Abstract
This review highlights the significance of the insulin receptor (IR) and insulin-like growth factor-1 receptor (IGF-1R) signaling pathway in cancer and assesses its potential as a therapeutic target. Our emphasis is on breast cancer, but this pathway is central to the behavior of many cancers. An understanding of how IR/IGF-1R signaling contributes to the function of the normal mammary gland provides a foundation for understanding its aberrations in breast cancer. Specifically, dysregulation of the expression and function of ligands (insulin, IGF-1 and IGF-2), receptors and their downstream signaling effectors drive breast cancer initiation and progression, often in a subtype-dependent manner. Efforts to target this pathway for the treatment of cancer have been hindered by several factors including a lack of biomarkers to select patients that could respond to targeted therapy and adverse effects on normal metabolism. To this end, we discuss ongoing efforts aimed at overcoming such obstacles.
Keywords: Insulin, Insulin-like Growth Factor (IGF), insulin receptor, IGF-1R, Breast Cancer, Signal Transduction, Therapy
A number of comprehensive reviews have been written about the insulin and IGF signaling pathway and its role in cancer (Belfiore et al., 2009; Gallagher and LeRoith, 2020; Pollak, 2008), and especially in breast cancer (Farabaugh et al., 2015), and they have elaborated the pre-clinical evidence to support the involvement of this signaling pathway in both cancer risk and progression. Unfortunately, efforts to inhibit this pathway in the clinic have been unsuccessful to date, in part due to adverse effects of therapies on normal physiology (Pollak, 2012; Yang and Yee, 2012). In this review we will provide an overview of the IR/IGF-1R signaling pathway and its importance for breast cancer and we will highlight the more recent discoveries that provide continued optimism for targeting insulin and IGF signaling for the treatment of breast cancer patients. The lessons learned at the bedside when targeting the IR/IGF-1R pathway need to be brought back to the bench for further basic research. A more rigorous understanding of the molecular mechanisms by which this pathway signals to regulate breast tumor progression is needed to facilitate the development of more selective and effective therapies.
The IR/IGF-1R signaling pathway in normal physiology
Before discussing the IR/IGF-1R signaling pathway in breast cancer, it is necessary to understand the players involved and their role in normal organismal growth and metabolism, because it is the dysregulation of these normal functions that contribute to breast cancer development and progression. In addition, issues that arise in response to targeting this pathway for cancer treatment are related to adverse effects of disrupting these normal functions.
Receptors and ligands
The insulin and insulin-like growth factor (IGF) signaling pathway is comprised of a family of cell surface receptor tyrosine kinases (RTKs) that arose from a common ancestral gene and share a high degree of homology (reviewed in (Barbieri et al., 2003)). Each receptor contains subunits of di-sulfide bonded extracellular α and transmembrane β chains that dimerize to form holoreceptors (Figure 1). The IR has two splice variants, IR-A and IR-B, that differ by the inclusion of exon 11 in IR-B that encodes a 12 amino acid region in the α chain (Frasca, 1999). In addition to forming IR and IGF-1R homodimers, the IR and IGF-1R can also heterodimerize to form hybrid receptors, increasing the complexity of this signaling pathway. The ligands for the IR and IGF-1R signaling pathway include insulin, IGF-1 and IGF-2, which differ in their affinity for each receptor dimer (Figure 1). At physiological levels, insulin binds primarily to the IR-A and IR-B and IR-A/IR-B hybrid receptors, and IGF-1 to the IGF-1R and IR/IGF-1R hybrids (Belfiore et al., 2009). IGF-2 binds to the IGF-1R and also the IR-A, as well as hybrid IGF1R/IR-A receptors (Frasca, 1999). The 12 amino acid region encoded by exon 11 reduces the affinity of IR-B for IGF2, making this ligand primarily selective for the IR-A isoform (Frasca et al., 1999). IGF-2 also binds to a separate receptor, the IGF-2R, that functions as a decoy receptor to inhibit IGF-2-dependent signaling through the IR/IGF-1R pathway (Brown et al., 2009; Kornfeld, 1992).
Figure 1: Schematic of the insulin/IGF signaling pathway receptors and ligands.

The insulin receptor (IR) is expressed as two splice isoforms (IR-A and IR-B) and the insulin-like growth factor-1 (IGF-1) receptor (IGF-1R) has one form. The IR-B differs from the IR-A receptor by the inclusion of a 12 amino acid region in the α chain. Each half receptor, which are comprised of disulfide bonded α and β chains, can form homodimers or heterodimers, resulting in 6 different receptors. Each receptor has differing affinities for the insulin, IGF-1 and IGF-2 ligands. − no binding, + low binding, ++ moderate binding, +++ high binding.
Normal Function of the IR/IGF-1R pathway: Metabolism vs. Mitogenesis
In normal physiological contexts, insulin stimulation of the IR regulates metabolic signaling, whereas IGF-1 and IGF-2 stimulation of the IGF-1R regulates mitogenic signaling (Figure 2). In adults, insulin-responsive tissues such as the liver, muscle and adipose tissue express primarily the IR-B isoform and the primary role of this signaling axis is to regulate glucose uptake to control organismal glucose homeostasis (Belfiore et al., 2017). Upon feeding, increased glucose levels stimulate insulin expression and secretion from the pancreas into the circulation. Insulin binds to IRs on cells in the target tissues, primarily muscle and adipose, stimulating glucose uptake and storage as glycogen and triglycerides, respectively, for later energetic needs. Transport of glucose is rate limiting and insulin signaling promotes glucose transporter-4 (GLUT-4) trafficking to the cell surface for glucose uptake (Klip et al., 2019). Gluconeogenesis is concurrently suppressed by insulin stimulation in the liver, which is the major site of insulin clearance.
Figure 2: Functional outcomes of the IR/IGF-1R/IRS pathway in normal and breast cancer settings.

Ligands are expressed both systemically (from the liver and pancreas) and locally in the breast in both normal and cancer and elevated ligand expression is observed in breast cancer. In normal tissues (on left), stimulation of the IR-B and IGF-1R regulates glucose homeostasis and growth, respectively. In breast cancer (on right), expression of IR-A and IGF-1R is increased. Systemic factors such as obesity and type 2 diabetes (T2D) promote pathway activity by upregulating ligand and receptor expression. Moreover, hypoxic tumor microenvironments enhance IRS-2 expression. The IR/IGF-1R pathway cross-talks with the estrogen receptor (ER) pathway to enhance signaling and promote tumor growth and progression. Targeted inhibitors are shown that disrupt pathway function (white boxes): FMD, fasting mimicking diet; IGFi, inhibitory IGF-1 and IGF-2 antibodies; IGF-1Ri, inhibitory IGF-1R antibodies; RTKi, IR/IGF-1R tyrosine kinase inhibitors; PI3Ki, PI3K inhibitors; SERM, selective estrogen receptor modulators; SERD, selective estrogen receptor degraders. T2D, Type 2 Diabetes; SHBG, sex-hormone binding globulin; IGFBP, insulin-like growth factor binding protein
IGF-1 and IGF-2 are expressed predominantly in the liver under control of growth hormone (GH) and released into the circulation where they travel to target tissues to bind IGF-1R for their growth regulatory action (Kleinberg et al., 2009). The importance of IGF-1 for normal growth regulation is demonstrated by individuals with Laron dwarfism, a condition that is the result of low IGF-1 expression (Laron and Werner, 2020). Igf1−/− and Ifg1r−/− mice are small in body size as well (Baker et al., 1993; Liu et al., 1993; Powell-Braxton et al., 1993). IGF-2-dependent embryonic growth has also been attributed in part to stimulation of IR-A, which is the predominant IR isoform expressed in fetal tissues and regulates mitogenic signaling (Frasca et al., 1999). The bioavailability of circulating IGF ligands is regulated by IGF-binding proteins (IGFBPs), which stabilize IGF expression and restrict binding to receptors to prevent signaling (Baxter, 2014). In contrast with insulin, which acts exclusively as a systemic hormone, the IGFs can also be produced locally and act in an autocrine or paracrine manner within tissues (Figure 2) (Kleinberg et al., 2009; Livingstone, 2013).
Upon stimulation by ligands, IR/IGF-1R receptors undergo a conformational change that activates intracellular tyrosine kinases in the β-chains, resulting in receptor trans-autophosphorylation (De Meyts and Whittaker, 2002). These phosphorylation events lead to the recruitment of effectors that are phosphorylated by the receptors, activating signaling pathways to modulate cellular functions that regulate organismal growth and metabolic homeostasis. Major signaling adaptors recruited to the IR and IGF-1R are the insulin receptor substrate (IRS) and Shc proteins, which activate the PI3K and MAPK signaling pathways, respectively (Figure 3) (Belfiore et al., 2009). As most cells express both IR and IGF-1R, the added complexity of hybrid receptor formation has made it difficult to decipher the specific signaling functions of each individual receptor. However, studies on chimeric receptors in which the extracellular domains of each receptor were swapped with the transmembrane and intracellular domains of the other receptor, and expressed in double IR−/−, IGF1R−/− knockout cells, provide evidence for unique effector interactions and signaling dynamics that may contribute to the distinct metabolic and mitogenic activities of these receptors (Cai et al., 2017).
Figure 3: Signaling via the IR and IGF-1R.
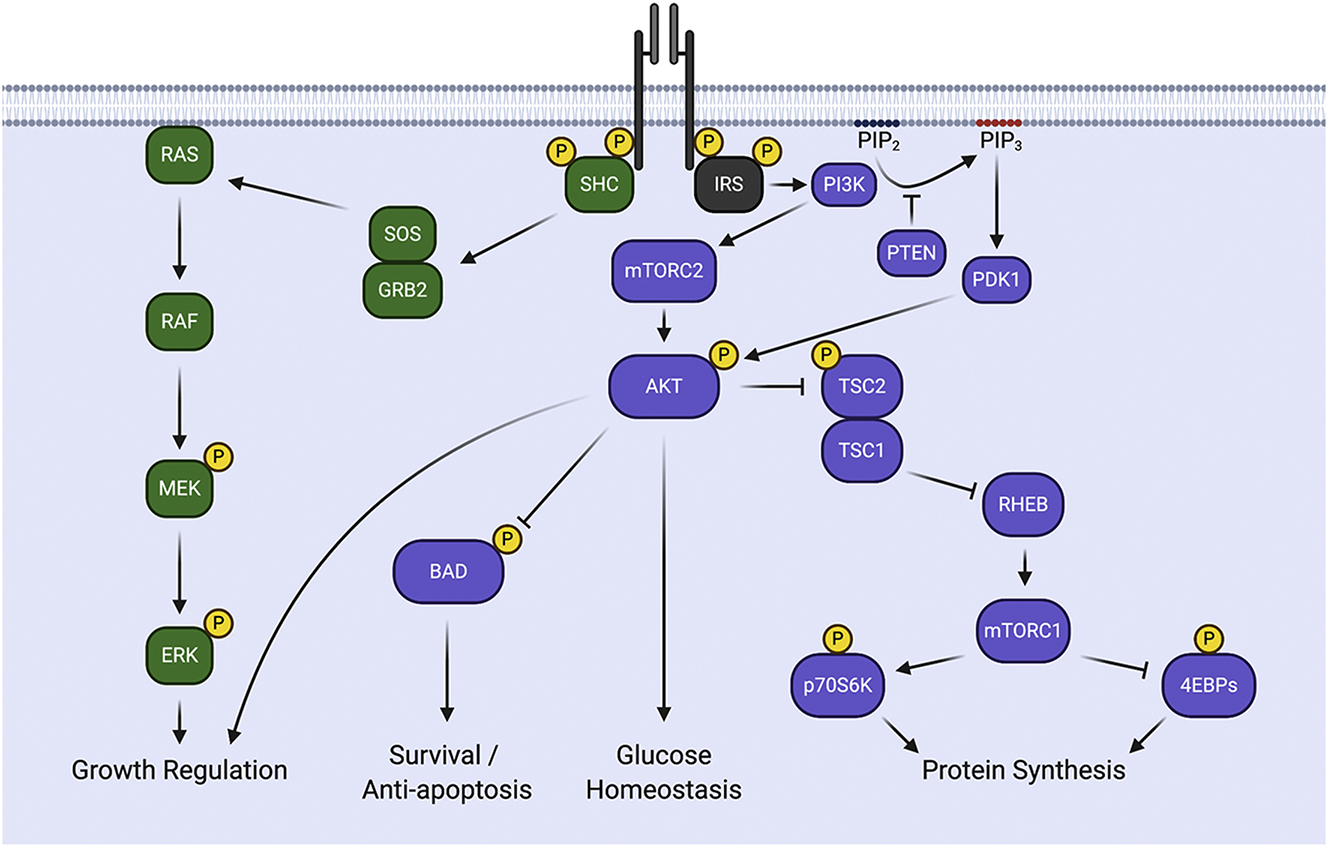
Upon stimulation with ligand, receptors undergo trans-autophosphorylation and recruit the downstream signaling adaptors SHC and the IRS proteins. Once bound, these adaptors are phosphorylated on tyrosine residues, creating docking sites for the recruitment of signaling effectors. Recruitment of SHC activates the RAS/MEK/ERK signaling pathway to stimulate growth. Recruitment of the IRS proteins activates PI3K and the downstream signaling effectors AKT and mTOR to regulate survival, glucose metabolism and protein synthesis.
