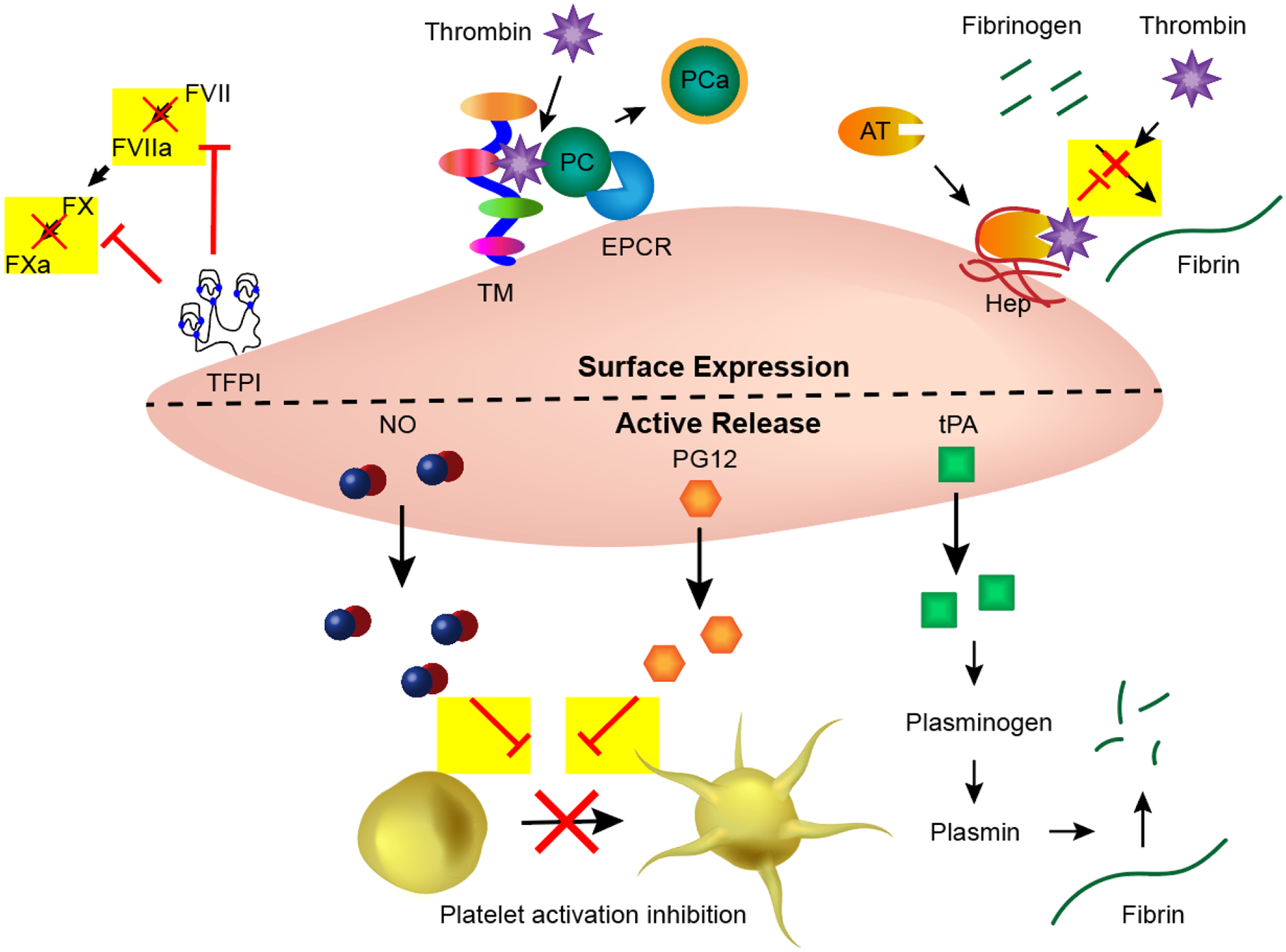
Endothelial cells employ many antithrombotic mechanisms. Tissue factor pathway inhibitor (TFPI); Thrombomodulin (TM); Endothelial protein C receptor (EPCR); Protein C (PC); Antithrombin (AT); Heparin-like molecules (Hep); Nitric oxide (NO); Prostacyclin (PG12); tissue-Plasminogen Activator (tPA).
As demonstrated by the endothelium’s mitigation system, the complexity of the coagulation cascade provides many targets for potential antithrombotic material designs. As protein adsorption and the initiation of contact activation are largely determined by the surface chemistry of foreign materials, there has been considerable research into surface modifications to prevent these events. Additionally, inhibition of the coagulation cascade via anticoagulants or other bioactive compounds can be employed for the same downstream effects. In general, the most common design strategies for decreasing thrombus formation are: 1) Immobilization of anticoagulants,15, 16 2) Surface chemistry/morphology alterations,17, 18 and 3) Active release of antithrombotic compounds.19, 20
Although these strategies have shown initial promise, improving the hemocompatibility of medical devices in vivo for long-term applications has had limited success. Because coagulation is a complex series of reactions, many materials still lack universal properties needed to prevent thrombosis. For example, although some surface modifications have minimized platelet adhesion and activation, foreign surfaces that do not readily prevent protein adsorption will still suffer from thrombotic complications. Similarly, antifouling materials that slow the adsorption and adhesion of proteins and platelets do not actively mediate and prevent thrombogenic factors from being activated. Recently, the field of hemocompatible materials has progressed to the combination of these strategies in order to address their individual shortcomings and enhance the design of endothelial-mimicking surfaces. Through the use of multiple strategies, several antithrombotic mechanisms can be employed in hopes of achieving synergistic anticoagulation effects. This mini review will discuss the emerging trend of combining antithrombotic design strategies for hemocompatible applications. We highlight the advantages that multifunctional hemocompatible materials seem to have over single strategies and their increased potential to mimic the endothelium. As the field of BCMDs is extremely vast, we will focus on impermanent, polymeric-based devices (catheters, extracorporeal membrane oxygenation (ECMO) circuits, etc.) or coatings/materials applicable for such devices. Designs for these devices concentrate on deterring surface-induced thrombosis, the topic of this review, whereas blood-contacting implants such as stents and vascular grafts may also employ endothelialization techniques, thoroughly discussed elsewhere.21–23 We first provide an overview of the three categories of antithrombotic design strategies (Table 1) and any multifunctionality work within them. We then progress into a discussion of combinations of the strategies, highlighting how multiple mechanisms of action can improve the antithrombotic abilities of blood-contacting coatings/surfaces (Fig. 3).
