Abstract
Background
Large scale physical distancing measures and movement restrictions imposed to contain COVID-19, often referred to as ‘lockdowns’, abruptly and ubiquitously restricted access to routine healthcare services. This study describes reported barriers and coping mechanisms to accessing healthcare among chronic care patients during the nationwide COVID-19 lockdown in Rwanda.
Methods
This cross-sectional study was conducted among chronic care patients enrolled in pediatric development, HIV/AIDS, non-communicable diseases, mental health, and oncology programs at 3 rural Rwandan districts. Active patients with an appointment scheduled between March–June 2020 and a phone number recorded in the electronic medical record system were eligible. Data were collected by telephone interviews between 23rd April and 11th May 2020, with proxy reporting by caregivers for children and critically ill-patients. Fisher’s exact tests were used to measure associations. Logistic regression analysis was also used to assess factors associated with reporting at least one barrier to accessing healthcare during the lockdown.
Results
Of 220 patient respondents, 44% reported at least one barrier to accessing healthcare. Barriers included lack of access to emergency care (n = 50; 22.7%), lack of access to medication (n = 44; 20.0%) and skipping clinical appointments (n = 37; 16.8%). Experiencing barriers was associated with the clinical program (p < 0.001), with oncology patients being highly affected (64.5%), and with increasing distance from home to the health facility (p = 0.031). In the adjusted logistic regression model, reporting at least one barrier to accessing healthcare was associated with the patient's clinical program and district of residence. Forty (18.2%) patients identified positive coping mechanisms to ensure continuation of care, such as walking long distances during suspension of public transport (n = 21; 9.6%), contacting clinicians via telephone for guidance or rescheduling appointments (n = 15; 6.8%), and delegating someone else for medication pick-up (n = 6; 2.7%). Of 124 patients who reported no barriers to accessing healthcare, 9% used positive coping mechanisms.
Conclusion
A large proportion of chronic care patients experienced barriers to accessing healthcare during the COVID-19 lockdown. However, many patients also independently identified positive coping mechanisms to ensure continuation of care - strategies that could be formally adopted by healthcare systems in Rwanda and similar settings to mitigate effects of future lockdowns on patients.
Keywords: COVID-19 lockdown, Chronic care, Chronic diseases, Barriers and coping mechanisms to accessing healthcare, Rwanda
Background
The coronavirus disease (COVID-19) pandemic has infected millions of people while also causing millions of deaths globally [1]. Historic health crises including the 2009 influenza A (H1N1) pandemic, the 2013–2016 West Africa Ebola outbreaks, and other natural disasters and humanitarian crises have overwhelmed health systems while disrupting the provision and access to routine healthcare [2–4]. These disruptions have been associated with poor patient outcomes, including increased morbidity, drug resistance, and mortality [4, 5]. Generally, efforts to contain the spread of COVID-19 have included national and regional lockdowns – defined as large scale physical distancing measures and movement restrictions. Current models suggest that disruption of routine health services due to COVID-19 pandemic and measures to control it could result in increased deaths from many non-COVID causes [6, 7]. These models estimate at least additional 253,500 child deaths and 12,200 maternal deaths [6], and 36, 20 and 10% increase in deaths due to malaria, TB and HIV, respectively [6, 7].
Health system disruption during the COVID-19 pandemic has been expected to particularly impact access to healthcare among chronic care patients in low- and middle-income countries (LMICs) [7, 8]. However, as has been seen in previous crises, these disruptions can be either exacerbated or mitigated through individual patient and health system responses. For instance, during political and economic crises and flood disasters in Zimbabwe and Namibia, patients receiving ART for HIV reported skipping drug doses, sharing and/or selling drugs and changing regimens [4, 9]. In contrast, during the 2008 political unrest in Kenya, patients and providers were able to create stockpiles of medication to mitigate treatment discontinuation [10]. During the COVID-19 pandemic, healthcare workers have adopted a variety of strategies, including telemedicine [11, 12] and home delivery of medication [13], to promote continuation of care without exposing patients to the risk of infection.
In Rwanda, the first case of COVID-19 was reported on March 14, 2020, and the Rwandan government rapidly enforced policies to stop local transmission, including the implementation of a national lockdown between March 22nd and May 3rd, 2020 [14]. During this lockdown, hospitals, clinics, health centers, pharmacies and other essential services remained open. However, patients’ access to health-care might have been highly affected by the geographical distribution of health facilities, tight restrictions on movements of people across subnational boundaries, suspension of public transport, and patients’ fear of COVID-19 infection [15]. During a rapid assessment conducted by the World Health Organization (WHO), national health organizations in all member states, including Rwanda, reported remarkable macro-level disruptions in non-communicable diseases services and resources due to COVID-19 [16]. However, this assessment did not capture chronic care patients’ individual experiences and did not assess experiences among rural patients, who may be most vulnerable to disruptions of care. During the March–April 2020 nationwide lockdown, Partners In Health/Inshuti Mu Buzima (PIH/IMB), a non-governmental organization that provides health system strengthening support to three rural districts in Rwanda, conducted a cross-sectional telephone survey among patients with chronic conditions who were receiving care from PIH/IMB-supported health facilities. In this paper, we describe patients’ barriers to accessing healthcare and coping mechanisms used by patients or their caregivers to ensure continuation of care during the lockdown in rural Rwanda.
Methods
Study setting
PIH/IMB is a non-profit organization that has been collaborating with the Rwanda Ministry of Health (MOH) to strengthen government owned health facilities since 2005 and currently supports facilities in Kayonza, Kirehe and Burera districts of Rwanda. These health facilities serve approximately one million people, including 24,635 patients with chronic conditions enrolled in five chronic care programs [17].
Study design and population
This cross-sectional study targeted patients enrolled in one of five chronic care programs: a) the HIV program; b) the non-communicable diseases (NCD) program, which provides care for patients with type 1 and type 2 diabetes, asthma, hypertension and heart failure; c) the mental health (MH) program, which treats patients with depression and other mental health disorders as well as epilepsy; d) the pediatric development clinics (PDC), which provide clinical and nutritional follow-up to vulnerable children under-5 with developmental delays associated with various conditions, including prematurity, low birth weight and hypoxic ischemic encephalopathy [18]; and e) the oncology program. The HIV, NCD, and MH programs are currently active in all three districts; the PDC program is active in Kirehe and Kayonza districts whilst the oncology program is located in Burera district. Across all programs, study inclusion criteria were: (1) having a clinical appointment scheduled between March–June 2020, (2) having a contact telephone number recorded in the electronic medical record (EMR) system, and (3) being recorded in EMR as a resident in one of the 3 districts (Kayonza, Kirehe and Burera). However, as the proportion of HIV patients with a phone number recorded in EMR was particularly low, so we contacted 39 HIV patients through their community health workers (CHWs).
