Abstract
The behaviour of high oleic sunflower oil in deep frying process of purple potato Purple Majesty has been evaluated simulating a fast food cooking process. This oil was used for 8h/day for 6 days, filling up from the 2nd day. A discontinuous and prolonged procedure was tested. Free Fatty Acidity (FFA), Peroxide Value (PV), Total Polar Compounds (TPC), Fatty Acid (FA) composition, Volatile Organic Compounds (VOC) have been determined at different times in thermo-oxidized (T-OX) oil, and in frying oil. The FFA in T-OX oil samples showed values in the range 0.09%–0.24%, whereas in the frying oil values varied in a range between 0.09% and 0.16%. TPCs values varied from 1.76% to 38.24% in T-OX oils, whereas in frying oil used for frying purple potatoes (FOPP) showed values in the range from 1.76 to 29.13%. The peroxides values did not follow a regular pattern, both during thermo-oxidation and during frying. Among the Long Chain Fatty Acids (LCFAs), oleic acid was the most represented (84.13%). Short chain fatty acids (SCFAs) amount was 0.34% (octanoic acid). Medium chain fatty acids (MCFA) amount was 4.45% (palmitic acid). During the thermo-oxidation, the poly-unsaturated fatty acids (PUFA) amount decreased during the 48 h of heat treatment, reaching an amount of 6.21%. This determined the increase in short chain fatty acids (SCFA). Trans fatty acids increased with the frying time. Unsaturated fatty acids (UFA) reached the value of 90.19%; SFA was 9.79%, and octanoic acid was 0.20%. A correlation between TPC vs UFA/SFA and TPC vs C18:2/C16:0 was observed in the frying oil. The most abundant volatile compounds in frying oil (from 0 to 48 h) were the aldehydes produced by decomposition of hydroperoxides of oleic and linoleic acids.
Keywords: High oleic sunflower oil, Purple potato “Purple Majesty”, Deep frying, Thermo-oxidation, Oxidative stability
high oleic sunflower oil; purple potato “Purple Majesty”; deep frying; thermo-oxidation; oxidative stability
1. Introduction
Oleic acid, a monounsaturated omega-9 fatty acid, 18:1 cis-9, with formula CH3(CH2)7CH = CH(CH2)7COOH, is found in many foods, but mainly in olive and seeds oils. Even if other mono-unsaturated fatty acids are present in olive and seed oil (Rotondo et al., 2020), oleic acid it is receiving great attention worldwide for its beneficial health properties (Sales-Campos et al., 2013). The FDA in 2018 has determined that exists realistic evidence to support a health claim associated to the oleic acid consuming, and to the reduction of coronary heart disease’ risk (FDA, 2018).
For this reason, in the recent decades, plants with a higher oleic acid content (up to 60% and more) have been selected, which opened up a new frontier to the possible uses of these crops taking advance of possible beneficial health effects, and triggering at the same time the market interest for its wider use (Ramadan et al., 2006; Dominguez Brando and Sarquis, 2012; Ramadan, 2013).
Interest is growing for the sunflower oil with a high oleic acid content, namely in the range between 70%-75% up to 90%. This oil is called “high oleic content” sunflower oil and presents a fatty acid composition similar to that of rapeseed and olive oil and has antioxidant potential similar to the one observed for the sesame cake extract which is interesting for the stabilization of sunflower and soybean oils (Mohdaly et al., 2011).
Deep frying or deep-fat frying is one of the most widespread and oldest food preparation processes used worldwide. There are many historical signs, dating back to 1600 BC, that document its use (Firestone, 2007). Frying, on the other hand, is a popular practice of food cooking for the peculiar sensory characteristics given to the food and for its relatively low cost. This cooking method (which requires a cooking temperature in the range from 160 °C to 180 °C) leads to possible alteration of the compounds present in the oil, depending mainly on its composition.
During the frying process, plenty of reactions occur, which results into the formation of new compounds, generated by the exposure of the oil to high temperatures, by the contact between food and the frying medium, by the water content of the food, and by atmospheric air in contact with the food surface. The primary chemical reactions involved in deep-fat frying that cause great changes to oil's fatty acid composition are: oxidation, hydrolysis, polymerization and isomerization, which lead to the production of free fatty acid, alcohols, aldehydes, ketones, diglycerides, monoglycerides, cyclic compounds, and epoxides (Choe and Min, 2007; Zhang et al., 2012; Romano et al., 2013a, Romano et al., 2013b; Li et al., 2017; Manzo et al., 2019). Frying cooking method is also related to the moisture evaporation, heat, and fat uptake.
Saturated and unsaturated fatty acids can influence the degradation of frying oils. In general, oils having high amounts of polyunsaturated fatty acids (i.e. corn, sunflower, soybean, rapeseed, and peanut oils) are suitable for domestic cooking. Nonetheless, the oils with a low content of linoleic acid and a high content of oleic acid (i.e. olive, almond, and canola oils) have been reported to be more stable during the frying process (Bou et al., 2012; Abdulkarim and Ghazali, 2012).
It has also been shown that the presence of large quantities of polyunsaturated fatty acids, mainly α-linolenic, is positively correlated to the oxidative degradation affecting the oxidation resistance between different oils. To increase the oxidative stability during deep-fat frying, it is recommended an oil or fat with a low unsaturation degree. Indeed, a higher percentage of oleic acid indicates a better resistance to thermal and oxidative degradation during refining and storage. This reflects on a better frying performance of these last seed oils being able to replace the more expensive olive oil (Raß et al., 2008; Aladedunyu et al., 2013; Romano et al., 2013a, Romano et al., 2013b; Yu et al., 2017).
Therefore, vegetable oils rich in oleic acid have gained ample area of interest not only for their important health benefits but also due to the characteristic of this monounsaturated fatty acid that help to counteract the generation of oxidation compounds during frying cooking.
The potato is a starchy tuber of the plant Solanum tuberosum, native to the Americas, and is a perennial in the Solanaceae family.
Even if the potato cultivated worldwide belongs to one botanical species, the tubers derived from thousands of cultivars present a great variability in shape, size, texture, color, taste, and cooking characteristics. In the framework of the European market, yellow-fleshed potatoes are widely diffused on the markets, but varieties of colored-fleshed potatoes have also recently appeared, and have been increasingly appreciated in the recent years. Purple Majesty is a potato cultivar with colored flesh registered in the British Potato Variety (Scotland). The interest for this cultivar is given by the amount of polyphenols, especially for the high content of anthocyanins, the main responsible compounds of the representative color of these varieties of potatoes (Xu et al., 2009; Lemos et al., 2015).
In this context, this work aims to evaluate the oxidative stability of high oleic sunflower oil during deep-frying process of purple potato Purple Majesty. In particular, the chemical characteristics of the oil have been investigated at different frying times evaluating free fatty acidity, peroxide value, total polar compounds, fatty acid composition, and volatile organic compounds.