Table 1:
Overview of the three antithrombotic design strategies.
| Design Strategy | Activity classification | Examples | Common Advantages | Common Shortcomings |
|---|---|---|---|---|
| Immobilized anticoagulants |
Bioactive (Inhibition of components of the coagulation cascade) & Passive (Can improve general biocompatibility26) |
|||
| Surface property modifications | Passive (Delay of thrombosis via deterring protein adsorption) |
|
||
| Bioactive (Platelet inhibition or clot disintegration) |
|
|||
| Release of antithrombotic compounds | Bioactive (Inhibition of components of the coagulation cascade or clot disintegration) |
|
Figure 3:
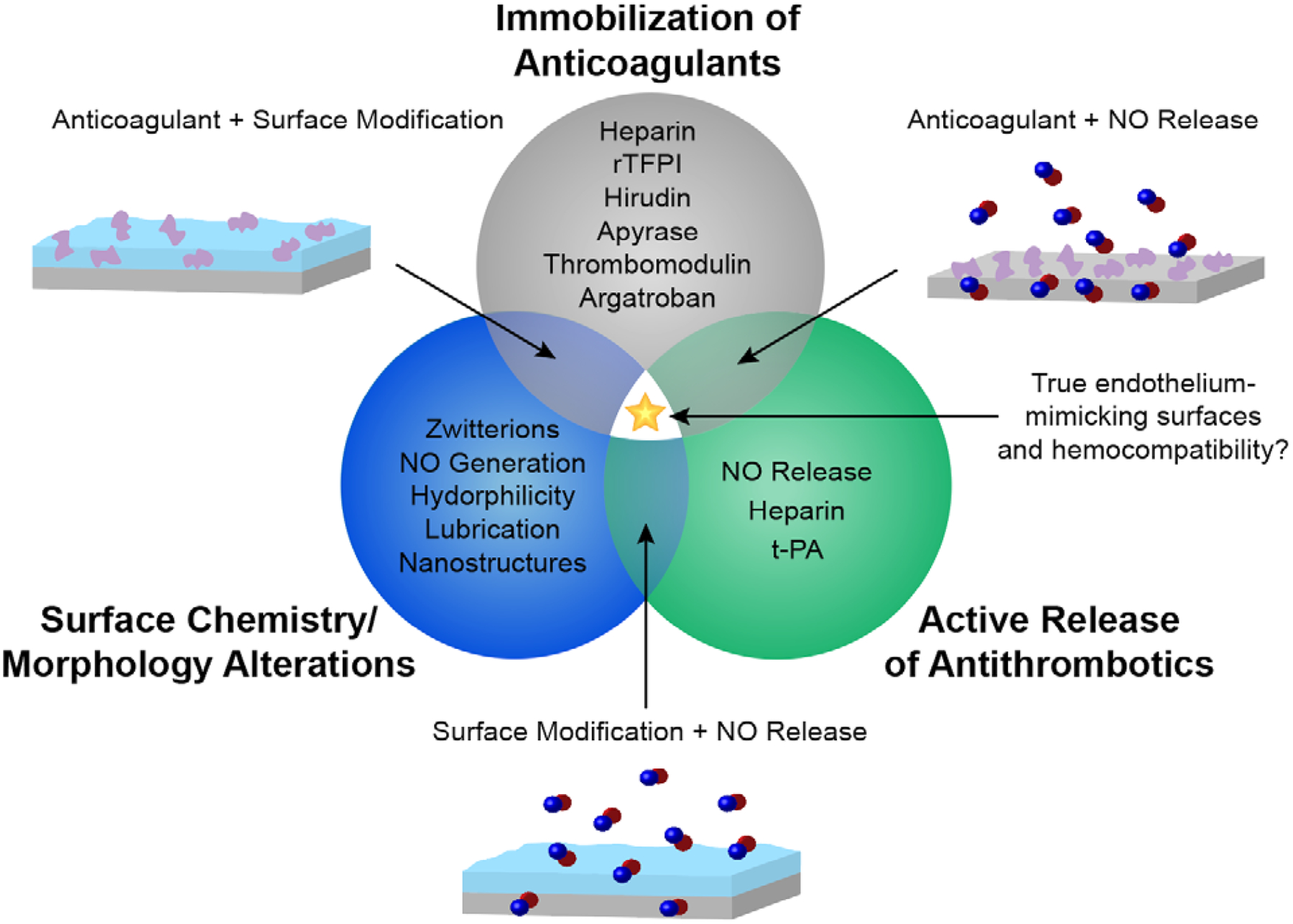
Current antithrombotic strategies and combinations.
2. Overview of Antithrombotic Strategies
2.1. Immobilized Anticoagulant Surface Designs
Systemic administration of anticoagulants is commonly used to prevent thrombosis during and after implantation of BCMD, but it is associated with potentially serious adverse effects such as major bleeding and hypersensitivity.4, 24, 25 The immobilization of anticoagulants has been widely explored to localize the antithrombotic actions and potentially avoid adverse effects seen with systemic administration.26 Pursuit of this strategy stretches back decades and has resulted in products reaching the market.15 The various anticoagulants that have been immobilized and investigated have many different mechanisms of action; however, they generally interact and inhibit specific parts of the coagulation cascade (e.g., heparin binds to antithrombin III, which inactivates thrombin and factor Xa27). Immobilized heparin has been the most thoroughly explored anticoagulant in this field of research, but other, less conventional, immobilized anticoagulants include thrombomodulin (with and without EPCR),16, 28, 29 apyrase,30 hirudin,31, 32 argatroban,33 and recombinant tissue factor pathway inhibitor (rTFPI)34 (Fig. 4A). While there has been considerable work done towards investigating novel or naturally occurring anticoagulant peptides,35, 36 their incorporation into biomaterials has not yet been explored.
