Abstract
Background and Purpose:
Mitochondrion is the main indicator of oocyte quality and one of the components of oocyte, which is sensitive to oxidative damage during the maturation process. Mitoquinone mesylate (MitoQ) is a strong antioxidant targeting mitochondria as well as anti-apoptotic agent. However, the effect of MitoQ on the quality of oocytes during in vitro maturation (IVM) is still unknown.
Objectives:
This study investigated the possible effects of MitoQ on maturation and developmental competency in mice oocytes.
Materials and Methods:
The oocytes were collected at germinal vesicle stage from 6-8-week old female NMRI mice and then cultured in TCM-199 medium supplemented with 0, 0.01, 0.02 and 0.04 µM MitoQ. The sham group was treated with DMSO (0.01% v.v). Then intracellular Glutathione (GSH), reactive oxygen species (ROS) levels, mitochondria membrane potential (ΔΨm), as well as in vitro fertilization (IVF) rate in the 18-20 h matured oocytes and metaphase II (MII) oocytes (in vivo-control), were assessed.
Results:
The results showed that between three dose of MitoQ, the 0.02 µM significantly increased nuclear maturation rate, GSH level, fertilization rate and blastulation (92.6, 231.7, 90.19 and 81.66%, respectively) than the in vitro-control (71.14, 152, 78.84 and 73.50%, respectively) and more comparable to that of the in vivo matured oocytes (100, 243.5, 92.10 and 83%, respectively). Also, the mitochondria membrane potential in the 0.02 µM MitoQ was significantly higher compared with those in the other groups (4.4). However, the intracellular ROS level in 0.02 µM MitoQ was significantly decreased (38.72%) compared to in vitro-control (82.2%) and was similar to the in vivo-control (33.5%).
Conclusion:
The results indicated that supplementation of IVM medium with MitoQ (specially 0.02 µM) enhance maturation and fertilization rate. In conclusion, MitoQ might be considered as a novel component that could be added to IVM media.
Keywords: Antioxidant, Assisted reproduction, Fertilization, MitoQ, Oocyte in vitro maturation
1. Introduction
Mitochondria are critical intracellular organelle in cell metabolism with an important dichotomous role in oocyte development ( 1 ) . During oocyte maturation, several cellular functions such as mitotic spindle formation and ATP production are regulated by mitochondria function and distribution ( 2 , 3 ) . Oocytes in vitro maturation (IVM) is an acceptable method for fertility preservation in patients who are in danger of ovarian hyperstimulation syndrome (OHSS) or those with cancer ( 4 , 5 ) . It has been shown that IVM oocytes suffer from low developmental capacity and fertilization ( 6 , 7 ) . Therefore, IVM oocytes are not usually used in infertility treatment ( 4 , 5 ) . During IVM, mitochondrial functional integrity is necessary for oocyte survival and fertilization outcome ( 8 ) . Mitochondrial function is largely influenced by external factors such as light, culture medium conditions and oxygen exposure. All of which may cause retardation in meiosis initiation, thereby influence cytoplasm and nuclear maturation, which are both crucial for fertilization ( 9 , 10 ) .
Also, the increasing interval time between ovulation and fertilization in IVM, reduces the mitochondrial membrane potential and subsequently increases the oxidative stress and reactive oxygen species (ROS) production ( 7 , 9 ) . Oxidative stress impairs mitochondrial function ( 2 ) . It results in increased expression of apoptotic factors and ROS production, decreased levels of ATP and Glutathione (GSH), DNA fragmentation, and calcium homeostasis disorder. Furthermore, oxidative stress leads to decreased fertilization rate, low-quality embryos and consequently higher rates of miscarriage compared to in vivo matured oocyte ( 2 , 11 ) . Therefore, IVM system must be optimized. Antioxidants such as resveratrol, melatonin and retinoic acid are generally added to IVM culture media to improve oocyte maturation ( 12 - 14 ) . Several well-known cellular effects have been indicated by these antioxidants.
Mitoquinone mesylate (MitoQ) is a well-known artificial antioxidant having ability to target mitochondrial dysfunction. It is rapidly and extensively taken up by mitochondria without any carrier. Structurally, MitoQ consists of lipophilic triphenylphosphonium (TTP) covalently linked to ubiquinone ( 15 , 16 ) . MitoQ has been successfully applied in a number of preclinical models suffered by the side effects of oxidative stress and apoptotic death. These results were obtained by modifying redox signaling pathways ( 16 , 17 ) .
2. Objective
Since oxidative stress during IVM is inevitable, there is a growing demand for mitochondria-targeted antioxidants aimed at preserving mitochondrial function. Herein, we have presented the first study investigating the potential role of MitoQ as a mitochondrial-targeted antioxidant on improving oocyte in vitro maturation and embryo development. The MitoQ was applied in the IVM medium, to investigate whether MitoQ could enhance the developmental competence of immature oocytes. Following IVM with and without MitoQ, we examined oocyte nuclear maturation, intracellular levels of GSH and ROS, mitochondrial membrane potential and embryonic development.
3. Materials and Methods
3.1. Animal Care
Female and male NMRI mice, 6-8 weeks old, were obtained from Royan Institute of Iran and kept under controlled conditions (12-h:12-h light/dark cycle, 22°C, and food and water ad lib). All animal protocols were approved by the Shahid Beheshti University of Medical Science Animal Ethics Committee based on the University guidelines.
