Abstract
Vitiligo is an autoimmune skin disease in which epidermal melanocytes are targeted for destruction by CD8+ T cells specific for melanocyte/melanoma-shared antigens. IFNγ is the central cytokine driving disease but the role of type I IFN in vitiligo remains unclear. We investigated the functional role of type I IFN during vitiligo progression using two different mouse models; one induced with a vaccinia virus (VV) vaccine and one induced with dendritic cells to prime autoimmune T cells. Induction of vitiligo by VV in IFNaR-deficient mice led to development of severe vitiligo compared to wild type (WT) mice and was characterized by a significantly enhanced effector CD8+ T cell response. Severe vitiligo in this model was a result of VV persistence, because exacerbation of disease in IFNaR-deficient mice was not observed when antigen-pulsed dendritic cells were used to induce vitiligo instead of virus. Treatment of B16F10 melanoma inoculated mice with VV vaccine therapy also induced a significantly enhanced anti-tumor response in IFNaR-deficient mice compared to WT. These results not only help define the pathways responsible for vitiligo progression but also suggest that blockade of type I IFNs following administration of a VV vaccine may provide increased immunogenicity and efficacy for melanoma immunotherapy.
Keywords: autoimmunity, vitiligo, melanoma, type I interferon, CD8 T cells, vaccine immunotherapy, vaccinia virus
Introduction
Vitiligo is a disfiguring skin disease in which autoreactive CD8+ T cells target and destroy melanocytes, the pigment-producing cells of the epidermis, leading to patchy depigmentation(Ogg, Dunbar, Romero, Chen, & Cerundolo, 1998; van den Boorn et al., 2009). Vitiligo affects roughly 1% of the population worldwide and currently there are no FDA approved medical treatments to reverse the disease (Alikhan, Felsten, Daly, & Petronic-Rosic, 2011; Ezzedine et al., 2015; J. E. Harris, 2017; Taïeb & Picardo, 2009). Our lab and others have shown that signaling through type II interferon, IFN-gamma (IFNγ), is critical for disease pathogenesis in human patients and in a mouse model of vitiligo (J. E. Harris, Harris, Weninger, Wherry, Hunter, & Turka, 2012a; Rashighi et al., 2014; Strassner, Rashighi, Ahmed Refat, Richmond, & Harris, 2017; van den Boorn et al., 2009). Previous work has identified epidermal IFNγ signaling and the induction of the chemokine ligands CXCL9 and CXCL10 in promoting migration of anti-melanocyte CXCR3-expressing CD8+ T cells to the epidermal-dermal junction where they mediate elimination of melanocytes (J. E. Harris, Harris, Weninger, Wherry, Hunter, & Turka, 2012a; Rashighi et al., 2014; Richmond, Bangari, et al., 2017a; Richmond, Masterjohn, et al., 2017b).
The role of type I interferon signaling in vitiligo pathogenesis is unclear. Vitiligo patients have an increased incidence of other type I interferon-driven autoimmune diseases, such as systemic lupus erythematous (SLE) and Sjogrens syndrome, and their association with vitiligo suggests an overlap in disease-associated pathways (Alkhateeb, Fain, Thody, Bennett, & Spritz, 2003; Cunliffe, Hall, Newell, & Stevenson, 1968; Gill et al., 2016; J. E. Harris, 2017). In addition, genome wide association studies identified variants in interferon-induced with helicase C domain 1 (IFIH1) and toll like receptor adaptor molecule 1 (TICAM1) as vitiligo susceptibility loci (Jin et al., 2016; Spritz, 2013). Activation of IFIH1 or TICAM1 leads to induction of type I IFNs and expression of interferon-stimulated genes. Finally, both IFNγ and type I IFN receptor signaling can lead to activation of STAT1 homodimers to induce transcription of multiple overlapping gene targets, including CXCL10, due to the sharing of promoter binding elements (Ivashkiv & Donlin, 2014; van Boxel-Dezaire, Rani, & Stark, 2006).
A few reports show a correlation between the presence of type I IFNs and vitiligo development. One study showed a modest increase in IFNB1 mRNA in vitiligo uninvolved skin compared to healthy control skin but there was no difference in gene expression compared to lesional skin (Reimann et al., 2012). This suggests that the expression of type I IFN may be more closely associated with immune tolerance in the skin rather than progressive disease. Another study reported increased numbers of CD123 positive cells in the skin of vitiligo patients and moderately increased levels of CXCL9 and MxA, and concluded that type I IFN was responsible for this induction (Bertolotti et al., 2014; Jacquemin et al., 2017), although these markers can also be associated with IFNγ. Furthermore, multiple case studies have reported the development of vitiligo in Hepatitis C Virus patients following therapy with PEG-IFNα and ribavirin (Anbar, Abdel-Rahman, & Ahmad, 2008; Hamadah, Binamer, Sanai, Abdo, & Alajlan, 2010; Nouri, Busso, & Machler, 1997). However, this rare phenomenon is not only linked to vitiligo but also to the development of other autoimmune diseases such as type I diabetes and SLE (Lodato et al., 2006; Onishi et al., 2010; C. Popescu, Popescu, & Arama, 2013).
It is well established that melanoma, an aggressive skin cancer that arises from mutations in melanocytes, can be targeted by CD8+ T cells that recognize tumor-specific antigens (Hodi et al., 2010; Schwartzentruber et al., 2011). In fact, immunotherapies for melanoma have focused on targeting CD8 T cell function (van Oijen et al., 2004). The association between vitiligo and melanoma has been long recognized after many case studies reported the development of vitiligo following melanoma immunotherapy (BURDICK & HAWK, 1964; J. L. Smith & Stehlin, 1965). This is because there is significant overlap between the antigens expressed by normal melanocytes and melanoma cells, antigens known as melanocyte differentiation antigens (MDA), which include the protein premelanosome (PMEL) (Houghton, 1984). In fact vitiligo is an independent positive prognostic factor for melanoma patients (Nordlund et al., 1983). Melanoma patients that developed vitiligo following treatment with anti-PD-1 (pembrolizumab) or anti-CTLA-4 (ipilimumab) immunotherapies have increased overall survival compared to patients that did not develop vitiligo (Hodi et al., 2010; Hua et al., 2016). Additional studies in mice reveal that the development of vitiligo increases the formation of memory CD8+ T cells, and that these memory cells provide protection against melanoma tumor re-challenge (Malik et al., 2017; Park et al., 2019).
Vaccinia virus have been used as a recombinant vaccine vector because of its large genome, which allows for insertion and high level expression of foreign genes, and because it replicates in the cytoplasm and poses no risk of integration of inserted DNA into the host genome (Jacobs et al., 2009). Vaccinia virus has been used for the treatment of melanoma either by direct injection into the tumor, or through vaccination of recombinant vaccinia virus expressing tumor associated antigens (Drexler et al., 1999; Oertli et al., 2002; Roenigk, Deodhar, St Jacques, & Burdick, 1974). Expression of MDAs increased specific anti-tumor CD8+ T cell frequencies and improved clinical responses. Other vaccination strategies infected dendritic cells with recombinant VACV, which revealed induction of strong anti-tumor CD8+ T cell responses (Di Nicola et al., 2003; Prabakaran et al., 2002; Yang et al., 2000). Although, vaccinia virus vaccines are safe and stimulate anti-tumor effects, understanding the signals driving VACV-induced anti-tumor responses will lead to the development of strategies that enhance the immunogenicity and durability of VACV therapies.
Here, we sought to determine whether there is a functional role of type I IFN in vitiligo. In this study, we used two different mouse models of human disease. The first model is dependent on activation of autoimmune T cells using a vaccinia virus vaccine. The second model is a novel mouse model that is dependent on adoptive transfer of bone marrow-derived dendritic cells to prime autoimmune T cells. We find that induction of vitiligo using vaccinia virus vaccine resulted in severe disease in IFNaR-deficient hosts because of vaccinia virus persistence and the subsequent hyperactivation of autoimmune effector CD8+ T cells. Consistent with this observation, we determined that vaccination with vaccinia virus improved anti-tumor immunity against melanoma in IFNaR-deficient hosts compared to treatment in WT hosts. We show functional evidence that type I IFN is not required during the effector phase of the vitiligo response, since induction of vitiligo with antigen-pulsed dendritic cells resulted in similar disease development in both WT and IFNaR-deficient hosts. Our results further define the pathways responsible for vitiligo progression and will inform new therapies. These studies highlight the role of type I IFN in viral vaccine-induced adaptive immune responses and suggest targeting IFNaR to benefit viral vaccine efficacy during melanoma immunotherapy.
Materials & Methods
Mice.