2. Material and methods
2.1. Materials and frying protocol
High oleic sunflower oil (HOSO) was obtained from the local Italian market, and frozen potatoes of the cultivar Purple Majesty from Albert Bartlett Inc. (Greenwood Village, CO, United States). In order to simulate a typical fast food cooking, the potatoes were subjected to deep-fat frying process where the oil was used for 8h/day for 6 days, with filling up from the 2nd day as discontinuous and prolonged method. The frying process of potatoes was carried out according to a previously described procedure (Romano et al., 2012a, Romano et al., 2012b), and potatoes were used to simulate the real use in fast food conditions. For this purpose they were allowed to reach final temperature of 15 ± 1 °C before the frying process. The cooking process was conducted in a thermostatically temperature-controlled fryer (Tefal, Milan, Italy) at 180 ± 5 °C. A parallel experiment has been conducted to evaluate the characteristics of the oil subjected to a thermo-oxidation (T-OX) process, for the same times and temperatures equal to those of the frying process, but in the absence of the food matrix.
The oil samples were taken regularly at 8 h intervals and analyzed. Therefore, the samples analyzed refer to the following hours of heat treatment: 0, 8, 16, 24, 32, 40, 48h, respectively. All reagents were of analytical or spectroscopic grade and were supplied by Sigma Aldrich (St. Louis, MO, Unites States).
2.2. Free fatty acidity
The Free Fatty Acidity (FFA) was determined by titration as described in the European Official Methods of Analysis (EECR 2568/91). Approximately 5 g of oil were dissolved in ethylic alcool/diethyl ether (1:2) mixture and titrated with a 0.1 N NaOH solution in presence of phenophtalein as indicator. The result was expressed as g of oleic acid in 100 g of oil (% w/w).
2.3. Peroxide value
The peroxide value (PV) determination was carried out according to the Regulation CE 1989/03 expoliting the method for determining the extent of oil oxidation and measure the formation of intermediate hydroperoxides. Five grams of the oil were dissolved in an acetic acid/chloroform (3:2) mixture, added with a supersaturated solution of KI and, after staying 5 min in the dark, titrated with a 0.01 N solution of Na2O3S2 in presence of starch as indicator. The results were expressed as meq of O2 per Kg of sample.
2.4. Fatty acids
Fatty acids (FA) analysis was performed by gas chromatography (GC) after derivatization to fatty acids methyl esters (FAMEs) using a 2 N KOH solution in methanol, according to the IUPAC standard method (IUPAC 2.301) and Romano et al., (2011). FAMEs, including methyl octanoate, were analyzed on a GC PerkinElmer AutoSystem XL (Perkin Elmer, MA, United States) equipped with a programmed temperature vaporizer, a flame ionization detector (FID), and a capillary column of 100 m × 0.25 mm ID; film thickness of 0.20 μm; stationary phase of 50% cianopropyl methyl silicone (Supelco, Bellofonte, PA, United States). Helium has been used as carrier gas, at a flow rate of 20 cm/s. The oven temperature program was as follows: 120 °C for 5 min, 5 °C/min ramp-up to 165 °C for 5 min, and then 10 °C/min ramp-up to 240 °C for 20 min. The split ratio was 1/60, and the FID temperature was 260 °C. Peaks have been identified by comparison with an external standard (SupelcoTM 37 component FAME MIX). The sample concentrations were calculated by comparison with the pure standard retention time and expressed as weight/weight percentages (% w/w).
2.5. Total polar compounds
Total Polar Compounds (TPCs) were separated using a preparative chromatography column as described by Dobarganes et al. (2000) and gravimetrically determined. One gram of oil was loaded on a silica gel column (Discovery SPE DSC-Si Silica Tube, 20 ml, 5g; Supelco Analytical, Bellefonte, PA, United States), and the polar components were eluted with a solvent mixture composed of 90% light petroleum and 10% diethyl ether. The results were expressed as g of TPC in 100 g of oil (% w/w).
2.6. Volatile organic compounds
Volatile Organic Compounds (VOCs) were isolated from the frying oil using the purge-and-trap DHS concentrator from Teledyne Tekmar Instruments (Manchester, UK) equipped with a Tenax trap. Each sample (2 mL) was spiked with 100 μg/L of undecane as an internal standard (IS). A purge vessel containing the sample was connected to the purge-and-trap unit. VOC analysis was performed using an Agilent 6890N gas-chromatograph equipped with an Agilent 5973N mass spectrometer and a capillary column with 5% phenylmethylpolysiloxane (30 m × 0.25 mm id × 0.25 μm) HP-5 MS (Agilent J&W, Santa Clara, CA, United States). Helium at a flow rate of 1.2 mL/min was used as carrier gas. The oven temperature program was as follows: 45 °C for 3 min, 10 °C/min ramp-up to 240 °C for 1 min, and 15 °C/min ramp-up to 270 °C for 1 min. Mass spectra were obtained at 70 eV in the range of 35–400 UMA. VOC identification was achieved by comparing the obtained mass spectra and GC retention times with those of the pure standards. The concentration of the detected compounds was obtained by comparing the peak areas with the area of the known amount of the IS (Romano et al., 2012a, Romano et al., 2012b) and was expressed as mg/Kg.
2.7. Statistical analysis
All determinations and experiments were performed in triplicate and the presented results are the average values of three determinations. Data were subjected to ANOVA analysis of variance (XLSTAT 2006; ADDINSOFT, Paris, France). Differences with P≤0.05 were considered significant.
3. Results and discussion
3.1. Free fatty acidity, peroxide values and total polar compounds
Table 1 reports the FFA values (g oleic acid/100g of oil) for the high oleic sunflower oil (HOSO) in T-OX oil and frying oil (FOPP). The FFA index represents the measure of hydrolysis degree of the oil. T-OX oil samples showed values ranged from 0.09% and 0.24%, whereas frying oil values varied in a range between 0.09% and 0.16%. Concerning T-OX, the increase of acidity value may be due to the thermal degradation of triglycerides with consequential release of free fatty acid. It can be observed a significant increase of values in FOPP samples only during the first stage of treatment (from 0 to 8h). This could be related to filling up operations that slow down the formation of mono-glycerides and di-glycerides as well as the presence of antioxidants released from purple potatoes. The PV values are reported in Table 1. The PV represents a quality parameter and it measures the quantity of oxygen dissolved in a food oil, indicating the rancidity degree of the oil. PV values varied in the range 1.10–4.38 meq O2/Kg oil in T-OX and in the range 1.63–6.73 meq O2/Kg oil in frying oil. As shown in Table 1, peroxides values do not follow a regular pattern, both during thermo-oxidation and during frying. A continuous formation and decomposition of primary oxidation products occurs. The discontinuous heat treatment, compared to a continuous one, is more destructive this is due to the cooling of the oil which promotes the formation of new hydroperoxides that in the next heating cycle can go through hydrolysis (Frankel, 2005).
Table 1.
