Abstract
Dental compounds and restorative materials undergo surface degradation and erosion from exposure to a variety of dietary substances. In this study we investigated changes in the surface properties of Rebaron, a hard denture reline material (HDRM), following timed immersion in carbonated soft drinks to determine its durability in a common acidic environment. Samples were prepared and immersed in a carbonated soft drink (or its components) for 6, 12, or 24 h. Surface structure and mechanical properties were characterized using Atomic Force Microscopy (AFM). Raman spectroscopy was used to identify changes in the HDRM surface chemistry following exposure to the test solutions. AFM revealed that prolonged exposure led to pit formation and a subsequent increase in surface roughness, from 302.02 ± 30.20 to 430.59 ± 15.07 nm Ra, following a 24 h exposure. Young's modulus values decreased from 9.3 ± 7.0 to 0.53 ± 0.26 GPa under the same conditions, demonstrating a softening and embrittlement of the HDRM sample. Raman results revealed that immersion in the carbonated soft drink or acidic solution changed the nature of the HDRM structure, converting the HDRM surface chemistry from primarily hydrophobic to hydrophilic. Our study indicates that sustainability and durability of Rebaron HDRM are significantly reduced by prolonged exposure to carbonated (acidic) soft drink, resulting in deformation and degradation of the material surface.
Keywords: Surface degradation, Surface roughness, Dental material, Carbonated soft drink, AFM, Raman spectroscopy
surface degradation; surface roughness; dental material; carbonated soft drink; AFM; Raman spectroscopy
1. Introduction
Restorative dental materials are an indispensable tool in dentistry, where they not only improve tooth aesthetics, but also help to withstand erosion in the oral cavity [1, 2]. Various materials have been used for such purposes, including metals, ceramic, plastics, and composite resins [3, 4, 5]. Early dental compounds consisted of silver amalgam, which had high durability and longevity, but also included mercury, making it a potential environmental and health hazard [6, 7]. Advances in materials and polymer technology have led to the discovery of a variety of resin-based acrylic polymers [8, 9]. For example, Poly (methyl methacrylate) (PMMA) exhibits superb mechanical properties, reduced polymer shrinking, and enhanced optical properties. It is also inexpensive and easily replaceable [10, 11]. Originally, MMA was polymerized to PMMA using heat. However, more recent versions can be auto-polymerized with a wide range of copolymers and stabilizing agents [12, 13]. Continued efforts are underway to develop innovative materials with improved wear resistance, fracture toughness, and biocompatibility [14, 15].
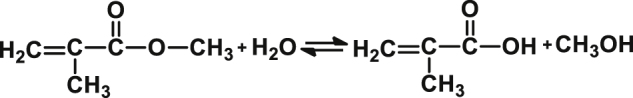
Despite improvements, dental filling materials and denture restorative materials remain susceptible to water sorption [16], insufficient polymerization, humidity, an individual's eating habits (e.g., vigorous chewing), and critical oral environment changes (e.g., long-term carbonated beverage consumption), which can result in abrasion and chemical degradation [17]. Among them, one of the most consequential for increased risk of dental caries and dental compound degradation is the long-term intake of carbonated soft drinks [18, 19]. These carbonated beverages usually contain added acidity regulators (phosphoric acid, malic acid, and citric acid, etc.), sweetener, and sugar [20]. Previously published studies have reported that the beverages' acidity causes gradual erosion and material degradation of dental composite surfaces, thereby reducing the lifespan [16, 21, 22]. A proposed mechanism for acidity-based degradation in dental composites is the hydrolysis of ester radicals present in resin monomers (eq1), i.e., PMMA, Bis-GMA, Bis-EMA, and UDMA [23, 24, 25, 26, 27].
(eq1).
A better understanding of the surface chemistry, structural, and mechanical properties of restorative dental compounds is essential for improved oral health and dental compound material optimization [28].
Although the occurrence of surface degradation is deterministic, it is not straight forward to measure and monitor material surfaces and interfaces. Characterization of mechanical properties using conventional methods would yield bulk property estimates but would not reveal insights into micro and nanoscale structural and mechanical properties or changes. Using AFM to measure surface structural and nanomechanical properties facilitates non-destructive comparison and evaluation under a range of experimental conditions [29, 30, 31, 32, 33]. Fourier-transform infrared spectrometry and Raman spectrometry provide tools to characterize the chemical effects of commercial carbonated beverages on the dental material's surface. In particular, Raman spectrometry is capable of monitoring changes in chemistry at room temperature, in the water phase/gas phase [34], and under non-destructive conditions [35]. It can reveal details regarding bond formation and dissociation when a chemical reaction takes place [36]. These spectra can be used as a fingerprint to characterize the composition and examine temporal changes to a material under controlled conditions [35, 37].
Hard denture reline materials are commonly used in full or partial denture remediation to repair and replace the critical conformal interface between the restorative and the patient's gum. This study investigates the degradation effects of carbonated soft drinks and their acidic ingredients on a commercially available HDRM (Rebaron). Structural and mechanical changes were characterized using AFM following sample immersion in a carbonated soft drink solution. Physico-chemical changes in the materials were monitored using Raman spectroscopy where immersion of the material in an acidic solution changed the molecules' nature, resulting in the weakening of bonds [27]. The degree of erosion on the dental restoration material surface and its temporal dependence on carbonated soft drink exposure time were analyzed in detail.
