Abstract
Hepatic stellate cells (HSCs) activation is a key step that promotes hepatic fibrosis. Emerging evidence suggests that aerobic glycolysis is one of its important metabolic characteristics. Our previous study has reported that CD147, a glycosylated transmembrane protein, contributes significantly to the activation of HSCs. However, whether and how it is involved in the aerobic glycolysis of HSCs activation is unknown. The objective of the present study was to validate the effect of CD147 in HSCs activation and the underlying molecular mechanism. Our results showed that the silencing of CD147 decreased the expression of α-smooth muscle-actin (α-SMA) and collagen I at both mRNA and protein levels. Furthermore, CD147 silencing decreased the glucose uptake, lactate production in HSCs, and repressed the lactate dehydrogenase (LDH) activity, the expression of hexokinase 2 (HK2), glucose transporter 1 (Glut1). The effect of galloflavin, a well-defined glycolysis inhibitor, was similar to CD147 siRNA. Mechanistically, CD147 silencing suppressed glycolysis-associated HSCs activation through inhibiting the hedgehog signaling. Moreover, the hedgehog signaling agonist SAG could rescue the above effect of CD147 silencing. In conclusion, CD147 silencing blockade of aerobic glycolysis via suppression of hedgehog signaling inhibited HSCs activation, suggesting CD147 as a novel therapeutic target for hepatic fibrosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10616-021-00460-9.
Keywords: CD147, Hepatic stellate cells(HSCs), Aerobic glycolysis, Hepatic fibrosis
Introduction
Hepatic fibrosis is a complex wound-repair process in response to diverse chronic liver injuries (Bataller and Brenner 2005). If untreated, hepatic fibrosis can lead to cirrhosis and even hepatocellular carcinoma (HCC). Cirrhosis is the final stage of hepatic fibrosis, which causes over 1 million deaths each year (Ebrahimi et al. 2016). Cirrhosis is irreversible, whereas hepatic fibrosis is reversible (Weiskirchen and Tacke 2016). Therefore, clarifying the molecular mechanisms of hepatic fibrosis and preventing the progression to cirrhosis is crucial.
In the mechanisms of hepatic fibrosis, HSCs activation after liver injury remains a dominant theme,and α-smooth muscle actin (α-SMA) overexpression is a key feature in HSCs activation (Tsuchida and Friedman 2017). Recent studies evidence that HSCs activation is closely related to aerobic glycolysis which is very similar to the “Warburg effect” that appeared in tumor cells (Ban et al. 2019; Gao et al. 2019; Lian et al. 2016). Glycolysis is a complex multi-step process involving a variety of complicated proteins and enzymes, such as glucose transporters (Glut), hexokinase (HK), and lactate dehydrogenase (LDH) (Li et al. 2015a, b). Aerobic glycolysis is a prominent hallmark during HSCs activation. Nevertheless, little is known about the detailed mechanism underlying this aerobic glycolysis in HSCs activation.
Hedgehog (Hh) signaling, an evolutionarily conserved pathway, has been documented in liver physiology and pathophysiology (Choi et al. 2011). Hedgehog signaling is propagated by the interaction of the Hh ligand, namely sonic Hh (SHH), to its membrane receptors patched, activating smoothened (SMO), a central transducer of Hedgehog signaling. The activated SMO promotes the Hedgehog target gene transcription by regulating the Glioblastoma (Gli) family nuclear translocation (Kim et al. 2017). However, hedgehog-interacting protein (Hhip) can block the binding of SHH to patched (Choi et al. 2011). Several studies have shown that Hedgehog signaling activation is essential for HSCs activation and hepatic fibrogenesis (Philips et al. 2011; Sicklick et al. 2005; Swiderska-Syn et al. 2013). Accumulating evidence suggests that Hedgehog signaling is closely associated with aerobic glycolysis in various physiological and pathological processes (Chen et al. 2012; Ge et al. 2015; Lian et al. 2015; Zheng et al. 2017). Nevertheless, the potential molecular mechanisms of aerobic glycolysis mediated by Hedgehog signaling have not been fully elucidated in the HSCs activation.
CD147, a glycosylated transmembrane protein, contributes significantly to the development of HCC and other epithelial tumors (Wu et al. 2011). It plays a crucial role in tumor growth, migration, metastasis, and angiogenesis. Interestingly, Huang et al. reported that CD147 can promote glucose uptake and lactate production in HCC cells (Huang et al. 2014). Our previous works found that CD147 is increased in the activation of HSCs in vitro and in vivo (Li et al. 2015a, b). However, whether and how CD147 is involved in the aerobic glycolysis of HSCs activation is unknown.
This study set out to gain further understanding of the CD147 effect in HSCs activation, and the underlying molecular events. We verified that there is a positive correlation between CD147 expression and HSCs activation. We demonstrate that the CD147 silencing significantly suppresses the activation of HSCs by inhibiting aerobic glycolysis. This is regulated by the Hedgehog signaling, which is important for glucose metabolism in a variety of cells and tissues. This study indicates that CD147 is required to regulate aerobic glycolysis in HSCs activation, and targeting CD147 mediated Hedgehog signaling may be a useful strategy to inhibit hepatic fibrosis.
