Abstract
Transcription activator‐like effectors (TALEs), which induce the expression of specific plant genes to promote infection, are the main pathogenic determinants of various Xanthomonas bacteria. However, investigation of TALEs from Xanthomonas campestris pv. campestris, which causes black rot disease of crucifers, received little attention. In this study, we used PCR‐based amplification followed by SMRT amplicon sequencing to identify TALE genes in several X. campestris pv. campestris strains. Computational prediction in conjunction with quantitative reverse transcription PCR analysis was used to find their targets in the Brassica oleracea genome. Transcription factor ERF121, from the AP2/ERF family, was identified as target gene for the conserved TALEs from multiple X. campestris pv. campestris strains. Several members of this family from diverse plants were previously identified as targets of TALEs from different Xanthomonas species. We propose that TALE‐dependent activation of AP2/ERF transcription factors promotes susceptibility to Xanthomonas through the misregulation of plant defence pathways.
Keywords: black rot of crucifers, susceptibility gene, susceptibility hub, transcription activator‐like effector, Xanthomonas campestris pv. campestris
Xanthomonas campestris pv. campestris, causative agent of the black rot disease of crucifers, up‐regulates transcription factor ERF121 in Brassica oleracea by the conserved TAL‐effector.
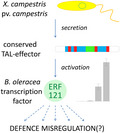
Transcription activator‐like effectors (TALEs) of Xanthomonas bacteria are transkingdom transcription factors that are translocated to plant cells via the type III secretion system, specifically binding to the promoters of certain host genes and activating their transcription (Hutin et al., 2015). The central part of TALEs consists of a series of quasi‐identical repeats typically comprising 33–35 amino acid residues, which differ mainly by residues at positions 12–13, known as repeat variable diresidues (RVDs). Each TALE repeat binds a single nucleotide, and repeats with different RVDs preferentially bind different nucleotides. The order of repeats in a TALE determines the sequence in the plant genomic DNA to which the TALE specifically binds. If this sequence, referred to as the effector‐binding element (EBE), is located within the promoter region of a gene, the TALE induces the expression of this gene on binding (Hutin et al., 2015). Hence, if the primary structure of a TALE and, accordingly, the order of the RVD are known, it is possible to predict TALE target genes in a plant genome.
TALE‐mediated up‐regulation of target genes promotes disease development. TALE target genes are also called susceptibility genes, or S genes (Hutin et al., 2015). TALE‐dependent induction of the expression of a single S gene can determine the difference between resistance and susceptibility in plant–Xanthomonas interactions (Streubel et al., 2013), and TALEs that activate such genes are often conserved and widely distributed among Xanthomonas species (Oliva et al., 2019). Some TALE‐activated S genes are conserved between different plant–Xanthomonas systems. The best‐studied examples are members of the SWEET gene family, in which TALE‐dependent activation was observed in the different plants (rice, citrus, cassava, cotton, pepper) on infection with different Xanthomonas species and strains (Hutin et al., 2015; Pérez‐Quintero et al., 2015). In some cases, this activation has been shown to be critically important for disease development (Streubel et al., 2013). Another example of conserved TALE targets involves transcription factors. For example, different TALEs from several pathovars of Xanthomonas citri activate the expression of the LOB1 transcription factor in citrus, and TALEs from numerous Xanthomonas oryzae pv. oryzae strains up‐regulate the expression of the TFX1 transcription factor (Hutin et al., 2015). Conserved S genes or S gene families are referred to as susceptibility hubs or pathogenicity hubs (Hutin et al., 2015; Mücke et al., 2019; Tran et al., 2018). Their identification is important not only for improving our understanding of the infection process, but also for developing resistant plants because the modification of conserved TALE targets can lead to more durable resistance.
Because of their role as the main pathogenic determinants, TALEs have been widely studied in Xanthomonas pathogens of several crop species, especially rice, citrus, and pepper. Xanthomonas campestris pv. campestris (Xcc) causes black rot, the most harmful and economically important disease of vegetable brassica crops (Vicente & Holub, 2013). The presence of TALEs has been shown for many Xcc strains of different geographical origins (Denancé et al., 2018; Kay et al., 2005; Mokryakov et al., 2010). However, the role of Xcc TALEs in black rot disease development has not been investigated in sufficient depth, and TALE target genes are largely unknown.
