Abstract
Pediatric mesotheliomas are rare and their pathogenesis remains undefined. In this study we report 5 cases of malignant mesothelioma in children, characterized by fusions involving the anaplastic lymphoma kinase (ALK) gene. Four cases occurred in females involving the abdominal cavity and were characterized by a pure epithelioid morphology. The fifth arose in the tunica vaginalis of a 15-year-old male and displayed a biphasic epithelioid-sarcomatoid phenotype. All cases demonstrated the classic morphologic and immunohistochemical features of malignant mesothelioma, including tubulopapillary architecture and cuboidal epithelioid cells with eosinophilic cytoplasm and uniform nuclei with vesicular chromatin. Immunohistochemically, all cases showed labeling for ALK, cytokeratins, WT-1, and calretinin, while lacking expression of adenocarcinoma immunomarkers. Four cases demonstrated weak-moderate labeling for PAX8 protein, which resulted in diagnostic challenges with primary peritoneal serous carcinoma. The ALK genetic abnormalities were investigated by a combination of molecular methods. Archer FusionPlex was performed in 2 cases, showing fusions between ALK with either STRN or TPM1 genes, resulting in a transcript that retained the ALK kinase domain. One case was further studied by DNA targeted sequencing, but no additional genetic alterations were observed. In one case cytogenetic analysis showed the presence of a t(2;15)(p23;q22), and FISH confirmed the ALK gene break-apart. In the remaining 2 cases, ALK gene rearrangements were demonstrated by FISH. Unlike adult mesotheliomas, which are tightly linked to asbestos exposure, often show loss of BAP1 expression and have complex karyotypes, ALK-rearranged mesothelioma appears to be similar to other fusion positive-mesotheliomas, such as those harboring EWSR1/FUS-ATF1 fusions, sharing significant morphologic overlap, occurring in young patients and displaying a simple, translocation-driven genetic profile.
Keywords: Mesothelioma, ALK, Translocation
INTRODUCTION
Malignant mesothelioma is a highly aggressive malignant neoplasm of the mesothelial lining, which can affect the pleura, and less commonly the peritoneum, pericardium, or tunica vaginalis testis in males. Median overall survival is around one year. Morphologically, these tumors can be purely epithelioid, sarcomatoid, or biphasic. These neoplasms typically harbor complex chromosomal alterations, with common inactivation of the CDKN2A, NF2 and BRCA1-associated protein-1 (BAP1) genes1–3. Current systemic therapies are largely ineffective.
The best-defined risk factors for malignant mesothelioma are asbestos exposure, prior radiation, and inherited mutations in the BAP1 gene. The latter increases susceptibility to asbestos exposure4,5, which has a stronger association with pleural-based rather than peritoneal malignant mesothelioma6–8. Because of the long latency (estimated at 30 years) of the carcinogenic effects of asbestos, malignant mesothelioma is mainly a disease of adults, with a median age of approximately 73 years in the United States (www.cdc.gov). Malignant mesothelioma in children, which is extremely rare and mainly limited to case reports and small case series, is typically not associated with asbestos exposure and is expected to have a distinct pathogenesis9–16. Indeed, clinicopathologic features have emerged that distinguish malignant mesotheliomas in young patients from those of adults17. First, while there is a striking 5:1 male to female predominance in adults, malignant mesotheliomas in children occur at a nearly equal gender distribution. Second, while pleural malignant mesotheliomas predominate in adults, there is a nearly equal distribution of peritoneal and pleural mesotheliomas in young patients. Third, malignant mesotheliomas in children and young adults have been associated with a more favorable outcome. The pathogenesis of pediatric and young adult mesotheliomas remains largely unclear, but recently recurrent chromosome translocations have been identified in a subset of cases. First, a group of mesotheliomas in young adults were found to harbor fusions between EWSR1/FUS genes with either ATF118 or YY119 genes, which are unrelated to asbestos exposure and BAP1 abnormalities. Second, few reports have described malignant mesotheliomas in the peritoneum of young patients associated with gene fusions involving the anaplastic lymphoma kinase (ALK) gene.20–23 In this study, we describe five additional cases of malignant mesothelioma of the peritoneal cavity or tunica vaginalis testis with ALK gene fusions, all occurring in children.
MATERIALS AND METHODS
Case Selection and Pathologic Examination
The five ALK-rearranged cases in this study originate from the consultation files of the authors (PA, AA, JIE, CRA) and the institutional files of the Johns Hopkins Medical Institutions and Memorial Sloan Kettering Cancer Center. The 5 ALK-rearranged cases above came from a cohort of 10 mesothelioma cases in our files in patients under the age of 40 years. One of the ALK-rearranged cases (case 3) was previously reported16 without genetic analysis. All cases were reviewed by 2 pathologists (PA, CRA), who assessed their microscopic features, including architectural patterns, cell type (epithelioid, spindle), cytoplasmic appearance, nuclear shape and degree of pleomorphism, mitotic activity, necrosis and psammomatous calcifications. The medical charts or correspondence to the original pathologists were investigated for possible asbestos exposure, presence of locoregional and distant metastasis, and survival. The study was approved by the Institutional Review Board at our institutions.
Immunohistochemistry
Immunohistochemical labeling was performed on the Benchmark XT autostainer (Ventana Medical Systems Inc, Tucson, AZ) using the I-View detection kit. The standard antibodies used, vendors, pretreatments, and dilutions included: ALK (Abcam ab17127, 5A4, steam, 1:50), PAX8 (Proteintech 10336–1-AP, polyclonal, steam, 1:800), CK5/6 (Ventana/Roche 790–4554, D5/16B4, steam, predilute), BRG1 (Abcam ab110641, EPNCIR111A, steam, 1:100), desmin (Dako M0760, clone D33, steam, 1:100), cytokeratin AE1/3 (Chemicon, steam, 1:4000), EMA (Ventana, 760–4259, steam, prediluted), CD99 (Leica, Clone 12E7, steam, prediluted), S100 protein (Ventana, 760–2914, stream, prediluted), WT-1 (Ventana, 6F-H2, steam, predilute), calretinin (Cell Marque, polyclonal, steam, predilute), BAP1 (Santa Cruz, clone C4, steam, 1:200), and INI1 (BD Transduction Laboratories, clone 25/BAP47, steam, 1:400).