2. Materials and methods
2.1. Preparation of dental compound material
A resin-based dental compound Rebaron (GC CORPORATION, Tokyo, Japan) was prepared using commercial standard formulations by mixing a powder agent A (1.5 g) consisting of polymer beads, indicator, and pigments (in which the major component is PMMA), and liquid agent B (1.0 ml) consisting of a monomer (MMA) in a 1:3 ratio for 30 s at ambient temperature to form a 60% dental compound. The final sample forms were prepared by placing the material in a hand spindle tablet press and applying a fixed pressure to form 2 × 1 mm ingots with a uniform surface.
2.2. Characterization
An MFP-3D™ AFM (Asylum Research, Santa Barbara, CA, USA) was used to characterize the dental compound surface topography, surface roughness, and to acquire force measurements to determine the Young's modulus. AFM imaging experiments were performed in AC (tapping) mode under ambient conditions. A silicon cantilever (Olympus, AC240TS) with a nominal spring constant of 2 N/m was used for all images with a scan rate of 0.75 Hz and an image resolution of 512 × 512 pixels. Force-displacement curves were acquired using a silicon cantilever (Olympus AC240TS) with a measured spring constant of 2.8 N/m. The spring constant was determined using the thermal noise calibration method [38]. Multiple curves were acquired (997–1024) for each sample and analyzed using the data analysis tools built into the AFM software (Igor pro 6.37). A detailed description of the force curve analysis and Young's modulus calculation is described in our previous work [39, 40]. All Raman experiments were performed on a Raman microscope (WiTec alpha 300R) with a 532 nm incident laser at 15 mW power. A holographic grating (1800 grooves mm−1) and a 1024 × 127 pixel back-illuminated CCD detector with a total accumulation time of 30 s were used. Water contact angles were acquired using a Gardco PGX+ (Testing Machines, Inc., USA) goniometer. A pH meter (Cyberscan 510 Thermo Scientific Eutech Ltd., USA) was used to measure the soft drink acidity.
2.3. Immersion test
Hard denture reline material (HDRM) samples prepared as described above were immersed in commercially available carbonated soft drink with a pH of 2.65. Changes in the surface morphology were monitored at different time intervals using AFM and Raman spectroscopy, and the results were compared with a freshly prepared HDRM sample. Similar conditions were repeated while immersing in sucrose, citric acid, and phosphoric acid solutions for comparison.
2.4. Statistical analysis
At least three replicates were performed for each of the immersion tests described above with duplicate tests performed. SPSS version 12.0 (Endicott, NY, USA) was used for data analysis. Surface roughness and Young's modulus data means were subject to a one-way ANOVA test and where significant differences among means were found, these were separated by Tukey's multiple range test at a significance level of p < 0.05. OriginPro, version 8.1 (OriginLab, Northampton, MA) was used for preparing figures.
3. Result
3.1. Surface morphology and roughness
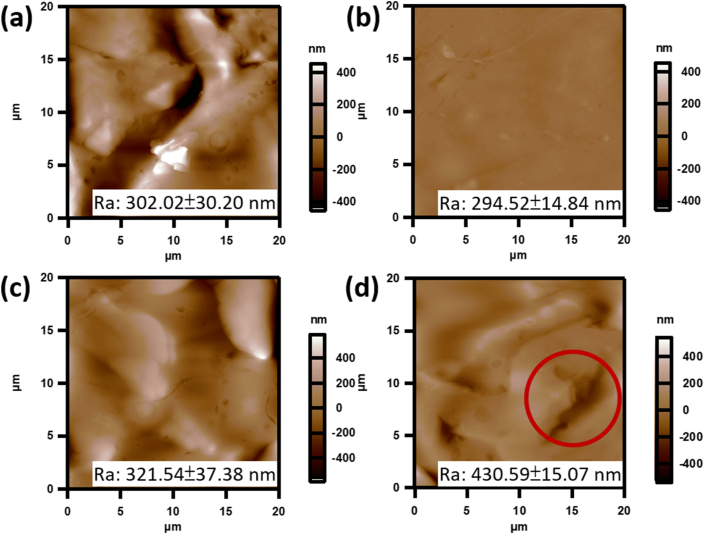
Surface topography and roughness measurements on a prepared HDRM sample were performed using AFM. The sample roughness (Ra) before treatment was 302.02 ± 30.2 nm, as shown in Figure 1a. In that image, large particles were present following the curing process and before treatment. Samples were then immersed in a carbonated beverage for various time intervals (6, 12, and after 24 h), and the surface topography and roughness were measured, as shown in Figure 1(b-d). Morphology changes were observed following a 6 h immersion in the carbonated beverage with surface roughness decreasing to (Ra) 294.52 ± 14.84 nm. As the immersion time increased to 12 h, slight erosion of HDRM was observed across the surface, increasing roughness to Ra 321.54 ± 37.38 nm. Following a 24 h exposure, degradation occurred with pits forming on the surface and a significant increase in roughness to Ra 430.59 ± 15.07 nm (Figure 1d).
