Abstract
Nonmyeloablative allogeneic hematopoietic cell transplantation (HCT) from HLA-identical related donors using cyclosporine (CSP) and mycophenolate mofetil (MMF) for post-grafting immunosuppression is effective therapy for hematologic cancers. However, graft-vs-host-disease (GVHD) remains a major cause of morbidity and mortality. Pilot data suggested lower acute GVHD incidence with tacrolimus/MMF compared to historical experience using CSP/MMF after nonmyeloablative HCT. In a phase II multicenter trial, we evaluated the effect of tacrolimus/MMF for GVHD prophylaxis after HLA-identical related donor peripheral blood HCT in patients with hematologic malignancies (n=150) using conditioning with 2 Gy total body irradiation (TBI) for patients with a preceding (within 6 months) planned autologous HCT (n=50), or combined with 90mg/m2 fludarabine for those without recent autologous HCT (n=100). Oral tacrolimus was given from days −3 to 56 (tapered by day +180 if no GVHD). Oral MMF was given from days 0 to 27. Patient median age was 57 (range 20–74) years. The cumulative incidences (CI) of day-100 grade II-IV and III-IV acute GVHD were 27% and 4%, respectively. With median follow-up of 10.3 (range, 3.1–14.5) years, the 5-year CI of chronic extensive GVHD was 48%. One and 5-year estimates of non-relapse mortality, relapse/progression, survival, and progression-free survival were 9% and 13%, 35% and 50%, 73% and 53%, and 56% and 37%, respectively. GVHD prophylaxis with tacrolimus/MMF resulted in a low risk of acute GVHD and compared favorably with results from a concurrent trial using CSP/MMF. A randomized phase III trial to investigate tacrolimus/MMF versus CSP/MMF in nonmyeloablative HCT is warranted.
Graphical Abstract
Introduction
Nonmyeloablative conditioning using low-dose total body irradiation (TBI) with or without fludarabine (FLU) and HLA-matched donor peripheral blood hematopoietic cell transplantation (HCT) is an effective therapy for many hematologic malignancies. Although this approach achieves reliable engraftment with reduced regimen-related toxicity, the major causes of non-relapse mortality (NRM) are the development of acute and chronic graft-versus-host-disease (GVHD).1,2 Historically, cyclosporine (CSP) has been used in combination with mycophenolate mofetil (MMF) for post-grafting immunosuppression in the nonmyeloablative setting due to demonstrated synergy in preventing GVHD and improvement in survival in the canine model.3,4
Tacrolimus (TAC) is an effective immunosuppressant that has been used in solid organ transplantation and HCT. Both TAC and CSP mediate their activity through formation of a drug-immunophilin complex, which then inhibits the phosphorylase activity of calcineurin, resulting in inhibition of IL-2 gene expression. TAC also blocks conversion of precursor helper T-lymphocytes to antigen-conditioned T-lymphocytes.5 On an equimolar basis, the immunosuppressive effects of TAC were approximated to be 100 times higher than CSP in early in vitro studies.6
In patients who received myeloablative HCT conditioning, several large prospective randomized studies have demonstrated that TAC combined with methotrexate resulted in significantly lower acute GVHD compared to CSP and methotrexate, although a survival benefit was not observed in these studies.7–9 After nonmyeloablative conditioning, pilot data suggested superior GVHD prophylaxis with TAC/MMF compared to our historical experience using CSP/MMF after nonmyeloablative HCT.10 Other studies using a different nonmyeloablative conditioning regimen and TAC/MMF for GVHD prophylaxis have also found low rates of acute GVHD.11
We conducted a phase II multicenter prospective study using nonmyeloablative conditioning for hematologic malignancies using post-grafting immunosuppression with TAC and MMF in recipients of unmodified peripheral blood stem cell (PBSC) grafts from HLA-identical siblings. The primary aims were to evaluate the incidence of grade III/IV acute GVHD and chronic extensive GVHD. Secondary aims were to estimate the incidence of graft rejection, grade II-IV acute GVHD, disease progression and/or relapse-related mortality, rate and duration of steroid use for the treatment of chronic GVHD, and 1-year overall survival.