Isolation of TALE genes is quite challenging due to their large size, high GC content, and presence of an array of quasi‐identical repeats. To isolate the TALE genes from different Xcc strains, we used two‐step high‐temperature PCR with conserved primers that anneal far from the central repeat region, which greatly facilitates amplification according to Hommelsheim et al. (2014). In the sample of Xcc strains from the different races, all four strains belonging to Xcc race 6 carried a single TALE gene, whereas strains from races 1, 3, and 4 carried none (Figure 1), which was further confirmed by PCR amplification of shorter TALE fragments (Figure S1). Because the primers used for full‐length TALE amplification anneal at the conserved regions of the TALE genes, they apparently can be used for the inexpensive and rapid identification and isolation of TALE genes from diverse Xanthomonas species.
FIGURE 1.
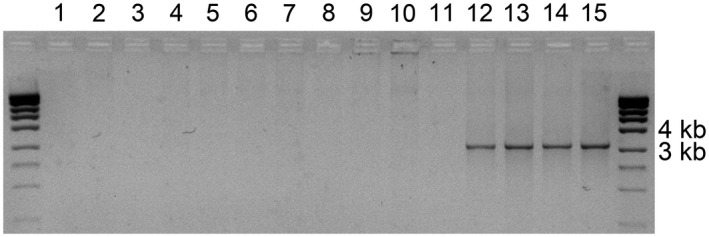
TALE gene identification in the Xanthomonas campestris pv. campestris (Xcc) strains from different races. DК‐1 (lane 1), Ram 3–2 (2), Ram 4–1 (3), 276NZ (4), and Tir‐2 (5) – race 1; Ram 1–3 (6), Ram 2–2 (7), 306NZ (8), and B‐1 (9) – race 3; Bun‐1 (10) and Xn‐13 (11) – race 4; XY1‐1 (12), XY1‐2 (13), XY2‐1 (14), and XY2‐2 (15) – race 6. The strains are referred to Xcc races 1, 3, 4, and 6 according to the report of Ha et al. (2014). Marker, MassRuler High‐Range DNA ladder (Thermo Fisher Scientific)
MinION (Oxford Nanopore Technologies) amplicon single‐molecule real‐time (SMRT) sequencing showed that the TALEs from all four strains contained 14 full repeats and a half repeat. According to the TALE class assignment algorithm from the AnnoTale suite (Grau et al., 2016), they were assigned to the TalEN class and named TalEN5 (accession number MT828881, strain XY1‐1), TalEN6 (MT828882, strain XY1‐2), TalEN7 (MT828883, strain XY2‐1), and TalEN8 (MT828884, strain XY2‐2). Phylogenetic relationships between TalEN5 to ‐8 and previously sequenced Xcc full‐length TALE genes (Denancé et al., 2018) were studied using the DistAL algorithm (Pérez‐Quintero et al., 2015). On the phylogenetic tree, TalEN5 to ‐8 were located closest to TalEN1, TalEN2, and TalEN3 from the Chinese strains CN‐12, CN‐17, and CN‐18, respectively (Figure 2a). These TALEs also belong to the TalEN class according to the AnnoTale suite and were referred to as members of the Tal15g group by Denancé et al. (2018).
FIGURE 2.