Study sample and data collection
Data analyzed here were extracted from a survey conducted between April 23rd and May 11, 2020 among chronic care patients to inform provision of social support to these patients during the COVID-19 pandemic. To ensure sufficient sample sizes to investigate patient responses by clinical program and district, the survey used stratified random sampling. Twelve strata were defined by district and clinical program. The sample size estimate to allow to report 95% confidence intervals for program-specific estimates with a precision of +/− 15% was at least 48 patients from each clinical program (16 patients across 3 districts for HIV, NCD, and MH program, 24 patients across 2 districts for PDC, and 48 patients in one district for oncology). Initially, 75 patients from each clinical program were sampled for inclusion on first-call lists. If we were unable to attain our target stratum-specific sample size after contacting everyone on the first-call list, we sampled replacement patients from the same district-clinical program strata until we either reached our target sample size or all eligible individuals from that stratum had been contacted. Six data collectors with a strong research experience and familiarity with the chronic care programs collected the data. Computer-assisted telephone interviews were conducted, where the interviewer administered the survey questions by calling patients or their caregivers, while reading and directly recording responses in an electronic online form developed in REDCap electronic data capture tools hosted at PIH/IMB [19, 20]. Proxy reporting by caregivers was allowed for children and critically ill-patients. Data collectors were instructed to call every sampled patient at different times on 3 separate days before deciding that the patient could not be reached.
Variables and data analysis
This study used data generated by the survey exploring effects of the COVID-19 lockdown on access to healthcare, patients’ psychological needs and patients’ or caregiver’s knowledge about the COVID-19 infection and its prevention as well as patients’ demographic and socio-economic characteristics. However, our analysis only focused on describing reported barriers and used strategies to accessing healthcare during the lockdown. Access to care questions were not validated scales, but instead reflected clinician and program managers’ perception about what barriers could be. Patients were invited to free-list barriers to care and strategies used to ensure continuation of care during the lockdown, but data collectors checked off pre-identified categories and provided patients with examples, as needed. “Other” was an option, but responses were evaluated and reclassified into one of the existing categories or created new categories. Socioeconomic status was measured using the 2015 Rwandan Government’s Ubudehe categorization of households - a four-level categorization ranging from 1 (poorest) to 4 (richest) [21, 22]. Category 1 included people who belong to families that don’t own a house and always struggle to afford basic needs. Families that own or are able to rent a house, but have members rarely getting fulltime jobs fall under the Ubudehe category 2. Category 3 included households that have members with a full time job or farmers who can go beyond subsistence farming. Families that own large-scale businesses or with individuals working with international organizations, industries, or as public servants are in Ubudehe category 4. Patients were asked to list all the effects that the lockdown had on their access to healthcare. “Reporting at least one barrier to accessing healthcare” was defined as the patient reporting one or more of the following effects of the lockdown: (i) lack of access to emergency care, (ii) skipping appointments, (iii) lack of access to medication, and (iv) difficulty getting (walking long distances due to suspension of public transport) to the health facility. Among patients who were prescribed at least one medication, interviewees were asked about their experiences in the 4 weeks prior to the survey that might have affected their ability to take their medication. “Reduced ability to take medication as prescribed” was explained by any of these reported experiences: a) feeling sad or depressed, b) running out of medication, c) unwillingness to take medication in the sight of other family members, and d) forgetting. Patients were also asked to name strategies they used to access health care during the lockdown. We classified these strategies as adverse coping mechanisms if they would prevent the continuity of care. “Exclusive use of positive coping mechanisms” was defined as the patient only reporting coping mechanisms that helped to maintain continuity of care. We used frequencies and percentages to describe patients and survey respondents’ characteristics, reported barriers, and coping mechanisms to accessing healthcare. In bivariate analyses, Fisher’s exact tests were used to measure associations between patient’s characteristics and reporting barriers to accessing healthcare. In addition, we conducted a multivariable logistic regression analysis to assess factors associated with reporting at least one barrier to accessing healthcare during the COVID-19 lockdown using Wald tests to calculate p-values for each variable in the logistic regression model. The association between utilized coping mechanisms to ensure continuation of care and reporting barriers to accessing healthcare during the lockdown was also assessed using Fisher’s exact test. The data were analyzed using Stata v.15.1 (Stata Corp, College Station, TX, USA).
Results
A total of 457 patients were identified from the electronic medical records (EMR) and contacted by telephone, including 369 patients from the first call-lists for interviews and 88 replacements resampled when the target sample size for the clinical program-district stratum had not been met (Fig. 1). Of these 457 patients, 220 (48.1%) responded. Response rates by clinical program were 43% for HIV, 67% for NCD, 42% for MH, 49% for PDC, and 45% for oncology. Non-response was due to either the patient not answering phone calls on 3 different attempts on 3 separate days (56.5%), to having incorrect phone numbers recorded in EMR (26.6%), the phone owner no longer living with the patient (13.1%) or the patient dying before the survey (3.8%).
Fig. 1.