Figure 1.
AFM topographic images of HDRM before and after immersion in a carbonated soft drink (a) 0 h, (b) 6 h, (c) 12 h, and (d) 24 h. Average roughness (Ra) values were calculated from each entire image (n = 3).
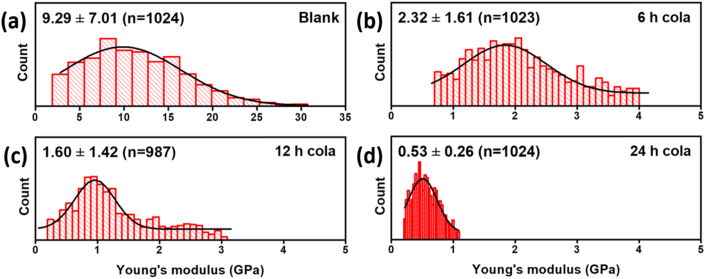
Changes in the mechanical properties of the HDRM were monitored by characterizing the Young's modulus (YM) of the sample. A pre-treatment YM value of the as-prepared HDRM was 9.3 ± 7.01 GPa, as shown in Figure 2a. Immersing the HDRM in carbonated soft drinks led to a decrease in YM value as a function of immersion time. We observed values of 2.3 ± 1.61, 1.60 ± 1.42, and 0.53 ± 0.26 GPa, for immersion times of 6, 12, and 24 h, respectively, as shown in Figure 2(b-d). Roughness and corresponding Young's modulus values of HDRM after immersion in carbonated soft drink for 0, 6, 12 and 24 h are mentioned in Table 1.
Figure 2.
Young's modulus of HDRM samples after immersion in a carbonated soft drink for (a) 0 h, (b) 6 h, (c) 12 h, and (d) 24 h.
Table 1.
Roughness and modulus data of HDRM after immersion in carbonated soft drink for 0, 6, 12, and 24 h.
| Time (h) | Roughness Ra (nm) | Young's modulus (GPa) |
|---|---|---|
| 0 | 302.02 ± 30.20aa | 9.29 ± 7.01a |
| 6 | 294.52 ± 14.84ab | 2.32 ± 1.61a |
| 12 | 321.54 ± 37.38abab | 1.60 ± 1.42a |
| 24 | 430.59 ± 15.07abab | 0.53 ± 0.26a |
All data are mean ± standard error of mean (n = 3); same letters indicate data that do not differ at significance level p < 0.05.
3.2. Raman analysis of HDRM
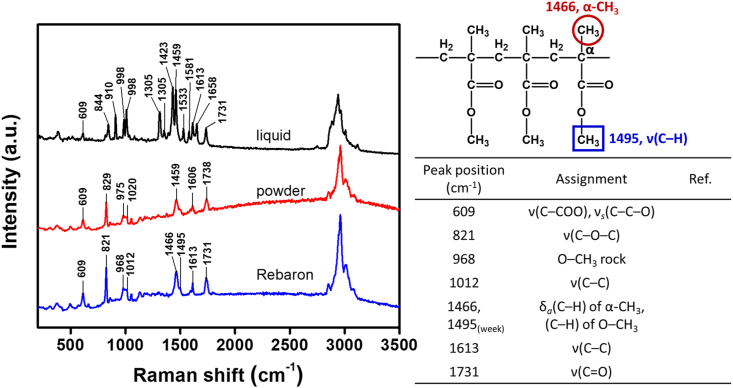
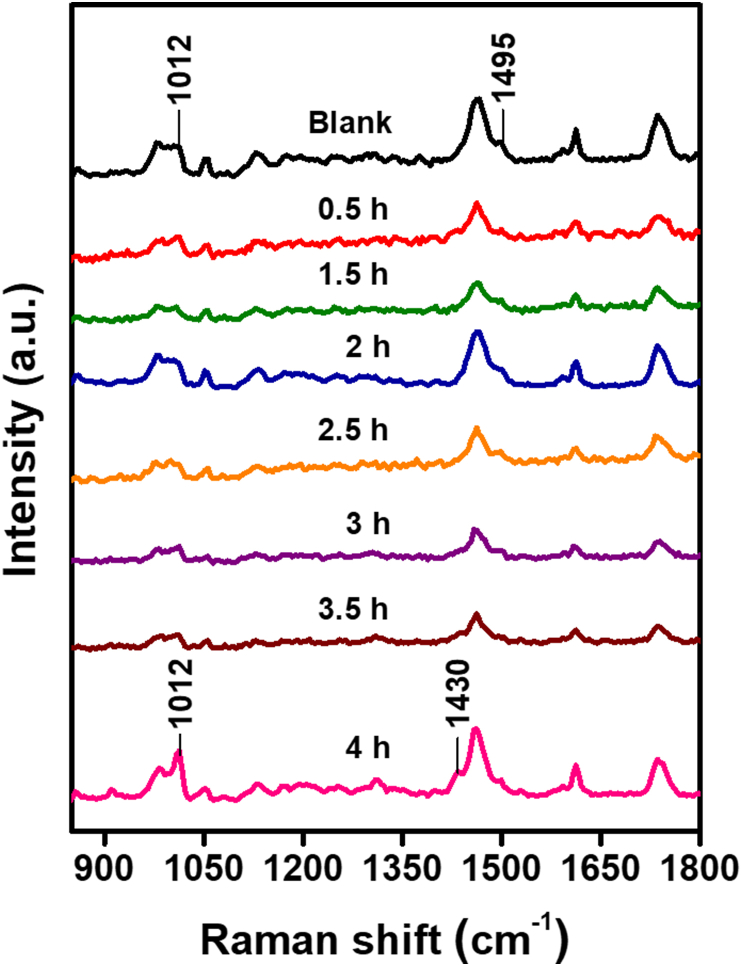
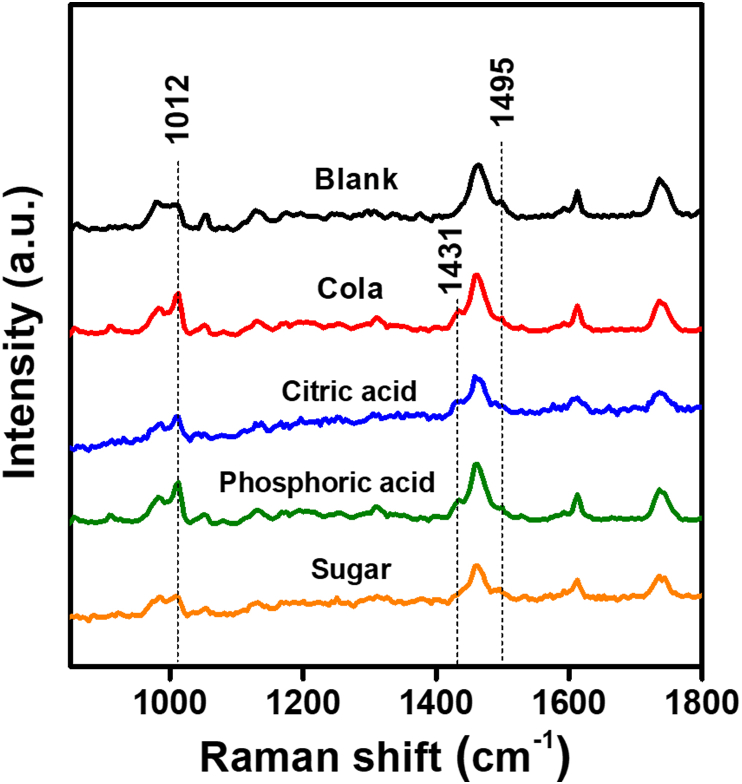