Materials and methods
Cell culture
LX-2 cells (a human HSCs cell line) and HSC-T6 cells (a rat HSC cell line) were routinely cultured in DMEM. CD147-siRNA and control siRNA were synthesized by Shanghai GenePharma Co, Ltd. si-CD147-1 (5′-GTACAAGATCACTGACTCT-3′) and si-CD147-2 (5′-GTTCTTCGTGAGTTCCTC-3′) were transfected using the lipofectamine 2000 transfection reagents (Invitrogen, Carlsbad CA USA).
Real‐time polymerase chain reaction (RT-PCR)
Real-time PCR was performed as described previously (Li et al. 2015a, b). All primers were carried out by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd: CD147: (forward) 5′-ACTCCTCACCTGCTCCTTGA-3′, (reverse) 5′-GCCTCCATGTTCAGGTTCTC-3′; α-SMA: (forward) 5′- GGCTCTGGGCTCTGTAAGG-3′ (reverse) 5′-CTCTTGCTCTGGGCTTCATC3′; collagen I : (forward) 5′- AACATGACCAAAAACCAAAA-3′, (reverse) 5′-CATTGTTTCCTGTGTCTTCTGGGTG-3′; HK2: (forward) 5′- F AGGTCCTGATGCGGTTGG-3′, (reverse) 5′-TCGCCTTTGTTCTCCTTGAT-3′; Glut1(forward) 5′-GCTCATCAACCGCAACGA-3′, (reverse) 5′-GACCTTCTTCTCCCGCATC-3′; GAPDH: (forward) 5′-GCACCGTCAAGGCTGAGAAC-3′ (reverse) 5′-TGGTGAAGACGCCAGTGGA-3′. Gene expression was analyzed by 2−∆∆Ct method, using GAPDH as endogenous reference.
Western blot analysis
Western blot analysis was conducted as previously mentioned (Li et al. 2015a, b). The primary antibodies: CD147, α-SMA, Collagen I, HK2 and Glut1 (Abcam, Cambridge, UK); patched, Gli1, and Hhip (Cell Signaling Technology, Danvers, MA, USA); Smo, (Sigma, St Louis, MO, USA); α-tubulin (Santa Cruz Technology, Santa Cruz, CA, USA).
Glucose uptake assay
LX-2 cells were cultured in 96-well plates for 24 h and subjected to various treatments. Glucose uptake was tested by the Glucose Uptake Assay Kit (Abnova, Taiwan, China) according to the specification.
Measurement of intracellular lactate
LX-2 cells were seeded in 6-well plates overnight and were treated with different reagents at indicated concentrations for 24 h. Intracellular lactate levels in lysates were determined using the Lactate Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s protocol.
Enzyme activity assay
The LX-2 cells LDH intracellular activity was detected using kits (Shanghai Meilian Biology Technology Co. Ltd., Shanghai, China) according to the manufacturer’s protocol.
Immunofluorescence staining
Immunofluorescence staining was carried out following as previously (Li et al. 2015a, b). The nucleus was stained with DAPI (Molecular probes, Eugene, USA). Primary antibody: Gli1 (Cell Signaling Technology, Danvers, MA, USA). The Gli1 expression was observed under a fluorescence microscope (Nikon Eclipse Ti, Nikon, Japan).
Statistical analysis
All the results from at least triplicate experiments were presented as the mean ± SD. GraphPad Prism 5 was used to analyze data. Means were analyzed by One-Way ANOVA. Statistical significance was stated for p < 0.05.
Results
CD147 is overexpressed in activated HSCs, and silencing of CD147 inhibits the HSCs activation
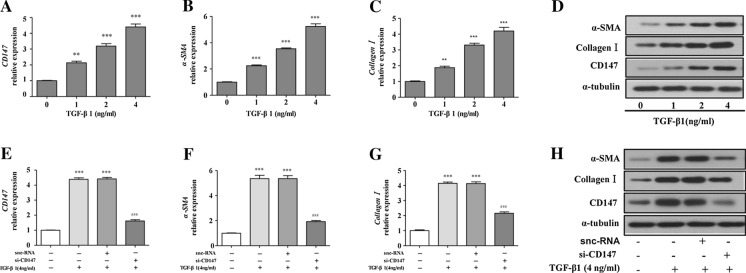
TGF-α1 is recognized as the most potent cytokine that drives hepatic fibrosis (Weng et al. 2009). HSCs activation models were established in human and rat HSCs line LX-2 and HSC-T6 cells with TGF-α1 stimulation. To explore the role of CD147 in HSCs activation, we evaluated CD147 and hepatic fibrosis markers at different concentrations of TGF-α1 in LX-2 and HSC-T6 cells. As shown in Fig. 1a–d, TGF-α1 significantly increased the CD147, α-SMA, and collagen I mRNA and protein levels in a dose-dependent manner in LX-2 cells. Western blots analyses of CD147, α-SMA, and collagen I showed the same results as above in HSC-T6 cells (Supplemental Fig. 1a). To confirm whether CD147 is crucial for HSCs activation, two different CD147 siRNAs (si-CD147) targeting CD147 was used to down-regulate CD147 expression in TGF-α1 pre-stimulated LX-2 and HSC-T6 cells. In both cell lines, we found that both siRNAs drastically reduced CD147 protein levels compared with the control groups (Supplemental Fig. 1b, c). As shown in Fig. 1e–h, CD147, α-SMA, and collagen I both mRNA and protein levels were markedly reduced in si-CD147-treated LX-2 and HSC-T6 cells (Supplemental Fig. 1d), as detected by real-time PCR and Western blots. Altogether, these data indicate that CD147-mediated HSCs activation is essential to the development of hepatic fibrosis.