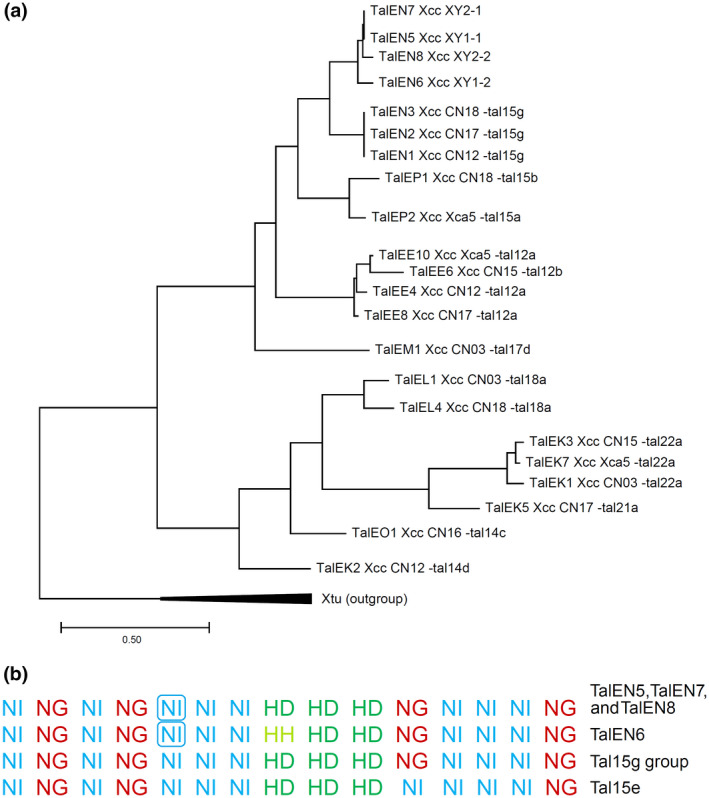
Multiple Xanthomonas campestris pv. campestris (Xcc) strains carry transcription activator‐like effectors (TALEs) with the conserved repeat variable diresidue (RVD) array. (a) Phylogenetic tree of the full‐length TALE genes sequenced in this study and in the study by Denancé et al. (2018). For each TALE gene from Denancé et al. (2018), the corresponding RVD group name is provided. TALEs from Xanthomonas translucens pv. undulosa (Xtu) were used as the outgroup. (b) RVD sequences of TALEs from this study and the Tal15 g group members and Tal15e from the study by Denancé et al. (2018). The RVDs from the fifth repeats of TalEN5 to ‐8, which are 35 amino acids in length, are shown in boxes
Differences in the nucleotide sequences between TalEN5 to‐8 and Tal15g group members occurred mainly at the 3′ part of the coding region downstream of the repeats (Figure S2). Additionally, all repeats in the central part of the Tal15g TALEs were 102 nucleotides in length and encoded 34 amino acid protein repeats, while in TalEN5 to ‐8, the fifth repeat was 105 nucleotides in length and coded for a 35 amino acid repeat. The combination of repeats of different lengths in a single protein is generally not very common for TALEs from diverse Xanthomonas species but is typical for Xcc TALEs (Denancé et al., 2018).
Despite some differences in both the nucleotide and amino acid sequences between TalEN5 to ‐8 and TALEs from the Tal15g group, the composition and order of RVDs were identical between them (Figure 2b). The only exception was TalEN6 from the XY1‐2 strain, which contains an HH RVD instead of an HD RVD in repeat 8; however, repeats with both HH and HD RVDs preferentially bind cytosine in the genomic DNA. The geographical origin of strains harbouring such an RVD combination varies: China and Belgium (Denancé et al., 2018) and Crimea (this work). TALEs from the Tal15g group were found in seven of the 22 Xcc strains that harbour any TALE genes according to Denancé et al. (2018). Also, Tal15 e had very similar RVD organization (Figure 2b). This suggests that this RVD combination is common among Xcc TALEs. Identification of a larger number of Xcc strains of different geographical origins is desirable to determine whether this RVD combination arose independently. Because the RVD composition defines a set of TALE target genes, the existence of identical RVD arrays in TALEs from multiple Xcc strains of different geographical origins suggests the importance of their targets as S genes upon infection.
