Abstract
Kidney transplantation is the ideal choice of kidney replacement therapy in children as it offers a low risk of mortality and a better quality of life. A wide variance in the access to kidney replacement therapies exists across the world with only 21% of low- and low-middle income countries (LLMIC) undertaking kidney transplantation. Pediatric kidney transplantation rates in these under-resourced regions are reported to be as low as < 4 pmcp [per million child population]. A robust kidney failure care program forms the cornerstone of a transplant program. Even the smallest transplant program entails a multidisciplinary workforce and expertise besides ensuring family commitment towards long-term care and economic burden. In general, the short-term graft survival rates from under-resourced regions are comparable to most high-income countries (HIC) and the challenge lies in the long-term outcomes. This review focuses on specific issues relevant to kidney transplants in children in under-resourced regions by highlighting limitations in the capacity and health workforce, regulatory norms, medical issues, economic burden, factors beyond financial hardship and ethical considerations relevant to these regions. Finally, the perspective of strengthening transplant programs in these regions should factor in the bigger challenges that exist in achieving the health-related sustainable development goals by 2030.
Keywords: Paediatric kidney transplantation, Low middle-income countries, Under-resourced regions, Developing nations, Challenges, Barriers, Outcomes
The ideal kidney replacement therapy
The choice of kidney replacement therapy for children with kidney failure should ensure a lower risk of morbidity and mortality and provide a better quality of life. In this regard, kidney transplantation is the ideal choice of kidney replacement therapy in children [1, 2]. Globally, in recent times, improved patient and graft survival have been observed with pre-emptive transplantation [3, 4]. Despite known advantages of living donor transplantation, with nearly 80% of living donors being parents of the child, the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) reveals that only one third of paediatric transplants arise from a living-related donor (LRD) [5]. Deceased organ transplant is beneficial to children in specific situations and in addition provides an opportunity for a potential second transplant with an LRD [4, 6]. While the world is striving to achieve the sustainable developmental goals (SDG) by 2030, child health care has taken centre stage. Investment towards achieving this goal has been proposed to be highly cost-effective in the long term [7], providing the unique opportunity to highlight various issues concerning the care of a child with kidney failure needing transplantation in low-resource settings.
Access to kidney transplantation
Amongst 215 countries across the globe, there are 82 countries which can be classified as low-middle- and low-income countries (LLMIC) [8]. These countries have a greater prevalence of chronic kidney disease (CKD) and death due to CKD in younger individuals (including adolescents) compared to high-income countries (HIC) on the global disease burden analysis [9].
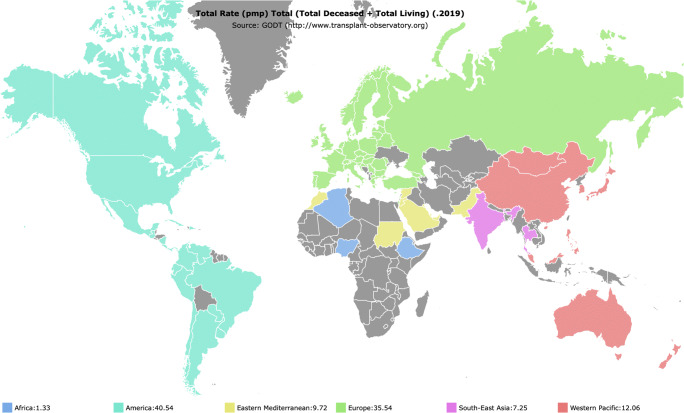
In addition, according to the Global Kidney Health Atlas, there is also a wide variation in access to kidney transplantation which is not available in 21% of countries (64% of African countries and 88% of LIC) [10, 11]. Kidney transplant rates in adults per million population (pmp) are 40.54 and 35.54 among HIC such as America and those in Europe, respectively, as shown in Fig. 1. In contrast, South-East Asian countries and those in Africa have the lowest rates of transplantation of 7.25 pmp and 1.33 pmp, respectively, and minimal deceased organ transplant activity in particular [12]. In Europe, rates of paediatric kidney transplants range from 0 to 13.5 per million children population (pmcp) [13]. Similar data for paediatric kidney transplants is very limited from LLMIC, some reporting rates of paediatric kidney transplantation as low as < 4 pmcp [14–16]. Moreover, the COVID-19 pandemic has added to the existing disparities in kidney transplantation rates across regions [12].
Fig. 1.
Total rate (pmp) of kidney transplants undertaken across regions from the global database on donation and transplantation website
Even a small kidney transplant program demands a multidisciplinary workforce, high levels of expertise, and family commitment and an ability for patient follow-up with drug monitoring. Several gaps involving resource availability, capacity building, strategy development, and regulatory oversight exist in LLMIC. Across 32 countries in Europe, a strong association was observed between country income based on the GDP per capita and the rates of paediatric kidney transplantation. For every US$1000 increase in GDP per capita, the kidney transplant rate increased by 0.2 [13]. However, analysis of geographic measures of socioeconomic status from the United Nations Organ Sharing (UNOS) registry data revealed that socioeconomic status did not predict 1-year paediatric kidney transplant outcomes [17]. In LLMIC, in addition to economic disparities, access to transplantation to an extent is influenced by racial, ethnic and geographical disparities [18–20]. Similar observations have been reported from a country-wide study in Brazil. Among 271 paediatric kidney transplants from Brazil, disparities in access to transplants across various regions were related to large differences in health care resources and economic status within the regions [21].
Kidney failure care—the stepping stone
Outcomes in transplantation rely on a sound kidney failure care program. LLMIC have unique challenges regarding kidney failure care in adults [22] and children [23]. To a large extent, the status of kidney failure care in children depends on the standards of adult kidney care existing in the region. The real challenge that under-resourced regions or LLMIC face is prompt detection of CKD in children, early referral and providing optimal CKD 5D care services. The ground reality is that despite considerable progress in health care in recent years, the proportion of children presenting for the first time in late stages of CKD persists at around 25% over the last two decades in India [24, 25]. Inequalities impact several steps in the overall process from diagnosis of kidney failure to receiving a transplant at country, institution and family levels [26].
Studies from a tertiary Indian centre report higher burden of CKD comorbidities and faster rates of CKD progression compared to HIC [25, 27]. Besides, an alarming observation from the same centre included a significant (42%) proportion of children with CKD 5 being lost to follow-up with discontinuation of care while only 20% underwent transplantation [28]. In resource-limited settings, many of these children present late with life-threatening complications or CKD 5 requiring urgent dialysis that makes pre-emptive transplantation impractical. These situations demand optimal resources, infrastructure and availability of trained health personnel [29, 30]. On a positive note, parental acceptance of manual chronic peritoneal dialysis especially in younger children in resource-constrained settings is improving, which may pave the way for transplantation in younger children and infants in these regions [31, 32].