Methods
Patient and donor selection
Patients <75 years-old with hematologic malignancies treatable with HCT who were not eligible for conventional myeloablative allogeneic HCT were included. Diseases included were diffuse large B-cell non-Hodgkin lymphoma (NHL) and other aggressive lymphomas, low-grade NHL, mantle cell NHL, chronic lymphocytic leukemia, Hodgkin lymphoma, multiple myeloma (following planned or failed prior autograft), acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia beyond first chronic phase, myelodysplastic syndrome (MDS), myeloproliferative neoplasms (MPN), and Waldenstrom’s macroglobulinemia. Patients who had received prior autologous or allogeneic HCT were eligible. Patients with rapidly progressive aggressive NHL, chronic myelomonocytic leukemia, and AML, ALL, MDS, or MPN with ≥5% marrow blasts were excluded. Other major exclusion criteria included HIV-positive patients, active central nervous system involvement by disease, presence of circulating blasts, active infections, Karnofsky (adults) or Lansky (pediatric) performance score <50, and significant organ dysfunction. The research was approved by the Fred Hutch Institutional Review Board (IRB) or at the local institutional IRB, and all participants gave written informed consent. The trial is registered at clinicaltrials.gov (NCT00089011: Tacrolimus and Mycophenolate Mofetil in Preventing Graft-Versus-Host Disease in Patients Who Have Undergone Total-Body Irradiation With or Without Fludarabine Phosphate Followed by Donor Peripheral Blood Stem Cell Transplant for Hematologic Cancer).
Only HLA-matched related donors were allowed on the study. The related donor was HLA-identical genotypically at a minimum at one haplotype and could be phenotypically or genotypically identical at the allele level at HLA-A, -B, -C, -DRB1, and -DQB1. G-CSF mobilized blood was the only source of stem cells allowed. Donors age <12 years and twin donors were excluded.
Treatment plan
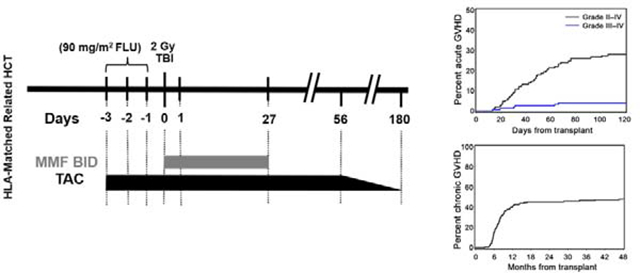
Patients at low risk of graft rejection (preceding planned autologous HCT within 6 months of conditioning for allogeneic HCT) received 2 Gy TBI alone (prior auto; n=50) while the remaining patients received 90 mg/m2 FLU and 2 Gy TBI conditioning (no auto; n=100). FLU 30mg/m2/day was given intravenously on days −4 through −2, followed by TBI 2 Gy on day 0 and subsequent infusion of unmanipulated G-CSF-mobilized PBSCs on day 0. TAC (targeted to 15–20 ng/ml for the first 28 days and 10–20 ng/ml subsequently while on full dose) was administered orally (0.06 mg/kg, Q12 hr) from days −3 to +56 and in the absence of GVHD tapered off by day +180. Blood tacrolimus levels were monitored at least weekly or more often as clinically indicated. MMF was given orally (15 mg/kg, Q12 hr) from day 0 until day 27. Diagnosis, grading and treatment of acute and chronic GVHD were performed according to previously established criteria.12,13 The study was initiated prior to adoption of the National Institutes of Health criteria for chronic GVHD.
Statistical analysis
The primary objective of this study was to evaluate the incidence of grade III/IV acute GVHD and chronic extensive GVHD after nonmyeloablative conditioning and post-grafting immunosuppression with TAC/MMF in patients with hematologic malignancies. Two groups of patients were defined based on prior treatment (with or without prior autologous HCT within 6 months of conditioning for allogeneic HCT). Although conditioning differed between these groups, there was no intent to compare outcomes between groups; rather, the conditioning was modified to not require FLU if they had an immediately preceding autologous HCT. Planned sample size of up to 75 patients per group was based on estimating the rate of grade III/IV GVHD with a standard error of no more than 5 percentage points. Due to changes in treatment practice over time (increased treatment options for high risk myeloma patients), accrual of patients with prior auto-HCT was less than expected, so additional accrual was allowed in the group without prior auto-HCT but without changing the total target accrual of 150 patients. Stopping rules were incorporated within each patient group for evidence that the rate of grade III/IV acute GVHD exceeded 15%, or the rate of graft rejection exceeded 5%. No stopping rule was triggered in either group.
Overall and progression-free survival were estimated using the Kaplan-Meier method. Cumulative incidences of other time-to-event endpoints were estimated using competing risk methods. Relapse was a competing risk for non-relapse morality; death was a competing risk for relapse, acute and chronic GVHD, and infection.