Figure 4:
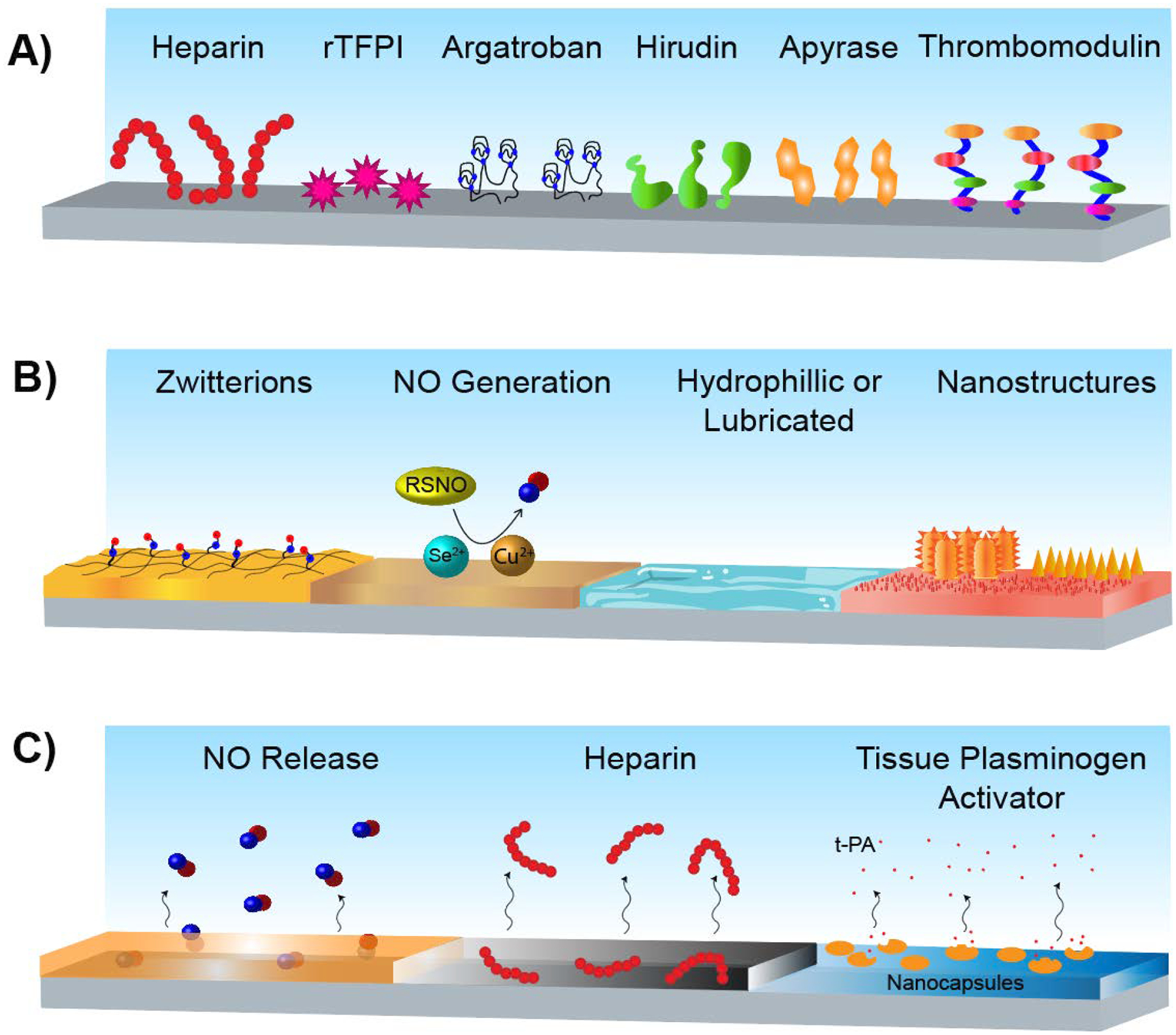
Antithrombotic material design strategies. A) Surface-immobilization of anticoagulants. B) Surface chemistry and/or morphology alterations. C) Active release of antithrombotic compounds.
Drawbacks of this surface modification strategy include its restricted mechanism of action; disruption of the coagulation cascade does not prevent plasma protein adsorption. In fact, protein adsorption has been shown to impair the antithrombotic effects of some surfaces such as albumin-heparin surfaces.37 Additionally, with immobilization, anticoagulants such as heparin, can be locked into a confirmation that may not be the most effective. Depending on the functional groups used for immobilization, anticoagulants may or may not retain their full antithrombotic actions.15
Limited work has been done in solely combining various immobilized anticoagulants; when used in combination, this strategy is generally combined with other modifications (discussed in 3.1 and 3.2). However, while not a traditional immobilized anticoagulant pairing, there has been investigation into the combination of thrombomodulin immobilization and protein C-activating surfaces.29, 38, 39 As briefly mentioned before, protein C is an endogenous coagulation mediator that is antithrombotic when activated. Thrombomodulin itself is another anticoagulant protein but is also a cofactor for thrombin-mediated protein C activation. As thrombomodulin’s efficiency at activating protein C is greatly increased when in a phospholipid membrane, the protein activating surfaces can have direct action (activating protein C) and can also increase the efficiency of thrombomodulin’s efficacy. Thus, this dual anticoagulant strategy provides multiple mechanisms of action and mimics antithrombotic actions of the endothelium.
2.2. Surface Chemistry/Morphology Alteration Strategies
Changes in surface properties and morphology can deter thrombosis through passive or active means (Fig. 4B). A large part of the research field is devoted to developing surfaces that decrease plasma protein adsorption (the initiation of the contact activation pathway), a phenomenon driven by hydrophobic and electrostatic interactions with BCMD.40, 41 Generally, hydrophilic surfaces have been shown to be more resistant to protein adsorption than hydrophobic ones;42 however, the potential of superhydrophobic surfaces for antifouling applications is a growing field.43 Surface modifications incorporating zwitterions, which form repeating positive and negative regions on the surface resulting in strong hydration forces, have also shown protein adsorption resistance.44 The effects of surface morphology on protein adsorption have additionally been investigated; some surface nanostructures show potential for controlling aspects of protein adsorption,45 but, largely, smooth surfaces have less protein adsorption than rough surfaces.46 Super slippery surfaces have resulted in exceptionally stable omni-repellent liquid layers at surface interfaces and are also being explored for hemocompatible and general antifouling purposes.47 Through less plasma protein adsorption, most of these strategies aim to trigger less contact activation response and therefore reduce thrombosis. However, protein adhesion is inevitable on all current materials even if it can be reduced or delayed.48 Surface designs that reduce protein adsorption passively deters thrombosis, but they do not mimic any of the active antithrombotic mechanisms that the endothelium employs.