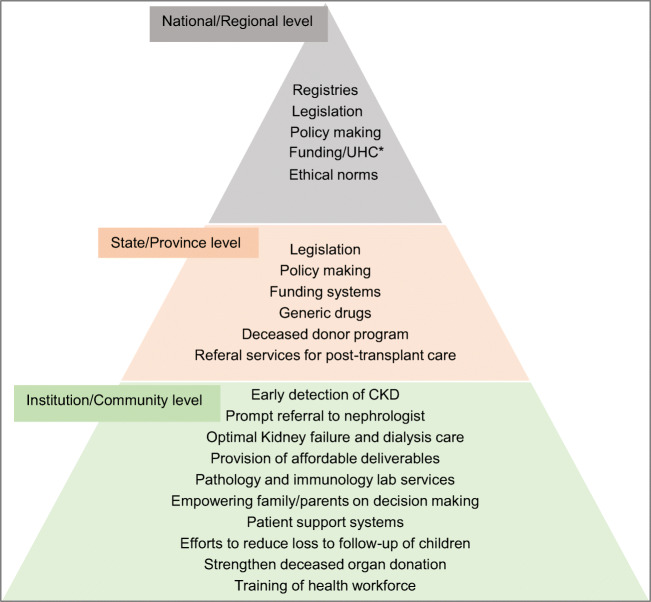
To facilitate better preparation for and to maximise sustainability of kidney replacement therapies in areas with resource-limited regions, the International Society of Nephrology (ISN) is developing guidance documents applicable to both adults and children for the World Health Organisation. A recent review addresses the various components involved in incorporating transplantation within integrated care for kidney care [33]. From a national level perspective, the key areas governing sustainable transplant services include legislation, regulation, health finance, health workforce, family and community involvement, maintenance of registries, strengthening deceased organ transplant programs and abiding by ethical standards. The essential components needed for a sustainable transplant program at specific levels of health care are depicted in Fig. 2.
Fig. 2.
Essential components of a sustainable paediatric transplant program at various levels of health care relevant to LLMIC based on reference 33. *Universal health coverage
Dearth of national registries
A major limitation that needs to be addressed regarding priorities is the lack of national registries for CKD 5, dialysis or kidney transplantation in the majority of under-resourced nations. The paucity of comprehensive health information systems curtails the potential to capture critical information on the burden, needs and provision of kidney replacement therapies including transplantation in these regions. Adult transplant registries exist in 44% of LMIC and none in LIC [10, 11]. In fact, populous nations like India and Germany, and parts of Africa, do not have a registry for kidney transplantation [34]. However, countries like Brazil, Thailand and South Africa have made appreciable progress by establishing paediatric kidney transplant registries [35–37]. The various dimensions and utility of registry data as a source of evidence beyond randomised controlled trials have been well discussed with regard to kidney and liver transplantation [38]. Registry management needs to be staffed by a committee that involves all stakeholders who ensure mandatory reporting, provision of adequate technology infrastructure, human workforce and sources for funding. Voluntary participation is the norm for most national registries which requires extensive “buy-in” with ongoing motivation and is most often governed by national societies or bodies rather than the government. Data collection can be a significant challenge but new electronic databases available on smart phone technology have made this easier.
Transplant outcomes in LMIC
The updated NAPRTCS data (2012–2017) reports graft survival rates at 1 year, 3 years and 5 years of 99.5%, 97.2% and 94.9% in living donor transplants and 97.6%, 94.4% and 90.1%, respectively, in deceased donor transplants [4]. The allograft survival rates from LLMIC are depicted in Table 1 [15, 39–55]. Though the 1-year allograft survival rates are quite similar to the NAPRTCS report, the outcomes at 5 years and beyond pose major concerns in these countries. Key factors associated with graft loss have been deceased donor transplant (with resultant increased cold ischaemic times and “less than perfect” older donors), invasive infections, cytomegalovirus disease and more than two rejection episodes, with sepsis being the key contributor to patient loss [41, 43–56]. The unfortunate event of patient loss with an underlying functional allograft is not an uncommon scenario. Predictors of chronic rejection are secondary to poor compliance, lack of induction therapy and multiple episodes of acute rejection [44]. Access to support services, including transplant histopathology, monitoring of viral load and drug level, access to vaccines, imaging and immunology tests are limited in most LLMIC that contribute to suboptimal outcomes [57]. Adolescent patients often fall between services and adolescent care is a challenging field especially in under-resourced regions. Planned transition and development of specific adolescent clinics with age-appropriate treatment and psychological support improve engagement with health services and ultimately improve compliance. This is really important for resource-limited adult services [58].
Table 1.
Kidney graft survival of live related transplants in LLMICs
| Country-wise published data | Graft survival % (LRD) | |||
|---|---|---|---|---|
| 1 yr | 5 yr | 10 yr | 15 yr | |
| Brazil [39] | 90 | 72 | 59 | |
| South Africa [40] | 82 | 44 | 23 | |
| India [41–47] | 90–98 | 80–92 | 66–85 | 77.6% (1 centre) |
| Pakistan [15] | 96 | 81 | - | |
| Thailand [48] | 100 | 86 | - | |
| Iran [49] | 90 | 81 | 62 | |
| Chile [50] | 87 | 78 | - | |
| Egypt [51] | 93 | 73 | - | |
| Turkey [52] | 91 | 67 | - | |
| Jordan [53] | 97 | 91 (3yr) | - | |
| Saudi Arabia [54] | 98 | 92 | - | |
| Kuwait [55] | 98 | - | 84 | |
Most LLMIC initiate transplant services through a living related donor program. Development of a deceased donor program is challenging in the developing world as it demands changes in legislation (brain death legislation), better infrastructure, community involvement, support from intensive care teams, rapid turn-around of transplant immunology services and interventions to address educational, social and religious barriers to organ donation. Currently of 54 countries in Africa, only 4 have brain death legislation (South Africa, Tunisia, Egypt and Sudan). Besides, due to a huge demand for organs amongst adults, there is resistance to enforcing prioritised allocation criteria for children in these regions, leading to prolonged waiting periods for children and adolescents. This often leads to poor growth and failure to thrive with developmental delay and chronic issues of rickets and anaemia. There needs to be a visionary move towards paediatric recipients receiving solid organs from paediatric deceased donors, which is currently not a practice even in the best of centres in LLMIC countries. Deceased donor donation has been the predominant approach to transplanting in children in countries like South Africa (58%), Chile (61%), Saudi Arabia (56%) and Brazil (32%) with limited data available from other LMIC [48, 59]. Thailand reported a comparable 5-year graft survival between children receiving living related and deceased donor transplants [48]. An Indian study of 37 children who received deceased donor kidney transplants at a government institution demonstrated 1-year, 5-year and 10-year graft survival rates of 90.4%,73.3% and 73.3%, respectively [60]. A public-private partnership model for a sustainable donor pool program relevant to developing countries has been set up by the Multi Organ Harvesting and Networking (MOHAN) foundation (http://www.mohanfoundation.org/), a philanthropic non-governmental organization aiming to promote organ donation and transplantation in India. Empowerment of trained transplant coordinators by the foundation led to an appreciable increase (65%) in the willingness among families of the deceased to donate organs [61]. Appointment of paediatric transplant co-ordinators can also increase the donation rate in children’s health care facilities.