Fluorescence in situ hybridization (FISH)
FISH on interphase nuclei from paraffin-embedded 4-micron sections was performed applying custom probes using bacterial artificial chromosomes (BAC), covering and flanking the ALK gene. Break-apart FISH was performed as previously described24. BAC clones were chosen according to UCSC genome browser (http://genome.ucsc.edu), see Supplementary Table 1. The BAC clones were obtained from BACPAC sources of Children’s Hospital of Oakland Research Institute (CHORI)(Oakland, CA)(https://bacpacresources.org/). DNA from individual BACs was isolated according to the manufacturer’s instructions, labeled with different fluorochromes in a nick translation reaction, denatured, and hybridized to pretreated slides. Slides were then incubated, washed, and mounted with DAPI in an antifade solution, as previously described. The genomic location of each BAC set was verified by hybridizing them to normal metaphase chromosomes. Two hundred successive nuclei were examined using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany), controlled by Isis 5 software (Metasystems, Newton, MA). A positive score was interpreted when at least 20% of the nuclei showed a break-apart signal. Nuclei with incomplete set of signals were omitted from the score.
RNA Sequencing
Two cases were tested by Archer™ FusionPlex Sarcoma Assay. Total RNA was extracted from macro-dissected tissue sections using the Promega ReliaPrep™ FFPE Total RNA Miniprep System (Promega, Madison, WI, USA) following the recommended protocol. The quantity of RNA extracted was measured using the QuantiFluor® RNA System (Promega, Madison, WI, USA). 50–250 ng of RNA was used for library preparation utilizing the Archer® FusionPlex® Sarcoma Panel kit (AK00328) according to the manufacturer’s protocol (ArcherDX, Boulder, CO, USA). The prepared library was sequenced using an Ion Torrent PGM™ next-generation sequencer. The Hi-Q™ sequencing kit and Hi-Q™ View CHEF kit were used according to the manufacturer’s protocol (Life Technologies, Forster City, CA, USA). Data obtained was analyzed by the Archer Data Analysis (version 5.0.6) portal (ArcherDX, Boulder, CO, USA). Archer results were confirmed further in both cases either by FISH or reverse transcriptase-polymerase chain reaction (RT-PCR) using appropriate primers flanking the breakpoint and Sanger sequencing.
Case 2 was also evaluated using MSKCC-Impact (Integrated Mutational Profiling of Actionable Cancer Targets), a targeted ultra-deep next generation sequencing platform designed to capture all exons and selected introns of over 400 oncogenes, tumor suppressor genes, and members of targetable pathways.25
Cytogenetics
For cytogenetic analysis, the tumor culture from case 4 was established by mincing and digestion with collagenase type I. Following exposure to colcemid for 3 hours 30 minutes, cells were harvested after at least 24 hours. Slides were prepared and G-banded using trypsin and Wright’s stain.
RESULTS
Patient Characteristics:
The clinicopathologic features of the cases are described in Table 1. There were 5 patients included in the study, all children, 4 females and one male, with a mean age of 13 years (median 15, range 8–16). The four female patients’ tumors arose in the peritoneal cavity, forming multifocal masses requiring debulking. Abdominal lymph nodes were involved by metastatic disease in all three cases in which they were excised. As previously described16, case 3 had initially been diagnosed as an ovarian serous carcinoma, while case 5 was submitted with the differential diagnosis of serous carcinoma versus mesothelioma.
Table 1:
Pediatric Mesothelioma with ALK fusions: Clinical and Pathologic Findings
| Case # | ALK fusion (method) | Age/Sex | Presentation | Histology | IHC Positive | IHC Negative | Management/ Follow-Up |
|---|---|---|---|---|---|---|---|
| 1 |
STRN exon 3-ALK exon 20 (Archer, RT-PCR, FISH) |
9/F | Abdominal mass requiring debulking, fever. | Epithelioid, tubulo-papillary | WT1, CRT, AE1/3, EMA, CK7, CK5/6, ALK granular BRG1 intact BAP1 non-contributory |
Desmin, BerEp4, inhibin, CEA, PAX8 | Primary resection (x2) and adjuvant Cisplatin/Gemcitabine; LR (60 mo) Cisplatin/Pemetrexed with stabilization 24 mo; Crizotinib 12 mo with no effect; AWD 96 mo |
| 2 |
TPM1 exon 8-ALK exon 20 (Archer, MSKCC IMPACT) |
8/F | Pelvic mass requiring debulking | Epithelioid, tubulo-papillary | WT1, CRT, mesothelin; PAX8 weak ALK granular BAP1 & INI1 retained |
Claudin4, pCEA, PD-L1 | Neoadjuvant chemotherapy 4 cycles and debulking |
| 3 | ALK rearrangement (FISH) | 16/F | Massive peritoneal disease, no asbestos exposure | Epithelioid, tubulo-papillary | WT1, CRT, CK5, CK7, Vimentin, D2–40, HBME; PAX8 weak ALK granular BAP1 & BRG1 retained |
BerEP4, CEA, ER/PR | Initially diagnosed as adenocarcinoma. Resection and HIPEC, Cisplatin/ Pemetrexed; NED 17 mo |
| 4 | t(2;15)(p23;q22) and ALK rearrangement (FISH) | 15/M | Painful left testicular mass, spermatic cord lesion | Biphasic | WT1*, CRT, AE1/3*, EMA, CK5/6, D2–40; PAX8 weak-moderate ALK granular* BAP1 & BRG1 retained |
HMB45, inhibin, myogenin, desmin | s/p HIPEC with lymph node dissection (9/9 positive nodes); AWD 12 mo |
| 5 | ALK rearrangement (FISH) | 15/F | Multifocal peritoneal disease | Epithelioid, tubulo-papillary | WT1, CRT, CK5/6, CK7, D2–40, PAX8; ALK granular (focal) BAP1 retained. |
MOC31 | Diagnosed as serous carcinoma versus mesothelioma |
also stained spindle cell component; LR, local recurrence; AWD-alive with disease; NED, no evidence of disease; HIPEC, Hyperthermic Intraperitoeneal Chemotherapy; mo, months.
