Abstract
We aimed to investigate the effect of propofol on the growth of human ovarian cancer cells ES2 and OVCAR-3 in vitro by regulating long non-coding RNA HOST2 (human ovarian cancer-specific transcript 2) and the inhibition of JAK2/STAT3 signaling pathway. In the present study, ES-2 and OVCAR-3 cells were firstly treated with different concentrations of propofol (0, 1, 5 and 10 μg/ml). The expression of HOST2 in ES-2 and OVCAR-3 cells were detected by quantitative reverse transcription-PCR (qRT-PCR). Then, the expression of HOST2 was changed by transfection of HOST2 overexpression plasmid into ES-2 and OVCAR-3 cells. Cell proliferation, migration, invasion and apoptosis were performed using CCK-8, wound-healing, Transwell assays and Flow Cytometry. Western blot analysis was performed to detect the expressions of apoptosis-associated proteins and JAK2/STAT3 pathway-related proteins. Results showed that cell viability and intracellular HOST2 expression in ES-2 and OVCAR-3 cells were decreased gradually with the increase of propofol concentration in a dose-dependent manner. Compared with the propofol group, overexpression of HOST2 significantly promoted the cell proliferation, migration, invasion and inhibited apoptosis, and ameliorated the inhibitory effect of propofol on the growth of tumor cells. Western blot analysis showed that compared with propofol group, the expression of Bcl-2 was significantly increased whereas Bax and the ratio of Cleaved caspase3/caspase3 were significantly decreased in pcDNA-HOST2 group. In addition, overexpression of HOST2 significantly enhanced the phosphorylation level of JAK2 and STAT3, and reduced the suppressive effect of propofol on JAK2/STAT3 signaling. Our results illustrated that propofol could significantly inhibit the proliferation, migration, invasion and induce apoptosis of ES-2 and OVCAR-3 cells by downregulating HOST2. The regulation mechanism may be achieved by inhibiting the activation of JAK2/STAT3 signaling pathway.
Keywords: Propofol, Ovarian cancer, HOST2, JAK2/STAT3, Proliferation, Apoptosis
Introduction
Ovarian cancer (OC) is the third most common malignant tumor in the female reproductive system, and its mortality ranks first in the malignant tumors of the female genital system, with a 5-year survival rate were only 30% and 40% (Kammar et al. 2019). According to statistics in 2018, there are more than 295,000 new cases and more than 184,000 deaths worldwide (Bray et al. 2018). At present, surgery is still the most effective method for the treatment of OC (Pujade-Lauraine (2017)). The safety of surgery depends on the rational use of anesthetics during the perioperative period. More and more basic and clinical studies have shown that anesthetics may affect the prognosis of patients with breast cancer (D'Agostino et al. 2018), colon cancer (Baptista-Hon et al. 2014), lung cancer (Cata et al. 2018) and ovarian cancer (Lin et al. 2011). However there are still few studies on the effect of anesthetics on various tumors in vitro. Therefore, an in-depth study of the effects of anesthetics on the biological characteristics of OC will be of great significance to the formulation of treatment plans for OC patients and to reduce the intervention of anesthetics on the treatment of OC.
Propofol is a kind of intravenous anesthetic, which has the characteristics of rapid onset, short action time, rapid awakening and less adverse reactions. So, propofol has been widely used in clinical induction and maintenance of anesthesia (Chidambaran et al. (2015); Oren-Ziv et al. 2015). With the deepening of research, more and more evidences indicated that propofol has a wide range of anticancer effects in addition to anesthetic effects. It plays an important role in inhibiting the proliferation and metastasis of tumors such as esophageal squamous cell carcinoma (Zhou et al. 2017), lung cancer (Liu and Liu 2018) and colorectal cancer (Xu et al. 2018). In OC, some studies have shown that propofol could promote apoptosis of OC cells by enhancing the expression of microRNA let-7i (Su et al. 2014). In addition, propofol significantly inhibited the proliferation of OC cells by suppressing miR-9/NF-κB signaling pathway (Huang et al. 2016). Although the inhibitory effect of propofol on the growth of OC cells has been confirmed, the research on propofol in OC are still rare and the molecular mechanism of its antitumor effect has not been clearly specified.
Long non-coding RNA (lncRNAs) are non-coding RNA molecules with the length of more than 200 nucleotides, which were found in organisms in recent years. LncRNAs could regulate the occurrence and development of many types of tumors including OC by transcriptional activation or interference (Zhou et al. 2016; Tripathi et al. 2018). HOST2 (human ovarian cancer-specific transcript 2) is one kind of RNA, overexpressed in OC cells, which is involved in the development of many kinds of malignant tumors (Kim et al. 2013). A previous study showed that HOST2 promoted the proliferation, migration and invasion of epithelial ovarian tumor cells (Gao et al. 2015a). However, no researchs have specifically addressed whether propofol could inhibit the proliferation, migration, invasion and apoptosis of OC cells by regulating HOST2.