The signaling outcomes of the IR and IGF-1R are influenced by the IRS proteins that play an essential role in determining the diversity of cellular responses to IR/IGF-1R stimulation (Mardilovich et al., 2009). IRS-1 and IRS-2 are ubiquitously expressed and are the primary mediators of insulin and IGF-dependent regulation of glucose metabolism and mitogenesis in most cell types (reviewed in (White, 2002)). Humans also express IRS-4, which has restricted expression primarily in the kidney, thymus and liver (Lavan et al., 1997a). An additional IRS protein, Irs-3, was identified in rodents, but it is not expressed in humans (Bjornholm et al., 2002; Lavan et al., 1997b). The IRS proteins share their highest degree of homology in their N-termini, which contain pleckstrin homology (PH) and phosphotyrosine binding (PTB) domains that mediate their recruitment to the IR and IGF-1R (Mardilovich et al., 2009). The IRS proteins are phosphorylated by the receptors on tyrosine residues in their C-termini, generating binding sites that recruit downstream effectors which include PI3K, Grb-2, SHP-2, Fyn, c-Crk, CrkII and Nck (Mardilovich et al., 2009). An additional motif in IRS-2 that contributes to receptor engagement, the kinase regulatory loop binding (KRLB) domain, may contribute to differential signaling downstream of distinct receptor dimers as it differentially interacts with the IR and IGF-1R and limits IRS-2 tyrosine phosphorylation by the IR (Sawka-Verhelle et al., 1996).
Despite sharing significant homology and activating many similar downstream pathways, the functional outcomes of signaling via IRS-1 or IRS-2 are diverse and non-redundant, as highlighted by knockout mouse studies. Irs1−/− mice are born small and remain runted throughout their lives, implicating a primary role for this IRS protein in somatic growth regulation (Araki et al., 1994; Tamemoto et al., 1994). Irs2−/− mice are born normal in size but have small brains and are infertile (Schubert et al., 2003; Withers et al., 1998). With regard to metabolic regulation, mice lacking either Irs-1 or Irs-2 develop insulin resistance, but only Irs2−/− mice develop early-onset diabetes due to a loss of pancreatic β-cell function (Withers et al., 1999; Withers et al., 1998). Irs4−/− mice are phenotypically normal, with only mild growth, reproductive and insulin sensitivity defects (Fantin et al., 2000), supporting a more restricted role for this family member. These differences in knockout phenotype indicate unique roles for the IRS proteins in IR/IGF-1R signaling in normal development and physiology, a finding that is also evident in cancer.
Regulation ofIR/IGF-1R pathway function
The IR/IGF-1R signaling pathway is tightly regulated in normal physiology to maintain growth control and metabolic homeostasis. Upon stimulation by ligands, receptors are internalized from the cell surface and their signaling longevity is controlled by endocytic trafficking either to the late endosome and lysosome for receptor/ligand degradation or to the recycling endosome for trafficking back to the cell surface where a subsequent round of signaling may occur (Romanelli et al., 2007). The metabolic and mitogenic signaling differences of the IR and IGF-1R, respectively, may be explained in part by differential trafficking of each receptor and the longevity of their signals (Chow et al., 1998; Zapf et al., 1994). Negative feedback regulation of the IRS proteins also controls insulin sensitivity and glucose homeostasis by limiting the magnitude and duration of signaling responses (Gual et al., 2005). Activation of downstream signaling pathways feedback to phosphorylate the IRS proteins on serine residues, causing either inhibition of interactions with upstream receptors and/or downstream effectors, or degradation via ubiquitin-mediated targeting to the proteasome (Lee et al., 2000; O’Reilly et al., 2006; Rui et al., 2001). The end result is a cessation of signaling. If sustained, this negative feedback leads to insulin resistance. Differential sensitivity of IRS-1 and IRS-2 to the effects of negative feedback regulation likely explains some of the distinct functional outcomes elicited by each IRS protein in response to insulin and IGF-1 stimulation.
IR/IGF-1R pathway function in the normal breast
In addition to systemic regulation of organismal metabolism and growth, the insulin and IGF-1 signaling pathway also plays a functional role locally in the normal breast, both during development and in the mature adult gland. Knockout and expression studies in mice reveal that Igf-1 ligand and the Igf-1r are required for terminal end bud (TEB) development and ductal morphogenesis during mammary gland development, and they are also required for alveolar differentiation during pregnancy and lactation (Bonnette and Hadsell, 2001; Ruan and Kleinberg, 1999; Sun et al., 2011). The Irs proteins have unique expression patterns in the developing and adult mammary gland, suggesting unique signaling functions (Lee et al., 2003). Irs-1 is expressed in a subset of luminal mammary epithelial cells in ducts and also in the body of the TEB of the developing gland. In contrast, Irs-2 is expressed homogeneously in the developing ductal luminal epithelial cells and also throughout the TEB, including the cap cell layer, which is highly proliferative. Proliferation is decreased in the cap cells of TEBs in Igf1r−/− mice, suggesting a selective role for Igf-1r/Irs-2 signaling in extension of the developing ductal structures (Sun et al., 2011). Interestingly, the cap cell layer also contains a population of multipotent mammary stem cells that contribute to both the luminal and basal lineages of the gland (Ruan and Kleinberg, 1999). In this regard, a recent study examining overexpression of a constitutively active IGF-1R in the mammary gland provided evidence that signaling by this receptor regulates luminal cell fate through the maintenance of luminal progenitor cell populations (Farabaugh et al., 2020). This regulation could underlie the aberrant development and defective adult glands in the Igf1−/− and Igf1r−/− mice. The involvement of the IR/IGF-1R signaling pathway in stem cell regulation in the normal mammary gland foreshadows a role for this pathway in breast cancer stem cells.
The IR/IGF-1R signaling pathway in breast cancer
IR/IGF-1R pathway function is tightly regulated to maintain normal organismal growth and metabolism. Dysregulation of this pathway leads to pathophysiological conditions including diabetes and cancer. With regard to the latter, aberrant IR/IGF-1R signaling has been implicated in the initiation and progression of many types of cancer, including breast cancer (reviewed in (Gallagher and LeRoith, 2020; Pollak, 2008)). Alterations in expression and function of ligands, receptors and signaling effectors in breast tumors drive enhanced activity of this signaling pathway and impact tumor behavior, often in a subtype-dependent manner (Figure 3). The significant associations of the IR/IGF-1R pathway with breast cancer risk and outcomes underscore the importance of understanding mechanistically how this pathway functions to regulate tumor growth and progression. In this section, we will discuss the progress that has been made in deciphering how IR/IGF-1R signaling contributes to breast tumor biology and the tumor-specific functions of this pathway that may be exploited for future therapeutic benefit.
The IR/IGF-1R pathway in breast cancer: links to obesity and cancer disparities
A significant amount of evidence to support the importance of the IR/IGF-1R signaling pathway in cancer comes from the connection between obesity and cancer. Obesity is linked to both the risk of developing cancer and reduced overall survival of cancer patients (Poloz and Stambolic, 2015). In breast cancer, obesity is associated with a 2-fold increased relative risk of death (Calle, 2003). Although there are many factors that contribute to breast cancer development and progression in obese states, including increased sex hormone production, altered adipokine expression and enhanced inflammation, a major consequence of obesity is insulin resistance, which results in systemic hyperinsulinemia (Gallagher and LeRoith, 2020; Lorincz and Sukumar, 2006). The fact that high insulin levels are an independent risk factor for breast cancer initiation and recurrence and are significantly associated with poor outcomes of breast cancer patients highlights the important role of the IR/IGF-1R pathway in the context of obesity driven breast cancer (Ferroni et al., 2016; Formica et al., 2012; Goodwin et al., 2002).
The ability of hyperinsulinemia to promote mammary tumor progression has been demonstrated experimentally using mouse models. Transgenic mice that express a kinase-dead IGF-1R in the skeletal muscle are hyperinsulinemic, but otherwise exhibit normal weight and lack other obesity characteristics (Fernandez et al., 2001). Mammary tumor growth and metastatic progression are enhanced in both spontaneous (MMTV-PyMT) and orthotopic tumor models in these mice, and this increased tumor progression is dependent upon IR function in the tumors (Novosyadlyy et al., 2010; Rostoker et al., 2015). The fractions of free IGF-1 and IGF-2 also increase in response to hyperinsulinemia due to suppression of systemic IGFBP expression, which enhances the bioavailability of these ligands to activate their receptors (Figure 2) (Clemmons and Underwood, 1991). The association of IGF-1 with breast cancer risk is indicated by a reduced overall cancer incidence in patients with Laron drawfism (Laron and Werner, 2020). Moreover, free IGF-1 is elevated in the serum of breast cancer patients and higher overall IGF-1 levels are associated with an increased risk of developing estrogen receptor positive (ER+) breast cancer (Murphy et al., 2020; Qian and Huo, 2020).