All mice were maintained in pathogen-free facilities at UMMS, and procedures were approved by the UMMS Institutional Animal Care and Use Committee and in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The following mouse strains were used for these studies and are available from The Jackson Laboratory: PMEL TCR transgenic mice (stock no. 005023), KRT14-Kitl*4XTG2Bjl (Krt14-Kitl*) mice (stock no. 009687), IFNgR1-deficient mice (stock no. 003288), and IFNAR1-deficient mice (provided by A. Rothstein, now available as stock no. 032045, MMRRC). All mice used for vitiligo studies were on a C57BL/6J background, or a mixed 129-B6 background that had been backcrossed for at least 10 generations. Age and sex-matched mice were used, and both male and female mice of all strains were tested to avoid gender bias.
Vitiligo induction using vaccinia virus.
Vitiligo was induced as previously described (Harris et al., 2012). Briefly, PMEL CD8+ T cells were isolated from the spleens of PMEL TCR transgenic mice through negative selection on microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Purified CD8+ T cells (1×106) were injected intravenously into sublethally irradiated (500 rads 1 day before transfer) Krt14-Kitl* hosts. Recipient mice also received i.p. injection of 1×106 pfu rVV-hPMEL (N Restifo, NCI, NIH) on the same day of transfer. Scoring of vitiligo progression in mice was done by a blinded investigator, using a point scale based on the estimated depigmentation on the ears, nose, tail, and footpads. Replicate experiments were performed at least twice.
Flow Cytometry.
Tissues were harvested at the indicated times and processed as previously described(Riding, Richmond, & Harris, 2018). Skin was incubated for 1 hour at 37°C in 5U/mL of Dispase II (Roche, Basel, Switzerland) for tail or 50U/mL Dispase II for ears or footpad skin. Epidermal skin was removed and mechanically dissociated into a single cell suspension for staining. Dermis was incubated in 1mg/mL collagenase IV and 2 mg/mL DNAse I (Sigma Aldrich, St. Louis, MO) for 1 hour at 37°C before mechanical dissociation. All murine flow cytometry samples were blocked with 2.4G2 following staining with antibodies.
Bone marrow chimeras.
Bone marrow was isolated from femurs and tibias of donor mice. Recipient mice were lethally irradiated (800 rads at least 5h before transplant) and administered 5×106 bone marrow cells intravenously. Mice were left untreated for 8 weeks for reconstitution of bone marrow cells prior to use in the vitiligo model. Chimerism was verified by performing PCRs or flow cytometry.
Generation of BMDCs for peptide-pulsed DC vaccine.
Bone marrow-derived dendritic cells (BMDCs) were generated according to a modified version of a previously described method(Helft et al., 2015; Lutz et al., 1999). Briefly, bone marrow cells isolated from the femurs and tibia of 7–14-week-old WT mice were filtered through a 70-μm nylon strainer, red blood cells lysed by ACK lysis buffer (Sigma Aldrich), and cultured in BMDC medium (RPMI-1640 containing 10% FBS, 100 U/mL PS, 2 mM l-glutamine (Gibco), 50 μM 2-mercaptoethanol (Sigma Aldrich), 20 ng/mL GM-CSF (PeproTech), and 10 ng/mL IL-4 (PeproTech)). The BMDC medium was replaced on days 3 and 6. On day 8, non-adherent cells were harvested and BMDC purity was assessed by flow cytometry to ensure staining for markers CD11c, MHC II, CD11b, and CD86. For DC vaccination, non-adherent cells were pulsed for 3 h at 37°C with 10 μM of the human gp10025–33 (hgp100) peptide (GenScript) in Opti-MEM media (Gibco) and washed three times with PBS before their use. For induction of vitiligo in mice, 1.0×106 CD11c+MHC II+ hgp100 pulsed BMDCs were co-injected intravenously into WT, IFNaR-deficient or IFNGR-deficient hosts.
Plaque Assay.
The day before infection, CV-1 cells were seeded in a 6 well plate at 1 million cells/well in 2 mLs DMEM + 10% fetal bovine serum (FBS) and incubated at 37°C overnight. The following day, serial dilutions of virus infected tissue supernatants was performed. Culture medium was removed from CV-1 cells and 600 ul-1mL of virus infected tissue supernatant was added to each well and incubated at 37°C for 1 hour. After incubation, 2 mLs of a heated agarose solution containing 0.6% agarose and 2X MEM+20% FBS mixed at 1:1 was added to each well. Plates were incubated at 37°C for 72 hours. Cells were fixed by adding 1.5 mLs of 10% formaldehyde. Following fixation, excess fixative and agar was removed carefully to avoid scraping the plate underneath. Cells were then stained with 200 ul of 2% Crystal violet diluted in water.
Tumor Model.
B16F10 melanoma cells (1.0×105) were resuspended in 100 μL of PBS and implanted subcutaneously into the right flank of 6–12-week-old WT and IFNaR deficient mice. Tumor size was measured in two dimensions by caliper and is expressed as the product of two perpendicular diameters. PMELs were isolated from the spleens of PMEL mice through negative selection on microbeads (Miltenyi Biotec) according to the manufacturer’s instructions. After 6–8 days of tumor injection, purified PMELs (1.0 ×106) were transferred by intravenous injection into sublethally irradiated (500 rad, day −1) hosts. On the same day mice were infected with 106 pfu of rVV-hPMEL to activate PMEL in vivo.
Statistical Analyses.
Statistical analyses were performed using GraphPad Prism software (GraphPad, La Jolla, CA). Dual comparisons were made with unpaired Student’s t test. Groups of three or more were analyzed by analysis of variance with Tukey or Dunnett posttests. P values less than 0.05 were considered significant.
Results
IFNaR-deficient hosts develop accelerated and severe viral-induced vitiligo compared to WT hosts
To directly compare the relative contribution of type I and type II interferon signaling in vitiligo pathogenesis, we induced vitiligo in WT, IFNaR-deficient, and IFNGR-deficient hosts as previously described (J. E. Harris, Harris, Weninger, Wherry, Hunter, & Turka, 2012a). In brief, vitiligo was induced by adoptive cell transfer of naïve CD8+ T cells specific for the melanocyte protein premelanosome (PMEL) and these cells were activated by same day infection with recombinant vaccinia virus (VV) expressing their cognate antigen. Effector PMEL CD8+ T cells traffic to the skin to target melanocytes and hosts develop epidermal depigmentation on the ears, nose, footpads, and tail between 5–7 weeks after PMEL transfer (J. E. Harris, Harris, Weninger, Wherry, Hunter, & Turka, 2012b; Riding et al., 2018).
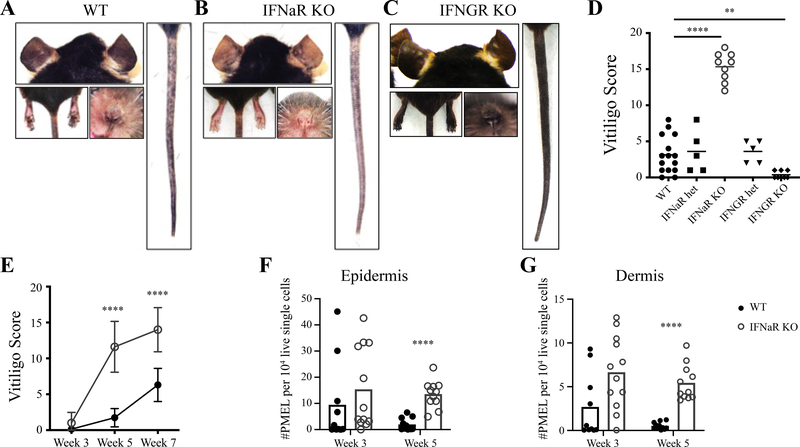
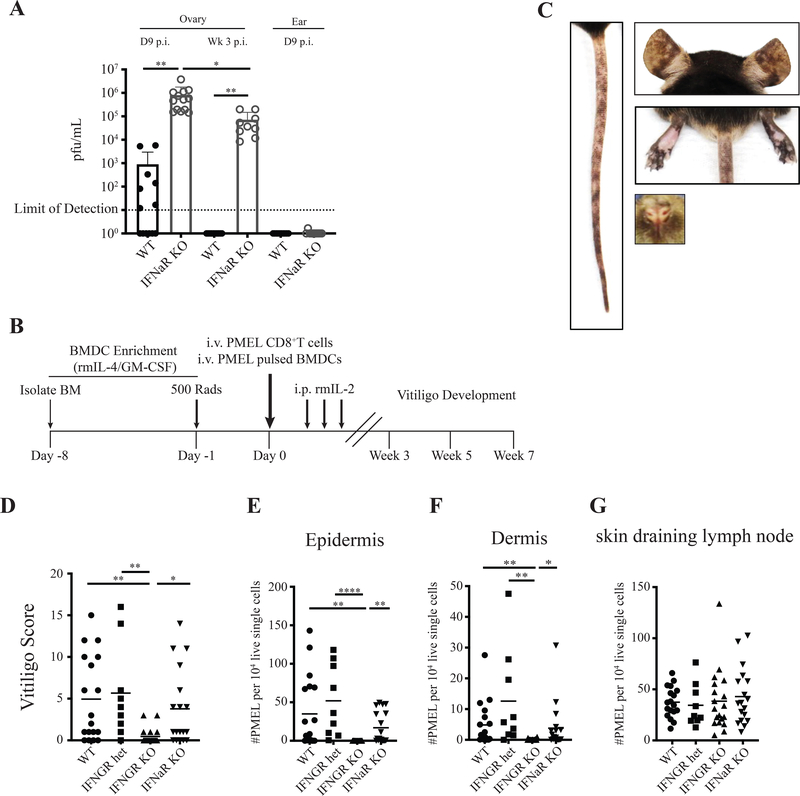
Surprisingly, induction of vitiligo in IFNaR-deficient hosts led to enhanced disease compared to wildtype hosts and resulted in complete depigmentation at some skin sites (Figure 1A–D). This was in stark contrast to IFNGR-deficient hosts, which were protected from disease (Figure 1C–D) and had significantly reduced PMEL numbers in the epidermis and dermis (Supplementary Figure 1A–C), consistent with our previous studies that targeted IFNγ using an antibody (J. E. Harris, Harris, Weninger, Wherry, Hunter, & Turka, 2012a). In IFNaR-deficient hosts, depigmentation was accelerated, with severe disease visible by 5 weeks post vitiligo induction (Figure 1E). We next asked whether IFNaR signaling affects PMEL trafficking into the skin. PMEL number was slightly increased in the skin at week 3, but significantly increased at week 5 post vitiligo induction in IFNaR-deficient mice (Figure 1F–G). The altered kinetics of PMEL infiltration into the skin of IFNaR-deficient mice correlate with the acceleration of vitiligo. These results suggest that in contrast to IFNGR signaling, IFNaR signaling is not required during the effector phase of the CD8+ T cell response to vitiligo.