The single male patient (case 4) presented with a history of a painful left testicular mass. However, the tumor was found to arise in the tunica vaginalis of the spermatic cord. Subsequent limited lymph node dissection revealed metastases in 9 of 9 resected lymph nodes.
No patient had a known clinical history of asbestos exposure; this was confirmed in four cases in which clinicians were contacted. Follow up was limited. Two patients are alive with the disease, one of whom had a local recurrence. One patient demonstrates no evidence of disease at 17 months, while 2 cases have no follow up.
Pathologic Findings:
The tumors occurring in females had similar morphologic findings, with tubulopapillary or focally solid architecture and epithelioid cytology (Figures 1–3). The neoplastic cells were cuboidal, with uniform nuclei and pale eosinophilic cytoplasm. The only tumor occurring in a male patient (case 4) revealed a classic biphasic phenotype, with both bland spindle cells and a tubulopapillary epithelial component. Mitotic figures were generally sparse; in four cases being 0–1 mitoses in 10 high power fields, while in the last case there were 2 mitoses per 10 high power fields. No necrosis was observed. Three cases (cases #3–5) contained psammomatous calcifications.
Figure 1. Pathologic findings of ALK-rearranged mesothelioma.
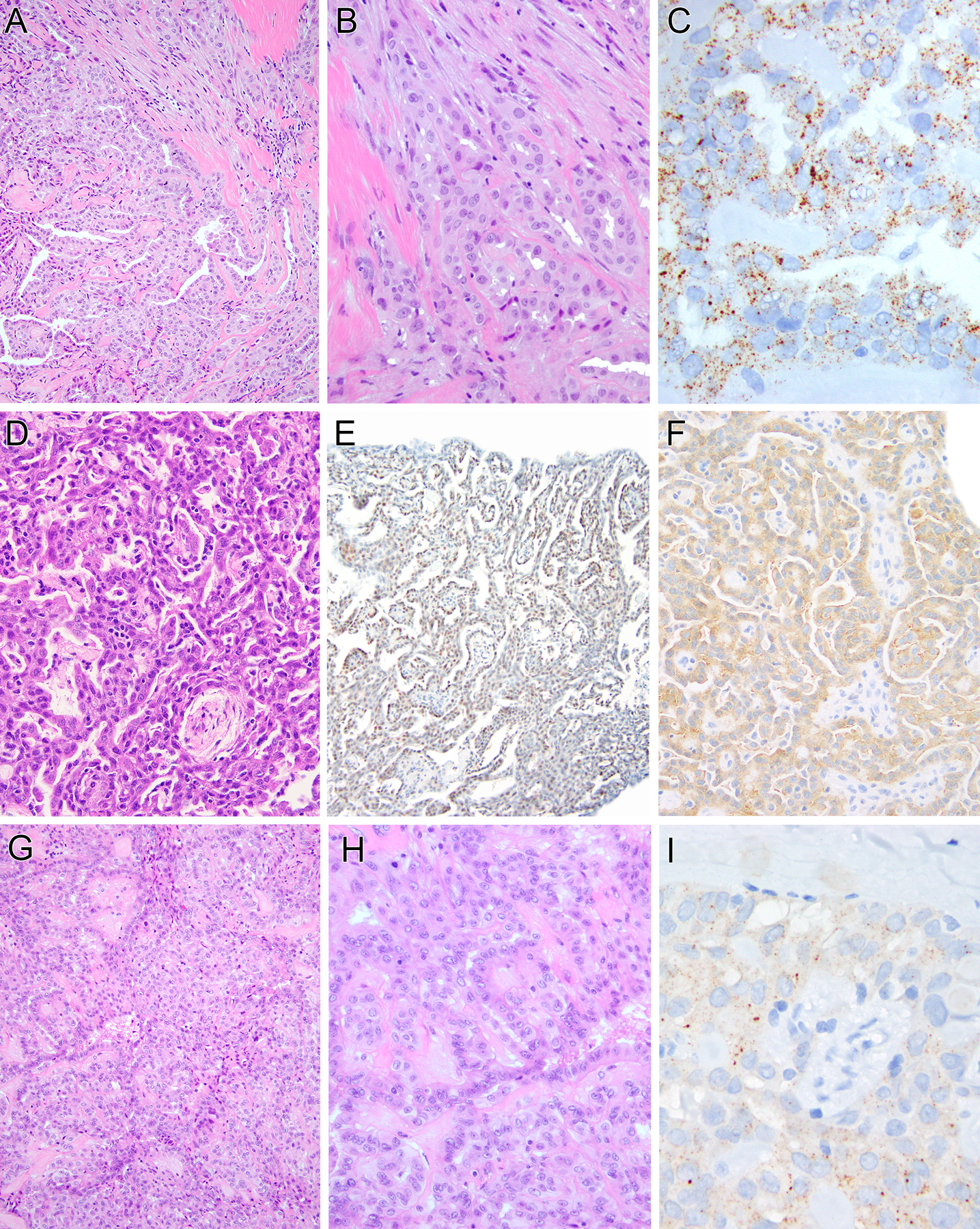
A–C (case 1): The neoplasm demonstrates prominent tubulopapillary architecture (A), composed of epithelioid cells with uniform round nuclei with vesicular chromatin and pinpoint nucleoli (B). Immunohistochemically, tumor shows granular cytoplasmic labeling for ALK (C). D–F (case 2): Tumor reveals a solid and tubulopapillary pattern (D). The neoplastic cells demonstrate moderate nuclear labeling for PAX8 (E) and granular cytoplasmic labeling for ALK (F). G–H (case 3): The epithelioid cells are arranged in a typical solid to papillary architecture (G), which at high power, reveals bland nuclei with open chromatin and small nucleoli, along with rare mitotic figures (H). Tumor shows granular cytoplasmic labeling for ALK (I).
Figure 3. Further morphologic features of epithelioid ALK-rearranged mesothelioma.
