Abstract
The study intends to investigate the regulation of syndecan-1 in human uterine leiomyoma cells. Human syndecan-1 levels were detected by Western blot in uterus leimyoma's tissue. The efficacy of syndecan-1 silencing on the cell proliferation, metalloproteinases and extracellular matrix were determined through Cell Counting Kit (CCK8) assay and Western blot assay, respectively. We compared the respective and combined effect of mifepristone and syndecan-1 on cell proliferation and the expression of metalloproteinases and extracellular matrix (ECM) in human uterine leiomyoma cells. The inhibitory effects of Syndecan-1 silencing on proliferation, ECM and Matrix Metalloproteinase (MMP) were observed in human uterine leiomyoma cells. Furthermore, syndecan-1 inhibition enhanced the effects of mifepristone against uterine leiomyoma cell proliferation. The expression of MMPs and ECM components in human uterine leiomyoma cells was decreased dramatically after syndecan-1 silencing, which was promoted after mifepristone treatment. Altogether, syndecan-1 silencing enhanced the efficacy of mifepristone on the uterine leiomyoma cell proliferation and ECM formation. Therefore, targeting syndecan-1 represents a novel therapeutic strategy to treat uterine leiomyoma.
Keywords: Syndecan-1, Mifepristone, Uterine leiomyoma, Metalloproteinases and extracellular matrix
Introduction
Uterine fibroids are the most common benign tumors of the female reproductive system (Laughlin et al. 2010). The main components of uterine fibroids are smooth muscle cells and extracellular matrix. There are multiple pathological types of fibroids due to different fibrous connective tissue content, which contains abundant extracellular matrix (ECM), and the main stromal cells are fibroblasts (Bhowmick et al. 2004). At present, the exact pathogenesis of uterine fibroids is not clear, but it is generally believed that uterine fibroids are closely related to ovarian hormones and hormone-dependent tumors (Maheux et al. 1985).
The abnormal expression of syndecan-1 was closely associated with unfavorable phenotypes of cancers and adverse outcomes of patients, including breast cancer and ovarian cancer (Liebersbach and Sanderson 1994; Kato et al. 1995; Barbareschi et al. 2003; Davies et al. 2004; Conejo et al. 2000). Syndecan-1 was reported to regulate cell viability and apoptosis in human endometrial cancer cell lines, the silencing of which suppressed extracellular regulated protein kinases and Akt-mediated cell proliferation (Choi et al. 2007). The co-localization of Syndecan-1 and transforming growth factor-β (TGF-β) was found in nasal mucosa and nasal polyp tissues (Go et al. 2010). Previous studies have suggested that TGF-β1, TGF-β2, and TGF-β3 can all induce the expression of Syndecan-1 (Hayashida et al. (2006)). A research demonstrated that TGF-β regulates the expression of Syndecan-1 in human breast stromal fibroblasts (Liakou et al. 2016).
Mifepristone is an anti-progesterone drug widely used in the treatment of uterine fibroids, which can shrink leiomyomas and improve symptoms. Additionally, mifepristone can inhibit the activity of uterine fibroids and deposition of extracellular matrix (Patel et al. 2016; Shen et al. 2019). These research results caught our attention to the role of syndecan-1 in uterine fibroids, particularly what is involved in cellular proliferation and ECM. Besides, we also focus on whether interfering syndecan-1 can promote the effect of clinical mifepristone.
Methods
Clinical data
All the patients with uterine fibroids were from the Department of Gynaecology of the Affiliated Maternity and Child Health Care Hospital of Nantong University, and those who needed surgical treatment for uterine fibroids were selected as the research objects. According to the clinical manifestations, uterine fibroids were diagnosed by gynecological examination and B-ultrasonography, with definite indications of hysterectomy or myomectomy. During the operations, leiomyoma was paired with adjacent normal muscle tissues as samples, and the expression of syndecan-1 in uterine leiomyoma and adjacent normal myometrium tissues was detected by Western blot. All paired uterine fibroids were fibroid tissues and adjacent normal myometrium tissues from the same patient. Samples were stored in – 80 °C refrigerator. The patients had no history of using hormones or other special drugs within three months before the operations. All these patients were pathologically confirmed as uterine fibroids after the operations. Patients were strictly informed and signed the informed consent prior to surgery as required by medical research. The study was approved by the Ethics Committee of the Affiliated Maternity and Child Health Care Hospital of Nantong University.