The PrediTALE tool (Erkes et al., 2019) was used to identify putative EBEs for TalEN5 to ‐8) in the genome of the Brassica oleracea line TO1000DH3, which is highly susceptible to Xcc strains XY1‐1, XY1‐2, XY2‐1, and XY2‐2 on vein inoculation (Figure S3). The EBE that received the highest score was located in the promoter region of the annotated B. oleracea gene GenBank XM_013739306.1, which codes for the putative ethylene‐responsive transcription factor ERF121. The nucleotide sequence of this EBE is optimal for TalEN5 to ‐8) binding (Figure 3a). This is quite unusual because (with very rare exceptions; Mücke et al., 2019) all known natural EBEs, including those from highly induced genes, harbour single or even multiple mismatches relative to the optimal TALE‐binding sequence. Because even a single mismatch can significantly reduce the extent of TALE‐dependent gene up‐regulation (Cohn et al., 2016; Erkes et al., 2017; Zaka et al., 2018), the perfect match between the TalEN5 to ‐8) RVD array and the EBE is especially suitable for the activation of ERF121. In addition to the optimal sequence, the placement of the EBE in the ERF121 promoter is also favourable. The EBE is located upstream of the coding sequence close to the putative start codon, which is typical for TALE‐activated genes (Grau et al., 2013; Pereira et al., 2014; Streubel et al., 2017). Additionally, TALE EBEs often overlap with TATA boxes (Cohn et al., 2016; Grau et al., 2013; Pereira et al., 2014), and the EBE in the ERF121 promoter comprises a TATA‐like sequence (TATAWA consensus; Bernard et al., 2010; Figure 3a).
FIGURE 3.
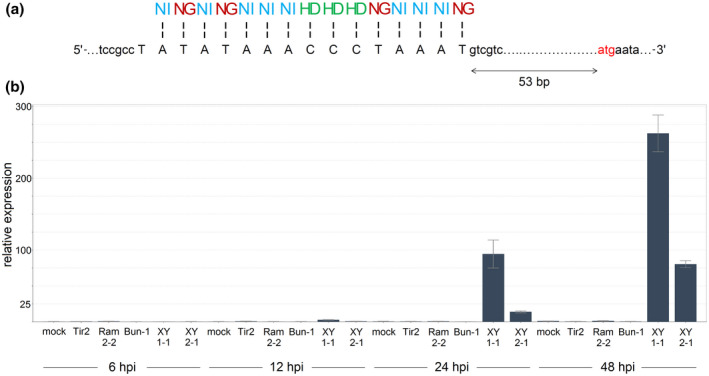
Xanthomonas campestris pv. campestris (Xcc) transcription activator‐like effectors (TALEs) activate the expression of the ERF121 gene. (a) Composition and location of the effector‐binding element (EBE) in the ERF121 promoter. The vertical dashes indicate matches between the EBE and repeat variable diresidues. The putative start codon is highlighted in red. (b) Expression level of ERF121 in the leaf tissues of Brassica oleracea line TO1000DH3 on syringe infiltration with TALE‐bearing (XY1‐1, XY2‐1) or TALE‐less (Tir2, Ram2‐2, Bun‐1) Xcc strains or mock at 6, 12, 24, and 48 hr postinoculation (hpi). Expression in the sample after mock inoculation at 48 hpi is considered a single expression unit. The bars show relative expression fold changes ± SEM. Three reference genes (Actin‐2, GAPDH, EF1α) were used to calculate relative expression
Due to the presence of the optimal EBE, ERF121 was considered as a target for TalEN5 to ‐8), and the expression of ERF121 was studied in B. oleracea TO1000DH3 on leaf inoculation with XY1‐1 (carrying TalEN5) and XY2‐1 (carrying TalEN7) strains. A substantial increase in expression of ERF121 was observed in response to both strains, while the inoculation with TALE‐less Xcc strains did not lead to ERF121 activation (Figure 3b). When plants for inoculation were grown under different environmental conditions (greenhouse instead of growth room), even higher ERF121 activation by XY1‐1 and XY2‐1 relative to mock was observed (Figure S4), which was not explained by the differences in the basal level of ERF121 expression between these conditions (Figure S5). One possible explanation of this finding is the unequal induction of a bacterial type III secretion system, which translocates effectors into plant cells, in the inoculated tissues of plants grown under different conditions. The type III secretion system is known to be activated or suppressed by various plant metabolites (Anderson et al., 2014; Tang et al., 2006; Wang et al., 2020; Yuan et al., 2020) whose content could be affected by plant growth conditions. Curiously, in both experiments ERF121 induction by XY1‐1 was observed earlier and was higher than by XY2‐1. Because the amino acid sequences are almost identical between TalEN5 and TalEN7, this effect could possibly be explained by some intrinsic physiological differences between XY1‐1 and XY2‐1 strains that led to different levels of synthesis and/or different effectiveness of translocation of TALEs into the host cells upon colonization of B. oleracea plants.
