Abstract
Investments in autism spectrum disorder (ASD) research, guided by the Interagency Autism Coordinating Committee (IACC), have focused disproportionately on etiology over a well-established stakeholder priority area: research to improve accessibility and quality of community-based services. This study analyzed National Institutes of Health ASD services research funding from 2008–2018 to examine funding patterns, evaluate the impact of IACC objectives, and identify future directions. Approximately 9% of total funds were allocated to services research. This investment remained relatively stable across time and lacked diversity across domains (e.g., area of focus, ages sampled, implementation strategies used). While advancements were observed, including increased prevalence of projects focused on adult samples and on dissemination/implementation and prevention areas, greater investment in service research is critically needed.
Keywords: Autism Spectrum Disorder, ASD services research, Dissemination and Implementation, ASD Policy, Community Mental Health Services, National Institutes of Health (U.S.)
The Centers for Disease Control and Prevention (CDC)’s Autism and Developmental Disabilities Monitoring (ADDM) Network now estimates that one in 54 children in the US have been identified with autism spectrum disorder (ASD; Maenner et al., 2020). Given the dramatic increase in ASD prevalence over the previous several decades, considerable public and private resources have been allocated to autism research. Legislatively, the Combating Autism Act (CAA) of 2006 was the first to expand federal involvement in and funding for ASD-specific research, surveillance, assessment and treatment services, and education programs. Most recently, the Autism Collaboration, Accountability, Research, Education, and Support (CARES) Act of 2019 was reauthorized and built on the ASD-related activities that were established under the CAA and continued under the Combating Autism Reauthorization Act (CARA) of 2011 and the Autism CARES Act of 2014 (CAA of 2006; CARA of 2011; Autism CARES Act of 2014; Autism CARES Act of 2019).
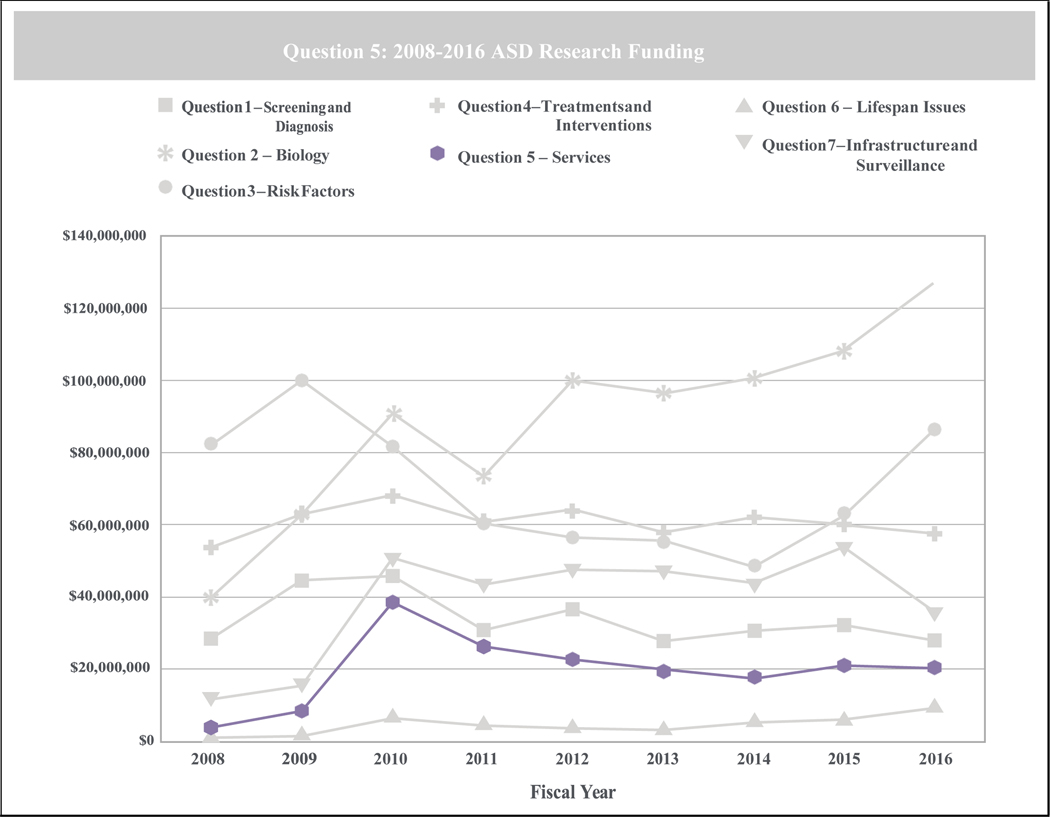
These legislative acts not only mandated substantial and consistent funding for autism research, but established the Interagency Autism Coordinating Committee (IACC), a federal advisory committee tasked with developing and updating strategic plans for ASD research, appraising and reporting on annual advances in ASD research, and monitoring federal activities related to ASD. Now over a decade old, the IACC has helped elucidate funding trends, highlight important research findings, and establish future research directions across seven topic areas, including: screening and diagnosis; biology; risk factors; treatments and interventions; services; lifespan issues; and, infrastructure and surveillance. According to the last ten years of IACC reports, the extensive program of research, funded through multiple public and private agencies, has largely focused on characterizing and defining ASD on behavioral and neurobiological levels, and recognizing etiological underpinnings of and risk factors for ASD. In fact, each report since the first Portfolio Analysis Report in 2008 indicated that both services research (i.e., research to improve accessibility and quality of services in the community) and research on lifespan issues are consistently allocated the fewest resources across the seven topic areas (See Figure 1). Based on the most recent results, services research made up 5% of total ASD research funding, and across reports since 2008, service research funding allocations averaged 7.2% of total funds (IACC, 2019; IACC, 2020).
Figure 1.
ASD Research Funding Allocations across Public and Private Funding Sources by IACC Topic Area - Adapted from the 2016 ASD Portfolio Analysis Report (IACC, 2019)
These IACC data suggest that there has been little change to funds allocated toward services research, despite a decade of IACC strategic plans detailing areas of research need. Across these years, the IACC has repeatedly emphasized the importance of improving access to and implementing evidence-based treatments in community settings. In the concluding budget recommendations of the latest IACC strategic plan, the committee reported that the three areas in “particular need of resource growth” were the development and delivery of new and improved treatments and interventions, the development and delivery of evidence-based services, and research on lifespan issues (IACC, 2017a, p. 101). In the 2018–2019 strategic plan update, the IACC noted again that the areas of services research and lifespan issues, have been “consistently less well funded than other research areas” (IACC, 2020). Lack of research in these areas, and particularly in the services research area, is an issue given that access to quality ASD care across the lifespan remains limited in much of the country and especially among underserved minority groups (Cantor et al., 2020; Daniels & Mandell, 2014; Farmer et al., 2014; K. A. Smith et al., 2020; Ziskind et al., 2020). Service systems across the nation also vary widely in resources available and in policies related to the delivery and coverage of ASD care (IACC, 2017a; Wood et al., 2015). Further, ASD supports and services are expensive, with the total costs of caring for the US ASD population estimated at $268 billion in 2015 and projected to be $461 billion by 2025 (Leigh & Du, 2015). Services research would directly address these issues, as this area of research focuses on developing evidence-based interventions and establishing improved methods for organizing, funding, and supporting delivery systems in the community to increase service availability (Hoagwood et al., 2018).