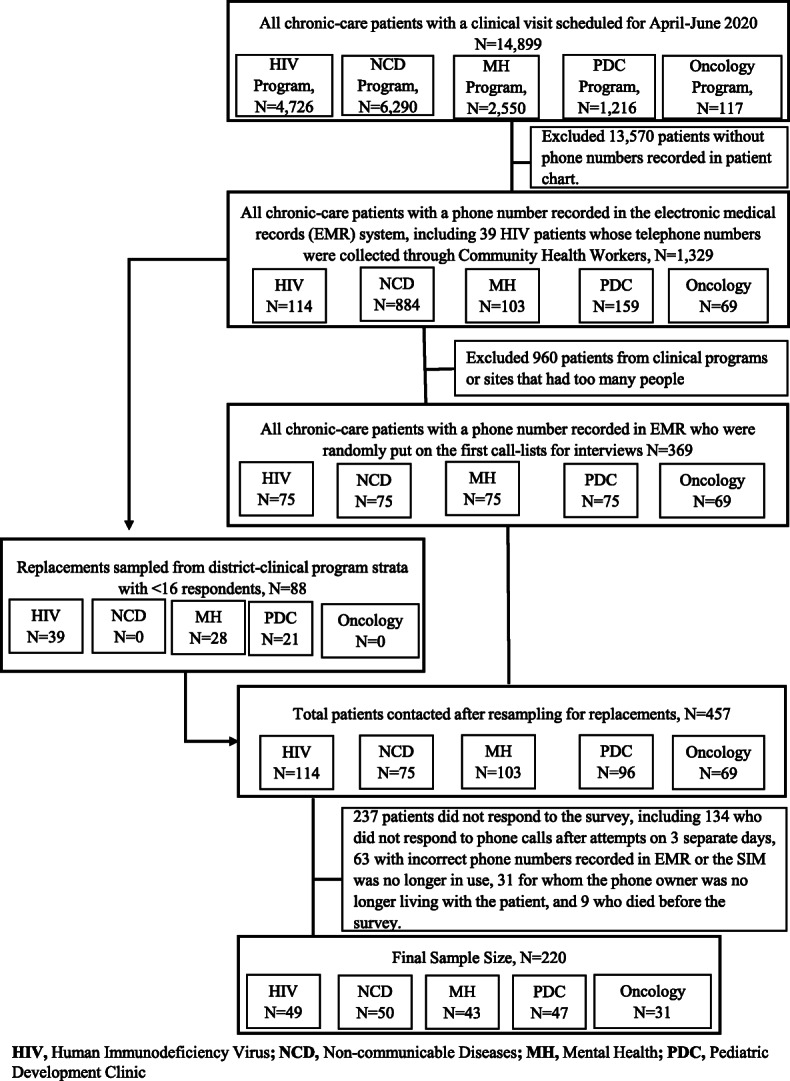
Flow chart of study participants
Of the 220 patients who responded to the survey, 50 (22.7%) were from the NCD program, 49 (22.3%) from HIV, 47 (21.4%) from PDC, 43 (19.6%) were from MH, and 31 (14.1%) from oncology (Table 1). Sixty-eight percent of patients responded to the survey on their own behalf. Of 70 patients who had a caregiver respond on their behalf, 47 (67.1%) were children in PDC, 20 were (28.6%) MH patients, 2 (2.9%) were oncology patients and 1 (1.4%) was an HIV patient. Of 218 patients with known socioeconomic status, 38 (17.4%) were in the poorest category 1, 82 (37.6%) in category 2 and 98 (45.0%) in category 3. The estimated time from home to the facility of usual care was < 1 h for 78 (35.4%) patients and > 2 h for 42 (19.1%) patients. The majority of patients (77.7%) were prescribed medication to take at home.
Table 1.
Respondents’ socio-demographic and patients’ clinical characteristics (N = 220)
| Characteristic | N | % |
|---|---|---|
| Survey respondent | ||
| Self | 150 | 68.2 |
| Caregiver | 70 | 31.8 |
| Patient/respondent’s level of education | ||
| None | 48 | 21.8 |
| Primary | 123 | 55.9 |
| Secondary or higher | 44 | 20.0 |
| Missing data | 5 | 2.3 |
| Patient’s district | ||
| Kayonza | 75 | 34.1 |
| Kirehe | 80 | 36.4 |
| Burera | 65 | 29.6 |
| Patient’s gender | ||
| Male | 83 | 37.7 |
| Female | 137 | 62.3 |
| Patient’s age (years) | ||
| < 5 | 28 | 12.7 |
| 5–17 | 9 | 4.1 |
| 18–35 | 34 | 15.5 |
| 36–59 | 79 | 35.9 |
| > =60 | 36 | 16.4 |
| Missing data | 34 | 15.5 |
| Patient’s marital status | ||
| Married | 81 | 36.8 |
| Cohabiting | 16 | 7.3 |
| Widowed | 28 | 12.7 |
| Divorced | 6 | 2.7 |
| Single - adult | 33 | 15.0 |
| Child | 56 | 25.5 |
| Socio-economic status (Ubudehe category) | ||
| 1 | 38 | 17.3 |
| 2 | 82 | 37.3 |
| 3 | 98 | 44.6 |
| Unknown | 2 | 0.9 |
| Patient’s clinical program | ||
| HIV/AIDS | 49 | 22.3 |
| Non-communicable Disease (NCD) | 50 | 22.7 |
| Mental Health (MH) | 43 | 19.6 |
| Pediatric Development Clinic (PDC) | 47 | 21.4 |
| Oncology | 31 | 14.1 |
| Patient’s diagnosesa | ||
| HIV/AIDS | 50 | 22.7 |
| Diabetes type 1 | 8 | 3.6 |
| Diabetes type 2 | 9 | 4.1 |
| Hypertension | 47 | 21.4 |
| Heart failure | 2 | 0.9 |
| Asthma | 14 | 6.4 |
| Cancer | 32 | 14.6 |
| Mental illnesses | 43 | 19.6 |
| PDC (Prematurity, low birth weight, hypoxic ischemic encephalopathy (HIE), etc.) | 47 | 21.4 |
| Was the patient prescribed medication to help manage health at home? | ||
| No | 49 | 22.3 |
| Yes | 171 | 77.7 |
| Estimated time (in hours) from the patient’s home to the health facility of usual health-care | ||
| < 1 h | 78 | 35.4 |
| 1–2 h | 100 | 45.5 |
| > 2 h | 42 | 19.1 |
| Was the patient living with someone who could help remind the patient to take medication or accompany the patient to the health facility? | ||
| No | 49 | 22.3 |
| Yes | 167 | 75.9 |
| Missing data | 4 | 1.8 |
aIt was possible for one patient to have multiple diagnoses
Forty-four percent of the patients reported at least one barrier to accessing healthcare during lockdown (Table 2). Reported barriers included lack of access to emergency care (n = 50, 22.7%), lack of access to medication (n = 44, 20.0%), skipping clinical appointments (n = 36, 16.4%) and lack of transport (n = 29, 13.2%). In the bivariate analyses, reporting barriers to accessing healthcare was associated with the clinical program (p = 0.001), with HIV/AIDS patients being least likely to report barriers to accessing healthcare (20.4%) and oncology patients being most likely to report barriers to accessing healthcare (64.5%). Reporting barriers to accessing healthcare was also associated with increased distance from home to the health facility of usual care (p = 0.031). In the adjusted logistic regression model, reporting at least one barrier to accessing healthcare was associated with the patient’s clinical program and district of residence. Higher odds of reporting at least one barrier to accessing healthcare were observed among patients in the oncology program [odds ratio (OR): 6.5; 95% confidence interval (CI): 1.9–21.8], NCD (OR: 3.7; 95% CI: 1.4–9.6), MH (OR: 3.6; 95% CI: 1.3–9.9) and PDC (OR: 3.4; 95% CI: 1.2–9.1) compared to HIV program patients. In addition, patients in Kirehe district had 2.1 times odds (95% CI: 1.0–4.4) of reporting at least one barrier to accessing healthcare compared to patients in Kayonza.