Medical issues
Native kidney diseases that are known to recur post-transplant, those that warrant genetic testing and those that need combined kidney-liver transplantation pose challenges to paediatric nephrologists in LLMIC. A multi-centre prospective study in 308 children with CKD 5 from the UK observed that barriers to transplantation included disease-related factors in 36%, donor unavailability in 27% and size of the child in 20% [62]. A retrospective study of 155 children with CKD 5 from a single LMIC centre revealed non-medical barriers to overshadow medical issues [28]. The medical issues included lack of timely access to deceased donor organs, incomplete bladder preparation among those with urological issues and lack of expertise with multi-organ transplantation as well as lack of expertise in managing transplantation in small children and infants.
Variable outcomes in children transplanted with lower urinary tract dysfunction have been reported from few LMIC, where specific concerns regarding repeated urinary infections, poorer long-term graft function and the need for frequent urological interventions are described [41, 63–65]. An interim analysis of transplanted children with (n = 23) and without abnormal lower urinary tract problems (n = 52) from the Department of Pediatric Nephrology, St John’s Medical College Hospital, revealed suboptimal long-term graft function [94%, 87%, 68% vs. 94%, 90%, 86%] and patient survival [100%, 84%, 65% vs. 98%, 91%, 91%] at 1 year, 5 years and 10 years, respectively (unpublished data).
Poor growth post-transplant has been a common concern with steroid sparing or avoidance protocols not being followed [44]. In the developed world, a reduction in cardiovascular and infection-related deaths has led to improved survival after paediatric kidney transplantation [66]. A recent study from an LMIC reported a high burden of masked and nocturnal hypertension in paediatric transplant recipients [67].
The spectrum of infections described in children post-transplant includes urinary tract infections, sepsis, pneumonia, tuberculosis, meningitis and viral infections [44, 68]. Infections were noted in 50% of children post-transplant while sepsis/severe infections contributed to two-thirds of deaths [44]. Death with graft function is common and occurs secondary to sepsis or severe infections among the majority of adults post-transplant [69]. With limited access to molecular diagnostic tests such as real-time polymerase chain reaction, nucleic acid tests and multiplex ligation-dependent probe amplification, the diagnosis of viral infections is delayed causing child morbidity and graft dysfunction. Therapies for viral disease are expensive, further compromising the social and economic security of families.
Capacity and health workforce
A survey of paediatric kidney transplant practices in India highlights the key concerns that are applicable to LMIC. In stark contrast to well-resourced nations, transplantation in children in the majority of centres was undertaken by adult nephrologists (61%) with greater numbers being done in private hospitals [70]. However, most transplants in children undertaken by adult nephrologists include a body weight of 20 kg and above. A skillful transplant surgeon is indispensable for a successful transplant, to the extent that in some LLMIC centres, there is dependence on transplant surgeons from overseas to facilitate the transplants. Enhancing workforce capacity through training of nurses in transplant and critical care nursing, having paediatric transplantation made mandatory in the curriculum for post-doctoral training and including medical social work services within the framework of multidisciplinary teams are some key steps to strengthen the workforce. A structured and collaborative approach between paediatric and adult nephrologists for transition of adolescents to adult renal services is lacking in most well-established centres.
In many countries in South Asia, public/government hospitals receive a large number of children with CKD and CKD 5 but many are unable to provide kidney replacement therapies for children in particular. Private centres that have the expertise and infrastructure in place have to shoulder the burden of raising funds and cutting costs to make kidney replacement therapies including transplantation affordable to all sectors of society. An example of a public-private partnership model to cater to providing kidney transplant to poorer sectors of society in a LMIC is described for adults [71].
Most successful private specialty hospitals are located in metropolitan cities and some have geared up to perform highly specialised transplant-related procedures like ABO incompatibility and paired organ donation in children [72, 73]. However, a paediatric ABO-incompatible kidney transplant has also been reported from a university teaching hospital in Nepal [74]. The hard fact remains that families belonging to lower-middle and lower socioeconomic segments of society struggle to cope with the challenges of preparing their child for transplantation, sustaining long-term expenses, maintaining close follow-up and transition of care of their children to adult service.
Economic barriers
The most important barrier to transplantation in LLMIC is the high costs involved. The concept that transplantation is not as expensive as long-term dialysis has been proven in adults but can be applied to children as well [75].
Table 2 demonstrates the approximate costs related to paediatric dialysis and transplantation from two centres in LLMIC. We observe a tendency for families to agree to some form of dialysis as an approach to manage the crisis of kidney failure. This happens more so due to children presenting late and needing emergent kidney replacement therapy. Most families thereafter continue with long-term dialysis for several reasons despite being cognizant of the fact that expenditure towards long-term dialysis exceeds that of transplantation. Without the support of insurance, families face out-of-pocket costs that can lead to catastrophic expenditures from any modality of kidney replacement therapy, leading to graft loss in case of transplantation [76, 77]. Public funding for kidney replacement therapy exists in most HIC but is minimal in LLMIC. The need for urgent funding support towards kidney transplantation in children has been recently highlighted in a study from Nigeria [78]. Though some LMIC are providing funding models for dialysis, there is no such support for transplantation as yet [79]. While LLMIC countries are moving towards universal health care, from a policy-making perspective, it becomes important to assess the feasibility and benefits involved in supporting dialysis or transplantation within regions [80]. Maintenance immunosuppression adds to the financial burden of transplantation and a transition from brand to generic drugs could cut costs of life-long therapy [81]. A community-government partnership model for funding paediatric kidney replacement therapies has been implemented in Pakistan [15].
Table 2.
Approximate costs related to paediatric kidney replacement therapies from two centres belonging to LMIC
| Kidney replacement therapies for a child | Indiaa Cost (USD ex rate = 69) Rounded to nearest USD |
South Africab USD Government subsidised |
|---|---|---|
| Maintenance dialysis monthly | ||
| CAPD costs (2 bags dialysate + medications + clinic visit) | 489 | 650 |
| HD (3 per week) + medications + clinic visit) | 311 | 450 |
| Transplant-related treatment | ||
| Cost of LRD transplantation | 9420 | 10,800 |
| Monthly immunosuppression | 145 | 390 |
| Monthly transplant clinic visit | 51 | 150 |
USD United States Dollar, CAPD continuous ambulatory peritoneal dialysis, HD hemodialysis
aSt John’s Medical College Hospital, Bangalore—a non-government, “not for profit” academic Institution
bRed Cross War Memorial Children’s Hospital, University of Cape Town–Government hospital
Barriers beyond financial hardship
Commuting from rural and remote areas to reach tertiary hospitals has been a major concern observed in developing nations for both pre- and post-transplant long-term care [14, 15]. As an example, from a community perspective in India, basic kidney function testing is not done in a village centre or a subcentre. Travel distance to the nearest primary health centre that cares for a population of 20,000–30,000 and has the facility to test for kidney function ranges from 5 to 10 km and travel to a district hospital for imaging studies may reach 35 km [82]. Moreover, substantial variability in serum creatinine assays exists in laboratories across levels of health care.