A decade has passed since the US Congress mandated a more coordinated and comprehensive approach to federal investment in ASD research, but it is clear that despite services needs being a well-established priority area for ASD stakeholders (Frazier et al., 2018; Pellicano et al., 2014), very little funding has been allocated to ASD services research. It is less clear how the National Institute of Health (NIH) services research funding specifically has evolved over time and how the IACC recommendations have impacted the focus of services research across the decade since it was established. As such, given the substantial role the NIH plays in establishing and funding the research agenda in ASD, this study was designed to: (1) conduct a detailed analysis of trends in services research funding for ASD over the last decade using data on NIH-funded services research projects extracted from the NIH Research Portfolio Online Reporting Tools (RePORTER) website; and (2) identify important future research directions.
Method
Project Search and Selection
To understand how funding patterns and the issues addressed by funded projects have evolved since the IACC’s first report, we examined data on NIH funding for ASD services research from 2008 through 2018. We examined exclusively NIH-funded projects for several reasons: the NIH plays a significant role in setting the ASD research agenda through the IACC, the majority of ASD research funding (e.g., 64% in 2016) has historically come from the NIH (IACC, 2019), and detailed information regarding funded projects is publicly available and easily accessible through NIH RePORTER. Search terms used in the current analysis were: (autism or “autism spectrum disorder” or asd or “autistic disorder” or “autistic children” or pdd or “pdd-nos” or “pervasive developmental disorder” or “pervasive developmental disorder-not otherwise specified” or “pervasive developmental disorders” or asperger or “asperger’s”) AND (“services research” or “systems research” or “implementation research” or “dissemination” or “health services” or “intervention research” or “implementation strategy” or implementation). The search was conducted on 04/15/2020 and limited to project abstracts, project titles, or project terms, in fiscal years 2008 through 2018.
The initial search yielded 1179 applications funded across all fiscal years. Two authors (PC, MM) independently assessed project titles and descriptions on NIH RePORTER for eligibility based on the following criteria. Projects were included if they: (1) had a primary focus on ASD and (2) addressed services-related topics including those outlined by the IACC; examples included: developing and evaluating training for service providers of children and adults with ASD; understanding characteristics across diverse, underserved ASD populations; improving access to care; and enhancing the efficacy, cost-effectiveness, implementation and dissemination of evidence-based practice. Services aimed at improving both core symptoms (i.e., social communication, social interaction, restricted, repetitive behavior) and associated symptoms, such as mental (e.g., anxiety, emotion dysregulation) and physical health (e.g., dental issues, obesity) and cognition/learning were included. Exclusion criteria included: (1) had a primary goal to identify etiology or risk factors for ASD, (2) primarily described neurological, biological, or cognitive processes associated with ASD, and (3) intervention under investigation was biomedical (e.g., pharmacological treatments). Disagreement regarding a project’s eligibility status was resolved through consensus. After excluding projects that did not meet eligibility criteria, 410 applications funded across all fiscal years remained (34.8% of resulting applications). Then, to determine the sensitivity of our initial search terms, a second search was conducted on 08/18/2020 replacing the term “services research” with “services”. Of the 2045 resulting entries from the second search, 522 met eligibility for inclusion in the study (25.5%). There were 116 eligible applications in the second search that were not captured in the original search. These projects were added to our dataset. Therefore, 526 total application entries, representing 173 unique projects, were included in the analyses (See Supplement A).
Data Extraction
Data were both exported directly from the search and extracted from the NIH RePORTER website. One author conducted the data export (MM) and collated all projects into a database. Four authors (PC, MM, JE, DS) then extracted additional data for each eligible project from the website using a pre-piloted data collection form. Information collected included: project title, administering institute/center, type of grant, fiscal year, total cost per fiscal year, brief description of the project, study subcategory (i.e., patient characteristics, policy, dissemination and implementation, treatment development and evaluation, prevention, and tool development), population of interest (i.e., at-risk population, exclusively individuals with ASD, ASD and related disorders), age of population of interest, number of linked publications (i.e., publications resulting from the grant that were included in the NIH RePORTER listing), and the state in which the study was conducted. Fifteen percent of the projects were re-categorized, with strong interrater reliability (κ = 0.87).
Because the IACC continues to recommend growth in the areas of dissemination and implementation and policy but has yet to meet many of their objectives (IACC, 2017b), the authors collected additional data on projects in these categories to identify both progress and gaps in the research portfolio. Specifically, one author (PC) coded the implementation strategies and implementation strategy clusters described in each project’s NIH RePORTER description. Implementation strategies, or the methods by which clinical programs/practices are adopted, implemented, and sustained (Proctor et al., 2013), were coded based on the Expert Recommendations for Implementing Change (ERIC) study, according to the 73 unique implementation strategies provided in Powell et al. (2015) and the nine implementation strategy clusters in Waltz et al. (2015). The senior author (SH) reviewed and confirmed all codes, with 98.5% agreement.
Finally, to provide further context to our findings and assess the priorities of the NIH during this time, we analyzed the funding opportunity announcements (FOAs) of all NIH-funded ASD projects from 2008–2018. To further expand our search of NIH priorities, we conducted a separate NIH RePORTER search with only ASD-related search terms (i.e., autism or ASD or autistic or “autism spectrum” or “autism spectrum disorder”) and pulled all unique FOAs that funded these projects into a database. Then, we coded the FOAs to determine if they: (1) were specific to ASD and (2) if the call was omnibus (i.e., could include services research as well as other research approaches), specific to more basic science (e.g., biology, neuroimaging, risk factors), or specific to services research (e.g., prevention, psychosocial treatments, service systems). Subsequently, we randomly sampled two-thirds of the omnibus FOAs to examine the proportion of funded applications that represented services research (e.g., prevention, psychosocial treatment, examination of service systems) compared to other types of research, such as biological and risk factor research (e.g., genetics, neuroimaging) and descriptive projects (e.g., examining social engagement and emotional processing, describing symptom stability across time) based on the information provided on NIH RePORTER.
Data Analyses
Data were analyzed descriptively using SPSS Statistical Software. To increase power for relevant statistical analyses, the decade was split into two five-year time periods (i.e., Early: 2009–2013 and Late: 2014–2018), omitting 2008 as this year was analyzed by the IACC within their first Portfolio Analysis report but occurred prior to the publication of their first strategic plan. Of note, funding allocated to services research was compared to total NIH autism research funding per fiscal year presented by NIH’s Research, Condition, and Disease Categorization (RCDC) system. The Research, Condition, and Disease Categorization system is a computerized reporting process that calculates NIH funding each fiscal year for nearly 300 research, condition, and disease categories (https://report.nih.gov/rcdc/), including autism. All monetary values (e.g., project costs per fiscal year, RCDC autism estimates) were adjusted using the Biomedical Research and Development Price Index, with 2018 as the reference year.