3.2. Collection of Immature Oocytes
The mice were administered intraperitoneal (I.P.) injections of 7.5 IU of PMSG (Sigma), in order to produce oocytes at prophase I. Forty-eight h post- injection, the mice were sacrificed and extracted ovaries were placed in pre-warmed (37 °C) TCM-199 (Invitrogen), with 10% (v.v) heat-inactivated fetal bovine serum (FBS; Sigma) under sterile conditions. Excess adipose tissue was removed from the ovary and cumulus-oocyte-complex (COCs) were aspirated from antral follicles using 28-G needles. Germinal vesicle (GV) oocytes enclosed within the intact compact cumulus layers were selected and washed three times in 20 μL drops of TCM-199 supplemented with 10% FBS at 37 °C before culture ( 18 ) .
3.3. In Vitro Maturation
COCs (cumulus thickness, compactness granulation and homogeneity of the ooplasm) (9) were collected and incubated in TCM-199 containing 20% (v.v) FBS, 7.5 IU hCG (Pregnyl, Organon) 100 mIU FSH (Sigma), and 1μg.ml-1 Beta-estradiol (Sigma), for 18-20 h at 37 °C in a humidified chamber with 5% CO2 (9) . For experimental groups, three different doses of MitoQ (Medkoo) (0.01, 0.02 and 0.04 µM) were added into IVM medium. For sham group, DMSO (0.01% v.v, as the MitoQ solvent) without any antioxidant was added into IVM medium. For in vitro-control group, COCs were cultured without any antioxidant interaction in IVM medium. After 18- 20 h, oocytes with one polar body (PB) were verified as metaphase II (MII oocytes). The number of MII oocytes with a round zona pellucida (ZP), a small perivitelline space and pale moderately granular cytoplasm without inclusions ( 19 ) was counted in each group and accordingly, IVF and other examinations were performed.
3.4.In Vivo Matured Oocyte Collection
Forty eight h after I.P. injection of PMSG, 7.5 units IU of HCG (Pregnyl, Organon) was prescribed, and 13-14 h later the mice were sacrificed by cervical dislocation and fallopian tubes were removed and placed into the collection medium containing Human Tubal Fluid (HTF; Sigma), supplemented with 5 mg.mL-1 bovine serum albumin (BSA; Sigma). After removing residual adipose tissue, the fallopian tubes were opened using a 28-gauge needle, and the cumulus egg mass was collected. Enzymatic digestion and mechanical dissociation of the cumulus mass were performed using 85 IU.mL hyaluronidase (Cook Medical) and mechanical dissociation. The extracted oocytes were placed in 20 μL drops of HTF medium. Only MII oocytes with one PB and normal morphology (with round ZP, a small perivitelline space and pale moderately granular cytoplasm without inclusions) ( 19 ) were selected and used in this study. All steps were performed under mineral oil (Sigma) and incubated at 37 °C in humidified air with 5% CO2 ( 20 ) .
3.5. In Vitro Fertilization
The epididymal spermatozoa were obtained from cauda epididymis of male NMRI mice. The obtained spermatozoa cells were preincubated for 1.5 h in capacitation medium (HTF supplemented with 15 mg.mL-1 BSA). Sperm at a final concentration of 2×106 sperms.mL was used to inseminate COCs. Fertilization plate was incubated at 37 °C in humidified air with 5%CO2. After 4-6 h, the inseminated oocytes with two pronuclei were washed twice by moderate pipetting and then placed in KSOM medium (Sigma) supplemented with 5 mg.mL-1 BSA. To assess fertilization, the development of the 2-cell stage embryos was measured one day after insemination. The 2-cell stage embryos were transferred into a fresh medium for further development until blastocyst formation on day 5 after insemination ( 18 ) .
3.6. Intracellular Measurement of ROS and GSH Levels
Denuded MII oocytes collected from all experimental and control groups prepared for evaluation of intracellular reactive oxygen species (ROS) and Glutathione (GSH) level. In short, 2′7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen (and 4-chloromethyl-6,8- difluoro-7-hydroxycoumarin (Cell Tracker Blue CMF2HC Molecular Probes; Invitrogen) were applied to detect intracellular ROS as green fluorescence and GSH as blue fluorescence, respectively. A total of 35-40 denuded MII oocytes from each group were incubated in PBS.PVA (PBS, which possessed 1 mg.mL-1 polyvinyl alcohol) containing 10 μM H2DCFDA or 10 μM Cell Tracker Blue in the dark for 30 minutes at 37 °C with 5% CO2. After incubation, the oocytes were washed with PBS.PVA drops several times, then placed in 50 μL PBS.PVA drops, under mineral oil and observed using a fluorescence microscope (Labomed Lx 400 fluorescence microscope; Labo America Inc., Roseville, CA, USA) with UV filters (460 nm for ROS and 370 nm for GSH) ( 21 ) . All the experiments were repeated three times in the dark. The fluorescent images were recorded in TIFF format and the fluorescence intensity was quantified according to control oocytes intensity by Image J (Version 1.40; National Institutes of Health) software for post acquisitions analysis.