Fig. 1.
CD147 is overexpressed in activated HSCs, and CD147 silencing inhibits the HSCs activation. Measurement of CD147, α-SMA, collagen I mRNA (a–c) and protein (d) levels in LX-2 cells stimulated with various doses of TGF-α1 by real-time PCR and western blots. Measurement of CD147, α-SMA, collagen I mRNA (e, f, h) and protein (i) levels in si-CD147 treated LX-2 cells exposed to 4 ng/ml TGF-α1. **p < 0.01 vs. control; ***p < 0.001 vs. control; ###p < 0.001 vs. TGF-α1+snc-RNA
Silencing of CD147 inhibits the HSCs activation through suppressing aerobic glycolysis
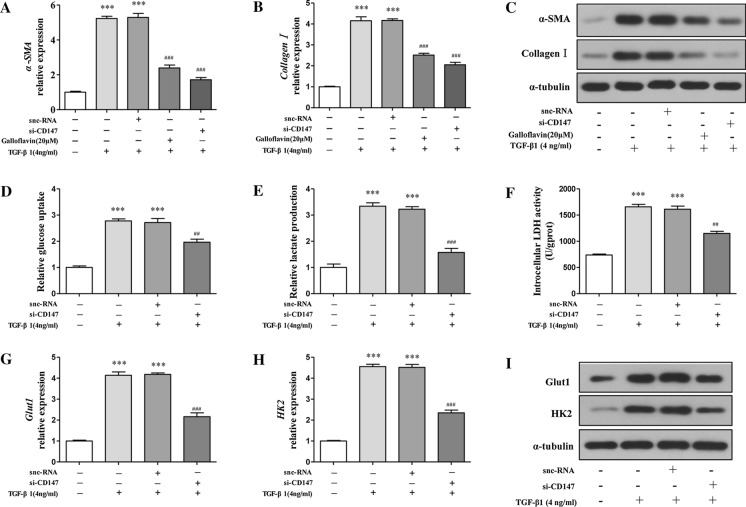
Previous researches have shown that aerobic glycolysis is significantly associated with hepatic fibrosis, we therefore further examined whether suppressing aerobic glycolysis could inhibit HSCs activation. We applied the lactate dehydrogenase inhibitor galloflavin to block aerobic glycolysis in TGF-α1 pre-stimulated LX-2 cells.
α-SMA and collagen I mRNA expression was suppressed with the treatment of galloflavin at 20 µM, detected by real-time PCR (Fig. 2a–b). Meanwhile, the protein expression of the two molecules was also suppressed by galloflavin (LX-2 Fig. 2c, HSC-T6 Supplemental Fig. 2a). To further confirm whether CD147 promotes HSCs activation through aerobic glycolysis, we examined the si-CD147 effect on HSCs’ aerobic glycolysis. We found that si-CD147 down-regulated glucose uptake, lactate production, and LDH activity (Fig. 2d–f). Moreover, si-CD147 markedly reduced the mRNA (LX-2 Fig. 2g–h) and protein (LX-2 Fig. 2 iHSC-T6 Supplemental Fig. 2b) levels of the glycolytic key proteins HK2 and Glut1. Taken together, these data showed that CD147 silencing-induced repression of aerobic glycolysis led to the inhibition of HSCs activation.
Fig. 2.
CD147 silencing inhibits the HSCs activation through suppressing aerobic glycolysis. TGF-α1, si-CD147, and/or galloflavin (a lactate dehydrogenase inhibitor) were applied to LX-2 cells at indicated concentrations for 24 h. Measurement of α-SMA, collagen I mRNA (a, b) and protein (c) levels by real-time PCR and western blots. Measurement of glycolysis parameters including glucose uptake (d), intracellular levels of lactate (e), and activities of LDH (f). Measurement of HK2, Glut1 mRNA (g, h) and protein (i) levels by real-time PCR and western blots. Statistical significance: ***p < 0.001 vs. control; ##p < 0.01 vs. TGF-α1+snc-RNA; ###p < 0.001 vs. TGF-α1+snc-RNA
Silencing of CD147 inhibits hedgehog signaling in activated HSCs
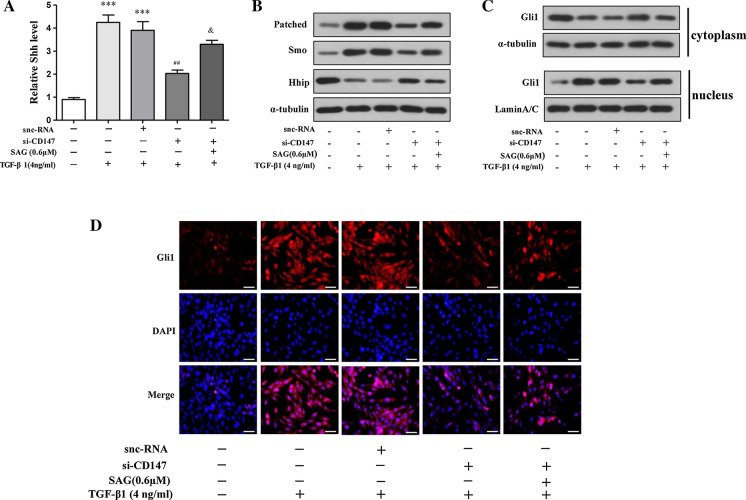
Since some studies reported HSCs glycolysis was activated by the Hedgehog signaling, the effect of CD147 in the mediation of Hedgehog signaling was investigated in the current study in LX-2 cells. The ELISA analysis indicated that si-CD147 significantly decreased Shh secretion compared with the control in LX-2 supernatant (Fig. 3a). Western blots showed that si-CD147 decreased Patched and Smo but increased Hhip at the protein level (LX-2 Fig. 3b, HSC-T6 Supplemental Fig. 3). Since Gli1 nuclear translocation is necessary for Hedgehog signaling, we evaluated Gli1 expression in both cytosolic and nuclear fractions. Expectedly, the detection of Gli1 distribution by western blots demonstrated that si-CD147 up-regulated Gli1 abundance in the cytoplasm and down-regulated its abundance in the nucleus (Fig. 3c). Simultaneously, immunofluorescence assay indicated that si-CD147 inhibits the nuclear translocation of Gli1 and more Gli1 was mainly concentrated in the cytoplasm. Furthermore, SAG, a well-established Hedgehog signaling activator, was able to rescue the Hedgehog signaling key protein expression (Shh, Patched, Smo, Hhip) or distribution (Gli1) by CD147 silencing in the context of TGF-α1 activated LX-2 cells (Fig. 3a–d). Collectively, these results consistently revealed that CD147 silencing inhibited Hedgehog signaling in activated HSCs.