Results
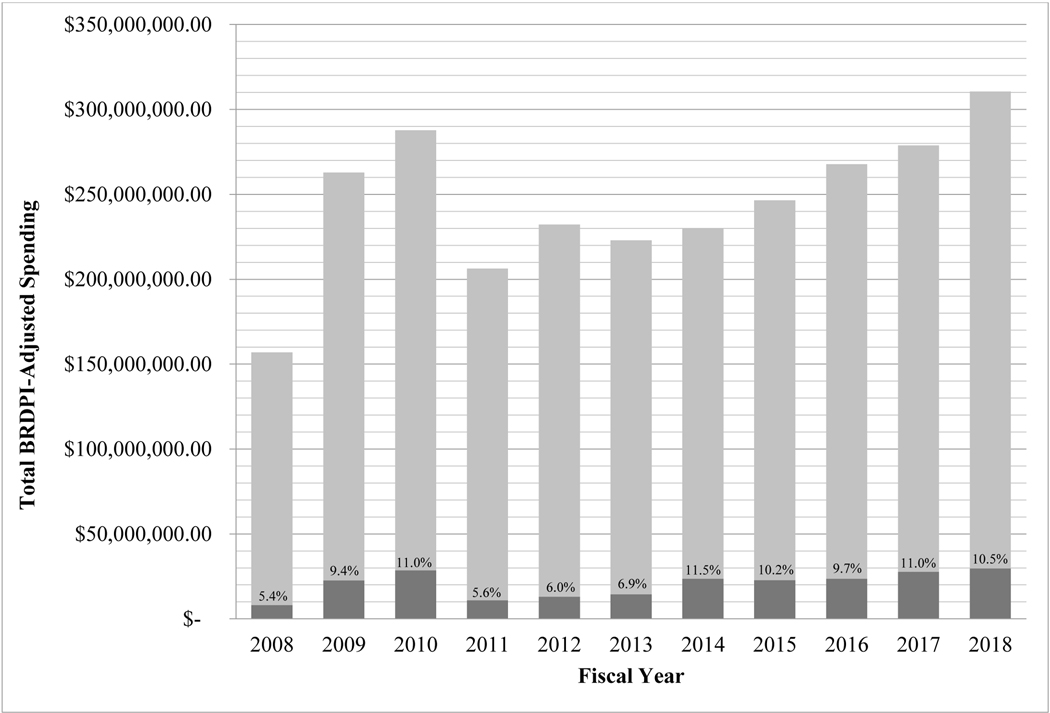
From 2008–2018, NIH allocated 9.1% of total funds, approximately $225 million of the nearly $2.5 billion devoted to ASD research, to services-related research. While there was a slight increase in the proportion of funds dedicated to services research from 2008–2010, corresponding with the American Recovery and Reinvestment Act (ARRA), a subsequent decrease from 2010–2011, and then another small increase from 2013–2014, values remained relatively stable, suggesting little change to NIH financial investment in services research across the decade (see Figure 2). A similar trend was evident in the total number of services-related research projects funded. After a significant jump from 2008–2009 (19 to 51 services-related projects funded), an average of 50.7 projects per year were funded between 2009–2018; 2018 had the largest number of services projects (N=70).
Figure 2.
NIH Funding for ASD Services Research v. NIH Funding for ASD Research Overall, FY 2008–2018
Source: NIH Services Research Funding data extracted from NIH RePORTER 04/15/2020 and 08/18/2020; includes all grant types; NIH Total Spending on ASD extracted from NIH Research, Condition, and Disease Categorization (RCDC) system for each fiscal year (FY); FY 2009 and FY 2010 includes ARRA research funds. All data are in FY 2018 dollars, adjusted using the NIH Biomedical Research and Development Price Index with 2018 as the reference year.
Funding Agency and Mechanism
The National Institute of Mental Health (NIMH) was primarily responsible for supporting services-related projects, funding 71.3% of the projects overall and an average of 34.1 projects per year. The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) funded 15.8% of total projects and averaged 7.5 projects per year. The National Institute on Deafness and Other Communication Disorders (NIDCD) funded 6.5% of total projects and averaged 3.1 projects per year. The Agency for Healthcare Research and Quality (AHRQ), the National Center on Birth Defects and Developmental Disabilities (NCBDDD), the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute of Environmental Health Sciences (NIEHS), the National Institute of Neurological Disorders and Stroke (NINDS), the Fogarty International Center (FIC), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and the National Institute of Nursing Research (NINR) were all represented in the funding of services-related projects, and on average, each funded one or fewer projects per year.
Almost half of all projects were funded through the R01 mechanism (39.7% of projects, M=19.0/year). R34 grants represented 13.3% of total projects (M=6.4/year), and R21 grants represented 10.5% of total project (M=5.0/year). All other grant types represented less than 10% of total projects funded and included F31, F32, K01, K23, K99, N01, P50, R03, R13, R18, R24, R33, R41, R42, R43, R44, R61, RC1, and U01 grants. Notably, the funding of training grants was infrequent; K and F grants together represented 10.5% of total projects between 2008–2018 and were funded at an average of 5.0 per year. The prevalence of funded training grants was not significantly different between early (i.e., 2009–2013) and late (i.e., 2014–2018) time periods, Χ2(1)=0.27, p=0.61.
Project Subcategory
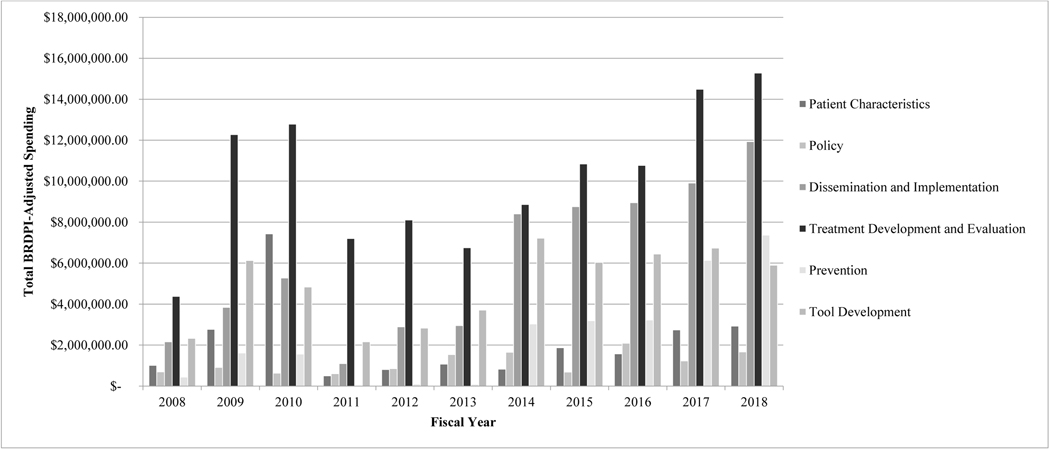
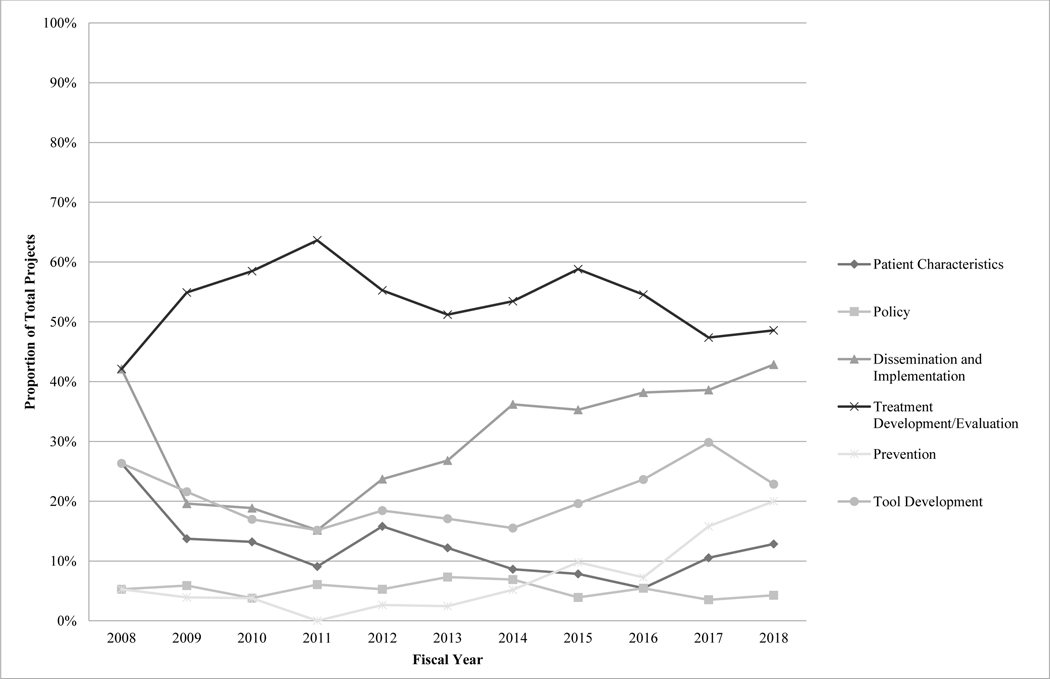
With regard to study focus, more than half of projects were categorized as treatment development/evaluation efforts (53.6% of total projects). Funding for treatment development/evaluation projects totaled approximately $111.7 million (49.7% of total funds), averaging $10.2 million per year. Substantial focus on and funding for treatment development/evaluation, disproportionate to other services-related research subcategories, remained consistent across the decade, with no differences in project prevalence across the early and late half of the decade, Χ2(1)=0.90, p=0.34.
Dissemination and implementation projects were second most prevalent, making up 31.4% of total funded projects, and were second highest funded (29.4% of total funds; $66.2 million). Focus on dissemination and implementation demonstrated an increasing trend across the decade, and the prevalence of dissemination and implementation projects was significantly higher in the later half of the decade compared to the earlier period, Χ2(1)=18.08, p<0.001. Similar funding trends were evident; funding for dissemination and implementation efforts totaled approximately $16 million from 2009–2013 (18.0% of funds) and increased to nearly $48 million from 2014–2018 (37.7% of funds).
Approximately 20% of total projects focused on tool development; these projects made up 24.2% of total funds awarded ($54.3 million). Differences in tool development project prevalence across the early and late periods of the decade were not found, Χ2(1)=1.39, p=0.24. Projects focused on tool development were evenly distributed across application to assessment and treatment and were primarily technological (86.2% of tool development projects).