Table 2.
Self-reported barriers to accessing healthcare for chronic-care patients during the COVID-19 lockdown in Rwanda (N = 220)
| Variables | Bivariate analysis of the factors associated with reporting barriers to accessing healthcare during the COVID-19 lockdown | Multivariable logistic regression analysis of the factors associated with reporting at least one barrier to accessing healthcare in lockdown | |||||
|---|---|---|---|---|---|---|---|
| Lack of access to emergency care, n (%) |
Lack of access to medication, n (%) |
Skipping clinical appointments, n (%) |
Difficulty getting to the health facility (walking long distances, lack of transport), n (%) |
At least one barrier to accessing health care reported, n (%) |
Odds ratio [95% confidence interval] |
p-value | |
| Overall | 50 (22.7) | 44 (20.0) | 37 (16.8) | 28 (12.7) | 96 (43.6) | – | – |
| Clinical program | (p = 0.239) | (p = 0.008) | (p < 0.001) | (p < 0.001) | (p = 0.001) | (p = 0.023) | |
| HIV/AIDS | 6 (12.2) | 6 (12.2) | 2 (4.1) | 0 (0.0) | 10 (20.4) | ref | |
| Non-communicable diseases | 12 (24.0) | 13 (26.0) | 3 (6.0) | 10 (20.0) | 24 (48.0) | 3.7 [1.4–9.6] | |
| Mental health | 9 (20.9) | 13 (30.2) | 9 (20.9) | 0 (0.0) | 19 (44.2) | 3.6 [1.3–9.9] | |
| Pediatric Development Clinic | 14 (29.8) | 3 (6.4) | 15 (31.9) | 6 (12.8) | 23 (48.9) | 3.4 [1.2–9.1] | |
| Oncology | 9 (29.0) | 9 (29.0) | 8 (25.8) | 11 (35.5) | 20 (64.5) | 6.5 [1.9–21.8] | |
| Estimated time (hours) from home to the health facility of usual health care | (p = 0.077) | (p = 0.465) | (p = 0.223) | (p = 0.006) | (p = 0.031) | (p = 0.243) | |
| < 1 h | 12 (15.4) | 13 (16.7) | 9 (11.5) | 6 (7.7) | 31 (39.7) | ref | |
| 1–2 h | 24 (24.0) | 20 (20.0) | 18 (18.0) | 10 (10.0) | 39 (39.0) | 0.9 [0.4–1.7] | |
| > 2 h | 14 (33.3) | 11 (26.2) | 10 (23.8) | 12 (28.6) | 26 (61.9) | 1.9 [0.7–4.7] | |
| District of residence | (p < 0.001) | (p = 0.001) | (p = 0.104) | (p < 0.001) | (p = 0.073) | (p = 0.040) | |
| Kayonza | 12 (16.0) | 7 (9.3) | 11 (14.7) | 16 (21.3) | 29 (38.7) | ref | |
| Kirehe | 33 (41.3) | 26 (32.5) | 19 (23.8) | 1 (1.3) | 43 (53.8) | 2.1 [1.0–4.4] | |
| Burera | 5 (7.7) | 11 (16.9) | 7 (10.8) | 11 (16.9) | 24 (36.9) | 0.8 [0.4–1.9] | |
| Socio-economic status (Ubudehe category)a | (p = 0.535) | (p = 0.690) | (p = 0.506) | (p = 0.058) | (p = 0.121) | (p = 0.068) | |
| 1 | 6 (15.8) | 7 (18.4) | 8 (21.1) | 3 (7.9) | 13 (34.2) | ref | |
| 2 | 21 (25.6) | 14 (17.1) | 11 (13.4) | 8 (9.8) | 32 (39.0) | 1.3 [0.5–3.0] | |
| 3 | 22 (22.5) | 22 (22.5) | 18 (18.4) | 17 (17.4) | 50 (51.0) | 2.4 [1.0–5.9] | |
| Living with someone who could help remind the patient to take medication or accompany the patient to the health facilityb | (p = 0.437) | (p = 0.416) | (p > 0.999) | (p = 0.469) | (p = 0.624) | (p = 0.843) | |
| No | 13 (26.5) | 12 (24.5) | 8 (16.3) | 8 (16.3) | 23 (46.9) | ref | |
| Yes | 35 (21.0) | 31 (18.6) | 27 (16.2) | 20 (12.0) | 71 (42.5) | 1.1 [0.5–2.3] | |
aTwo patients didn’t have data on socio-economic status
bFour patients had missing data on whether they were living with someone who could remind the patient to take medication/accompany the patient to the health facility or not
Of 171 patients who were prescribed medication to take at home during the lockdown, 89 (52.1%) reported reduced ability of taking that medication as prescribed (Table 3). The most common reasons for not adhering to their medication prescription included self-reported feeling sad or depressed (n = 61, 35.7%), running out of medication (n = 42, 24.6%) and being unwilling to take medication in the sight of other family or household members (n = 27, 15.8%). Reporting reduced ability to take medication as prescribed during the lockdown was significantly associated with the patient’s district of residence (p < 0.001), with patients living in Kirehe district being highly affected (n = 45, 77.6%). Living with someone who could help remind the patient to take medication or accompany the patient to the health facility did not make a significant difference with regard to the patient’s ability to take medication as prescribed.
Table 3.