Cell culture
The human uterine leiomyoma cells were purchased from Coriell Institute for Medical Research (GM10964, Camden, NJ) and cultured in DMEM/F12 medium (Gibco, USA) with 10% fetal calf serum at 37 °C with 5% CO2. Cells were passaged by trypsin digestion for further experiments (Gibco, USA).
Plasmid transfection
Human uterine leiomyoma cells were transfected with different plasmids containing different shRNA sequence targeting syndecan (ShRNA- syndecan-1–1, ShRNA- syndecan-1–2) or scramble shRNA sequence (Gemephamacy, Shanghai, China), respectively. 24 h after transfection, the cells were treated using 10 µm Mifepristone for 24 h and then used to perform further study.
Western blot
The total protein in human uterine leiomyoma or human uterine leiomyoma cells were extracted, respectively. Then, proteins underwent 10% SDS-PAGE gel electrophoresis and transferred onto PVDF membranes. 5% skimmed milk powder was added to incubate with the protein band for 1 h. Subsequently, the protein band was incubated with the primary antibody (Abcam, England) overnight at 4 °C after being rinsed with PBS. Following being washed using PBS, the blots were incubated with HRP-conjugated secondary antibodies (Abcam, England) at room temperature for 1 h. Enhanced chemiluminescence (ThermoFisher Scientific. Inc.) was used to visualize the bands, the grey value of which was analyzed using Image 1.46r software. The relative expression of target protein was calculated relative to GADPH as an internal reference.
RT-qPCR
Total RNA in human uterine leiomyoma cells was extracted using TRIzol method (Promega, Madison, WI) in accordance with the manufacturer’s protocol. Subsequently, total RNA was reversed into cDNA using Titan™ One Tube RT-PCR Kit (Roche, Swiss). The amplification of cDNA was amplified using FastStart Universal SYBR Green Master (Roche, Swiss). The relative expression of syndecan-1 was calculated using 2−△△Ct method. The primers used in this experiment are as following: Syndecan-1 forward: 5′- GGCTGTAGTCCTGCCAGAAG-3′, reverse: 5′ -GTCGTTGAGGCCTGATGAGT-3′. GAPDH forward: 5′-CAGGAGGCATTGCTGATGAT-3′, reverse: 5′- AAGGCTGGGGCTCATTT-3’.
CCK8 assay
Human uterine leiomyoma cells were seeded into 96-well plates (5*103 cells/well). After cells underwent different experimental treatments for 24 h, 48 h, 72 h or 96 h, 10µL CCK-8 solution was added to each well, and the occurrence of bubbles should be avoided (CCK8 solution, Abcam, England). The cells were incubated in the incubator for 4 h, and the absorbance value (OD value) of each hole at 450 nm was measured with a microplate reader.
Statistical analysis
The experimental data was expressed as mean ± SD of at least three independent experiments. One-way ANOVA was used to conduct statistical comparisons among different groups, followed by turkey’s test. p < 0.05 was considered as statistically significant.
Result
TGFβ3 signal regulated the expression of syndecan-1
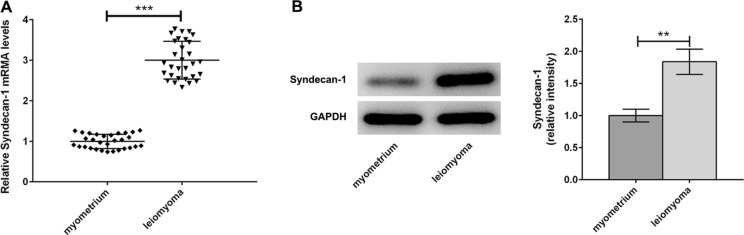
Surgical specimens were collected from uterine fibroids patients and used for the detection of Syndecan-1 expression by qPCR and Western blot (Fig. 1a, b). We observed marked difference in basal Syndecan-1 at mRNA and protein levels of uterine fibroids as compared to adjacent normal myometrium tissues. After treatment of human uterine leiomyoma cells with TGF β3 (5 ng/mL) or SB431542 (10 ng/ML), the protein and mRNA levels of Syndecan-1 were respectively detected through Western blot and qPCR. TGF β3 significantly increased leiomyoma expression compared with control group (Fig. 2a, b). On the contrary, a notable decrease in leiomyoma expression was observed in SB431542 group. These findings suggested that TGF β3 signal changed transcriptional and translational levels of Syndecan-1.
