Abstract
Background and Aim
With the increasing prevalence of persons without Helicobacter pylori (HP) infection, cases of HP‐negative gastric cancer are increasing. Although rare, cases of differentiated adenocarcinoma of the antrum have been reported in HP‐negative patients. We collected cases with such lesions and investigated their endoscopic and histological features.
Methods
Of 1965 consecutive patients with early gastric cancer who underwent endoscopic resection between January 2009 and December 2017, we extracted 9 cases of HP‐negative differentiated adenocarcinoma located in the antrum (HPN‐DAA). The clinical data, endoscopic findings, and histopathological findings were reviewed.
Results
Of the nine patients with HPN‐DAA, seven were male, and the median age was 53.8 years. The tumor arose from the pyloric gland mucosa in all cases. According to the endoscopic findings, the lesions were flat‐elevated or depressed, mimicking varioliform gastritis. Magnifying endoscopy with narrow‐band imaging showed the absence of a clear demarcation line or an irregular microvessel/surface pattern. As for the histopathological findings, eight of the nine lesions were diagnosed as high‐grade dysplasia/intraepithelial neoplasia, while the remaining case was diagnosed as tubular adenocarcinoma with submucosal infiltration. The findings of immunohistochemistry confirmed that three cases were of the intestinal mucin phenotype and six were of the mixed gastric and intestinal mucin phenotype.
Conclusion
HPN‐DAA is a very rarely occurring cancer that had never been recognized earlier. They belong to the new category of HP‐negative cancers, and there seems to be a certain number of such cases.
Keywords: diagnosis, differentiated‐type cancer, endoscopic resection, gastric cancer, Helicobacter pylori
With the increasingly prevalence of persons without Helicobacter pylori (HP) infection, cases of HP‐negative gastric cancer are increasing. Although rare, cases of differentiated adenocarcinoma of the antrum have been reported in HP‐negative patients. In this study, we collected cases with such lesions and investigated their endoscopic and histological features.
Introduction
Chronic gastritis caused by persistent Helicobacter pylori (HP) infection is known to be strongly associated with the development of gastric cancer, and HP infection has been certified as a “definite carcinogen” by the World Health Organization. 1 With the high prevalence of HP infection, Japan is well known as a country with a high incidence of gastric cancer. 2 Uemura et al. reported in their study that there were no cases of gastric cancer in the group without HP infection in their case series. 3 However, in clinical practice, we sometimes encounter a small number of gastric cancers in patients without HP infection. As the number of persons without HP infection increases, cases with gastric cancer in this population is also expected to increase. These are recognized as cases of HP‐negative gastric cancer. The reported incidence of HP‐negative gastric cancer is in the range of 0.5–3.1%, which is quite a significant number. 4 , 5 , 6
HP‐negative gastric cancers can be classified into several phenotypes. The most common type is signet‐ring cell carcinoma, which is a discolored lesion, frequently identified in the middle or lower third of the stomach. 7 Meanwhile, a variety of differentiated‐type gastric cancers has also been identified in patients without HP infection (e.g. gastric adenocarcinoma of the fundic gland type 8 ; low‐grade differentiated cancer of the gastric phenotype 9 ; and foveolar‐type dysplasia, endoscopically showing a raspberry‐like appearance and recognized as a low‐grade differentiated type of adenocarcinoma in Japan 10 ). More recently, although rare, a differentiated type of adenocarcinoma mimicking varioliform gastritis has been reported as occurring in the gastric antrum of persons without HP infection. There are only a few case reports, and the characteristics of these lesions remain unclear. 11 , 12 , 13
In the present study, we collected a number of cases of HP‐negative differentiated adenocarcinoma located in the antrum (HPN‐DAA) and revealed the associations between their endoscopic and histological features.