Self-reported factors negatively affecting the patient’s ability of taking medication at home as prescribed during COVID-19 lockdown (N = 171)
| Variables | Reduced ability to take medication at home as prescribed, n (%) |
Feeling sad or depressed, n (%) |
Ran out of medication, n (%) |
Unwilling to take medication in the sight of other family/household members, n (%) |
Forgetting, n (%) |
|---|---|---|---|---|---|
| Overall | 89 (52.1) | 61 (35.7) | 42 (24.6) | 27 (15.8) | 22 (12.9) |
| Clinical program | (p = 0.920) | (p = 0.487) | (p = 0.022) | (p = 0.080) | (p = 0.232) |
| HIV/AIDS | 25 (51.0) | 18 (36.7) | 6 (12.2) | 14 (28.6) | 9 (18.4) |
| Non-communicable diseases | 26 (54.2) | 20 (41.7) | 11 (22.9) | 4 (8.3) | 3 (6.3) |
| Mental health | 22 (51.2) | 15 (34.9) | 13 (30.2) | 5 (11.6) | 7 (16.3) |
| Pediatric Development Clinic | 3 (75.0) | 2 (50.0) | 3 (75.0) | 0 (0.0) | 1 (25.0) |
| Oncology | 13 (48.2) | 6 (22.2) | 9 (33.3) | 4 (14.8) | 2 (7.4) |
| Estimated time (hours) from home to the health facility of usual health care | (p = 0.317) | (p = 0.482) | (p = 0.557) | (p = 0.891) | (p = 0.549) |
| < 1 h | 24 (43.6) | 16 (29.1) | 13 (23.6) | 8 (14.6) | 6 (10.9) |
| 1–2 h | 45 (55.6) | 31 (38.3) | 18 (22.2) | 14 (17.3) | 13 (16.1) |
| > 2 h | 20 (57.1) | 14 (40.0) | 11 (31.4) | 5 (14.3) | 3 (8.6) |
| District of residence | (p < 0.001) | (p < 0.001) | (p < 0.001) | (p < 0.001) | (p = 0.002) |
| Kayonza | 15 (27.8) | 6 (11.1) | 7 (13.0) | 1 (1.9) | 3 (5.6) |
| Kirehe | 45 (77.6) | 35 (60.3) | 26 (44.8) | 18 (31.0) | 15 (25.9) |
| Burera | 29 (49.2) | 20 (33.9) | 9 (15.3) | 8 (13.6) | 4 (6.8) |
| Socio-economic status (Ubudehe category)a | (p = 0.863) | (p = 0.892) | (p = 0.613) | (p = 0.485) | (p = 0.324) |
| 1 | 17 (53.1) | 12 (37.5) | 7 (21.9) | 3 (9.4) | 3 (9.4) |
| 2 | 34 (54.0) | 23 (36.5) | 13 (20.6) | 12 (19.1) | 11 (17.5) |
| 3 | 37 (49.3) | 25 (33.3) | 21 (28.0) | 11 (14.7) | 7 (9.3) |
| Living with someone who could help remind the patient to take medication or accompany the patient to the health facilityb | (p = 0.598) | (p > 0.999) | (p = 0.288) | (p = 0.336) | (p = 0.440) |
| No | 24 (55.8) | 15 (34.9) | 12 (27.9) | 9 (20.9) | 7 (16.3) |
| Yes | 64 (50.4) | 46 (36.2) | 29 (22.8) | 18 (14.2) | 15 (11.8) |
aTwo patients didn’t have data on socio-economic status
bFour patients had missing data on whether they were living with someone who could remind the patient to take medication/accompany the patient to the health facility or not
A total of 77 (35%) patients reported using either adverse or positive coping mechanisms in response to the effects of the lockdown (Table 4). Patients who reported at least one barrier to health care were more likely to adopt negative coping mechanisms, such as skipping or delaying appointments than those who did not (24.0% vs. 11.3%). Eighteen percent of patients used exclusively positive coping mechanisms to ensure continuation of care during the lockdown. These positive coping mechanisms included walking long distances due to suspension of public transport (n = 21, 9.6%), contacting the usual clinician via telephone for guidance or rescheduling the appointment (n = 15, 6.8%), delegating relatives or neighbor clinicians to pick up medication for the patient (n = 6, 2.7%), going to a community health worker (CHW) (n = 2, 0.9%), and buying medication from a nearest pharmacy (n = 1, 0.5%). Of 124 patients who reported no barriers to accessing healthcare, 11 (8.9%) reported using any of the positive coping mechanisms. Patients reporting barriers to accessing healthcare were still more likely to use positive coping mechanisms than those who did not. There was no association between the patient’s socio-economic status and using positive/negative coping strategies (results not presented in tables).
Table 4.
Self-reported coping mechanisms and association with reported barriers to accessing health-care during the COVID-19 lockdown
| Reported coping mechanisms | Overall, N = 220 |
Among patients reporting no barrier to accessing health care, N = 124 |
Among patients reporting at least 1 barrier to accessing health care, N = 96 |
p-value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Adverse coping mechanisms: | ||||
| Stopped treatment, skipped, or delayed treatment | 37 (16.8) | 14 (11.3) | 23 (24.0) | 0.018 |
| Positive coping mechanisms | ||||
| Went on foot or used other alternative forms of transport to go to the health facility | 21 (9.6) | 7 (5.7) | 14 (14.6) | 0.036 |
| Contacted usual clinician via phone for guidance/to reschedule appointment | 15 (6.8) | 2 (1.6) | 13 (13.5) | 0.001 |
| Delegated son/daughter/neighbour clinician to pick up medication for me | 6 (2.7) | 0 (0.0) | 6 (6.3) | 0.006 |
| Went to a community health worker (CHW) | 2 (0.9) | 2 (1.6) | 0 (0.0) | 0.506 |
| Bought medication from a nearest pharmacy | 1 (0.5) | 0 (0.0) | 1 (1.0) | 0.436 |
| Used exclusively positive coping mechanisms | 40 (18.2) | 11 (8.9) | 29 (30.2) | < 0.001 |
Discussion
In this study, we described barriers and coping mechanisms to accessing healthcare among rural patients with chronic diseases who required chronic care during a nationwide COVID-19 lockdown in Rwanda. A large proportion (44%) of patients reported barriers to accessing healthcare, while about 18% of patients were able to identify positive coping mechanisms that helped to ensure continuation of care during the lockdown. Despite the adoption of positive coping mechanisms, patients who reported barriers to healthcare access were still more likely to skip appointments or delay treatment than those who did not. Avoiding barriers to healthcare access during lockdowns remains critical for chronic care patients since non-adherence to medical treatment is particularly associated with worse treatment outcomes for these patients [23].