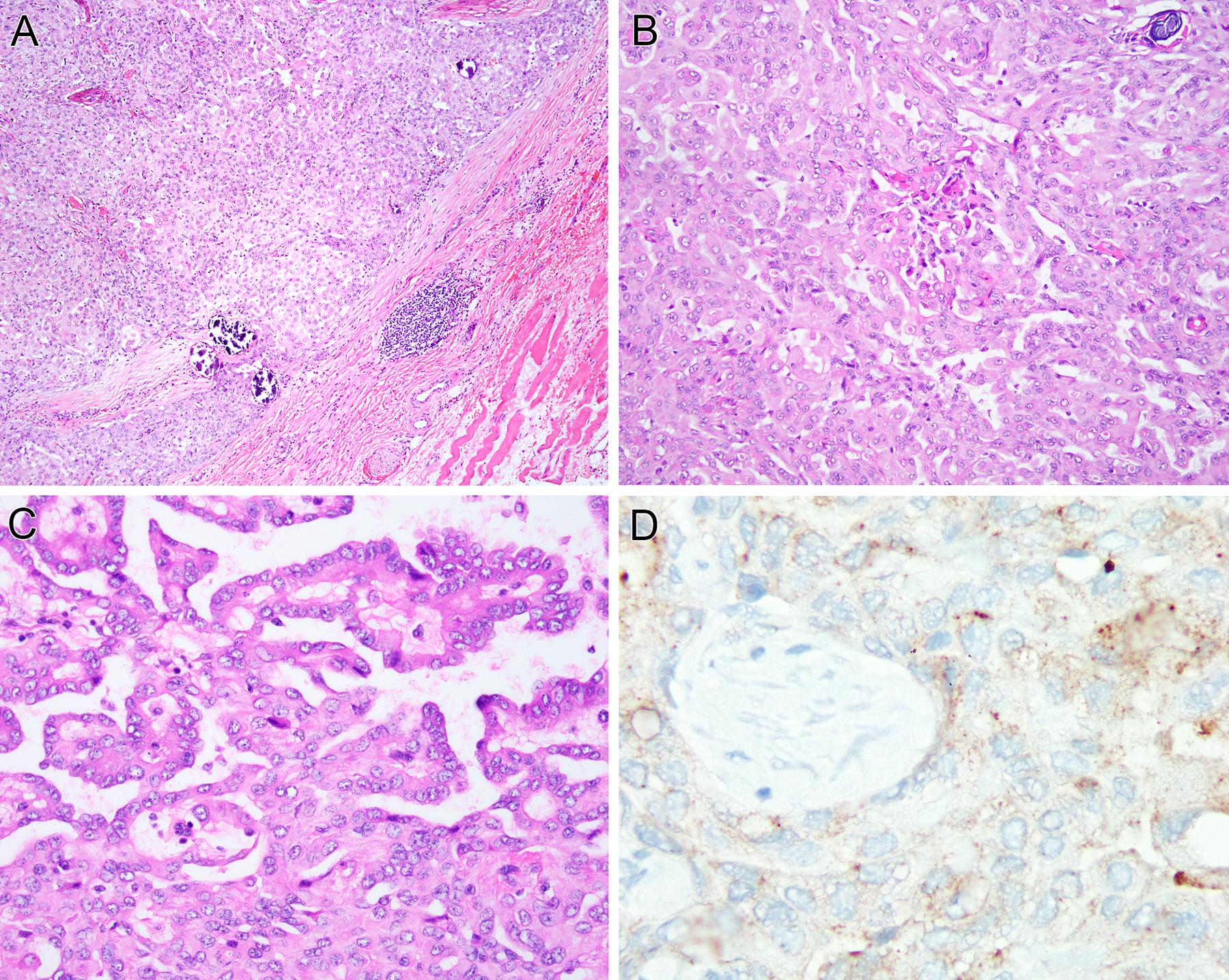
(case 5). This solid tubulopapillary epithelial neoplasm is associated with extensive psammoma bodies, which raised the differential diagnosis of a primary peritoneal serous carcinoma (A, B). The neoplastic cells demonstrate uniform nuclei with vesicular chromatin and pinpoint nucleoli (C). Tumor cells show granular cytoplasmic labeling for ALK protein (D).
All five cases demonstrated diffuse strong immunoreactivity for cytokeratins AE1/3, Cam5.2, and CK5/6 along with diffuse labeling for WT1 and calretinin. D2–40 was positive in all three cases tested. ALK was positive in all 5 cases with a granular cytoplasmic pattern. None of the cases tested demonstrated immunoreactivity for markers of adenocarcinoma (CEA, BerEp4, MOC31), markers of sex cord stromal tumor (inhibin, estrogen receptor), or desmin. BAP1 was retained in all four evaluable cases, while staining in case 1 was equivocal (neither tumor cells nor the internal control stromal tissue demonstrated nuclear labeling). All except one of the cases (case 1) tested demonstrated nuclear labeling for PAX8, though in two cases staining was weak.
Molecular Studies
Two cases were tested by Archer FusionPlex (Table 1). Case 1 demonstrated a fusion between STRN exon 3 and ALK exon 20 fusion, while case 2 revealed a TPM1 exon 8-ALK exon 20 fusion (Figure 4). Both fusions retained the kinase domain of ALK in the predicted fusion oncoprotein (Figure 4). Of note, MSKCC IMPACT identified no mutations or copy number alterations in this case 2 (Tumor Mutation Burden 0 mutations/megabase), but did demonstrate the same TPM1 exon 8-ALK exon 20 fusion, supporting the ALK fusion as the molecular driver of the disease. Case 4 demonstrated a t(2;15)(p23;q22) translocation by cytogenetics, in which the ALK break-apart was confirmed by FISH. In the remaining 2 cases, the presence of ALK gene rearrangement was identified by FISH.
Figure 4.