Methods
Study design and patients
This retrospective cohort study was conducted to investigate the endoscopic and histopathological features of HPN‐DAA. Among 1965 consecutive patients with early gastric cancer who underwent endoscopic resection at the NTT Medical Center Tokyo between January 2009 and December 2017, we extracted cases without HP infection. HP‐negative status was confirmed by the following criteria: (i) no atrophy or gastritis as assessed by endoscopy, (ii) no histologic evidence of HP infection, (iii) negative results of two consecutive tests for HP infection, and (iv) negative history of HP eradication therapy. The endoscopic findings were reviewed using the Kimura‐Takemoto Classification 14 and Kyoto classification of gastritis. 15 HP‐negative patients met these inclusion criteria: no mucosal atrophy and histological confirmation of a regular arrangement of collecting venules on the lesser curvature of the lower gastric body. Resected specimens were histologically evaluated according to the updated Sydney system. 16 A patient was defined as HP‐negative when there was no mucosal atrophy, intestinal metaplasia, presence of HP, or infiltration by neutrophils and mononuclear cells. With regard to the test for HP infection status, we use the anti‐HP serum IgG antibody kit (BML, INC., Tokyo, Japan) and 13C‐labeled urea breath test (UBIT® tablets 100 mg, Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan). HP‐negative patients met the criteria of a negative result in both the former (<3.0 U/ml) and the latter (<2.5%). Finally, 39 cases (2.0%) were identified as cases of HP‐negative cancers. Then, we divided the cases into several types according to their endoscopic findings: (i) signet‐ring cell carcinoma; (ii) gastric adenocarcinoma of the fundic gland type; (iii) low‐grade differentiated cancer of the gastric phenotype and foveolar‐type dysplasia, endoscopically showing a raspberry‐like appearance; and (iv) HPN‐DAA. Finally, nine patients with HPN‐DAA were enrolled in this study. The clinical data, including age; gender; and endoscopic findings such as the tumor location, tumor size, tumor morphology, preoperative diagnosis, as well as histopathological findings, were reviewed. To elucidate HPN‐DAA characteristics, we extracted patients with HP‐positive differentiated adenocarcinomas located in the antrum (HPP‐DAA) who underwent endoscopic resection during the same period and compared HPN‐DAA and HPP‐DAA clinicopathological findings. We obtained written informed consent for the endoscopic resection and investigation of the long‐term outcomes at the time of the initial treatment. This study was conducted with the approval of the ethics committee of NTT Medical Center Tokyo (20‐22).
Procedure of endoscopy
We use magnification video endoscope systems (GIF‐H260Z or GIF‐H290Z; Olympus Medical Systems Co. Ltd., Tokyo, Japan) and a standard optical video endoscope system (EVIS LUCERA ELITE system; Olympus Medical Systems). We obtained the images of the lesions, not only by white‐light endoscopy (WLE) but also by magnifying endoscopy with narrow‐band imaging (ME‐NBI). The diagnostic strategy adopted for the findings of ME‐NBI was in accordance with the Magnifying Endoscopy Simple Diagnostic Algorithm for early Gastric cancer (MESDA‐G). 17 To ensure precise results of the pathological examination, all the lesions were resected by endoscopic submucosal dissection (ESD). All the procedures were performed by endoscopists certified by the Japan Gastroenterological Endoscopy Society with 10 years of experience or longer in endoscopy.
Pathological examination
The tumor size was determined by measuring the resected specimen prior to tissue fixation in formalin. The specimens were processed and cut into sections at a thickness of 4 μm, which were stained with hematoxylin and eosin. Then, the differentiation degree, invasion depth, presence/absence of lymphovascular invasion, and the lateral and vertical resection margins were assessed. Cell differentiation (based on the staining status for MUC2, MUC5AC, MUC6, CDX‐2, and CD10), proliferation (based on the Ki‐67 index), and genetic status (based on the staining status for MLH1, MSH2, MSH6, and PMS2) were evaluated immunohistochemically using monoclonal antibodies to MUC2 (Novocastra), MUC5AC (Novocastra/Leica Biosystems, Newcastle, UK), MUC6 (Novocastra), CDX‐2 (Dako/Agilent Technologies, Glostrup, Denmark), CD10 (Novocastra), Ki‐67 (clone MIB‐1; Dako), MLH1 (FALCO biosystems, Kyoto, Japan), MSH2 (FALCO), MSH6 (FALCO), and PMS2 (FALCO). The histopathological examinations were performed by an experienced pathologist specialized in pathology of the gastrointestinal tract (S.I.).
Statistical analyses
The statistical significance of differences in clinical parameters were evaluated using the χ2 test or unpaired Student's t‐test. A P value <0.05 was considered statistically significant.
Results
Clinical features and endoscopic findings
The clinical features and endoscopic findings of the patients are shown in Table 1. Among the nine patients, seven were male, and the median age was 53.8 years (range 32–64 years). None of the patients had a history or family history of gastric cancer. None of the patients showed atrophy or gastritis in the background mucosa. The lesions in all cases were located in the antrum. Morphologically, they were flat‐elevated or depressed‐type lesions, like varioliform gastritis (Fig. 1). ME‐NBI showed the absence of a clear demarcation line and of the irregular microvessel/surface pattern (IMVP/IMSP) in almost all the cases. Hence, according to MESDA‐G, all the lesions were diagnosed as noncancer. Biopsy specimens had been obtained in all the cases previously. Only three patients were categorized as group 5 (Carcinoma) in advance, while the remaining were classified as group 2 (Indefinite for neoplasia; material for which diagnosis of neoplastic or nonneoplastic lesion is difficult), group 3 (Adenoma), or group 4 (Neoplastic lesion that is suspected to be carcinoma) based on the “Group classification.” 18 Furthermore, despite the lesions having been diagnosed several years (4–11 years) earlier in three of the cases, the patients had been followed up as the lesions were diagnosed as not being neoplastic. Hence, endoscopic resection was performed for the purpose of diagnosis in some of the cases. Representative WLE and ME‐NBI images are shown in Figure 2.