In general, the purpose of this study was to study the effects of propofol on the proliferation, migration, invasion and apoptosis of ES-2 and OVCAR-3 cells by regulating the expression of HOST2 in vitro and to explore its possible signal transduction pathway. These results will provide a new reference point for propofol to be an ideal anesthetic in OC surgery.
Methods
Cell culture and treatment
Human ovarian cancer cell lines (ES-2 and OVCAR-3) were purchased from ATCC (Manassas, VA, USA). Cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS), 1000U/ml penicillin and 100 mg/ml streptomycin. Then the cell culture bottle was cultured in a constant temperature incubator of 5% CO2 at 37 °C. After the cells were passaged, the logarithmic phase cells were taken for later experiment.
Propofol was obtained from Astra Zeneca Inc. Untreated ES-2 and OVCAR-3 cells were labeled as 0 μg/ml group. ES-2 and OVCAR-3 cells were treated with different concentrations of propofol (1, 5 and 10 μg/ml) for 24 h. These cells were then labeled as 1, 5 and 10 μg/ml groups according to the concentration of propofol.
Cell transfection
The overexpressed HOST2 vector plasmid (pcDNA-HOST2) and the corresponding pcDNA3.1 empty vector plasmid (pcDNA-NC) were purchased from GenePharma (GenePharma Co., Ltd., Shanghai, China). OC cells were inoculated into 6-well plates (1 × 105 cells/well), and the pcDNA3.1 empty plasmid and HOST2 overexpression plasmid were transfected into ES-2 and OVCAR-3 cells using Lipofectamine 3000 transfection reagent (Invitrogen) according to the manufacturer's instructions. The transfection efficiency was detected by qRT-PCR after 48 h.
CCK-8 assay
The transfected ES-2 and OVCAR-3 cells were inoculated in 96-well plates with the density of 2.5 × 104 cells/ml, 100 μl per well, and cultured in 5%CO2 and 97% humidity incubator 37 °C. After 48 h, 10 μl CCK-8 solution was added into each well and cultured for 2 h. The 96-well plate was placed on the automatic enzyme labeling instrument (BIO-RAD) and the absorbance value was measured at the 450 nm wavelength.
Cell migration assay
The transfected ES-2 and OVCAR-3 cells were inoculated in 6-well plates with the density of 5 × 105 cells/ml. Use the tip of the aseptic pipette to scratch in each hole. The cells were washed with sterile PBS to remove the cell fragments, and then cultured in the medium without FBS. At the time point of 24 h, the migration status was recorded at the same position of each hole.
Cell invasion assay
The serum-free ES-2 and OVCAR-3 cell suspensions were inoculated in 24-well plates with the density of 5 × 105 cells/ml. The Matrigel Invasion Chamber (Corning, NY) was performed to analyze the cell invasion. 750 μl medium containing 10% FBS was added in the lower chamber, and 500 μl serum-free cell suspension was added in the upper chamber, and continued to be cultured in the incubator for 24 h. At the end of culture, the cells were fixed with methanol and stained with DAPI. The invaded cells were observed and counted with microscope.
Cell apoptosis assay
The cells of each group were collected and washed with PBS for 3 times. According to the operation instructions of AnnexinV-fluorescein isothiocyanate (AnnexinV-FITC)/propidium iodide (PI) Apoptosis Detection kit (Invitrogen), the cells were first resuspended with 400 μl binding buffer, and then 10 μl Annexin V-FITC and 5 μl PI were added in turn to incubate 15 min in the dark. The level of apoptosis was detected by flow cytometry.
qRT-PCR analysis
Cells in logarithmic phase were collected and total RNA was extracted by Trizol reagent (Takara, Dalian, China). Afterwards, Reverse Transcription Kit (Takara, Japan) was conducted to reverse transcription of the RNA into cDNA. Real-time fluorescence quantitative PCR was conducted to using the ABI 7500 PCR instrument. Reaction conductions were as follows: 95 °C for 2 min, 95 °C for 15 s, 60 °C for 20 s, 72 °C for 20 s, for a total of 40 cycles. The expression level of HOST2 was calculated by 2−ΔΔCt (Livak and Schmittgen 2001) method. GAPDH was used as the internal reference gene of HOST2. The experiment was repeated 3 times and the average value was taken.