Some surface modifications can be considered to be active approaches with mechanisms outside of preventing plasma protein adsorption. As NO is a known platelet inhibitor produced by the endothelium,49 NO-generating materials have been developed as one such active antithrombotic strategy.50 These materials liberate NO from endogenous NO donors, localizing its antiplatelet actions.51 Additionally, there has been investigation into the use of fibrinolytic surfaces. For example, a layer-by-layer substrate containing gold, chitosan, and a copolymer of sodium 4-vinylbenzenesulfonate and 1-adamantan-1-ylmethyl methacrylate with lysine-containing β-cyclodextrin derivatives attached has been shown to deter the formation of nascent clots.52
There has been preliminary exploration of combined strategies within this approach. For general antifouling properties, Kim et al. (2013) investigated the combination of surface micro- and nano-features with lubricant infusion.53 Surfaces with lubricated uniform nanostructures were found to perform better in high-shear stress environments compared to those with hierarchically structures (previously considered to perform better). For specific hemocompatible applications, He et al. (2016) developed dual layered graphene oxide and sulfonated polyanions composite membranes.54 This study combined the antithrombotic and hemocompatible properties of the sulfonated polyanions (structurally similar to some heparin analogues55) and the unique structural and biocompatible properties of graphene oxide to make an improved antithrombotic surface, particularly demonstrated via longer clotting times. Additionally, and most notably, Cai et al. (2011) created carboxyl-ebselen immobilized polyethylenimine and alginate layer-by-layer films that are hydrophilic and can generate NO from endogenous donors.56 Although the authors focused on the material’s potential as a multi-functional surface (both antithrombotic and antimicrobial) instead of thoroughly demonstrating the enhanced, dual antithrombotic strategies of hydrophilicity and NO generation, it still suggests that combinations such as these have excellent potential for blood-contacting applications.
2.3. Active-Release Antithrombotic Material Designs
The release of anticoagulants and other antithrombotic compounds from materials is another field of investigation for blood-contacting surfaces (Fig. 4C). The most commonly released antithrombotic compound is the aforementioned NO. NO release mimics the endothelium, temporarily inhibiting platelet activation and preventing platelet adhesion.57 As NO has a very short half-life, NO donor molecules have been incorporated into numerous polymeric constructs to provide sustained NO release for hemocompatible applications.20, 58 Other investigated release strategies involve heparin or heparin conjugates. The localized release of heparin and other clinical anticoagulants can minimize the adverse effects seen with systemic administration and not have the decreased bioactivity of immobilized heparin.59 In an early investigation of this strategy, heparin-prostaglandin E1 complexes were synthesized for release from blood-contacting materials, successfully deterring fibrin formation and platelet aggregations in a rabbit model when released from polyurethane.60 Heparin itself has been incorporated into cleavable hydrogels19 and self-titrating peptide-polysaccaride nanocomplexes61 to selectively release the anticoagulant in thrombotic conditions. A responsive coating that releases t-PA has also been designed.62 Nanocapsules consisting of thrombin-degradable hydrogels containing t-PA were immobilized on various surfaces and demonstrated fibrinolytic actions in the presence of thrombin.
This strategy has great potential for hemocompatible clinical applications, and as a result, many patents regarding NO-releasing coatings and materials have been filed with the United States Patent and Trademark Office.63–65 However, the longevity of antithrombotic-releasing materials is limited to their loading capacity, and achieving steady, sustained release at relevant concentrations can be a challenge.68
3. Combinations of Antithrombotic Design Strategies
3.1. Combinations of Immobilized Anticoagulants and Surface Chemistry/Morphology Alteration Strategies
Through the combination of immobilized anticoagulants and changes in surface chemistries and/or morphologies, multiple passive mechanisms of action to deter surface induced thrombosis can be employed. Like surfaces solely modified with anticoagulant immobilization, heparin is commonly used in combination strategies as well.69–73 Early studies investigated the use of albumin-heparin multilayer coatings.73 Albumin-coated surfaces had been established to have antiplatelet effects,74 thus combining albumin and heparin provides antiplatelet and antithrombin actions. Interestingly, the albumin-coated samples showed significantly less protein adsorption and slightly better platelet conservation than the combination samples, although it should be noted that the heparin used was either depolymerized low molecular weight heparin (LMWH) or unfractionated heparin, and unmodified LMWH may have better effects. Another heparin combination included chitosan in a design for hemodialyzing membranes.71 While chitosan has some thrombogenic properties, it was shown that the combination of surface heparin and chitosan prolonged clotting times compared to either heparin or chitosan samples.
The immobilization of polyethylene glycol (PEG) is another common strategy used in antithrombotic combinations.75–77 PEG is well known to generally improve biocompatibility when attached to surfaces and nanoparticles.78 When in contact with blood, PEG coatings can reduce nonspecific protein adsorption,79 combating thrombosis similarly to most other surface modifications. A combination of PEG and hirudin demonstrated that the presence of PEG decreased protein adsorption, but this effect was slightly compromise by the addition of hirudin, particularly with thrombin.75 The efficacy of the combination in deterring thrombosis, however, was not explored. Dai et al. (2019), on the other hand, did thoroughly investigated the combination of PEG with agratroban.76 The addition of PEG to argatroban-immobilized films decreased the hemolysis ratio and maintained increased thromboplastin times. Platelet adhesion was also decreased, but there was no statistical difference between the argatroban and PEG-argatroban samples.
A combination of anticoagulant and zwitterion immobilization involved citric acid and alkynyl-poly(sulfobetaine methacrylate) and azide-poly(sulfobetaine methacrylate) on a polysulfone membrane.80 Interestingly, the addition of citric acid to the layer-by-layer films increased platelet adhesion, although this did not affect the activated partial thromboplastin time (APTT) as it was approximately equivalent to the zwitterion samples. Better antithrombic properties with the addition of citric acid were only demonstrated with the addition of calcium chloride (as the anticoagulant actions of citric acid are dependent on Ca2+). However, this improvement is only seen when compared to the zwitterion samples; when observing the trend in APTT with calcium chloride, the APTTs decreased as the concentration of Ca2+ increased. While this particular combination of immobilized anticoagulant and zwitterion did not prove to be complementarily antithrombotic, other combinations employing anticoagulant and decreased protein adsorption mechanisms of action have been, warranting further research of potential zwitterion-anticoagulant combinations.