It should be noted that hyperinsulinemia, which results from obesity and other factors, not only drives systemic increases in free IGF-1 and IGF-2, but it also promotes their expression locally in the tumor microenvironment (Figure 2). This elevated expression impacts autocrine tumor cell signaling and paracrine cross-talk with stromal cells to promote tumor growth and progression (De Vincenzo et al., 2019; Gui et al., 2019; Sciacca et al., 1999). IGF2 transcription and IGF-2 protein expression are also commonly increased locally in tumors through loss of imprinting of the IGF2 locus as a result of demethylation of the differentially methylated region (DMR) of the maternal allele (DeChiara et al., 1991; Kaneda et al., 2007; Wu et al., 1997). Evidence that dysregulation of IGF-2 can promote breast cancer initiation comes from transgenic mouse studies in which overexpression of IGF-2 in the mammary epithelium drives tumor formation, and these tumors are suppressed by concurrent expression of the inhibitory IGF-2R (Bates et al., 1995; Wise and Pravtcheva, 2006). In summary, all three ligands of the IR/IGF-1R pathway are upregulated in obese/hyperinsulinemic states and activate their cognate receptors to drive breast tumor growth and progression.
Obesity and Type-2 Diabetes (T2D) share the common feature of insulin resistance and hyperinsulinemia and they link the IR/IGF-1R signaling pathway to health disparities in breast cancer (Gallagher et al., 2016). African American women have a higher incidence of obesity and T2D than Caucasian women (Calle et al., 2003; Peairs et al., 2011). Although their incidence of breast cancer development is similar, African American women are more likely to develop aggressive triple negative breast cancer (TNBC) and do so at a younger age than Caucasian women and this is associated with a higher mortality rate (Scott et al., 2019). Insulin resistance is positively associated with poor prognosis in African American breast cancer patients (Gallagher et al., 2020). Tumors in these patients exhibit higher IR expression and a higher ratio of IR:IGF-1R than in tumors from Caucasian women, supporting a role for IR signaling in disparities of breast cancer outcomes. IGF-2 is also associated with disparities in breast cancer risk and survival outcomes in African American women. IGF-2 expression is elevated in the normal breast tissue of African American women when compared with Caucasian women, and this higher level of IGF-2 expression persists in aggressive breast tumors in younger African American women (Kalla Singh et al., 2010).
IR andIGF-1R function: redundant or diverse roles in breast cancer?
The specific role of each receptor/ligand pair in driving breast cancer initiation and progression is difficult to determine due to common expression of both IR and IGF-1R in tumors and the resultant complexity of hybrid receptor formation, as well as the promiscuous binding of each ligand to multiple receptor dimers (Pandini et al., 1999). Moreover, currently available antibody reagents do not discriminate between phosphorylated receptor forms, preventing the determination of the individual activity of each receptor dimer within a tumor. However, some common themes emerge from the studies that have evaluated receptor expression and activity in breast tumors. The IGF-1R is frequently over-expressed in breast tumors, with higher expression in luminal A and B tumors and lower expression in HER2+ and basal/triple negative breast cancer (TNBC) (Shimizu et al., 2004). Early studies revealed the contribution of IGF-1R to tumor growth through the use of inhibitory antibodies that blocked ligand binding to the receptor and reduced mitogenic responses (Cullen et al., 1990). However, the expression of IGF-1R is not associated with prognosis for most breast cancer subtypes, with the exception of luminal B tumors in which IGF-1R expression correlates with better outcomes (Yerushalmi et al., 2012). Although expression levels are a poor predictor of prognosis, an IGF-1 gene signature is associated with poor disease outcomes, indicating that IGF-1R pathway activity is a more accurate measure of function in tumors than relative expression (Creighton et al., 2008; Litzenburger et al., 2011). However, IGF-1R signaling may inhibit tumor progression in some contexts, specifically in Wnt1-driven TNBC (Rota et al., 2014).
IR expression is also altered in breast tumors, although studies have been conflicting on its association with prognosis. The impact of the IR on tumor growth and progression may be more related to isoform ratios than total expression levels, with a higher ratio of IR-A:IR-B most commonly observed in tumors, but this varies with molecular subtype (Harrington et al., 2012). Luminal breast tumors express higher overall IR levels and a greater IR-A:IR-B ratio than HER2+ and basal/TNBC tumors due to the elevated luminal expression of the CUGBP1 splicing factor that favors IR-A splicing (Huang et al., 2020). A reduction in IR-B expression in tumors may also drive the elevated IR-A:IR-B ratio (Huang et al., 2011). Of clinical relevance, higher IR-A:IR-B ratios correlate positively with OncotypeDX proliferation scores (Huang et al., 2011), supporting the contribution of IR-A to tumor growth.
Although the total level of IR and IGF-1R expression varies across breast cancer subtypes, phosphorylated IR/IGF-1R is observed in luminal, HER2 and basal/TNBC tumors and this phosphorylation event is associated with poor patient survival (Law et al., 2008). The staining pattern of strong IGF-1R, moderate/strong IR and pIR/pIGF-1R positivity together is associated with poor breast cancer patient outcomes, highlighting that it may be the coincident expression of IR and IGF-1R, resulting in hybrid receptor formation, and not individual homodimer expression, that determines the prognostic outcomes of this pathway (Bjorner et al., 2017). This has significant implications for targeting this pathway in cancer as it suggests that both IR and IGF-1R signaling drive tumor progression and need to be co-inhibited for therapeutic benefit.
The IRS proteins and breast cancer
As described for normal breast function, the IRS proteins also play essential roles in regulating the response of tumors to IR and IGF-1R signaling in breast cancer, with impact on patient outcomes. IRS-1 is regulated by ER-α and it is expressed at moderate to strong levels in more well-differentiated, ER-positive (ER+), luminal cell lines and tumors where it is localized diffusely in the cytoplasm or in the nucleus (Schnarr et al., 2000; Sisci et al., 2007). In the nucleus, IRS-1 interacts with ER-α to modulate gene expression (Morelli et al., 2004). The nuclear localization of IRS-1 is associated with positive tamoxifen response and improved patient survival, likely because expression of IRS-1 in the nucleus is a biomarker for active ER signaling, which renders tumors more sensitive to a selective estrogen receptor modulator (SERM) such as tamoxifen (Migliaccio et al., 2010). IRS-1 expression decreases in more poorly differentiated, higher-grade tumors that lack ER expression (Schnarr et al., 2000).
In contrast with IRS-1, IRS-2 is expressed at higher levels in ER negative (ER−) basal/TNBC cells and tumors (Porter et al., 2013). IRS-2 is excluded from the nucleus and is instead localized either in the cytoplasm or at the plasma membrane (Clark et al., 2011). IRS-2 membrane staining is significantly associated with decreased overall survival of breast cancer patients, in particular in patients with progesterone receptor (PR) negative tumors (Clark et al., 2011). Although IRS-2 expression is positively regulated by the PR (Cui et al., 2003; Vassen et al., 1999), alternative mechanisms of regulation drive expression in these tumors that lack functional PR. Epidermal growth factor receptor (EGFR) signaling promotes IRS-2 expression in a JNK-dependent manner through the AP-1 transcription factor (Cui et al., 2006). IRS-2 expression is also positively regulated by hypoxia in the tumor microenvironment, and this transcriptional regulation is dependent upon hypoxia-inducible factor-1 (HIF-1) and HIF-2 (Figure 2) (Mardilovich and Shaw, 2009).
Genetic modifications of the IR/IGF-1R pathway in breast cancer
In addition to expression changes of components of the IR/IGF-1R signaling pathway in breast cancer, genetic alterations may also contribute to the enhanced activity of this pathway. Analysis of somatic mutation data from a genome sequencing study of a rare but aggressive form of lobular breast cancer, pleomorphic invasive lobular carcinoma (PILC), identified frequent mutations in IR/IGF1R/IRS2 signaling pathway components (Zhu et al., 2018). Top hits for this sequencing analysis were ‘IRS-related events triggered by IGF1R’ and ‘Insulin receptor signaling cascade’. When downstream PI3K effectors were also included in the analysis, components of the IR/IGF1R/IRS2/PI3K signaling pathway were mutated in 15/17 (88%) of the PILC tumors that were sequenced. Patients with PILC tend to have poor clinical outcomes with short relapse times, a high risk of recurrence and decreased overall survival, supporting the contribution of the IR/IGF-1R pathway to this aggressive behavior.
Relationship of IR/IGF-1R signaling and the ER in breast cancer
There is significant cross-talk between the ER and the IR/IGF-1R pathway in breast cancer (Figure 2). As mentioned previously, IGF-1, IGF-1R and IRS-1 are all ER-α target genes and their expression is upregulated by estrogen in ER+, luminal breast cancers (Huynh et al., 1996; Lee et al., 1999). Enhanced IR/IGF-1R signaling and promotion of proliferation are observed in ER+ cells treated with both estrogen and insulin/IGF-1, but cooperation is not observed in ER− cells (Hamelers et al., 2002). In turn, IGF-1 and insulin signaling can regulate ER activity through estrogen-dependent and independent mechanisms. Stimulation of IR/IGF-1R signaling phosphorylates ER in the presence or absence of estrogen, and this phosphorylation event is dependent upon downstream PI3K activation (Martin et al., 2000). This phosphorylation (Ser167) enhances ER transcriptional activity. An additional cross-talk of these pathways occurs in an estrogen-dependent manner. Elevated circulating levels of insulin and IGF-1 suppress the expression of sex-hormone-binding globulin (SHBG), which binds to testosterone and estrogen with high affinity (Figure 2) (Pugeat et al., 1991). As a result, the bioavailability of estrogen is increased and can stimulate ER+ breast tumor growth. In post-menopausal women, levels of SHBG are inversely correlated with breast cancer risk, supporting the important contribution of this cross-talk to breast cancer promotion, especially in the obese setting (Zeleniuch-Jacquotte et al., 2004).
Functional outcomes of IR/IGF-1R signaling in breast cancer
The IR/IGF-1R signaling pathway promotes breast cancer initiation and progression by regulating many of the hallmarks of cancer including proliferation, survival, metabolism and migration/invasion (Hanahan and Weinberg, 2011). Although some of these functional responses to pathway stimulation are observed across all breast tumor subtypes, other outputs occur more selectively. Some of this specificity is contributed by differential receptor function, such as the preferential regulation of proliferation by the IGF-1R and IR-A receptors and metabolic pathways by the IR-B receptor. As a result, extrinsic factors that impact the relative expression levels of each receptor, for example, ER-α regulation of IGF-1R expression or splicing factors that alter IR-A:IR-B ratios, will determine the outcomes of stimulation of this pathway and confer subtype-specific differences in tumor behavior.
As discussed previously, IGF-1R signaling maintains normal mammary luminal progenitor cells to regulate mammary gland development and function of the mature gland. In breast cancer, the IR/IGF-1R signaling pathway has also been implicated in cancer stem cell (CSC) regulation. CSCs are a subpopulation of tumor cells with the properties of self-renewal and pluripotency, the ability to differentiate and repopulate the heterogeneity of a tumor (Brooks et al., 2015). CSCs contribute to primary tumor initiation, metastatic colonization and drug resistance (Lawson et al., 2015; Lorincz and Sukumar, 2006). Pre-clinical studies support a role for IR/IGF-1R signaling in breast CSC self-renewal and both receptors have been implicated in maintaining CSC populations (Chang et al., 2013; Rostoker et al., 2015; Rostoker et al., 2016). Analysis of published breast CSC gene expression datasets reveals decreased IGF-1R expression in some contexts (Dox-resistant MCF-7 cells), but no significant change for IGF-1R or IR in other datasets (Calcagno et al., 2010). It should be noted, however, that the IR-A and IR-B isoforms are not distinguished by total mRNA analysis and the ratio of IR-A:IR-B may be more important than total expression for CSC regulation. IGF-2, which can stimulate both the IGF-1R and IR-A, is elevated in Dox-MCF-7 and CD44+,CD24− CSC datasets and has been implicated in the regulation of breast CSCs, suggesting that the activity of these receptors, rather than their expression, may be altered in CSCs (Liu et al., 2007; Pandini et al., 1999; Sciacca et al., 1999; Tominaga et al., 2017). The fact that IGF-2/IR-A are both associated with more aggressive TNBC tumors in African American women may reflect their role in CSC regulation.