3.7. Evaluation of Mitochondrial Membrane Ptential
Mitochondria membrane potential (ΔΨm) was measured using tetrachloro-1,1′,3,3′ tetraethylbenzamideazolyl- carbocyanine iodide (JC-1, Invitrogen), in all 35-40 Denuded MII oocytes collected from each experimental group. All oocytes were stained with 5µg.mL-1 JC-1 added to HTF and incubated in the dark for 30 minutes at 37 °C with 5% CO2. After incubation, the oocytes were washed several times in HTF drops and then placed in 20 μL HTF drops under mineral oil. The images were captured using a fluorescence microscope with UV filters (485 nm filters are in green fluorescence and 590 nm are represented in red). The fluorescent images recorded in TIFF format along with all the graphics files were saved on the computer and analyzed with ImageJ software. At high potentials, JC-1 monomers form J-aggregates which are recognized by red fluorescence, while at a low potential, JC-1 generally exists as a monomer that can be recognized by green fluorescence. All the experiments were repeated three times and all performed in dark ( 22 ) .
3.8. Statistical Analysis
All statistical tests were executed on GraphPad Prism (GraphPad Software, Inc.) and they were repeated three times. The results are presented as the mean ± SEM. The statistical significance was determined by One- way ANOVA and Tukey post-test. A p value <0.05 was considered statistically significant.
4. Results
4.1. Maturation Rate of Oocytes
The results of oocytes nuclear maturation rate were shown in Table 1. The percentage of MII oocytes in the IVM medium treated with MitoQ (0.01, 0.02 and 0.04µM) was significantly higher (89.01, 92.6 and 83.05%, respectively) than in vitro-control (71.14%) and sham (69.25%) (P< 0.05). Moreover, the nuclear maturation rate of oocytes in the 0.02 µM was higher than two other MitoQ supplemented groups.
Table 1.
Effect of different concentration of MitoQ in maturation media on mouse oocytes developmental competence, compared to in vivo-control, in vitro-control and sham groups.
| In vivo-control | In vitro-control | Sham | MitoQ (0.01 µM) | MitoQ (0.02 µM) | MitoQ (0.04 µM) | |
|---|---|---|---|---|---|---|
| Maturation rate | ---- | 71.14 ± 0.91 | 69.25 ± 1.38 | 89.01 ± 1.40 a | 92.6 ± 0.92a | 83.05 ± 0.91c |
| Matured (per initiated) | 178 | 156 | 163 | 169 | 153 | 171 |
| Fertilization rate (%) | 92.1±1.90a | 78.84 ± 1.54 | 77.91 ± 1.31 | 85.79 ± 2.20 | 90.19 ± 2.01a | 80.7 ± 1.58 |
| Cleavage rate (%) | 88 ± 2.48 a | 65.75 ± 2.49 | 63.75 ± 1.31 | 76.66 ± 1.75 cd | 80.62 ± 1.67ad | 68 ± 1.47 |
| Blastulation (%) | 83 ± 2.48 b | 58 ± 2.16 | 57.25 ± 2.56 | 73.5 ± 2.62 cd | 81.66 ± 1.85b | 64 ± 3.02 |
Each experiment involved four replicates. Mean ± SEM
Each experiment involved four replicates. Mean ± SEM
aP ≤ 0.01 versus in vitro-control and sham
bP ≤ 0.01 versus in vitro-control, sham, 0.01 and 0.04 μM MitoQ
cP ≤ 0.05 versus in vitro-control and sham
dP ≤ 0.05 versus 0.04 μM MitoQ
4.2. Cytoplasmic Levels of ROS and GSH in IVM Oocytes
To demonstrate the effect of MitoQ on oocytes oxidative stress in IVM conditions, the amount of cytoplasmic ROS and GSH were measured (Fig. 1 A, B, C, D). The level of ROS in the 0.01 and 0.02 µM MitoQ supplemented groups significantly decreased (60.11 ± 3.33% and 38.72 ± 1.40%), compared with 0.04 µM MitoQ (70.27 ± 2.16%), in vitro-control (82.63±3.79%) and sham (83.2±4.59%) groups (p<0.05).
Figure 1.
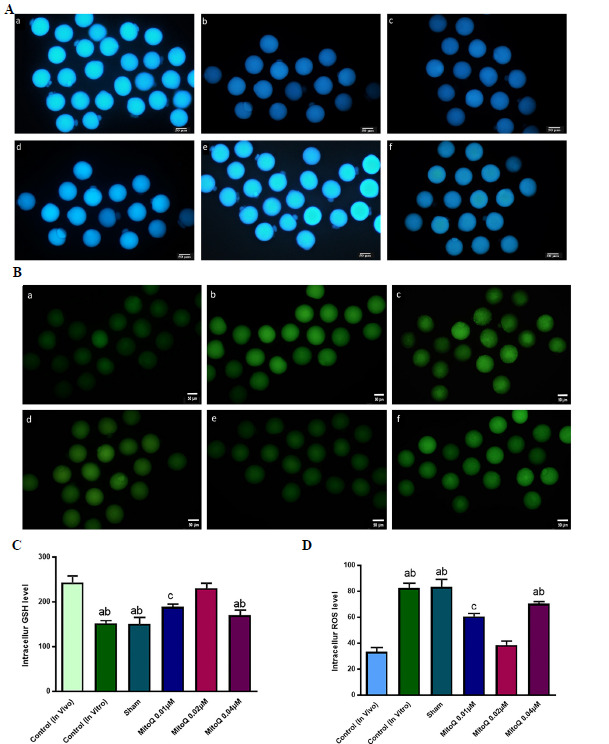
Representative images of all experimental groups to detect intracellular ROS (Stained with D H2DCFDA) as green fluorescence (A) and GSH (Stained with Cell Tracker Blue) as blue fluorescent (B). All images captured in 20X magnification. In the 0.01 and 0.02 µM MitoQ supplemented groups, compared with other IVM groups, the level of ROS significantly decreased and on the other side, the GSH level had a significant increase. Although there were a lower intracellular ROS and a higher intracellular GSH level in the in vivo-control group compared to 0.02 µM. Scale bar = 50 µm a, in vivo-control, ba, in vitro-control, c, sham, d. 0.01 µM MitoQ, e. 0.02 µM MitoQ and f, 0.04 µM MitoQ. C and D are the quantification data of acquired fluorescence images and representing the ROS and GSH levels and each column in these graphs indicates the significant difference between the control, sham and treated groups (Mean ± SEM). a, versus in vivo- control and 0.02 µM MitoQ (p <0.01); b, versus 0.01 µM MitoQ (p <0.05); c, versus in vivo-control and 0.02 µM MitoQ (p <0.05).