Although health facilities in Rwanda remained open for both emergency and routine services throughout the duration of the lockdown, 22.7% of patients still reported being unable to access emergency care and 16.8% reported being unable to attend regular clinical appointments. These results indicate a reduced access to emergency care and attendance of scheduled medical appointments due to the COVID-19 lockdown that is consistent with evidence from other settings [24–29]. Perceived reasons for a dramatic decline in the utilization of emergency care services and low attendance of medical visits during the COVID-19 pandemic varied by setting, and included absence of public transport or increased costs of transport [24], shortage of care providers due to reallocation of staff to the COVID-19 response [25], and fear of COVID-19 infection [26–28].
This study also revealed a significant association between experiencing barriers to accessing healthcare and the patient’s clinical program. The lowest proportion of patients reporting barriers to accessing healthcare was observed among patients in the HIV program, while patients in all other chronic care programs had higher odds of reporting barriers to accessing healthcare during the COVID-19 lockdown compared to HIV program patients. None of the HIV patients reported lack of transport as an issue and HIV patients were also the least likely to report running out of medication as a barrier to taking medications as prescribed. Unlike PDC, Oncology, MH and NCD clinics, HIV treatment program has been decentralized to the health center level in all PIH/IMB-supported districts, which is typically within walking distance for patients [30]. Additionally, in 2018 PIH/IMB-supported districts implemented the HIV differentiated service delivery model (DSDM) launched by the Rwanda Ministry of Health. This strategy entails having stable HIV patients attend one clinical visit per 6 months and receive ARVs and OIs prophylaxis medications for a 3-month period. The low effect of the COVID-19 lockdown on HIV program patients also suggests the importance of DSDM that aimed at more frequent services for higher-risk HIV patients and less frequent routine appointments for stable, low-risk HIV patients by offering them drug refill every 3 months and appointments every 6 months.
In contrast, access to oncology was badly affected by the lockdown. Until the onset of COVID-19, oncology services in PIH/IMB-supported districts were still centralized and only available at Butaro District Hospital. Therefore, patients in the oncology program traveled longer distances to access care compared with patients in other programs. Suspension of public transport during the lockdown would have also made it difficult for oncology patients to access emergency care and attend scheduled clinical appointments. In general, we observed that increasing distance to the health facility was also associated with experiencing barriers to accessing healthcare. These findings suggest that decentralized treatment programs may be less vulnerable to disruptions due to COVID-19 and join a larger body of research highlighting the importance of treatment decentralization in promoting patient’s access to health care [31, 32].
In contrast to a previous study in Nigeria, which reported that 35% of patients with chronic diseases had difficulties accessing medication during the COVID-19 lockdown [33], we found that a relatively low rate (20.0%) of patients with chronic conditions reported lack of access to medication due to the COVID-19 lockdown [33, 34]. However, 50.3% of patients who were prescribed medication still reported reduced ability to take their medication at home as prescribed. The most common reason for reduced ability of taking medication at home was feeling sad or depressed. It has been expected that the COVID-19 pandemic could increase the levels of anxiety and depression [35], and there is a strong evidence that depression among chronic care patients negatively affects adherence to medical treatment [36, 37]. During the pandemic, PIH/IMB and other care providers may need to provide additional socio-economic and psychological support to improve the mental health of patients and therefore increase medication adherence.
Many patients facing barriers to accessing healthcare independently identified coping mechanisms to ensure continuation of care during the lockdown. Two of these strategies, contacting clinicians via telephone and delegating relatives or neighbors to pick up medication for the patient, could be formalized to promote continuity of care during future crises. Although telephone consultations cannot replace clinical consultations, given the increase in access to mobile phones in low-income countries, health systems could leverage the use of these tools to accelerate the provision and access to care through telemedicine [11, 12, 38]. In our setting, as in other rural areas in Rwanda, access to mobile phones is still relatively low (58.6% in rural vs. 87.9% in urban) [39] and patients’ contact information change often, so physician-initiated phone calls is not currently feasible. However, establishing a clinical program helpline for patients and sharing this number with patients and their community health workers, who are provided with phones, could formalize this line of communication. Additionally, programming the EMR to provide annual reminders to update patients’ telephone numbers may improve the feasibility of future clinician-initiated communication. Similarly, allowing patients to designate formal delegates to obtain medication refills could be especially helpful for patients on multi-month prescriptions, who must still visit the pharmacy for monthly refills due to medical insurance requirements.
Although many patients reported walking long distances due to suspension of public transport, this positive coping mechanism is not a solution that should be formalized, since this strategy would not be accessible to all patients, especially the elderly or disabled. Health providers may instead work with local authorities to provide transport passes for patients’ appointments or increase decentralization of services.
This study had several limitations. First, our survey respondents’ population may not be representative of our patient population. Due to safety considerations and travel restrictions during the COVID-19 lockdown, our study population was limited to patients with active phone numbers, and only 9% of eligible patients had a phone number recorded in EMR. We hypothesize that this selection bias may have resulted in a respondent population that was wealthier than the overall patient population served by PIH/IMB. However, the magnitude of this bias may not be large because the proportion of survey respondents in the lowest Ubudehe category 1 is very similar to rates reported in the general population in Rwanda (17.3% vs. 16.0%) [40]. Second, this study only included patients who were residents in three districts supported by PIH/IMB and the effects of COVID-19 on patients in PIH/IMB-supported areas may differ from effects in other areas of Rwanda. These biases would likely result in an underestimate of the negative effects of the lockdown on chronic care patients, and our results should be viewed as a lower bound of the likely negative effects of the COVID-19 pandemic on rural Rwandan populations. These results may also not be generalizable to patients living in urban areas, who would have been living in areas with higher COVID-19 transmission but would have also had easier access to a wider variety of public and private health facilities within walking distance as well as better access to private transport options.
Our findings reveal that during the nationwide COVID-19 lockdown, a significant proportion of chronic care patients in rural Rwanda experienced barriers to accessing healthcare and challenges adhering to medication. In general, our data suggests the impact of the lockdown were smallest for patients from highly decentralized programs, like HIV, and worst for highly centralized programs that required patients to travel long distance, like oncology. To promote resilience and help mitigate effects of future lockdowns or other crises, we recommend healthcare systems in Rwanda or other similar settings consider a) continue to pursue decentralization to enhance access to health services b) leverage mobile phones as a formal mean of communication between patients and clinicians, and c) allow patients to designate delegates to obtain medication refills. We also recommend further research to explore the patients’ experience and motivation to engage in the behavior that helped to cope with the barriers to accessing healthcare during the COVID-19 lockdown.