Fig. 1.
The expression of Syndecan-1 was upregulated in leiomyoma. a, b The mRNA and protein levels of Syndecan-1 were assessed in surgical specimens of leiomyoma and matched myometrial tissues by quantitative real-time polymerase chain reaction (qPCR). ***p < 0.001
Fig. 2.
TGF β3 regulated the expression of Syndecan-1. a, b After human primary leiomyoma cells were treated with TGF β3 (5 ng/mL) or SB431542 (10 ng/ML), the mRNA and protein levels of Syndecan-1 were measured by Western blot and qPCR analysis. **p < 0.01, ***p < 0.001
Syndecan-1 silencing suppressed the proliferation of leiomyoma cells
To test the role of syndecan-1 in human leiomyoma cells, we firstly reduced the protein and mRNA levels of Syndecan-1 after exposure to ShRNA- syndecan-1–1 and ShRNA- syndecan-1–2 vectors, respectively (Fig. 3a, b). The result suggested that ShRNA- syndecan-1–1 had greater effects on silencing syndecan-1 expression versus ShRNA- syndecan-1–2 vector. Therefore, in all the subsequent experiments, we treated these cells with ShRNA- syndecan-1–1 vector. Next, human leiomyoma cells were incubated in vitro with ShRNA- syndecan-1–1 vector for 24, 48, 72 and 96 h, respectively. We performed CCK8 assay and found that syndecan-1 silencing significantly suppressed the proliferation of human leiomyoma cells in a time-dependent manner (Fig. 3c).
Fig. 3.
Syndecan-1 silencing suppressed the cell viability of leiomyoma cells. a, b The protein and mRNA levels of Syndecan-1 were detected in cells transfected with ShRNA-syndecan-1–1 and ShRNA-syndecan-1–2 vectors, respectively, through Western blot and qPCR analysis. c After 24, 48, 72 and 96 h of treatment with ShRNA-syndecan-1–1, respectively, the proliferation abilities of human leiomyoma cells were detected by CCK8 assay. ***p < 0.001
Syndecan-1 silencing altered the protein production of MMPs and ECM components
In order to analyze the pathophysiological functions of syndecan-1, we silenced syndecan-1 and analyzed its influence on cells. Firstly, we evaluated the expression level of MMP2 and MMP9 in leiomyoma primary cells after syndecan-1 silencing. As shown in Fig. 4a, the expression levels of MMP2 and MMP11 were significantly lower in leiomyoma cells, compared with syndecan-1 silencing group. Next, we also observed decreases in fibronectin, collagen type 1 and α-SMA protein levels, showing that syndecan-1 silencing markedly suppressed ECM formation (Fig. 4b).
Fig. 4.
Syndecan-1 silencing reduced the levels of MMPs and ECM components. a The protein and mRNA levels of MMP2 and MMP9 were measured by Western blot and qPCR after Sh-syndecan-1. b The protein and mRNA levels of representative ECM markers, such as fibronectin, collagen type 1, vimentin, and α-SMA were analyzed by Western blot and qPCR after syndecan-1 silencing. ***p < 0.001
The efficacy of Mifepristone was enhanced via silencing syndecan-1
Mifepristone is an anti-progesterone drug widely used in the treatment of uterine fibroids, which could reduce uterine leiomyoma and improve patients’ symptoms. This study continued to hypothesize that inhibition of syndecan-1 could further inhibit the proliferation of leiomyoma cells and extracellular matrix deposition. To test this hypothesis, human leiomyoma cells were incubated with ShRNA- syndecan-1–1 followed by exposure to 10 µm or 20 µm of mifepristone or vehicle. We observed that mifepristone (20 µm) suppressed the proliferation of human leiomyoma cells compared with untreated control cells (Fig. 5a). Interestingly, the proliferation of human leiomyoma cells was further suppressed upon silencing syndecan-1. Subsequently, MMP2 and MMP11 expression was reduced by mifepristone in leiomyoma cells at the concentration of 20 µm compared with control group, which was further reduced after silencing endogenous syndecan-1 expression by shRNA in leiomyoma cells (Fig. 5b). In addition, in leiomyoma cells treated only with 20 µm of mifepristone, fibronectin, collagen type 1, vimentin, and α-SMA levels were significantly reduced compared with control cells (Fig. 5c). Furthermore, the decreased amount of these proteins was further reduced after inhibition of syndecan-1 (Fig. 5c).