In contrast, the level of GSH in those groups treated by 0.01 and 0.02 µM MitoQ significantly increased (189.1 ± 6.0 and 231.7 ± 4.28) compared with the 0.04 µM MitoQ, in vitro-control and sham groups (170.18 ± 3.32, 152 ± 2.62 and 151.4 ± 1.70%, respectively (p<0.05)).
Assessment of intracellular levels of ROS and GSH in the in vivo-control group showed 33.5 ± 2.5% and 243.5± 1.1%, respectively, which were closed to the 0.02 µM MitoQ supplemented group. The obtained data cleared that the function and quality of IVM oocyte improved after treated by 0.02µM MitoQ.
4.3. Mitochondrial Membrane Potential
For further investigation of the role of MitoQ on oocyte development, mitochondrial membrane potential was measured using JC-1 stain. Fluorescence images were stored in TIFF format and then the intensity of the fluorescence pixels of each oocyte was analyzed using ImageJ software and then the red:green fluorescence ratio was evaluated in the images. The results showed that oocytes red:green fluorescence ratio was significantly higher in 0.02 µM MitoQ supplemented group compared to 0.01 and 0.04 µM MitoQ supplemented, in vitro-control and sham groups (4.4 ± 0.06, 1.9 ± 0.05, 1.55 ± 0.04, 1.3 ± 0.08 and 1.2 ± 0.6 respectively (p< 0.01)). Also, the oocytes with red: green fluorescence ratio in 0.02 µM group was higher than in vivo-control group (4.1 ± 0.06), but the difference was not significant. These results confirmed that supplementation of IVM medium with 0.02 µM MitoQ preserved the integrity of mitochondria membrane in the IVM oocyte (Fig. 2, 3).
Figure 2.
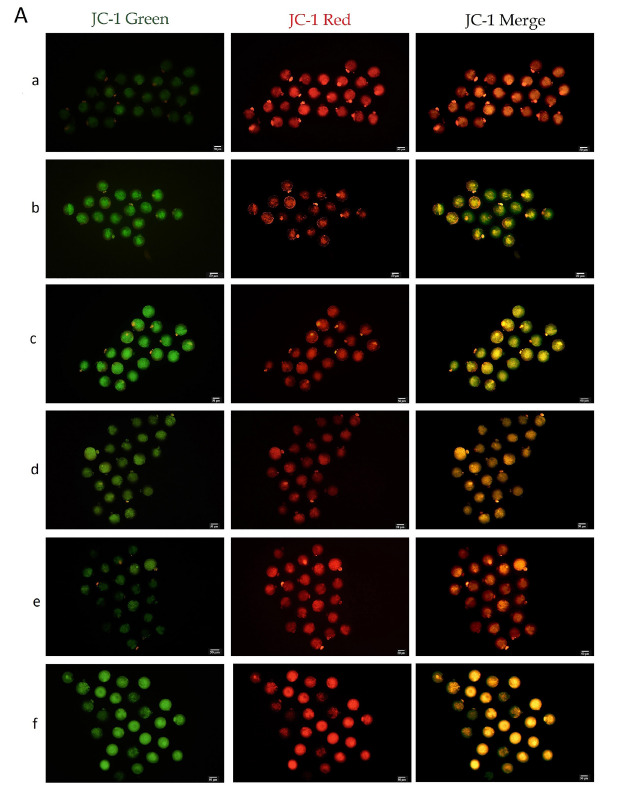
JC-1 Representative images of all experimental groups, all images captured in 20X magnification. ΔΨm was significantly increased in the 0.02 µM MitoQ and in vivo-control groups compared to other IVM groups (p<0.001). Scale bar = 20 µm, a, in vivo-control, b, in vitro-control, c, sham, d. 0.01µM MitoQ, e. 0.02 µM MitoQ and f, 0.04 µM MitoQ.
Figure 3.