Role of the IRS proteins in the tumor response to IR/IGF-1R signaling
As key signaling nodes downstream of the IR/IGF-1R, the IRS proteins play a major role in determining the functional outputs of these receptors in tumors (Gibson et al., 2007). Although some functions are commonly regulated by IRS-1 and IRS-2, such as the promotion of IR/IGF-1R-dependent tumor cell survival (Cesarone et al., 2006; Nagle et al., 2004), other functions are uniquely regulated by each adaptor protein (Figure 4). In ER+, luminal breast carcinoma cells, IRS-1 is the predominant IRS family member that is expressed and activated by IGF-1 stimulation and this signaling adaptor mediates IGF-1-dependent proliferation in these cells (Byron et al., 2006; Jackson et al., 1998). In contrast, ER− breast carcinoma cells that express predominantly IRS-2 do not proliferate when stimulated with IGF-1 (Byron et al., 2006). Instead, ER− tumor cells exhibit enhanced migration and invasion in response to IGF-1 stimulation (Jackson et al., 2001; Mercado-Matos et al., 2018; Nagle et al., 2004; Zhang et al., 2004). Therefore, similar to IR-A:IR-B ratios influencing the outcomes of insulin stimulation, the ratio of IRS-1:IRS-2 expression modifies the response to receptor stimulation, and in so doing significantly impacts tumor outcomes. As a result, autocrine and paracrine factors in the tumor microenvironment that impact the relative expression of each adaptor protein influence the functional outcome of signaling when the IR/IGF-1R pathway is stimulated.
Figure 4: The IRS proteins determine IR/IGF-1R signaling outcomes.
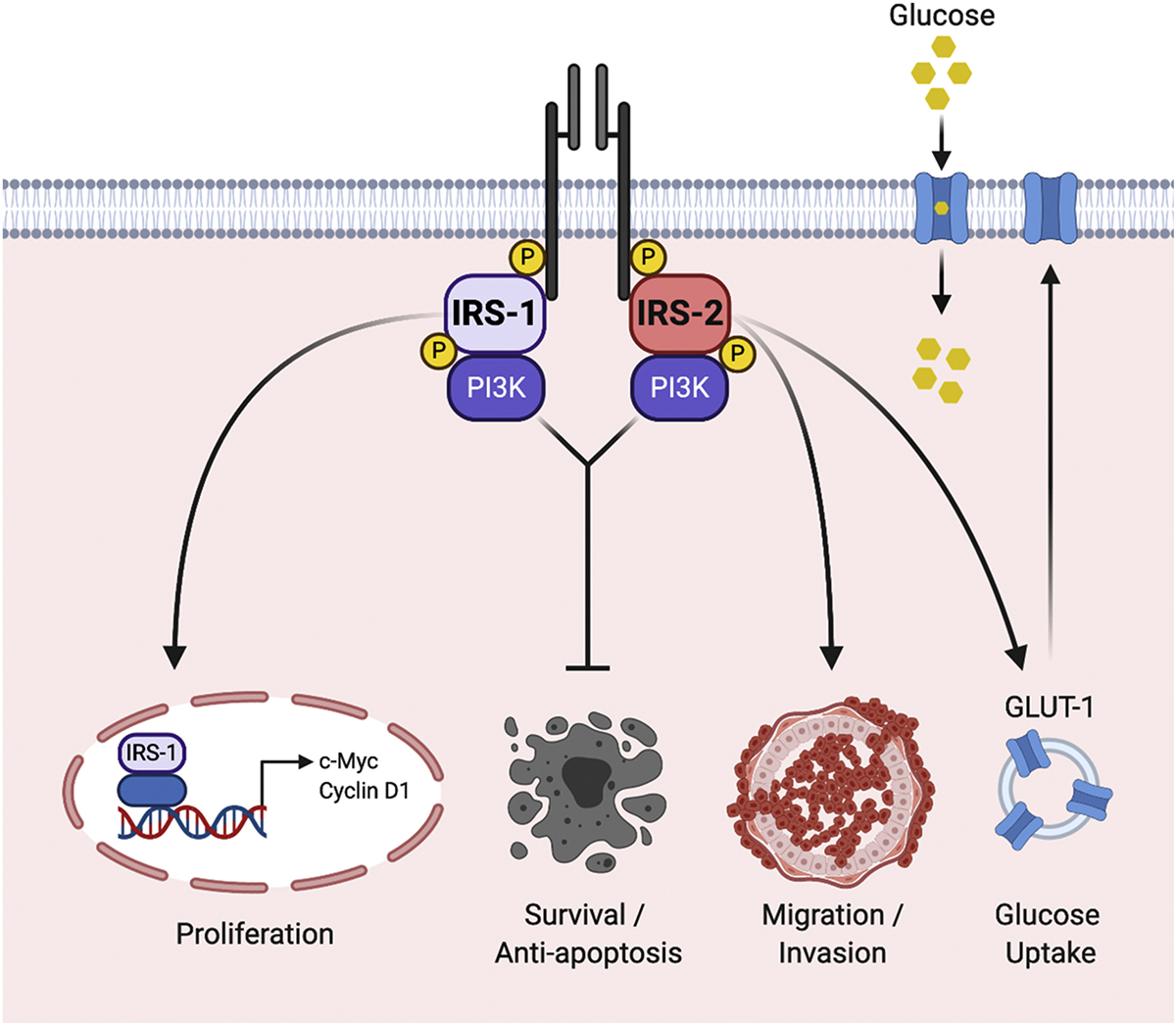
The IRS proteins are essential signaling intermediates of the IR and IGF-1R and they influence the functional response to pathway stimulation. Both IRS-1 and IRS-2 promote tumor cell survival by activating anti-apoptotic pathways in response to IR/IGF-1R stimulation. However, IRS-1 and IRS-2 also regulate distinct functions and their relative expression determines these outcomes. Breast carcinoma cells that express predominantly IRS-1 proliferate in response to IR/IGF-1R stimulation. IRS-1 localizes to the nucleus where it promotes the transcription of cell cycle regulatory genes. Breast carcinoma cells that express predominantly IRS-2 migrate and invade in response to IR/IGF-1R stimulation. IRS-2 signaling also regulates glucose uptake through GLUT-1, which enhances invasion.
As breast tumors lose ER expression and function and become more poorly differentiated and invasive, IRS-1 expression decreases and IRS-2 expression increases, changing the balance to favor IRS-2 as the dominant adaptor protein in these tumors. Disruption of IGF-1R function in ER− breast carcinoma cells does not inhibit tumor growth, as it does in ER+ tumors, but it prevents metastasis of these cells in mouse xenograft models (Dunn et al., 1998; Sachdev et al., 2004; Sachdev et al., 2010). Mammary tumor metastasis is also significantly diminished in PyMT:Irs2−/− mice, whereas PyMT:Irs1−/− tumors that express elevated levels of active (i.e. tyrosine phosphorylated) Irs-2 have enhanced metastatic rates (Ma et al., 2006; Nagle et al., 2004), supporting that IRS-2 plays an essential role in metastatic progression. The ability of IRS-2 to promote invasion, an early pre-requisite step for the dissemination of metastatic cells to secondary organs, is one mechanism by which it contributes to tumor progression (Mercado-Matos et al., 2018; Nagle et al., 2004). Moreover, the selective regulation of aerobic glycolysis by IRS-2, via maintenance of the GLUT-1 glucose transporter on the tumor cell surface and increased glucose uptake, is required for its ability to promote invasion (Figure 4) (Landis and Shaw, 2014; Pankratz et al., 2008).
The differential functional outcomes of signaling through IRS-1 and IRS-2 raises the question of how these similar proteins regulate divergent tumor cell responses. The localization of IRS-1 and IRS-2 within distinct intracellular compartments likely plays a role in their distinct functions. The targeting of IRS-1 and IRS-2 to unique intracellular compartments would facilitate their access to distinct subsets of downstream effectors, altering their functional output. For example, in the nucleus, IRS-1 interacts with transcription factors such as ER-α, β-catenin, the androgen receptor, and upstream binding factor-1 to positively regulate target gene expression (Chen et al., 2005; Lanzino et al., 2009; Morelli et al., 2004). IRS-1-dependent regulation of Cyclin D1 and c-Myc expression is likely to play a role in the stimulation of tumor proliferation (Wu et al., 2007). In contrast, IRS-2 is excluded from the nucleus and instead this adaptor is localized in the cytoplasm or at the cell membrane, which puts IRS-2 in proximity to downstream effectors that are involved in regulating dynamic adhesive and cytoskeletal rearrangements that are required for cell migration and invasion (Clark et al., 2011). IRS-2 interacts with microtubules and the ability of IRS-2, but not IRS-1, to activate AKT is dependent upon an intact microtubule cytoskeleton, suggesting that this interaction controls access of IRS-2 to its downstream effectors (Mercado-Matos et al., 2017). Membrane recruitment of IRS-2 would also localize its signaling to regulate the surface expression of glucose transporter 1 (GLUT1) to promote aerobic glycolysis (Landis and Shaw, 2014; Pankratz et al., 2008). The fact that both IRS-1 and IRS-2 localization are associated with clinical outcomes of breast cancer patients underscores the importance of intracellular localization to their activities (Clark et al., 2011; Migliaccio et al., 2010).
IRS-1 and IRS-2 share approximately 35% identity in their C-termini where they are phosphorylated by the IR/IGF-1R and recruit downstream effectors to initiate their signaling cascades (Mardilovich et al., 2009). Many phosphotyrosine motifs are conserved between IRS-1 and IRS-2 and they activate some common signaling pathways including PI3K and the Erk1/2 kinases in a variety of cancer model systems. However, the fact that PI3K has been implicated in the regulation of both proliferation and invasion by IRS-1 and IRS-2, respectively, underscores that unique pathways that either modify the outcomes of PI3K signaling or cooperate with this signaling pathway must be regulated by each adaptor protein to promote divergent functions (Mercado-Matos et al., 2018; Myers et al., 1996). To date, relatively few unique interacting proteins have been described that would explain the distinctive downstream intracellular compartmentalization or functional outcomes of IRS-1 and IRS-2. Proteomic analysis has identified potential interacting partners of IRS-1 and IRS-2, but these effectors have not been validated by functional studies (Hanke and Mann, 2009). Finally, IRS-1 and IRS-2 are differentially regulated by post-translational modifications such as serine phosphorylation, ubiquitination and acetylation which control effector interactions and signaling duration and could contribute to their distinct downstream functions (Fukushima et al., 2015; Gual et al., 2005; Kaiser and James, 2004; Rui et al., 2001; Yoshihara et al., 2012; Zhang, 2007). Additional study of the signaling mechanisms of the IRS proteins is needed to understand fully their divergent mechanisms of action, and importantly to elucidate their potential as therapeutic targets to inhibit pathway signaling.