Western blot analysis
Total protein was extracted by RIPA lysate (Thermo Fisher Scientific) and the protein concentration was determined by BCA protein assay (Bio-Rad). Afterwards, protein lysates were separated by SDS-PAGE and transferred to PVDF membrane (BioRad). These membranes were sealed with 5% skimmed milk powder at room temperature for 1 h and incubated with primary antibodies against Bcl-2 (cat. no. Ab32124; 1:1000 dilution; Abcam), Bax (cat. no. Ab32503; 1:1000 dilution; Abcam), Cleaved caspase-3 (cat. no. Ab32042; 1:500 dilution; Abcam), caspase-3 (cat. no. Ab13847; 1:500 dilution; Abcam), JAK2 (cat. no. Ab108596; 1:5000 dilution; Abcam), p-JAK2 (cat. no. Ab32101; 1:1000 dilution; Abcam), STAT3 (cat. no. Ab68153; 1:1000 dilution; Abcam), p-STAT3 (cat. no. Ab76315; 1:2000 dilution; Abcam) and GAPDH (cat. no. Ab8245; 1:1000 dilution; Abcam) at 4 °C overnight. These membranes were washed with TBST for three times. Then, the secondary antibodies conjugated to horseradish peroxidase (1:5000; Santa Cruz Biotechnology, Inc.) was incubated at room temperature for 1 h. Immunoreactive bands were detected using the ECL detection reagent (GE Healthcare). The bands in the gel were determined using a LAS4010 Biomolecular Imager. GAPDH was used as internal reference to analyze the gray value of target band.
Statistical analysis
SPSS 20.0 software was used to analyze the experimental data. The measurement data were expressed by the mean ± standard deviation (SD). One-wsay ANOVA followed by Tukey´s post hoc test was used for comparison between groups, and the difference was statistically significant (P < 0.05).
Results
Propofol inhibits the activity and HOST2 expression of ES-2 and OVCAR-3 cells
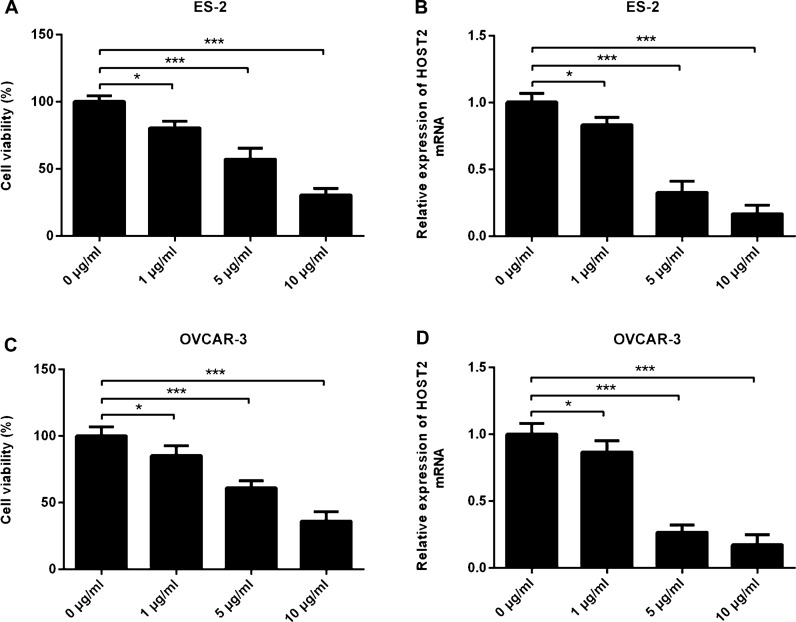
Firstly, we treated ES-2 and OVCAR-3 cells with different concentrations of propofol (0, 1, 5 and 10 μg/ml). CCK-8 results showed that the activity of ES-2 and OVCAR-3 cells decreased gradually with the increase of propofol concentration, showing a dose-dependent relationship (Fig. 1a and c). In addition, the results of PCR showed that compared with 0 μg/ml group, the expression level of HOST2 decreased gradually with the increase of propofol concentration, and reached the minimum value at 10 μg/ml (Fig. 1b and d). These results indicated that propofol suppressed the viability and HOST2 expression of ES-2 and OVCAR-3 cells in a dose-dependent manner.
Fig. 1.