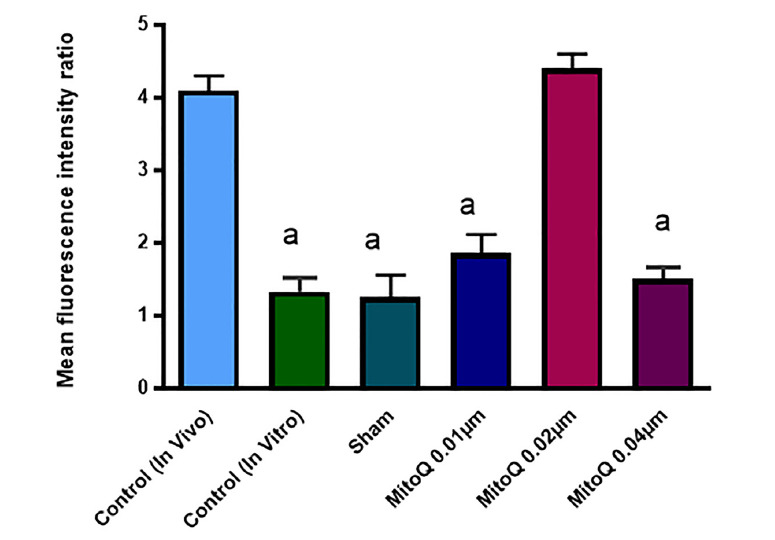
green ratio. Each column in these graphs indicates the significant difference between control, sham and treated groups (Mean ± SEM). a, versus in vivo-control and 0.02 µM MitoQ (p <0. 01)
4.4. IVF Rate
The fertilization rate was significantly higher in the 0.02 µM MitoQ (90.19%) than in vitro-control and sham groups (78.84 and 77.91%, (P< 0.01)), as well as was higher than other MitoQ treated groups (85.79 and 80.70%), The cleavage rate in the 0.01 and 0.02 µM MitoQ groups was significantly higher (76.66 and 88%) than 0.04 µM MitoQ (68%), in vitro-control (65.75%) and sham groups (63.75%) (P< 0.05). The blastocyst-formation rate in the 0.01 and 0.02 µM MitoQ groups (73.50 and 81.66%) was significantly higher than 0.04 µM MitoQ, in vitro-control and sham groups (73.50, 64, 58 and 57.25% respectively (p<0.05)). The developmental competence in the 0.02 µM MitoQ oocytes were higher than the 0.01 µM MitoQ, but not significantly. And also, the fertilization, cleavage and blastocyst-formation rate were similar in the 0.02 µM MitoQ group compared with in vivo-control group (92.1 and 88 and 83%).
In whole, these results showed that the treatment of oocytes with 0.02 µM MitoQ throughout the IVM increased the embryonic development potential by improving the cleavage rate and blastocyst formation. The comparative details of all groups are demonstrated in Table 1.
5. Discussion
In this study, we attempted to find the most suitable concentration of MitoQ during IVM of mouse oocytes. Here, we investigated the effect of three concentrations (0.01, 0.02 and 0.04µM) of MitoQ during IVM through assaying nuclear maturation, intracytoplasmic GSH and ROS levels, mitochondrial membrane potential and developmental competence of oocytes up to blastocyst stage.
In the 0.02 µM MitoQ treated oocytes, GSH level increased, while ROS level decreased compared with the other in vitro matured groups. These findings in turn indicated induction of cytoplasmic maturation of oocytes and inhibition of oxidative stress. In addition, significant enhanced mitochondrial membrane potential was assayed in the treated oocytes with 0.02 µM MitoQ compared to other IVM groups. The maturation, fertilization and blastulation rates improved following 0.02 µM MitoQ administration. Also, the results were closed to in vivo matured oocytes.
Previous researches showed that balance between pro-oxidants and anti-oxidants is required for having competent oocyte and embryo and high concentrations of antioxidants disrupts meiotic progression ( 23 , 24 ) . Concordantly, our results revealed the dose-dependent effects of MitoQ on maturation and fertilization of oocytes. While the lower concentration of MitoQ did not have any significant impact on oocyte maturation, the higher dose of 0.04 µM MitoQ resulted in deterioration of maturation and fertilization rates.
Despite the unfavorable effect of in vitro condition on oocyte maturation, IVM technique should be considered as a therapeutic treatment in some infertile patients ( 4 ) . IVM oocytes fail to acquire full competency to develop normal embryo due to adverse effects of culture condition ( 9 , 10 ) . Adding antioxidant in the culture medium augments oocyte development through scavenging free radicals and activating several antioxidant systems, such as catalase, superoxide dismutase and enhancing GSH content ( 24 ) . Also, some antioxidants can influence mitochondria activity indirectly and inhibit mitochondrial damage and oocyte apoptosis ( 6 , 9 , 12 ) Among different antioxidants, MitoQ as a unique mitochondrial antioxidant with the ability to enter mitochondria, improves redox signaling pathway and prevents mitochondrial damage via targeting membrane lipid peroxidation ( 15 , 16 ) . Here, we have investigated for the first time, the detailed role of MitoQ in mice oocyte maturation and developmental competency during IVM.
Oocyte maturation has been considered as synchronization between nuclear and cytoplasmic maturation ( 25 , 26 ) . Nuclear maturation is a dynamic process which is related to mitochondria potential and distribution (1, 3). In GV stage oocytes, the mitochondria are spread at the periphery and redistributed into the central pattern during maturation. This mitochondrial redistribution state is essential to provide enough energy to construct mitotic spindle and PB extrusion ( 2 , 3 ) . Our results revealed that higher PB extrusion in MitoQ-treated oocytes (specially in 0.02 µM) might be related to the increased mitochondria membrane potential, which ensures enough energy production for mitotic maturation. This is in agreement with previous studies, which revealed that L-carnitine promotes nuclear maturation by modulation of mitochondrial function ( 9 ) .