Table 1.
Clinicopathological features of Helicobacter pylori‐negative differentiated adenocarcinoma located in the antrum
| Case | Gender | Age | Background mucosa | Location | Morphology | Size (mm) | Preoperative diagnosis | Differentiation | Depth |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 51 | Pyloric | Less | IIa + IIc | 10 | Group 4 | tub1 | M |
| 2 | Male | 57 | Pyloric | Ant | IIa | 6 | Group 3 | tub1 | M |
| 3 | Male | 56 | Pyloric | Post | IIa | 12 | Group 2 | tub1 | M |
| 4 | Male | 51 | Pyloric | Gre | IIa | 14 | Group 3 | tub1 | M |
| 5 | Male | 32 | Pyloric | Gre | IIa | 8 | Group 5 | tub1 | M |
| 6 | Female | 48 | Pyloric | Gre | IIc | 10 | Group 3 | tub1 | M |
| 7 | Female | 61 | Pyloric | Gre | IIa + IIc | 18 | Group 5 | tub1 | SM1 |
| 8 | Male | 64 | Pyloric | Post | IIa | 2 | Group 2 | tub1 | M |
| 9 | Male | 64 | Pyloric | Gre | IIa | 3 | Group 5 | tub1 | M |
Ant, anterior wall; Gre, greater curvature; Less, lesser curvature; M, intramucosal cancer; Post, posterior wall; SM1, invasion depth < 500 μm from muscularis mucosa.
Figure 1.
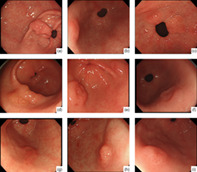
White‐light endoscopy of 10 Helicobacter pylori (HP)‐negative differentiated adenocarcinoma located in the antrum cases. The lesions were recognized as a single erosion in the antrum of the stomach in the absence of HP infection. Unlike typical gastric cancers, the border line was unclear. A–I corresponds to cases 1–9 in Tables 1 and 2.
Figure 2.
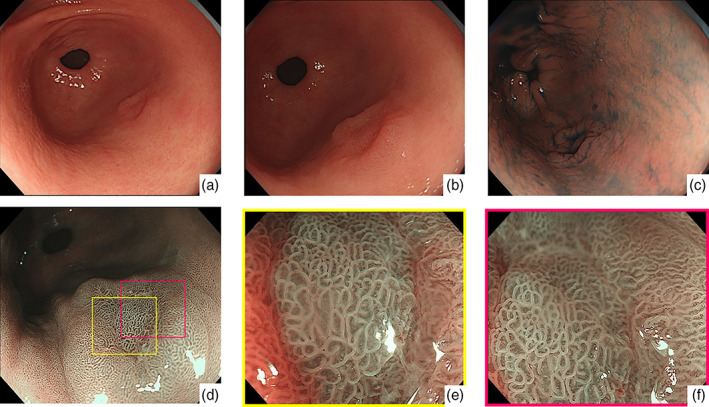
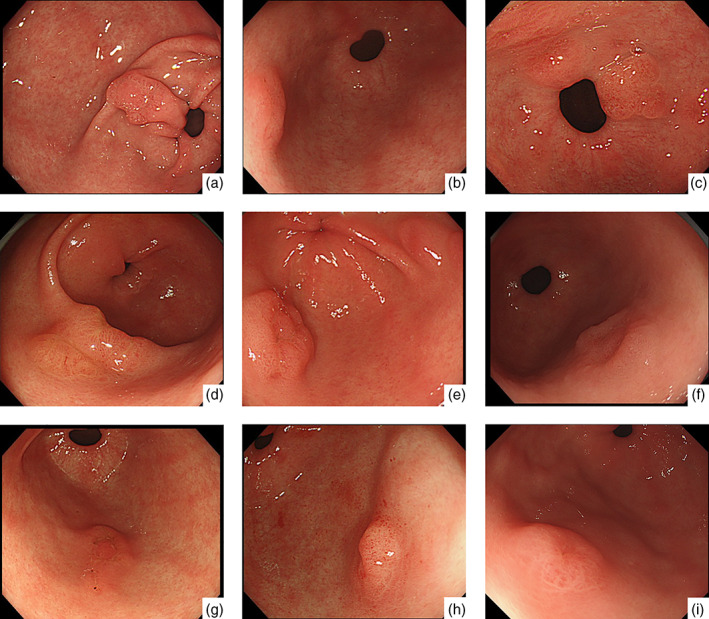
Representative endoscopic findings of Helicobacter pylori‐negative differentiated adenocarcinoma located in the antrum (case 6). (a,b) White‐light endoscopy revealed a 0–IIa + IIc‐type lesion located in the greater curvature of the antrum. (c) Chromoendoscopy showed the morphology of the lesion more distinctly. (d–f) Narrow‐band imaging (NBI) findings of the lesion. magnifying endoscopy‐NBI did not show a clear demarcation line or irregular microvessel/surface pattern. According to Magnifying Endoscopy Simple Diagnostic Algorithm for early Gastric cancer, the lesion was diagnosed as noncancer.