Results
Patient characteristics
From 2004 to 2013, 150 patients were enrolled and received unmodified PBSC (median dose of 8.1 × 106 CD34+ cells/kg and 3.5 × 108 CD3+ cells/kg) from HLA-identical sibling donors, with the exception of one patient whose donor was an HLA-matched child. Patient characteristics are summarized in Table 1. Fifty-eight percent of patients had an HCT comorbidity index (HCT-CI) score of 3 or greater. In the no auto group, 27% of patients had failed a prior autologous HCT (>6 months from study enrollment), 2% had failed prior allogeneic HCT, and 1 patient was transplanted for secondary MDS/AML after prior autologous HCT for B-cell malignancy. Median follow-up was 10.3 (range, 3.1–14.5) years.
Table 1.
Patient Characteristics.
| Total (n=150) | No Auto (FLU/TBI) (n=100) | Prior Auto (TBI only) (n=50) | |
|---|---|---|---|
| Patient age, median (range), years | 57 (20–74) | 60 (20–74) | 54 (28–70) |
| Male patient gender, no. (%) | 87 (58) | 54 (54) | 33 (66) |
| Donor age, median (range), years | 54 (23–76) | 57 (23–76) | 51 (24–68) |
| Sex of patient/donor | |||
| Male / Female, no. (%) | 41 (27) | 25 (25) | 16 (32) |
| Male / Male, no. (%) | 46 (31) | 29 (29) | 17 (34) |
| Female / Male, no. (%) | 23 (15) | 16 (16) | 7 (14) |
| Female / Female, no. (%) | 40 (27) | 30 (30) | 10 (20) |
| CMV serostatus of patient/donor | |||
| Negative/negative, no. (%) | 26 (17) | 20 (20) | 6 (12) |
| Negative/positive, no. (%) | 21 (14) | 15 (15) | 6 (12) |
| Positive/negative, no. (%) | 39 (26) | 26 (26) | 13 (26) |
| Positive/positive, no. (%) | 61 (41) | 37 (37) | 24 (48) |
| Positive/unknown, no. (%) | 3 (2) | 2 (2) | 1 (2) |
| Prior (failed) high-dose HCT | |||
| Autologous, no. (%) | 43 (29) | 281 (28) | 15 (29) |
| Allogeneic, no. (%) | 2 (1) | 2 (2) | 0 |
| Diagnoses | |||
| Acute myeloid leukemia, no. (%) | 42 (28) | 42 (42) | 0 |
| MDS/MPN, no. (%) | 12 (8) | 12 (12) | 0 |
| Acute lymphoblastic leukemia, no. (%) | 6 (4) | 6 (6) | 0 |
| Non-Hodgkin lymphoma, no. (%) | 25 (17) | 222 (22) | 3 (6) |
| Chronic lymphocytic leukemia, no. (%) | 2 (1) | 2 (2) | 0 |
| Hodgkin lymphoma, no. (%) | 8 (5) | 7 (7) | 1 (2) |
| Multiple myeloma, no. (%) | 55 (37) | 9 (9) | 46 (92) |
| Relapse risk (DRI) | |||
| Low, no. (%) | 14 (9) | 13 (13) | 1 (2) |
| Intermediate, no. (%) | 117 (78) | 71 (71) | 46 (92) |
| High/Very High, no. (%) | 19 (13) | 16 (16) | 3 (6) |
| HCT comorbidity index, no. (%) | |||
| 0 | 25 (17) | 15 (15) | 10 (20) |
| 1,2 | 36 (24) | 23 (23) | 13 (26) |
| 3+ | 87 (58) | 61 (61) | 26 (52) |
| unknown | 2 (1) | 1 (1) | 1 (2) |
| CD34+ dose, 106 cells kg, median (range) | 8.1 (1.0–25.3) | 8.1 (1.0–25.3) | 8.5 (3.1–22.2) |
| CD3+ dose, 108 cells/kg, median (range) | 3.5 (0.3–10.8) | 3.6 (0.3–10.2) | 3.5 (1.0–10.8) |
| Karnofsky performance status, no. (%) | |||
| 90–100 | 85 (57) | 51 (51) | 34 (68) |
| 80–85 | 35 (23) | 29 (29) | 6 (12) |
| 60–70 | 21 (14) | 18 (18) | 3 (6) |
| unknown | 9 (6) | 2 (2) | 7 (14) |
Includes two with multiple failed autos and 1 with prior auto for B-cell neoplasm transplanted for secondary MDS/AML
Includes two Waldenstrom’s disease
Abbreviations: FLU, fludarabine; TBI, total body irradiation; CMV, cytomegalovirus; HCT, hematopoietic cell transplant; MDS/MPD, myelodysplastic syndrome/myeloproliferative neoplasms; DRI, disease risk index
Engraftment and chimerism
The median number of days that the absolute neutrophil count was less than 500 cells/μL was 1 day (range 0–97 days). Sixty-nine patients (46%) never reached ANC <500 cells/μL. The median minimum platelet count was 62,000/μL (range 9–226 × 103/μL). Seventy patients (47%) and 108 patients (72%) did not require red blood cell or platelet transfusions in the first 100 days post-HCT, respectively. For the purposes of this study, graft rejection was defined as the inability to detect or loss of detection of greater than 5% donor T cells (CD3+) as a proportion of the total blood T-cell population after HCT. Graft failure was defined as grade IV thrombocytopenia and neutropenia after day 21 that lasts >2 weeks and refractory to growth factor support. One graft rejection was observed at day 300 posttransplant in a patient with AML in remission conditioned with FLU/TBI, despite donor lymphocyte infusion (DLI) given on day 268 posttransplant for low CD3 chimerism (described below). One graft failure was observed and occurred at day 345 posttransplant in a patient with AML in remission conditioned with FLU/TBI. The bone marrow biopsy was consistent with aplastic anemia. The patient underwent a second nonmyeloablative allogeneic HCT.