Fig. 5.
a Human primary leiomyoma cells were transfected with ShRNA- syndecan-1–1 vector, followed by treatment of 10 µm or 20 µm of mifepristone for CCK8 proliferation assay. ***p < 0.001 comparisons between Mifepristone 10 µM and control group. @@@p < 0.001 comparisons between Mifepristone 10 µM + shRNA-syndecan-1 and Mifepristone 10 µM + shRNA-NC. ###p < 0.001 comparisons between Mifepristone 10 µM and Mifepristone 20 µM. △△△p < 0.001 comparisons between Mifepristone 20 µM + shRNA-syndecan-1 and Mifepristone 20 µM + shRNA-NC. b Human primary leiomyoma cells after syndecan-1 silencing or mifepristone treatment were analyzed for MMP2 and MMP11 expression. c The expression of fibronectin, collagen type 1, vimentin, and α-SMA was determined after syndecan-1 silencing or mifepristone treatment. *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
The transformation of the uterine smooth muscles cells and ECM dysfunction play vital roles in the development of uterine fibroid. TGF-β pathway is implicated in development of the fibrotic phenotype and TGF-β3 mRNA levels showed significant upregulation in leiomyoma (Lewis et al. (2019)). It has been reported that both TGF β3 and TGF β1 could induce syndecan-1 expression (Hayashida et al. 2006; Worapamorn et al. 2002). Notably, the present study found that exogenous TGF-β3 led to the upregulation of syndecan-1 in human uterine leiomyoma cells while SB431542 caused opposite effect in the expression of syndecan-1 as compared to control group. These results confirmed that TGF β3 signals regulated syndecan-1 expression and implied that the function of TGF β3 could be mediated by syndecan-1 in leiomyoma. The mechanism of leiomyoma regulating TGF β3 was recently reported to be associated with miR-29c (Chuang and Khorram 2019). The study indicated that TGF-β3 could cross-talk with miR-29c via an epigenetic mechanism in leiomyoma. Given that TGF-β3 altered MMPs levels and ECM formation, syndecan-1, a downstream of TGF-β3, possibly displayed a similar function on these molecules (Cardozo et al. 2018; Joseph et al. 2010). Further experiments found that syndecan-1 silencing decreased the levels of metalloproteinases including MMP2 and MMP11, and extracellular matrix that are recognized to affect tumor growth, indicating that syndecan-1 silencing affected uterine leiomyoma cells growth (Lin et al. 2019). The inhibitory effects of mifepristone on cell proliferation, metalloproteinases and extracellular matrix were enhanced in the cells silenced by syndecan-1.