Projects characterizing understudied groups represented 11.4% of total projects and received 10.5% of total funds ($23.5 million). Differences in the prevalence of projects characterizing understudied groups across the early and late periods of the decade were not found, Χ2(1)=1.74, p=0.19. Projects in this category frequently examined unique clinical subgroups (e.g., individuals with psychiatric/medical comorbidity, individuals who experienced delayed diagnosis; 35.0% of total patient characteristic projects) and adults (33.3%). Racial/ethnic minorities were the primary focus in 26.7% of these projects, and females were the focus in 5.0% of projects.
Policy and prevention projects were much less prevalent, representing 5.1% and 8.0% of total projects across years, respectively. Prevention projects received 11.9% of total funds ($26.7 million), and number of and funding for prevention efforts saw substantial increases over the decade. There have been statistically significantly more prevention efforts in the later compared to earlier half of the decade, Χ2(1)=14.27, p<0.001. Funding for prevention efforts totaled $3.4 million from 2009–2013 (3.8% of funds) and rose to $22.9 million from 2014–2018 (18.0% of funds). Focus on policy remained consistently low, with no change in prevalence of policy efforts across the decade, Χ2(1)=0.14, p=0.71. Policy projects also received the fewest resources overall (5.6% of total funds; $12.6 million) and averaged only $1.1 million per year. See Figures 3 and 4.
Figure 3.
Project Subcategory Funding Across Years
Figure 4.
Project Subcategory: Proportion of Total Projects by Year
Implementation Strategies Employed: Dissemination and Implementation and Policy Projects
Because dissemination/implementation and policy have been recognized by the IACC as areas of needed growth, and given the importance of this line of work for increasing service accessibility and quality in the community, we then evaluated the strategies described in NIH RePORTER descriptions for each unique dissemination and implementation and policy project (N=66). Results demonstrated that a range of implementation strategies was employed. Nearly all of the projects (N=57; 86.4%) noted the Use of Evaluative and Iterative Strategies, most frequently for quality monitoring (i.e., 81.8% developed and organized quality monitoring systems and 81.8% developed and implemented tools for quality monitoring). Over 25% of projects assessed for readiness/identified barriers and facilitators. Fewer projects employed other strategies within this cluster, with 10.6% obtaining and using patients/consumers and family feedback, 9.1% conducting a local need assessment, and 3.0% developing a formal implementation blueprint.
Nearly 70% of projects Developed Stakeholder Interrelationships. Expectedly, provided these projects resulted from a search of NIH services research, community-academic partnerships (54.5%) were most frequent. Other strategies within this cluster were less frequent and included the use of advisory boards and workgroups (12.1%); promoting network weaving (4.5%); conducting local consensus discussions (4.5%); capturing and sharing local knowledge (3.0%); recruiting, designating, and training for leadership (3.0%); identifying and preparing champions (1.5%); and building a coalition (1.5%). Strategies to Train and Educate Stakeholders were next most prevalent (N=23; 34.8%). Projects often developed and distributed educational materials (24.2% and 16.7%, respectively), and conducted educational meetings (6.1%) and educational outreach visits (3.0%). Three projects made training dynamic (i.e., vary information delivery methods and shape training to be interactive; 4.5%), two provided ongoing consultation (3.0%), and one used train-the-trainer strategies (1.5%).
Approximately 30% projects (N=21) used strategies to Engage Consumers. Over 20% of projects involved patients/consumers and family members, and 15.2% prepared patients/consumers to be active participants. Less frequently, projects intervened with patients/consumers to enhance uptake and adherence (4.5%) and used mass media (1.5%). Relatedly, no strategies within the Support Clinicians cluster were reported, and few reported using strategies to Provide Interactive Assistance (3.0%). One project used facilitation (1.5%), and one provided local technical assistance (1.5%). Within the Adapt and Tailor to Context cluster, used in 22.7% of projects, eight projects promoted adaptability (12.1%) and six tailored strategies (9.1%).
Seven projects (10.6%) incorporated strategies falling under the Utilize Financial Strategies cluster, all of which were policy projects examining the effects of insurance policy variations. Similarly, all but one project utilizing strategies within the Change Infrastructure cluster were policy projects. Seven policy projects examined mandated changes to insurance policies (10.6%), whereas one dissemination and implementation project started a dissemination organization (1.5%).