Pathological findings
All the lesions were resected by ESD without any complications. The histopathological findings of the nine cases are shown in Tables 1 and 2. Even though the lesions in all cases showed similar endoscopic findings, their pathological findings were not identical. With regard to the background mucosa, the tumors arose from the pyloric gland mucosa in all cases. All the lesions were diagnosed as well‐differentiated tubular adenocarcinoma based on the glandular structure and cellular atypia using the Japanese gastric cancer treatment guidelines. 19 Except for one case, the remaining eight cases were of intramucosal cancer (“carcinoma in situ”). These were classified as noninvasive high‐grade neoplasia (category 4) according to the Vienna classification 20 and as high‐grade dysplasia/intraepithelial neoplasia according to the WHO classification. 21 As for the single case, namely, case 7, the lesion showed submucosal invasion (250 μm from the muscularis mucosa, diagnosed as SM1 according to the Japanese gastric cancer treatment guidelines), which was diagnosed as invasive neoplasia (category 5) according to Vienna classification and tubular adenocarcinoma by the WHO classification. In addition, they often had nonneoplastic epithelium on the surface layer or within the lesion. Lymphovascular invasion was not confirmed in any of the cases, and all cases were resected with negative lateral and vertical margins. Interestingly, it was notable that all cases had fibromuscular obliteration, such as in the case of rectal mucosal prolapse syndrome, of the lamina propria around the lesions. Immunohistochemistry revealed that three of the cases were of the intestinal type, and six were of the mixed type (Table 2). Of the six cases of mixed‐type HPN‐DAA, two were gastric mucin phenotype dominant (MUC5AC(+), MUC6(+)), although they also showed CDX2(+), while four cases were intestinal mucin phenotype dominant (MUC2(+), CDX2(+)), and they were also MUC6(+). The Ki‐67 labeling index of the tumor cells was over 90%, but the superficial nonneoplastic foveolar epithelial cells were negative for Ki‐67, with a Ki‐67 labeling index in the range of 49.4%–66.8%. With regard to the expression of mismatch repair (MMR) proteins such as MLH1, MSH2, MSH6, and PMS2, no downregulation in the expression of any of these proteins was observed in any of the cases. Representative pathological images are shown in Figure 3.
Table 2.
Mucin phenotype of Helicobacter pylori‐negative differentiated adenocarcinoma located in the antrum
| Case | MUC5AC | MUC6 | MUC2 | CDX‐2 | CD10 | MMR | Ki‐67 (%) | Mucin phenotype |
|---|---|---|---|---|---|---|---|---|
| 1 | − | − | + | + | + | − | 54.8 | Intestinal |
| 2 | − | − | + | + | + | − | 55.8 | Intestinal |
| 3 | − | − | + | + | + | − | 60.2 | Intestinal |
| 4 | ± | + | + | + | ± | − | 65.2 | Mixed (intestinal) |
| 5 | ± | + | + | + | + | − | 49.4 | Mixed (intestinal) |
| 6 | − | + | + | + | + | − | 59.2 | Mixed (intestinal) |
| 7 | ± | + | + | + | − | − | 66.8 | Mixed (intestinal) |
| 8 | + | + | − | + | − | − | 63.6 | Mixed (gastric) |
| 9 | + | + | − | + | − | − | 66.5 | Mixed (gastric) |
Figure 3.