Figure 1: Type I interferon receptor-deficient mice develop accelerated and severe vitiligo.
Representative images of mouse ears, nose, footpads, and tail 7 weeks post vitiligo induction for A) WT, B) IFNaR KO, and C) IFNGR KO hosts. D) Vitiligo score at 7 weeks post vitiligo induction. E) Vitiligo scores of WT (black) and IFNaR KO (grey) mice at weeks 3, 5 and 7 pooled from 2 to 3 separate experiments; mean ± standard deviation of the mean. Normalized number of PMEL in the footpad epidermis (F) and dermis (G) of WT and IFNaR KO mice at weeks 3 and 5 post vitiligo induction.
IFNaR signaling on host radioresistant cells is important for controlling disease development
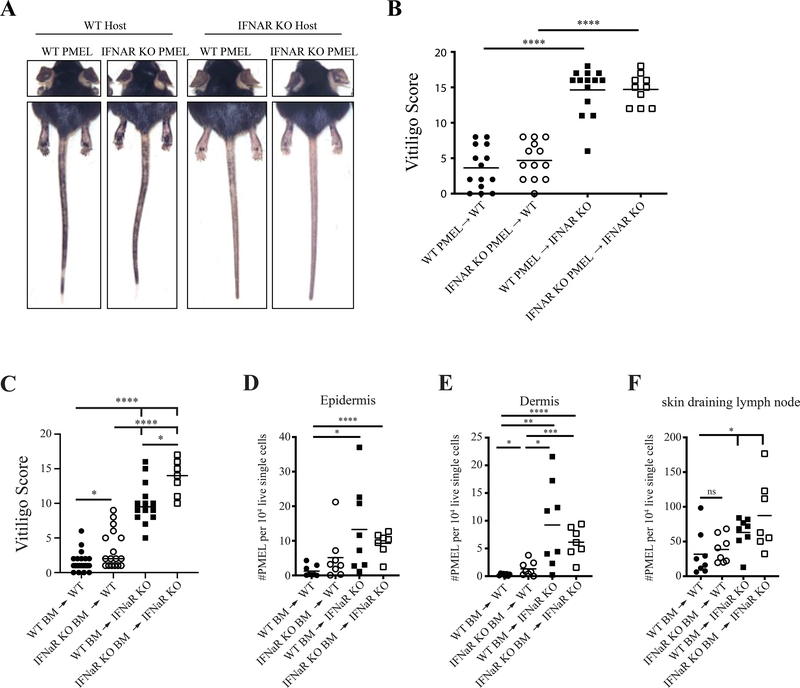
In wild type and IFNaR-deficient vitiligo hosts, transferred wild type PMELs can respond to type I IFNs. Since type I IFN signaling can directly enhance the proliferation and effector capabilities of CD8+ T cells (Kolumam, Thomas, Thompson, Sprent, & Murali-Krishna, 2005; Le Bon et al., 2006), we asked whether type I IFNs acted directly on PMELs to mediate severe disease. To test this, we transferred wild-type or IFNaR-deficient PMELs into wild type and IFNaR-deficient hosts. Whether the T cells were IFNaR-deficient or IFNaR-sufficient had no impact on the course of the disease (Figure 2A–B). However, IFNaR expression on host cells played a critical role as both WT and IFNaR-deficient T cells into IFNaR-deficient hosts developed significantly more severe vitiligo than the corresponding wild type hosts (Figure 2 A–B). These results suggest that type I IFN signaling directly on autoreactive PMEL does not modulate disease severity.
Figure 2: IFNaR signaling on host radioresistant cells is important for controlling disease development.
A) Representative mouse images at 7 weeks post vitiligo induction. B) Vitiligo scores at week 7 post vitiligo induction. C) Vitiligo scores of chimeric mice 7 weeks post vitiligo induction. Normalized PMEL numbers in the epidermis (D), dermis (E), and draining lymph node (F) of chimeric mice at 5 weeks post vitiligo induction.
To determine whether IFNaR deficiency on host hematopoietic or radioresistant cells was required for enhanced vitiligo, we generated bone marrow chimeric mice by reconstituting lethally irradiated WT or IFNaR-deficient mice with WT or IFNaR-deficient BM stem cells. Chimeric mice that lacked IFNaR on radioresistant cells (IFNaRRC) developed significantly more severe disease compared to wild type recipients (Figure 2C). IFNaR-deficiency in the hematopoietic cells had more modest effects (Figure 2C). There was increased PMEL trafficking to the epidermis and dermis of IFNaRRC mice at 3 weeks post vitiligo induction (Figure 2D–F). Thus, IFNaR deficiency on radioresistant cells and, to a lesser extent, hematopoietic cells is responsible for the accelerated migration of autoimmune CD8+ T cells in hosts with vitiligo.
IFNaR-deficient mice have increased circulating CD44+ effector memory PMEL with enhanced effector function
In addition to increased migration of PMEL into the skin of IFNaR-deficient mice, we observed significantly increased numbers of circulating PMEL in the skin draining lymph nodes and spleen of IFNaR-deficient hosts as early as 3 weeks after vitiligo induction (Supplementary Figure 2A–B), and the number of T cells remained significantly increased relative to wild type mice for up to 7 weeks. Phenotypic analyses of circulating PMEL revealed a significant increase in effector memory, CD44+CD62L− PMEL in IFNaR-deficient host mice compared to WT (Supplementary Figure 2C–D). Since recirculating memory PMEL are important in mediating melanocyte death in vitiligo mice (Richmond et al., 2018), these results suggest that increased numbers of recirculating memory PMEL are likely to contribute to disease severity.
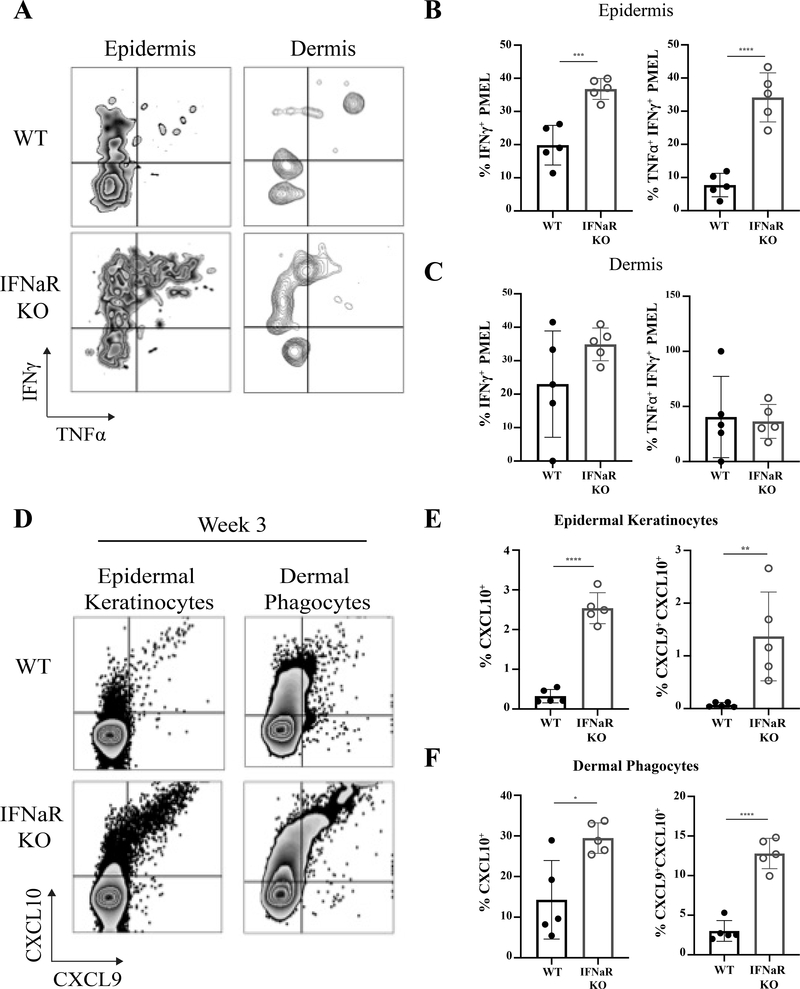
We next asked whether PMEL in IFNaR-deficient hosts exhibited increased effector potential. Vitiligo was induced in mice and then tissue cell suspensions were stimulated with anti-CD3/CD28 overnight. Epidermal PMEL in IFNaR-deficient hosts produced significantly more TNFα and IFNγ compared to WT controls (Figure 3A–C). Importantly, poly functional TNFα+ IFNγ+ PMEL were significantly increased in the epidermis of IFNaR-deficient host mice (Figure 3B). Thus, development of severe vitiligo in IFNaR-deficient hosts likely results from increased PMEL IFNγ production.