3.2. Combinations of Immobilized Anticoagulants and Active Release Antithrombotic Surface Strategies
The combination of immobilized anticoagulants and the release of antithrombotic compounds provides two mechanisms of bioactive actions to inhibit thrombosis. To date, the only actively released compound within strategy combinations is NO (Fig. 5). NO’s ability to locally inhibit platelet activation combined with the various coagulation cascade targets of anticoagulants creates a multifaceted defense against clotting. This combination may also address a major shortcoming of active release strategies: even after the load of NO (or other antithrombotic compounds) within the material is depleted, an antithrombotic strategy remains.
Figure 5:
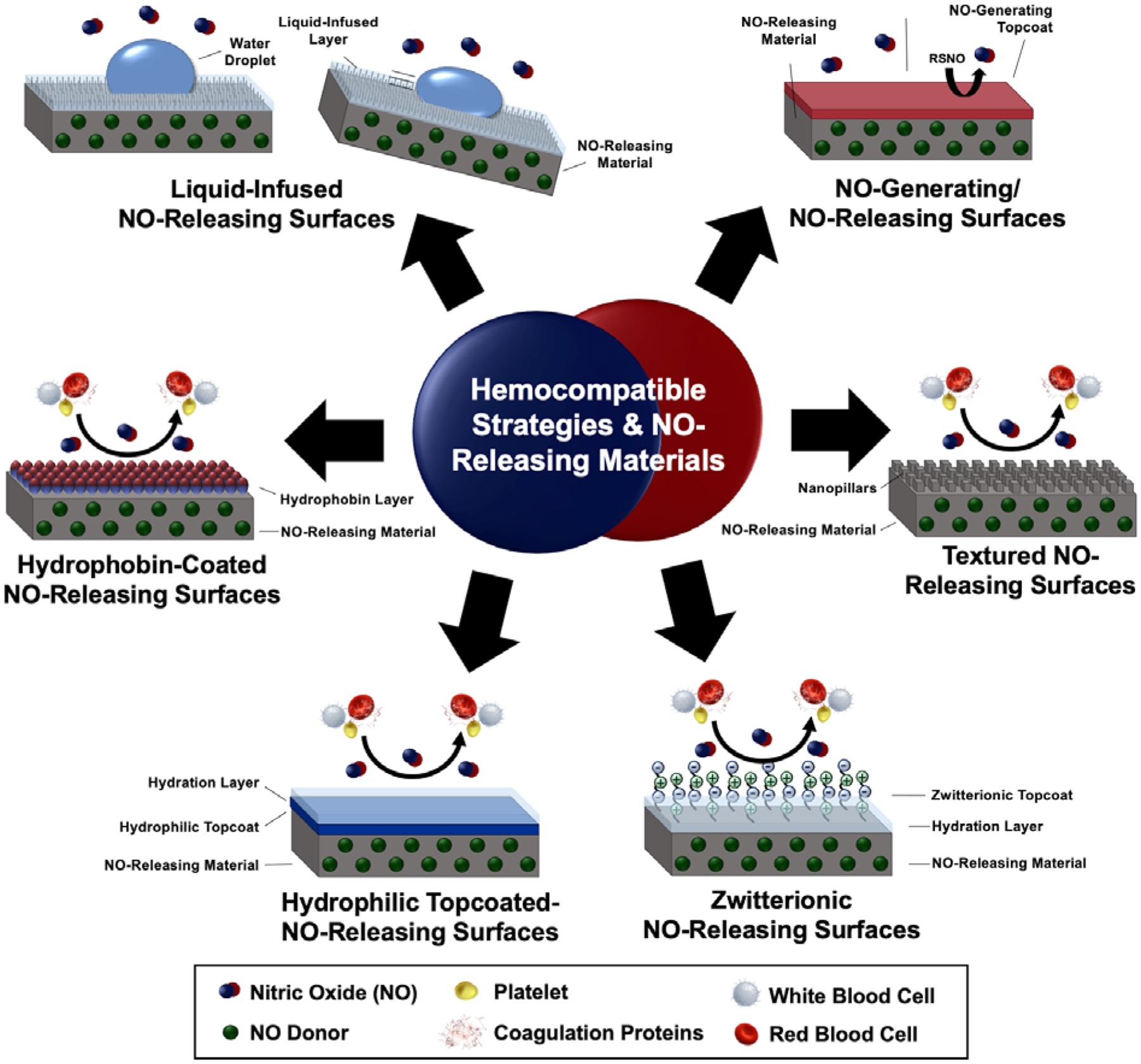
NO release has been combined with several surface modifications for multiple mechanisms of antithrombotic actions and improved hemocompatibility.
Heparin and NO-releasing technologies have been combined in several material and therapeutic designs for hemocompatible applications.27, 81–83 Zhou et al. (2005) were the first to synthesize and characterize combined NO release and immobilized heparin in polymeric coatings.83 In a different construct, Devine et al. (2020) demonstrated this combination’s efficacy in vitro and in vivo as an extracorporeal circuit (ECC) in a rabbit model.81 When used in vivo, the platelet consumption of the dual functionalized ECC was significantly less than either hemocompatible strategy alone or the unmodified control, demonstrating less thrombotic response with the combination of strategies. Wu et al. (2007) attempted to improve heparin-NO combination materials by adding a third component - immobilized thrombomodulin.84 The tri-functional material demonstrated stable NO release and retained the anticoagulants’ activity after immobilization. However, the material’s antithrombotic efficacy has not been thoroughly explored in vivo.
Combining NO and argatroban has demonstrated similar advantages as NO and heparin.85 Immobilized argatroban on a NO-releasing polymer within an ECC was shown to reduce clot formation 15% more than the NO-releasing ECC in a 4 h arteriovenous shunt rabbit model. NO releasing surfaces have also been combined with immobilized bivaldirudin.86 Yang et al. (2020) clearly demonstrated that the combination performed better than either strategy alone; platelet adhesion/activation on the combined coating was reduced by 2.1/32.5 folds and 1.2/25.2 folds compared to those determined on lone bivaldirudin and NO coatings. The significantly improved hemocompatibility seen when used in vivo (compared to the individual strategies and controls) in a rabbit model with an arteriovenous shunt is attributed to these synergistic antithrombotic and antiplatelet effects.
3.3. Combinations of Surface Chemistry/Morphology Alterations and Active Release Antithrombotic Strategies
The combination of physical or chemical antithrombotic surface designs and the release of antithrombotic compounds from the surfaces likewise provides a multifaceted strategy, generally one passive and one active. Similar to 3.2, the only active release compound incorporated into these dual-functionalized materials as of yet is NO. Combinations of this sort generally seek to mimic the endothelium by reducing plasma protein adsorption and/or platelet adhesion to a surface and through the antithrombotic actions of NO release.