Targeting the IR/IGF1R signaling pathway in breast cancer
The strong association of the IR/IGF-1R pathway with clinical outcomes in cancer and the extensive pre-clinical data supporting the functional role of this pathway in cancer progression have led to efforts to develop targeted drugs to inhibit this signaling axis in tumors (Figure 2) (King et al., 2014).
IGF-1R pathway inhibition
Given the importance of the IR for normal metabolic homeostasis and concern that disrupting normal insulin signaling would lead to adverse metabolic outcomes, initial efforts to inhibit the IR/IGF-1R pathway in tumors focused on targeting IGF-1R function selectively. The first drugs to be developed were inhibitory antibodies specific for the IGF-1R (Figure 2; IGF-1Ri). These antibodies not only block receptor ligand binding, but also cause internalization to deplete receptors from the cell surface (Sachdev et al., 2003). Neutralizing antibodies that target IGF-1 and IGF-2 to block receptor interactions have also been developed and showed promise in pre-clinical studies (Figure 2; IGFi) (Haluska et al., 2006). However, few positive responses to these targeted agents have been observed in clinical trials and outcomes for breast cancer and most other cancers have been disappointing.
A major reason for the poor response to selective IGF-1R targeting agents may be compensation by the IR when IGF-1R function is inhibited. IR signaling in tumors sustains growth promoting signals and promotes tumor progression when IGF-1R expression or function are selectively targeted in pre-clinical studies. Evidence of this IR compensation was also observed in one phase II human study. Breast cancer patients with low total expression of IR had significantly longer progression free and overall survival when treated with the anti-IGF-1R drug Cixutumumab (Gradishar et al., 2016). To address this compensation by IR signaling, small molecule inhibitors that dually target both IR and IGF-1R have been developed (Figure 2; RTKi). These drugs inhibit the tyrosine kinase activity of both receptors and they have been shown in many pre-clinical studies to inhibit tumor growth and viability with greater efficacy than targeting IGF-1R function alone (Haluska et al., 2006; Hou et al., 2011). As an example of one such pre-clinical mouse study, a small molecule inhibitor of IR/IGF-1R activity was more effective at inhibiting tumor growth in endocrine resistant tumors than agents that targeted IGF-1R alone, supporting an important role for IR in this therapeutic context (Kruger et al., 2020). Unfortunately, as anticipated, adverse systemic metabolic issues resulting from the inhibition of normal insulin receptor function have limited the efficacy of these small molecular inhibitors in human clinical trials. The poor outcomes of IR/IGF-1R targeted therapies in human trials emphasizes the need to develop alternative approaches to inhibit IR/IGF-1R signaling in tumors, while preserving normal systemic metabolic functions of this pathway.
Impact of diet on the tumor response to IR/IGF-1R pathway inhibition
One recent approach that has been investigated to improve the systemic response to IR/IGF-1R inhibition is the use of diet to suppress the feedback upregulation of hyperglycemia and hyperinsulinemia (Nencioni et al., 2018). As discussed previously, normal insulin signaling is tightly regulated to control the uptake of glucose from the circulation and maintain glucose homeostasis. When insulin signaling is disrupted in mice by therapies that target the pathway, glucose levels rise and stimulate insulin secretion to compensate, resulting in hyperinsulinemia and consequent elevation of IGF expression. Basal glucose levels can be reduced by altering diet, either through complete fasting or a fasting mimicking diet (FMD) that is low in carbohydrate (Nencioni et al., 2018). Lower basal glucose levels suppress the spike in insulin and IGF-1 expression that occurs when the pathway is disrupted and prevent hyperstimulation of the pathway in tumors.
The efficacy of this dietetic approach for cancer treatment has been demonstrated in several pre-clinical studies in mice, providing hope that this approach could improve clinical outcomes in tumors that are responsive to the IR/IGF-1 pathway. For example, PI3K is a frequently mutated gene in cancer and activity of this kinase is also upregulated by additional molecular alterations in tumors such as loss of the tumor suppressor PTEN or activation of growth factor receptors including IR/IGF-1R (Thorpe et al., 2015). For this reason, considerable effort has gone into developing PI3K targeted inhibitors for cancer treatment (Figure 2; PI3Ki). However, inhibition of PI3K prevents insulin-dependent glucose uptake, which causes systemic hyperglycemia and subsequent hyperinsulinemia. As a result, IR/IGF-1R signaling is hyperactivated in tumors and their growth is not diminished by these inhibitors (Hopkins et al., 2018). The hyperglycemic response to PI3K inhibition is suppressed in mice fed a ketogenic diet that is low in carbohydrates. Breast tumor growth becomes sensitive to inhibition of PI3K in mice fed this diet (Hopkins et al., 2018). A fasting or a fasting mimicking diet (FMD) also improved the inhibition of ER+ breast tumor growth in mice by ER-targeting drugs (Caffa et al., 2020). When fulvestrant was combined with the CDK4/6 inhibitor Palbociclib and mice were fed in periodic cycles of FMD, tumor regression was observed and acquired resistance to treatment was prevented.
In light of these positive pre-clinical results, alternative diets have started to be evaluated in humans undergoing breast cancer treatment. In a small cohort of patients with ER+ breast cancer receiving endocrine therapy, cycles of a FMD led to reduction in circulating levels of insulin, IGF-1 and leptin, mimicking the metabolic effects observed in the pre-clinical mouse studies (Caffa et al., 2020). Although this cohort was limited in size, clinical outcomes were promising with several patients showing clinically controlled disease and prolonged progression free survival. Similarly, a recent Phase II clinical trial evaluated the impact of a FMD on response to neoadjuvant chemotherapy in HER2- stage II/III breast cancer. This study reported that a radiological complete or partial response occurred more often in patients on the FMD diet (de Groot et al., 2020). Together, these studies provide encouraging evidence that restrictive diets may be effective as an approach to suppress IR/IGF-1R signaling in breast tumors and improve responses in a variety of therapeutic contexts. However, compliance of patients with fasting and/or strict dietary restrictions is a concern, and for this reason there remains a need for the development of novel targeted drugs to disrupt IR/IGF-1R signaling in a tumor-specific manner.
The IRS proteins as novel therapeutic targets
One potential approach for selectively inhibiting tumor-specific functions of the IR/IGF-1R pathway is through targeting the IRS proteins. As key determinants of IR/IGF-1R signaling outcomes, the IRS proteins represent nodes of the pathway that could be targeted to inhibit tumor functions while maintaining essential normal physiological functions of the pathway. A small molecule inhibitor that indirectly targets both IRS-1 and IRS-2 for degradation has been described, but the dual suppression of both adaptors would likely also impact normal metabolic signaling (Reuveni et al., 2013). The IRS proteins share their highest degree of homology in their structured N-terminal domains (PH and PTB) that are required for recruitment to upstream receptors, followed by long predominantly disordered tails which are less conserved (Mardilovich et al., 2009). In a recent study to identify unique structural features of IRS-2 that are required for the ability to promote invasion, deletion mutants were generated that preserved the ability of IRS2 to be recruited to upstream receptors and activate PI3K, functions that are shared by IRS-1 and are required for normal glucose regulation (Mercado-Matos et al., 2018). A region in the IRS2 C-terminal tail was identified that is necessary and sufficient for promoting invasion but is not required for the IRS2-dependent regulation of glucose uptake. The results of this study provide proof of concept that a targeting strategy to disrupt tumor-specific functions of the IR/IGF-1R pathway while maintaining essential metabolic functions in normal cells is feasible.
Future Directions
The evidence to support an important role for the IR/IGF-1R signaling pathway in breast cancer promotion and progression is compelling. Unfortunately, early excitement about the potential for targeting the IR/IGF-1R signaling axis as a therapeutic approach in breast cancer has been diminished by disappointing results from clinical trials, and many drug programs have been halted. One lesson that has been learned from efforts to target the IGF-1R pathway in cancer is that improved biomarkers that can predict response to disruption of the pathway are needed. Unlike other oncogenic receptors such as HER2 or ER, where expression correlates with response to targeted therapies, IGF-1R expression alone has not been predictive as a biomarker for response to targeted inhibitors. The IR and the IRS proteins impact tumor response to pathway stimulation and their expression should be explored further as predictive biomarkers for efficacy of pathway inhibition. In this regard, it should be noted that tumor growth is most often the endpoint monitored to determine successful outcomes in clinical trials. However, disruption of IR/IGF-1R signaling in ER− breast tumors that express primarily IRS-2 does not inhibit tumor growth, but instead inhibits metastasis (Dunn et al., 1998; Sachdev et al., 2004; Sachdev et al., 2010). Therefore, consideration for alternative endpoints to monitor outcomes of these targeted therapies is warranted.
The IRS proteins represent an important, but relatively unexplored area with regard to development of targeted therapies to inhibit IR/IGF-1R signaling in a tumor-specific manner. Relatively little is known regarding the structure of the IRS proteins and how they mediate their distinct signaling functions. Structural studies of both IRS-1 and IRS-2 are necessary to understand their unique mechanisms of action, a pre-requisite to selectively targeting their tumor-promoting functions for therapeutic benefit. Further identification and interrogation of IRS effectors that play tumor-selective roles in IR/IGF-1R signaling will also open new avenues for novel drug development. In this regard, bone morphogenic protein-2-inducible kinase (BMP2K) binds to the invasion-promoting region in IRS-2 and it contributes to IRS2-dependent invasion (Mercado-Matos et al., 2018). The potential of targeting this kinase or other effectors to selectively prevent tumor functions of IR/IGF-1R signaling requires additional investigation. As continued research illuminates a greater understanding of the molecular mechanisms of insulin receptor (IR) and IGF-1 receptor (IGF-1R) function and signaling, novel possibilities for targeting this pathway will be revealed, with the hope of improving outcomes for breast cancer patients.
Acknowledgments
This work was supported by NIH grants CA240655 and CA229910 to LMS. We thank Art Mercurio for helpful comments on the manuscript. The Figures were created using BioRender.com.