Acknowledgements
We acknowledge the contributions of Placide Habinshuti, Todd Anderson, Deogratias Ndagijimana, Jean Marie Vianney Dusengimana, Symaque Dusabeyezu, Pacifique Uwamahoro, Anne Niyigena and Gedeon Ngoga and community health workers (CHWs) in Kayonza, Kirehe and Burera districts for supporting data collection.
Authors’ contributions
AN, DAB, VKC and FK conceived and designed the study. AN led data analysis and literature search, wrote the first draft of the manuscript and updated subsequent versions of the paper with co-author comments and inputs. DAB, VKC, FK, CS, DN, JMVD, SD, GU, NK and JMM contributed to the interpretation of results, revised the manuscript, suggested policy implications from the findings and edited the final draft. The author(s) read and approved the final manuscript.
Funding
The authors received no specific funding for this study. However, data collection activities were funded by Partners In Health/Inshuti Mu Buzima (PIH/IMB). DAB is supported by the Harvard Medical School Global Health Equity Research Fellowship, funded by Jonathan M. Goldstein and Kaia Miller Goldstein. Funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript that should be declared.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study received approval from the PIH/IMB research committee and was conducted under umbrella protocols of all involved clinical programs (MH: 196/RNEC/2020; NCD: 910/RNEC/2019; HIV: 015/RNEC/2020; Oncology: 805/RNEC/2019 and PDC: 713/RNEC/2019) as approved by the Rwanda National Ethics Committee (RNEC). All methods were carried out in accordance with relevant guidelines and regulations for research involving human subjects. Patients (or their primary caregivers) aged 18 years or older were given a detailed explanation about the purpose of the study and asked to provide a verbal consent for their voluntary participation. Informed verbal consent was obtained from all participants or from a parent/legal guardian for participants who were under 18 years of age.
Consent for publication
Not applicable.
Competing interests
None declared.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. 2021. Available from: https://covid19.who.int/. [cited 2021 Apr 09]
- 2.Wilhelm JA, Helleringer S. Utilization of non-Ebola health care services during Ebola outbreaks: a systematic review and meta-analysis. J Global Health. 2019;9(1):010406. 10.7189/jogh.09.010406. [DOI] [PMC free article] [PubMed]
- 3.Brolin Ribacke KJ, Saulnier DD, Eriksson A, von Schreeb J. Effects of the West Africa Ebola Virus Disease on Health-Care Utilization – A Systematic Review. Front Public Health. 2016;4. [cited 2020 Sep 18] Available from: http://journal.frontiersin.org/article/10.3389/fpubh.2016.00222/full [DOI] [PMC free article] [PubMed]
- 4.Veenstra N, Whiteside A, Lalloo D, Gibbs A. Unplanned antiretroviral treatment interruptions in southern Africa: how should we be managing these? Glob Health. 2010;6(1):4. doi: 10.1186/1744-8603-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parpia AS, Ndeffo-Mbah ML, Wenzel NS, Galvani AP. Effects of response to 2014–2015 Ebola outbreak on deaths from malaria, HIV/AIDS, and tuberculosis, West Africa. Emerg Infect Dis. 2016;22(3):433–441. doi: 10.3201/eid2203.150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberton T, Carter ED, Chou VB, Stegmuller AR, Jackson BD, Tam Y, Sawadogo-Lewis T, Walker N. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(7):e901–e908. doi: 10.1016/S2214-109X(20)30229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogan AB. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. 2020;8:e1132–41. 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed]
- 8.Kugbey N, Ohene-Oti N, Vanderpuye V. COVID-19 and its ramifications for cancer patients in low-resource settings: Ghana as a case study. ecancer. 2020 20 [cited 2020 Sep 18];14. Available from: https://ecancer.org/en/journal/editorial/99-covid-19-and-its-ramifications-for-cancer-patients-in-low-resource-settings-ghana-as-a-case-study [DOI] [PMC free article] [PubMed]
- 9.Anthonj C, Nkongolo OT, Schmitz P, Hango JN, Kistemann T. The impact of flooding on people living with HIV: a case study from the Ohangwena region, Namibia. Global Health Action. 2015;8(1):26441. doi: 10.3402/gha.v8.26441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bamrah S, Mbithi A, Mermin JH, Boo T, Bunnell RE, Sharif S, et al. The impact of post-election violence on HIV and other clinical services and on mental health—Kenya, 2008. Prehospital Disaster Med. 2013;28(1):9. doi: 10.1017/S1049023X12001665. [DOI] [PubMed] [Google Scholar]
- 11.Kamulegeya LH, Bwanika JM, Musinguzi D, Bakibinga P. Continuity of health service delivery during the COVID-19 pandemic: the role of digital health technologies in Uganda. Pan Afr Med J. 2020;35(Supp 2). [cited 2020 Sep 18] Available from: https://www.panafrican-med-journal.com/content/series/35/2/43/full [DOI] [PMC free article] [PubMed]
- 12.Chudasama YV, Gillies CL, Zaccardi F, Coles B, Davies MJ, Seidu S, Khunti K. Impact of COVID-19 on routine care for chronic diseases: a global survey of views from healthcare professionals. Diab Metab Syndr Clin Res Reviews. 2020;14(5):965–967. doi: 10.1016/j.dsx.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brey Z, Mash R, Goliath C, Roman D. Home delivery of medication during Coronavirus disease 2019, Cape Town, South Africa: Short report. African Journal of Primary Health Care & Family Medicine. 2020;12(1). [cited 2020 Sep 18] Available from: http://www.phcfm.org/index.php/PHCFM/article/view/2449 [DOI] [PMC free article] [PubMed]
- 14.Rwanda partially lifts coronavirus lockdown. The East African. 2020 [cited 2020 Oct 22]. Available from: https://www.theeastafrican.co.ke/tea/news/east-africa/rwanda-partially-lifts-coronavirus-lockdown-1440606
- 15.Rwanda expands drone use to deliver medicine. The East African. 2020 [cited 2020 Sep 18]. Available from: https://www.theeastafrican.co.ke/tea/news/east-africa/rwanda-expands-drone-use-to-deliver-medicine-1440756
- 16.World Health Organization . The impact of the COVID-19 pandemic on noncommunicable disease resources and services: results of a rapid assessment. Geneva: World Health Organization; 2020. [Google Scholar]
- 17.National Institute of Statistics of Rwanda (NISR) [Rwanda], Ministry of Finance and Economic Planning (MINECOFIN) [Rwanda]. Rwanda Fourth Population and Housing Census. Thematic Report: Population size, structure and distribution. Kigali, Rwanda; 2012.