Figure 3: Increased PMEL function in IFNaR-deficient hosts leads to enhanced CXCL9 and CXCL10 chemokine ligand expression.
A) Representative flow plots of IFNy and TNFa production by PMEL following ex vivo stimulation with 5 ug/mL anti-CD3 and 2 ug/mL anti-CD28 in the epidermis and dermis of WT and IFNaR KO mice. Percentage of IFNy+, and IFNy+ TNFa+ PMEL in the epidermis (B) and dermis (C). D) CXCL9 and CXCL10 reporter expression by epidermal keratinocytes and dermal MHC II+ phagocytes in the ear skin of WT and IFNaR KO mice at week 3 post vitiligo induction. Percent CXCL10 single positive and CXCL9+CXCL10+ double positive keratinocytes (E) and dermal phagocytes (F) in WT and IFNaR KO mice.
Increased expression of the CXCR3 ligands CXCL9 and CXCL10 in IFNaR-deficient hosts
The IFN-induced chemokine ligands CXCL9 and CXCL10 promote migration of CXCR3+ PMEL T cells into the skin to destroy melanocytes during vitiligo (Rashighi et al., 2014; Richmond, Masterjohn, et al., 2017b). Because we observed increased IFNγ production by PMEL in IFNaR-deficient mice, we monitored CXCL9 and CXCL10 expression levels in the skin over the course of disease using reporter REX3 mice (Groom et al., 2012). We previously described that chemokine production by epidermal keratinocytes (Richmond, Bangari, et al., 2017a) is important for disease progression, and we found that epidermal keratinocyte expression of CXCL9 and CXCL10 was significantly higher in IFNaR-deficient mice compared to WT mice (Figure 3 D–F), as well as production by dermal phagocytes. These results suggest that increased IFNγ production by PMEL in IFNaR-deficient mice amplifies expression of the chemokines CXCL9 and CXCL10, thereby enhancing the migration of effector PMEL into the skin.
Expression of the negative immune regulators PD-1 and PD-L1 are reduced in IFNaR-deficient hosts
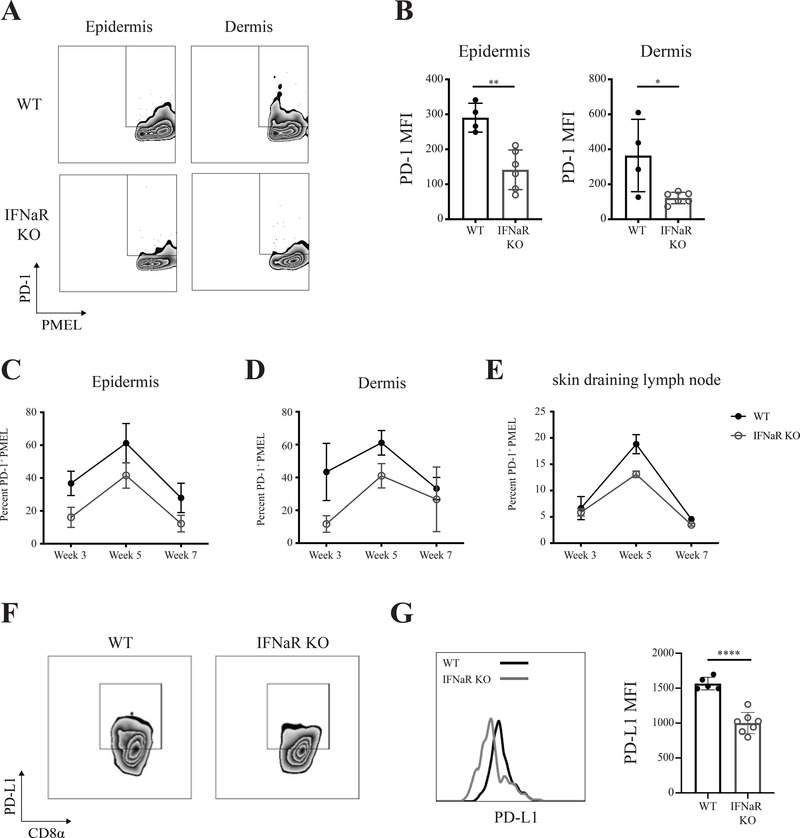
Since type I IFNs induce expression of negative immune regulators such as programmed cell death-1 and programmed cell death ligand 1 (PD-L1), which act to suppress T cell immune responses (L. Chen et al., 2007; Schreiner et al., 2004; Teijaro et al., 2013), we sought to determine whether inhibitory molecule expression was altered in IFNaR-deficient mice. Both the percentage of PD-1 expressing PMEL and the mean fluorescent intensity (MFI) of PD-1 was significantly reduced on PMELs in the epidermis and dermis of IFNaR-deficient hosts compared to wild type hosts (Figure 4A–D). However, there was no difference in PD-1 expression on PMEL in the lymph nodes or spleen (Figure 4E). In addition, IFNaRRC chimeras revealed significantly reduced PD-1 expression on PMEL (Supplementary Figure 3A–C). Examination of PD-L1 on cross priming CD8⍺ dendritic cells revealed a significant reduction in IFNaR-deficient hosts compared to wild type (Figure 4F–G). These results suggest that enhanced vitiligo in IFNaR-deficient mice may result from dysregulated inhibitory molecule expression on both T cells and antigen presenting cells.
Figure 4: Reduced expression of PD-1 and PD-L1 in IFNaR-deficient hosts.
A) Representative flow plots of epidermal and dermal PD-1 expression on PMEL in WT and IFNaR KO host mice. B) PD-1 MFI on PMEL in the skin epidermis and dermis. Each data point represents an individual animal; n = 5 with 2–3 replicate experiments performed. Percentage of PD-1 on epidermal (C) dermal (D) and draining lymph node (E) PMEL at weeks 3, 5, and 7 post vitiligo induction pooled from 2 to 3 separate experiments; mean ± standard deviation of the mean. F) PD-L1 expression by CD8a dendritic cells in the spleen of WT and IFNaR KO mice at week 3 post vitiligo induction. G) Representative histogram and quantification of PD-L1 expression as determined by flow cytometry on WT and IFNaR KO CD8a DCs at week 3 post vitiligo induction. Each data point represents an individual animal with 2–3 replicate experiments performed.
Severe disease in IFNaR-deficient hosts requires activation of PMEL CD8+ T cells by exposure to vaccinia virus
Early studies show that both type I IFN and IFNγ are required for defense against vaccinia virus as mice deficient for either IFNaR or IFNGR showed increased susceptibility to infection compared to wild type mice(Müller et al., 1994). Although our mouse model of vitiligo is induced with a highly attenuated vaccinia virus strain and we inject virus-specific CD8+ T cells (PMELs) into mice that have the capability to clear infected cells, we asked whether IFNaR-deficient mice had reduced viral clearance. IFNaR-deficient host mice had significantly increased viral loads in the ovary at 9 days and 3 weeks post-infection compared to wild type hosts (Figure 5A), however, no virus was detected in the skin of wild type or IFNaR-deficient mice (Figure 5A). These results suggest that increased viral replication in IFNaR-deficient hosts may be responsible for increasing the activation of PMELs and thus the development of severe autoimmune vitiligo.
Figure 5: Severe vitiligo in IFNaR-deficient hosts is dependent on vaccinia virus persistence.