Barriers to accomplishing transplant in an under-resourced setting includes the unique issue of loss to follow-up or disappearance of children and their families from the treatment centre [28]. This is associated with low socioeconomic status, longer time and distance to travel, parent’s education and parent’s occupation. Negative attitudes towards transplantation and organ donation among parents and families are also encountered. Lack of interest in transplantation was observed in 61% which included reasons like the father being unwilling to pursue transplantation, focus on the birth of the next child, hope that kidney failure will recover miraculously and competing family health issues. Another issue seen in 15% was neither parent being fit or willing to donate and unwilling to miss work as daily wage labourers. A qualitative study of children and adolescents found that negative emotions regarding transplantation among children and adolescents included fear, anxiety, guilt for burdening siblings and parent as donors [83]. Children and adolescents did view kidney transplantation as freedom from dialysis while knowing that transplantation is not necessarily a return to full health.
Ethical issues
It is imperative to consider equity in the provision of kidney replacement therapy in LLMIC and this is governed by factors like availability of resources, affordability of treatments and acceptability concerning ethical and societal standards [84]. Issues regarding unregulated organ trade and transplant tourism prevail in these regions [14, 85, 86]. Gender disparities have been recognised with respect to donors and recipients across LMIC [87, 88]. A pragmatic and critical ethical issue is consideration of family resources while making medical recommendations for transplantation (an expensive treatment with no guarantee of success) to a child belonging to a poor family. This discussion includes consideration of principles of beneficence, maleficence, justice and autonomy [89]. A preliminary guidance document on the ethical framework and approach to ethical challenges in kidney failure care, including kidney replacement therapies, has been developed for LMIC but requires further refinement [90].
Steps towards bridging gaps
Legislation (regarding donors and recipients), regulatory norms, policy advocacy at local hospitals and government departments at health care levels for both children and adolescents, registries, health financing, adherence to ethical standards and community and patient engagement are the key domains that under-resourced nations need to work on to build effective transplant programs. Templates for development of transplant programs as a component of integrated kidney failure care in LLMIC have been put forth by the ISN [33].
Scientific bodies such as the ISN, The Transplantation Society (TTS), the International Pediatric Nephrology Association (IPNA), the International Society of Peritoneal Dialysis (ISPD) and the International Pediatric Transplant Association (IPTA) have invested in unique exchange training programs and educational collaborations. These initiatives have gone a long way in empowering under-resourced countries to undertake transplantation in both adults and children with an additional responsibility of engaging in efforts to solve regional challenges. An example of a collaboration that not only reflects the strengths of collaboration but also projects the specific challenges relevant to an under-resourced region is a centre in Myanmar. A centre in Yangon Hospital, Myanmar, undertook the first paediatric transplantation under the umbrella of the ISN Sister renal centre collaboration in 2017. Under the guidance and mentorship of the team from Children Kidney Centre, National University Hospital, Singapore, kidney failure care services were implemented, pre-transplant preparation was undertaken and ten children received transplants successfully. Key challenges noted were convincing the community and medical fraternity about the need for and feasibility of undertaking transplantation in children, raising funds for the surgery, supporting post-transplant immunosuppression and dealing with various issues of sustaining follow-up care. Medical professionals had to shoulder responsibilities beyond the purview of nephrology care through home visits, ensuring basic water supply, housing and sanitation for the children following transplantation (personal communication with Prof. Yi Yi Khin, Pediatric Nephrologist, Myanmar).
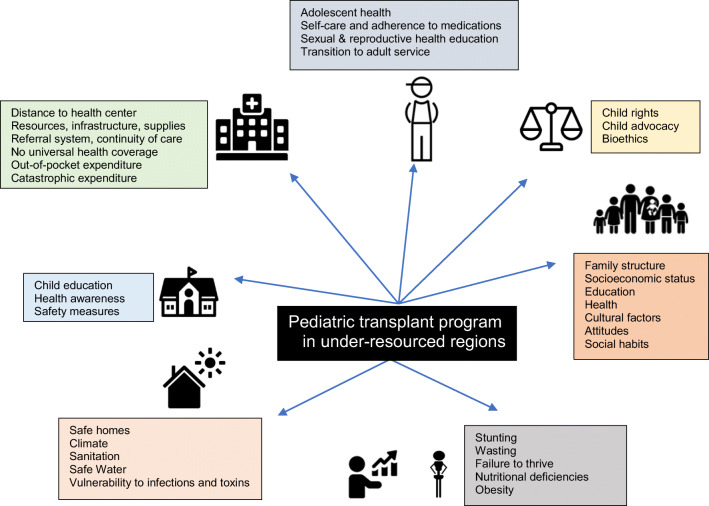
This brings us back to reality. If we take a step back from the transplant scene, we see a panoramic view of the under-resourced world that is faced with very basic and complex challenges related to health. Figure 3 illustrates the various health-related SDGs that pose challenges to kidney failure care and transplantation in under-resourced regions. The under-resourced world in particular is battling against health-related SDGs like maternal, neonatal and under-five mortality, striving to find ways to deal with a huge burden of basic needs like clean water supply, sanitation hygiene, safe homes and other environmental hazards towards the timeline of 2030 [7, 91, 92]. In this context, it becomes crucial to understand and accept the fact that unless progress towards solving these elementary issues and inequalities impacting child health takes place with the required momentum, kidney failure care and kidney replacement therapies will continue to remain a big challenge in under-resourced regions. However, on a brighter note, as it demands a multidisciplinary approach, development of transplant programs in under-resourced regions will have positive influences on the entire health care system, serving as an impetus to develop and sustain higher standards of health care at all levels in the region.
Fig. 3.