Propofol inhibits the activity and HOST2 expression of ES-2 and OVCAR-3 cells. a Effects of different doses of propofol (0, 1, 5 and 10 μg/ml) on viability of ES-2 cells were detected by CCK-8. b Effects of different doses of propofol (0, 1, 5 and 10 μg/ml) on the expression levels of HOST2 in ES-2 cells were determined by real-time PCR. c Effects of different doses of propofol (0, 1, 5 and 10 μg/ml) on viability of OVCAR-3 cells were detected by CCK-8. (D) Effects of different doses of propofol (0, 1, 5 and 10 μg/ml) on the expression levels of HOST2 in OVCAR-3 cells were determined by real-time PCR. *p < 0.05 and ***p < 0.001
Propofol inhibits the proliferation, migration and invasion of ES-2 and OVCAR-3 cells by downregualting HOST2 expression
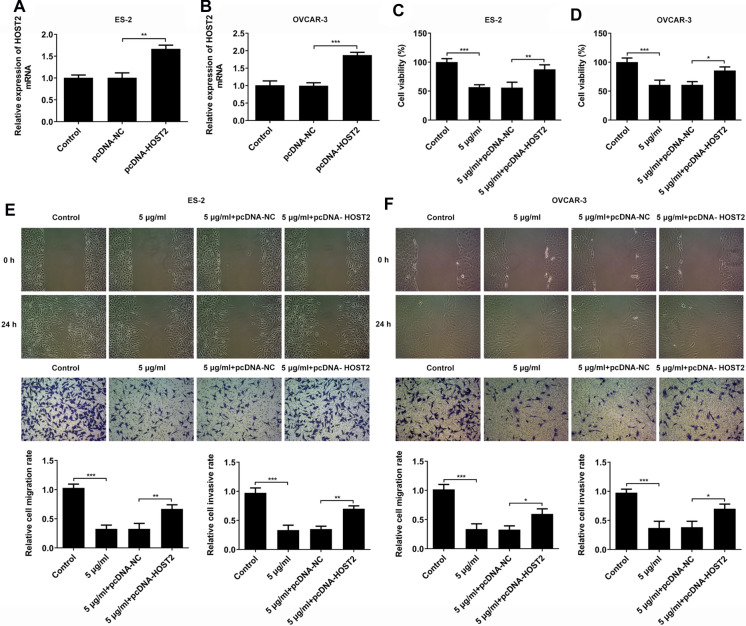
Then, CCK-8, scratch wound healing and Transwell assays were used to detect the effect of propofol on the proliferation, migration and invasion abilities of ES-2 and OVCAR-3 cells by regulating HOST2. We successfully constructed the HOST2 overexpression vector plasmid. PCR results showed that the expression level of HOST2 in pcDNA-HOST2 group was significantly higher than that in control group (Fig. 2a and b). Subsequently, CCK-8 results showed that compared with the control group, 5 μg/ml propofol significantly reduced cell viability, but this change could be alleviated by HOST2 overexpression (Fig. 2c and d). The results of migration and invasion assays showed that compared with the control group, 5 μg/ml propofol significantly reduced cell migration and invasion, but this inhibitory effect could be alleviated by HOST2 overexpression (Fig. 2e and f). These results suggested that HOST2 was involved in the effects of propofol on the proliferation, migration and invasion of ES-2 and OVCAR-3 cells.
Fig. 2.
Propofol inhibits the proliferation, migration and invasion of ES-2 and OVCAR-3 cells by downregualting HOST2 expression. a mRNA levels of HOST2 overexpression in ES-2 cells were detected by real-time PCR. b mRNA levels of HOST2 overexpression in OVCAR-3 cells were detected by real-time PCR. c Effects of HOST2 overexpression on the inhibitory effect of 5 μg/ml propofol on the activity of ES-2 cells were detected by CCK-8. d Effects of HOST2 overexpression on the inhibitory effect of 5 μg/ml propofol on the activity of OVCAR-3 cells were detected by CCK-8. e Effects of HOST2 overexpression on the inhibitory effect of 5 μg/ml propofol on the migration and invasiveness of ES-2 cells were detected by Scratch wound-healing and Transwell assays. f Effects of HOST2 overexpression on the inhibitory effect of 5 μg/ml propofol on the migration and invasiveness of OVCAR-3 cells were detected by Scratch wound-healing and Transwell assays. *p < 0.05, **p < 0.01 and ***p < 0.001