Free fatty acidity (FFA), peroxide value (PV) and total polar compounds (TPC) trends in thermo-oxidized oil (TOX) and in frying oil used for frying purple potatoes (FOPP) at different treatment times.
| Time (h) | FFA |
PV (meq O2/Kg oil) |
TPC (g/100 g oil) |
|||
|---|---|---|---|---|---|---|
| TOX | Frying oil | TOX | Frying oil | TOX | Frying oil | |
| 0 | 0.09e ± 0.01 | 0.09d ± 0.01 | 4.38a ± 0.04 | 4.38c±0.04 | 1.76f±0.05 | 1.76g ± 0.01 |
| 8 | 0.15d ± 0.01 | 0.13c±0.01 | 1.10d ± 0.14 | 6.73a±0.04 | 10.54e±0.15 | 9.53f±0.01 |
| 16 | 0.20c±0.01 | 0.15b ± 0.01 | 2.90b ± 0.14 | 4.63b ± 0.04 | 11.27d ± 0.01 | 18.77e±0.01 |
| 24 | 0.24b ± 0.01 | 0.16a±0.01 | 1.90c ± 0.14 | 3.05d ± 0.07 | 15.93c±0.03 | 21.59d ± 0.01 |
| 32 | 0.21c±0.01 | 0.16a±0.01 | 1.20d ± 0.01 | 2.20e±0.01 | 17.74c±0.01 | 26.85c±0.01 |
| 40 | 0.27a±0.01 | 0.16a±0.01 | 1.80c ± 0.01 | 1.63g ± 0.04 | 27.58b ± 0.09 | 28.17b ± 0.01 |
| 48 | 0.24b ± 0.01 | 0.16a±0.01 | 1.40d ± 0.01 | 1.95f ± 0.01 | 38.24a ± 0.09 | 29.13a ± 0.01 |
a-g: different letters in the same column indicate significant differences (p ≤ 0.05) among treatment times.
Since hydroperoxides are reported to be unstable products, the measurement of the polar materials can be regarded as a key test for determining the degradation level of an oil. The TPCs values are reported in Table 1: they include substances such as monoglycerides, diglycerides, and free fatty acids, naturally occurring in fats or formed during frying or also heating. The maximum limit value of TPC allowed by the current European regulation, valid in many Countries, is 25% (Aladedunyu et al., 2013; Stier et al., 2013). As expected, the percentage of total polar compounds increased in both in thermo-oxidized and frying oil. TPCs values varied from 1.76% to 38.24% in T-OX oils, whereas in TOFF oils the range is 1.76–29.13%. Moreover, T-OX oil reached the value allowed by law at 40h, whereas FOPP at 32h. Hassanien and Sharoba (2014), by studying sunflower oil, cottonseed oil, and palm olein during deep frying for 4–16 h, reported an increase during frying of total phenolic content in different oils, and this was strongly correlated to the frying time.
3.2. Fatty acids
Ten fatty acids were identified in T-OX as shown in Table 2. Long chain fatty acids (LCFA, from C18:0 to C22:0), were the most abundant (96.47%). Among the LCFAs, oleic acid (C18: 1c) was the most representative one with values that reached the 84.13%. Short chain fatty acids (SCFA) reached a percentage of 0.34%, with C8:0 (octanoic acid) as the only representative. Medium chain fatty acids (MCFA) reached percentages of 4.45%, with C16: 0 (palmitic acid) as the only one present.
Table 2.
Fatty acid composition (%) of thermo-oxidized high oleic sunflower oil at different treatment times.
| Fatty acids | Time (h) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 | 16 | 24 | 32 | 40 | 48 | |
| C8:0 | nd | 0.07c ± 0.02 | 0.11b.c±0.01 | 0.06c ± 0.03 | 0.26a.b±0.03 | 0.35a ± 0.04 | 0.34a ± 0.01 |
| C16:0 | 3.55d ± 0.04 | 3.76d ± 0.01 | 3.92b.c.d±0.01 | 3.76c.d±0.01 | 4.28a.b.c±0.15 | 4.48a ± 0.34 | 4.45a.b±0.01 |
| C18:0 | 3.01b ± 0.04 | 2.94b ± 0.11 | 3.18a.b±0.01 | 2.98b ± 0.08 | 3.21a.b±0.26 | 3.34a.b±0.25 | 3.59a ± 0.02 |
| C18:1t | nd | 0.22b ± 0.01 | 0.3b ± 0.13 | 0.28b ± 0.02 | 0.95a ± 0.22 | 1.21a ± 0.29 | 1.33a ± 0.05 |
| C18:1c | 84.11a ± 0.11 | 84.00a ± 0.34 | 83.94a.b±0.03 | 84.00a ± 0.39 | 84.12a ± 0.36 | 84.2a ± 0.34 | 84.13a ± 0.10 |
| C18:2t | nd | 0.01 ± 0.02 | 0.03 ± 0.01 | nd | 0.25 ± 0.33 | 0.23 ± 0.33 | 0.03 ± 0.01 |
| C18:2c | 8.08d ± 0.02 | 11.59a ± 0.19 | 9.91c ± 0.02 | 10.01b ± 0.49 | 7.32e ± 0.16 | 6.26f ± 0.09 | 5.96f ± 0.09 |
| C20:0 | 0.26 ± 0.02 | 0.42 ± 0.24 | 0.28 ± 0.00 | 0.27 ± 0.01 | 0.25 ± 0.07 | 0.27 ± 0.07 | 0.32 ± 0.01 |
| C18:3 | 0.27 ± 0.01 | 0.3 ± 0.08 | 0.34 ± 0.01 | 0.35 ± 0.05 | 0.2 ± 0.05 | 0.23 ± 0.06 | 0.22 ± 0.0d |
| C22:0 | 0.96 ± 0.01 | 0.71 ± 0.02 | 0.83 ± 0.0d | 0.68 ± 0.10 | 0.77 ± 0.13 | 0.8 ± 0.11 | 0.89 ± 0.01 |
| ∑SFA | 7.77c ± 0.02 | 7.90b.c±0.07 | 8.32b ± 0.00 | 7.75b.c±0.05 | 8.77a ± 0.11 | 9.24a ± 0.11 | 9.59a ± 0.02 |
| ∑MUFA | 84.11 ± 0.04 | 84.22 ± 0.08 | 84.24 ± 0.04 | 84.28 ± 0.11 | 85.07 ± 0.12 | 85.41 ± 0.15 | 85.46 ± 0.04 |
| ∑PUFA | 8.35c ± 0.01 | 11.90a ± 0.06 | 10.28b ± 0.01 | 10.36b ± 0.11 | 7.77c ± 0.11 | 6.72d ± 0.10 | 6.21d ± 0.02 |
| ∑TRANS | 0.00c ± 0.02 | 0.23b.c±0.01 | 0.33b ± 0.07 | 0.28b.c±0.01 | 1.20a ± 0.18 | 1.44a ± 0.11 | 1.36a ± 0.03 |
| ∑UFA | 92.46a ± 0.02 | 96.12b ± 0.07 | 94.52c ± 0.03 | 94.64c ± 0.11 | 92.84d ± 0.12 | 92.13e ± 0.13 | 91.67e ± 0.03 |
| c18:1C/c18:2c | 10.41e ± 0.01 | 7.25d ± 0.04 | 8.47c ± 0.02 | 8.39c ± 0.02 | 11..49b ± 0.1 | 13.45a ± 0.11 | 14.12a ± 0.04 |
| C18:2C/C16:0 | 2.28a.b±0.03 | 3.08a ± 0.05 | 2.53c.b±0.05 | 2.66a ± 0.01 | 1.71c ± 0.08 | 1.40d ± 0.04 | 1.34d ± 0.05 |
| MUFA/SFA | 10.83a ± 0.05 | 10.66a.b±0.01 | 10.13b ± 0.06 | 10.87a.b±0.06 | 9.70c ± 0.05 | 9.24c ± 0.07 | 8.91c ± 0.04 |
| PUFA/SFA | 1.07a ± 0.01 | 1.51b ± 0.03 | 1.24c ± 0.01 | 1.34a ± 0.04 | 0.89d.e±0.06 | 0.73e ± 0.01 | 0.65e ± 0.06 |
| UFA/SFA | 11.90a.b±0.04 | 12.17a ± 0.06 | 11.36b ± 0.03 | 12.21a ± 0.05 | 10.59b ± 0.09 | 9.97c ± 0.06 | 9.56c ± 0.05 |
a-d: different letters in the same row indicate significant differences (p ≤ 0.05) among treatment times. nd: not detected.