The presence of the optimal EBE and the strong TALE‐dependent induction indicate that ERF121 is a direct TALE target, while the presence of TALEs with this RVD combination in multiple Xcc strains of different geographical origins suggests that ERF121 activation is a widespread Xcc pathogenicity strategy. Transcription factors are promising TALE targets because activation of a single such gene can lead to downstream changes in the expression of numerous genes and global shifts in the cellular environment. ERF121 belongs to the large ERF group within the AP2/ERF transcription factor family (Licausi et al., 2013). TALE‐dependent activation of AP2/ERF transcription factors occurs in various plants, such as rice (Pérez‐Quintero et al., 2013; Tariq et al., 2019; Tran et al., 2018; Wang et al., 2017), wheat (Peng et al., 2019), and kale, on infection with diverse Xanthomonas species. For example, expression of the rice ERF123 gene is activated by TALEs from multiple X. oryzae pv. oryzae and X. oryzae pv. oryzicola strains, and ERF123 is considered to be a susceptibility hub in the rice–Xanthomonas interaction (Tran et al., 2018).
Different TALE‐activated AP2/ERFs have limited homology to each other and fall into distinct groups within the AP2/ERF family (Table S1), which suggests dissimilar functions. Plants commonly harbour more than 100 AP2/ERF transcription factors that generally have low sequence similarity and exert diverse functions; however, many of them are associated with resistance to stress factors, including numerous pathogens, which is especially true for ERFs (Licausi et al., 2013). Interestingly, rice ERF123 is up‐regulated under chilling stress (Tran et al., 2018), while the expression of ERF121 relatives from Arabidopsis thaliana changes under different stress conditions and treatment with defence‐associated plant hormones (Table S2) (Caarls et al., 2017; Feng et al., 2005; McGrath et al., 2005; Pierce & Rey, 2013; Postnikova et al., 2011). Curiously, in the study of Denancé et al. (2018) other defence‐associated genes, which code for TGG myrosinases, were found to be the targets for TALEs from the Tal15g group. Myrosinases are well‐known defence proteins that participate in the protection of cruciferous plants against herbivores and diverse pathogens (Piasecka et al., 2015; Poveda et al., 2020). According to our data, inoculation with XY1‐1 and XY2‐1 strains also elevated the expression of myrosinase genes in TO‐1000 plants (data not shown).
TALE‐induced overexpression of seemingly defence‐associated genes may seem counterintuitive. However, activation of some plant defence pathways can be beneficial for pathogenic bacteria, for example through the inhibition of competing microbes in plant tissues (Wu et al., 2019) under field conditions. Also, it is well known that plant responses to biotic and abiotic stresses, and to pathogens with different lifestyles often act antagonistically to each other. Phytopathogenic bacteria use various type III effectors to activate certain plant biotic and abiotic defence pathways, which leads to the repression of antagonistic defence responses (Kazan & Lyons, 2014; Sowden et al., 2018). Recently, TALE‐mediated activation of host factors involved in abscisic acid signalling was demonstrated in different plant–Xanthomonas systems (Mücke et al., 2019; Peng et al., 2019). The objective of TALE‐dependent ERF activation may be the inhibition of responses most useful on Xanthomonas attack through the misactivation of competing defence pathways. Indeed, overexpression of certain ERFs inversely regulated resistance to different pathogens and abiotic stress factors (Broekaert et al., 2006; Li et al., 2018; Lu et al., 2020; Tsutsui et al., 2009).