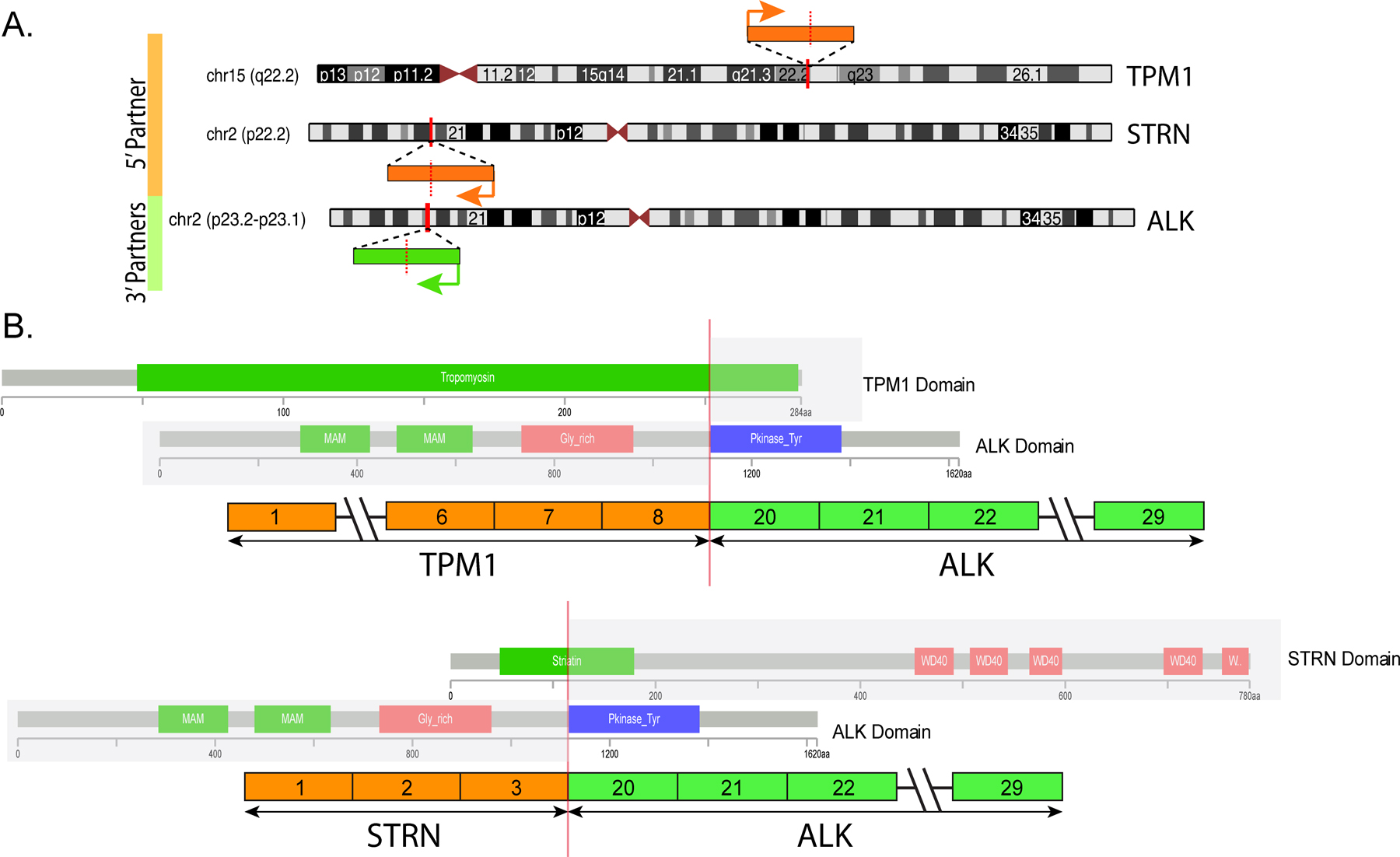
Diagrammatic representation of the chromosomal locations of ALK and its two fusion gene partners, TPM1 and STRN (A). Vertical red bars indicate the exact genomic break, while orange and green arrows indicate the direction of transcription for each gene. (B) Schematic illustration of the TPM1-ALK and STRN-ALK fusion transcripts, revealing similar breakpoints on ALK, which retains the kinase domain in the fusion oncoprotein. Protein domains are also represented.
DISCUSSION
We report the largest series of malignant mesotheliomas harboring ALK fusions. All cases in our cohort occurred in children (mean age of 13 years), all except one were females and arose in the peritoneal cavity. To date, nine other cases of ALK-rearranged malignant mesothelioma have been reported, all except one occurred in females and seven of them in the peritoneal cavity20–23 (Table 2). The patients ranged in age from 10 to 51 years (median 17 years; mean 24 years), which is approximately 50 years younger than the median age of onset for mesothelioma in the United States. Our current series further support that ALK fusion-positive mesotheliomas have a predilection for children, females, and peritoneal cavity.
Table 2:
Summary of the ALK-Rearranged Mesothelioma Reported in the Literature
| Ref# | Age/Sex | Location/Asbestos exposure | ALK Fusion | Pathology | IHC | Tx/FU |
|---|---|---|---|---|---|---|
| #20 | 10/F | Peritoneum (19 cm) / none | ALK gene rearrangement | Biphasic | ALK pos | Surgery and adjuvant cisplatin & gemcitabine AWD 6 mo |
| #21 | 17/F 36/F 51/F |
Peritoneum / none |
STRN-ALK ATG16L1-ALK TPM1-ALK |
2 epithelial, 1 biphasic | ALK pos BAP1 intact |
Surgery (x3), adjuvant chemo (x2), HIPEC (x1) FU median survival 79 mo AWD 2, DOD 1 |
| #22 | 13/F | Peritoneum / none | STRN-ALK | Epithelial | ALK pos BAP1 intact |
Neoadjuvant Cisplatin and Pemetrexed surgery & HIPEC Ceritinib (good response) AWD 3 mo |
| #23 | 14/F | Peritoneum / none | STRN-ALK | Epithelial | ALK neg | Neoadjuvant Cisplatin & Pemetrexed; surgery with HIPEC; adjuvant Cisplatin and Pemetrexed LR, clinical trial NED 60 mo |
| #23 | 27/M | Peritoneum |
ALK gene rearrangement (no fusion detected by NGS) |
Epithelial | ALK neg |
Surgery & HIPEC; LR, Cisplatin& Pemetrexed; antimesothelin tx with SS1P & Anetumab Ravtansine; Pembrolizumab; Crizotinib (4 mo, side effects) DOD 72 mo |
| #26 | NA | Pleura | EML4-ALK | Sarcomatoid | NA | NA |
| #38 | 45/F | Pleura | EML4-ALK | Epithelial | ALK pos PD-L1 pos |
Neoadjuvant Cisplatin and Pemetrexed; surgery; Carboplatin & Gemcitabine for brain mets; Pembrolizumab - good response AWD 6 mo |
Pos, positive; Neg, negative; LR, local recurrence; mo, months; NA, not available
Microscopically, all except one of our cases showed classic epithelial morphology, being arranged in a tubulopapillary or solid-papillary architecture. Three cases showed psammomatous calcifications. The tumors overall had uniform cytomorphology, with bland nuclei and low mitotic activity, lacking necrosis. Similarly, all except two cases from the 9 reported ALK-rearranged mesotheliomas in the literature had epithelial morphology. A single case in our series occurred in a male child within the tunica vaginalis and had a distinctive biphasic appearance, with alternating tubulopapillary epithelial areas and sarcomatoid components. Two other reported cases in the literature were described as biphasic, occurring in the peritoneal cavity of female patients20,21. A single sarcomatoid mesothelioma was reported arising in the pleura26. All of our cases showed ALK immunolabeling with a granular cytoplasmic pattern; however, 2 cases reported in the literature were described as being negative, one of them harboring a STRN-ALK fusion while for the other the fusion partner was unknown23.