During the thermo-oxidization, unsaturated fatty acids (UFA) represented the 91.67%. Among these, the monounsaturated fatty acids (MUFA) are the most abundant (85.46%), with the C18:1c as the most abundant fatty acid (84.13 %). Polyunsaturated fatty acids (PUFA) are present at 8,35%, with C18:2c as the most abundant acid (8,08%). The PUFA value underwent a decrease during the 48 h of heat treatment, reaching values of up to 6.21%. Its diminution could imply an increase in SCFA. In particular, the C8:0 reached values of 0.34%
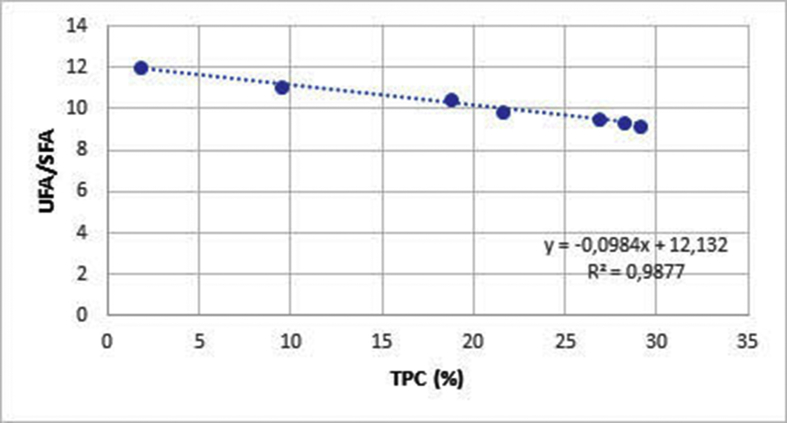
In Table 3, the fatty acid composition (%) of frying High Oleic Sunflower Oil for purple potato at different treatment times has been reported. As expected, there has been an increment of the percentage of trans fatty acids with increasing of the frying time. The amount of trans reached 0.81% for the FOPP. Unsaturated fatty acids reached the value of 90.19%, whereas SFA of 9.79%. The amount of octanoic acid (C8:0) was 0.20%. Octanoic acid (C8:0) is a significant product of fatty acid oxidation of methyl esters (Frankel 2005). It is synthesized from 9-hydroperoxide of oleic acid and linolenic acid. Short chain fatty acids can be considered good markers of the thermal behaviours due to their stability and can be used to evaluate the oxidation degree. During the frying process, volatile compounds (secondary oxidation products) are continuously moved away from the oil for the high temperature and for the influence of the steam, while short chain fatty acids remain bonded to the triglycerides as a "marker" of the oxidation process. An high correlation between TPC vs UFA/SFA and TPC vs C18:2/C16:0 has been observed in the frying oil as shown in Figures 1 and 2, respectively. Ramadan et al. (2006), by studying two oil blends, namely a mixture (1:1, w/w) of sunflower seed oil and palm olein (SO/PO) and a mixture (1:1, w/w) of cottonseed oil and palm olein (CO/PO), showed proportional correlation and positive relationship between the fatty acids levels and the physicochemical characteristics of the vegetable oils blends. A recent work of Sayyad (2017), studied the effect of deep-fat frying parameters on the oxidative stability, tocopherol isomeric content and alterations of the main fatty acid during the preparation of French fries. It has been shown that an amount of more than 9% of linoleic acid present in sunflower oil leads to an improvement of its stability during deep-fat frying process.
Table 3.
Fatty acid composition (%) of frying High Oleic Sunflower Oil for purple potato at different treatment times.
| Fatty acids | Time (h) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 | 16 | 24 | 32 | 40 | 48 | |
| C8:0 | nd | 0.05e ± 0.04 | 0.10 d ± 0.01 | 0.13c ± 0.03 | 0.19b ± 0.01 | 0.2a ±0.02 | 0.20a ± 0.01 |
| C16:0 | 3.55f ± 0.06 | 3.83e ± 0.18 | 4.05 d ± 0.02 | 4.17c ± 0.15 | 4.28b ± 0.02 | 4.35ab ± 0.07 | 4.39a ± 0.01 |
| C18:0 | 3.01f ± 0.02 | 3.20e ± 0.11 | 3.33 d ± 0.04 | 3.53d ± 0.07 | 3.61b ± 0.01 | 3.67b ± 0.13 | 3.75a ± 0.01 |
| C18:1t | nd | 0.12e ± 0.13 | 0.33 d ± 0.04 | 0.49c ± 0.16 | 0.63b ± 0.01 | 0.75a ±0.12 | 0.75a ± 0.01 |
| C18:1c | 84.11a ± 0.39 | 84.33b ± 0.37 | 84.53ab ± 0.03 | 84.54ab ± 0.12 | 84.53ab ± 0.01 | 84.52ab ± 0.01 | 84.35b ± 0.01 |
| C18:2t | nd | 0.04a ± 0.02 | 0.05a ± 0.02 | 0.04a ± 0.01 | 0.06a ± 0.01 | 0.07a ± 0.01 | 0.06 ± 0.01a |
| C18:2c | 8.08a ± 0.07 | 6.88b ± 0.89 | 6.04c ± 0.01 | 5.41d ± 0.60 | 4.98e ± 0.01 | 4.68f ± 0.45 | 4.73 ± 0.01f |
| C20:0 | 0.26d ± 0.02 | 0.27cd ± 0.02 | 0.28bcd ± 0.01 | 0.31abc ±0.02 | 0.33a ± 0.01 | 0.33ab ± 0.03 | 0.33a ± 0.01 |
| C18:3 | 0.27a ± 0.02 | 0.27a ± 0.01 | 0.27a ± 0.01 | 0.29a ± 0.01 | 0.29a ± 0.01 | 0.28a ± 0.02 | 0.29a ± 0.01 |
| C22:0 | 0.96cd ± 0.02 | 0.94d ± 0.02 | 0.99c ± 0.01 | 1.06b ± 0.03 | 1.06b ± 0.01 | 1.07b ± 0.05 | 1.11a ± 0.01 |
| ∑SFA | 7.77g ± 0.08 | 8.29f ± 0.33 | 8.74e ± 0.01 | 9.20d ± 0.31 | 9.46c ± 0.02 | 9.62b ± 0.31 | 9.79a ± 0.01 |
| ∑MUFA | 84.11a ± 0.39 | 84.45b ± 0.25 | 84.86ab ± 0.01 | 85.03ab ± 0.28 | 85.16a ± 0.01 | 85.28a ± 0.12 | 85.10a ±0.01 |
| ∑PUFA | 8.35a ± 0.05 | 7.20b ± 0.85 | 6.36c ± 0.04 | 5.73d ± 0.61 | 5.33e ± 0.01 | 5.03f ± 0.43 | 5.09f ± 0.01 |
| ∑TRANS | nd | 0.17e ± 0.15 | 0.38d ± 0.02 | 0.53c ± 0.15 | 0.69b ± 0.02 | 0.83a ± 0.12 | 0.81a ± 0.01 |
| ∑UFA | 92.46a ± 0.34 | 91.65b ± 1.10 | 91.22bc±0.02 | 90.76cd ± 0.33 | 90.49de ± 0.01 | 90.31e±0.31 | 90.19e ± 0.01 |
| C18:1C/C18:2C | 10.53g ± 0.14 | 12.25f ± 1.29 | 14.00e ± 0.01 | 15.64d ± 1.24 | 16.99c ± 0.01 | 18.07a ± 1.16 | 17.83b ± 0.01 |
| C18:2C/C16:0 | 2.28a ± 0.02 | 1.80b ± 0.34 | 1.49c ± 0.01 | 1.30d ± 0.22 | 1.16e ± 0.01 | 1.08f ± 0.13 | 1.08f ± 0.01 |
| MUFA/SFA | 10.96a ± 0.17 | 10.18b ± 0.46 | 9.71c ± 0.01 | 9.25d ± 0.32 | 9.00de ± 0.02 | 8.87e.f ±0.31 | 8.69f ±0.01 |