Fig. 3.
Silencing of CD147 inhibits Hedgehog signaling in activated HSCs. TGF-α1, si-CD147, and/or SAG (a Hedgehog signaling activator) were applied to LX-2 cells at indicated concentrations for 24 h. a ELISA analyseis of Shh levels in cell supernatant. b Western blots analyses of protein levels of Patched, Smo, and Hhip. c Western blots analyses of protein levels of Gli in the cytosol, and nuclear extracts, respectively. d Immunofluorescence analyses of Gli distribution, scale bar: 50 μm. ***p<0.001vs.control; ##p<0.01vs.TGF-α1+snc-RNA; &p<0.05vs. TGF-α1+si-CD147
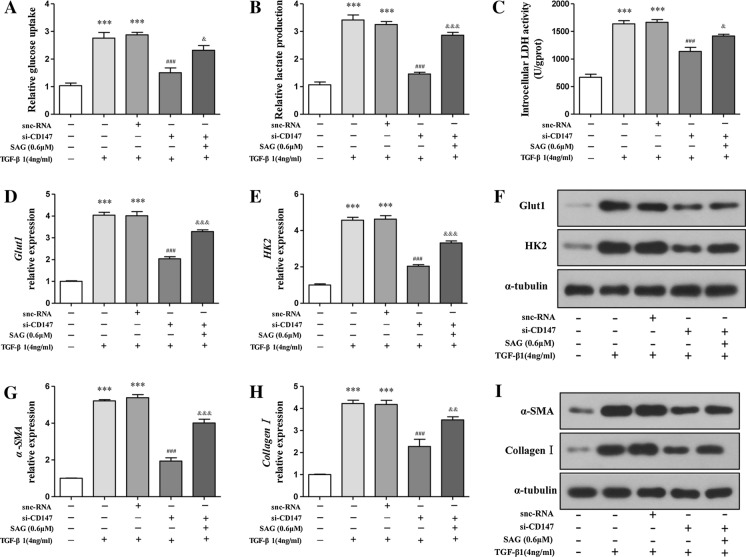
CD147 regulated glycolysis‐mediated HSCs activation through hedgehog signaling
In the above experiments, we demonstrated that CD147 silencing inhibited HSCs activation through down-regulation of glycolysis. We finally examined the role of Hedgehog signaling in the CD147 effect on HSCs glycolysis and activation. SAG was used to research whether the effect of CD147 was related to the regulation of Hedgehog signaling. As shown in Fig. 4a–c, si-CD147 inhibition of glucose uptake, lactate production, and LDH activity was rescued by SAG. Meanwhile, si-CD147 reduction of mRNA and protein expressions of HK2, Glut1 were also weakened by SAG (Fig. 4d–f). Even further, real-time PCR analyses illustrated that α-SMA and collagenImRNA levels were suppressed by si-CD147, but enhanced by SAG (Fig. 4g–h). Consistently, western blots demonstrated that si-CD147 down-regulation of α-SMA and collagenIprotein levels were abolished by SAG (Fig. 4i). Collectively, these data revealed that CD147 regulated glycolysis-mediated HSCs activation through Hedgehog signaling.
Fig. 4.