Goudie et al. (2017) infused both silicone oil and a NO donor into medical grade tubing to create “liquid-infused NO-releasing” silicone for the prevention of biofouling.87 The combination material did not demonstrate significantly less fibrinogen adsorption or platelet adhesion than the solely lubricated or NO-releasing materials respectively, suggesting that the two modifications were not mechanistically complimentary. However, as it was established to have both antibiofouling mechanisms of action, it is therefore better suited for antithrombotic applications than the unmodified and singly modified materials. Another antibiofouling surface is a hydrophobin-coated NO-releasing material, utilizing hydrophobin as a hydrophilic layer for an NO-releasing polymer film.88 The films demonstrated the complementary actions of the two strategies through significantly decreased platelet adhesion compared to each of the modifications alone and an unmodified control. Further dual-functional, antifouling designs include NO release with hydrophilic polymer topcoats.89 In this study, Singha et al. (2017) combined a NO-donor molecule with various hydrophilic, medical-grade polymers. A hydrophilic polymer top-coating was found to be effective at preventing protein adsorption, and the combination with NO release makes the material of interest for general antifouling purposes. Reduction of viable bacterial adhesion was demonstrated with the combination material, but applications for specific hemocompatible applications have not yet been explored. Additionally, Singha et al. (2020) constructed a NO-releasing catheter with a zwitterionic coating that demonstrated its potential in vivo in a 7 d rabbit model.90 Less thrombus formation on the dual-functionalized catheter compared to the mono-functionalized and control catheters was visually obvious, and decreased fouling was confirmed via scanning electron microscopy.
The combination of surface texturing and NO release has also been shown to be complimentary.91 Pillared films impregnated with NO donors had significantly better platelet adhesion reduction than solely textured or NO-releasing films. In a similar dual active/passive strategy, Goudie et al. (2019) explored the antibiofouling properties of silicon grafted with silanes with free amines that could then be used for the immobilization of NO donor molecules.92 The grafting alone reduced fibrinogen adsorption, but the combination of the surface modification and NO release led to greater platelet adhesion reduction. Although these actions were dependent on grafting density, it demonstrated the advantages of multiple mechanisms of action for hemocompatibility.
The exception to the “reduced protein/platelet adhesion with NO release” trend is a dual NO releasing/generating surface strategy. Mondal et al. (2019) created a NO-generating and releasing material incorporating catalytic selenium that releases NO from endogenous NO donors and additional NO donors within the material.93 In a 4 h ECC in vivo rabbit model, the combination material showed 85.5% platelet adhesion reduction compared the unmodified control, which was a statistically significant decrease compared to the lone selenium or NO-releasing samples. While a 4 h study is the accepted length for ECC models,94 it leaves questions about long-term materials characterization. As of yet, it is not clear if NO-generating surfaces have strong potential for longer-term antithrombotic actions compared to NO-releasing materials or if their activity is hindered by plasma protein adsorption and/or platelet adhesion that is inevitable with prolonged blood contact.
4. Conclusions
To prevent thrombotic complications and/or issues with systemic anticoagulant therapies, the development of hemocompatible surfaces for BCMD is paramount. Strides have been made by attempting to mimic the endothelium through the investigation of immobilized anticoagulants, surface chemistry/morphology alterations, and the release of antithrombotic compounds for these applications. In numerous fashions, it has been demonstrated that combining multiple antithrombotic surface design strategies provides a means to accommodate one strategy’s shortcoming or supply an additional means to prevent thrombosis, successfully resulting in better polymeric hemocompatible materials.
However, much work is still needed to achieve a truly hemocompatible surface, particularly one suitable for clinical applications. While the combination of all three antithrombotic categories has been seen on titanium surfaces,95 it has not yet been explored for polymeric materials, which are extensively used for blood-contacting applications. Moreover, in order to translate this growing field into clinical settings, sterilization stability, shelf life, and length of use should be taken into consideration.
As the endothelium employs numerous antithrombotic strategies, presumably so should blood-contacting surfaces. While much work remains, this new and growing trend in multifunctional material design recognizes that through the use of multiple mechanisms of action, the field advances towards developing a truly endothelial-mimicking, hemocompatible material for BCMD.
Acknowledgements:
We thank Dr. Donna Ashcraft (Clarion University) for assistance with graphical design and Mark Garren (University of Georgia) for topical insight.
Funding:
This work was supported by the National Institutes of Health [grant numbers R01HL134899, R01HL140301]; and the National Science Foundation [grant number NSF 1842396].
Footnotes
Conflicts of Interest: Hitesh Handa is a founder and maintains a financial interest in inNOveta Biomedical LLC. The company is exploring possibilities of using nitric oxide releasing materials for medical applications.