The anaplastic lymphoma kinase (ALK) gene encodes a receptor tyrosine kinase that mediates signal transduction in a regulated fashion based upon ligand binding. In neoplastic conditions driven by ALK fusions, the ALK tyrosine kinase domain is fused to a ubiquitously expressed fusion partner that provides a strong promoter and the ability to self-oligomerize. The process results in constitutive ligand-independent activation of the ALK kinase, which in turn leads to unregulated activation of downstream signaling pathways that promote cell survival and replication, such as JAK3/STAT3, Ras/Mek/Erk, and PI3K/AKT27,28. A growing list of neoplastic conditions are driven by oncogenic ALK gene fusions, including anaplastic large cell lymphoma (ALCL)29, inflammatory myofibroblastic tumor30, a group of lung adenocarcinomas31, a subset of renal cell carcinomas32,33, a group of melanocytic tumors with Spitzoid morphology34, epithelioid fibrous histiocytoma35, and others.
Numerous ALK gene fusion partners have been described. In the context of ALK-fusion positive mesotheliomas, our results identified STRN and TPM1 genes, also found in other reported cases (Table 2). In addition, one case with ATG16L1-ALK fusion has been described21. One of the reported cases harboring STRN-ALK, which showed ALK overexpression by IHC, demonstrated a clinical response to ceritinib, an ALK inhibitor, suggesting a potential role for targeted therapy in these neoplasms22. However the only patient in our cohort who received ALK-targeted therapy did not show a clinical benefit (case 1). Factors determining the efficacy of ALK-targeted therapy remain to be determined. While we could not establish the germline status of BAP1 in our cases, the retained BAP1 immunoreactivity argues against a germline or somatic BAP1 loss4,5,18. Consistent with the simple genetic profile of translocation-associated neoplasms, the TPM1-ALK fusion was the sole genetic alteration identified in the one case studied by targeted next generation sequencing (MSKCC IMPACT), which recapitulates other ALK-fusion positive mesotheliomas reported in the literature, lacking other mutations or copy number changes.
ALK fusions are not common genetic events in malignant pleural mesotheliomas. While a subset of pleural mesotheliomas show immunoreactivity for ALK, two large series examining 234 total cases found no evidence of ALK rearrangements36,37. Moreover, none of 87 malignant mesotheliomas studied by the TCGA demonstrated ALK rearrangements (www.cbioportal.org). One case of epithelioid mesothelioma of the pleura with a reported EML4-ALK fusion was recently described in a 45-year-old female, though surprisingly the fusion was not found in the brain metastasis (an unusual site for mesothelioma metastasis). This may indicate genetic heterogeneity, or potentially a false positive ALK fusion in the pleural specimen 38. Interestingly, the lesion demonstrated PD-L1 immunoreactivity, and responded to anti-PD1 therapy. Another single case of pleural sarcomatoid mesothelioma with an EML4-ALK gene fusion was recently reported, without further details26.
The differential diagnosis for mesotheliomas in children is broad. In females, serous carcinoma and serous borderline tumors are the main concern, as exemplified by the fact that one of our cases was originally misclassified as such, and another was submitted with the differential diagnosis of serous carcinoma and mesothelioma. PAX8 immunoreactivity as seen in most of our cases further suggests serous carcinoma, though it is known that peritoneal mesotheliomas (particularly well differentiated papillary mesothelioma) often express PAX839. The young age of our patients would be unusual for serous carcinoma but is also unusual for mesothelioma. Serous carcinomas would typically label for BerEp4 and MOC31, unlike the current cases. More recently, our group has described a series of neoplasms with EWSR1-CREM gene fusions that have epithelioid morphology, often label diffusely for cytokeratin and WT1, and arise in the peritoneal cavity of children. In contrast to the current cases, the CREM-rearranged neoplasms are negative for calretinin, and do not demonstrate mesothelial features ultrastructurally. They frequently have a prominent lymphoid cuff and cystic architecture, reminiscent of angiomatoid fibrous histiocytoma40.
In summary, we report a series of malignant mesotheliomas in children harboring ALK gene fusions, supporting the concept that these represent a distinctive clinicopathologic entity with a different pathogenesis from their adult counterparts. It is striking that a malignant neoplasm that in adults is associated with complex genetic alterations is caused by a simple chromosome translocation in young patients.
Supplementary Material
Figure 2. Pathologic findings of the tunica vaginalis ALK-rearranged mesothelioma.