At present, it is believed that mifepristone has a strong ability to bind to the progesterone receptor and has obvious antagonistic effects on progesterone in vivo, resulting in a significant reduction in the hormone's effect and shrinking fibroids' volume (Yoshida et al. 2010). The role of syndecan-1 in regulating the expression of extracellular matrix has been found in fibrogenesis pathology (Cui et al. 2020; Manon-Jensen et al. 2010; Regős et al. 2018). Next, it has been shown that mifepristone can inhibit the activity of uterine fibroids and the deposition of their extracellular matrix, which was in line with a previous study (Patel et al. 2016). Surprisingly, syndecan-1 silencing could increase the efficacy of mifepristone, suggesting a synergistic inhibitory effect of syndecan-1 silencing on cell growth with mifepristone. A study demonstrated that targeting syndecan-1 by microRNA repressed epithelial endometriotic cell invasiveness through reducing metalloproteinase activity and interleukin-6 secretion (Ibrahim et al. 2012). Additionally, mifepristone implicated in the regulation of microRNA (Liu et al. 2016). Taken together, microRNA could involve in the synergy effects of syndecan-1 for mifepristone. This study provides a potential therapeutical approach for uterine leiomyoma. Taken together, the study indicated the function role of sydecan-1 in uterine fibroids cell proliferation, ECM production and MMPs, as well as its synergistic role with mifepristone in inhibiting these biological processes. Collectively together, the aforementioned findings lend support to the notion that upregulation of syndecan-1 may be a critical element for uterine leiomyoma in maintaining their viability, and thus it can serve as a cancer specific therapeutic and diagnostic marker.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Barbareschi M, Maisonneuve P, Aldovini D, Cangi MG, Pecciarini L, Angelo Mauri F, Veronese S, Caffo O, Lucenti A, Palma PD, Galligioni E, Doglioni C. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer. 2003;98:474–483. doi: 10.1002/cncr.11515. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo ER, Foster R, Karmon AE, Lee AE, Gatune LW, Rueda BR, Styer AK. MicroRNA 21a–5p overexpression impacts mediators of extracellular matrix formation in uterine leiomyoma. Reprod Biol Endocrinol. 2018;16:46. doi: 10.1186/s12958-018-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Kim JH, Ryu HS, Kim HC, Han JH, Lee JS, Min CK. Syndecan-1, a key regulator of cell viability in endometrial cancer. Int J Cancer. 2007;121:741–750. doi: 10.1002/ijc.22713. [DOI] [PubMed] [Google Scholar]
- Chuang TD, Khorram O. Cross-talk between miR-29c and transforming growth factor-β3 is mediated by an epigenetic mechanism in leiomyoma. Fertil Steril. 2019;112:1180–1189. doi: 10.1016/j.fertnstert.2019.07.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejo JR, Kleeff J, Koliopanos A, Matsuda K, Zhu ZW, Goecke H, Bicheng N, Zimmermann A, Korc M, Friess H, Büchler MW. Syndecan-1 expression is up-regulated in pancreatic but not in other gastrointestinal cancers. Int J Cancer. 2000;88:12–20. doi: 10.1002/1097-0215(20001001)88:1<12::AID-IJC3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Cui J, Jin S, Jin C, Jin Z. Syndecan-1 regulates extracellular matrix expression in keloid fibroblasts via TGF-β1/Smad and MAPK signaling pathways. Life Sci. 2020;254:117326. doi: 10.1016/j.lfs.2020.117326. [DOI] [PubMed] [Google Scholar]
- Davies EJ, Blackhall FH, Shanks JH, David G, McGown AT, Swindell R, Slade RJ, Martin-Hirsch P, Gallagher JT, Jayson GC. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clin Cancer Res. 2004;10:5178–5186. doi: 10.1158/1078-0432.CCR-03-0103. [DOI] [PubMed] [Google Scholar]
- Go K, Ishino T, Nakashimo Y, Miyahara N, Ookubo T, Takeno S, Hirakawa K. Analysis of syndecan-1 and TGF-beta expression in the nasal mucosa and nasal polyps. Auris Nasus Larynx. 2010;37:427–435. doi: 10.1016/j.anl.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Johnston DR, Goldberger O, Park PW. Syndecan-1 expression in epithelial cells is induced by transforming growth factor beta through a PKA-dependent pathway. J Biol Chem. 2006;281:24365–24374. doi: 10.1074/jbc.M509320200. [DOI] [PubMed] [Google Scholar]