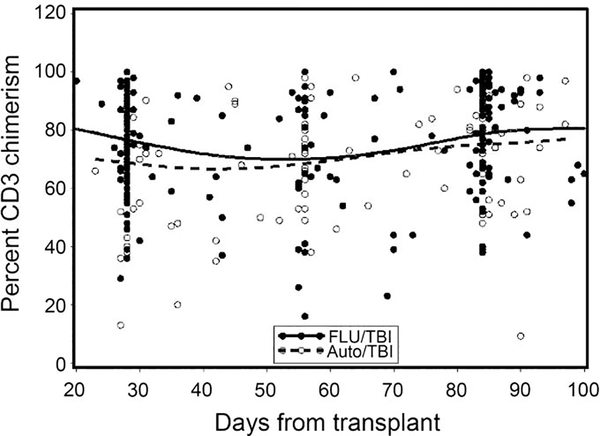
Peripheral blood CD3 donor chimerism at days 28, 56, and 84 were not significantly different between the prior auto and no auto groups (Figure 1). Four patients received DLI for low donor chimerism at a median of 163 (range 67–268) days post-HCT. All 4 patients were conditioned with FLU/TBI and received a median of 5.8 × 106 (range, 4.0–7.5) CD34+ cells/kg at the time of HCT. The median day 28 donor CD3 chimerism in this group was 39% (range 29–77%). Two of the 4 patients experienced disease relapse/progression after DLI without graft rejection, another patient rejected the graft (see above), and the fourth patient remains with stable engraftment, alive and in remission at last follow-up 5 years posttransplant.
Figure 1. Peripheral blood CD3 donor chimerism at day 28, 56, and 84 posttransplant.
There were no significant differences between the No Auto and Prior Auto groups.
GVHD
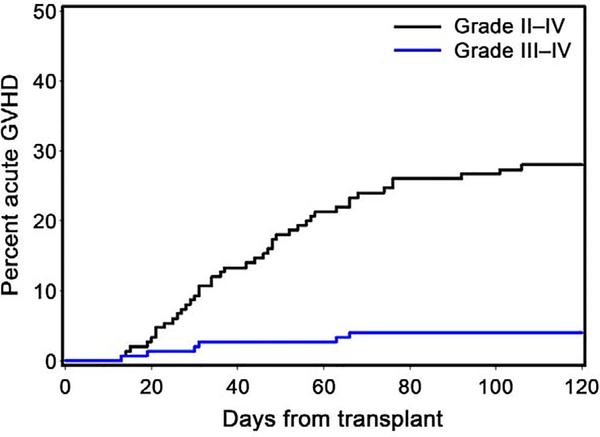
The cumulative incidences of grade II-IV and grade III-IV acute GVHD at 100 days were 27% and 4%, respectively (Figure 2).
Figure 2. Cumulative incidence of grade II-IV and III-IV acute GVHD.
The cumulative incidences of grade II-IV and grade III-IV acute GVHD at 100 days were 27% and 4%, respectively. Only one patient developed grade IV acute GVHD. See Table 2 for outcomes according to conditioning regimen.