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki E, Lipes MA, Patti ME, Bruning JC, Haag B 3rd, Johnson RS, and Kahn CR 1994. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 372:186–190. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, and Efstratiadis A 1993. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 75:73–82. [PubMed] [Google Scholar]
- Barbieri M, Bonafe M, Franceschi C, and Paolisso G 2003. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol EndocrinolMetab. 285:E1064–1071. [DOI] [PubMed] [Google Scholar]
- Bates P, Fisher R, Ward A, Richardson L, Hill DJ, and Graham CF 1995. Mammary cancer in transgenic mice expressing insulin-like growth factor II (IGF-II). Br J Cancer. 72:1189–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RC 2014. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer. 14:329–341. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Frasca F, Pandini G, Sciacca L, and Vigneri R 2009. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocrine reviews. 30:586–623. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Malaguarnera R, Vella V, Lawrence MC, Sciacca L, Frasca F, Morrione A, and Vigneri R 2017. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocrine reviews. 38:379–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorner S, Rosendahl AH, Simonsson M, Markkula A, Jirstrom K, Borgquist S, Rose C, Ingvar C, and Jernstrom H 2017. Combined and individual tumor-specific expression of insulin-like growth factor-I receptor, insulin receptor and phospho-insulin-like growth factor-I receptor/insulin receptor in primary breast cancer: Implications for prognosis in different treatment groups. Oncotarget. 8:9093–9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornholm M, He AR, Attersand A, Lake S, Liu SC, Lienhard GE, Taylor S, Arner P, and Zierath JR 2002. Absence of functional insulin receptor substrate-3 (IRS-3) gene in humans. Diabetologia. 45:1697–1702. [DOI] [PubMed] [Google Scholar]
- Bonnette SG, and Hadsell DL 2001. Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology. 142:4937–4945. [DOI] [PubMed] [Google Scholar]
- Brooks MD, Burness ML, and Wicha MS 2015. Therapeutic Implications of Cellular Heterogeneity and Plasticity in Breast Cancer. Cell Stem Cell. 17:260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Jones EY, and Forbes BE 2009. Keeping IGF-II under control: lessons from the IGF-II-IGF2R crystal structure. Trends Biochem Sci. 34:612–619. [DOI] [PubMed] [Google Scholar]
- Byron SA, Horwitz KB, Richer JK, Lange CA, Zhang X, and Yee D 2006. Insulin receptor substrates mediate distinct biological responses to insulin-like growth factor receptor activation in breast cancer cells. Br J Cancer. 95:1220–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffa I, Spagnolo V, Vernieri C, Valdemarin F, Becherini P, Wei M, Brandhorst S, Zucal C, Driehuis E, Ferrando L, Piacente F, Tagliafico A, Cilli M, Mastracci L, Vellone VG, Piazza S, Cremonini AL, Gradaschi R, Mantero C, Passalacqua M, Ballestrero A, Zoppoli G, Cea M, Arrighi A, Odetti P, Monacelli F, Salvadori G, Cortellino S, Clevers H, De Braud F, Sukkar SG, Provenzani A, Longo VD, and Nencioni A 2020. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature. 583:620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Sakaguchi M, Kleinridders A, Gonzalez-Del Pino G, Dreyfuss JM, O’Neill BT, Ramirez AK, Pan H, Winnay JN, Boucher J, Eck MJ, and Kahn CR 2017. Domain-dependent effects of insulin and IGF-1 receptors on signalling and gene expression. Nat Commun. 8:14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno AM, Salcido CD, Gillet JP, Wu CP, Fostel JM, Mumau MD, Gottesman MM, Varticovski L, and Ambudkar SV 2010. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J Natl Cancer Inst. 102:1637–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, and Thun MJ 2003. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England journal of medicine. 348:1625–1638. [DOI] [PubMed] [Google Scholar]
- Cesarone G, Garofalo C, Abrams MT, Igoucheva O, Alexeev V, Yoon K, Surmacz E, and Wickstrom E 2006. RNAi-mediated silencing of insulin receptor substrate 1 (IRS-1) enhances tamoxifen-induced cell death in MCF-7 breast cancer cells. J Cell Biochem. 98:440–450. [DOI] [PubMed] [Google Scholar]
- Chang WW, Lin RJ, Yu J, Chang WY, Fu CH, Lai A, Yu JC, and Yu AL 2013. The expression and significance of insulin-like growth factor-1 receptor and its pathway on breast cancer stem/progenitors. Breast Cancer Res. 15:R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wu A, Sun H, Drakas R, Garofalo C, Cascio S, Surmacz E, and Baserga R 2005. Functional significance of type 1 insulin-like growth factor-mediated nuclear translocation of the insulin receptor substrate-1 and beta-catenin. J Biol Chem. 280:29912–29920. [DOI] [PubMed] [Google Scholar]
- Chow JC, Condorelli G, and Smith RJ 1998. Insulin-like growth factor-I receptor internalization regulates signaling via the Shc/mitogen-activated protein kinase pathway, but not the insulin receptor substrate-1 pathway. J Biol Chem. 273:4672–4680. [DOI] [PubMed] [Google Scholar]
- Clark JL, Dresser K, Hsieh CC, Sabel M, Kleer CG, Khan A, and Shaw LM 2011. Membrane localization of insulin receptor substrate-2 (IRS-2) is associated with decreased overall survival in breast cancer. Breast Cancer Res Treat. 130:759–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR, and Underwood LE 1991. Nutritional regulation of IGF-I and IGF binding proteins. Annu RevNutr. 11:393–412. [DOI] [PubMed] [Google Scholar]
- Creighton CJ, Casa A, Lazard Z, Huang S, Tsimelzon A, Hilsenbeck SG, Osborne CK, and Lee AV 2008. Insulin-like growth factor-I activates gene transcription programs strongly associated with poor breast cancer prognosis. J Clin Oncol. 26:4078–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Kim HJ, Kuiatse I, Kim H, Brown PH, and Lee AV 2006. Epidermal Growth Factor Induces Insulin Receptor Substrate-2 in Breast Cancer Cells via c-Jun NH2-Terminal Kinase/Activator Protein-1 Signaling to Regulate Cell Migration. Cancer Res. 66:5304–5313. [DOI] [PubMed] [Google Scholar]
- Cui X, Lazard Z, Zhang P, Hopp TA, and Lee AV 2003. Progesterone crosstalks with insulin-like growth factor signaling in breast cancer cells via induction of insulin receptor substrate-2. Oncogene. 22:6937–6941. [DOI] [PubMed] [Google Scholar]
- Cullen KJ, Yee D, Sly WS, Perdue J, Hampton B, Lippman ME, and Rosen N 1990. Insulin-like growth factor receptor expression and function in human breast cancer. Cancer Res. 50:48–53. [PubMed] [Google Scholar]
- de Groot S, Lugtenberg RT, Cohen D, Welters MJP, Ehsan I, Vreeswijk MPG, Smit V, de Graaf H, Heijns JB, Portielje JEA, van de Wouw AJ, Imholz ALT, Kessels LW, Vrijaldenhoven S, Baars A, Kranenbarg EM, Carpentier MD, Putter H, van der Hoeven JJM, Nortier JWR, Longo VD, Pijl H, Kroep JR, and Dutch G Breast Cancer Research. 2020. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat Commun 11:3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyts P, and Whittaker J 2002. Structural biology of insulin and IGF1 receptors: implications for drug design. Nat Rev Drug Discov. 1:769–783. [DOI] [PubMed] [Google Scholar]
- De Vincenzo A, Belli S, Franco P, Telesca M, Iaccarino I, Botti G, Carriero MV, Ranson M, and Stoppelli MP 2019. Paracrine recruitment and activation of fibroblasts by c-Myc expressing breast epithelial cells through the IGFs/IGF-1R axis. Int J Cancer. 145:2827–2839. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Robertson EJ, and Efstratiadis A 1991. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 64:849–859. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Ehrlich M, Sharp NJ, Reiss K, Solomon G, Hawkins R, Baserga R, and Barrett JC 1998. A dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res. 58:3353–3361. [PubMed] [Google Scholar]
- Fantin VR, Wang Q, Lienhard GE, and Keller SR 2000. Mice lacking insulin receptor substrate 4 exhibit mild defects in growth, reproduction, and glucose homeostasis. Am J Physiol EndocrinolMetab. 278:E127–133. [DOI] [PubMed] [Google Scholar]
- Farabaugh SM, Boone DN, and Lee AV 2015. Role of IGF1R in Breast Cancer Subtypes, Stemness, and Lineage Differentiation. Front Endocrinol (Lausanne). 6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh SM, Litzenburger BC, Elangovan A, Pecar G, Walheim L, Atkinson JM, and Lee AV 2020. IGF1R constitutive activation expands luminal progenitors and influences lineage differentiation during breast tumorigenesis. Dev Biol. 463:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, Filmore J, Shulman GI, and Le Roith D 2001. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 15:1926–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferroni P, Riondino S, Laudisi A, Portarena I, Formica V, Alessandroni J, D’Alessandro R, Orlandi A, Costarelli L, Cavaliere F, Guadagni F, and Roselli M 2016. Pretreatment Insulin Levels as a Prognostic Factor for Breast Cancer Progression. Oncologist. 21:1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formica V, Tesauro M, Cardillo C, and Roselli M 2012. Insulinemia and the risk of breast cancer and its relapse. Diabetes Obes Metab. 14:1073–1080. [DOI] [PubMed] [Google Scholar]
- Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, and Vigneri R 1999. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 19:3278–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T, Yoshihara H, Furuta H, Kamei H, Hakuno F, Luan J, Duan C, Saeki Y, Tanaka K, Iemura S, Natsume T, Chida K, Nakatsu Y, Kamata H, Asano T, and Takahashi S 2015. Nedd4-induced monoubiquitination of IRS-2 enhances IGF signalling and mitogenic activity. Nat Commun. 6:6780. [DOI] [PubMed] [Google Scholar]
- Gallagher EJ, Fei K, Feldman SM, Port E, Friedman NB, Boolbol SK, Killelea B, Pilewskie M, Choi L, King T, Nayak A, Franco R, Cruz D, Antoniou IM, LeRoith D, and Bickell NA 2020. Insulin resistance contributes to racial disparities in breast cancer prognosis in US women. Breast Cancer Res. 22:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher EJ, and LeRoith D 2020. Hyperinsulinaemia in cancer. Nat Rev Cancer. 20:629–644. [DOI] [PubMed] [Google Scholar]
- Gallagher EJ, LeRoith D, Franco R, Antoniou IM, Nayak A, Livaudais-Toman J, and Bickell NA 2016. Metabolic syndrome and pre-diabetes contribute to racial disparities in breast cancer outcomes: hypothesis and proposed pathways. Diabetes Metab Res Rev. 32:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SL, Ma Z, and Shaw LM 2007. Divergent roles for IRS-1 and IRS-2 in breast cancer metastasis. Cell Cycle. 6:631–637. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, and Hood N 2002. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 20:42–51. [DOI] [PubMed] [Google Scholar]
- Gradishar WJ, Yardley DA, Layman R, Sparano JA, Chuang E, Northfelt DW, Schwartz GN, Youssoufian H, Tang S, Novosiadly R, Forest A, Nguyen TS, Cosaert J, Grebennik D, and Haluska P 2016. Clinical and Translational Results of a Phase II, Randomized Trial of an Anti-IGF-1R (Cixutumumab) in Women with Breast Cancer That Progressed on Endocrine Therapy. Clin Cancer Res. 22:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gual P, Le Marchand-Brustel Y, and Tanti JF 2005. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 87:99–109. [DOI] [PubMed] [Google Scholar]