Components of health-related SDG that potentially impact paediatric transplantation in under-resourced regions [7, 87]
Acknowledgements
We are grateful to Dr. Valerie Luyckx MBBch, MSc, PhD (nephrologist with a primary interest in global health and kidney disease in underserved populations and low-resource regions, University of Zurich) for providing valuable input to this review.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gillen DL, Stehman-Breen CO, Smith JM, McDonald RA, Warady BA, Brandt JR, Wong CS. Survival advantage of pediatric recipients of a first kidney transplant among children awaiting kidney trans-plantation. Am J Transplant. 2008;8:2600–2606. doi: 10.1111/j.1600-6143.2008.02410.x. [DOI] [PubMed] [Google Scholar]
- 2.Yadav P, Nunia S, Bansal A, Sureka SK, Jena R, Ansari MS, Srivastava A. Multidimensional assessment of quality of life of children and problem of parents in India Society after pediatric renal transplant: beyond the conventional thoughts. Pediatr Transplant. 2017;21:e13001. doi: 10.1111/petr.13001. [DOI] [PubMed] [Google Scholar]
- 3.Amaral S, Sayed BA, Kutner N, Patzer RE. Pre-emptive kidney transplantation is associated with survival benefits among pediatric patients with end-stage renal disease. Kidney Int. 2016;90:1100–1108. doi: 10.1016/j.kint.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chua A, Cramer C, Moudgil A, Martz K, Smith J, Blydt-Hansen T, Neu A, Dharnidharka VR, NAPRTCS investigators Kidney transplant practice patterns and outcome benchmarks over 30 years: the 2018 report of the NAPRTCS. Pediatr Transplant. 2019;23:e13597. doi: 10.1111/petr.13597. [DOI] [PubMed] [Google Scholar]
- 5.North American Pediatric Renal Trials and Collaborative Studies (2014) NAPRTCS 2014 Annual Transplant Report. https://naprtcs.org/system/files/2014_Annual_Transplant_Report.pdf. Accessed 30 March 2021
- 6.Sigurjonsdottir VK, Grimm PC. Living or deceased donor kidney transplantation in children. Curr Opin Pediatr. 2019;31:232–236. doi: 10.1097/mop.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 7.Clark H, Coll-Seck AM, Banerjee A, Peterson S, Dalglish SL, Ameratunga S, Costello A, Balabanova D, Bhan MK, Bhutta ZA. A future for the world’s children? A WHO–UNICEF–Lancet Commission. Lancet. 2020;395:605–658. doi: 10.1016/s0140-6736(19)32540-1. [DOI] [PubMed] [Google Scholar]
- 8.ChartsBin statistics collector team (2016) Country Income Groups (World Bank Classification). http://chartsbin.com/view/2438. Accessed 4 March 2020
- 9.Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, Al-Aly Z, Maddukuri G, Tsai CY, Floyd T, Al-Aly Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Bello AK, Levin A, Tonelli M, Okpechi IG, Feehally J, Harris D, et al. (2017) Global Kidney Health Atlas: A Report by the International Society of Nephrology on the Current State of Organization and Structures for Kidney Care Across the Globe. International Society of Nephrology, Brussels, Belgium
- 11.Bello AK, Levin A, Tonelli M, Okpechi IG, Feehally J, Harris D, Jindal K, Salako BL, Rateb A, Osman MA, Qarni B, Saad S, Lunney M, Wiebe N, Ye F, Johnson DW. Assessment of global kidney health care status. JAMA. 2017;317:1864–1881. doi: 10.1001/jama.2017.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Observatory on Donation and Transplantation (2020) Organ Donation and Transplantation Activities 2020. http://www.transplant-observatory.org/2020-activity-data. Accessed March 2021
- 13.Harambat J, van Stralen KJ, Schaefer F, Grenda R, Jankauskiene A, Kostic M, Macher MA, Maxwell H, Puretic Z, Raes A, Rubik J, Sørensen SS, Toots U, Topaloglu R, Tönshoff B, Verrina E, Jager KJ. Disparities in policies, practices and rates of pediatric kidney transplantation in Europe. Am J Transplant. 2013;13:2066–2074. doi: 10.1111/ajt.12288. [DOI] [PubMed] [Google Scholar]
- 14.Spearman CW, McCulloch MI. Challenges for paediatric transplantation in Africa. Pediatr Transplant. 2014;18:668–674. doi: 10.1111/petr.12333. [DOI] [PubMed] [Google Scholar]
- 15.Rizvi SA, Sultan S, Zafar MN, Naqvi SA, Lanewala AA, Hashmi S, Aziz T, Hassan AS, Ali B, Mohsin R, et al. (2013) Pediatric kidney transplantation in the developing world: challenges and solutions. Am J Transplant 13:2441–2449 [DOI] [PubMed]
- 16.Garcia C, Pestana JM, Martins S, Nogueira P, Barros V, Rohde R, Camargo M, Feltran L, Esmeraldo R, Carvalho R. Collaborative Brazilian pediatric renal transplant registry (CoBrazPed-RTx): a report from 2004 to 2013. Transplant Proc. 2015;47:950–953. doi: 10.1016/j.transproceed.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Miller R, Akateh C, Thompson N, Tumin DH, Jr, Black SM, Tobias JD. County socioeconomic characteristics and pediatric renal transplantation outcomes. Pediatr Nephrol. 2018;33:1227–1234. doi: 10.1007/s00467-018-3928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chesnaye NC, Schaefer F, Groothoff JW, Caskey F, Heaf JG, Kushnirenko S, Lewis M, Mauel R, Maurer E, Merenmies J. Disparities in treatment rates of paediatric end-stage renal disease across Europe: insights from the ESPN/ERA-EDTA registry. Nephrol Dial Transplant. 2015;30:1377–1385. doi: 10.1093/ndt/gfv064. [DOI] [PubMed] [Google Scholar]
- 19.Reese PP, Hwang H, Potluri V, Abt PL, Shults J, Amaral S. Geographic determinants of access to pediatric deceased donor kidney transplantation. J Am Soc Nephrol. 2014;25:827–835. doi: 10.1681/ASN.2013070684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patzer RE, Mohan S, Kutner N, McClellan WM, Amaral S. Racial and ethnic disparities in pediatric renal allograft survival in the United States. Kidney Int. 2015;87:584–592. doi: 10.1038/ki.2014.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nogueira PC, de Carvalho MF, de Santis Feltran L, Konstantyner T, Sesso R. Inequality in pediatric kidney transplantation in Brazil. Pediatr Nephrol. 2015;31:501–507. doi: 10.1007/s00467-015-3226-z. [DOI] [PubMed] [Google Scholar]