Raman spectroscopy was used to study the chemical and molecular changes to HDRM samples exposed to carbonated soft drink and its constituents. Peaks associated with HDRM precursors were characterized and assigned for each of the components in HDRM, as shown in Figure 3. Raman peaks and peak shifts were used to characterize changes in HDRM due to immersion in the carbonated soft drink. The Raman signal was monitored to identify when degradation initiated and to follow its course. The HDRM sample was immersed, and Raman signals were recorded at regular time intervals (0, 0.5, 1.5, 2, 2.5, 3, 3.5, and 4h), as shown in Figure 4. Changes were observed in the peak near 1012 cm−1 for ν(C–C) and 1459 cm−1 (ν(C–H) of O–CH3) with a new shoulder peak appearing at 1431 cm−1. No further changes to the peaks were observed at longer immersion times (6, 12, and 24h) as shown in Figure 5. To analyze the degradation mechanism of HDRM in more detail, the main ingredients of carbonated soft drinks such as (sugar, phosphoric acid, citric acid) were also examined with Raman spectroscopy by immersing an HDRM sample in a solution of each component over a period of 4 h. Raman signals were recorded, as shown in Figure 6. No changes were observed when samples were immersed in sugar solution. But similar changes appeared for the peak around 1012 cm−1 and 1459 cm−1 with a shoulder peak observed at 1431 cm−1 when immersed in citric acid and in phosphoric acid.
Figure 3.
Raman spectra for individual components: liquid, powder, HDRM.
Figure 4.
Raman spectra of HDRM after immersion in a carbonated soft drink for 0, 0.5, 1.5, 2, 2.5, 3, 3.5, and 4 h.
Figure 5.
Raman spectra of HDRM after immersion in carbonated soft drink solution for 6, 12, and 24 h.
Figure 6.
Raman spectra of HDRM treated with different solutions for 24 h.