In regard to the relationship between prevalence of implementation strategies and expert ratings of strategy importance and feasibility from Waltz et al. (2015), approximately 15% of the strategies assigned an average rating of 4 or higher on a 5-point Likert scale were reported in a majority of project NIH RePORTER descriptions (N=2; develop and organize quality monitoring systems and develop and implement tools for quality monitoring). Four of the 13 strategies rated as highly important (30.8%) were not represented in any project, and six (46.2%) were included in less than 10% of projects. Strategies with higher feasibility ratings did not appear to be more prevalent in ASD dissemination and implementation and policy projects. See Table 1.
Table 1.
Implementation Strategies Employed: Dissemination and Implementation and Policy Projects
| N=66 | N(%) | Importancea | Feasibilitya |
|---|---|---|---|
| Use evaluative and iterative strategies | 57 (86.4%) | 4.19 | 4.01 |
| Assess for readiness and identify barriers and facilitators | 17 (25.8%) | 4.60 | 4.57 |
| Audit and provide feedback | 0 (0%) | 4.40 | 4.13 |
| Purposefully reexamine the implementation | 0 (0%) | 4.40 | 4.03 |
| Develop and implement tools for quality monitoring | 54 (81.8%) | 4.37 | 3.63 |
| Develop and organize quality monitoring systems | 54 (81.8%) | 4.33 | 3.37 |
| Develop a formal implementation blueprint | 2 (3.0%) | 4.30 | 4.47 |
| Conduct local need assessment | 6 (9.1%) | 4.27 | 4.33 |
| Stage up implementation scale up | 0 (0%) | 3.97 | 3.77 |
| Obtain and use patients/consumers and family feedback | 7 (10.6%) | 3.67 | 3.80 |
| Conduct cyclical small tests of change | 0 (0%) | 3.63 | 4.03 |
| Provide interactive assistance | 2 (3.0%) | 3.67 | 3.29 |
| Facilitation | 1 (1.5%) | 4.13 | 3.77 |
| Provide local technical assistance | 1 (1.5%) | 3.97 | 3.20 |
| Provide clinical supervision | 0 (0%) | 3.83 | 3.10 |
| Centralize technical assistance | 0 (0%) | 2.73 | 3.10 |
| Adapt and tailor to context | 15 (22.7%) | 3.59 | 3.30 |
| Tailor strategies | 6 (9.1%) | 4.37 | 4.00 |
| Promote adaptability | 8 (12.1%) | 3.90 | 3.57 |
| Use data experts | 0 (0%) | 3.23 | 3.13 |
| Use data warehousing techniques | 1 (1.5%) | 2.87 | 2.50 |
| Develop stakeholder interrelationships | 44 (66.7%) | 3.47 | 3.64 |
| Identify and prepare champions | 1 (1.5%) | 4.20 | 3.77 |
| Organize clinical implementation team meetings | 0 (0%) | 3.97 | 3.53 |
| Recruit, designate, and train for leadership | 2 (3.0%) | 3.93 | 3.20 |
| Inform local opinion leaders | 0 (0%) | 3.90 | 4.03 |
| Build a coalition | 1 (1.5%) | 3.77 | 3.63 |
| Obtain formal commitments | 0 (0%) | 3.77 | 3.17 |
| Identify early adopters | 0 (0%) | 3.70 | 3.70 |
| Conduct local consensus discussions | 3 (4.5%) | 3.63 | 4.07 |
| Capture and share local knowledge | 2 (3.0%) | 3.63 | 3.87 |
| Use advisory boards and workgroups | 8 (12.1%) | 3.40 | 3.87 |
| Use an implementation advisor | 0 (0%) | 3.30 | 3.70 |
| Model and simulate change | 0 (0%) | 3.30 | 3.20 |
| Visit other sites | 0 (0%) | 3.17 | 3.73 |
| Involve executive boards | 0 (0%) | 2.97 | 3.63 |
| Develop an implementation glossary | 0 (0%) | 2.87 | 4.57 |
| Develop academic partnerships | 36 (54.5%) | 2.83 | 3.40 |
| Promote network weaving | 3 (4.5%) | 2.70 | 2.77 |
| Train and educate stakeholders | 23 (34.8%) | 3.43 | 3.93 |
| Conduct ongoing training | 0 (0%) | 4.17 | 3.87 |
| Provide ongoing consultation | 2 (3.0%) | 4.17 | 3.63 |
| Develop educational materials | 16 (24.2%) | 3.80 | 4.83 |
| Make training dynamic | 3 (4.5%) | 3.67 | 4.00 |
| Distribute educational materials | 11 (16.7%) | 3.50 | 4.77 |
| Use train-the-trainer strategies | 1 (1.5%) | 3.33 | 3.50 |
| Conduct educational meetings | 4 (6.1%) | 3.27 | 4.50 |
| Conduct educational outreach visits | 2 (3.0%) | 3.10 | 4.07 |
| Create a learning collaborative | 0 (0%) | 3.10 | 3.43 |
| Shadow other experts | 0 (0%) | 2.87 | 3.37 |
| Work with educational institutions | 0 (0%) | 2.73 | 3.30 |
| Support clinicians | 0 (0%) | 3.23 | 3.06 |
| Facilitate relay of clinical data to providers | 0 (0%) | 4.17 | 3.43 |
| Remind clinicians | 0 (0%) | 3.23 | 3.77 |
| Develop resource sharing agreements | 0 (0%) | 3.07 | 3.13 |
| Revise professional roles | 0 (0%) | 3.00 | 2.30 |
| Create new clinical teams | 0 (0%) | 2.67 | 2.67 |
| Engage consumers | 21 (31.8%) | 3.25 | 2.95 |
| Involve patients/consumers and family members | 14 (21.2%) | 3.87 | 3.63 |
| Intervene with patients/consumers to enhance uptake and adherence | 3 (4.5%) | 3.50 | 3.07 |
| Prepare patients/consumers to be active participants | 10 (15.2%) | 3.40 | 3.03 |
| Increase demand | 0 (0%) | 3.30 | 2.33 |
| Use mass media | 1 (1.5%) | 2.17 | 2.70 |
| Utilize financial strategies | 7 (10.6%) | 2.86 | 2.09 |
| Fund and contract for the clinical innovation | 0 (0%) | 3.67 | 2.43 |
| Access new funding | 0 (0%) | 3.57 | 2.40 |
| Place innovation on fee for service lists/formularies | 0 (0%) | 3.40 | 2.10 |
| Alter incentive/allowance structures | 0 (0%) | 3.17 | 2.23 |
| Make billing easier | 0 (0%) | 2.93 | 1.77 |
| Alter patient/consumer fees | 0 (0%) | 2.60 | 2.03 |
| Use other payment schemes | 7 (10.6%) | 2.30 | 1.87 |
| Develop disincentives | 0 (0%) | 2.17 | 2.13 |
| Use capitated payments | 0 (0%) | 1.97 | 1.80 |
| Change infrastructure | 8 (12.1%) | 2.40 | 2.01 |
| Mandate change | 7 (10.6%) | 3.23 | 2.63 |
| Change record systems | 0 (0%) | 2.83 | 2.23 |
| Change physical structure and equipment | 0 (0%) | 2.60 | 2.27 |
| Create or change credentialing and/or licensure standards | 0 (0%) | 2.23 | 1.47 |
| Change service sites | 0 (0%) | 2.20 | 2.20 |
| Change accreditation or membership requirements | 0 (0%) | 2.17 | 1.80 |
| Start a dissemination organization | 1 (1.5%) | 2.03 | 2.13 |
| Change liability laws | 0 (0%) | 1.87 | 1.33 |
Ratings extracted from Waltz et al. (2015); experts were asked to rate each strategy for importance and feasibility on a 5-point Likert scale, where 1=relatively unimportant/not at all feasible and 5=extremely important/extremely feasible.