- 22.Luyckx VA, Smyth B, Harris DCH, Pecoits-Filho R. Dialysis funding, eligibility, procurement, and protocols in low- and middle-income settings: results from the International Society of Nephrology collection survey. Kidney Int Suppl. 2020;10:e10–e18. doi: 10.1016/j.kisu.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalji R, Francis A, Johnson DW, McCulloch M. Health disparities in access to kidney replacement therapy amongst children and adolescents with end-stage kidney disease in low- and lower-middle-income countries. Kidney Int. 2020;97:463–465. doi: 10.1016/j.kint.2019.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Hari P, Singla IK, Mantan M, Kanitkar M, Batra B, Bagga A. Chronic renal failure in children. Indian Pediatr. 2003;40:1035–1042. [PubMed] [Google Scholar]
- 25.Kamath N, Iyengar A. Chronic kidney disease (CKD): an observational study of etiology, severity and burden of comorbidities. Indian J Pediatr. 2017;84:822–825. doi: 10.1007/s12098-017-2413-2. [DOI] [PubMed] [Google Scholar]
- 26.Harambat J, Ekulu PM. Inequalities in access to pediatric ESRD care: a global health challenge. Pediatr Nephrol. 2015;31:353–358. doi: 10.1007/s00467-015-3263-7. [DOI] [PubMed] [Google Scholar]
- 27.Kamath N, Iyengar A, George N, Luyckx VA. Risk factors and rate of progression of CKD in children. Kidney Int Rep. 2019;4:1472–1477. doi: 10.1016/j.ekir.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pais P, Blydt-Hansen TD, Michael Raj JA, Dello Strologo L, Iyengar A Low renal transplantation rates in children with end-stage kidney disease: a study of barriers in a low-resource setting. Pediatr Transplant 25:e13867. 10.1111/petr.13867 [DOI] [PubMed]
- 29.Niang A, Iyengar A, Luyckx VA. Hemodialysis versus peritoneal dialysis in resource-limited settings. Curr Opin Nephrol Hypertens. 2018;27:463–471. doi: 10.1097/mnh.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 30.Savla D, Ahmed S, Yeates K, Matthew A, Anand S. Barriers to increasing use of peritoneal dialysis in Bangladesh: a survey of patients and providers. Perit Dial Int. 2017;7:234–237. doi: 10.3747/pdi.2016.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamath N, Reddy HV, Iyengar A. Clinical and dialysis outcomes of manual chronic peritoneal dialysis in low-body-weight children from a low-to-middle-income country. Perit Dial Int. 2020;40:6–11. doi: 10.1177/0896860819873541. [DOI] [PubMed] [Google Scholar]
- 32.Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23:533–544. doi: 10.1681/ASN.2011060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Connell PJ, Brown M, Chan TM, Claure-Del Granado R, Davies SJ, Eiam-Ong S, Hassa MH, Kalantar-Zadeh K, Levin A, Martin DE. The role of kidney transplantation as a component of integrated care for chronic kidney disease. Kidney Int Suppl. 2020;10:e78–e85. doi: 10.1016/j.kisu.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.See EJ, Alrukhaimi M, Ashuntantang GE, Bello AK, Bellorin-font E, Benghanem Gharbi M, Braam B, Feehally J, Harris DC, Jha V. Global coverage of health information systems for kidney disease: availability, challenges, and opportunities for development. Kidney Int Suppl. 2018;8:74–81. doi: 10.1016/j.kisu.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Souza VC, Garcia CD, Pestana JM, Stopa Martins SB, Porini Custódio L d F, Bittencourt V, Rohde R, Simoes Pires I, Camargo MF, Koch Nogueira P, et al. (2019) Collaborative Brazilian pediatric renal transplant registry (CoBrazPed-RTx): a report from 2004 to 2018. Pediatr Transplant 23:e13463. 10.1111/petr.13463 [DOI] [PubMed]
- 36.Noppakun K, Ingsathit A, Pongskul C, Premasthian N, Avihingsanon Y, Lumpaopong A, Vareesangthip K, Sumethkul V, Subcommittee for kidney transplant registry, Thai Transplantation Society A 25-year experience of kidney transplantation in Thailand: Report from the Thai Transplant Registry. Nephrology. 2015;20:177–183. doi: 10.1111/nep.12378. [DOI] [PubMed] [Google Scholar]
- 37.Davids MR, Eastwood JB, Selwood NH, Arogundade FA, Ashuntantang G, Benghanem GharbiM Jarraya F, McCulloch M, Plange-Rhule J, Swanepoel CR, Adu D. A renal registry for Africa: first steps. Clin Kidney J. 2016;9:162–167. doi: 10.1093/ckj/sfv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wadström J, Ericzon BG, Halloran PF, Bechstein WO, Opelz G, Serón D, Grinyó J, Loupy A, Kuypers D, Mariat C. Advancing transplantation. Transplantation. 2017;101(Suppl 2S):S1–S41. doi: 10.1097/tp.0000000000001563. [DOI] [PubMed] [Google Scholar]
- 39.Garcia CD, Bittencourt VB, Tumelero A, Antonello JS, Moura DM, Vitola SP, Didone E, Guerra E, Pires F, Garcia VD. 300 pediatric renal transplantations: a single-center experience. Transplant Proc. 2006;38:3454–3455. doi: 10.1016/j.transproceed.2006.10.086. [DOI] [PubMed] [Google Scholar]
- 40.Pitcher GJ, Beale PG, Bowley DM, Hahn D, Thomson PD. Pediatric renal transplantation in a South African teaching hospital: a 20-year perspective. Pediatr Transplant. 2006;10:441–448. doi: 10.1111/j.1399-3046.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- 41.Chacko B, Rajamanickam T, Neelakantan N, Tamilarasi V, John GT. Pediatric renal transplantation - a single center experience of 15yr from India. Pediatr Transplant. 2007;11:844–849. doi: 10.1111/j.1399-3046.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- 42.Vasudevan A, Iyengar A, Jose B, Phadke K. Pediatric Renal Transplantation: A Single-Center Experience. Transplant Proc. 2008;40:1095–1098. doi: 10.1016/j.transproceed.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Sinha A, Hari P, Guleria S, Gulati A, Dinda AK, Mehra NK, Srivastava RN, Bagga A. Outcome of pediatric renal transplantation in north India. Pediatr Transplant. 2010;14:836–843. doi: 10.1111/j.1399-3046.2010.01394.x. [DOI] [PubMed] [Google Scholar]
- 44.Meena J, Sinha A, Hari P, Dinda AK, Khandelwal P, Goswami S, Srivastava RN, Guleria S, Gulati A, Bansal VK, et al. (2018) Pediatric kidney transplantation: experience over two decades. Asian J Pediatr Nephrol 1:22–28
- 45.Srivastava A, Prabhakaran S, Sureka SK, Kapoor R, Kumar A, Sharma RK, Prasad N, Ansari MS. The challenges and outcomes of living donor kidney transplantation in pediatric and adolescent age group in a developing country: a critical analysis from a single center of north India. Indian J Urol. 2015;31:33–37. doi: 10.4103/0970-1591.145290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phadke K, Iyengar A, Karthik S, Kumar A, Olakkengil S. Pediatric renal transplantation: the Bangalore experience. Indian Pediatr. 2006;43:44–48. [PubMed] [Google Scholar]