| PUFA/SFA | 1.07a ± 0.01 | 0.87b ± 0.15 | 0.73c ± 0.01 | 0.62d ± 0.10 | 0.56e ± 0.00 | 0.52f ± 0.07 | 0.52f ± 0.01 |
| UFA/SFA | 12.03a ± 0.01 | 11.05b ± 0.01 | 10.44c ± 0.01 | 9.87d ± 0.01 | 9.56e ± 0.02 | 9.39e.f ±0.01 | 9.21f ± 0.02 |
a-f: different letters in the same row indicate significant differences (p ≤ 0.05) among treatment times. nd: not detected.
Figure 1.
Correlation unsaturated/saturated fatty acids (UFA/SFA) vs total polar compounds (TPC) in frying oil at different times.
Figure 2.
Correlation linoleic/palmitic acid (C18:2c/C16:0) vs total polar compounds (TPC) in frying oil at different times.
3.3. Volatile aromatic compounds
Volatile compounds (VOCs) are formed both from thermal and oxidative decomposition of the food lipids, and from the interaction between fried food's components (in this case the potatoes matrix) and/or between food and frying medium. Gas chromatography results allowed to identify the most stable volatile compounds that are not distilled with the steam at the different thermal treatment times. The onset of volatile compounds is strictly affected by different factors, such as frying oils (i.e. oil typology and fatty acid composition), other food components (i.e. sugar, and amino acids), frying parameters (i.e. temperature, frying time, oxygen concentration, moisture content, and pH), and frying typology (i.e. air frying, and vacuum frying) (Hammouda et al., 2017; Chang et al., 2019).
In particular, as remarked in a recent review of Cheng et al. (2019), a great variety of volatile compounds (e.g. aldehydes, ketones, alcohols, carboxylic acids, hydrocarbons, furans, pyridines, and pyrazines) characterize deep-fried flavor, that come from a complex mechanisms of development, resulting in a strong tempting flavor stimulating the taste of the consumers. In Table 4 and Table 5 are reported respectively VOCs concentration (μg/L) of thermo-oxidized high oleic sunflower oil and frying High Oleic Sunflower Oil used to fry purple potato at different treatment times.
Table 4.
Volatile organic compounds (VOCs) concentration (mg/Kg) of thermo-oxidized high oleic sunflower oil at time 0, 8, 16, 32 and 48 h.
| Time (h) |
|||||
|---|---|---|---|---|---|
| 0 | 8 | 16 | 32 | 48 | |
| Octane | - | 192.68c ± 7.07 | 260.66a ± 6.75 | 241.87b ± 16.61 | 256.07a ± 2.01 |
| Nonane | - | 1.40a ± 0.32 | 1.12a ± 0.22 | 1.16a ± 0.13 | 1.38a ± 0.14 |
| Cyclohexane propyl | - | 1.57a ± 0.30 | 1.35a ± 0.39 | 1.32a ± 0.26 | 1.52a ± 0.02 |
| Cyclohexane butyl | - | 4.54a ± 0.92 | 4.04a ± 1.03 | 3.92a ± 0.46 | 4.38a ± 0.26 |
| Total alkanes | - | 200.19c ± 7.01 | 267.17a ± 6.07 | 248.27b ± 16.01 | 263.35a ± 2.07 |
| Octene | - | 4.29b ± 5.41 | 5.16b ± 0.72 | 5.16b ± 0.08 | 7.13a ± 0.19 |
| Cyclohexene 1-methyl-4 -(1-methylethyl)-(R) | - | 0.34a ± 0.16 | 4.77a ± 6.74 | 0.36a ± 0.05 | - |
| Cyclohexene 1-methyl-4-(1-methyl-4-(1-methylethenyl)-(S) | - | 1.84a ± 1.31 | 0.18a ± 0.25 | 0.24a ± 0.01 | - |
| Total alkenes | - | 6.47ab ± 5.01 | 10.11a ± 5.11 | 5.76ab ± 0.41 | 7.13ab ± 0.11 |
| heptanol | - | - | - | - | 0.68 ± 0.96 |
| 1-pentanol | - | 10.87b ± 1.73 | 8.28c ± 2.65 | - | 16.70a ± 0.36 |
| 1-hexanol | - | - | - | - | 6.48 ± 0.06 |
| Total alcohols | - | 10.87b ± 1.73 | 8.28c ± 2.63 | - | 23.86a±0.93 |
| Pentanal | - | 6.76a ±3.04 | 5.05a ±0.95 | - | 3.42ab ± 0.71 |
| Heptanal | - | 7.80a ± 2.03 | 5.87a ± 1.71 | 5.38a ± 0.07 | 6.21a ± 0.53 |
| Octanal | - | 4.98a ± 1.32 | 4.67a ± 0.39 | 4.08a ± 0.38 | 4.97a ± 0.59 |
| Nonanal | - | 20.09a ±1.61 | 18.47ab ± 1.50 | 11.77c ± 0.25 | 14.02bc ±1.88 |
| 2 hexenal | - | 1.92a ± 0.39 | 0.95a ± 1.20 | - | - |
| 2 Z heptenal | - | 8.13a ± 2.28 | 6.10a ± 2.53 | 6.78a ± 0.57 | 5.47b ± 0.33 |
| 2 E octenal | - | 0.32b ± 0.46 | - | 0.92a ± 0.01 | 1.08a ± 0.09 |
| 2 E decenal | - | - | 1.75a ± 0.59 | 1.74a ± 0.07 | 1.26a ± 1.78 |
| 2.4 E decadienal | - | - | 0.04 ± 0.05 | - | - |
| Total aldehydes | - | 50.00a ± 2.61 | 42.86b ± 1.50 | 30.67d ± 0.38 | 36.43c ± 1.86 |
| 1-Propone-2-Bromo-Phenyl (Ketones) | - | 0.50a ± 0.13 | 0.33b ± 0.22 | 0.47a ± 0.09 | 0.21c ± 0.30 |
| Hydro Peroxide. | - | 0.75ab ± 0.20 | 2.34a ± 0.77 | - | - |
| Hexyl furan 2-pentyl | - | 0.79b ± 0.28 | - | 0.94a ± 0.28 | 0.50c ± 0.70 |
| Total eterocyclic organic compounds | - | 1.54b ± 0.25 | 2.34a ± 0.77 | 0.94c ± 0.28 | 0.50d ± 0.70 |
| Hydroxylamine | - | 0.12a ± 0.17 | - | 0.12a ± 0.04 | - |
| O-(2-methylpropyl)-Pyrrolidine Methanamine | - | - | - | 0.25 ± 0.01 | - |
| N-methyl ethenyl-S Propanamide | - | - | 0.04 ± 0.05 | - | - |
| Total amines | - | 0.12b ± 0.17 | 0.04bc±0.05 | 0.37a ± 0.02 | - |
a-d: Different letters in the same row indicate significant differences (p ≤ 0.05) among treatment times.