- Gui Y, Aguilar-Mahecha A, Krzemien U, Hosein A, Buchanan M, Lafleur J, Pollak M, Ferrario C, and Basik M 2019. Metastatic Breast Carcinoma-Associated Fibroblasts Have Enhanced Protumorigenic Properties Related to Increased IGF2 Expression. Clin Cancer Res. 25:7229–7242. [DOI] [PubMed] [Google Scholar]
- Haluska P, Carboni JM, Loegering DA, Lee FY, Wittman M, Saulnier MG, Frennesson DB, Kalli KR, Conover CA, Attar RM, Kaufmann SH, Gottardis M, and Erlichman C 2006. In vitro and in vivo antitumor effects of the dual insulin-like growth factor-I/insulin receptor inhibitor, BMS-554417. Cancer Res. 66:362–371. [DOI] [PubMed] [Google Scholar]
- Hamelers IH, van Schaik RF, van Teeffelen HA, Sussenbach JS, and Steenbergh PH 2002. Synergistic proliferative action of insulin-like growth factor I and 17 beta-estradiol in MCF-7S breast tumor cells. Exp Cell Res. 273:107–117. [DOI] [PubMed] [Google Scholar]
- Hanahan D, and Weinberg RA 2011. Hallmarks of cancer: the next generation. Cell. 144:646–674. [DOI] [PubMed] [Google Scholar]
- Hanke S, and Mann M 2009. The phosphotyrosine interactome of the insulin receptor family and its substrates IRS-1 and IRS-2. Mol CellProteomics. 8:519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington SC, Weroha SJ, Reynolds C, Suman VJ, Lingle WL, and Haluska P 2012. Quantifying insulin receptor isoform expression in FFPE breast tumors. Growth Horm IGF Res. 22:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, Amadiume SC, Goncalves MD, Hodakoski C, Lundquist MR, Bareja R, Ma Y, Harris EM, Sboner A, Beltran H, Rubin MA, Mukherjee S, and Cantley LC 2018. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 560:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Huang F, Macedo LF, Harrington SC, Reeves KA, Greer A, Finckenstein FG, Brodie A, Gottardis MM, Carboni JM, and Haluska P 2011. Dual IGF-1R/InsR inhibitor BMS-754807 synergizes with hormonal agents in treatment of estrogen-dependent breast cancer. Cancer Res. 71:7597–7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Song C, Wang N, Qin T, Sui S, Obr A, Zeng L, Wood TL, Leroith D, Li M, and Wu Y 2020. RNA-binding protein CUGBP1 controls the differential INSR splicing in molecular subtypes of breast cancer cells and affects cell aggressiveness. Carcinogenesis. 41:1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Morehouse C, Streicher K, Higgs BW, Gao J, Czapiga M, Boutrin A, Zhu W, Brohawn P, Chang Y, Viner J, LaVallee T, Richman L, Jallal B, and Yao Y 2011. Altered expression of insulin receptor isoforms in breast cancer. PLoS One. 6:e26177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh H, Nickerson T, Pollak M, and Yang X 1996. Regulation of insulin-like growth factor I receptor expression by the pure antiestrogen ICI 182780. Clin Cancer Res. 2:2037–2042. [PubMed] [Google Scholar]
- Jackson JG, White MF, and Yee D 1998. Insulin receptor substrate-1 is the predominant signaling molecule activated by insulin-like growth factor-I, insulin, and interleukin-4 in estrogen receptor-positive human breast cancer cells. J Biol Chem. 273:9994–10003. [DOI] [PubMed] [Google Scholar]
- Jackson JG, Zhang X, Yoneda T, and Yee D 2001. Regulation of breast cancer cell motility by insulin receptor substrate-2 (IRS-2) in metastatic variants of human breast cancer cell lines. Oncogene. 20:7318–7325. [DOI] [PubMed] [Google Scholar]
- Kaiser C, and James SR 2004. Acetylation of insulin receptor substrate-1 is permissive for tyrosine phosphorylation. BMC Biol. 2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla Singh S, Tan QW, Brito C, De Leon M, Garberoglio C, and De Leon D 2010. Differential insulin-like growth factor II (IGF-II) expression: A potential role for breast cancer survival disparity. Growth Horm IGF Res. 20:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda A, Wang CJ, Cheong R, Timp W, Onyango P, Wen B, Iacobuzio-Donahue CA, Ohlsson R, Andraos R, Pearson MA, Sharov AA, Longo DL, Ko MS, Levchenko A, and Feinberg AP 2007. Enhanced sensitivity to IGF-II signaling links loss of imprinting of IGF2 to increased cell proliferation and tumor risk. Proceedings of the National Academy of Sciences of the United States of America. 104:20926–20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King H, Aleksic T, Haluska P, and Macaulay VM 2014. Can we unlock the potential of IGF-1R inhibition in cancer therapy? Cancer Treat Rev. 40:1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberg DL, Wood TL, Furth PA, and Lee AV 2009. Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions. Endocrine reviews. 30:51–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip A, McGraw TE, and James DE 2019. Thirty sweet years of GLUT4. J Biol Chem. 294:11369–11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S 1992. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu RevBiochem. 61:307–330. [DOI] [PubMed] [Google Scholar]
- Kruger DT, Alexi X, Opdam M, Schuurman K, Voorwerk L, Sanders J, van der Noort V, Boven E, Zwart W, and Linn SC 2020. IGF-1R pathway activation as putative biomarker for linsitinib therapy to revert tamoxifen resistance in ER-positive breast cancer. Int J Cancer. 146:2348–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis J, and Shaw LM 2014. Insulin receptor substrate 2-mediated phosphatidylinositol 3-kinase signaling selectively inhibits glycogen synthase kinase 3beta to regulate aerobic glycolysis. J Biol Chem. 289:18603–18613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzino M, Garofalo C, Morelli C, Le Pera M, Casaburi I, McPhaul MJ, Surmacz E, Ando S, and Sisci D 2009. Insulin receptor substrate 1 modulates the transcriptional activity and the stability of androgen receptor in breast cancer cells. Breast Cancer Res Treat. 115:297–306. [DOI] [PubMed] [Google Scholar]
- Laron Z, and Werner H 2020. Laron syndrome - A historical perspective. Rev Endocr Metab Disord. [DOI] [PubMed] [Google Scholar]
- Lavan BE, Fantin VR, Chang ET, Lane WS, Keller SR, and Lienhard GE 1997a. A novel 160-kDa phosphotyrosine protein in insulin-treated embryonic kidney cells is a new member of the insulin receptor substrate family. JBiol Chem. 272:21403–21407. [DOI] [PubMed] [Google Scholar]
- Lavan BE, Lane WS, and Lienhard GE 1997b. The 60-kDa phosphotyrosine protein in insulin-treated adipocytes is a new member of the insulin receptor substrate family. J Biol Chem. 272:11439–11443. [DOI] [PubMed] [Google Scholar]
- Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, Park E, Gee JM, Finlay P, Jones HE, Nicholson RI, Carboni J, Gottardis M, Pollak M, and Dunn SE 2008. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 68:10238–10246. [DOI] [PubMed] [Google Scholar]
- Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, Zhou A, Eyob H, Balakrishnan S, Wang CY, Yaswen P, Goga A, and Werb Z 2015. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 526:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AV, Gooch JL, Oesterreich S, Guler RL, and Yee D 2000. Insulin-like growth factor I-induced degradation of insulin receptor substrate 1 is mediated by the 26S proteasome and blocked by phosphatidylinositol 3’-kinase inhibition. Mol Cell Biol. 20:1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AV, Jackson JG, Gooch JL, Hilsenbeck SG, Coronado-Heinsohn E, Osborne CK, and Yee D 1999. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Molecular endocrinology. 13:787–796. [DOI] [PubMed] [Google Scholar]
- Lee AV, Zhang P, Ivanova M, Bonnette S, Oesterreich S, Rosen JM, Grimm S, Hovey RC, Vonderhaar BK, Kahn CR, Torres D, George J, Mohsin S, Allred DC, and Hadsell DL 2003. Developmental and hormonal signals dramatically alter the localization and abundance of insulin receptor substrate proteins in the mammary gland. Endocrinology. 144:2683–2694. [DOI] [PubMed] [Google Scholar]
- Litzenburger BC, Creighton CJ, Tsimelzon A, Chan BT, Hilsenbeck SG, Wang T, Carboni JM, Gottardis MM, Huang F, Chang JC, Lewis MT, Rimawi MF, and Lee AV 2011. High IGF-IR activity in triple-negative breast cancer cell lines and tumorgrafts correlates with sensitivity to anti-IGF-IR therapy. Clin Cancer Res. 17:2314–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, and Efstratiadis A 1993. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 75:59–72. [PubMed] [Google Scholar]
- Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, Sherlock G, Lewicki J, Shedden K, and Clarke MF 2007. The prognostic role of a gene signature from tumorigenic breast-cancer cells. The New Englandjournal of medicine. 356:217–226. [DOI] [PubMed] [Google Scholar]
- Livingstone C 2013. IGF2 and cancer. Endocrine-related cancer. 20:R321–339. [DOI] [PubMed] [Google Scholar]
- Lorincz AM, and Sukumar S 2006. Molecular links between obesity and breast cancer. Endocrine-related cancer. 13:279–292. [DOI] [PubMed] [Google Scholar]
- Ma Z, Gibson SL, Byrne MA, Zhang J, White MF, and Shaw LM 2006. Suppression of insulin receptor substrate 1 (IRS-1) promotes mammary tumor metastasis. Mol Cell Biol. 26:9338–9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardilovich K, Pankratz SL, and Shaw LM 2009. Expression and function of the insulin receptor substrate proteins in cancer. Cell communication and signaling : CCS. 7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardilovich K, and Shaw LM 2009. Hypoxia regulates insulin receptor substrate-2 expression to promote breast carcinoma cell survival and invasion. Cancer Res. 69:8894–8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MB, Franke TF, Stoica GE, Chambon P, Katzenellenbogen BS, Stoica BA, McLemore MS, Olivo SE, and Stoica A 2000. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 141:4503–4511. [DOI] [PubMed] [Google Scholar]
- Mercado-Matos J, Clark JL, Piper AJ, Janusis J, and Shaw LM 2017. Differential involvement of the microtubule cytoskeleton in insulin receptor substrate 1 (IRS-1) and IRS-2 signaling to AKT determines the response to microtubule disruption in breast carcinoma cells. J Biol Chem. 292:7806–7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Matos J, Janusis J, Zhu S, Chen SS, and Shaw LM 2018. Identification of a novel invasion-promoting region in Insulin Receptor Substrate 2 (IRS2). Mol Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio I, Wu MF, Gutierrez C, Malorni L, Mohsin SK, Allred DC, Hilsenbeck SG, Osborne CK, Weiss H, and Lee AV 2010. Nuclear IRS-1 predicts tamoxifen response in patients with early breast cancer. Breast Cancer Res Treat. 123:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli C, Garofalo C, Sisci D, del Rincon S, Cascio S, Tu X, Vecchione A, Sauter ER, Miller WH Jr., and Surmacz E 2004. Nuclear insulin receptor substrate 1 interacts with estrogen receptor alpha at ERE promoters. Oncogene. 23:7517–7526. [DOI] [PubMed] [Google Scholar]