GSH is a non-enzymatic intrinsic antioxidant with a critical role in the developmental competence of oocytes through inducting male pronucleus formation after fertilization ( 27 ) . Previous studies showed that the decrease in GSH production is accompanied with an increase of ROS production, which could cause inhibition of maturation in GV stage oocytes and even fertilization failure of mature oocytes ( 28 ) . Therefore, GSH is introduced as a key parameter for evaluation of cytoplasmic maturation ( 29 ) . In this study, we exhibited that GSH level in 0.01 and 0.02 µM MitoQ was significantly higher than other groups during IVM and also GSH level in 0.02 µM MitoQ was similar to in vivo-control group. This result was in accordance with previous study, which revealed that supplementation of culture medium with 0.1 – 1.0 µM MitoQ increased GSH level in pronuclear cells ( 30 ) .
Increased intracytoplasmic GSH level protects oocyte and embryo against ROS damage during oocyte maturation and early stage in development ( 31 ) . Although ROS as a key signaling molecule has a critical role in biological systems; however, high level of ROS possesses adverse effects on oocyte mitochondrial function by disturbing cytoplasmic maturation and promoting embryo fragmentation ( 32 , 33 ) . Our results showed that intracytoplasmic ROS level significantly decreased in the 0.01 and 0.02 µM MitoQ groups, indicating that MitoQ has beneficial effects on maintaining redox homeostasis under in vitro condition. Consistent with our finding, MitoQ attenuated liver fibrosis in mice hepatocytes by inhibiting oxidative stress ( 34 ) . Moreover, The presence of MitoQ at 0.5 – 1.0 µM concentration improved endogenous oxidative DNA damages of mononuclear cells, which were exposed to exogenous ROS ( 30 ) . However, further research is required to elucidate the detailed mechanism by which MitoQ decrease oxidative stress during IVM. Any disturbance in mitochondrial membrane integrity would lead to a failure in oocyte maturation and developmental competence ( 11 , 35 ) . Mitochondrial membrane potential plays a key role in ATP generation and maintenance of the metabolic regulation during oocytes maturation ( 2 , 36 ) . However, in mitochondria, ATP synthesis is associated with ROS production, which may pose a threat to the integrity of the mitochondria membrane during oxidative phosphorylation ( 2 , 37 ) . To further investigate the role of MitoQ on oocyte development, instead of evaluating intra-oocyte ATP levels, mitochondrial membrane potentials (ΔΨm) were measured using JC-1 as an appropriate indicator of oocyte damage, and oocytes red: green fluorescence ratio was evaluated ( 38 , 39 ) . In this study, the 0.02 µM MitoQ significantly increased mitochondrial membrane potential, in comparison with other groups. Consistent with our results, mitochondrial membrane potential was protected by MitoQ from an oxidative stress injury in the endothelial cell model of sepsis ( 40 ) . Similarly, the viability rate of post-thaw yellow catfish sperm was improved through the increase in mitochondrial membrane integrity when tested with MitoQ in culture medium ( 41 ) .
Developing embryo is a fast organism with high- energy demands, which obtain most of it from ATP via mitochondrial oxidative phosphorylation. Therefore, the mitochondria membrane integrity, and subsequently enough ATP production promote fertilization and embryo development ( 42 , 43 ) . In our study, the 0.01 and 0.02 µM MitoQ improved mouse oocyte developmental competence when added to the maturation medium. Moreover, intracytoplasmic GSH content is a crucial factor for decondensation of the sperm nucleus though replacement of protamines by histones proteins after fertilization ( 27 ) . Therefore, the positive effect of MitoQ treatment during IVM on embryo development is likely due to the increased mitochondrial membrane potential, production of the energy and GSH content and reduced ROS level in ooplasm. several recently reports also showed that addition of L-carnitine, melatonin, vitamins E and C during IVM improved the cytoplasmic maturation of oocytes and subsequent embryo development via modulating oxidative stress in mice oocytes ( 9 , 28 , 44 ) . Additional studies are needed to investigate the link between fertilization rate and MitoQ supplementation.
6. Conclusion
In conclusion, we demonstrated that the addition of MitoQ to the IVM media, especially at 0.02 µM, was advantageous for fertilization and subsequent blastocyst development by increasing oocytes GSH levels and mitochondrial membrane potentials and reducing intracellular ROS concentrations. In the 0.02 µM MitoQ treated group, the results were close to the values for oocytes matured in vivo. therefore, our results suggest that MitoQ exerts positive effects on mouse oocyte in vitro maturation by modifying mitochondrial function and oxidative stress. Future investigation should focus on ATP production mechanism of MitoQ regulation meiosis signaling.
Acknowledgement
The authors would like to acknowledge Shahid Beheshti University of Medical Sciences for financial support and the National Institute of Genetic Engineering and Biotechnology (NIGEB) for technical and also financial support under contract grant number of 960115-I-620 .