The - sign indicates that the value is below the detection limit (LOD<0.01%).
Table 5.
Volatile organic compounds (VOCs) concentration (mg/Kg) of frying High Oleic Sunflower Oil used to fry purple potato at time 0, 8, 16, 32 and 48 h.
| Time (h) |
|||||||
|---|---|---|---|---|---|---|---|
| 0 | 8 | 16 | 24 | 32 | 40 | 48 | |
| Heptane (Alkanes) | 0.87 ± 0.01 | - | - | - | - | - | - |
| 8-heptadecene (Alkenes) | - | 0.82ab ± 0.03 | 0.94a ± 0.09 | 0.79abc ±0.17 | 0.66 abc±0.18 | 0.50bc±0.06 | 0.40cd ± 0.04 |
| 1-tetradecine (Alkynes) | - | 1.08a ± 0.04 | 1.05a ± 0.06 | 1.33a ± 0.21 | 1.30a ± 0.10 | 1.26a ± 0.01 | 1.06a ± 0.07 |
| Eptanol | - | 1.47a ± 0.06 | 1.32a ±0.05 | 1.43a ± 0.41 | 1.51a ± 0.20 | 1.28a ± 0.01 | 1.13a ± 0.14 |
| 1-octen-3-ol | - | 0.90a ± 0.06 | 0.65a ±0.11 | 0.78a ± 0.19 | 0.73a ± 0.10 | 0.62a ± 0.09 | 0.54a ± 0.07 |
| 1-octanol | - | 2.21a ± 0.06 | 2.26a ±0.53 | 2.25a ± 0.73 | 2.03a ± 0.24 | 1.96a ± 0.20 | 1.68a ± 0.05 |
| Total alcohols | - | 4.58a ± 0.06 | 4.23bc±0.33 | 4.46ab ± 0.50 | 4.27bc±0.20 | 3.86d ± 0.15 | 3.35d ± 0.05 |
| Pentanal | - | 1.11a ± 0.21 | 1.14a ± 0.17 | 1.26a ± 0.39 | 1.58a ± 0.55 | 1.49a ± 0.05 | 1.04ab ± 0.09 |
| Exanal | 2.71c ± 0.06 | 2.90c ± 0.41 | 3.24c ± 0.18 | 3.64b ± 0.75 | 4.72a ± 1.23 | 3.77b ± 0.36 | 2.90c ± 0.56 |
| 2-exanal | - | 0.40a ± 0.03 | 0.39a ± 0.059 | 0.37a ± 0.11 | 0.40a ± 0.12 | 0.34a ± 0.03 | 0.29a ± 0.015 |
| Eptanal | 0.14b ± 0.05 | 1.76a ± 0.084 | 1.67a ± 0.30 | 1.84a ± 0.65 | 2.16a ± 0.55 | 1.90a ± 0.05 | 1.42ab ± 0.09 |
| 2-eptenal | - | 6.02a ± 0.44 | 5.15a ± 0.50 | 5.04a ± 1.30 | 4.69a ± 0.73 | 4.25a ± 0.05 | 3.62a ± 0.48 |
| Octanal | - | 3.63a ± 0.10 | 3.94a ± 0.83 | 4.45a ± 1.56 | 4.76a ± 1.12 | 4.30a ± 0.11 | 3.65a ± 0.30 |
| 2-octenal | - | 1.90a ± 0.21 | 2.12a ± 0.45 | 1.86a ± 0.63 | 2.24a ± 0.42 | 2.24a ± 0.27 | 1.87a ± 0.12 |
| Nonanal | 0.81b ± 0.22 | 18.83a ± 1.50 | 19.75a ± 5.60 | 21.09a ±8.06 | 19.76a±3.64 | 19.81a ± 1.00 | 17.98a ± 1.23 |
| 2-nonenal | - | 4.14a ± 0.38 | 4.23a ± 0.77 | 4.43a ± 1.14 | 4.25a ± 0.31 | 4.23a ± 0.30 | 3.66a ± 0.09 |
| cis-4-decenal | - | 1.70a ± 0.14 | 1.67a ± 0.06 | 1.84a ± 0.38 | 1.76a ± 0.07 | 1.80a ± 0.30 | 1.37a ± 0.13 |
| Decanal | - | 1.00b ± 0.05 | 1.11b ± 0.15 | 9.04a ± 0.88 | 1.05b ± 0.06 | 1.04b ± 0.06 | 0.97b ± 0.01 |
| 2.4-nonadienal | - | 0.82ab ± 0.06 | 0.90a ± 0.04 | 0.92a ± 0.14 | 0.93a ± 0.02 | 0.82ab ± 0.09 | 0.61b ± 0.01 |
| 2-decenal | - | 1.40ab ± 0.02 | 1.24ab ± 0.04 | 1.23ab ± 0.01 | 1.20ab ± 0.05 | 1.15bc ±0.05 | 0.94c ± 0.02 |
| trans-2-decenal | - | 49.83a ± 1.34 | 49.35a ± 1.08 | 49.71a ± 2.74 | 48.03a ± 2.41 | 44.66a ± 4.35 | 34.91b ± 0.14 |
| 2.4-decadienal | - | 23.26a ± 0.62 | 19.84ab ± 1.64 | 19.96ab ± 1.36 | 18.19ab ± 2.17 | 15.96bc±2.41 | 11.75c ± 1.24 |
| trans-undecen-4-al | - | 2.09a ± 0.01 | 1.94a ± 0.06 | 1.99a ± 0.06 | 2.07a ± 0.29 | 2.07a ± 0.42 | 1.45a ± 0.80 |
| 2-undecenal | - | 42.31a ± 0.13 | 41.67a ± 0.41 | 41.46a ± 2.49 | 40.41a ± 5.49 | 36.47ab ± 3.93 | 27.12b ± 1.16 |
| Total aldehydes | 3.66f ± 0.10 | 163.01b ± 0.40 | 159.35c ± 0.35 | 170.13a ± 5.06 | 158.20c ± 4.40 | 146.30d ± 3.01 | 115.55e ± 1.01 |
| 2-heptanone | 0.17 ± 0.05 | - | - | - | - | - | - |
| 5-decanone | - | 1.73ab ± 0.06 | 1.92ab ± 0.19 | 1.96ab ± 0.27 | 2.05a ± 0.07 | 1.72ab ± 0.29 | 1.26b ± 0.13 |
| Total ketones | 0.17c ± 0.05 | 1.73ab ± 0.06 | 1.92ab ± 0.19 | 1.96ab ± 0.27 | 2.05a ± 0.07 | 1.72ab ± 0.29 | 1.26b ± 0.13 |
| α-pinene | 0.77 ± 0.01 | - | - | - | - | - | - |
| o-cimene | 0.14 ± 0.01 | - | - | - | - | - | - |
| D-limonene | 0.10 ± 0.01 | - | - | - | - | - | - |
| Total terpenes | 1.01 ± 0.01 | ||||||
a-f: Different letters in the same row indicate significant differences (p ≤ 0.05) among treatment times. The - sign indicates that the value is below the detection limit (<0.01%).