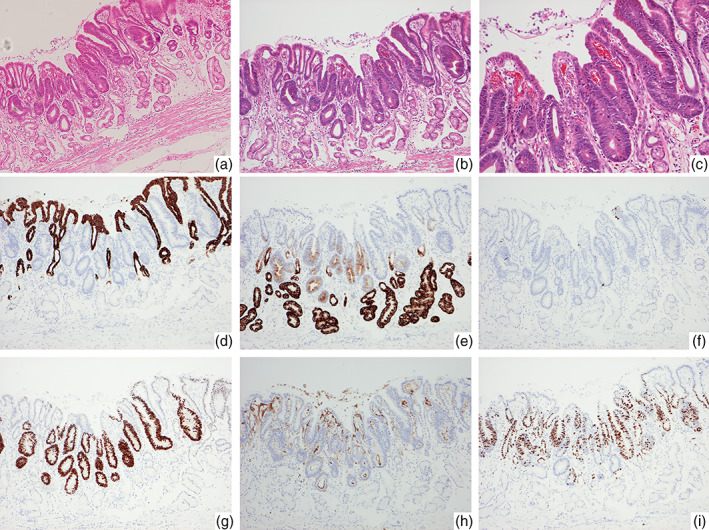
Histopathological images of Helicobacter pylori‐negative differentiated adenocarcinoma located in the antrum (case 6). (a) The lesion arose from the pyloric glands in the absence of intestinal metaplasia. Fibromuscular obliteration of the lamina propria is observed in the background mucosa. (b,c) The neoplastic gland showing irregular glandular arrangement with low‐grade cellular atypia. There is some nonneoplastic epithelium on the surface layer of the lesion: (b) low power field and (c) high power field. (d–i) Immunohistochemistry: The neoplastic cells showing negative staining for MUC5AC (d) and positive staining for MUC6 (e), MUC2 (f), CDX‐2 (g), and CD10 (h). (i) Ki‐67 labeling index is 59.2%.
Comparison of HPN‐DAA and HPP‐DAA
Clinicopathological findings of HPN‐DAA and HPP‐DAA are summarized in Table 3. Patients with HPN‐DAA were significantly younger than those with HPP‐DAA. No gender difference was found. Average HPN‐DAA tumor size was significantly smaller than that of HPP‐DAA. With respect to morphology, HPN‐DAA were more often observed to protrude compared with HPP‐DAA. No difference between groups was found in the prevalence of submucosal invasion.
Table 3.
Comparison of Helicobacter pylori‐negative differentiated adenocarcinoma located in the antrum (HPN‐DAA) and H. pylori‐positive (HPP)‐DAA patient characteristics
| HPN‐DAA (n = 9) | HPP‐DAA (n = 558) | P value | |
|---|---|---|---|
| Age, years | 53.8 ± 10.0 | 71.3 ± 8.6 | <0.01 |
| Gender, male | 7 (77.8%) | 411 (73.7%) | 0.56 |
| H. pylori status | N/A | ||
| Negative | 9 (100%) | 0 | |
| Positive | 0 | 242 (43.4%) | |
| Eradicated | 0 | 316 (56.6%) | |
| Diameter, mm | 9.2 ± 5.1 | 13.8 ± 9.7 | 0.01 |
| Macroscopic morphology | 0.39 | ||
| Protruded | 4 (44.4%) | 195 (34.9%) | |
| Flat, depressed | 5 (55.6%) | 363 (65.1%) | |
| Tumor depth | 0.15 | ||
| Mucosal | 8 (88.9%) | 533 (95.5%) | |
| Submucosal <500 μm | 1 (11.1%) | 11 (2.0%) | |
| Submucosal ≥500 μm | 0 | 14 (2.5%) | |
| Histological type | 0.36 | ||
| tub1 | 9 (100%) | 453 (81.2%) | |
| tub2 | 0 | 85 (15.2%) | |
| pap | 0 | 20 (3.6%) |
Values are mean ± standard deviation or n (%).
pap, papillary adenocarcinoma; tub1, well‐differentiated adenocarcinoma; tub2, moderate‐differentiated adenocarcinoma.
Discussion
With the decline in the number of people with HP infection owing to improved sanitary environments and spread of eradication therapy, the incidence of gastric cancers in persons without HP infection is expected to show a relative increase in Japan. 22 There have been some previous reports of HP‐negative cancers, such as signet‐ring cell carcinoma; gastric adenocarcinoma of the fundic gland type; low‐grade differentiated cancer with the gastric mucin phenotype; and foveolar‐type dysplasia, endoscopically showing a raspberry‐like appearance. These lesions are usually known to arise from the fundic gland area. Other types of gastric cancer are rarely experienced in HP‐negative patients. In contrast, the lesions in our cases arose from the pyloric glands in the gastric antrum. Although there have been three reports of differentiated adenocarcinoma arising from the antrum, 11 , 12 , 13 this is the first report to examine a certain number of such cases in detail.