Project Characteristics
Project Sample
Across all project subcategories, a majority of projects sampled individuals with ASD exclusively (77.2%). Approximately 18% of projects sampled children at-risk for ASD, and 5.3% sampled individuals with ASD and related developmental disorders. When age range was indicated in the NIH RePORTER description, the proportion of total projects devoted to each age group was fairly evenly distributed. Slightly more attention and much more funding were allocated to younger age groups overall. Infants and toddlers (<3 years old) were sampled in 41.4% of total projects and received $89.1 million in funding. Youth in early childhood (3–5 years old) were sampled in 31.9% of projects and received $43.0 million, and school-aged children (6–12 years old) were sampled in 33.6% of total projects and received $40.4 million. In contrast, adolescents (13–17 years old) were sampled in 23.6% of total projects and received $26.0 million across years, and adults (18+ years old) were sampled in 23.1% of total projects and received $21.9 million in total. There were statistically significant increases in projects focused on adults, Χ2(1)=4.29, p=0.04, and significant decreases in projects focused on early childhood, Χ2(1)=10.82, p=0.001, when comparing the early and late halves of the decade. There was no change in the prevalence of projects focused on infants and toddlers, Χ2(1)=1.37, p=0.24, school-aged children, Χ2(1)=2.41, p=0.12, or adolescents, Χ2(1)=3.31, p=0.07. Further, projects focused on infants and toddlers saw the largest increase in total funds from the early ($25.6 million) to late ($60.6 million) periods, followed by more modest increases for projects focused on adults (early: $6.8 million; late: $15.1 million) and projects focused on adolescents (early: $9.1 million; late: $16.5 million). Total funding for projects focused on school-aged children remained relatively stable (early: $18.2 million; late: $20.5 million), and total funding for projects focused on early childhood decreased from the early ($26.1 million) to the late ($16.7 million) half of the decade.
Project Location
Across all projects, 29 states and Washington DC (58.8% of US states) were represented; there were no funded projects in 21 states. Nearly 60% of the funding went to investigators in just four states (CA, PA, FL, and MA), and 52.5% of total projects were from these four states. Nearly 70% of dissemination and implementation projects came from three states (PA, CA, and MA), with CA most frequently represented (35.2%). Notably, almost 75% of policy projects came from just one state (PA). Further, a minority of projects were multisite efforts (28.5%). These multisite efforts were predominantly treatment development/evaluation projects (73.3%).
Linked Publications
The number of linked publications varied widely across projects (range: 0–135). The mean number was 4.9 (SD=11.3), and the median was 2. The mode was 0 linked publications.
ASD FOAs
From 2008–2018, there were 36 active FOAs specific to ASD. Five were calls for biological/risk factor research, seven were calls for services research, and 24 were omnibus (See Supplement B). Further examination of applications funded under 16 randomly selected omnibus FOAs (66.7%) was conducted. After removing applications for center cores (N=94) from analyses, 152 of the 1007 project applications funded through the omnibus FOAs represented services research (15.1%). The majority of funded applications represented biological/risk factor research (N=758; 75.3%). Sixteen funded applications (1.6%) were studies on pharmacological interventions, and 81 (8.0%) were research projects aimed at understanding processes associated with ASD.
Discussion
Consistent with earlier IACC data from multiple private and public funding bodies (Figure 1; IACC, 2019), the current analyses found that from 2008–2018, NIH allocated only 9.1% of total ASD funds to services research (i.e., research to improve accessibility and quality of services in the community related to characterizing understudied groups, policy, dissemination and implementation, treatment development and evaluation, prevention, and tool development). Further, despite the increasing prevalence of ASD and growing attention to the well-established need for higher quality and more accessible community-based services, there has been little change to the NIH financial investment in services-related research across the decade. While IACC budget recommendations have encouraged resource growth in this area, there has been little movement to increase investment and prioritize the research that would directly impact the current service infrastructure and those individuals currently living with challenges associated with ASD.
This conclusion was supported by the analysis of omnibus ASD-specific FOAs, as results demonstrated that there was clear, disproportionate support for biological and risk factor research over services research. This suggests that this area was prioritized at a much lower rate when committees reviewed grant applications and/or when NIH agencies made funding decisions. Of course, to make firm conclusions, it would be important to know how many unfunded applications to these omnibus calls represented services research versus other research domains. It would also be important to understand the representation of scientific review committee members with expertise in ASD services research, as their inclusion would be imperative to appraise the quality of services-related projects and to advocate for their funding.
Across NIH agencies, the NIMH funded a majority of the services research projects, and most projects were funded through the R01 mechanism. Very few projects represented training grants (10.5%), and there was no change in the prevalence of training grants across the decade. This not only holds implications for our investment in preparing the next generation of ASD services researchers, but is also in opposition to IACC recommendations to grow the service workforce (IACC, 2017a), of whom services researchers are essential, and to encourage the training of researchers in implementation and dissemination (IACC, 2014). Additionally, there were no funded projects in 21 states and over half of projects were from just four states. Limited representation of states in the funding of dissemination and implementation projects and policy projects was particularly notable. These findings prompt concern for the generalizability of project findings, given the variation in state-level policies and service delivery systems that impact ASD service access and utilization. Further, there were few multisite efforts, despite concerns noted by the IACC regarding the abundance of small-scale treatment studies and limitations in statistical power (IACC, 2014). While multisite projects are challenging and more expensive to launch, they are integral for increasing power and the generalizability of results, and for examining contextual variations. This is particularly true for community-based research, as treatments may have differential effectiveness and adoption, implementation, and sustainment rates across sites.
Treatment development/evaluation projects represented more than half of the total projects within the services research category, and received nearly half of the total services research funds. Attention to this area remained stable across the decade, aligning with continued IACC emphasis on the development and improvement ASD intervention services (IACC, 2017a). While important, the focus on developing and improving treatments at the expense of other services research areas, namely dissemination and implementation and policy, is questionable when viewed through the lens of public health value. While the autism field would benefit from more and better treatment options, several evidence-based ASD interventions for children and for adults exist (National Autism Center, 2015), but unfortunately there are significant barriers for families trying to access these services (Cantor et al., 2020; Farmer et al., 2014; K. A. Smith et al., 2020; Ziskind et al., 2020) and for community providers trying to implement them with fidelity (Suhrheinrich et al., 2013). Focused attention on these issues of access, quality, and fidelity are critical for sustaining the impact of any effective treatment or service. Developing these kinds of interventions absent attention to issues of access, quality and fidelity risks diluting their public health impact. In addition, attention to the policy context is critical for both deploying and sustaining effective treatments and services in states and communities, and for enhancing both implementation effectiveness and disseminability. Unfortunately, policy projects have not increased in prevalence over the years, and this area continues to receive both the least attention and the fewest resources.