Propofol promotes apoptosis of ES-2 and OVCAR-3 cells by inhibiting HOST2
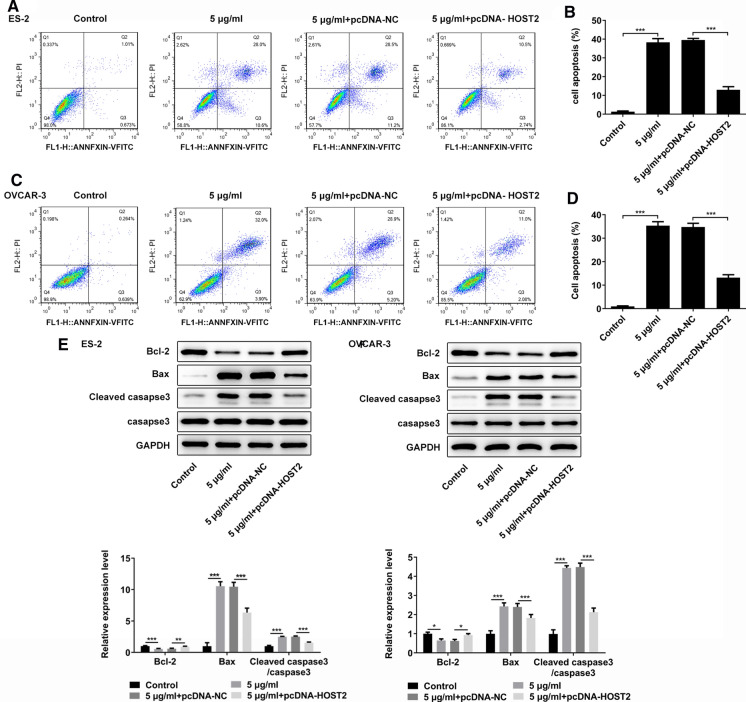
In addition, flow cytometry and western blot assay were performed to detected the effect of propofol on apoptosis of ES-2 and OVCAR-3 cells by regulating HOST2. The results of flow cytometry assay showed that compared with 5 μg/ml + pcDNA-NC group, HOST2 overexpression could significantly reduce the apoptosis rate (Fig. 3a–d). Western blot results showed that compared with 5 μg/ml + pcDNA-NC group, HOST2 overexpression could significantly reduce the expression level of Bax and Cleaved-caspase3 whereas increase the expression level of Bcl-2 (Fig. 3e and f). These results suggested that HOST2 was involved in the effects of propofol on apoptosis of ES-2 and OVCAR-3 cells.
Fig. 3.
Propofol promotes apoptosis of ES-2 and OVCAR-3 cells by inhibiting HOST2. a, b Detection of ES-2 cell apoptosis by flow cytometry. c, d Detection of OVCAR-3 cell apoptosis by flow cytometry. e Western blot was used for exploring apoptosis-related protein (Bcl-2, Bax and Caspase-3) expression level in ES-2 cells. f Western blot was used for exploring apoptosis-related protein (Bcl-2, Bax and Caspase-3) expression level in OVCAR-3 cells. *p < 0.05, **p < 0.01 and ***p < 0.001
Propofol regulates the phosphorylation levels of JAK2 and STAT3 by inhibiting HOST2
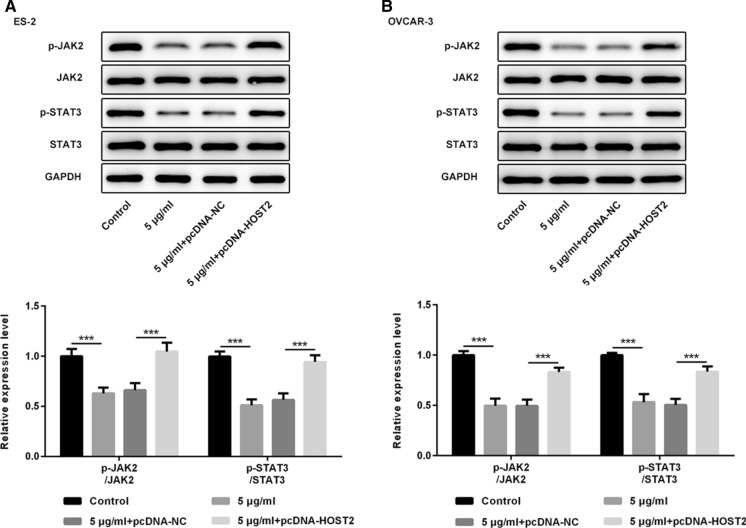
To further study the molecular mechanism of the effects of propofol on the proliferation, migration and invasion in ES-2 and OVCAR-3 cells, western blot assay were used to determine the activation of JAK2/STAT3 signal pathway. Results showed that compared with the control group, 5 μg/ml propofol could significantly inhibit the phosphorylation levels of JAK2 and STAT3 and HOST2 overexpression could reverse the inhibitory effect of propofol on the phosphorylation of JAK2 and STAT3 (Fig. 4a and b). To sum up, propofol inhibited the cell proliferation, migration and invasion via downregulating the expression of HOST2 by inhibiting the activation of JAK2/STAT3 in ES-2 and OVCAR-3 cells.
Fig. 4.