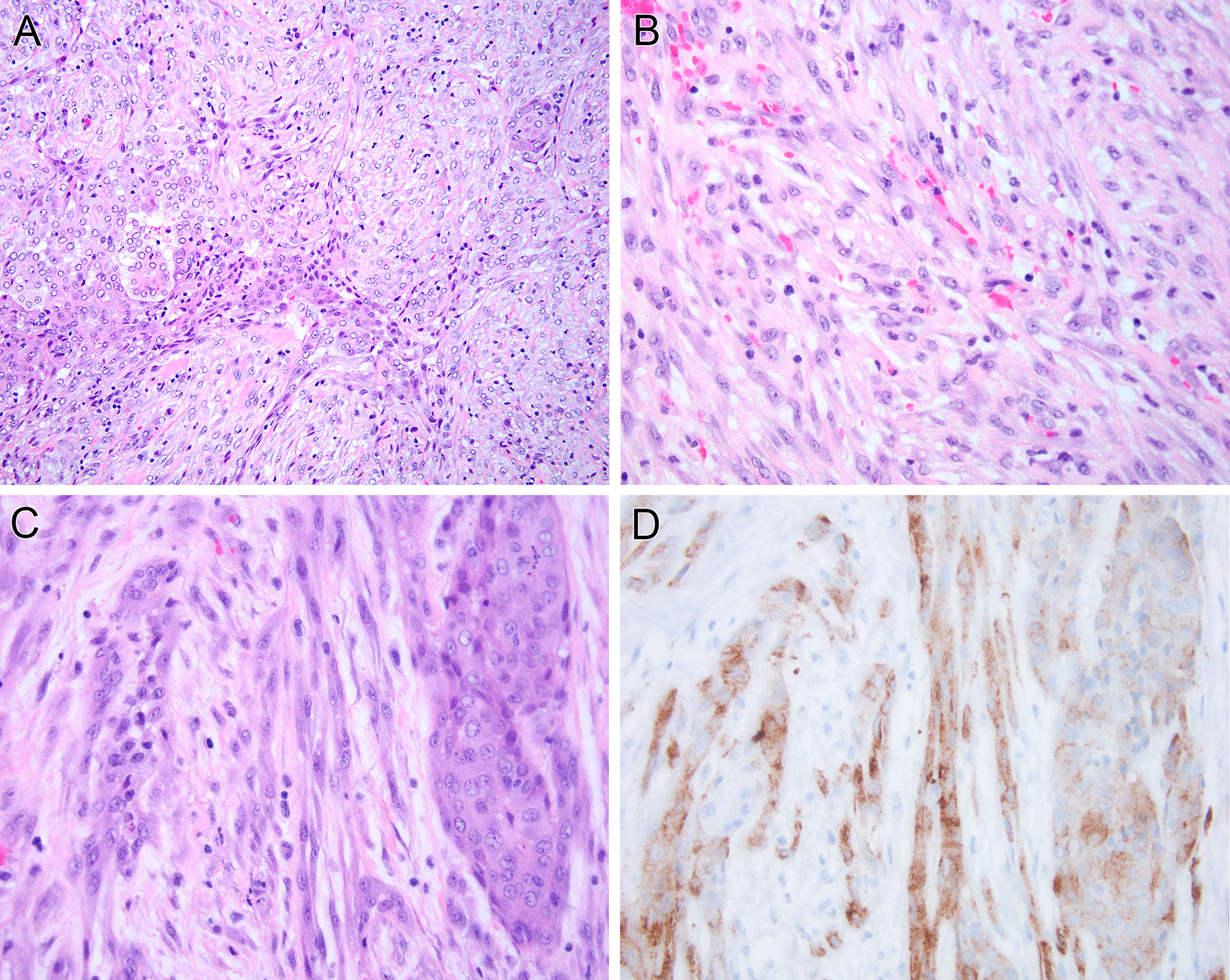
(case 4). Tumor shows a biphasic phenotype, with tubulopapillary epithelial components (left) and a prominent spindle component (right) (A). The spindle cell component is similar cytologically to the epithelial component in that it demonstrates vesicular chromatin with minute nucleoli and lacks significant pleomorphism (B). Both the sarcomatoid (left) and epithelioid (right) (C) components of the neoplasm demonstrate granular cytoplasmic labeling for ALK protein (D).
Acknowledgement:
We thank Norman Barker MA, MS, RBP for expert photographic assistance.
Disclosures: Supported in part by: P50 CA217694 (CRA), P50 CA140146 (CRA), P30 CA008748 (CRA), Cycle for Survival (CRA), Kristin Ann Carr Foundation (CRA), Dahan Translocation Carcinoma Fund (PA)
REFERENCES
- 1.Hylebos M, Van Camp G, van Meerbeeck JP, et al. The Genetic Landscape of Malignant Pleural Mesothelioma: Results from Massively Parallel Sequencing. J Thorac Oncol. 2016;11:1615–1626. [DOI] [PubMed] [Google Scholar]
- 2.Attanoos RL, Churg A, Galateau-Salle F, et al. Malignant Mesothelioma and Its Non-Asbestos Causes. Arch Pathol Lab Med. 2018;142:753–760. [DOI] [PubMed] [Google Scholar]
- 3.Leblay N, Lepretre F, Le Stang N, et al. BAP1 Is Altered by Copy Number Loss, Mutation, and/or Loss of Protein Expression in More Than 70% of Malignant Peritoneal Mesotheliomas. J Thorac Oncol. 2017;12:724–733. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Kadariya Y, Cheung M, et al. Germline mutation of Bap1 accelerates development of asbestos-induced malignant mesothelioma. Cancer Res. 2014;74:4388–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan R, Morrow B, Thomas A, et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc Natl Acad Sci U S A. 2019;116:9008–9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao S, Jin S, Cao J, et al. Advances in malignant peritoneal mesothelioma. Int J Colorectal Dis. 2015;30:1–10. [DOI] [PubMed] [Google Scholar]
- 7.Broeckx G, Pauwels P. Malignant peritoneal mesothelioma: a review. Transl Lung Cancer Res. 2018;7:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Fadrique A, Mehta A, Mohamed F, et al. Clinical presentation, diagnosis, classification and management of peritoneal mesothelioma: a review. J Gastrointest Oncol. 2017;8:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffin CM, Dehner LP. Mesothelial and related neoplasms in children and adolescents: a clinicopathologic and immunohistochemical analysis of eight cases. Pediatr Pathol. 1992;12:333–347. [DOI] [PubMed] [Google Scholar]
- 10.Cooper SP, Fraire AE, Buffler PA, et al. Epidemiologic aspects of childhood mesothelioma. Pathol Immunopathol Res. 1989;8:276–286. [DOI] [PubMed] [Google Scholar]
- 11.Fraire AE, Cooper S, Greenberg SD, et al. Mesothelioma of childhood. Cancer. 1988;62:838–847. [DOI] [PubMed] [Google Scholar]
- 12.Oberto C, Schwarz KB, Zambidis E, et al. Malignant peritoneal mesothelioma in a pediatric patient mimicking inflammatory bowel disease. Dig Dis Sci. 2004;49:434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugalski A, Davis M, Prasannan L, et al. Clinical, histologic, and genetic features of mesothelioma in a 7-year-old child. Pediatr Blood Cancer. 2013;60:146–148. [DOI] [PubMed] [Google Scholar]
- 14.Wolff-Bar M, Dujovny T, Vlodavsky E, et al. An 8-Year-Old Child with Malignant Deciduoid Mesothelioma of the Abdomen: Report of a Case and Review of the Literature. Pediatr Dev Pathol. 2015;18:327–330. [DOI] [PubMed] [Google Scholar]
- 15.Tsai LY, Yang YL, Lu MY, et al. Deciduoid mesothelioma of the pleura in an adolescent boy. Pediatr Hematol Oncol. 2010;27:132–137. [DOI] [PubMed] [Google Scholar]
- 16.Brecht IB, Agaimy A, Besendorfer M, et al. Malignant peritoneal mesothelioma in a 16-year-old girl: presentation of a rare disease. Klin Padiatr. 2012;224:170–173. [DOI] [PubMed] [Google Scholar]
- 17.Thomas A, Chen Y, Yu T, et al. Distinctive clinical characteristics of malignant mesothelioma in young patients. Oncotarget. 2015;6:16766–16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desmeules P, Joubert P, Zhang L, et al. A Subset of Malignant Mesotheliomas in Young Adults Are Associated With Recurrent EWSR1/FUS-ATF1 Fusions. Am J Surg Pathol. 2017;41:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panagopoulos I, Thorsen J, Gorunova L, et al. RNA sequencing identifies fusion of the EWSR1 and YY1 genes in mesothelioma with t(14;22)(q32;q12). Genes Chromosomes Cancer. 2013;52:733–740. [DOI] [PubMed] [Google Scholar]