As for our cases, or cases of HPN‐DAA, the lesions were observed as superficial elevated or depressed lesions close to the pyloric ring in all cases. Unlike typical gastric cancers, the border line was unclear. In addition, even with ME‐NBI, the demarcation line or IMVP/IMSP was not observed in most of the lesions. Hence, they were diagnosed as nonneoplastic lesions according to conventional diagnostics. Moreover, as it was difficult to make a definite diagnosis due to the low‐grade atypia, only three cases (33.3%) were diagnosed as cancer in advance; another three cases with lesions that were not diagnosed as being neoplastic underwent follow‐up examinations for several years (4–11 years). Fortunately, none of them showed rapid growth. Of the nine cases, eight were categorized as intramucosal cancer. However, if a single erosion, like varioliform gastritis, in the antrum of the stomach in the absence of HP infection is noted in the future, it is important to be aware that, although unlikely, the lesion could be a cancer. In that case, described above, as it is difficult to diagnose HPN‐DAA endoscopically, it is thought that biopsy should be actively performed. Even if the result was indefinite for neoplasia, the possibility of cancer cannot be ruled out, so endoscopic resection for the purpose of diagnosis is considered to be acceptable.
As for the histopathological diagnosis, HPN‐DAA showed weak atypia—not only structural atypia but cellular atypia as well. In addition, they often had a nonneoplastic epithelium inside the lesion as in gastric cancers detected after HP eradication. 23 These histopathological features may make the endoscopic diagnosis of these lesions more difficult, and as a result, the lesions were often diagnosed as nonneoplastic lesions even by ME‐NBI. Besides, interestingly, all lesions showed fibromuscular obliteration of the lamina propria in the background mucosa. Owing to this, the background mucosa around the lesion often protruded; therefore, it was considered that, morphologically, HPN‐DAA mimicked varioliform gastritis. This fibromuscular obliteration was usually demonstrated in the distal antrum, 24 and we thought that this phenomenon was due to gastric peristalsis (like in rectal mucosal prolapse syndrome). While fibromusculosis was not a specific finding of HPN‐DAA, this feature is considered important for understanding the endoscopic findings of HPN‐DAA.
As for the mucin phenotype, it has been reported that HPN‐DAA are of the intestinal mucin phenotype. 11 , 12 , 13 As most HP‐negative cancers, such as gastric adenocarcinoma of the fundic gland type, low‐grade differentiated cancer of the gastric phenotype, and foveolar‐type dysplasia, are known to be of the gastric mucin phenotype, 4 , 8 , 9 , 10 this is also a point that merits further attention. In the present study, three lesions were diagnosed as being of the intestinal mucin phenotype, while the others were diagnosed as being of the mixed phenotype: two were gastric mucin dominant, while the remaining were intestinal mucin dominant. Although the endoscopic findings were similar, the lesions showed different mucin phenotypes. It would be necessary to examine a larger number of cases for a more precise evaluation. In addition, it remains unclear how cancer with the intestinal mucin phenotype arose from the stomach in the absence of intestinal metaplasia. Kakinoki et al. reported that a phenotypic shift in gastric cancer cells from gastric to the intestinal type occurs with tumor progression. 5 As most of the lesions in this study were intramucosal cancers, it is unlikely that any phenotypic shift occurred. Therefore, further investigation with a larger number of cases is needed.
Recently, a new histological classification based on the molecular characterization of gastric cancer was suggested. 25 According to this classification, gastric cancers are classified as tumors positive for Epstein–Barr virus (EBV), microsatellite instability tumors, genomically stable tumors, and tumors with chromosomal instability according to the mechanism of carcinogenesis. With regard to HP‐negative cancer, genetic disorders such as CDH1 are known in cases like hereditary diffuse gastric cancer 26 and GNAS in cases of gastric adenocarcinoma of the fundic gland type. 27 As for tumors with microsatellite instability, although they are often known to occur in female patients and arise from the antrum, 28 no decrease in the expression of MMR was observed in the lesions in this study. However, it would still be difficult to rule out the possibility of involvement of some genetic abnormality in the carcinogenetic pathway of HPN‐DAA.
With regard to other risk factors for carcinogenesis, in addition to genetic mutations, EBV infection and autoimmune gastritis are also known risk factors. As for cancer associated with EBV infection, it has the characteristics of moderate to poorly differentiated cancer with lymphoid cell invasion, most commonly involving the upper to middle third of the stomach. 29 We did not perform tests for the presence of EBV infection as the lesions in this study showed characteristics that were clearly different from the above‐described features. Similarly, gastric cancers associated with autoimmune gastritis are assumed to show atrophic gastritis in the background mucosa, thus being obviously different from the lesions in this study.
In addition, considering that there was one case which showed submucosal invasion, HPN‐DAA does appear to have the potential to transform into invasive cancer. With regard to HP‐negative cancer, reports of intramucosal cancer have accounted for the majority so far; however, there have been a few reports of advanced cancers. 30 Examination of a larger number of cases of HPN‐DAA in the future is warranted for a more precise elucidation of their characteristics.