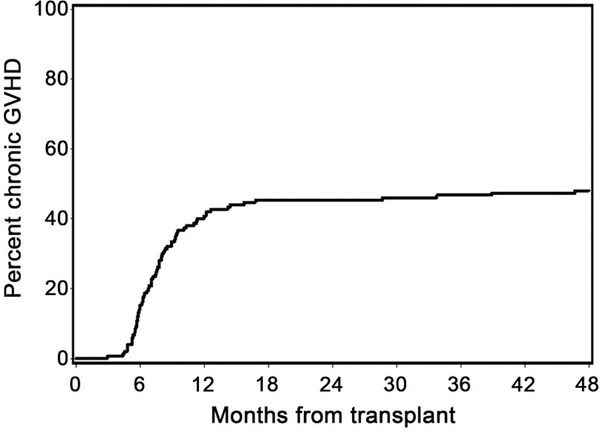
Our study was not designed to compare HCT outcomes between the no auto and prior auto cohorts, though results of the separate arms are presented in Table 2. Only one patient developed grade IV acute GVHD. Forty-eight percent of patients developed chronic extensive GVHD by 5 years (54% prior auto; 44% no auto) (Table 2; Figure 3). Median time to development of grade II-IV acute GVHD among affected patients was 44 days (range 13–127 days). Median time to development of chronic extensive GVHD among affected patients was 227 days (range 90–1423 days). Acute GVHD overall grading with organ staging of all patients is summarized in Table 3. Of the 72 patients who developed chronic extensive GVHD, 21 (29%) had prior diagnosis of acute GVHD and 51 (71%) had de novo chronic GVHD. The most common target organs involved in patients who developed chronic extensive GVHD were mouth (65%), skin (64%) and liver (56%) (Table 4).
Table 2.
Transplant outcome according to no auto or prior auto HCT
| Total (n=150) | No Auto (n=100) | Prior Auto (n=50) | |
|---|---|---|---|
| Grade II-IV acute GVHD at day 100, % | 27 | 25 | 30 |
| Grade III-IV acute GVHD at day 100, % | 4 | 2 | 8 |
| Chronic extensive GVHD (5 years), % | 48 | 44 | 56 |
| NRM (1/5 years), % | 9/13 | 12/16 | 4/6 |
| Relapse/Progression (1/5 years), % | 35/50 | 31/43 | 42/64 |
| Overall Survival (1/5 years), % | 73/53 | 73/55 | 74/48 |
| PFS (1/5 years), % | 56/37 | 57/41 | 54/30 |
Data are survival or cumulative incidence percentage. Study was not designed to compare outcomes between no auto and prior auto groups.
Abbreviations: GVHD, graft-versus-host-disease; NRM, non-relapse mortality; PFS, progression-free survival
Figure 3. Cumulative incidence of chronic GVHD.
48% of all patients was diagnosed with chronic extensive GVHD by 5 years. See Table 2 for outcomes according to conditioning regimen.
Table 3.
Acute GVHD overall grading with organ staging of all patients
| Overall Grade | (n) | Stage | Gut (n) | Liver (n) | Skin (n) |
|---|---|---|---|---|---|
| 0 | 103 | 0 | 116 | 148 | 125 |
| I | 6 | 1 | 28 | 1 | 9 |
| II | 35 | 2 | 2 | 0 | 5 |
| III | 5 | 3 | 3 | 1 | 11 |
| IV | 1 | 4 | 1 | 0 | 0 |
Table 4.
Chronic extensive GVHD organ involvement in 72 patients who developed chronic extensive GVHD.
| Site | n | % |
|---|---|---|
| Mouth | 47 | 65 |
| Skin | 46 | 64 |
| Liver | 40 | 56 |
| GI tract | 35 | 49 |
| Eyes | 32 | 44 |
| Genital tract | 11 | 15 |
| Lungs | 10 | 14 |
| Joints and fascia | 7 | 10 |
| Nails | 4 | 6 |
| Hair | 1 | 1 |
| Serosa | 1 | 1 |
Duration of immunosuppression
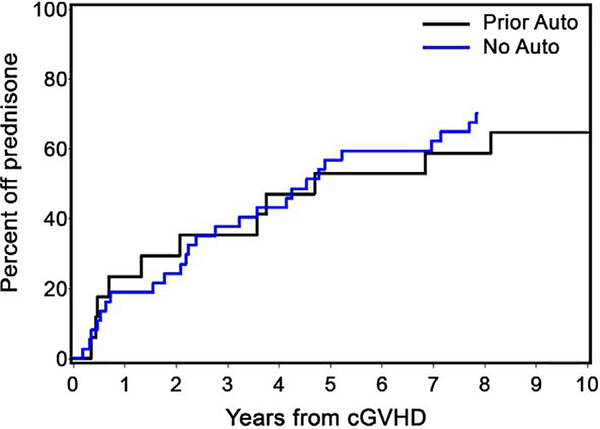
Of the 72 patients diagnosed with chronic extensive GVHD, 61 (84.7%) required systemic prednisone therapy for treatment. Among patients who successfully discontinued prednisone (while alive and in remission), the median time to discontinuation was 31 months (prior auto: 34 months; no auto: 31 months). The time at which the cumulative incidence of patients successfully discontinuing prednisone reached 50% was 54 months (prior auto: 56 months; no auto: 54 months) (Figure 4).