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Takahashi Y, Hashimoto S, Yamochi T, Goto H, Yamanaka M, Amo A, et al. Dynamic changes in mitochondrial distribution in human oocytes during meiotic maturation. J Assist Reprod Genet. 2016;33(7):929–938. doi: 10.1007/s10815-016-0716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hut- zler P, Gonçalves PB, et al. Mitochondrial distribution and ade- nosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and de- velopmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64(3):904–909. doi: 10.1095/biolreprod64.3.904. [DOI] [PubMed] [Google Scholar]
- 3.Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisat uro ML, et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16(5):909–917. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- 4.Ata B, Shalom Paz E, Chian R c, Tan SL. In vitro maturation of oocytes as a strategy for fertility preservation. Clin Obstet Gyne- col. 2010;53(4):775–786. doi: 10.1097/GRF.0b013e3181f9718f. [DOI] [PubMed] [Google Scholar]
- 5.Huang JY, Chian R-C, Tan SL, editors. Ovarian hyperstimula- tion syndrome prevention strategies: in vitro maturation. Semin Reprod Med. 2010; 28(06):519–531. doi: 10.1055/s-0030-1265680. [DOI] [PubMed] [Google Scholar]
- 6.Lord T, Nixon B, Jones KT, Aitken RJ. Melatonin prevents pos- tovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol reprod. 2013;88(3) doi: 10.1095/biolreprod.112.106450. [DOI] [PubMed] [Google Scholar]
- 7.Tatone C, Di Emidio G, Barbaro R, Vento M, Ciriminna R, Artini PG. Effects of reproductive aging and postovulatory aging on the maintenance of biological competence after oo- cyte vitrification: insights from the mouse model. Theriog- enology. 2011;76(5):864–873. doi: 10.1016/j.theriogenolo-gy.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Jansen RP, Burton GJ. Mitochondrial dysfunction in repro- duction. Mitochondrion. 2004;4(5):577–600. doi: 10.1016/j.mito.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 9.Zare Z, Farahani RM, Salehi M, Piryaei A, Novin MG, Fathabadi FF, et al. Effect of L-carnitine supplementation on maturation and early embryo development of immature mouse oocytes se- lected by brilliant cresyle blue staining. J Assist reprod Genet. 2015;32(4):635–643. doi: 10.1007/s10815-015-0430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton M, Gilchrist R, Thompson J. Effects of in-vivo and in-vi- tro environments on the metabolism of the cumulus–oocyte complex and its influence on oocyte developmental capacity. Hum Reprod Update. 2003;9(1):35–48. doi: 10.1093/humupd/dmg009. [DOI] [PubMed] [Google Scholar]
- 11.Mtango N, Harvey A, Latham K, Brenner C. Molecular control of mitochondrial function in developing rhesus monkey oocytes and preimplantation-stage embryos. Reprod Fert Develop. 2008;20(7):846–859. doi: 10.1071/RD08078. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, He X-Q, Huang X, Ding L, Xu L, Shen Y-T, et al. Res- veratrol protects mouse oocytes from methylglyoxal-induced oxidative damage. PloS one. 2013;8(10):e77960. doi: 10.1371/journal.pone.0077960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and im- proves fertilization rate. J Pineal Res. 2008;44(3):280–287. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 14.Tahaei LS, Eimani H, Yazdi PE, Ebrahimi B, Fathi R. Effects of retinoic acid on maturation of immature mouse oocytes in the presence and absence of a granulosa cell co-culture sys- tem. J Assist Reprod Genet. 2011;28(6):553–558. doi: 10.1007/s10815-011-9579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells antioxidant and anti- apoptotic properties. J Bio Chem. 2001;276(7):4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 16.Smith RA, Murphy MP. Animal and human studies with the mitochondria‐targeted antioxidant MitoQ. Ann N Y Sci. 2010;1201(1):96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 17.McManus MJ, Murphy MP, Franklin JL. The mitochondria-tar- geted antioxidant MitoQ prevents loss of spatial memory re- tention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J Neurosci Res. 2011;31(44):15703– 15715. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zare Z, Abouhamzeh B, Farahani RM, Salehi M, Mohammadi M. Supplementation of L-carnitine during in vitro maturation of mouse oocytes affects expression of genes involved in oocyte and embryo competence: An experimental study. Int J Reprod. Biomed 2017;15(12):779. doi: 10.29252/ijrm.15.12.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ubaldi F, Rienzi L. Morphological selection of gametes. Pla- centa. 2008;29:115–120. doi: 10.1016/j.placenta.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Jo JW, Jee BC, Suh CS, Kim SH. Addition of lysophosphatidic acid to mouse oocyte maturation media can enhance fertilization and developmental competence. Hum Reprod. 2013;29(2):234– 241. doi: 10.1093/humrep/det427. [DOI] [PubMed] [Google Scholar]
- 21.He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, et al. Mito- chondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int. J Mol Sci 2016;17(6):939. doi: 10.3390/ijms17060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhary GR, Yadav PK, Yadav AK, Tiwari M, Gupta A, Sharma A, et al. Necrosis and necroptosis in germ cell depletion from mammalian ovary. J cell physiol. 2019 ;234(6):8019–27. doi: 10.1002/jcp.27562. [DOI] [PubMed] [Google Scholar]
- 23.Farahavar A, Shahne AZ. Effect of melatonin on in vitro mat- uration of bovine oocytes. Afr J Biotechnol. 2010;9(17):2579– 2583. doi: 10.5897/AJB2010.000-3074. [DOI] [Google Scholar]