Propofol regulates the phosphorylation levels of JAK2 and STAT3 by inhibiting HOST2. a The levels of JAK2 and STAT3 phosphorylation and their respective protein levels in ES-2 cells were determined by western blot. b The levels of JAK2 and STAT3 phosphorylation and their respective protein levels in OVCAR-3 cells were determined by western blot. ***p < 0.001
Discussion
Ovarian cancer (OC) is one of the most common malignant tumors in the female reproductive system, and the mortality rate ranks first among the gynecological malignant tumors (Kammar et al. 2019). Surgery-based comprehensive treatment mode is still the main treatment of OC, but whether anesthetics have anti-cancer effect during operation and how to play its role are gradually attracting people's attention. In the present study, human OC cell lines (ES-2 and OVCAR-3) were treated with different concentrations of propofol (0, 1, 5 and 10 μg/ml). It was found that propofol could inhibit cell activity and HOST2 expression in a dose-dependent manner. In addition, in vitro studies, 5 μg/ml propofol significantly inhibited the proliferation, migration and invasion of ES-2 and OVCAR-3 cells while promoted apoptosis and inhibition of JAK2/STAT3 signaling pathway. HOST2 overexpression could partially reverse these above changes.
Propofol is a commonly used intravenous anesthetic in clinic, which has been proved to have a variety of non-anesthetic effects in addition to sedation and anesthetic effects (Vasileiou et al. 2009). In recent years, propofol has been reported to have many potential anti-cancer properties, such as inhibition of cancer cell proliferation, adhesion, metastasis and induction of apoptosis (Wang et al. 2015). Therefore, propofol has gradually become an ideal anesthetic in cancer surgery. In the field of OC, there are few studies on the regulatory mechanism of propofol on OC cells. Only a few studies have shown that propofol could inhibit the proliferation and induce apoptosis of OC cells in a dose-dependent manner (Su et al. 2014; Huang et al. 2016). Consistent with the above conclusions, our results indicated that propofol significantly reduced the proliferation, migration and invasion of ES-2 and OVCAR-3 cells in a dose-dependent manner. These results further confirmed that propofol can exert its anti-cancer effect by inhibiting the growth of OC cells.
Although the effect of propofol on proliferation of OC cells has been reported, it is not clear whether propofol can inhibit OC by regulating the expression of lncRNAs. LncRNA HOST2 is a member of the long non-coding RNA family, about 2.9 kb in length, which has no obvious open reading frame and has been reported to be highly expressed in OC for the first time (Wu et al. 2014). HOST2 has been confirmed to be abnormally expressed in triple-negative breast cancer (Zhang et al. 2019), liver cancer (Wu et al. 2018), and glioma (Wang et al. 2019), and is involved in regulating a variety of tumor biological processes including proliferation, migration, invasion and apoptosis. In epithelial ovarian cancer, downregulation of HOST2 could significantly inhibit the proliferation, migration and invasion of OC cells (D'Agostino et al. 2018). In addition, LncRNA-HOST2 can regulate cell biological behaviors in epithelial ovarian cancer through a mechanism involving microRNA let-7b (Gao et al. 2015b). However, whether propofol has a regulatory effect on HOST2 and the specific mechanism of its action in OC has not been reported. Therefore, we boldly speculated in this paper that propofol may influence the malignant process of OC through regulating the expression of HOST2. In our study, we further explored the function of HOST2 in OC cells. We found that propofol significantly decreased the expression of HOST2 in ES-2 and OVCAR-3 cells. In addition, HOST2 overexpression partially reversed the inhibitory effects of propofol on proliferation, migration and invasion and the promotion of apoptosis in ES-2 and OVCAR-3 cells. These results indicated that propofol significantly inhibited the growth of ES-2 and OVCAR-3 cells by downregulating the expression of HOST2.
Recently, JAK/STAT signaling pathway has been found to be involved in many biological behaviors including cell proliferation and differentiation, which plays an important role in affecting the efficacy and prognosis of patients with malignant tumors (Reyes et al. 2017). Compared with the normal ovarian epithelial cells, OC cells in vitro showed a higher level of phosphorylation of STAT3. Persistent abnormal activation of JAK2/STAT3 signaling pathway can eventually lead to the formation of malignant phenotype. So far, STAT3 has been defined as an oncogene (Levy and Inghirami 2006). With the further study of genes downstream of STAT3 pathway, more and more evidence shows that JAK2/STAT3 signaling pathway plays an important role in tumor cell proliferation, apoptosis inhibition, vascular remodeling, tumor metastasis and drug resistance. JAK2/STAT3 signaling pathway could also promote tumor cell proliferation and inhibit apoptosis (Pandey et al. 2009; Yang et al. 2010; Colomiere et al. 2009). Some studies have shown that propofol enhanced the effect in cisplatin chemotherapy by inhibiting the phosphorylation of JAK2/STAT3 pathway (Li et al. 2017), and HOST2 promoted the proliferation, invasion and migration of hepatocellular carcinoma cells by activating JAK2/STAT3 pathway (Wu 2018). It shows that HOST2 can regulate the expression level of JAK2/STAT3 to control the occurrence and development of the disease. Therefore, we speculated whether propofol can inhibit the malignant progression of OC cells by regulating HOST2/JAK2/STAT3 signaling pathway. So then we studied whether propofol could regulate the phosphorylation levels of JAK2 and STAT3, and then affect the progression of OC. Our results revealed that propofol could significantly suppress activation of JAK2/STAT3 pathway, and HOST2 overexpression partially reversed the inhibitory effect of propofol on JAK2/STAT3 pathway. Overall, these results draw a preliminary conclusion that propofol inhibited proliferation, migration, invasion and promoted apoptosis by regulating HOST2/JAK2/STAT3 signaling pathway in ES-2 and OVCAR-3 cells. The limitation of our paper is that it does not further demonstrate that the JAK2/STAT3 pathway is involved in the inhibitory effect of propofol on OC. In the following experiments, our laboratory will further prove that the JAK2/STAT3 pathway participates in the inhibitory function of propofol by adding JAK2/STAT3 pathway inhibitors or agonists.