A) Vaccinia virus PFU in WT and IFNaR KO mouse ovaries and ear skin determined by plaque assay at day 9 and week 3 post infection. Each data point represents an individual animal, pooled from 2 separate experiments. B) Schematic of the experimental time line for induction of vitiligo in mice using peptide-pulsed bone marrow dendritic cells (BMDCs). C) Representative mouse images showing spots of depigmentation of the ears, nose, footpads, and tail of WT mice 7 weeks post vitiligo induction. D) Vitiligo mouse scores 7 weeks post vitiligo induction. Normalized PMEL numbers in the epidermis (E), dermis (F), and draining lymph node (G) 7 weeks post vitiligo induction.
To determine whether IFNaR-mediated restraint of the autoimmune T cell response was a result of increased vaccinia virus replication, we developed a novel mouse model of vitiligo that is independent of induction with a vaccinia virus vaccine. In this model, vitiligo is induced by sublethal irradiation of Krt14-Kitl* host mice the day before adoptive T cell transfer. The following day, bone marrow derived dendritic cells (BMDCs) pulsed with PMEL peptide are co-transferred with PMEL CD8+ T cells into hosts (Figure 5B). Hosts are injected with rmIL-2 for the following 3 days to support PMEL CD8+ T cell engraftment (Figure 5B). Following induction of disease, hosts develop epidermal depigmentation on the nose, ears, footpads and tail, similar to vitiligo induced with a vaccinia viral vector (Figure 5C).
To determine whether IFNγ signaling is also required for disease pathogenesis in BMDC-induced vitiligo, we used IFNGR-deficient mice as hosts. Vitiligo induced in IFNGR-deficient hosts with BMDC-PMEL showed protection from disease and a significant reduction of PMEL in the epidermis and dermis (Figure 5D–G). These results reveal IFNγ as the shared pathogenic pathway in both BMDC-induced and vaccinia virus-induced vitiligo and confirm its importance in driving vitiligo progression.
To determine the role of type I IFN in vitiligo using a mouse model independent of the vaccinia virus vaccine, we induced vitiligo using BMDC-PMEL in IFNaR-deficient and wild type hosts. Disease severity was comparable between the two groups, revealing that type I IFN is not required for vitiligo induction or progression (Figure 5D). These results demonstrate that the extent of disease activity exhibited by CD8+ PMEL effector cells in the vaccinia virus-induced model was directly linked to the viral induction phase of the disease. Delayed clearance of vaccinia virus in IFNaR-deficient mice extends the priming phase of the disease, possibly resulting in greater activation of the effector T cells through a variety of mechanisms. These studies further show that timing and balance of type I and type II interferons during anti-viral immune responses is critical to prevent pathogenic immune responses to self.
Vaccinia virus vaccine therapy in IFNaR-deficient mice significantly enhances the anti-tumor response to melanoma
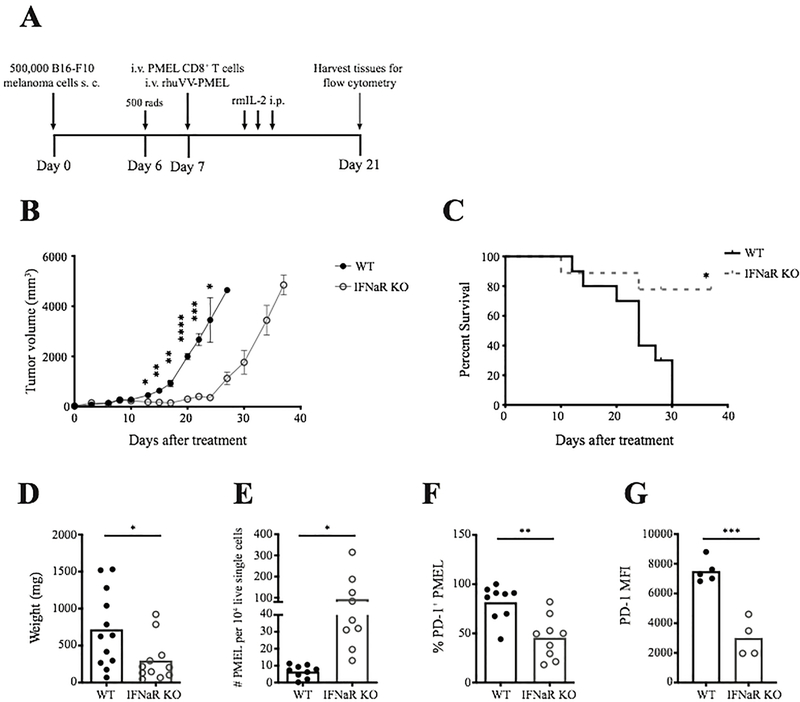
Our results reveal that type I IFN limits viral persistence and vaccinia virus vaccine delivery in IFNaR-deficient hosts enhances the activation of anti-melanocyte T cells. Since vaccinia vectors have also been used to prime T cells for tumor vaccination immunotherapies (Li et al., 2011; Oertli et al., 2002; Schütz et al., 2001; Zajac et al., 2003), we decided to ask whether a similar loss of type I IFN would improve tumor control in a model of adoptive T cell therapy enhanced by vaccinia virus vaccine (Figure 6A). B16-F10 melanomas were injected into WT or IFNaR-deficient mice. Six to eight days after melanoma injection, mice were infected with recombinant vaccinia virus expressing gp100 in combination with adoptive transfer of PMEL CD8+ T cells. IFNaR-deficient mice showed improved tumor control compared to wild type hosts and had significantly increased overall survival (Figure 6B–C). Complete regression of tumors was not observed in IFNaR-deficient hosts and approximately 25 days post infection, IFNaR-deficient hosts relapsed. We hypothesize that tumor outgrowth occurs following vaccinia virus clearance. Significant reduction in tumor weight in IFNaR-deficient host mice correlated with an increase in tumor infiltrating PMEL CD8+ T cells (Figure 6D–E) and a significant reduction in the expression of the immune checkpoint inhibitor PD-1 on PMEL T cells (Figure 6F–G). These results reveal that type I IFN production following vaccinia virus vaccination restricts anti-melanocyte CD8+ T cell response in both autoimmunity and melanoma immunotherapy.
Figure 6: Vaccinia virus vaccine therapy in IFNaR-deficient mice significantly enhances the anti-tumor response to melanoma.
A) Schematic of the experimental design for treatment of B16-F10 melanomas in WT and IFNaR KO hosts. B) Tumor volume post treatment in WT and IFNaR KO mice. Data shown is one representative experiment, repeated 3 times. C) Overall survival of WT (n=10) and IFNaR KO (n=9) hosts following treatment pooled from 2 experiments. D) Tumor weight 14 days after adoptive T cell and vaccinia virus vaccine treatment. E) Normalized tumor infiltrating PMEL numbers 14 days post treatment in WT and IFNaR KO host mice. F) Percentage of PD-1 positive PMEL in WT and IFNaR KO host tumors. G) PD-1 MFI on PMEL in WT and IFNaR KO host tumors. Each data point represents an individual animal pooled from 2–3 experiments.
Discussion
Here we describe the role of type I IFN in vitiligo using two different mouse models of disease. We report that in contrast to IFNγ, type I IFN signaling is not required for the effector phase of the T cell response during vitiligo development in mice. These results are important to inform future therapies for vitiligo patients. Our results further reveal that administration of a vaccinia virus vaccine in IFNaR-deficient mice led to enhanced priming of CD8+ T cells and development of more severe vitiligo compared to IFNaR-sufficient hosts. Type I IFNs are critical for controlling virus infections, and we report that type I IFN signaling is necessary to control the replication of even an attenuated vaccinia virus strain.
Type I IFNs can contribute to impaired host adaptive immune responses during chronic infections (Cheng et al., 2017; Teijaro et al., 2013). Blockade of type I IFN during chronic LCMV and HIV reactivates the anti-viral CD8+ T cell response leading to clearance of the virus through reduction of PD-1 on T cells and PD-L1 on dendritic cells (Cheng et al., 2017; Teijaro et al., 2013). Similar to these observations, we show that type I IFN signaling on radioresistant cells during vaccinia virus persistence limits T cell expansion and activation. Increased activation of T cells in IFNaR-deficient hosts following vaccinia virus vaccine may result from prolonged antigen exposure, because of persistent antigen presentation. Persistent infection may also impact the regulatory activity of dendritic cells, as we observed reduced PD-L1 on the CD8α dendritic cells responsible for T cell priming.
Previous clinical studies report that injection of type I IFN into the skin has induced local, and sometimes distant depigmentation consistent with vitiligo(Anbar et al., 2008; Hamadah et al., 2010; Nouri et al., 1997). These reports demonstrate that overexposure of the skin to increased doses of type I IFN are capable of promoting depigmentation, although this does not necessarily indicate that type I IFN plays a role in normal vitiligo pathogenesis and we show functional evidence that type I IFN is not required for the autoimmune CD8+ T cell effector response. Thus, the reported development of autoimmunity following IFN treatment may be dependent on chronic infection or an individuals’ predisposition to autoimmunity. Administration of type I IFNs in the setting of chronic infection may lead to the activation of cross-reactive T cells that cause autoimmune pathology, or alternatively that type I IFN-induced inflammation in the right context can recruit autoreactive T cells into the skin, similar to koebner phenomenon(van Geel et al., 2012).