3.3. Contact angle measurement
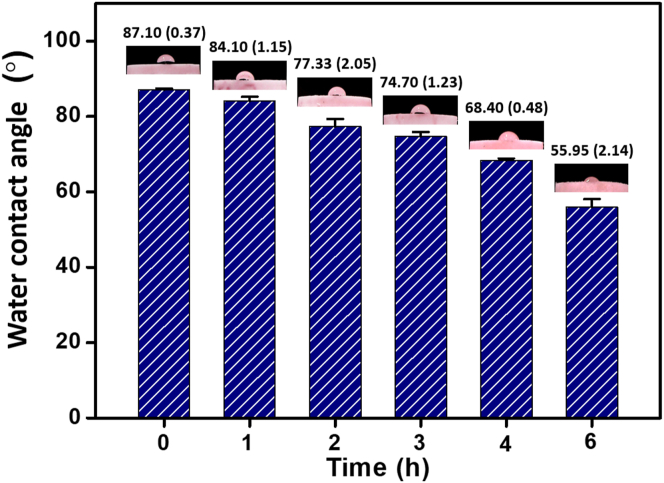
The PMMA in HDRM is a hydrophobic material as it needs to withstand harsh conditions in the oral cavity. The hydrophobicity of the material helps to retain the form of the restoration material by minimizing crack and cavity formation. Experiments were performed by monitoring the surface's contact angle every hour after immersion in soft drinks. Over a total of 6 h, the contact angle changed from 87.1 (0.37) ° to 55.95 (2.14) °, as shown in Figure 7.
Figure 7.
Water contact angle measurements of HDRM immersed in a carbonated soft drink at 1-hour intervals for a total of 6 h.
4. Discussion
In this study, we investigated changes in the surface properties of HDRM during due to immersion in carbonated beverages. Using AFM, we were able to image and visualize the topography of the samples. Differences in the roughness of HDRM was measured on freshly prepared samples and on treated samples. When immersed in the carbonated soft drink for over 6 h, the surface became more uniform as the roughness value decreased from 302.02 ± 30.2 nm to 294.52 ± 14.84 nm. This was primarily due to the removal of large bumps from the surface. With longer immersion times, surface degradation was more prominent. Following a 12 h immersion time, the roughness increased to 321.54 ± 37.38 nm, and after 24 h, the roughness increased significantly to 430.59 ± 15.07 nm. Over the 24 h period, cavities formed and were observed across the HDRM surface due to degradation [41, 42].
Young's modulus results showed that immersing the HDRM in a carbonated soft drink for 6 h resulted in significant changes to the surface microstructure. the YM value decreased from 9.3 ± 7.0 to 2.3 ± 1.6 GPa. Upon longer exposures, the YM value further decreased to 1.60 ± 1.42, and 0.53 ± 0.26 GPa, over 12 and 24 h, respectively, as structural deterioration occurred, making the material weak and brittle [43].
Raman spectroscopy was used to analyze and monitor the chemical nature of the HDRM samples during timed immersion experiments. The primary component in HDRM is PMMA in the form of a cross-linked polymer obtained by mixing powder (A), consisting of PMMA, and liquid (B), consisting of MMA. The primary characteristic peaks at 609 cm−1 corresponded to ν(C–COO)/νs (C–C–O), with peaks at 821 and 968 cm−1 belonging to ν(C–O–C) and the O–CH3 rocking mode, respectively. The peaks at 1012 and 1613 cm−1 are assigned to ν(C–C) from the CH2 groups in the polymer chains. The peaks at 1466 and 1495 cm−1 are assigned to δa (C–H) of α-CH3 and (C–H) of O–CH3, respectively. The peak at 1731 cm−1 belongs to the ν(C=O) mode. Our peak positions and values agree with previous literature reports [44, 45].
According to literature reports, the strength of molecular bonding is related to polymerization [46]. During the HDRM immersion experiments, no significant changes were observed for up to 3 h. Small changes started to appear at 3.5 h, where the peak near 1012 cm−1 for ν(C–C) sharpened as the polymeric bond weakened. A more significant change was observed after 4 h as the peak intensity around 1012 cm−1 was enhanced. A new shoulder peak appeared at 1431 cm−1 and the Raman intensity of the peak at 1459 cm−1 (ν(C–H) of O–CH3) was simultaneously weakened, which can be caused by CH3 deformation modes of the polymer main chain structures when a HRDM surface is eroded. These results indicate that the appearance of the peak at 1431 cm−1 signaled the beginning of polymer degradation in HDRM. With longer immersion times of 6, 12, and 24 h, the 1431 cm−1 peak intensity increased further while the peak intensity around 1012 cm−1 and 1431 cm−1 remained constant. No further significant peak shifts were observed for ester to acid conversion.
The signal intensity of Raman spectroscopy at 1012 cm−1 increased for HDRM samples when immersed in citric acid and phosphoric acid. Similarly, a new shoulder peak appeared at 1431 cm−1, and the peak intensity at 1459 cm−1 decreased. The results obtained from citric acid and phosphoric acid in Figure 6 were consistent with the commercial beverage results. No significant changes were observed in the sample immersed in sugar solution. Hence, we conclude that the acidic components in carbonated soft drinks are primarily responsible for the degradation of HDRM.