As long as the molecular mechanism of action of most ERFs, including B. oleracea ERF121, remains unknown, without further mechanistic studies we can only speculate about presumable defence pathways induced by TALE‐activated ERFs. Generally, ERFs are the major mediators of ethylene signalling (Broekaert et al., 2006). Although ethylene plays an important role in plant defence against pathogens, its role in plant–Xanthomonas interactions is controversial (Kim et al., 2013; Shen et al., 2011; Van Loon et al., 2006). TALE‐dependent up‐regulation of some ERFs can activate branches of the ethylene response that contribute more to susceptibility than to defence on Xanthomonas attack. We believe that future high‐throughput studies of effector‐activated ERF regulons could reveal the molecular mechanisms underlying ERF‐mediated plant susceptibility. It is likely that the clues can be found at the intersections of the regulons of different ERFs activated on plant–Xanthomonas interactions.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
FIGURE S1 (a) Positions of primer annealing sites for the amplification of short fragments of the TALE gene. (b) Amplification of TALE gene fragments in Xanthomonas campestris pv. campestris strains from different races
FIGURE S2 Alignment of coding regions of the TALE genes from strains XY1‐1, XY1‐2, XY2‐1, and XY2‐2 (this work) and CN12, CN17, and CN18
FIGURE S3 Black rot symptoms developed at 12–14 days after inoculation of Brassica oleracea TO‐1000DH3 plants with TALE‐bearing Xanthomonas campestris pv. campestris strains (XY1‐1, XY1‐2, XY2‐1, XY2‐2)
FIGURE S4 Relative expression of ERF121 in the leaf tissues of Brassica oleracea TO1000DH3 plants grown in a growth room (top diagram) or greenhouse (bottom diagram) on syringe infiltration with Xanthomonas campestris pv. campestris strains or mock at 6, 12, 24, and 48 hr postinoculation
FIGURE S5 Relative expression of ERF121 in the leaf tissues of Brassica oleracea TO1000DH3 plants grown in a greenhouse or growth room after mock inoculation with 10 mM MgCl2 at 6, 12, 24, and 48 hr postinoculation
TABLE S1 Similarity between amino acid sequences of the different TALE‐activated ERFs
TABLE S2 Expression of ERF120, ERF121, and ERF122 genes from Arabidopsis thaliana changes under stress conditions and treatment with defence‐associated plant hormones
FILE S1 Supplementary materials and methods. Bacterial strains, growth conditions, and Xanthomonas campestris pv. campestris genomic DNA extraction; TALE gene amplification and sequencing; phylogenetic analyses, EBE prediction; plant material, growth conditions and leaf inoculation; TALE target validation assays by quantitative reverse transcription PCR
ACKNOWLEDGEMENTS
The authors acknowledge Aspen Orynbayev and Fevzi Dzhalilov for providing Xcc strains. We apologize to our colleagues whose work was not cited in this article due to limited space. The work was done using the scientific equipment of the Center for Collective Use “Biotechnology” at the All‐Russia Research Institute of Agricultural Biotechnology (Moscow, Russia; agreement RFMEFI62114 × 0003). This study was supported by the RFBR (grant no. 18‐316‐00134) and State task (grant no. 0574‐2019‐0001).
Zlobin N, Lebedeva M, Monakhova Y, Ustinova V, Taranov V. An ERF121 transcription factor from Brassica oleracea is a target for the conserved TAL‐effectors from different Xanthomonas campestris pv. campestris strains. Mol Plant Pathol. 2021;22:618–624. 10.1111/mpp.13048
DATA AVAILABILITY STATEMENT
Sequences of TALE genes from this study were deposited in GenBank at https://www.ncbi.nlm.nih.gov/genbank/ under accession numbers MT828881, MT828882, MT828883, and MT828884.