Compared to HPP‐DAA, HPN‐DAA tumor size was smaller. Similar results have been reported for HP‐negative signet‐ring cell carcinoma. 31 Further investigation is needed to clarify whether this is due to differences in tumor proliferative capacity or whether HPN‐DAA just happened to be discovered at early stages, supported by the lack of gastritis or intestinal metaplasia in the background mucosa. Considering that several cases of HPN‐DAA did not change significantly over several years of follow‐up, they may be slowly progressing lesions. In addition, patients with HPN‐DAA were younger than those with HPP‐DAA. Several similar reports have observed that patients with HP‐negative cancer are younger than those with HP‐positive cancer.. 9 , 31 , 32 If further studies confirm that patients with HP‐negative cancer are younger than those with HP‐positive cancer, we may expect the average age of gastric cancer patients to decrease as the HP‐negative population increases.
The present study had several limitations. First, the sample size was very small as this study was a retrospective observational analysis performed at a single institution besides the fact that the lesion is rare. Further investigation in a larger population, such as through a multicenter analysis, is necessary. Second, the effects of bile acids were not investigated in this study. It is known that bile acids can damage DNA or cause carcinogenesis. 33 A further detailed investigation of this point would be desirable. Third, although the endoscopic findings were similar, it is difficult to exclude the possibility that the disease may not have been the same in all cases as they had different mucin phenotypes. A more detailed examination in a larger population is warranted in the future to elucidate the characteristics, as well as pathogenesis, of these tumors in the future.
In conclusion, HPN‐DAA is a very rare cancer that has never been recognized previously. The lesions have the characteristics of well‐differentiated carcinoma and lack any specific endoscopic findings; these are some of the reasons they may have been overlooked so far. They belong to the new category of HP‐negative cancers, and there seems to be a certain number of such cases. With the estimated increase in the population without HP infection, more attention will need to be paid to the possibility of such lesions when performing endoscopic examinations.
Acknowledgments
The authors thank all their colleagues at NTT Medical Center Tokyo who supported the study. They also thank IMIC (https://www.imic.or.jp/) for editing a draft of this manuscript.
Declaration of conflict of interest: None
References
- 1. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Schistosomes, liver flukes and Helicobacter pylori. In: IARC Monogr Eval Carcinog Risks Hum 1994, Vol. 61. Lyon Publication: IARC; 1994; 177–240. [Google Scholar]
- 2. Kobayashi T, Kikuchi S, Lin Y et al. Trends in the incidence of gastric cancer in Japan and their associations with Helicobacter pylori infection and gastric mucosal atrophy. Gastric Cancer. 2004; 7: 233–9. [DOI] [PubMed] [Google Scholar]
- 3. Uemura N, Okamoto S, Yamamoto S et al. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001; 345: 784–9. [DOI] [PubMed] [Google Scholar]
- 4. Kato S, Matsukura N, Tsukada K et al. Helicobacter pylori infection‐negative gastric cancer in Japanese hospital patients: incidence and pathological characteristics. Cancer Sci. 2007; 98: 790–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kakinoki R, Kushima R, Matsubara A et al. Reevaluation of histogenesis of gastric carcinomas: a comparative histopathological study between Helicobacter pylori‐negative and H. pylori‐positive cases. Dig. Dis. Sci. 2009; 54: 614–20. [DOI] [PubMed] [Google Scholar]
- 6. Ono S, Kato M, Suzuki M et al. Frequency of Helicobacter pylori‐negative gastric cancer and gastric mucosal atrophy in a Japanese endoscopic submucosal dissection series including histological, endoscopic and serological atrophy. Digestion. 2012; 86: 59–65. [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto Y, Fujisaki J, Omae M, Hirasawa T, Igarashi M. Helicobacter pylori‐negative gastric cancer: characteristics and endoscopic findings. Dig. Endosc. 2015; 27: 551–61. [DOI] [PubMed] [Google Scholar]
- 8. Ueyama H, Yao T, Nakashima Y et al. Gastric adenocarcinoma of fundic gland type(chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am. J. Surg. Pathol. 2010; 34: 609–19. [DOI] [PubMed] [Google Scholar]
- 9. Yamada A, Kaise M, Inoshita N et al. Characterization of Helicobacter pylori – Naïve Early Gastric Cancers. Digestion. 2018; 98: 127–34. [DOI] [PubMed] [Google Scholar]