Figure 4. Duration of systemic prednisone use in patients with chronic extensive GVHD.
The cumulative incidences of patients successfully discontinuing prednisone reached 50% at 56 and 54 months in prior auto and no auto groups, respectively.
Overall and PFS, NRM, and relapse/progression
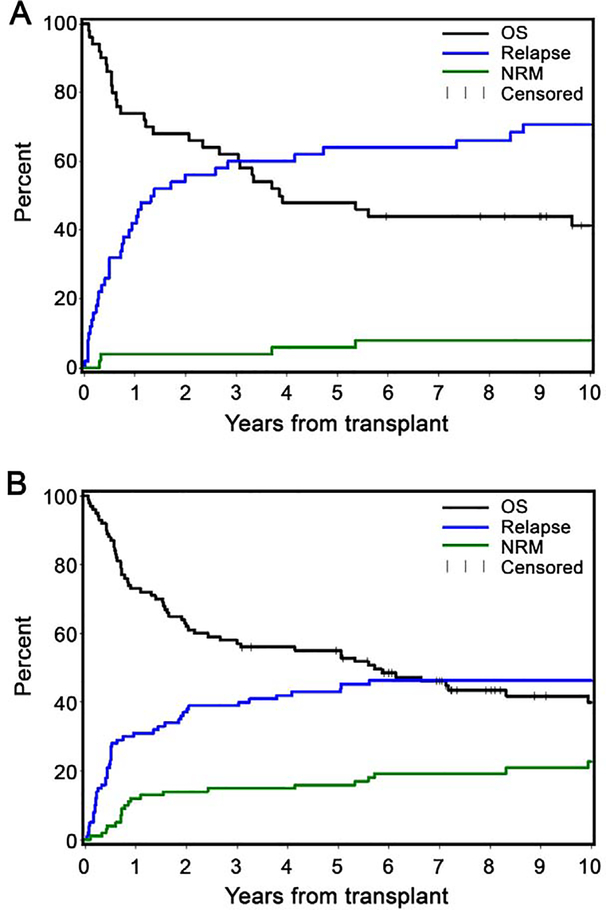
For the prior auto group, the 1-year cumulative incidences of NRM, relapse/progression, survival, and progression-free survival (PFS) were 4%, 42%, 74%, and 54%, respectively. Same 1-year estimates for the no auto group were 12%, 31%, 73%, and 57%, respectively. Five-year cumulative incidences of NRM, relapse/progression, survival and PFS were 6%, 64%, 48%, and 30%, respectively (prior auto) and 16%, 43%, 55%, and 41%, respectively (no auto) (Table 2; Figure 5).
Figure 5. Cumulative incidence of non-relapse mortality, relapse, and survival for prior auto (A) and no prior auto (B).
Five-year cumulative incidences of NRM, relapse/progression, survival and progression-free survival were 6%, 64%, 48%, and 30%, respectively (prior auto) and 16%, 43%, 55%, and 41%, respectively (no auto).
Infectious complications
Eleven (7%) patients were treated for proven or presumptive fungal pulmonary infections. Two patients developed candidemia and 1 patient developed nasal aspergillosis. CMV reactivation meeting threshold for treatment was observed in 42 (28%) patients at a median of 38 (range 1–212) days posttransplant. Six (4%) patients were diagnosed with CMV disease involving the lung (n=4) and gastrointestinal tract (n=3). EBV viremia occurred in 3 patients (2%), and all were treated with rituximab. Seven patients (4.7%) developed BK cystitis.
Secondary malignant neoplasms
Fourteen patients (9%) developed 15 secondary malignancies after HCT, excluding squamous cell carcinoma (SCC) of the skin and basal cell carcinoma (BCC). One patient developed 2 gastrointestinal malignancies of different histology at 10.8 years (pancreatic neuroendocrine tumor) and 12.7 years (small bowel adenocarcinoma) posttransplant. The median time to development of non-SCC of skin/non-BCC secondary malignancy was 5.4 (range, 0.4–13.0) years, calculated from time of allogeneic HCT. Five of the 14 patients were diagnosed with melanoma. Two patients with underlying disease of Hodgkin and non-Hodgkin lymphoma developed presumed host-derived therapy-related AML; however, the possibility of donor-derived AML could not be excluded based on available data. Six of the 14 diagnosed with secondary malignancies have died due to the secondary malignancy.