- 47.Bijalwan P, Sanjeevan KV, Mathew A, Nair TB. Outcome and complications of living donor pediatric renal transplantation: experience from a tertiary care center. Indian J Urol. 2017;33:221–225. doi: 10.4103/iju.IJU_382_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chantarogh S, Tangnararatchakit K, Tirapanich W, Viseshsindh W, Saisawat P, Pirojsakul K. Clinical outcomes in pediatric renal transplant recipients who received steroid-based immunosuppressive regimen. Transplant Proc. 2017;49:971–976. doi: 10.1016/j.transproceed.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 49.Naderi G, Latif A, Karimi S, Tabassomi F, Esafahani ST. The long-term outcome of pediatric kidney transplantation in Iran: results of a 25-year single-center cohort study. Int J Organ Transplant Med. 2017;8:85–96. [PMC free article] [PubMed] [Google Scholar]
- 50.Rosati P, Pinto V, Delucchi A, Salas P, Cano F, Zambrano P, Lagos E, Rodriguez E, Hevia P, Ramirez K, et al. (2005) Pediatric renal transplantation: 13 years of experience—report from the Chilean Cooperative Multicenter Group. Transplant Proc 37:1569–1573 [DOI] [PubMed]
- 51.El-Husseini AA, Foda MA, Bakr MA, Shokeir AA, Sobh MA, Ghoneim MA. Pediatric live-donor kidney transplantation in Mansoura Urology & Nephrology Center: a 28-year perspective. Pediatr Nephrol. 2006;21:1464–1470. doi: 10.1007/s00467-006-0150-2. [DOI] [PubMed] [Google Scholar]
- 52.Sözen H, Dalgic A, Karakayali H, Baskin E, Saatci U, Arslan G, Haberal M, et al. (2006) Renal transplantation in children. Transplant Proc 38:426–429 [DOI] [PubMed]
- 53.Shilbayeh S, Hazza I. Pediatric renal transplantation in the Jordanian population: the clinical outcome measures during long-term follow-up period. Pediatr Neonatol. 2012;53:24–33. doi: 10.1016/j.pedneo.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Souqiyyeh MZ, Al-Khader AA, Shaheen FA, Huraib SO, Al-Harbi M. Pediatric renal transplantation in Saudi Arabia. Saudi J Kidney Dis Transpl. 1997;8:302–309. [PubMed] [Google Scholar]
- 55.Samhan M, Fathi T, Al-Kandari N, Buresley S, Nampoory MR, Niar P, Halim M, AI-Mousawi M, et al. Renal transplantation in children. Transplant Proc. 2007;39:911–913. doi: 10.1016/j.transproceed.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 56.Akoh JA. Renal transplantation in developing countries. Saudi J Kidney Dis Transpl. 2011;22:637–650. [PubMed] [Google Scholar]
- 57.Mubarak M. Practicing renal transplant pathology in a developing country: challenges and opportunities. J Transplant Technol Res. 2012;2:e118. doi: 10.4172/2161-0991.1000e118. [DOI] [Google Scholar]
- 58.Davidson B, Okpechi I, McCulloch M, Wearne N. Adolescent nephrology: an emerging frontier for kidney care in sub-Saharan Africa. Nephrology. 2017;22:933–939. doi: 10.1111/nep.13135. [DOI] [PubMed] [Google Scholar]
- 59.Moosa MR. The state of kidney transplantation in South Africa. S Afr Med J. 2019;109:235. doi: 10.7196/samj.2019.v109i4.13548. [DOI] [PubMed] [Google Scholar]
- 60.Kute VB, Trivedi HL, Vanikar AV, Shah PR, Gumber MR, Patel HV, Munjappa BC, Modi PR, Gera DN. Long-term outcome of deceased donor renal transplantation in pediatric recipients: a single-center experience from a developing country. Pediatr Transplant. 2012;16:651–657. doi: 10.1111/j.1399-30146.2012.01746x. [DOI] [PubMed] [Google Scholar]
- 61.Abraham G, Vijayan M, Gopalakrishnan N, Shroff S, Amalorpavanathan J, Yuvaraj A, Nair S, Sundarrajan S. State of deceased donor transplantation in India: a model for developing countries around the world. World J Transplant. 2016;6:331–335. doi: 10.5500/wjt.v6.i2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JS, Marlais M, Balasubramanian R, Muorah M, Inward C, Smith GC, Reynolds BC, Yadav P, Morgan H, Shenoy M, Tse Y, Hussain F, Grylls S, Kessaris N, Sinha MD, Marks SD, British Association for Paediatric Nephrology UK national study of barriers to renal transplantation in children. Arch Dis Child. 2020;106:384–386. doi: 10.1136/archdischild-2019-318272. [DOI] [PubMed] [Google Scholar]
- 63.Ali-El-Dein B, Abol-Enein H, El-Husseini A, Osman Y, Shehab El-Din AB, Ghoneim MA. Renal transplantation in children with abnormal lower urinary tract. Transplant Proc. 2004;36:2968–2973. doi: 10.1016/j.transproceed.2004.11.095. [DOI] [PubMed] [Google Scholar]
- 64.Maison POM, Smit S, McCulloch M, Gajjar P, Nourse P, Thomson D, Muller E, Millar A, Numanoglu A, Khan D. Urological complications following unstented pediatric renal transplantation. Pediatr Transplant. 2017;21:e13045. doi: 10.1111/petr.13045. [DOI] [PubMed] [Google Scholar]
- 65.Mishra SK, Muttu V, Rajapurkar MM, Desai MR. Kidney transplantation in abnormal bladder. Indian J Urol. 2007;23:299–304. doi: 10.4103/0970-1591.33728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Francis A, Johnson DW, Melk A, Foster BJ, Blazek K, Craig JC, Wong G. Survival after kidney transplantation during childhood and adolescence. Clin J Am Soc Nephrol. 2020;15:392–400. doi: 10.2215/CJN.07070619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pais P, Dello Strologo L, Iyengar A, Velusamy V, Greenbaum LA. Nocturnal hypertension and left ventricular hypertrophy in pediatric renal transplant recipients in South India. Pediatr Transplant. 2020;24:e13710. doi: 10.1111/petr.13710. [DOI] [PubMed] [Google Scholar]
- 68.Ullah K, Reza S, Ahmed P, Chaudhry QU, Satti TM, Ahmed S, Mirza SH, Akhtar F, Kamal K, Akhtar FM. Posttransplant infections: single centre experience from the developing world. Int J Infect Dis. 2008;12:203–214. doi: 10.1016/j.ijid.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 69.Prakash J, Ghosh B, Singh S, Soni A, Rathore SS. Causes of death in renal transplant recipients with functioning allograft. Indian J Nephrol. 2012;22:264–268. doi: 10.4103/0971-4065.101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sethi SK, Sinha R, Rohatgi S, Kher V, Iyengar A, Bagga A. Pediatric renal transplant practices in India. Pediatr Transplant. 2017;21:e12892. doi: 10.1111/petr.12892. [DOI] [PubMed] [Google Scholar]