CD147 regulated glycolysis-mediated HSCs activation through Hedgehog signaling. TGF-α1,si-CD147, and/or SAG were applied to LX-2 cells at indicated concentrations for 24 h. Measurement of glycolysis parameters including glucose uptake (a), intracellular levels of lactate (b), and activities of LDH (c). Measurement of HK2, Glut1 mRNA (d, e) and protein (f) levels by real-time PCR and western blots. Measurement of α-SMA, collagen I mRNA (g, h) and protein (i) levels by real-time PCR and western blots.***p < 0.001 vs. control; ###p < 0.001 vs. TGF-α1+snc-RNA; &p < 0.05, &&p < 0.01, &&&p < 0.001 vs. TGF-α1+si-CD147
Discussion
Numerous studies have established that HSCs activation is a hallmark of hepatic fibrogenesis (Tsuchida and Friedman 2017). Accordingly, suppression of HSCs activation is an important intervention strategy for hepatic fibrosis or cirrhosis. However, the molecular mechanism of HSCs activation are not fully elucidated, which resulted in the involved molecular targets were still ambiguous. TGF-α1, the primary member of the TGF-α superfamily, is considered as the main mediator in the development of hepatic fibrosis. It is the strongest factor involved in HSC activation via the canonical ALK/SMAD and Rho GTPase pathways, and the TGF-α1-activated hepatic stellate cells model is a well-recognized culture model (Huang et al. 2011; Liu et al. 2016; Lv et al. 2020; Ohyama et al. 2012; Shimada et al. 2011). Besides, TGF-α1 was identified to regulate a series of glycolytic genes to promote glycolysis in many diseases such as renal fibrosis, osteoarthritis, and glioblastoma (Ding et al. 2017; Rodríguez-García et al. 2017; Wang et al. 2018). However, it remains unclear whether TGF-α1 promotes hepatic stellate cells activation by regulating glycolysis. Furthermore, TGF-α1 is also regarded as an important inducer that triggers Hedgehog signaling activation (Thayer et al. 2003). TGF-α1 upregulates major Hedgehog signaling molecules shh and Gli expression in many diseases such as interstitial renal fibrosis, ovarian and oral squamous cell carcinomas. The role of TGF-α1 in the regulation of Hedgehog signaling in the development of hepatic fibrosis is yet to be explored. Our previous study revealed that TGF-α1 elevated CD147 expression in HSCs. In this study, we verified again that CD147 expression was significantly increased in TGF-α1 activated HSCs. Further investigation found that the silencing of CD147 markedly inhibited levels of α-SMA, collagen I in activated HSCs. Consistent with our previous reports that CD147 over-expression promoted HSCs transdifferentiation (Li et al. 2015a, b). These findings lead us to aim at CD147 how to mediate HSCs activation. Interestingly, our experimental data proved the regulation of aerobic glycolysis was a vital molecular event and an essential step in CD147’ profibrotic process.
Aerobic glycolysis is observed in rapidly proliferating tumor cells, and this phenomenon is called the “Warburg effect”. Similar to the tumor cells Warburg effect, several recent studies suggest that induction of aerobic glycolysis is a driving force of the quiescent HSCs differentiate into myofibroblast (Gao et al. 2019; Lian et al. 2016; Wang et al. 2019). CD147, a tumor-associated antigen, contributes to regulating glucose metabolism in HCC, lung adenocarcinoma (LUAD), and thyroid cancer (TC) (Huang et al. 2014, 2018; Zhang et al. 2020). Huang et al. (2014) reported CD147 promotes glucose metabolism reprogramming and cell proliferation by inhibiting p53-dependent signaling, which indicates the CD147 effect in maintaining the aerobic glycolysis process related to HCC. Zhang et al. (2020) found that CD147 promoted aerobic glycolysis depending on MCT1 and MCT4 in LUAD. However, there is no exact evidence, which supports aerobic glycolysis of HSCs have a positive relationship with CD147 in hepatic fibrosis. In our study, we hypothesized that HSCs activation could be regulated by aerobic glycolysis, and silencing CD147 could inhibit HSC activation through decreasing aerobic glycolysis. We uncovered that α-SMA and collagen I expression were downregulated after the use of glycolysis inhibitors in HSCs. Our observations verified again that HSCs activation is potently suppressed by inhibition of aerobic glycolysis. Furthermore, si-CD147 effectively blocked the aerobic glycolysis, evidenced by reduced glucose uptake, decreased lactate production, and downregulation of two key rate-limiting enzymes. Taken together, The HSCs activation was disrupted by blockade of aerobic glycolysis, which was mediated by CD147.
Hedgehog signaling is involved in regulating embryogenesis and tissue regeneration and contributes to fibrogenesis in various tissues (Hu et al. 2015). It has been reported that a positive correlation between hepatic fibrosis degree and activation of the Hedgehog signaling (Guy et al. 2012; Swiderska-Syn et al. 2013). Moreover, Yan et al. have shown that Hedgehog signaling can activate HSCs to promote hepatic fibrosis while inhibiting the Hedgehog signaling can prevent the trans-differentiation from HSCs to myofibroblasts (Yan et al. 2020). Furthermore, multiple latest studies show that blockage of the Hedgehog signaling can inhibit HSCs activation and decreased hepatic fibrosis (Feng et al. 2019; Gao et al. 2019; Hu et al. 2019; Shen et al. 2020). Interestingly, several researchers have found that the Hedgehog signaling during HSCs activation contributed to mediating the reprogramming of glucose metabolic (Chen et al. 2012; Gao et al. 2019; Lian et al. 2016). In our study, we have identified that si-CD147 inhibited HSCs activation, which is induced by aerobic glycolysis. Therefore, we supposed whether CD147 regulated HSCs activation and HSCs aerobic glycolysis via the Hedgehog signaling. Our data have revealed that CD147 silencing significantly reduced Shh, Patched, and Smo but increased Hhip expression in HSCs. Meanwhile, CD147 silencing suppressed Gli1 translocation from cytoplasm to nuclear in activated HSCs. Moreover, the Hedgehog pathway agonist SAG markedly rescued the Hedgehog signaling key protein, HSC fibrosis marker molecule, and glycolysis-related factor expression in si-CD147-treated activated HSCs. These results indicate that CD147 regulated glycolysis-mediated HSCs activation through Hedgehog signaling. However, the exact molecular mechanism of CD147 on regulating the Hedgehog signaling promoted aerobic glycolysis in HSCs remains unclear and requires further investigations.