- Murphy N, Knuppel A, Papadimitriou N, Martin RM, Tsilidis KK, Smith-Byrne K, Fensom G, Perez-Cornago A, Travis RC, Key TJ, and Gunter MJ 2020. Insulin-like growth factor-1, insulin-like growth factor-binding protein-3, and breast cancer risk: observational and Mendelian randomization analyses with approximately 430 000 women. Ann Oncol. 31:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG Jr., Zhang Y, Aldaz GA, Grammer T, Glasheen EM, Yenush L, Wang LM, Sun XJ, Blenis J, Pierce JH, and White MF 1996. YMXM motifs and signaling by an insulin receptor substrate 1 molecule without tyrosine phosphorylation sites. Mol Cell Biol. 16:4147–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle JA, Ma Z, Byrne MA, White MF, and Shaw LM 2004. Involvement of insulin receptor substrate 2 in mammary tumor metastasis. Mol Cell Biol. 24:9726–9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencioni A, Caffa I, Cortellino S, and Longo VD 2018. Fasting and cancer: molecular mechanisms and clinical application. Nat Rev Cancer. 18:707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, Carboni JM, Gottardis MM, Pennisi PA, Molinolo AA, Kurshan N, Mejia W, Santopietro S, Yakar S, Wood TL, and LeRoith D 2010. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 70:741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, and Rosen N 2006. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66:1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandini G, Vigneri R, Costantino A, Frasca F, Ippolito A, Fujita-Yamaguchi Y, Siddle K, Goldfine ID, and Belfiore A 1999. Insulin and insulin-like growth factor-I (IGF-I) receptor overexpression in breast cancers leads to insulin/IGF-I hybrid receptor overexpression: evidence for a second mechanism of IGF-I signaling. Clin Cancer Res. 5:1935–1944. [PubMed] [Google Scholar]
- Pankratz SL, Tan EY, Fine Y, Mercurio AM, and Shaw LM 2008. Insulin receptor substrate-2 regulates aerobic glycolysis in mouse mammary tumor cells via glucose transporter 1. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, Brancati FL, and Wolff AC 2011. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 29:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M 2008. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 8:915–928. [DOI] [PubMed] [Google Scholar]
- Pollak M 2012. The insulin receptor/insulin-like growth factor receptor family as a therapeutic target in oncology. Clin Cancer Res. 18:40–50. [DOI] [PubMed] [Google Scholar]
- Poloz Y, and Stambolic V 2015. Obesity and cancer, a case for insulin signaling. Cell Death Dis. 6:e2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter HA, Perry A, Kingsley C, Tran NL, and Keegan AD 2013. IRS1 is highly expressed in localized breast tumors and regulates the sensitivity of breast cancer cells to chemotherapy, while IRS2 is highly expressed in invasive breast tumors. Cancer Lett. 338:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, and Stewart TA 1993. IGF-I is required for normal embryonic growth in mice. Genes Dev. 7:2609–2617. [DOI] [PubMed] [Google Scholar]
- Pugeat M, Crave JC, Elmidani M, Nicolas MH, Garoscio-Cholet M, Lejeune H, Dechaud H, and Tourniaire J 1991. Pathophysiology of sex hormone binding globulin (SHBG): relation to insulin. J Steroid Biochem Mol Biol. 40:841–849. [DOI] [PubMed] [Google Scholar]
- Qian F, and Huo D 2020. Circulating Insulin-Like Growth Factor-1 and Risk of Total and 19 Site-Specific Cancers: Cohort Study Analyses from the UK Biobank. Cancer Epidemiol Biomarkers Prev. 29:2332–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni H, FlashnER-αbramson E, Steiner L, Makedonski K, Song R, Shir A, Herlyn M, Bar-Eli M, and Levitzki A 2013. Therapeutic destruction of insulin receptor substrates for cancer treatment. Cancer Res. 73:4383–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli RJ, LeBeau AP, Fulmer CG, Lazzarino DA, Hochberg A, and Wood TL 2007. Insulin-like growth factor type-I receptor internalization and recycling mediate the sustained phosphorylation of Akt. J Biol Chem. 282:22513–22524. [DOI] [PubMed] [Google Scholar]
- Rostoker R, Abelson S, Bitton-Worms K, Genkin I, Ben-Shmuel S, Dakwar M, Orr ZS, Caspi A, Tzukerman M, and LeRoith D 2015. Highly specific role of the insulin receptor in breast cancer progression. Endocrine-related cancer. 22:145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostoker R, Ben-Shmuel S, Rashed R, Shen Orr Z, and LeRoith D 2016. CD24 cell surface expression in Mvt1 mammary cancer cells serves as a biomarker for sensitivity to anti-IGF1R therapy. Breast Cancer Res. 18:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota LM, Albanito L, Shin ME, Goyeneche CL, Shushanov S, Gallagher EJ, LeRoith D, Lazzarino DA, and Wood TL 2014. IGF1R inhibition in mammary epithelia promotes canonical Wnt signaling and Wnt1-driven tumors. Cancer Res. 74:5668–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan W, and Kleinberg DL 1999. Insulin-like growth factor I is essential for terminal end bud formation and ductal morphogenesis during mammary development. Endocrinology. 140:5075–5081. [DOI] [PubMed] [Google Scholar]
- Rui L, Fisher TL, Thomas J, and White MF 2001. Regulation of insulin/insulin-like growth factor-1 signaling by proteasome-mediated degradation of insulin receptor substrate-2. J Biol Chem. 276:40362–40367. [DOI] [PubMed] [Google Scholar]
- Sachdev D, Hartell JS, Lee AV, Zhang X, and Yee D 2004. A dominant negative type I insulin-like growth factor receptor inhibits metastasis of human cancer cells. J Biol Chem. 279:5017–5024. [DOI] [PubMed] [Google Scholar]
- Sachdev D, Li SL, Hartell JS, Fujita-Yamaguchi Y, Miller JS, and Yee D 2003. A chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-I. Cancer Res. 63:627–635. [PubMed] [Google Scholar]
- Sachdev D, Zhang X, Matise I, Gaillard-Kelly M, and Yee D 2010. The type I insulin-like growth factor receptor regulates cancer metastasis independently of primary tumor growth by promoting invasion and survival. Oncogene. 29:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawka-Verhelle D, Tartare-Deckert S, White MF, and Van Obberghen E 1996. Insulin receptor substrate-2 binds to the insulin receptor through its phosphotyrosine-binding domain and through a newly identified domain comprising amino acids 591–786. J Biol Chem. 271:5980–5983. [DOI] [PubMed] [Google Scholar]
- Schnarr B, Strunz K, Ohsam J, Benner A, Wacker J, and Mayer D 2000. Down-regulation of insulin-like growth factor-I receptor and insulin receptor substrate-1 expression in advanced human breast cancer. Int J Cancer. 89:506–513. [DOI] [PubMed] [Google Scholar]
- Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, Farhang-Fallah J, Dikkes P, Warot XM, Rio C, Corfas G, and White MF 2003. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci. 23:7084–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciacca L, Costantino A, Pandini G, Mineo R, Frasca F, Scalia P, Sbraccia P, Goldfine ID, Vigneri R, and Belfiore A 1999. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene. 18:2471–2479. [DOI] [PubMed] [Google Scholar]
- Scott LC, Mobley LR, Kuo TM, and Il’yasova D 2019. Update on triple-negative breast cancer disparities for the United States: A population-based study from the United States Cancer Statistics database, 2010 through 2014. Cancer. 125:3412–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu C, Hasegawa T, Tani Y, Takahashi F, Takeuchi M, Watanabe T, Ando M, Katsumata N, and Fujiwara Y 2004. Expression of insulin-like growth factor 1 receptor in primary breast cancer: immunohistochemical analysis. Hum Pathol. 35:1537–1542. [DOI] [PubMed] [Google Scholar]
- Sisci D, Morelli C, Garofalo C, Romeo F, Morabito L, Casaburi F, Middea E, Cascio S, Brunelli E, Ando S, and Surmacz E 2007. Expression of nuclear insulin receptor substrate 1 in breast cancer. J Clin Pathol. 60:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Shushanov S, LeRoith D, and Wood TL 2011. Decreased IGF type 1 receptor signaling in mammary epithelium during pregnancy leads to reduced proliferation, alveolar differentiation, and expression of insulin receptor substrate (IRS)-1 and IRS-2. Endocrinology. 152:3233–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, and et al. 1994. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 372:182–186. [DOI] [PubMed] [Google Scholar]
- Thorpe LM, Yuzugullu H, and Zhao JJ 2015. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 15:7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K, Shimamura T, Kimura N, Murayama T, Matsubara D, Kanauchi H, Niida A, Shimizu S, Nishioka K, Tsuji EI, Yano M, Sugano S, Shimono Y, Ishii H, Saya H, Mori M, Akashi K, Tada KI, Ogawa T, Tojo A, Miyano S, and Gotoh N 2017. Addiction to the IGF2-ID1-IGF2 circuit for maintenance of the breast cancer stem-like cells. Oncogene. 36:1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassen L, Wegrzyn W, and Klein-Hitpass L 1999. Human insulin receptor substrate-2 (IRS-2) is a primary progesterone response gene. Molecular endocrinology. 13:485–494. [DOI] [PubMed] [Google Scholar]
- White MF 2002. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 283:E413–422. [DOI] [PubMed] [Google Scholar]
- Wise TL, and Pravtcheva DD 2006. Delayed onset of Igf2-induced mammary tumors in Igf2r transgenic mice. Cancer Res. 66:1327–1336. [DOI] [PubMed] [Google Scholar]
- Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, and White MF 1999. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet. 23:32–40. [DOI] [PubMed] [Google Scholar]
- Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, and White MF 1998. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 391:900–904. [DOI] [PubMed] [Google Scholar]
- Wu A, Chen J, and Baserga R 2007. Nuclear insulin receptor substrate-1 activates promoters of cell cycle progression genes. Oncogene. [DOI] [PubMed] [Google Scholar]
- Wu HK, Squire JA, Catzavelos CG, and Weksberg R 1997. Relaxation of imprinting of human insulin-like growth factor II gene, IGF2, in sporadic breast carcinomas. Biochem Biophys Res Commun. 235:123–129. [DOI] [PubMed] [Google Scholar]
- Yang Y, and Yee D 2012. Targeting insulin and insulin-like growth factor signaling in breast cancer. Journal of mammary gland biology and neoplasia. 17:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerushalmi R, Gelmon KA, Leung S, Gao D, Cheang M, Pollak M, Turashvili G, Gilks BC, and Kennecke H 2012. Insulin-like growth factor receptor (IGF-1R) in breast cancer subtypes. Breast Cancer Res Treat. 132:131–142. [DOI] [PubMed] [Google Scholar]
- Yoshihara H, Fukushima T, Hakuno F, Saeki Y, Tanaka K, Ito A, Yoshida M, Iemura S, Natsume T, Asano T, Chida K, Girnita L, and Takahashi S 2012. Insulin/insulin-like growth factor (IGF) stimulation abrogates an association between a deubiquitinating enzyme USP7 and insulin receptor substrates (IRSs) followed by proteasomal degradation of IRSs. Biochem Biophys Res Commun. 423:122–127. [DOI] [PubMed] [Google Scholar]
- Zapf A, Hsu D, and Olefsky JM 1994. Comparison of the intracellular itineraries of insulin-like growth factor-I and insulin and their receptors in Rat-1 fibroblasts. Endocrinology. 134:2445–2452. [DOI] [PubMed] [Google Scholar]
- Zeleniuch-Jacquotte A, Shore RE, Koenig KL, Akhmedkhanov A, Afanasyeva Y, Kato I, Kim MY, Rinaldi S, Kaaks R, and Toniolo P 2004. Postmenopausal levels of oestrogen, androgen, and SHBG and breast cancer: long-term results of a prospective study. Br J Cancer. 90:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J 2007. The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem. 282:34356–34364. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kamaraju S, Hakuno F, Kabuta T, Takahashi S, Sachdev D, and Yee D 2004. Motility response to insulin-like growth factor-I (IGF-I) in MCF-7 cells is associated with IRS-2 activation and integrin expression. Breast Cancer Res Treat. 83:161–170. [DOI] [PubMed] [Google Scholar]
- Zhu S, Ward BM, Yu J, Matthew-Onabanjo AN, Janusis J, Hsieh CC, Tomaszewicz K, Hutchinson L, Zhu LJ, Kandil D, and Shaw LM 2018. IRS2 mutations linked to invasion in pleomorphic invasive lobular carcinoma. JCI Insight. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]