References
- 1.Jaffer IH, Fredenburgh JC, Hirsh J and Weitz JI, J Thromb Haemost, 2015, 13 Suppl 1, S72–81. [DOI] [PubMed] [Google Scholar]
- 2.van Hinsbergh VW, Semin Immunopathol, 2012, 34, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffer IH and Weitz JI, Acta Biomaterialia, 2019, 94, 2–10. [DOI] [PubMed] [Google Scholar]
- 4.Bircher AJ, Harr T, Hohenstein L and Tsakiris DA, Allergy, 2006, 61, 1432–1440. [DOI] [PubMed] [Google Scholar]
- 5.Vogler EA and Siedlecki CA, Biomaterials, 2009, 30, 1857–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naudin C, Burillo E, Blankenberg S, Butler L and Renne T, Semin Thromb Hemost, 2017, 43, 814–826. [DOI] [PubMed] [Google Scholar]
- 7.Smith SA, Travers RJ and Morrissey JH, Crit Rev Biochem Mol Biol, 2015, 50, 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grunkemeier JM, Tsai WB, McFarland CD and Horbett TA, Biomaterials, 2000, 21, 2243–2252. [DOI] [PubMed] [Google Scholar]
- 9.Afshar-Kharghan V, Blood, 2017, 129, 2214–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mast AE, Arterioscler Thromb Vasc Biol, 2016, 36, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin FA, Murphy RP and Cummins PM, Am J Physiol Heart Circ Physiol, 2013, 304, H1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhamme P and Hoylaerts MF, Acta Clin Belg, 2006, 61, 213–219. [DOI] [PubMed] [Google Scholar]
- 13.Hamilos M, Petousis S and Parthenakis F, Cardiovasc Diagn Ther, 2018, 8, 568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mican J, Toul M, Bednar D and Damborsky J, Comput Struct Biotechnol J, 2019, 17, 917–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biran R and Pond D, Adv Drug Deliv Rev, 2017, 112, 12–23. [DOI] [PubMed] [Google Scholar]
- 16.Sperling C, Salchert K, Streller U and Werner C, Biomaterials, 2004, 25, 5101–5113. [DOI] [PubMed] [Google Scholar]
- 17.de Mel A, Cousins BG and Seifalian AM, International Journal of Biomaterials, 2012, 2012, 707863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchouta LN and Bonde PN, ASAIO J, 2015, 61, 623–634. [DOI] [PubMed] [Google Scholar]
- 19.Maitz MF, Zitzmann J, Hanke J, Renneberg C, Tsurkan MV, Sperling C, Freudenberg U and Werner C, Biomaterials, 2017, 135, 53–61. [DOI] [PubMed] [Google Scholar]
- 20.Wo Y, Brisbois EJ, Bartlett RH and Meyerhoff ME, Biomater Sci, 2016, 4, 1161–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melchiorri AJ, Hibino N and Fisher JP, Tissue Eng Part B Rev, 2013, 19, 292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedair TM, ElNaggar MA, Joung YK and Han DK, J Tissue Eng, 2017, 8, 2041731417731546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radke D, Jia W, Sharma D, Fena K, Wang G, Goldman J and Zhao F, Adv Healthc Mater, 2018, 7, e1701461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wall C, Moore J and Thachil J, J Intensive Care Soc, 2016, 17, 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffer IH and Weitz JI, Acta Biomater, 2019, 94, 2–10. [DOI] [PubMed] [Google Scholar]
- 26.Murugesan S, Xie J and Linhardt RJ, Curr Top Med Chem, 2008, 8, 80–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suchyta DJ, Handa H and Meyerhoff ME, Mol Pharm, 2014, 11, 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han HS, Yang SL, Yeh HY, Lin JC, Wu HL and Shi GY, J Biomater Sci Polym Ed, 2001, 12, 1075–1089. [DOI] [PubMed] [Google Scholar]
- 29.Kador KE, Mamedov TG, Schneider M and Subramanian A, Acta Biomater, 2011, 7, 2508–2517. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson PH, Engberg AE, Back J, Faxalv L, Lindahl TL, Nilsson B and Ekdahl KN, Biomaterials, 2010, 31, 4484–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li F, Wang J and Huang N, Journal of Wuhan University of Technology-Mater. Sci. Ed, 2011, 26, 950–954. [Google Scholar]
- 32.Li J, Liu F, Qin Y, He J, Xiong Z, Deng G and Li Q, Journal of Membrane Science, 2017, 523, 505–514. [Google Scholar]
- 33.Yu J, Brisbois E, Handa H, Annich G, Meyerhoff M, Bartlett R and Major T, J Mater Chem B, 2016, 4, 2264–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandiwal A, Zaman FS, Mast AE and Hall CL, J Biomater Sci Polym Ed, 2006, 17, 1025–1037. [DOI] [PubMed] [Google Scholar]
- 35.Syed AA and Mehta A, International Journal of Peptide Research and Therapeutics, 2018, 24, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Horani RA and Desai UR, Expert Opin Ther Pat, 2016, 26, 323–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Delden CJ, Engbers GH and Feijen J, J Biomater Sci Polym Ed, 1996, 7, 727–740. [DOI] [PubMed] [Google Scholar]
- 38.Tseng PY, Jordan SW, Sun XL and Chaikof EL, Biomaterials, 2006, 27, 2768–2775. [DOI] [PubMed] [Google Scholar]
- 39.Feng J, Tseng P-Y, Faucher KM, Orban JM, Sun X-L and Chaikof EL, Langmuir, 2002, 18, 9907–9913. [Google Scholar]
- 40.Lichtenberg JY, Ling Y and Kim S, Sensors (Basel), 2019, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallace A, Albadawi H, Patel N, Khademhosseini A, Zhang YS, Naidu S, Knuttinen G and Oklu R, Cardiovasc Diagn Ther, 2017, 7, S246–S257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogler EA, Biomaterials, 2012, 33, 1201–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avramescu RE, Ghica MV, Dinu-Pirvu C, Prisada R and Popa L, Materials (Basel), 2018, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X, Yuan L, Li D, Tang Z, Wang Y, Chen G, Chen H and Brash JL, Journal of Materials Chemistry B, 2014, 2, 5718–5738. [DOI] [PubMed] [Google Scholar]
- 45.Firkowska-Boden I, Zhang X and Jandt KD, Advanced Healthcare Materials, 2018, 7, 1870001. [DOI] [PubMed] [Google Scholar]
- 46.Xu LC, Bauer JW and Siedlecki CA, Colloids Surf B Biointerfaces, 2014, 124, 49–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Ueda E, Paulssen D and Levkin PA, Advanced Functional Materials, 2019, 29, 1802317. [Google Scholar]
- 48.Brash JL, Horbett TA, Latour RA and Tengvall P, Acta Biomater, 2019, 94, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin RC and Loscalzo J, J Blood Med, 2010, 2010, 147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Qi PK, Yang ZL and Huang N, Biosurface and Biotribology, 2015, 1, 177–201. [Google Scholar]
- 51.Major TC, Brant DO, Burney CP, Amoako KA, Annich GM, Meyerhoff ME, Handa H and Bartlett RH, Biomaterials, 2011, 32, 5957–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin S, Gu H, Chen X, Liu X, Zhan W, Wei T, Sun X, Ren C and Chen H, Colloids and Surfaces B: Biointerfaces, 2018, 167, 28–35. [DOI] [PubMed] [Google Scholar]
- 53.Kim P, Kreder MJ, Alvarenga J and Aizenberg J, Nano Letters, 2013, 13, 1793–1799. [DOI] [PubMed] [Google Scholar]
- 54.He C, Shi ZQ, Cheng C, Lu HQ, Zhou M, Sun SD and Zhao CS, Biomater Sci, 2016, 4, 1431–1440. [DOI] [PubMed] [Google Scholar]
- 55.Mohamed S and Coombe DR, Pharmaceuticals (Basel), 2017, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai W, Wu J, Xi C, Ashe AJ 3rd and Meyerhoff ME, Biomaterials, 2011, 32, 7774–7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gordge MP and Xiao F, Br J Pharmacol, 2010, 159, 1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang H, Nacharaju P, Friedman A and Friedman JM, Future Sci OA, 2015, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei H, Han L, Ren J and Jia L, ACS Applied Materials & Interfaces, 2013, 5, 12571–12578. [DOI] [PubMed] [Google Scholar]
- 60.Jacobs H, Okano T, Lin JY and Kim SW, Journal of Controlled Release, 1985, 2, 313–319. [Google Scholar]
- 61.Lin KY, Lo JH, Consul N, Kwong GA and Bhatia SN, ACS Nano, 2014, 8, 8776–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li C, Du H, Yang A, Jiang S, Li Z, Li D, Brash JL and Chen H, Advanced Functional Materials, 2017, 27, 1703934. [Google Scholar]
- 63.De Oliveira MG, Moreira AM, Seabra AB, d. MM Simões SGand Morato SP, US Pat., 20100112033A1, 2010.