- 71.Abraham G, John GT, Sunil S, Fernando EM, Reddy YNV. Evolution of renal transplantation in India over the last four decades. NDT Plus. 2009;3:203–207. doi: 10.1093/ndtplus/sfp178. [DOI] [Google Scholar]
- 72.Sethi SK, Bansal SB, Wadhwani N, Tiwari A, Arora D, Sharma R, Nandwani A, Yadav DK, Mahapatra AK, Jain M, Jha P, Ghosh P, Bhan A, Dhaliwal M, Raghunathan V, Kher V. Pediatric ABO-incompatible kidney transplantation: evolving with the advancing apheresis technology: a single-center experience. Pediatr Transplant. 2018;22:e13138. doi: 10.1111/petr.13138. [DOI] [PubMed] [Google Scholar]
- 73.Kute VB, Agarwal SK, Sahay M, Kumar A, Rathi M, Prasad N, Sharma RK, Gupta KL, Shroff S, Saxena SK. Kidney-paired donation to increase living donor kidney transplantation in India: Guidelines of Indian Society of Organ Transplantation - 2017. Indian J Nephrol. 2018;28:1–9. doi: 10.4103/ijn.IJN_365_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kafle MP, Poudyal AK, Chalise PR, Shah DS. Pediatric kidney transplantation in Nepal. Pediatr Transplant. 2019;23:e13588. doi: 10.1111/petr.13588. [DOI] [PubMed] [Google Scholar]
- 75.Wong G, Howard K, Chapman JR, Chadban S, Cross N, Tong A, Webster AC, Craig JC. Comparative survival and economic benefits of deceased donor kidney transplantation and dialysis in people with varying ages and co-morbidities. PLoS One. 2012;7:e29591. doi: 10.1371/journal.pone.0029591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jha V, Martin DE, Bargman JM, Davies S, Feehally J, Finkelstein F, Harris D, Misra M, Remuzzi G, Levin A, International Society of Nephrology Ethical Dialysis Task Force Ethical issues in dialysis therapy. Lancet. 2017;389:1851–1856. doi: 10.1016/S0140-6736(16)32408-4. [DOI] [PubMed] [Google Scholar]
- 77.Ramachandran R, Jha V. Kidney transplantation is associated with catastrophic out of pocket expenditure in India. PLoS One. 2013;8:e67812. doi: 10.1371/journal.pone.0067812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eke FU, Ladapo TA, Okpere AN, Olatise O, Anochie I, Uchenwa T, Okafor H, Ibitoye P, Ononiwu U, Adebowale A, Akuse R, Oniyangi S. The current status of kidney transplantation in Nigerian children: still awaiting light at the end of the tunnel. Pediatr Nephrol. 2021;36:693–699. doi: 10.1007/s00467-020-04753-7. [DOI] [PubMed] [Google Scholar]
- 79.Harris DCH, Davies SJ, Finkelstein F. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int. 2019;95:S1–S33. doi: 10.1016/j.kint.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 80.Teerawattananon Y, Dabak SV, Khoe LC, Bayani DBS, Isaranuwatchai W. To include or not include: renal dialysis policy in the era of universal health coverage. BMJ. 2020;368:m82. doi: 10.1136/bmj.m82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Helmuth ME, Liu Q, Turenne MN. Secular trends in the cost of immunosuppressants after solid organ transplantation in the United States. Clin J Am Soc Nephrol. 2019;14:421–430. doi: 10.2215/cjn.10590918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumar S, Dansereau EA, Murray CJL. Does distance matter for institutional delivery in rural India? Appl Econ. 2014;46:4091–4103. doi: 10.1080/00036846.2014.950836.75. [DOI] [Google Scholar]
- 83.Walker RC, Naicker D, Kara T, Palmer SC. Children’s experiences and expectations of kidney transplantation: a qualitative interview study. Nephrology. 2019;24:647–653. doi: 10.1111/nep.13405. [DOI] [PubMed] [Google Scholar]
- 84.Van Biesen W, Jha V, Abu-Alfa AK, Andreoli SP, Ashuntantang G, Bernieh B, Brown E, Chen Y, Coppo R, Couchoud C, Cullis B, Douthat W, Eke FU, Hemmelgarn B, Hou FF, Levin NW, Luyckx VA, Morton RL, Moosa MR, Murtagh FEM, Richards M, Rondeau E, Schneditz D, Shah KD, Tesar V, Yeates K, Garcia Garcia G. Considerations on equity in management of end-stage kidney disease in low- and middle-income countries. Kidney Int Suppl. 2020;10:e63–e71. doi: 10.1016/j.kisu.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Odetunde OI, Okafor HU, Uwaezuoke SN, Ezeonwu BU, Ukoha OM (2014) Renal replacement therapy in children in the developing world: challenges and outcome in a tertiary hospital in Southeast Nigeria. ScientificWorldJournal:903151. 10.1155/2014/903151 [DOI] [PMC free article] [PubMed]
- 86.Jafar TH. Organ trafficking: global solutions for a global problem. Am J Kidney Dis. 2009;54:1145–1157. doi: 10.1053/j.ajkd.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 87.Melk A, Babitsch B, Borchert-Mörlins B, Claas F, Dipchand AI, Eifert S, Eiz-Vesper B, Epping J, Falk CS, Foster B, Geyer S, Gjertson D, Greer M, Haubitz M, Lau A, Maecker-Kolhoff B, Memaran N, Messner HA, Ostendorf K, Samuel U, Schmidt BMW, Tullius SG, West L, Wong G, Zimmermann T, Berenguer M. Equally interchangeable? How sex and gender affect transplantation. Transplantation. 2019;103:1094–1110. doi: 10.1097/tp.0000000000002655. [DOI] [PubMed] [Google Scholar]
- 88.Sahay M. Men are from mars, women are from venus: gender disparity in transplantation. Indian J Transplant. 2019;13:237–239. doi: 10.4103/ijot.ijot_72_19. [DOI] [Google Scholar]
- 89.Iyengar A, Lewin S, Lantos JD. Considering family resources when making medical recommendations. Pediatrics. 2018;141:e20171198. doi: 10.1542/peds.2017-1198. [DOI] [PubMed] [Google Scholar]
- 90.Luyckx VA, Martin DE, Moosa MR, Bello AK, Bellorin-Font E, Chan TM, Claure-Del Granado R, Douthat W, Eiam-Ong S, Eke FU, et al. (2020) Developing the ethical framework of end stage kidney disease care: from practice to policy. Kidney Int Suppl 10:e72–e77. 10.1016/j.kisu.2019.11.003 [DOI] [PMC free article] [PubMed]
- 91.Luyckx VA, Tonelli M, Stanifer JW. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018;96:414–422D. doi: 10.2471/BLT.17.206441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luyckx VA, Al-Aly Z, Bello AK, Bellorin-Font E, Carlini RG, Fabian J, Garcia-G, Iyengar A, Sekkarie M, Van Biesen W. Sustainable development goals relevant to kidney health: an update on progress. Nat Rev Nephrol. 2021;17:15–32. doi: 10.1038/s41581-020-00363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]