- 20.Loharamtaweethong K, Puripat N, Aoonjai N, et al. Anaplastic lymphoma kinase (ALK) translocation in paediatric malignant peritoneal mesothelioma: a case report of novel ALK-related tumour spectrum. Histopathology. 2016;68:603–607. [DOI] [PubMed] [Google Scholar]
- 21.Hung YP, Dong F, Watkins JC, et al. Identification of ALK Rearrangements in Malignant Peritoneal Mesothelioma. JAMA Oncol. 2018;4:235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruschoff J, Grandhang E, Kahraman Ay N, et al. STRN-ALK rearranged malignant peritoneal mesothelioma with dramatic response following ceritinib treatment. JCO Precision Oncology. 2019. [DOI] [PMC free article] [PubMed]
- 23.Mian I, Abdullaev Z, Morrow B, et al. Anaplastic Lymphoma Kinase Gene Rearrangement in Children and Young Adults With Mesothelioma. J Thorac Oncol. 2020;15:457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao YC, Sung YS, Zhang L, et al. EWSR1 Fusions With CREB Family Transcription Factors Define a Novel Myxoid Mesenchymal Tumor With Predilection for Intracranial Location. Am J Surg Pathol. 2017;41:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leal JL, Peters G, Szaumkessel M, et al. NTRK and ALK rearrangements in malignant pleural mesothelioma, pulmonary neuroendocrine tumours and non-small cell lung cancer. Lung Cancer. 2020;146:154–159. [DOI] [PubMed] [Google Scholar]
- 27.Mano H ALKoma: a cancer subtype with a shared target. Cancer Discov. 2012;2:495–502. [DOI] [PubMed] [Google Scholar]
- 28.Gunby RH, Sala E, Tartari CJ, et al. Oncogenic fusion tyrosine kinases as molecular targets for anti-cancer therapy. Anticancer Agents Med Chem. 2007;7:594–611. [DOI] [PubMed] [Google Scholar]
- 29.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence B, Perez-Atayde A, Hibbard MK, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000;157:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. [DOI] [PubMed] [Google Scholar]
- 32.Smith NE, Deyrup AT, Marino-Enriquez A, et al. VCL-ALK renal cell carcinoma in children with sickle-cell trait: the eighth sickle-cell nephropathy? Am J Surg Pathol. 2014;38:858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuroda N, Trpkov K, Gao Y, et al. ALK rearranged renal cell carcinoma (ALK-RCC): a multi-institutional study of twelve cases with identification of novel partner genes CLIP1, KIF5B and KIAA1217. Mod Pathol. 2020. [DOI] [PubMed]
- 34.Ronchi A, Montella M, Cozzolino I, et al. The potential diagnostic and predictive role of anaplastic lymphoma kinase (ALK) gene alterations in melanocytic tumors. Eur Rev Med Pharmacol Sci. 2020;24:3829–3838. [DOI] [PubMed] [Google Scholar]
- 35.Doyle LA, Marino-Enriquez A, Fletcher CD, et al. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod Pathol. 2015;28:904–912. [DOI] [PubMed] [Google Scholar]
- 36.Monch D, Bode-Erdmann S, Kalla J, et al. A subgroup of pleural mesothelioma expresses ALK protein and may be targetable by combined rapamycin and crizotinib therapy. Oncotarget. 2018;9:20781–20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salvi S, Varesano S, Boccardo S, et al. FISH Analysis of Crizotinib Target Genes ROS1/ALK/MET in Malignant Mesothelioma. J Thorac Oncol. 2017;12:e116–e118. [DOI] [PubMed] [Google Scholar]
- 38.Bronte G, Delmonte A, Burgio MA, et al. Impressive clinical response to anti-PD-1 therapy in epithelioid mesothelioma with high clonal PD-L1 expression and EML4-ALK rearrangement. Lung Cancer. 2020;142:47–50. [DOI] [PubMed] [Google Scholar]
- 39.Xing D, Banet N, Sharma R, et al. Aberrant Pax-8 expression in well-differentiated papillary mesothelioma and malignant mesothelioma of the peritoneum: a clinicopathologic study. Hum Pathol. 2018;72:160–166. [DOI] [PubMed] [Google Scholar]
- 40.Argani P, Harvey I, Nielson G, et al. EWSR1/FUS-CREM Fusion Defines a Distinctive Malignant Epithelioid Neoplasm with Epithelial Differentiation and Predilection for Mesothelial-Lined Cavity. Mod Pathol. 2020;33: 2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