Since we intentionally activate transferred PMEL CD8+ T cells either using vaccinia virus that expresses their cognate antigen or peptide pulsed DCs, our model may bypass the role of innate immune activation and type I IFN production in the development of vitiligo. Activation downstream of innate immune pattern recognition receptors in DCs and production of type I IFN results in maturation of DCs (Cella et al., 1999). This includes DC migration into the lymph nodes, upregulation of MHC molecules, costimulatory molecules, and production of cytokines that all impact the presentation of antigens and the priming of CD8+ T cells (Banchereau et al., 2000). Thus, type I IFN production may play a role during the initiation of disease or during the development of autoimmunity through the priming of endogenous autoreactive CD8+ T cells, but more studies are required to answer this question.
The generation of a robust cytotoxic T lymphocyte (CTL) response against melanoma is critical for the success of immunotherapy. Similar to the immune-suppressive effects of type I IFN during chronic viral infections, prolonged type I IFNs can supress tumor immunity and induce resistance to treatment (Medrano, Hunger, Mendonça, Barbuto, & Strauss, 2017; Snell, McGaha, & Brooks, 2017). After observing more severe vitiligo in IFNaR-deficient hosts following administration of a vaccinia virus vaccine, we sought to determine whether stimulation of the anti-tumor response to melanoma could be enhanced in IFNaR-deficient mice. Our results demonstrate that in the absence of type I IFN signaling, adoptive CD8+ T cell therapy in combination with a vaccinia virus vaccine enhances the anti-melanoma response.
Immune related adverse side effects (irAEs) or autoimmune toxicity occur in some patients following immunotherapy administration as seen with anti-PD-1 and anti-CTLA4 treatment (Okada et al., 2019; Rogado et al., 2019). Autoimmune toxicity can occur as a consequence of shared antigen expression between the tumor and normal tissue. This has been observed in patients with melanoma where those responding develop autoimmune vitiligo (Hua et al., 2016; Nordlund et al., 1983). Autoimmune toxicity or irAEs may also develop as a result of activation of T cells present in organs with pre-existing inflammation or as a result of inflammatory cytokine production that activates bystander cross-reactive T cells (Das & Johnson, 2019). Our model system requires adoptive transfer of T cells specific for the antigen PMEL, combined with a vaccinia virus vaccine that expresses PMEL to specifically activate these cells, thus toxicity against normal tissue expressing PMEL is possible. We did not observe any irEAs in IFNaR-deficient host mice following treatment with extended observation. However, administration of vaccinia virus vaccine in immunocompromised individuals in combination with IFNaR blockade may pose a risk of dissemination of the virus.
A major limitation to the use of viral vaccines in cancer immunotherapy has been their lack of immunogenicity (C. L. Smith et al., 2005). Our results reveal that absence of type I IFNs during vaccinia virus administration can improve the immunogenicity of the vaccine, likely resulting from increased CD8+ T cell priming during vector persistence. Studies using oncolytic viruses for cancer immunotherapy have also reported benefits of blocking type I IFNs to increase infection and improve responses to therapy (Budhwani, Mazzieri, & Dolcetti, 2018). A recent study reported increased tumor regression after IFNaR blockade during oncolytic virus therapy in combination with adoptive T cell therapy in mice (Walsh et al., 2019). Another report revealed that type I IFN following oncolytic virus infection reduced the efficacy of a combination therapy with CAR T cells, highlighting an important observation that type I IFN production following virus infection can suppress the anti-tumor response(Evgin et al., 2020). Our findings suggest that blockade of IFNaR during vaccinia virus vaccine for cancer immunotherapy may enhance both immunogenicity and persistence of the vector, thus improving vaccine efficacy, and may induce long-term benefits for tumor eradication.
Supplementary Material
Supplementary Figure 3: PD-1 expression by PMEL is significantly reduced in hosts lacking IFNaR on radioresistant cells. A) Representative flow cytometry plots of PD-1 expression on epidermal PMEL in chimeric mice. B) Percentage of PMEL expressing PD-1 in the epidermis and dermis of chimeric mice 5 weeks post vitiligo induction. C) PD-1 MFI on PMEL in the epidermis and dermis of chimeric mice 5 weeks post vitiligo induction.
Supplementary Figure 2: IFNaR-deficient mice have increased circulating CD44+ effector memory PMEL. Normalized PMEL numbers in the skin draining lymph nodes (A) and spleen (B) at weeks 3, 5, and 7 post vitiligo induction. C) Representative flow cytometry plots pre-gated on PMEL showing CD44 and CD62L marker expression. D) Percent of CD44+CD62L− PMEL in the spleen of WT and IFNaR KO mice at weeks 3, 5, and 7 post vitiligo induction.
Supplementary Figure 1: Significantly reduced PMEL numbers in the tail skin of IFNGR KO mice at 7 weeks post vitiligo induction. A) Epidermal (B) Dermal, and (C) inguinal lymph node PMEL numbers normalized to 10,000 live single cells.
Significance.
Vitiligo is an autoimmune skin disease mediated by CD8+ T cells specific for shared melanocyte/melanoma antigens. Vitiligo is an independent positive prognostic factor for melanoma patients following immunotherapy. We report that administration of a vaccinia virus vaccine in the absence of type I IFN signaling leads to viral persistence and robust activation of both autoimmune/anti-tumor CD8+ T cells. Vaccinia virus vaccine delivered to IFNaR-deficient mice resulted in more severe vitiligo and an enhanced anti-tumor response to melanoma, reflected by improved tumor regression. Our results indicate that type I IFN blockade will enhance the potency of vector-vaccines in cancer immunotherapy.
Acknowledgements:
We thank B.J. Longley for Krt14-Kitl* mice, U. von Andrian for DPE GFP mice, N. Restifo for recombinant vaccinia virus, and A. Rothstein for insightful comments on the manuscript. We thank members of the Harris Lab including J. Strassner, L. Zapata, L. Lajoie, E. Kim, M. Garg and M. Rashighi for technical assistance. This study was supported by a Research Grant and Calder Research Scholar Award from the American Skin Association (to JMR); the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number AR069114 (to JEH); and research grants from the Kawaja Vitiligo Research Initiative, Vitiligo Research Foundation, and Dermatology Foundation Stiefel Scholar Award (to JEH); and Training Grant AI007349 (to RLR). Flow cytometry equipment used for this study is maintained by the UMMS Flow Cytometry Core Facility.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- Alikhan A, Felsten LM, Daly M, & Petronic-Rosic V (2011). Vitiligo: A comprehensive overview. Journal of American Dermatology, 65(3), 473–491. 10.1016/j.jaad.2010.11.061 [DOI] [PubMed] [Google Scholar]
- Alkhateeb A, Fain PR, Thody A, Bennett DC, & Spritz RA (2003). Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Research, 16(3), 208–214. [DOI] [PubMed] [Google Scholar]
- Anbar TS, Abdel-Rahman AT, & Ahmad HM (2008). Vitiligo occurring at site of interferon-α 2b injection in a patient with chronic viral hepatitis C: a case report. Clinical and Experimental Dermatology, 33(4), 503–503. 10.1111/j.1365-2230.2008.02719.x [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. (2000). Immunobiology of dendritic cells. Annual Review of Immunology, 18(1), 767–811. 10.1146/annurev.immunol.18.1.767 [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Boniface K, Vergier B, Mossalayi D, Taïeb A, Ezzedine K, & Seneschal J (2014). Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res, 27(3), 398–407. 10.1111/pcmr.12219 [DOI] [PubMed] [Google Scholar]
- Budhwani M, Mazzieri R, & Dolcetti R (2018). Plasticity of Type I Interferon-Mediated Responses in Cancer Therapy: From Anti-tumor Immunity to Resistance. Frontiers in Oncology, 8, 322. 10.3389/fonc.2018.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURDICK KH, & HAWK WA (1964). VITILIGO IN A CASE OF VACCINIA VIRUS-TREATED MELANOMA. Cancer, 17(6), 708–712. [DOI] [PubMed] [Google Scholar]
- Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, & Lanzavecchia A (1999). Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. Journal of Experimental Medicine, 189(5), 821–829. 10.1084/jem.189.5.821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhang Z, Chen W, Zhang Z, Li Y, Shi M, et al. (2007). B7-H1 up-regulation on myeloid dendritic cells significantly suppresses T cell immune function in patients with chronic hepatitis B. The Journal of Immunology, 178(10), 6634–6641. [DOI] [PubMed] [Google Scholar]