Table 4 shows the main chemical groups of volatile compounds in thermo-oxidized oil reported as: alkanes, alkenes, alcohols, aldehydes, ketones, eterocyclic organic compounds, ammines. As instance, for eterocyclic organic compounds, hydro peroxide hexyl and furan 2-pentyl were quantified, whereas the amines present are: hydroxylamine, O-(2-methylpropyl)-pyrrolidine methanamine, N-methyl ethenyl-S propanamide.
Monitoring the trend of the volatile compounds (from 0 to 48 h) in frying oil as shown in Table 5, the identified molecules can be divided into 7 chemical groups: alcohols, aldehydes, ketones, alkynes, alkenes, alkanes, and terpenes. The most abundant class is represented by the aldehydes, which are produced by the decomposition of the hydroperoxides of the oleic and linoleic acid, the main unsaturated fatty acid constituting the oil matrix.
Among the aldehydes, high concentrations of nonanal (with values that reached 21.09 mg/kg), trans-2-decenal (with values that reached 49.83 mg/kg), 2.4-decadienal (with values at the end of the process of 23.26 mg/kg), 2-undecenal (with values at the end of the heat treatment of 42.31 mg/kg) have been observed. Other aldehydes such as 2-E-heptenal, 2-E-octal, 2,4-E E-nonadienal, 2,4-EE-decadienal, are characteristic of the frying flavor. Pentanal, hexanal, heptane, 2-hexanal, heptane and 2-decenal provide unpleasant odors during frying. The 2,4-decadienal, in addition to the homolytic β-cleavage of the 9-hydroperoxide of linoleic acid, can also be formed from oleic acid, in particular from its decomposition product, the 2-decenal. The 2,4-alkadienals can derive by the hydroperoxidation or hydroxylation reactions on the methylene carbon in the allylic position of the 2-alkenals, generated by the decomposition of the hydroperoxide of the oleic acid, followed by the loss of hydrogen peroxide or water. Moreover, the amount of alkadienals produced throughout this mechanism is far lower than the one derived from the cleavage of the hydroperoxides. This may explain why foods fried in oils with a high oleic acid content have a less intense frying flavor. These oils during oxidative and thermal degradation, produce large quantities of 2-nonenal and 2-decenal, which give some green type fruity flavor and fatty and waxy type flavor, respectively. At high frying temperatures, other degradation products are formed in addition to hydroperoxides, such as ketoderivatives, epoxy derivatives, and non-volatile dimers, which in turn can undergo degradation developing volatile compounds characterized by the typical fried food smell.
Another relevant class of volatile compounds are the alcohols, whose production is linked to the cleavage products of hydroperoxide methyl oleate (Hammouda et al., 2017). In the frying oil the following compounds were identified: heptanol (with values of 1.51 mg/Kg), 1-oct-ol (with values of 0.90 mg/Kg) and the-octanol (with values 2.26 mg/Kg).
Terpenes compounds identified were: α-pinene (with values of 0.77 mg/Kg), o-cymene (with values of 0.14 mg/Kg) and D-limonene (with values 0.1 mg/kg). The amounts of these compounds, which are naturally present in the oil, have been identified only at time 0, probably because they are subsequently degraded by the high frying cooking temperatures (Hammouda et al., 2017). To better understand the trend of total volatile compounds in thermo-oxidized and frying oil, the analysis of the main components (PCA) was used as shown in Figure 3, indicating that aldehydes and alkadienals are the main compounds present in the frying time range explored.
Figure 3.
Principal component analysis (PCA) of volatile organic compounds in thermo-oxidized (T-OX) and frying (F) oils at time 0, 8, 16, 32 and 48 h.
4. Conclusions
During the frying process, volatile compounds which are secondary oxidation products continuously move away from the oil due the high temperature and to the influence of the steam. Short chain fatty acids remain bounded to the triglycerides and can be considered as a marker of the oxidation process. Concerning FFA, in the T-OX the increase of acidity value may be due to the thermal degradation of triglycerides with consequent release of free fatty acid, whereas in frying oil samples the increase only during the first stage of thermal treatment (from 0 to 8h) could be related to filling up operations and to the presence of antioxidants naturally part on the oil itself. The percentage of the total polar compounds increases in both thermo-oxidized and frying oil, whereas peroxides values do not follow a regular pattern, both during thermo-oxidation and during frying.
An high correlation between TPC vs UFA/SFA and TPC vs C18:2/C16:0 has been observed in the frying sunflower oil used rich in high oleic fatty acids. It can be concluded that oxidative stability increase during deep-fat frying, and that an oil or fat with a low unsaturation degree is the best choice. Monitoring the trend of the volatile compounds (from 0 to 48 h) in frying, allowed to identify seven chemical groups of molecules, namely: aldehydes, alcohols, ketones, alkynes, alkenes, alkanes, and terpenes. The most abundant class was represented by the aldehydes, produced by the decomposition of hydroperoxides of oleic and linoleic acid, which are the main unsaturated fatty acid of the oil matrix evaluated in the deep frying. The trend of total volatile compounds in thermo-oxidized and frying oil, evaluated using the analysis of the main components (PCA) revealed that the aldeydes (the main representative one being the nonanal) and alkanes are the most abundant compounds in the frying time range explored.
Declarations
Author contribution statement
Raffaele Romano, Gioacchino Filosa, Fabiana Pizzolongo, Alessandra Durazzo, Massimo Lucarini, Patricia Severino, Eliana B. Souto, Antonello Santini: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare the following conflict of interests: Antonello Santini; [is an Associate Editor for the Food Science and Nutrition section of Heliyon.]
Additional information
No additional information is available for this paper.