There were several notable advancements in the services research portfolio across the decade. First, projects on infants and toddlers were highly prevalent and funding for projects focused on this age group saw a significant increase across the decade. Because early identification of ASD continues to improve and there are substantial data to support the importance of early treatment (Eldevik et al., 2009; D. P. Smith et al., 2019; Zwaigenbaum, 2010), garnering evidence for the effectiveness and the implementation of intervention for this age cohort is critical. Likely relatedly, prevention efforts also increased significantly from the early to late half of the decade. This may have been in response to the US Preventative Services Task Force (USPSTF) guidelines drafted in 2015 that stated there was insufficient evidence available to recommend for or against universal ASD screening, a practice that had long been encouraged to improve early detection of ASD and link at-risk youth to essential diagnostic and treatment services (Mandell & Mandy, 2015; Robins et al., 2016). It is important to note, however, that although our ability to research younger populations has improved, children with ASD have always been prioritized in services research, while adults with ASD have been neglected. Since its first report, the IACC has highlighted the need to improve understanding of and services for adult populations. Results of our study suggest that these recommendations have been effective; the prevalence of NIH-funded services projects on adults with ASD has increased significantly across the decade. However, this group continues to receive the fewest funds. Continued resource growth and research attention on adults are essential.
The prevalence of and funding for dissemination and implementation projects also saw encouraging increases across time, again aligning with the IACC objectives. However, as disparities in access and quality of care in the community remain a critical issue (Cantor et al., 2020), continued investment is needed. Further, while the dissemination and implementation and policy projects used a variety of implementation strategies, several strategies were rarely, if ever, employed, including many that were rated as highly important by experts in the field (Waltz et al., 2015). Strategies used were primarily related to stakeholder training, corresponding to IACC recommendations to address workforce needs. However, without the use of strategies to support clinicians and provide interactive assistance, gains in competence and behavior change from training are unlikely to be sustained. Further, without addressing financial and infrastructure factors—that is, service system issues—access to these trained clinicians will remain limited. Projects using financial strategies and strategies in the Change Infrastructure cluster were largely evaluative, for example, describing service use trends when specific policies were or were not in place, but none enacted change in infrastructure or utilized financial strategies for a particular clinical innovation. This finding is reflective of gaps in the larger implementation science field, where costs associated with implementation strategies are rarely examined in research (Cidav et al., 2020). It also reflects IACC recommendations that cited the need to more frequently evaluate service cost-effectiveness (IACC, 2014).
As indicated by the analysis of linked publications, the dissemination of project findings was disappointing, with most projects having no publications listed on NIH RePORTER. This calls into serious question the impact these projects are having on individuals with ASD and their families. However, we recognize that analyzing NIH RePORTER linked publications to serve as a proxy for the dissemination of project findings may be questionable. Results appeared somewhat over-represented for some projects, where linked publications often included loosely-related manuscripts (e.g., neuroimaging studies within a primarily dissemination and implementation focused project) or manuscripts completed prior to project start date. Further, for some projects, linked publications appeared under-represented. Due to the high prevalence of no linked publications, the authors conducted a PubMed search for approximately 10% of projects using principal investigator name and/or project title and/or grant number as search terms. Although it would be difficult to determine with full accuracy whether a manuscript resulting from the PubMed search should have been linked, it appeared likely that several of the projects had closely related publications that may have resulted from the project but were not linked to the NIH RePORTER listing. As this may indicate failure to follow NIH guidelines regarding linking publications, the NIH should consider providing heightened oversight on linking practices to ensure that those interested have easy access to research findings. Further, it remains an issue if even a proportion of the projects with zero linked publications truly did not publish following project completion and would indicate a considerable need for improvement to optimize the federal dollars allocated to this research.
This study is not without limitations. First, the method used to estimate total NIH services research funding was different from the method used to obtain the estimate for total NIH ASD funding overall. The NIH RePORTER search, which generated the NIH services research funding data, like all projects using search strategies, is limited by its search terms and may not be inclusive of all services projects, whereas the RCDC system autism funding estimate for overall NIH ASD expenditures, which is computer-generated, may be more accurate. Next, categorization and codes were assigned according to the information presented on each project’s NIH RePORTER listing, which may not be fully representative or include all pertinent details. This is particularly true for coded implementation strategies, as these have generally been criticized as being poorly described and detailed in the implementation science literature (Cidav et al., 2020; Proctor et al., 2013) and may not have been fully reported in brief NIH RePORTER descriptions. Additionally, the adult age group code was assigned for projects sampling individuals aged 18 years and older; this range is wide given that adults of different ages likely have different primary concerns (e.g., physical health needs versus transition services) and interact with different service systems. Finally, as previously noted, this analysis was focused only on NIH, as the largest contributor to ASD research funding. However, other funding bodies contribute to ASD research, and their services research funding patterns may differ from these findings.
Conclusion
NIH investment in services research for ASD over the past ten years is limited and it lacks diversity. While attention to the development and evaluation of treatments remains strong, there is much less attention given to dissemination, implementation and policy contexts within which these treatments and services are delivered. Treatments and services are unlikely to be sustained with their intended effects unless there is increased attention to accessibility, quality and fidelity within diverse communities. Continued advancements are required in evidence-based services for adults, and more resources should be allocated to this cohort. Geographic diversity in the states conducting research is also needed to increase the breadth of topic areas covered, because policies and service systems are vastly different from state to state. Finally, research on implementation strategies should vary and expand, as barriers to successful implementation need to be addressed, particularly related to cost-effectiveness and policy. Increasing ASD services research funding and diversifying services research projects are crucial for meeting IACC services objectives and improving the lives of individuals with ASD and their families.
Supplementary Material
Acknowledgments
Funding: This study was funded by the National Institute of Mental Health (P50MH113662). The authors thank the NIMH for their support but acknowledge that the findings and conclusions are those of the authors and do not necessarily reflect the opinions of the NIMH.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Cidav Z, Mandell D, Pyne J, Beidas R, Curran G, & Marcus S. (2020). A pragmatic method for costing implementation strategies using time-driven activity-based costing. Implementation Science, 15(1), 28. 10.1186/s13012-020-00993-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combating Autism Act (CAA) of 2006, Pub. L. 109–416.
- Combating Autism Reauthorization Act (CARA) of 2011, Pub. L. 112–32.
- Autism Collaboration, Accountability, Research, Education, and Support (CARES) Act of 2014, Pub. L. 113–157.
- Autism Collaboration, Accountability, Research, Education, and Support (CARES) Act of 2019, Pub. L. 116–60.