- Cheng L, Ma J, Li J, Li D, Li G, Li F, et al. (2017). Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. Journal of Clinical Investigation, 127(1), 269–279. 10.1172/JCI90745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe WJ, Hall R, Newell DJ, & Stevenson CJ (1968). Vitiligo, thyroid disease and autoimmunity. British Journal of Dermatology, 80(3), 135–139. [DOI] [PubMed] [Google Scholar]
- Das S, & Johnson DB (2019). Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. Journal for Immunotherapy of Cancer, 7(1), 306. 10.1186/s40425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Anichini A, Mortarini R, Guidetti A, Tragni G, et al. (2003). Clinical protocol. Immunization of patients with malignant melanoma with autologous CD34(+) cell-derived dendritic cells transduced ex vivo with a recombinant replication-deficient vaccinia vector encoding the human tyrosinase gene: a phase I trial. Human Gene Therapy, 14(14), 1347–1360. 10.1089/104303403322319426 [DOI] [PubMed] [Google Scholar]
- Drexler I, Antunes E, Schmitz M, Wölfel T, Huber C, Erfle V, et al. (1999). Modified vaccinia virus Ankara for delivery of human tyrosinase as melanoma-associated antigen: induction of tyrosinase- and melanoma-specific human leukocyte antigen A*0201-restricted cytotoxic T cells in vitro and in vivo. Cancer Research, 59(19), 4955–4963. [PubMed] [Google Scholar]
- Evgin L, Huff AL, Wongthida P, Thompson J, Kottke T, Tonne J, et al. (2020). Oncolytic virus-derived type I interferon restricts CAR T cell therapy. Nature Communications, 11(1), 3187–15. 10.1038/s41467-020-17011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzedine K, Sheth V, Rodrigues M, Eleftheriadou V, Harris JE, Hamzavi IH, et al. (2015). Vitiligo is not a cosmetic disease. Journal of the American Academy of Dermatology, 73(5), 883–885. 10.1016/j.jaad.2015.07.039 [DOI] [PubMed] [Google Scholar]
- Gill L, Zarbo A, Isedeh P, Jacobsen G, Lim HW, & Hamzavi I (2016). Comorbid autoimmune diseases in patients with vitiligo: A cross-sectional study. Journal of the American Academy of Dermatology, 74(2), 295–302. 10.1016/j.jaad.2015.08.063 [DOI] [PubMed] [Google Scholar]
- Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, et al. (2012). CXCR3 Chemokine Receptor-Ligand Interactions in the Lymph Node Optimize CD4+ T Helper 1 Cell Differentiation. Immunity, 37(6), 1091–1103. 10.1016/j.immuni.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadah I, Binamer Y, Sanai FM, Abdo AA, & Alajlan A (2010). Interferon-induced vitiligo in hepatitis C patients: a case series. Pharmacology and Therapeutics, 1–5. [DOI] [PubMed] [Google Scholar]
- Harris JE (2017). Optimizing Vitiligo Management: Past, Present, and Future. Dermatologic Clinics, 35(2), xi. 10.1016/j.det.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, & Turka LA (2012a). A Mouse Model of Vitiligo with Focused Epidermal Depigmentation Requires IFN-γ for Autoreactive CD8+ T-Cell Accumulation in the Skin. Journal of Investigative Dermatology, 132(7), 1869–1876. 10.1038/jid.2011.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, & Turka LA (2012b). A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8⁺ T-cell accumulation in the skin. The Journal of Investigative Dermatology, 132(7), 1869–1876. 10.1038/jid.2011.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, et al. (2015). GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c(+)MHCII(+) Macrophages and Dendritic Cells. Immunity, 42(6), 1197–1211. 10.1016/j.immuni.2015.05.018 [DOI] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. The New England Journal of Medicine, 363(8), 711–723. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton AN (1984). Identification of differentiation antigens of melanoma and melanocytes by mouse and human monoclonal antibodies. Transplantation Proceedings, 16(2), 351–354. [PubMed] [Google Scholar]
- Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. (2016). Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatology, 152(1), 45–51. 10.1001/jamadermatol.2015.2707 [DOI] [PubMed] [Google Scholar]
- Ivashkiv LB, & Donlin LT (2014). Regulation of type I interferon responses. Nature Publishing Group, 14(1), 36–49. 10.1038/nri3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Langland JO, Kibler KV, Denzler KL, White SD, Holechek SA, et al. (2009). Vaccinia virus vaccines: past, present and future. Antiviral Research, 84(1), 1–13. 10.1016/j.antiviral.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin C, Rambert J, Guillet S, Thiolat D, Boukhedouni N, Doutre M-S, et al. (2017). Heat shock protein 70 potentiates interferon alpha production by plasmacytoid dendritic cells: relevance for cutaneous lupus and vitiligo pathogenesis. British Journal of Dermatology, 177(5), 1367–1375. 10.1111/bjd.15550 [DOI] [PubMed] [Google Scholar]
- Jin Y, Andersen G, Yorgov D, Ferrara TM, Ben S, Brownson KM, et al. (2016). Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nature Genetics, 48(11), 1418–1424. 10.1038/ng.3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, & Murali-Krishna K (2005). Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. Journal of Experimental Medicine, 202(5), 637–650. 10.1084/jem.20050821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, et al. (2006). Direct Stimulation of T Cells by Type I IFN Enhances the CD8+ T Cell Response during Cross-Priming. The Journal of Immunology, 176(8), 4682–4689. 10.4049/jimmunol.176.8.4682 [DOI] [PubMed] [Google Scholar]
- Li J, O’Malley M, Urban J, Sampath P, Guo ZS, Kalinski P, et al. (2011). Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Molecular Therapy : the Journal of the American Society of Gene Therapy, 19(4), 650–657. 10.1038/mt.2010.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato F, Tame M-R, Colecchia A, Racchini C, Azzaroli F, D’Errico A, et al. (2006). Systemic lupus erythematosus following virological response to peginterferon alfa-2b in a transplanted patient with chronic hepatitis C recurrence. World Journal of Gastroenterology, 12(26), 4253–4255. 10.3748/wjg.v12.i26.4253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, & Schuler G (1999). An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. Journal of Immunological Methods, 223(1), 77–92. 10.1016/s0022-1759(98)00204-x [DOI] [PubMed] [Google Scholar]
- Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, et al. (2017). Resident memory T cells in the skin mediate durable immunity to melanoma. Science Immunology, 2(10), eaam6346. 10.1126/sciimmunol.aam6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano RFV, Hunger A, Mendonça SA, Barbuto JAM, & Strauss BE (2017). Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget, 8(41), 71249–71284. 10.18632/oncotarget.19531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, & Aguet M (1994). Functional role of type I and type II interferons in antiviral defense. Science, 264(5167), 1918–1921. 10.1126/science.8009221 [DOI] [PubMed] [Google Scholar]
- Nordlund JJ, Kirkwood JM, Forget BM, Milton G, Albert DM, & Lerner AB (1983). Vitiligo in patients with metastatic melanoma: a good prognostic sign. Journal of American Dermatology, 9(5), 689–696. 10.1016/s0190-9622(83)70182-9 [DOI] [PubMed] [Google Scholar]
- Nouri K, Busso M, & Machler BC (1997). Vitiligo associated with alpha-interferon in a patient with chronic active hepatitis C. Cutis, 60(6), 289–290. [PubMed] [Google Scholar]
- Oertli D, Marti WR, Zajac P, Noppen C, Kocher T, Padovan E, et al. (2002). Rapid induction of specific cytotoxic T lymphocytes against melanoma-associated antigens by a recombinant vaccinia virus vector expressing multiple immunodominant epitopes and costimulatory molecules in vivo. Human Gene Therapy, 13(4), 569–575. 10.1089/10430340252809856 [DOI] [PubMed] [Google Scholar]
- Ogg GS, Dunbar PR, Romero P, Chen J-L, & Cerundolo V (1998). High Frequency of Skin-homing Melanocyte-specific Cytotoxic T Lymphocytes in Autoimmune Vitiligo. Journal of Experimental Medicine, 188, 1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N, Kawazoe H, Takechi K, Matsudate Y, Utsunomiya R, Zamami Y, et al. (2019). Association Between Immune-Related Adverse Events and Clinical Efficacy in Patients with Melanoma Treated With Nivolumab: A Multicenter Retrospective Study. Clinical Therapeutics, 41(1), 59–67. 10.1016/j.clinthera.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Onishi S, Nagashima T, Kimura H, Matsuyama Y, Yoshio T, & Minota S (2010). Systemic lupus erythematosus and Sjögren’s syndrome induced in a case by interferon-alpha used for the treatment of hepatitis C. Lupus, 19(6), 753–755. 10.1177/0961203309353172 [DOI] [PubMed] [Google Scholar]