Discussion
Our prospective study showed that GVHD prophylaxis with TAC/MMF after nonmyeloablative conditioning and HLA-identical related peripheral blood HCT resulted in a low risk of acute GVHD in patients with hematologic malignancies. Although confirmation by a randomized study is needed, our results using TAC/MMF compare favorably with our experience in a concurrent trial using CSP/MMF wherein patients were randomized to conditioning with 2Gy TBI alone or in combination with 90 mg/m2 FLU prior to HLA-matched related peripheral blood HCT (NCT00075478).14 In this trial using CSP/MMF with the same timing of immunosuppression duration and discontinuation, the cumulative incidence of grades II to IV and grades III to IV acute GVHD at 120 days was 46% and 7% in the FLU/TBI arm. The TBI alone arm had lower initial CD3 chimerism which was associated with lower rates of acute GVHD (grades II to IV and grades III to IV acute GVHD were 32% and 11%, respectively) but higher rates of relapse and inferior survival; thus the relevant comparator arm to the current study using TAC/MMF was the FLU/TBI arm.14
From a pharmacokinetic perspective, studies have demonstrated differences in the clearance of mycophenolic acid (MPA), the active metabolite of MMF, when using CSP and TAC. Li et al.15,16 showed concomitant CSP use was associated with a 34% increase in total MPA clearance compared with concomitant TAC. Furthermore, we recently reported that the active metabolite of MMF (MPA) concentration at steady state (MPA Css) was lower in patients who received concomitant CSP than patients receiving TAC. Low total MPA Css was associated with an increased risk of severe acute GVHD following nonmyeloablative HCT.17 Therefore, the GVHD-protective effect of TAC could be mediated by delayed MMF clearance.
The duration of systemic prednisone use in patients who developed chronic extensive GVHD in this study was somewhat longer compared to previous reports after myeloablative conditioning. For example, a post-hoc analysis of a randomized phase III trial comparing chronic GVHD features after HLA-matched related HCT using mobilized peripheral blood stem cells versus bone marrow with methotrexate and CSP for GVHD prophylaxis after myeloablative conditioning showed that median time to discontinuation of glucocorticoid treatment was approximately 2.5 years in patients receiving peripheral blood.18 A large retrospective analysis of patients with chronic GVHD who received HCT after myeloablative conditioning and methotrexate and calcineurin-inhibitor for GVHD prophylaxis showed median duration of immunosuppressive therapy of 23 months in patients with chronic GVHD with resolution of symptoms before recurrent malignancy or death.19 The longer duration of prednisone use in our cohort may reflect differences in recipient age or use of MMF. Strategies to improve rates of chronic GVHD and prednisone use may include longer duration of prophylactic immunosuppressive therapy, increasing the dosing of MMF, or the use of post-transplant cyclophosphamide (PTCy).
Acute infectious complications and rates of secondary malignancy was favorable in our study. In comparison, a previous report showed cumulative incidence of secondary malignancies of 23.6% at 20 years posttransplant for patients older than 50 years of age. 20 Further, no significant acute toxicities attributable to TAC was observed. While our study enrolled nearly 60% of patients with HCT-CI scores of ≥3, rates of 1-year and 5-year NRM were low, highlighting the safety of this immunosuppressive combination for GVHD prophylaxis after nonmyeloablative conditioning.
Finally, although this prospective study enrolled two cohorts of patients receiving different conditioning regimens based on prior treatment (prior autologous treatment within 6 months vs. not) and thus risk of graft rejection, there was no intent to compare outcomes between groups. There are intrinsic differences in disease risk between the two cohorts, with aggressive lymphoma and high-risk myeloma patients constituting most patients receiving prior autologous HCT. This likely explains the differences in disease progression/relapse incidence between the two groups. Addition of posttransplant targeted agents or maintenance therapy could be considered to address high relapse risk in these patients.
The results of this multi-center prospective Phase II study show low rates of acute GVHD using post-grafting immunosuppression with TAC/MMF after HLA-identical nonmyeloablative HCT. Although our results compare favorably to a concurrent trial using CSP/MMF, a randomized study comparing TAC/MMF to CSP/MMF is needed to draw conclusions about the optimal calcineurin inhibitor for GVHD prophylaxis in this setting. Chronic GVHD and long-term systemic steroid use remains a major challenge, and novel approaches to and clinical investigation of chronic GVHD prevention after nonmyeloablative HCT for older individuals and those with co-morbidities is greatly needed. For example, we are currently investigating the use of PTCy in combination with cyclosporine and sirolimus after nonmyeloablative conditioning in patients after HLA-matched and mismatched unrelated donor transplantation in a phase II randomized clinical trial (NCT03246906). Nonetheless, our long-term follow-up data demonstrating overall favorable survival in a primarily older and infirm population with high-risk malignancies reinforce the durable success of nonmyeloablative allogeneic HCT for hematologic malignancies using this approach.