- 24.Kala M, Shaikh MV, Nivsarkar M. Equilibrium between anti‐ oxidants and reactive oxygen species: a requisite for oocyte de- velopment and maturation. Reprod Med Biol. 2017;16(1):28–35. doi: 10.1002/rmb2.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanhoutte L, De Sutter P, Nogueira D, Gerris J, Dhont M, Vander Elst J. Nuclear and cytoplasmic maturation of in vitro ma- tured human oocytes after temporary nuclear arrest by phos- phodiesterase 3-inhibitor. Hum Reprod. 2007;22(5):1239–1246. doi: 10.1093/humrep/dem007. [DOI] [PubMed] [Google Scholar]
- 26.Virant-Klun I, Bauer C, Ståhlberg A, Kubista M, Skutella T. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod biomed online. 2018;36(5):508–523. doi: 10.1016/j.rbmo.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Brad AM, Bormann CL, Swain JE, Durkin RE, Johnson AE, Clifford AL, Krisher RL. Glutathione and adenosine triphos- phate content of in vivo and in vitro matured porcine oo- cytes. Mol Reprod Dev. 2003 Apr;64(4):492–8. doi: 10.1002/mrd.10254. [DOI] [PubMed] [Google Scholar]
- 28.Lian H-Y, Gao Y, Jiao G-Z, Sun M-J, Wu X-F, Wang T-Y, et al. Antioxidant supplementation overcomes the deleterious effects of maternal restraint stress-induced oxidative stress on mouse oocytes. Reproduction. 2013;146(6):559–568. doi: 10.1530/REP-13-0268. [DOI] [PubMed] [Google Scholar]
- 29.Choi J-Y, Kang J-T, Park S-J, Kim S-J, Moon J-H, Saadeldin IM, et al. Effect of 7, 8-dihydroxyflavone as an antioxidant on in vitro maturation of oocytes and development of parthenoge- netic embryos in pigs. J Reprod Dev. 2013;59(5):450–456. doi: 10.1262/jrd.2012-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marthandan S, Murphy MP, Billett E, Barnett Y. An inves- tigation of the effects of MitoQ on human peripheral mono- nuclear cells. Free Radic Res. 2011;45(3):351–358. doi: 10.3109/10715762.2010.532497. [DOI] [PubMed] [Google Scholar]
- 31.Park SH, Cho HS, Yu IJ. Effect of Bovine Follicular Fluid on Reactive Oxygen Species and Glutathione in Oocytes, Apopto- sis and Apoptosis‐related Gene Expression of In vitro‐Produced Blastocysts. Reprod Dome Anim. 2014;49(3):370–377. doi: 10.1111/rda.12281. [DOI] [PubMed] [Google Scholar]
- 32.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochon- drion. 2011;11(5):797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal A, Gupta S, Sharma R. Oxidative stress and its impli- cations in female infertility–a clinician’s perspective. Reprod. Biomed online 2005;11(5):641–650. doi: 10.1016/S1472-6483(10)61174-1. [DOI] [PubMed] [Google Scholar]
- 34.Rehman H, Liu Q, Krishnasamy Y, Shi Z, Ramshesh VK, Haque K, et al. The mitochondria-targeted antioxidant MitoQ attenu- ates liver fibrosis in mice. Int J Physiol Pathophysiol Pharma- col. 2016;8(1):14–27. [PMC free article] [PubMed] [Google Scholar]
- 35.Babayev E, Seli E. Oocyte mitochondrial function and repro- duction. Curr Opin Obstet Gyn. 2015;27(3):175. doi: 10.1097/GCO.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarazona A, Rodriguez J, Restrepo L, Olivera‐Angel M. Mi- tochondrial activity, distribution and segregation in bovine oo- cytes and in embryos produced in vitro. Reprod Domest Anim. 2006;41(1):5–11. doi: 10.1111/j.1439-0531.2006.00615.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu S, Li Y, Gao X, Yan J-H, Chen Z-J. Changes in the distri- bution of mitochondria before and after in vitro maturation of human oocytes and the effect of in vitro maturation on mito- chondria distribution. Fertil Steril. 2010;93(5):1550–1555. doi: 10.1016/j.fertnstert.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 38.Liu Q, Kang L, Wang L, Zhang L, Xiang W. Mitofusin 2 regu- lates the oocytes development and quality by modulating meio- sis and mitochondrial function. Sci Rep. 2016;29(6):30561. doi: 10.1038/srep30561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jo JW, Lee JR, Jee BC, Suh CS, Kim SH. Exposing mouse oo- cytes to necrostatin 1 during in vitro maturation improves mat- uration, survival after vitrification, mitochondrial preservation, and developmental competence. Reprod Sci. 2015;22(5):615– 625. doi: 10.1177/1933719114556482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowes DA, Thottakam BM, Webster NR, Murphy MP. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide–peptidoglycan model of sepsis. Free Radic Biol Med. 2008;45(11):1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Fang L, Bai C, Chen Y, Dai J, Xiang Y, Ji X, et al. Inhibition of ROS production through mitochondria-targeted antioxidant and mitochondrial uncoupling increases post-thaw sperm via- bility in yellow catfish. Cryobiology. 2014;69(3):386–393. doi: 10.1016/j.cryobiol.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Dumollard R, Carroll J, Duchen M, Campbell K, Swann K, editors. Mitochondrial function and redox state in mammali- an embryos. Semin Cell Dev Biol. 2009;20(3):346–353. doi: 10.1016/j.semcdb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Wang L-y, Wang D-h, Zou X-y, Xu C-m. Mitochondrial func- tions on oocytes and preimplantation embryos. J Zhejiang Univ. Sci B. 2009;10(7):483–492. doi: 10.1631/jzus.B0820379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikmard F, Hosseini E, Bakhtiyari M, Ashrafi M, Amidi F, Afla- toonian R. Effects of melatonin on oocyte maturation in PCOS mouse model. Anim Sci J. 2017;88(4):586–392. doi: 10.1111/asj.12675. [DOI] [PubMed] [Google Scholar]