Certainly, our study has several limitations. First, further in vivo and clinical researches are required to verify our experimental results in vitro. Second, the specific molecular mechanism between CD147 and Hedgehog signaling in HSCs aerobic glycolysis needs to be investigated. These limitations will be further improved in our next work.
In summary, our work illustrates that CD147 is overexpressed in activated HSCs, and silencing of CD147 significantly suppresses the activation of HSCs, which is related to the inhibition of HSCs aerobic glycolysis. Moreover, these effects are closely related to the regulation of Hedgehog signaling mediated by CD147. In conclusion, Our experimental results show that the regulation of HSCs aerobic glycolysis by CD147 may represent novel molecular basis and therapeutic target for hepatic fibrosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Fig. 1. CD147 is overexpressed in activated HSCs, and CD147 silencing inhibits the HSCs activation. Measurement of CD147, α-SMA, collagen protein levels (A) in HSC-T6 cells stimulated with various doses of TGF-β1 by western blots. The protein levels of CD147 knocked down by 2 different siRNAs were measured in LX-2 (B) and HSC-T6 (C) cells by western blots. Measurement protein levels of CD147 (D) in si-CD147 treated HSC-T6 cells exposed to 4ng/ml TGF-β1Supplemental Fig. 1. CD147 is overexpressed in activated HSCs, and CD147 silencing inhibits the HSCs activation. Measurement of CD147, α-SMA, collagen protein levels (A) in HSC-T6 cells stimulated with various doses of TGF-β1 by western blots. The protein levels of CD147 knocked down by 2 different siRNAs were measured in LX-2 (B) and HSC-T6 (C) cells by western blots. Measurement protein levels of CD147 (D) in si-CD147 treated HSC-T6 cells exposed to 4ng/ml TGF-β1 (TIF 887 kb)
Supplemental Fig. 2. CD147 silencing inhibits the HSCs activation through suppressing aerobic glycolysis. TGF-β1, si-CD147, and/or galloflavin(a lactate dehydrogenase inhibitor) were applied to HSC-T6 cells at indicated concentrations for 24 h. A. Measurement of α-SMA, collagen I protein levels by western blots. B. Measurement of HK2, Glut1 protein levels by western blots (TIF 258 kb)
Supplemental Fig. 3. Silencing of CD147 inhibits Hedgehog signaling in activated HSCs. TGF-β1,si-CD147, and/or SAG (a Hedgehog signaling activator) were applied to HSC-T6 cells at indicated concentrations for 24 h. Western blots analyses of protein levels of Patched, Smo, and Hhip (TIF 563 kb)
Acknowledgements
This work was supported by grants from the National Natural Science Fund of China (Grant No. 81700546),Scientific Research Project of Xi’an Medical University (Grant Nos. 2017PT13; 2018PT68), Key Research Project in Shaanxi Province (Grant Nos. 2018SF-168; 2020SF-349), Natural Science Basic Research Program of Shaanxi (Grant No. 2019JQ-886), National Science and Technology Fund research Program of Xi’an Medical University (Grant No. 2017GFY18).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ban D, Hua S, Zhang W, Shen C, Miao X, Liu W. Costunolide reduces glycolysis-associated activation of hepatic stellate cells via inhibition of hexokinase-2. Cell Mol Biol Lett. 2019;24:1. doi: 10.1186/s11658-019-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Investig. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, Xie G, Moylan CA, Garibaldi F, Premont R, et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319–1329. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SS, Omenetti A, Syn W-K, Diehl AM. The role of Hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol. 2011;43:238–244. doi: 10.1016/j.biocel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan Q, Luo J, Zen K, Yang J. Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis. Am J Physiol Renal Physiol. 2017;313:F561–F575. doi: 10.1152/ajprenal.00036.2017. [DOI] [PubMed] [Google Scholar]
- Ebrahimi H, Naderian M, Sohrabpour AA. New concepts on pathogenesis and diagnosis of liver fibrosis; a review article. Middle East J Dig Dis. 2016;8:166–178. doi: 10.15171/mejdd.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Wang CF, Liu T, Li JJ, Wu LW, Yu Q, Li SN, Zhou YT, Zhang J, Chen JJ, et al. Procyanidin B2 inhibits the activation of hepatic stellate cells and angiogenesis via the Hedgehog pathway during liver fibrosis. J Cell Mol Med. 2019;23:6479–6493. doi: 10.1111/jcmm.14543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Sun J, Wang F, Lu Y, Wen C, Bian Q, Wu H. Deoxyelephantopin suppresses hepatic stellate cells activation associated with inhibition of aerobic glycolysis via hedgehog pathway. Biochem Biophys Res Commun. 2019;516:1222–1228. doi: 10.1016/j.bbrc.2019.07.015. [DOI] [PubMed] [Google Scholar]