- 64.Zhang H and Meyerhoff ME, US Pat., 6841166B1, 2005.
- 65.Batchelor MM, Oh BK and Meyerhoff ME, US Pat., 7763283B2, 2010.
- 66.Borhani S, Hassanajili S, Ahmadi Tafti SH and Rabbani S, Prog Biomater, 2018, 7, 175–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yin RX, Yang DZ and Wu JZ, Theranostics, 2014, 4, 175–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanzi MC, Expert Rev Med Devices, 2005, 2, 473–492. [DOI] [PubMed] [Google Scholar]
- 69.Elahi MF, Guan G, Wang L and King MW, Materials (Basel), 2014, 7, 2956–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Houska M, Brynda E, Solovyev A, Brouckova A, Krizova P, Vanickova M and Dyr JE, J Biomed Mater Res A, 2008, 86, 769–778. [DOI] [PubMed] [Google Scholar]
- 71.Lin W-C, Liu T-Y and Yang M-C, Biomaterials, 2004, 25, 1947–1957. [DOI] [PubMed] [Google Scholar]
- 72.Zhang K, Li JA, Deng K, Liu T, Chen JY and Huang N, Colloids Surf B Biointerfaces, 2013, 108, 295–304. [DOI] [PubMed] [Google Scholar]
- 73.Brynda E, Houska M, Jiroušková M and Dyr JE, Journal of Biomedical Materials Research, 2000, 51, 249–257. [DOI] [PubMed] [Google Scholar]
- 74.Ryu G, Han D, Kim Y and Min B, ASAIO J, 1992, 38, M644–648. [DOI] [PubMed] [Google Scholar]
- 75.Alibeik S, Zhu S and Brash JL, Colloids and Surfaces B: Biointerfaces, 2010, 81, 389–396. [DOI] [PubMed] [Google Scholar]
- 76.Dai Y, Dai S, Xie X and Ning J, Journal of Biomaterials Science, Polymer Edition, 2019, 30, 608–628. [DOI] [PubMed] [Google Scholar]
- 77.Zhao J, Bai L, Muhammad K, Ren X.-k., Guo, Xia S, Zhang W and Feng Y, ACS Biomaterials Science & Engineering, 2019, 5, 2846–2857. [DOI] [PubMed] [Google Scholar]
- 78.D’Souza AA and Shegokar R, Expert Opin Drug Deliv, 2016, 13, 1257–1275. [DOI] [PubMed] [Google Scholar]
- 79.Bernard M, Jubeli E, Pungente MD and Yagoubi N, Biomaterials Science, 2018, 6, 2025–2053. [DOI] [PubMed] [Google Scholar]
- 80.Xiang T, Wang R, Zhao W-F, Sun S-D and Zhao C-S, Langmuir, 2014, 30, 5115–5125. [DOI] [PubMed] [Google Scholar]
- 81.Devine R, Goudie M, Singha P, Douglass M, Schmiedt CW, Brisbois EJ and Handa H, ACS Applied Materials & Interfaces, 2020, DOI: 10.1021/acsami.9b22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo R, Zhang J, Zhuang W, Deng L, Li L, Yu H, Wang J, Huang N and Wang Y, Journal of Materials Chemistry B, 2018, 6, 5582–5595. [DOI] [PubMed] [Google Scholar]
- 83.Zhou Z and Meyerhoff ME, Biomaterials, 2005, 26, 6506–6517. [DOI] [PubMed] [Google Scholar]
- 84.Wu B, Gerlitz B, Grinnell BW and Meyerhoff ME, Biomaterials, 2007, 28, 4047–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Major TC, Brisbois EJ, Jones AM, Zanetti ME, Annich GM, Bartlett RH and Handa H, Biomaterials, 2014, 35, 7271–7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang T, Du Z, Qiu H, Gao P, Zhao X, Wang H, Tu Q, Xiong K, Huang N and Yang Z, Bioact Mater, 2020, 5, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goudie MJ, Pant J and Handa H, Sci Rep, 2017, 7, 13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Devine R, Singha P and Handa H, Biomater Sci, 2019, 7, 3438–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singha P, Pant J, Goudie MJ, Workman CD and Handa H, Biomaterials Science, 2017, 5, 1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singha P, Goudie MJ, Liu Q, Hopkins S, Brown N, Schmiedt CW, Locklin J and Handa H, ACS Applied Materials & Interfaces, 2020, 12, 9070–9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu LC, Meyerhoff ME and Siedlecki CA, Acta Biomater, 2019, 84, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goudie MJ, Singha P, Hopkins SP, Brisbois EJ and Handa H, ACS Applied Materials & Interfaces, 2019, 11, 4523–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mondal A, Douglass M, Hopkins SP, Singha P, Tran M, Handa H and Brisbois EJ, ACS Applied Materials & Interfaces, 2019, 11, 34652–34662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Major TC, Handa H, Annich GM and Bartlett RH, J Biomater Appl, 2014, 29, 479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simon-Walker R, Romero R, Staver JM, Zang Y, Reynolds MM, Popat KC and Kipper MJ, ACS Biomaterials Science & Engineering, 2017, 3, 68–77. [DOI] [PubMed] [Google Scholar]
- 96.Zia F, Zia KM, Zuber M, Tabasum S and Rehman S, International Journal of Biological Macromolecules, 2016, 84, 101–111. [DOI] [PubMed] [Google Scholar]