- 10. Shibagaki K, Fukuyama C, Mikami H et al. Gastric foveolar‐type adenoma endoscopically showing a raspberry‐like appearance in the Helicobacter pylori‐uninfected stomach. Endoscopy International Open. 2019; 07: E784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ozaki Y, Suto H, Nosaka T et al. A case of Helicobacter pylori‐negative intramucosal well differentiated gastric adenocarcinoma with intestinal phenotype. Clin J Gastroenterol. 2015; 8: 18–21. [DOI] [PubMed] [Google Scholar]
- 12. Kotani S, Miyaoka Y, Fujiwara A et al. Intestinal‐type gastric adenocarcinoma without Helicobacter pylori infection successfully treated with endoscopic submucosal dissection. Clin J Gastroenterol. 2016; 9: 228–32. [DOI] [PubMed] [Google Scholar]
- 13. Yoshii S, Hayashi Y, Takehara T. Helicobacter pylori negative early gastric adenocarcinoma with complete intestinal mucus phenotype mimicking verrucous gastritis. Dig. Endosc. 2017; 29: 235–6. [DOI] [PubMed] [Google Scholar]
- 14. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969; 3: 87–97. [Google Scholar]
- 15. Kamada T, Haruma K, Inoue K, Shiotani A. Helicobacter pylori infection and endoscopic gastritis ‐Kyoto classification of gastritis. Nihon Shokakibyo Gakkai Zasshi. 2015; 112: 982–93 (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 16. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis: the updated Sydney System. International workshop on the histopathology of gastritis, Houston 1994. Am. J. Surg. Pathol. 1996; 20: 1161–81. [DOI] [PubMed] [Google Scholar]
- 17. Muto M, Yao K, Kaise M et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA‐G). Dig. Endosc. 2016; 28: 379–93. [DOI] [PubMed] [Google Scholar]
- 18. Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14: 101–12. [DOI] [PubMed] [Google Scholar]
- 19. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guideline 2010: 3rd English edition. Gastric Cancer. 2011; 14: 113–23.21573742 [Google Scholar]
- 20. Schlemper RJ, Riddell RH, Kato Y et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000; 47: 251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lauwers G, Carneiro F, Graham D et al. WHO Classification of Tumors of the Digestive System, 4th edn. JARC: Lyon, 2010. [Google Scholar]
- 22. Shiota S, Murakami K, Suzuki R, Fujioka T, Yamaoka Y. Helicobacter pylori infection in Japan. Expert Rev. Gastroenterol. Hepatol. 2013; 7: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito M, Tanaka S, Takata S et al. Morphological changes in human gastric tumours after eradication therapy of Helicobacter pylori in a short‐term follow‐up. Aliment. Pharmacol. Ther. 2005; 21: 559–66. [DOI] [PubMed] [Google Scholar]
- 24. Owen DA. Lamina propria, Stomach. In: Histology for the Pathologists, 5th edn. Philadelphia: Wolters Kluwer, 2020; 607. [Google Scholar]
- 25. Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014; 513: 202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Funakoshi T, Miyamoto S, Kakiuchi N et al. Genetic analysis of a case of Helicobacter pylori‐uninfected intramucosal gastric cancer in a family with hereditary diffuse gastric cancer. Gastric Cancer. 2019; 22: 892–8. [DOI] [PubMed] [Google Scholar]
- 27. Nomura R, Saito T, Mitomi H et al. GNAS mutation as an alternative mechanism of activation of the Wnt /β‐catenin signaling pathway in gastric adenocarcinoma of the fundic gland type. Hum. Pathol. 2014; 45: 2488–96. [DOI] [PubMed] [Google Scholar]
- 28. Sugimoto R, Sugai T, Habano W et al. Clinicopathological and molecular alterations in early gastric cancers with the microsatellite instability‐high phenotype. Int. J. Cancer. 2016; 138: 1689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fukayama M. Epstein‐Barr virus and gastric carcinoma. Pathol. Int. 2010; 60: 337–50. [DOI] [PubMed] [Google Scholar]
- 30. Okano A, Kato S, Ohana M. Helicobacter pylori‐negative gastric cancer: Advanced‐stage undifferentiated adenocarcinoma located in the pyloric gland area. Clin J Gastroenterol. 2017; 10: 13–17. [DOI] [PubMed] [Google Scholar]
- 31. Horiuch Y, Fujisaki J, Ishizuka N et al. Study on clinical factors involved in Helicobacter pylori‐uninfected, undifferentiated‐type early gastric cancer. Digestion. 2017; 96: 213–19. [DOI] [PubMed] [Google Scholar]
- 32. Matsuo T, Ito M, Takata S, Tanaka S, Yoshihara M, Chayama K. Low prevalence of Helicobacter pylori‐negative gastric cancer among Japanese. Helicobacter. 2011; 16: 415–19. [DOI] [PubMed] [Google Scholar]
- 33. Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res. 2005; 589: 47–65. [DOI] [PubMed] [Google Scholar]