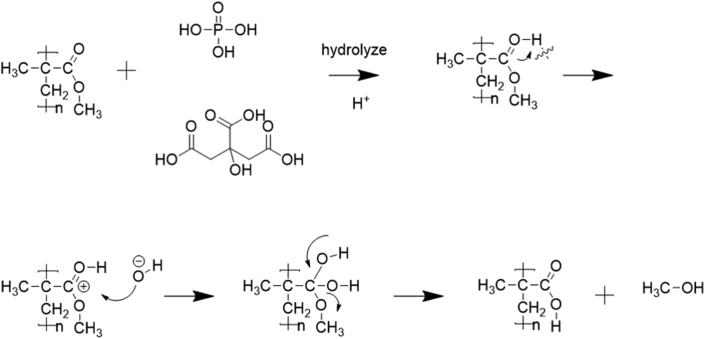
We propose that H+ in the citric and phosphoric acid acts as a driving force making the carbonyl carbon more electrophilic. This is followed by a nucleophilic attack on the electrophilic carboxylic group in PMMA, facilitating the ester-to-acid reaction to eliminate a small amount of methanol (as shown in Figure 8) [47]. This process induces persistent structural changes and causes HDRM to become soft with long-term exposure to acidic solutions. Because a soft drink's acidic environment chemically alters the surface, we used contact angle measurements to further characterize these chemical changes in HDRM.
Figure 8.
The reaction mechanism of HDRM material with phosphoric and citric acid.
The contact angle measurement of HDRM changed from 87.1 (0.37) ° to 55.95 (2.14) ° as time progressed, indicating that the nature of the material converted from hydrophobic to hydrophilic. This may be due to the ester group's conversion in PMMA to carboxylic acid in the presence of the acidic components in soft drinks, making the material more hydrophilic [48].
5. Conclusion
The degradation of HDRM dental compound immersed in carbonated soft drink and it's components was analyzed. With increased immersion time, changes in the surface hardness led to erosion and cavity (pit) formation in the HDRM. The roughness value increase from 302.02 ± 30.2 nm to 430.59 ± 15.07 nm over a 24 h immersion period. AFM-based Young's modulus values went from 9.3 ± 7.0 to 0.53 ± 0.26 GPa, indicating a significant decrease in surface elasticity. A peak shift was observed at 1459 cm−1 by Raman spectroscopy and a new shoulder peak at 1431 cm−1 suggesting that O–CH3 deformation had occurred. The proposed mechanism shows that in the presence of an acid, ester hydrolysis takes place in PMMA. This concept was further verified by monitoring changes in the contact angle, which confirmed a gradual shift from hydrophobic character to hydrophilic. Thus, prolonged exposure or frequent consumption of carbonated soft drinks results in structural deformation and surface degradation of HDRM, ultimately leading to restoration failure.
Declarations
Author contribution statement
Chung-Chih Tseng: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Pei-Ying Lin, Rajendranath Kirankumar: Analyzed and interpreted the data; Wrote the paper.
Zi-Wei Chuang, I-Hsuan Wu: Performed the experiments; Analyzed and interpreted the data.
Shuchen Hsieh: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Kaohsiung Armed Forces General Hospital (KAFGH-ZY-A-109021), and Ministry of Science and Technology, Taiwan (MOST 109-2113-M-110-001).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Jandt K.D., Sigusch B.W. Future perspectives of resin-based dental materials. Dent. Mater. 2009;25(8):1001–1006. doi: 10.1016/j.dental.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi R.L., Powers J.M., editors. Craig's Restorative Dental Materials. thirteenth ed. Mosby; Saint Louis: 2012. Chapter 9 - restorative materials—composites and polymers; pp. 161–198. [Google Scholar]
- 3.Yu P., Xiong Y., Zhao P., Xu Z., Yu H., Arola D., Gao S. On the wear behavior and damage mechanism of bonded interface: ceramic vs resin composite inlays. J. Mech. Behav. Biomed. Mater. 2020;101:103430. doi: 10.1016/j.jmbbm.2019.103430. [DOI] [PubMed] [Google Scholar]
- 4.Chadwick B., Dummer P., Dunstan F., Gilmour A., Jones R., Phillips C., Rees J., Richmond S., Stevens J., Treasure E. What type of filling? Best practice in dental restorations. Qual. Health Care : QHC. 1999;8:202–207. doi: 10.1136/qshc.8.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khurshid Z., Zafar M., Qasim S., Shahab S., Naseem M., AbuReqaiba A. Advances in nanotechnology for restorative dentistry. Materials (Basel) 2015;8(2):717–731. doi: 10.3390/ma8020717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin W.M. Comparative study of the sealing efficacy of various bonding systems to Class V dental amalgam restorations. Int. J. Adhesion Adhes. 2006;26(3):145–150. [Google Scholar]
- 7.Eley B.M. The future of dental amalgam: a review of the literature. Part 4: mercury exposure hazards and risk assessment. Br. Dent. J. 1997;182(10):373–381. doi: 10.1038/sj.bdj.4809393. [DOI] [PubMed] [Google Scholar]
- 8.Ravi R., Alla R.K., Mohammed S., Devarhubli A. Dental composites - a versatile restorative material: an overview. Indian J. Dent. Sci. 2013;5:111–115. [Google Scholar]