- Ge X, Lyu P, Gu Y, Li L, Li J, Wang Y, Zhang L, Fu C, Cao Z. Sonic hedgehog stimulates glycolysis and proliferation of breast cancer cells Modulation of PFKFB3 activation. Biochem Biophys Res Commun. 2015;464:862–868. doi: 10.1016/j.bbrc.2015.07.052. [DOI] [PubMed] [Google Scholar]
- Guy CD, Suzuki A, Zdanowicz M, Abdelmalek MF, Burchette J, Unalp A, Diehl AM. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–1721. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Lin X, Lu H, Chen B, Bai Y. An overview of hedgehog signaling in fibrosis. Mol Pharmacol. 2015;87:174–182. doi: 10.1124/mol.114.095141. [DOI] [PubMed] [Google Scholar]
- Hu ZQ, Li L, Ran JH, Chu G, Gao HQ, Guo LC, Chen JP. miR-125b acts as anti-fibrotic therapeutic target through regulating Gli3 in vivo and in vitro. Ann Hepatol. 2019;18:825–832. doi: 10.1016/j.aohep.2019.06.016. [DOI] [PubMed] [Google Scholar]
- Huang S, Chang SJ, Yang M, Chen JJ, Chang WH. Nanoscale hepatoprotective herbal decoction attenuates hepatic stellate cell activity and chloroform-induced liver damage in mice. Int J Nanomed. 2011;6:1365–1371. doi: 10.2147/IJN.S19503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Li J, Xing J, Li W, Li H, Ke X, Zhang J, Ren T, Shang Y, Yang H, et al. CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the p53-dependent signaling pathway. J Hepatol. 2014;61:859–866. doi: 10.1016/j.jhep.2014.04.035. [DOI] [PubMed] [Google Scholar]
- Huang P, Mao LF, Zhang ZP, Lv WW, Feng XP, Liao HJ, Dong C, Kaluba B, Tang XF, Chang S. Down-regulated miR-125a-5p promotes the reprogramming of glucose metabolism and cell malignancy by increasing levels of CD147 in thyroid cancer. Thyroid. 2018;28:613–623. doi: 10.1089/thy.2017.0401. [DOI] [PubMed] [Google Scholar]
- Kim J, Hyun J, Wang S, Lee C, Lee JW, Moon EY, Cha H, Diehl AM. Thymosin beta-4 regulates activation of hepatic stellate cells via hedgehog signaling. Sci Rep. 2017;7:3815. doi: 10.1038/s41598-017-03782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-b, Gu J-d, Zhou Q-h. Review of aerobic glycolysis and its key enzymes - new targets for lung cancer therapy. Thoracic Cancer. 2015;6:17–24. doi: 10.1111/1759-7714.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Ju D, Zhang DW, Li H, Kong LM, Guo Y, Li C, Wang XL, Chen ZN, Bian H. Activation of TGF-beta1-CD147 positive feedback loop in hepatic stellate cells promotes liver fibrosis. Sci Rep. 2015;5:16552. doi: 10.1038/srep16552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian N, Jiang Y, Zhang F, Jin H, Lu C, Wu X, Lu Y, Zheng S. Curcumin regulates cell fate and metabolism by inhibiting hedgehog signaling in hepatic stellate cells. Lab Investig. 2015;95:790–803. doi: 10.1038/labinvest.2015.59. [DOI] [PubMed] [Google Scholar]
- Lian N, Jin H, Zhang F, Wu L, Shao J, Lu Y, Zheng S. Curcumin inhibits aerobic glycolysis in hepatic stellate cells associated with activation of adenosine monophosphate-activated protein kinase. IUBMB Life. 2016;68:589–596. doi: 10.1002/iub.1518. [DOI] [PubMed] [Google Scholar]
- Liu XY, Liu RX, Hou F, Cui LJ, Li CY, Chi C, Yi E, Wen Y, Yin CH. Fibronectin expression is critical for liver fibrogenesis in vivo and in vitro. Mol Med Rep. 2016;14:3669–3675. doi: 10.3892/mmr.2016.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv F, Li N, Kong M, Wu J, Fan Z, Miao D, Xu Y, Ye Q, Wang Y. CDKN2a/p16 Antagonizes Hepatic Stellate Cell Activation and Liver Fibrosis by Modulating ROS Levels. Front Cell Dev Biol. 2020;8:176. doi: 10.3389/fcell.2020.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Sato K, Kishimoto K, Yamazaki Y, Horiguchi N, Ichikawa T, Kakizaki S, Takagi H, Izumi T, Mori M. Azelnidipine is a calcium blocker that attenuates liver fibrosis and may increase antioxidant defence. Br J Pharmacol. 2012;165:1173–1187. doi: 10.1111/j.1476-5381.2011.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips GM, Chan IS, Swiderska M, Schroder VT, Guy C, Karaca GF, Moylan C, Venkatraman T, Feuerlein S, Syn W-K, et al. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS ONE. 2011;6:e23943. doi: 10.1371/journal.pone.0023943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-García A, Samsó P, Fontova P, Simon-Molas H, Manzano A, Castaño E, Rosa JL, Martinez-Outshoorn U, Ventura F, Navarro-Sabaté À, et al. TGF-α1 targets Smad, p38 MAPK, and PI3K/Akt signaling pathways to induce PFKFB3 gene expression and glycolysis in glioblastoma cells. FEBS J. 2017;284:3437–3454. doi: 10.1111/febs.14201. [DOI] [PubMed] [Google Scholar]
- Shen JY, Yan JY, Wei XZ, Liu ZP, Ni J, Hu BW, Jin LF (2020) Gant61 ameliorates CCl4-induced liver fibrosis by inhibition of Hedgehog signaling activity. Toxicol Appl Pharmacol 387:114853 [DOI] [PubMed]