- Ibrahim SA, Yip GW, Stock C, Pan JW, Neubauer C, Poeter M, Pupjalis D, Koo CY, Kelsch R, Schule R, Rescher U, Kiesel L, Gotte M. Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer cell motility and invasiveness via a Rho-GTPase- and E-cadherin-dependent mechanism. Int J Cancer. 2012;131:E884–E896. doi: 10.1002/ijc.27629. [DOI] [PubMed] [Google Scholar]
- Joseph DS, Malik M, Nurudeen S, Catherino WH. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril. 2010;93:1500–1508. doi: 10.1016/j.fertnstert.2009.01.081. [DOI] [PubMed] [Google Scholar]
- Kato M, Saunders S, Nguyen H, Bernfield M. Loss of cell surface syndecan-1 causes epithelia to transform into anchorage-independent mesenchyme-like cells. Mol Biol Cell. 1995;6:559–576. doi: 10.1091/mbc.6.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SK, Schroeder JC, Baird DD. New directions in the epidemiology of uterine fibroids. Semin Reprod Med. 2010;28:204–217. doi: 10.1055/s-0030-1251477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TD, Malik M, Britten J, Parikh T, Cox J, Catherino WH. Ulipristal acetate decreases active TGF-β3 and its canonical signaling in uterine leiomyoma via two novel mechanisms. Fertil Steril. 2019;111:806–815. doi: 10.1016/j.fertnstert.2018.12.026. [DOI] [PubMed] [Google Scholar]
- Liakou E, Mavrogonatou E, Pratsinis H, Rizou S, Evangelou K, Panagiotou PN, Karamanos NK, Gorgoulis VG, Kletsas D. Ionizing radiation-mediated premature senescence and paracrine interactions with cancer cells enhance the expression of syndecan 1 in human breast stromal fibroblasts: the role of TGF-β. Aging (Albany NY) 2016;8:1650–1669. doi: 10.18632/aging.100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebersbach BF, Sanderson RD. Expression of syndecan-1 inhibits cell invasion into type I collagen. J Biol Chem. 1994;269:20013–20019. doi: 10.1016/S0021-9258(17)32120-8. [DOI] [PubMed] [Google Scholar]
- Lin PH, Kung HL, Chen HY, Huang KC, Hsia SM. Isoliquiritigenin suppresses E2-induced uterine leiomyoma growth through the modulation of cell death program and the repression of ECM accumulation. Cancers (Basel) 2019;11:1131. doi: 10.3390/cancers11081131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Shi P, Nie Z, Liang H, Zhou Z, Chen W, Chen H, Dong C, Yang R, Liu S, Chen C. Mifepristone suppresses basal triple-negative breast cancer stem cells by down-regulating KLF5 expression. Theranostics. 2016;6:533–544. doi: 10.7150/thno.14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheux R, Guilloteau C, Lemay A, Bastide A, Fazekas AT. Luteinizing hormone-releasing hormone agonist and uterine leiomyoma: a pilot study. Am J Obstet Gynecol. 1985;152:1034–1038. doi: 10.1016/0002-9378(85)90554-X. [DOI] [PubMed] [Google Scholar]
- Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. Febs J. 2010;277:3876–3889. doi: 10.1111/j.1742-4658.2010.07798.x. [DOI] [PubMed] [Google Scholar]
- Patel A, Malik M, Britten J, Cox J, Catherino WH. Mifepristone inhibits extracellular matrix formation in uterine leiomyoma. Fertil Steril. 2016;105:1102–1110. doi: 10.1016/j.fertnstert.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Regős E, Abdelfattah HH, Reszegi A, Szilák L, Werling K, Szabó G, Kiss A, Schaff Z, Kovalszky I, Baghy K. Syndecan-1 inhibits early stages of liver fibrogenesis by interfering with TGFβ1 action and upregulating MMP14. Matrix Biol. 2018;68–69:474–489. doi: 10.1016/j.matbio.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Shen Q, Zou S, Sheng B, Zhao M, Sun LZ, Zhu X. Mifepristone inhibits IGF-1 signaling pathway in the treatment of uterine leiomyomas. Drug Des Dev Ther. 2019;13:3161–3170. doi: 10.2147/DDDT.S212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worapamorn W, Tam SP, Li H, Haase HR, Bartold PM. Cytokine regulation of syndecan-1 and -2 gene expression in human periodontal fibroblasts and osteoblasts. J Periodontal Res. 2002;37:273–278. doi: 10.1034/j.1600-0765.2002.01610.x. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ohara N, Xu Q, Chen W, Wang J, Nakabayashi K, Sasaki H, Morikawa A, Maruo T. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Semin Reprod Med. 2010;28:260–273. doi: 10.1055/s-0030-1251483. [DOI] [PubMed] [Google Scholar]