- 9.Pratap B., Gupta R.K., Bhardwaj B., Nag M. Resin based restorative dental materials: characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019;55(1):126–138. doi: 10.1016/j.jdsr.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szczesio-Wlodarczyk A., Sokolowski J., Kleczewska J., Bociong K. Ageing of dental composites based on methacrylate resins-A critical review of the causes and method of assessment. Polymers (Basel) 2020;12(4):882. doi: 10.3390/polym12040882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heo H.J., Han D.J., Sohn E.-H. Impact of trifluoromethyl groups on the control of surface and optical properties of poly(methyl methacrylate) J. Fluor. Chem. 2019;219:92–97. [Google Scholar]
- 12.Chan K.H., Mai Y., Kim H., Tong K.C., Ng D., Hsiao J.C. Review: resin composite filling. Materials. 2010;3(2):1228–1243. [Google Scholar]
- 13.Rakhshan V. Marginal integrity of provisional resin restoration materials: a review of the literature. Saudi J. Dent. Res. 2015;6(1):33–40. [Google Scholar]
- 14.Muraybid Al Azmi M., Hashem M.I., Assery M.K., Al Sayed M.S., Kumar A. An in-vitro evaluation of mechanical properties and surface roughness of bulk fill vs incremental fill resin composites. Int. J. Prev. Clin. Dent. Res. 2017;4(1):37–42. [Google Scholar]
- 15.Zafar M.S. Prosthodontic applications of polymethyl methacrylate (PMMA): an update. Polymers (Basel) 2020;12(10):2299. doi: 10.3390/polym12102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahim T.N.A.T., Mohamad D., Md Akil H., Ab Rahman I. Water sorption characteristics of restorative dental composites immersed in acidic drinks. Dent. Mater. 2012;28(6):e63–e70. doi: 10.1016/j.dental.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Nedeljkovic I., Teughels W., De Munck J., Van Meerbeek B., Van Landuyt K.L. Is secondary caries with composites a material-based problem? Dent. Mater. 2015;31(11):e247–e277. doi: 10.1016/j.dental.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Giacaman R.A., Pailahual V., Diaz-Garrido N. Cariogenicity induced by commercial carbonated beverages in an experimental biofilm-caries model. Eur. J. Dent. 2018;12(1):27–35. doi: 10.4103/ejd.ejd_188_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Attin T., Wegehaupt F.J. Impact of erosive conditions on tooth-colored restorative materials. Dent. Mater. 2014;30(1):43–49. doi: 10.1016/j.dental.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Pinto S.C.S., Bandeca M.C., Silva C.N., Cavassim R., Borges A.H., Sampaio J.E.C. Erosive potential of energy drinks on the dentine surface. BMC Res. Notes. 2013;6 doi: 10.1186/1756-0500-6-67. 67-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbosa C.S., Kato M.T., Buzalaf M.A. Effect of supplementation of soft drinks with green tea extract on their erosive potential against dentine. Aust. Dent. J. 2011;56(3):317–321. doi: 10.1111/j.1834-7819.2011.01338.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheng R., Yang H., Shao M.Y., Hu T., Zhou X.D. Dental erosion and severe tooth decay related to soft drinks: a case report and literature review. J. Zhejiang Univ. - Sci. B. 2009;10(5):395–399. doi: 10.1631/jzus.B0820245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayre W.N., Denyer S.P., Evans S.L. Ageing and moisture uptake in polymethyl methacrylate (PMMA) bone cements. J. Mech. Beh. Biomed. Mat. 2014;32:76–88. doi: 10.1016/j.jmbbm.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semen J., Lando J.B. The acid hydrolysis of isotactic and syndiotactic poly(methyl methacrylate) Macromolecules. 1969;2(6):570–575. [Google Scholar]
- 25.Chadwick R.G., McCabe J.F., Walls A.W.G., Storer R. The effect of storage media upon the surface microhardness and abrasion resistance of three composites. Dent. Mater. 1990;6(2):123–128. doi: 10.1016/s0109-5641(05)80042-9. [DOI] [PubMed] [Google Scholar]
- 26.Söderholm K.J., Zigan M., Ragan M., Fischlschweiger W., Bergman M. Hydrolytic degradation of dental composites. J. Dent. Res. 1984;63(10):1248–1254. doi: 10.1177/00220345840630101701. [DOI] [PubMed] [Google Scholar]
- 27.Paula Mathias T.R.A. An overview of the impact of lifestyle behaviors on the operative dentistry. JBR J. Interdiscip. Med. Dent. Sci. 2014;2(4):1000128. [Google Scholar]
- 28.Gupta R., Madan M., Dua P., Saini S., Mangla R., Kainthla T., Dupper A. Comparative evaluation of microhardness by common drinks on esthetic restorative materials and enamel: an in vitro study. Int. J. Clin. Pediatr. Dent. 2018;11(3):155–160. doi: 10.5005/jp-journals-10005-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peskersoy C., Culha O. Comparative evaluation of mechanical properties of dental nanomaterials. J. Nanomater. 2017;2017:6171578. [Google Scholar]