In summary, HOST2 plays a negative role in the inhibitory effect of propofol on the progression of ES-2 and OVCAR-3 cells. Propofol can inhibit the JAK2/STAT3 signal pathway by downregulating the expression of HOST2, and finally reduce the proliferation, migration and invasion as well as induced apoptosis of ES-2 and OVCAR-3 cells.
Author contributions
XS and JP made substantial contributions to the conception and design of the present study. XS designed the research; XS, DW and XC performed the research; XS and DW analyzed the data and wrote the paper.
Funding
No funding was received.
Data availability
All data generated or analyzed during this study are included in this published article.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baptista-Hon DT, Robertson FM, Robertson GB, et al. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and NaV1. 5 channel function. Br J Anaesth. 2014;113:39–48. doi: 10.1093/bja/aeu104. [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clinic. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Cata JP, Lasala J, Mena GE, et al. Anesthetic considerations for mediastinal staging procedures for lung cancer. J Cardiothorac Vasc Anesth. 2018;32:893–900. doi: 10.1053/j.jvca.2017.08.041. [DOI] [PubMed] [Google Scholar]
- Chidambaran V, Costandi A, D’Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015;29:543–563. doi: 10.1007/s40263-015-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomiere M, Ward AC, Riley C, et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial–mesenchymal transition in ovarian carcinomas. Br J Cancer. 2009;100:134–144. doi: 10.1038/sj.bjc.6604794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino G, Saporito A, Cecchinato V, et al. Lidocaine inhibits cytoskeletal remodelling and human breast cancer cell migration. Br J Anaesth. 2018;121:962–968. doi: 10.1016/j.bja.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Gao Y, Meng H, Liu S, et al. LncRNA-HOST2 regulates cell biological behaviors in epithelial ovarian cancer through a mechanism involving microRNA let-7b. Hum Mol Genet. 2015;24:841–852. doi: 10.1093/hmg/ddu502. [DOI] [PubMed] [Google Scholar]
- Gao Y, Meng H, Liu S, Hu J, Zhang Y, Jiao T, et al. LncRNA-HOST2 regulates cell biological behaviors in epithelial ovarian cancer through a mechanism involving microRNA let-7b. Hum Mol Genet. 2015;24:841–852. doi: 10.1093/hmg/ddu502. [DOI] [PubMed] [Google Scholar]
- Huang X, Teng Y, Yang H, et al. Propofol inhibits invasion and growth of ovarian cancer cells via regulating miR-9/NF-κB signal. Braz J Med Biol Res. 2016;49:e5717. doi: 10.1590/1414-431x20165717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammar P, Bhatt A, Anam J, et al. Correlation between pelvic peritoneal disease and nodal metastasis in advanced ovarian cancer: can intraoperative findings define the need for systematic nodal dissection? Indian J Surg Oncol. 2019;10:84–90. doi: 10.1007/s13193-019-00881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Jutooru I, Chadalapaka G, et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Inghirami G. STAT3: a multifaceted oncogene. Proc Natl Acad Sci USA. 2006;103:10151–10152. doi: 10.1073/pnas.0604042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lu Y, Pang Y, Li M, Cheng X, Chen J. Propofol enhances the cisplatin-induced apoptosis on cervical cancer cells via EGFR/JAK2/STAT3 pathway. Biomed Pharmacother. 2017;86:324–333. doi: 10.1016/j.biopha.2016.12.036. [DOI] [PubMed] [Google Scholar]
- Lin L, Liu C, Tan H, et al. Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: a retrospective analysis. Br J Anaesth. 2011;106:814–822. doi: 10.1093/bja/aer055. [DOI] [PubMed] [Google Scholar]