References
- Abdulkarim S.M., Ghazali H.M. Fatty acid ratios and their relative amounts as indicators of oil stability and extent of oil deterioration during frying. J. Food Agric. Environ. 2012;10(2):33–38. [Google Scholar]
- Aladedunye F., Przybylski R. Performance of palm olein and modified rapeseed, sunflower, and soybean oils in intermittent deep-frying. Eur. J. Lipid Sci. 2013;116:144–152. [Google Scholar]
- Bou R., Navas J.A., Tres A., Codony R., Guardiola F. Quality assessment of frying fats and fried snacks during continuous deep-fat frying at different large scale producers. Food Contr. 2012;27(1):254–267. [Google Scholar]
- Chang C., Wu G., Zhang H., Jin Q., Wang X. Deep-fried flavor: characteristics, formation mechanisms, and influencing factors. Crit. Rev. Food Sci. Nutr. 2019;11:1–19. doi: 10.1080/10408398.2019.1575792. [DOI] [PubMed] [Google Scholar]
- Cheng S., Tu M., Liu H., Zhao G., Du M. Food-derived antithrombotic peptides: preparation, identification, and interactions with thrombin. Crit. Rev. Food Sci. Nutr. 2019:1–15. doi: 10.1080/10408398.2018.1524363. [DOI] [PubMed] [Google Scholar]
- Choe E., Min D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007;72(5):R77–R86. doi: 10.1111/j.1750-3841.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- Dobarganes C., Màrquez-Ruiz G., Velasco J. Interaction between fat and food during deep-frying. Eur. J. Lipid Sci. Technol. 2000;102:521–528. [Google Scholar]
- Dominguez Brando J., Sarquis A.V. Proceedings of the 18th International Sunflower Conference, Mar del Plata, Argentina. 2012. Challenges for the sunflower oil market for 2020. [Google Scholar]
- FDA . 2018. Completes Review of Qualified Health Claim Petition for Oleic Acid and the Risk of Coronary Heart Disease. [Google Scholar]
- Firestone D. Regulation of frying fat and oil. Deep frying. Chem. Nutr. Prac. Appl. 2007:373–385. [Google Scholar]
- Frankel E.N. The Oily Press; Dundee (UK): 2005. Lipid Oxidation; pp. 161–186. [Google Scholar]
- Hammouda I.B., Freitas F., Ammar S., Gomes Da Silva M.D.R, Bouaziz M. Comparison and characterization of volatile compunda as markers of oils stablity during frying by HS-SPME-GC/MS and chemometric analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1068-1069:322–334. doi: 10.1016/j.jchromb.2017.10.063. [DOI] [PubMed] [Google Scholar]
- Hassanien M.F.R., Sharoba A.M. Rheological characteristics of vegetable oils as affected by deep frying of French fries. J. Food Meas. Char. 2014;8(3):171–179. [Google Scholar]
- Lemos M.A., Aliyu M.M., Hungerford G. Influence of cooking on the levels of bioactive compounds in Purple Majesty potato observed via chemical and spectroscopic means. Food Chem. 2015;173:462–467. doi: 10.1016/j.foodchem.2014.10.064. [DOI] [PubMed] [Google Scholar]
- Li X., Li J., Wang Y., Cao P., Liu Y. Effects of frying oils' fatty acids profile on the formation of polar lipids components and their retention in French fries over deep-frying process. Food Chem. 2017;237:98–105. doi: 10.1016/j.foodchem.2017.05.100. [DOI] [PubMed] [Google Scholar]
- Manzo N., Santini A., Pizzolongo F., Aiello A., Romano R. Effects of α-tocopherol and oleic acid content in sunflower oil subjected to discontinuous and prolonged frying process. Prog. Nutr. 2019;21(3):686–692. [Google Scholar]
- Mohdaly A.A.A., Smetanska I., Ramadan M.F., Sarhan M.A., Mahmoud A. Antioxidant potential of sesame (Sesamum indicum) cake extract in stabilization of sunflower and soybean oils. Ind. Crop. Prod. 2011;34(1):952–959. [Google Scholar]
- Raß M., Schein C., Matthäus B. Virgin sunflower oil. Eur. J. Lipid Sci. Technol. 2008;110(7):618–624. [Google Scholar]
- Ramadan M.F. Healthy blends of high linoleic sunflower oil with selected cold pressed oils: functionality, stability and antioxidative characteristics. Ind. Crop. Prod. 2013;43:65–72. [Google Scholar]
- Ramadan M.F., Amer M.M.A., Sulieman A.E.-R.M. Correlation between physicochemical analysis and radical-scavenging activity of vegetable oil blends as affected by frying of French fries. Eur. J. Lipid Sci. Technol. 2006;108(8):670–678. [Google Scholar]
- Romano R., Giordano A., Chianese L., Addeo F., Spagna Musso S. Triacylglycerols, fatty acids and conjugated linoleic acids in Italian Mozzarella di Bufala Campana cheese. J. Food Compos. Anal. 2011;24:244–249. [Google Scholar]
- Romano R., Giordano A., Le Grottaglie L., Manzo N., Paduano A., Sacchi R., Santini A. Volatile compounds in intermittent frying by gas chromatography and nuclear magnetic resonance. Eur. J. Lipid Sci. Technol. 2013;115:764–773. [Google Scholar]
- Romano R., Giordano A., Vitiello S., Le Grottaglie L., Spagna Musso S. Comparison of the frying performance of olive oil and palm superolein. J. Food Sci. 2012;77(5):C519–C531. doi: 10.1111/j.1750-3841.2012.02663.x. [DOI] [PubMed] [Google Scholar]
- Romano R., Le Grottaglie L., Manzo N., Giordano A., Vitiello S., Santini A. Comparison of frying performance of olive oil, bi-fractionated palm oil and sunflower oil. Prog. Nutr. 2012;14(3):199–218. [Google Scholar]
- Romano R., Manzo N., Le Grottaglie L., Giordano A., Romano A., Masi P. Comparison of the frying performance of high oleic oils subjected to discontinuous and prolonged thermal treatment. J. Am. Oil Chem. Soc. 2013;90:965–975. [Google Scholar]
- Rotondo A., La Torre G.L., Dugo G., Cicero N., Santini A., Salvo A. Oleic acid is not the only relevant mono-unsaturated fatty ester in olive oil. Foods. 2020;9(4):384. doi: 10.3390/foods9040384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales-Campos H., Reis de Souza P., Crema Peghini B., Santana da Silva J., Ribeiro Cardoso C. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013;13(2):201–210. [PubMed] [Google Scholar]
- Sayyad R. Effects of deep-fat frying process on the oil quality during French fries preparation. J. Food Sci. Technol. 2017;54(8):2224–2229. doi: 10.1007/s13197-017-2657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier R.F. Ensuring the health and safety of fried foods. Eur. J. Lipid Sci. Technol. 2013;115:956–964. [Google Scholar]
- Xu X., Li W., Lu Z., Beta T., Hydamaka A.W. Phenolic content, composition, antioxidant activity, and their changes during domestic cooking of potatoes. J. Agric. Food Chem. 2009;57(21):10231–10238. doi: 10.1021/jf902532q. [DOI] [PubMed] [Google Scholar]
- Yu K.S., Cho H., Hwang K.T. Physicochemical properties and oxidative stability of frying oils during repeated frying of potato chips. Food Sci. Biotechnol. 2017;27(3):651–659. doi: 10.1007/s10068-017-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Saleh A.S.M., Chen J., Shen Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: a review. Chem. Phys. Lipids. 2012;165(6):662–681. doi: 10.1016/j.chemphyslip.2012.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.