- Cantor J, McBain RK, Kofner A, Stein BD, & Yu H. (2020). Fewer than half of US mental health treatment facilities provide services for children with autism spectrum disorder: Results from a survey of US mental health treatment facilities on the availability of behavioral health care services for children with autism spectrum disorder. Health Affairs, 39(6), 968–974. 10.1377/hlthaff.2019.01557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels AM, & Mandell DS (2014). Explaining differences in age at autism spectrum disorder diagnosis: A critical review. Autism, 18(5), 583–597. 10.1177/1362361313480277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldevik S, Hastings RP, Hughes JC, Jahr E, Eikeseth S, & Cross S. (2009). Meta-analysis of early intensive behavioral intervention for children with autism. Journal of Clinical Child &Adolescent Psychology, 38(3), 439–450. 10.1080/15374410902851739 [DOI] [PubMed] [Google Scholar]
- Farmer JE, Clark MJ, Mayfield WA, Cheak-Zamora N, Marvin AR, Law JK, & Law PA (2014). The relationship between the medical home and unmet needs for children with autism spectrum disorders. Maternal and Child Health Journal, 18(3), 672–680. 10.1007/s10995-013-1292-z [DOI] [PubMed] [Google Scholar]
- Frazier TW, Dawson G, Murray D, Shih A, Sachs JS, & Geiger A. (2018). Brief report: A survey of autism research priorities across a diverse community of stakeholders. Journal of Autism and Developmental Disorders, 48(11), 3965–3971. 10.1007/s10803-018-3642-6 [DOI] [PubMed] [Google Scholar]
- Hoagwood KE, Atkins M, Kelleher K, Peth-Pierce R, Olin S, Burns B, Landsverk J, & Horwitz SM (2018). Trends in children’s mental health services research funding by the national institute of mental health from 2005 to 2015: A 42% reduction. Journal of the American Academy of Child & Adolescent Psychiatry, 57(1), 10–13. 10.1016/j.jaac.2017.09.433 [DOI] [PubMed] [Google Scholar]
- Interagency Autism Coordinating Committee (IACC). (2017a). 2016–2017 Interagency Autism Coordinating Committee Strategic Plan For Autism Spectrum Disorder. Retrieved from the U.S. Department of Health and Human Services Interagency Autism Coordinating Committee website: https://iacc.hhs.gov/publications/strategic-plan/2017/ [Google Scholar]
- Interagency Autism Coordinating Committee (IACC). (2014). IACC Strategic Plan for Autism Spectrum Disorder (ASD) Research —2013 Update. Retrieved from the U.S. Department of Health and Human Services Interagency Autism Coordinating Committee website: http://iacc.hhs.gov/strategic-plan/2013/index.shtml. [Google Scholar]
- Interagency Autism Coordinating Committee (IACC). IACC Strategic Plan for Autism Spectrum Disorder (ASD) 2018–2019 Update. July 2020. Retrieved from the U.S. Department of Health and Human Services Interagency Autism Coordinating Committee website: http://iacc.hhs.gov/strategic-plan/2019/. [Google Scholar]
- Leigh JP, & Du J. (2015). Brief report: Forecasting the economic burden of autism in 2015 and 2025 in the united states. Journal of Autism and Developmental Disorders, 45(12), 4135–4139. 10.1007/s10803-015-2521-7 [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Shaw KA, Baio J, et al. (2020). Prevalence of autism spectrum disorder among children aged 8 years — Autism and developmental disabilities monitoring network, 11 sites, united states, 2016. MMWR Surveillance Summaries, 69(No. SS-4), 1–12. DOI: 10.15585/mmwr.ss6904a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell D, & Mandy W. (2015). Should all young children be screened for autism spectrum disorder? Autism, 19(8), 895–896. 10.1177/1362361315608323 [DOI] [PubMed] [Google Scholar]
- National Autism Center. (2015). Findings and conclusions: National standards project, phase 2. Randolph, MA: Author. [Google Scholar]
- Office of Autism Research Coordination, National Institute of Mental Health, on behalf of the Interagency Autism Coordinating Committee (IACC). (2017b). 2014–2015 IACC Autism Spectrum Disorder Research Portfolio Analysis Report. Retrieved from the U.S. Department of Health and Human Services Interagency Autism Coordinating Committee website: https://iacc.hhs.gov/portfolio-analysis/2015/index.shtml [Google Scholar]
- Office of Autism Research Coordination, National Institute of Mental Health, on behalf of the Interagency Autism Coordinating Committee (IACC). (2019). 2016 IACC Autism Spectrum Disorder Research Portfolio Analysis Report. Retrieved from the U.S. Department of Health and Human Services Interagency Autism Coordinating Committee website: https://iacc.hhs.gov/portfolio-analysis/2016/index.shtml [Google Scholar]
- Pellicano E, Dinsmore A, & Charman T. (2014). What should autism research focus upon? Community views and priorities from the United Kingdom. Autism, 18(7), 756–770. 10.1177/1362361314529627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, Proctor EK, & Kirchner JE (2015). A refined compilation of implementation strategies: Results from the Expert Recommendations for Implementing Change (ERIC) project. Implementation Science, 10(1), 21. 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor EK, Powell BJ, & McMillen JC (2013). Implementation strategies: Recommendations for specifying and reporting. Implementation Science, 8(1), 139. 10.1186/1748-5908-8-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DL, Adamson LB, Barton M, Jr JEC, Dumont-Mathieu T, Dworkin PH, Fein D, Greenstein MA, Hsu H-W, Kerns C, Newschaffer C, Plumb J, Shattuck P, Turchi R, & Vivanti G. (2016). Universal autism screening for toddlers: Recommendations at odds. Journal of Autism and Developmental Disorders, 1–3. 10.1007/s10803-016-2697-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DP, Hayward DW, Gale CM, Eikeseth S, & Klintwall L. (2019). Treatment gains from early and intensive behavioral intervention (EIBI) are maintained 10 years later. Behavior Modification, 0145445519882895. 10.1177/0145445519882895 [DOI] [PubMed] [Google Scholar]
- Smith KA, Gehricke J-G, Iadarola S, Wolfe A, & Kuhlthau KA (2020). Disparities in service use among children with autism: A systematic review. Pediatrics, 145(Supplement 1), S35–S46. 10.1542/peds.2019-1895G [DOI] [PubMed] [Google Scholar]
- Suhrheinrich J, Stahmer AC, Reed S, Schreibman L, Reisinger E, & Mandell D. (2013). Implementation challenges in translating pivotal response training into community settings. Journal of Autism and Developmental Disorders, 43(12). 10.1007/s10803-013-1826-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz TJ, Powell BJ, Matthieu MM, Damschroder LJ, Chinman MJ, Smith JL, Proctor EK, & Kirchner JE (2015). Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: Results from the Expert Recommendations for Implementing Change (ERIC) study. Implementation Science, 10(1), 109. 10.1186/s13012-015-0295-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, McLeod BD, Klebanoff S, & Brookman-Frazee L. (2015). Toward the implementation of evidence-based interventions for youth with autism spectrum disorders in schools and community agencies. Behavior Therapy, 46(1), 83–95. 10.1016/j.beth.2014.07.003 [DOI] [PubMed] [Google Scholar]
- Ziskind D, Bennett A, Jawad A, & Blum N. (2020). Therapy and psychotropic medication use in young children with autism spectrum disorder. Pediatrics, 145(Suppl 1), S99–S107. 10.1542/peds.2019-1895M [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L. (2010). Advances in the early detection of autism. Current Opinion in Neurology, 23(2), 97–102. 10.1097/WCO.0b013e3283372430 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.