- 18.Ngabireyimana E, Mutaganzwa C, Kirk CM, Miller AC, Wilson K, Dushimimana E, et al. A retrospective review of the Pediatric Development Clinic implementation: a model to improve medical, nutritional and developmental outcomes of at-risk under-five children in rural Rwanda. Matern Health Neonatol Perinatol. 2017;3(1):13. doi: 10.1186/s40748-017-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed]
- 21.Better living standards see Ubudehe categories revised. The EastAfrican. 2014. Available from: https://www.theeastafrican.co.ke/tea/rwanda-today/news/better-living-standards-see-ubudehe-categories-revised-1338360. [cited 2021 Apr 09]
- 22.Ezeanya-Esiobu, Chika. The rise of homegrown ideas and grassroots voices: New directions in social policy in Rwanda. UNRISD Working Paper, No. 2017-6. Geneva: United Nations Research Institute for Social Development (UNRISD); 2017. Available at: http:hdl.handle.net/10419/18609
- 23.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Abdela SG, Berhanu AB, Ferede LM, van Griensven J. Essential healthcare Services in the Face of COVID-19 prevention: experiences from a referral Hospital in Ethiopia. Am J Trop Med Hygiene. 2020;103(3):1198–1200. doi: 10.4269/ajtmh.20-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barone MTU, Harnik SB, de Luca PV, Lima BL de S, Wieselberg RJP, Ngongo B, et al. The impact of COVID-19 on people with diabetes in Brazil. Diabetes Res Clin Pract. 2020;166:108304. doi: 10.1016/j.diabres.2020.108304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aledo-Serrano Á, Mingorance A, Jiménez-Huete A, Toledano R, García-Morales I, Anciones C, Gil-Nagel A. Genetic epilepsies and COVID-19 pandemic: lessons from the caregiver perspective. Epilepsia. 2020;61(6):1312–1314. doi: 10.1111/epi.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dopfer C, Wetzke M, Zychlinsky Scharff A, Mueller F, Dressler F, Baumann U, Sasse M, Hansen G, Jablonka A, Happle C. COVID-19 related reduction in pediatric emergency healthcare utilization – a concerning trend. BMC Pediatr. 2020;20(1):427. doi: 10.1186/s12887-020-02303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldenberg MM. Overview of drugs used for epilepsy and seizures: etiology, diagnosis, and treatment. Pharm Ther. 2010;35(7):392–415. [PMC free article] [PubMed] [Google Scholar]
- 29.Wong LE, Hawkins JE, Langness S, Iris P, Sammann A. Where are all the patients? Addressing covid-19 fear to encourage sick patients to seek emergency care. 2020. 10.1056/CAT.20.0193.
- 30.Drobac PC, Basinga P, Condo J, Farmer PE, Finnegan KE, Hamon JK, Amoroso C, Hirschhorn LR, Kakoma JB, Lu C, Murangwa Y, Murray M, Ngabo F, Rich M, Thomson D, Binagwaho A. Comprehensive and integrated district health systems strengthening: the Rwanda population health implementation and training (PHIT) partnership. BMC Health Serv Res. 2013;13(S2):S5. doi: 10.1186/1472-6963-13-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyer S, Eboko F, Camara M, Abé C, Nguini MEO, Koulla-Shiro S, et al. Scaling up access to antiretroviral treatment for HIV infection: the impact of decentralization of healthcare delivery in Cameroon. AIDS. 2010;24(Suppl 1):S5–15. doi: 10.1097/01.aids.0000366078.45451.46. [DOI] [PubMed] [Google Scholar]
- 32.McGuire M, Pinoges L, Kanapathipillai R, Munyenyembe T, Huckabee M, Makombe S, et al. Treatment Initiation, Program Attrition and Patient Treatment Outcomes Associated with Scale-Up and Decentralization of HIV Care in Rural Malawi. Myer L, editor. PLoS ONE. 2012;7(10):e38044. doi: 10.1371/journal.pone.0038044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emmanuel Awucha N, Chinelo Janefrances O, Chima Meshach A, Chiamaka Henrietta J, Ibilolia Daniel A, Esther Chidiebere N. Impact of the COVID-19 Pandemic on Consumers’ Access to Essential Medicines in Nigeria. Am J Trop Med Hygiene. 2020 [cited 2020 Sep 18]; Available from: http://www.ajtmh.org/content/journals/10.4269/ajtmh.20-0838 [DOI] [PMC free article] [PubMed]
- 34.Van Hees S, Siewe Fodjo JN, Wijtvliet V, Van den Bergh R, Faria de Moura Villela E, da Silva CF, et al. Access to healthcare and prevalence of anxiety and depression in persons with epilepsy during the COVID-19 pandemic: A multicountry online survey. Epilepsy Behavior. 2020;112:107350. doi: 10.1016/j.yebeh.2020.107350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong L, Bouey J. Public mental health crisis during COVID-19 pandemic, China. Emerg Infect Dis. 2020;26(7):3. doi: 10.3201/eid2607.200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 37.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep. 2014;11(3):291–307. doi: 10.1007/s11904-014-0220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith AC, Thomas E, Snoswell CL, Haydon H, Mehrotra A, Clemensen J, Caffery LJ. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19) J Telemed Telecare. 2020;26(5):309–313. doi: 10.1177/1357633X20916567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Institute of Statistics of Rwanda (NISR), Rwanda Poverty Profile Report, 2013/14, August 2015. Available from: https://www.statistics.gov.rw/publication/rwanda-poverty-profile-report-results-eicv-4.
- 40.World Food Programme. Rwanda 2018 | Comprehensive Food Security Analysis. Kigali, Rwanda: World Food Programme, Vulnerability Analysis and Mapping (VAM); 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