- Shimada H, Staten NR, Rajagopalan LE. TGF-α1 mediated activation of Rho kinase induces TGF-α2 and endothelin-1 expression in human hepatic stellate cells. J Hepatol. 2011;54:521–528. doi: 10.1016/j.jhep.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Sicklick JK, Li YX, Choi SS, Qi Y, Chen W, Bustamante M, Huang JW, Zdanowicz M, Camp T, Torbenson MS, et al. Role for Hedgehog signaling in hepatic stellate cell activation and viability. Lab Investig. 2005;85:1368–1380. doi: 10.1038/labinvest.3700349. [DOI] [PubMed] [Google Scholar]
- Swiderska-Syn M, Suzuki A, Guy CD, Schwimmer JB, Abdelmalek MF, Lavine JE, Diehl AM. Hedgehog pathway and pediatric nonalcoholic fatty liver disease. Hepatology. 2013;57:1814–1825. doi: 10.1002/hep.26230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- Wang C, Silverman RM, Shen J, O’Keefe RJ. Distinct metabolic programs induced by TGF-α1 and BMP2 in human articular chondrocytes with osteoarthritis. J Orthop Transl. 2018;12:66–73. doi: 10.1016/j.jot.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Jia Y, Li M, Wang L, Shao J, Guo Q, Tan S, Ding H, Chen A, Zhang F, et al. Blockade of glycolysis-dependent contraction by oroxylin a via inhibition of lactate dehydrogenase-a in hepatic stellate cells. Cell Commun Signal. 2019;17:11. doi: 10.1186/s12964-019-0324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskirchen R, Tacke F. Liver fibrosis: from pathogenesis to novel therapies. Dig Dis (Basel, Switzerland) 2016;34:410–422. doi: 10.1159/000444556. [DOI] [PubMed] [Google Scholar]
- Weng HL, Liu Y, Chen JL, Huang T, Xu LJ, Godoy P, Hu JH, Zhou C, Stickel F, Marx A, et al. The etiology of liver damage imparts cytokines transforming growth factor beta1 or interleukin-13 as driving forces in fibrogenesis. Hepatology. 2009;50:230–243. doi: 10.1002/hep.22934. [DOI] [PubMed] [Google Scholar]
- Wu J, Ru NY, Zhang Y, Li Y, Wei D, Ren Z, Huang XF, Chen ZN, Bian H. HAb18G/CD147 promotes epithelial-mesenchymal transition through TGF-beta signaling and is transcriptionally regulated by Slug. Oncogene. 2011;30:4410–4427. doi: 10.1038/onc.2011.149. [DOI] [PubMed] [Google Scholar]
- Yan JY, Huang HR, Liu ZP, Shen JY, Ni J, Han JW, Wang RJ, Lin DR, Hu BW, Jin LF. Hedgehog signaling pathway regulates hexavalent chromium-induced liver fibrosis by activation of hepatic stellate cells. Toxicol Lett. 2020;320:1–8. doi: 10.1016/j.toxlet.2019.11.017. [DOI] [PubMed] [Google Scholar]
- Zhang YF, Liu JJ, Sun YC, Yu XZ, Wang J, Dai D, Zhu YJ, Song XY, Zhu L, Li XF, et al. Enhanced glucose metabolism mediated by CD147 is associated with F-18-FDG PET/CT imaging in lung adenocarcinoma. Thoracic Cancer. 2020;11:1245–1257. doi: 10.1111/1759-7714.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Han H, Liu G-P, Ma Y-X, Pan R-L, Sang L-J, Li R-H, Yang L-J, Marks JR, Wang W, et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. Embo J. 2017;36:3325–3335. doi: 10.15252/embj.201797609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. CD147 is overexpressed in activated HSCs, and CD147 silencing inhibits the HSCs activation. Measurement of CD147, α-SMA, collagen protein levels (A) in HSC-T6 cells stimulated with various doses of TGF-β1 by western blots. The protein levels of CD147 knocked down by 2 different siRNAs were measured in LX-2 (B) and HSC-T6 (C) cells by western blots. Measurement protein levels of CD147 (D) in si-CD147 treated HSC-T6 cells exposed to 4ng/ml TGF-β1Supplemental Fig. 1. CD147 is overexpressed in activated HSCs, and CD147 silencing inhibits the HSCs activation. Measurement of CD147, α-SMA, collagen protein levels (A) in HSC-T6 cells stimulated with various doses of TGF-β1 by western blots. The protein levels of CD147 knocked down by 2 different siRNAs were measured in LX-2 (B) and HSC-T6 (C) cells by western blots. Measurement protein levels of CD147 (D) in si-CD147 treated HSC-T6 cells exposed to 4ng/ml TGF-β1 (TIF 887 kb)
Supplemental Fig. 2. CD147 silencing inhibits the HSCs activation through suppressing aerobic glycolysis. TGF-β1, si-CD147, and/or galloflavin(a lactate dehydrogenase inhibitor) were applied to HSC-T6 cells at indicated concentrations for 24 h. A. Measurement of α-SMA, collagen I protein levels by western blots. B. Measurement of HK2, Glut1 protein levels by western blots (TIF 258 kb)
Supplemental Fig. 3. Silencing of CD147 inhibits Hedgehog signaling in activated HSCs. TGF-β1,si-CD147, and/or SAG (a Hedgehog signaling activator) were applied to HSC-T6 cells at indicated concentrations for 24 h. Western blots analyses of protein levels of Patched, Smo, and Hhip (TIF 563 kb)