Highlights.
TAC/MMF after nonmyeloablative HLA-identical HCT results in low risk of aGVHD
Long-term outcomes show low rates of NRM and acceptable relapse rates
Chronic GVHD is a still a major problem; novel approaches for prevention are needed
Acknowledgments
The authors would like to thank the patients who participated in the clinical trial, research staff and clinical staff.
This study was supported by research funding from the National Cancer Institute, National Institutes of Health (CA78902 and CA15704).
Footnotes
Conflict of Interest Disclosures
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102(6):2021–2030. [DOI] [PubMed] [Google Scholar]
- 2.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–3400. [DOI] [PubMed] [Google Scholar]
- 3.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89(8):3048–3054. [PubMed] [Google Scholar]
- 4.Yu C, Seidel K, Nash RA, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood. 1998;91(7):2581–2587. [PubMed] [Google Scholar]
- 5.Jacobson P, Uberti J, Davis W, Ratanatharathorn V. Tacrolimus: a new agent for the prevention of graft-versus-host disease in hematopoietic stem cell transplantation (Review). Bone Marrow Transplantation. 1998;22(3):217–225. [DOI] [PubMed] [Google Scholar]
- 6.Kino T, Hatanaka H, Miyata S, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo). 1987;40(9):1256–1265. [DOI] [PubMed] [Google Scholar]
- 7.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–2068. [PubMed] [Google Scholar]
- 8.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (Prograf, FK506) with methotrexate and cyclosporine for graft-versus-host-disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–2314. [PubMed] [Google Scholar]
- 9.Hiraoka A, Ohashi Y, Okamoto S, et al. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Japanese FK506 BMT Study Group. Bone Marrow Transplantation. 2001;28(2):181–185. [DOI] [PubMed] [Google Scholar]
- 10.Nieto Y, Patton N, Hawkins T, et al. Tacrolimus and mycophenolate mofetil after nonmyeloablative matched-sibling donor allogeneic stem-cell transplantations conditioned with fludarabine and low-dose total body irradiation. Biol Blood Marrow Transplant. 2006;12(2):217–225. [DOI] [PubMed] [Google Scholar]
- 11.Sabry W, Le Blanc R, Labbe AC, et al. Graft-versus-host disease prophylaxis with tacrolimus and mycophenolate mofetil in HLA-matched nonmyeloablative transplant recipients is associated with very low incidence of GVHD and nonrelapse mortality. Biol Blood Marrow Transplant. 2009;15(8):919–929. [DOI] [PubMed] [Google Scholar]
- 12.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplantation. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 13.Thomas’ Hematopoietic Cell Transplantation. Vol. Second. Boston, MA: Blackwell Science, Inc.; 1999. [Google Scholar]
- 14.Kornblit B, Maloney DG, Storb R, et al. Fludarabine and 2 Gy TBI is superior to 2 Gy TBI as conditioning for HLA-matched related hematopoietic cell transplantation: a phase III randomized trial. Biology of Blood and Marrow Transplantation. 2013;19(9):1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Mager DE, Sandmaier BM, Maloney DG, Bemer MJ, McCune JS. Population pharmacokinetics and dose optimization of mycophenolic acid in HCT recipients receiving oral mycophenolate mofetil. Journal of Clinical Pharmacology. 2013;53(4):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Mager DE, Sandmaier BM, et al. Pharmacokinetic and pharmacodynamic analysis of inosine monophosphate dehydrogenase activity in hematopoietic cell transplantation recipients treated with mycophenolate mofetil. Biol Blood Marrow Transplant. 2014;20(8):1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott CL, Sandmaier BM, Storer B, et al. Nonrelapse mortality and mycophenolic acid exposure in nonmyeloablative hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2013;19(8):1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flowers MED, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100(2):415–419. [DOI] [PubMed] [Google Scholar]
- 19.Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104(12):3501–3506. [DOI] [PubMed] [Google Scholar]
- 20.Baker KS, Leisenring WM, Goodman PJ, et al. Total body irradiation dose and risk of subsequent neoplasms following allogeneic hematopoietic cell transplantation. Blood. 2019;133(26):2790–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]