- 30.Azmi M., Hashem M., Assery M., Sayed M. An in-vitro evaluation of mechanical properties and surface roughness of bulk fill vs incremental fill resin composites. Int. J. Prev. Clin. Dent. Res. 2017;4:37–42. [Google Scholar]
- 31.Uskoković V., Bertassoni L.E. Nanotechnology in dental sciences: moving towards a finer way of doing dentistry. Materials. 2010;3(3):1674–1691. doi: 10.3390/ma3031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma S., Cross S.E., Hsueh C., Wali R.P., Stieg A.Z., Gimzewski J.K. Nanocharacterization in dentistry. Int. J. Mol. Sci. 2010;11(6):2523–2545. doi: 10.3390/ijms11062523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lippert F., Parker D.M., Jandt K.D. In vitro demineralization/remineralization cycles at human tooth enamel surfaces investigated by AFM and nanoindentation. J. Colloid Interface Sci. 2004;280(2):442–448. doi: 10.1016/j.jcis.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh S., Lin P.-Y., Chu L.-Y. Improved performance of solution-phase surface-enhanced Raman scattering at Ag/CuO nanocomposite surfaces. J. Phys. Chem. C. 2014;118(23):12500–12505. [Google Scholar]
- 35.Ramakrishnaiah R., Rehman G.u., Basavarajappa S., Al Khuraif A.A., Durgesh B.H., Khan A.S., Rehman I.u. Applications of Raman spectroscopy in dentistry: analysis of tooth structure. Appl. Spectrosc. Rev. 2015;50(4):332–350. [Google Scholar]
- 36.Okulus Z., Buchwald T., Szybowicz M., Voelkel A. Study of a new resin-based composites containing hydroxyapatite filler using Raman and infrared spectroscopy. Mater. Chem. Phys. 2014;145(3):304–312. [Google Scholar]
- 37.Khan A.S., Khalid H., Sarfraz Z., Khan M., Iqbal J., Muhammad N., Fareed M.A., Rehman I.U. Vibrational spectroscopy of selective dental restorative materials. Appl. Spectrosc. Rev. 2016;52(6):507–540. [Google Scholar]
- 38.Hutter J.L., Bechhoefer J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993;64(7):1868–1873. [Google Scholar]
- 39.Hsieh S., Lin P.-Y., Hsieh C.-W., Li I.T., Hsieh S.-L., Wu C.-C., Huang Y.-S., Wang H.-M., Tu L.-W., Cheng K.-H., Wang H.-Y.J., Wu D.-C. Probing the adhesion of hepatocellular carcinoma HepG2 and SK-Hep-1 cells. J. Chin. Chem. Soc. 2012;59(8):929–933. [Google Scholar]
- 40.Hsieh S., Li I.T., Hsieh C.-W., Kung M.-L., Hsieh S.-L., Wu D.-C., Kuo C.-H., Tai M.-H., Wang H.-M., Wu W.-J., Yeh B.-W. Advances in cellular nanoscale force detection and manipulation. Arab. J. Chem. 2019;12(8):3163–3171. [Google Scholar]
- 41.Valinoti A.C., Neves Bg Fau - da Silva E.M., da Silva Em Fau - Maia L.C., Maia L.C. Surface degradation of composite resins by acidic medicines and pH-cycling. J. Appl. Oral Sci. 2008;16(4):1678–7765. doi: 10.1590/S1678-77572008000400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hengtrakool C., Kukiattrakoon B., Kedjarune-Leggat U. Effect of naturally acidic agents on microhardness and surface micromorphology of restorative materials. Eur. J. Dermatol. 2011;5(1):89–100. [PMC free article] [PubMed] [Google Scholar]
- 43.Munchow E.A., Ferreira A.C., Machado R.M., Ramos T.S., Rodrigues-Junior S.A., Zanchi C.H. Effect of acidic solutions on the surface degradation of a micro-hybrid composite resin. Braz. Dent. J. 2014;25(4):321–326. doi: 10.1590/0103-6440201300058. [DOI] [PubMed] [Google Scholar]
- 44.Thakur V.K., Vennerberg D., Madbouly S.A., Kessler M.R. Bio-inspired green surface functionalization of PMMA for multifunctional capacitors. RSC Adv. 2014;4(13):6677–6684. [Google Scholar]
- 45.Chen J., Li J., Xu L., Hong W., Yang Y., Chen X. The glass-transition temperature of supported PMMA thin films with hydrogen bond/plasmonic interface. Polymers (Basel) 2019;11(4):601. doi: 10.3390/polym11040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan A.A., Zafar M.S., Ali A Ghubayri A., AlMufareh N.A., Binobaid A., Eskandrani R.M., Al-Kheraif A.A. Polymerisation of restorative dental composites: influence on physical, mechanical and chemical properties at various setting depths. Mater. Technol. 2020:1–7. [Google Scholar]
- 47.Ye L., Meng X.-Y., Ji X., Li Z.-M., Tang J.-H. Synthesis and characterization of expandable graphite–poly(methyl methacrylate) composite particles and their application to flame retardation of rigid polyurethane foams. Polym. Degrad. Stabil. 2009;94(6):971–979. [Google Scholar]
- 48.Ferracane J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006;22(3):211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.