- Park SL, Buzzai A, Rautela J, Hor JL, Hochheiser K, Effern M, et al. (2019). Tissue-resident memory CD8+ T cells promote melanoma-immune equilibrium in skin. Nature, 565(7739), 366–371. 10.1038/s41586-018-0812-9 [DOI] [PubMed] [Google Scholar]
- Popescu C, Popescu G-A, & Arama V (2013). Type 1 diabetes mellitus with dual autoimmune mechanism related to pegylated interferon and ribavirin treatment for chronic HCV hepatitis. Journal of Gastrointestinal and Liver Diseases : JGLD, 22(1), 101–104. [PubMed] [Google Scholar]
- Prabakaran I, Menon C, Xu S, Gómez-Yafal A, Czerniecki BJ, & Fraker DL (2002). Mature CD83(+) dendritic cells infected with recombinant gp100 vaccinia virus stimulate potent antimelanoma T cells. Annals of Surgical Oncology, 9(4), 411–418. 10.1007/bf02573878 [DOI] [PubMed] [Google Scholar]
- Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, et al. (2014). CXCL10 Is Critical for the Progression and Maintenance of Depigmentation in a Mouse Model of Vitiligo. Science Translational Medicine, 6(223), 223ra23–223ra23. 10.1126/scitranslmed.3007811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann E, Kingo K, Karelson M, Reemann P, Loite U, Sulakatko H, et al. (2012). The mRNA expression profile of cytokines connected to the regulation of melanocyte functioning in vitiligo skin biopsy samples and peripheral blood mononuclear cells. Human Immunology, 73(4), 393–398. 10.1016/j.humimm.2012.01.011 [DOI] [PubMed] [Google Scholar]
- Richmond JM, Bangari DS, Essien KI, Currimbhoy SD, Groom JR, Pandya AG, et al. (2017a). Keratinocyte-Derived Chemokines Orchestrate T-Cell Positioning in the Epidermis during Vitiligo and May Serve as Biomarkers of Disease. Journal of Investigative Dermatology, 137(2), 350–358. 10.1016/j.jid.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JM, Masterjohn E, Chu R, Tedstone J, Youd ME, & Harris JE (2017b). CXCR3 Depleting Antibodies Prevent and Reverse Vitiligo in Mice. The Journal of Investigative Dermatology, 137(4), 982–985. 10.1016/j.jid.2016.10.048 [DOI] [PubMed] [Google Scholar]
- Richmond JM, Strassner JP, Rashighi M, Agarwal P, Garg M, Essien KI, et al. (2018). Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. The Journal of Investigative Dermatology. 10.1016/j.jid.2018.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riding RL, Richmond JM, & Harris JE (2018). Mouse Model for Human Vitiligo. Current Protocols in Immunology, 135, e63. 10.1002/cpim.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenigk HH, Deodhar S, St Jacques R, & Burdick K (1974). Immunotherapy of malignant melanoma with vaccinia virus. Archives of Dermatology, 109(5), 668–673. [PubMed] [Google Scholar]
- Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Leví A, et al. (2019). Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. European Journal of Cancer (Oxford, England : 1990), 109, 21–27. 10.1016/j.ejca.2018.10.014 [DOI] [PubMed] [Google Scholar]
- Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung H-P, Weller M, & Wiendl H (2004). Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. Journal of Neuroimmunology, 155(1–2), 172–182. 10.1016/j.jneuroim.2004.06.013 [DOI] [PubMed] [Google Scholar]
- Schütz A, Oertli D, Marti WR, Noppen C, Padovan E, Spagnoli GC, et al. (2001). Immunogenicity of nonreplicating recombinant vaccinia expressing HLA-A201 targeted or complete MART-1/Melan-A antigen. Cancer Gene Therapy, 8(9), 655–661. 10.1038/sj.cgt.7700351 [DOI] [PubMed] [Google Scholar]
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. (2011). gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. The New England Journal of Medicine, 364(22), 2119–2127. 10.1056/NEJMoa1012863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Mirza F, Pasquetto V, Tscharke DC, Palmowski MJ, Dunbar PR, et al. (2005). Immunodominance of poxviral-specific CTL in a human trial of recombinant-modified vaccinia Ankara. The Journal of Immunology, 175(12), 8431–8437. 10.4049/jimmunol.175.12.8431 [DOI] [PubMed] [Google Scholar]
- Smith JL, & Stehlin JS (1965). Spontaneous regression of primary malignant melanomas with regional metastases. Cancer, 18(11), 1399–1415. [DOI] [PubMed] [Google Scholar]
- Snell LM, McGaha TL, & Brooks DG (2017). Type I Interferon in Chronic Virus Infection and Cancer. Trends in Immunology, 38(8), 542–557. 10.1016/j.it.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritz RA (2013). Modern vitiligo genetics sheds new light on an ancient disease. The Journal of Dermatology, 40(5), 310–318. 10.1111/1346-8138.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassner JP, Rashighi M, Ahmed Refat M, Richmond JM, & Harris JE (2017). Suction blistering the lesional skin of vitiligo patients reveals useful biomarkers of disease activity. Journal of American Dermatology, 76(5), 847–855.e5. 10.1016/j.jaad.2016.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taïeb A, & Picardo M (2009). Vitiligo Clinical practice. New England Journal of Medicine, 160–169. [DOI] [PubMed] [Google Scholar]
- Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KCF, Welch M, et al. (2013). Persistent LCMV Infection Is Controlled by Blockade of Type I Interferon Signaling. Science, 340(6129), 199–202. 10.1126/science.1235047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxel-Dezaire AHH, Rani MRS, & Stark GR (2006). Complex modulation of cell type-specific signaling in response to type I interferons. Immunity, 25(3), 361–372. 10.1016/j.immuni.2006.08.014 [DOI] [PubMed] [Google Scholar]
- van den Boorn JG, Konijnenberg D, Dellemijn TAM, van der Veen JPW, Bos JD, Melief CJM, et al. (2009). Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. The Journal of Investigative Dermatology, 129(9), 2220–2232. 10.1038/jid.2009.32 [DOI] [PubMed] [Google Scholar]
- van Geel N, Speeckaert R, Mollet I, De Schepper S, De Wolf J, Tjin EPM, et al. (2012). In vivo vitiligo induction and therapy model: double-blind, randomized clinical trial. Pigment Cell Melanoma Res, 25(1), 57–65. 10.1111/j.1755-148X.2011.00922.x [DOI] [PubMed] [Google Scholar]
- van Oijen M, Bins A, Elias S, Sein J, Weder P, de Gast G, et al. (2004). On the role of melanoma-specific CD8+ T-cell immunity in disease progression of advanced-stage melanoma patients. Clinical Cancer Research, 10(14), 4754–4760. 10.1158/1078-0432.CCR-04-0260 [DOI] [PubMed] [Google Scholar]
- Walsh SR, Bastin D, Chen L, Nguyen A, Storbeck CJ, Lefebvre C, et al. (2019). Type I IFN blockade uncouples immunotherapy-induced antitumor immunity and autoimmune toxicity. Journal of Clinical Investigation, 129(2), 518–530. 10.1172/JCI121004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Kittlesen D, Slingluff CL, Vervaert CE, Seigler HF, & Darrow TL (2000). Dendritic cells infected with a vaccinia vector carrying the human gp100 gene simultaneously present multiple specificities and elicit high-affinity T cells reactive to multiple epitopes and restricted by HLA-A2 and -A3. The Journal of Immunology, 164(8), 4204–4211. 10.4049/jimmunol.164.8.4204 [DOI] [PubMed] [Google Scholar]
- Zajac P, Oertli D, Marti W, Adamina M, Bolli M, Guller U, et al. (2003). Phase I/II clinical trial of a nonreplicative vaccinia virus expressing multiple HLA-A0201-restricted tumor-associated epitopes and costimulatory molecules in metastatic melanoma patients. Human Gene Therapy, 14(16), 1497–1510. 10.1089/104303403322495016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 3: PD-1 expression by PMEL is significantly reduced in hosts lacking IFNaR on radioresistant cells. A) Representative flow cytometry plots of PD-1 expression on epidermal PMEL in chimeric mice. B) Percentage of PMEL expressing PD-1 in the epidermis and dermis of chimeric mice 5 weeks post vitiligo induction. C) PD-1 MFI on PMEL in the epidermis and dermis of chimeric mice 5 weeks post vitiligo induction.
Supplementary Figure 2: IFNaR-deficient mice have increased circulating CD44+ effector memory PMEL. Normalized PMEL numbers in the skin draining lymph nodes (A) and spleen (B) at weeks 3, 5, and 7 post vitiligo induction. C) Representative flow cytometry plots pre-gated on PMEL showing CD44 and CD62L marker expression. D) Percent of CD44+CD62L− PMEL in the spleen of WT and IFNaR KO mice at weeks 3, 5, and 7 post vitiligo induction.
Supplementary Figure 1: Significantly reduced PMEL numbers in the tail skin of IFNGR KO mice at 7 weeks post vitiligo induction. A) Epidermal (B) Dermal, and (C) inguinal lymph node PMEL numbers normalized to 10,000 live single cells.