- Liu WZ, Liu N. Propofol inhibits lung cancer A549 cell growth and epithelial-mesenchymal transition process by upregulation of MicroRNA-1284. Oncol Res Featuring Preclin Clin Cancer Therap. 2018;27:1–8. doi: 10.3727/096504018X15172738893959. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Oren-Ziv A, Hoppenstein D, Shles A, et al. Sedation methods for intra-articular corticosteroid injections in juvenile idiopathic arthritis: a review. Pediatr Rheumatol. 2015;13:28. doi: 10.1186/s12969-015-0021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey MK, Sung B, Ahn KS, et al. Butein suppresses constitutive and inducible signal transducer and activator of transcription (STAT) 3 activation and STAT3-regulated gene products through the induction of a protein tyrosine phosphatase SHP-1. Mol Pharmacol. 2009;75:525–533. doi: 10.1124/mol.108.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujade-Lauraine E. New treatments in ovarian cancer. Ann Oncol. 2017;28:viii57–viii60. doi: 10.1093/annonc/mdx442. [DOI] [PubMed] [Google Scholar]
- Reyes R, Wani NA, Ghoshal K, et al. Sorafenib and 2-deoxyglucose synergistically inhibit proliferation of both sorafenib-sensitive and-resistant HCC cells by inhibiting ATP production. Gene Expr J Liver Res. 2017;17:129–140. doi: 10.3727/105221616X693855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Hou XK, Wen QP. Propofol induces apoptosis of epithelial ovarian cancer cells by upregulation of microRNA let-7i expression. Eur J Gynaecol Oncol. 2014;35:688–691. [PubMed] [Google Scholar]
- Tripathi MK, Doxtater K, Keramatnia F, et al. Role of lncRNAs in ovarian cancer: defining new biomarkers for therapeutic purposes. Drug Discov Today. 2018;23:1635–1643. doi: 10.1016/j.drudis.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileiou I, Xanthos T, Koudouna E, et al. Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol. 2009;605(1–3):1–8. doi: 10.1016/j.ejphar.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Gong HY, Zheng F, et al. Propofol suppresses proliferation and invasion of gastric cancer cells via downregulation of microRNA-221 expression. Genet Mol Res. 2015;14:8117–8124. doi: 10.4238/2015.July.17.20. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhuang ZW, Cheng YM, et al. An in vitro and in vivo study of the role of long non-coding RNA-HOST2 in the proliferation, migration, and invasion of human glioma cells. IUBMB Life. 2019;71:93–104. doi: 10.1002/iub.1943. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang L, Wang Y, et al. Long noncoding RNA HOTAIR involvement in cancer. Tumor Biol. 2014;35:9531–9538. doi: 10.1007/s13277-014-2523-7. [DOI] [PubMed] [Google Scholar]
- Wu Y, et al. Long Noncoding RNA HOST2 Promotes epithelial-mesenchymal transition, proliferation, invasion and migration of hepatocellular carcinoma cells by activating the JAK2-STAT3 signaling pathway. Cell Physiol Biochem. 2018;51:301–314. doi: 10.1159/000495231. [DOI] [PubMed] [Google Scholar]
- Wu Y, Yuan T, Wang WW, et al. Long noncoding RNA HOST2 promotes epithelial-mesenchymal transition, proliferation, invasion and migration of hepatocellular carcinoma cells by activating the JAK2-STAT3 signaling pathway. Cell Physiol Biochem. 2018;51:301–314. doi: 10.1159/000495231. [DOI] [PubMed] [Google Scholar]
- Xu K, Tao W, Su Z. Propofol prevents IL-13-induced epithelial–mesenchymal transition in human colorectal cancer cells. Cell Biol Int. 2018;42:985–993. doi: 10.1002/cbin.10964. [DOI] [PubMed] [Google Scholar]
- Yang G, Rosen DG, Liu G, et al. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clin Cancer Res. 2010;16:3875–3886. doi: 10.1158/1078-0432.CCR-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Kang H, et al. Knockdown of long non-coding RNA HOST2 inhibits the proliferation of triple negative breast cancer via regulation of the let-7b/CDK6 axis. Int J Mol Med. 2019;43:1049–1057. doi: 10.3892/ijmm.2018.3995. [DOI] [PubMed] [Google Scholar]
- Zhou M, Wang X, Shi H, et al. Characterization of long non-coding RNA-associated ceRNA network to reveal potential prognostic lncRNA biomarkers in human ovarian cancer. Oncotarget. 2016;7:12598. doi: 10.18632/oncotarget.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Li J, Ji P. Propofol suppresses esophageal squamous cell carcinoma cell migration and invasion by down-regulation of sex-determining region Y-box 4 (SOX4) Med Sci Monit Int Med J Exp Clin Res. 2017;23:419. doi: 10.12659/MSM.899732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.