REFERENCES
- Anderson, J.C. , Wan, Y. , Kim, Y.M. , Pasa‐Tolic, L. , Metz, T.O. & Peck, S.C. (2014) Decreased abundance of type III secretion system‐inducing signals in Arabidopsis mkp1 enhances resistance against Pseudomonas syringae . Proceedings of the National Academy of Sciences of the United States of America, 111, 6846–6851. 10.1073/pnas.1403248111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, V. , Brunaud, V. & Lecharny, A. (2010) TC‐motifs at the TATA‐box expected position in plant genes: A novel class of motifs involved in the transcription regulation. BMC Genomics, 11, 166. 10.1186/1471-2164-11-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert, W.F. , Delauré, S.L. , De Bolle, M.F. & Cammue, B.P. (2006) The role of ethylene in host–pathogen interactions. Annual Review of Phytopathology, 44, 393–416. 10.1146/annurev.phyto.44.070505.143440 [DOI] [PubMed] [Google Scholar]
- Caarls, L. , Van der Does, D. , Hickman, R. , Jansen, W. , Verk, M.C.V. , Proietti, S. et al. (2017) Assessing the role of ETHYLENE RESPONSE FACTOR transcriptional repressors in salicylic acid‐mediated suppression of jasmonic acid‐responsive genes. Plant and Cell Physiology, 58, 266–278. 10.1093/pcp/pcw187 [DOI] [PubMed] [Google Scholar]
- Cohn, M. , Morbitzer, R. , Lahaye, T. & Staskawicz, B.J. (2016) Comparison of gene activation by two TAL effectors from Xanthomonas axonopodis pv. manihotis reveals candidate host susceptibility genes in cassava. Molecular Plant Pathology, 17, 875–889. 10.1111/mpp.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denancé, N. , Szurek, B. , Doyle, E.L. , Lauber, E. , Fontaine‐Bodin, L. , Carrère, S. et al. (2018) Two ancestral genes shaped the Xanthomonas campestris TAL effector gene repertoire. New Phytologist, 219, 391–407. 10.1111/nph.15148 [DOI] [PubMed] [Google Scholar]
- Erkes, A. , Mücke, S. , Reschke, M. , Boch, J. & Grau, J. (2019) PrediTALE: A novel model learned from quantitative data allows for new perspectives on TALE targeting. PLoS Computational Biology, 15, e1007206. 10.1371/journal.pcbi.1007206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkes, A. , Reschke, M. , Boch, J. & Grau, J. (2017) Evolution of transcription activator‐like effectors in Xanthomonas oryzae . Genome Biology and Evolution, 9, 1599–1615. 10.1093/gbe/evx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, J.‐X. , Liu, D.I. , Pan, Y.I. , Gong, W. , Ma, L.‐G. , Luo, J.‐C. et al. (2005) An annotation update via cDNA sequence analysis and comprehensive profiling of developmental, hormonal or environmental responsiveness of the Arabidopsis AP2/EREBP transcription factor gene family. Plant Molecular Biology, 59, 853–868. 10.1007/s11103-005-1511-0 [DOI] [PubMed] [Google Scholar]
- Grau, J. , Reschke, M. , Erkes, A. , Streubel, J. , Morgan, R.D. , Wilson, G.G. et al. (2016) AnnoTALE: Bioinformatics tools for identification, annotation, and nomenclature of TALEs from Xanthomonas genomic sequences. Scientific Reports, 6, 21077. 10.1038/srep21077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau, J. , Wolf, A. , Reschke, M. , Bonas, U. , Posch, S. & Boch, J. (2013) Computational predictions provide insights into the biology of TAL effector target sites. PLoS Computational Biology, 9, e1002962. 10.1371/journal.pcbi.1002962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha, V.T.N. , Dzhalilov, F.S. , Vinogradova, S. , Kyrova, E. & Ignatov, A. (2014) Гeнeтичecкoe paзнooбpaзиe вoзбyдитeля cocyдиcтoгo бaктepиoзa в Poccии: peaкция pacтeний. [Genetic diversity of black rot pathogen in Russia: Plant reaction]. Zashchita Kartofelya, 2, 26–28. [Google Scholar]
- Hommelsheim, C.M. , Frantzeskakis, L. , Huang, M. & Ülker, B. (2014) PCR amplification of repetitive DNA: A limitation to genome editing technologies and many other applications. Scientific Reports, 4, 5052. 10.1038/srep05052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutin, M. , Pérez‐Quintero, A.L. , Lopez, C. & Szurek, B. (2015) MorTAL Kombat: The story of defense against TAL effectors through loss‐of‐susceptibility. Frontiers in Plant Science, 6, 535. 10.3389/fpls.2015.00535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, S. , Boch, J. & Bonas, U. (2005) Characterization of AvrBs3‐like effectors from a Brassicaceae pathogen reveals virulence and avirulence activities and a protein with a novel repeat architecture. Molecular Plant‐Microbe Interactions, 18, 838–848. 10.1094/MPMI-18-0838 [DOI] [PubMed] [Google Scholar]
- Kazan, K. & Lyons, R. (2014) Intervention of phytohormone pathways by pathogen effectors. The Plant Cell, 26, 2285–2309. 10.1105/tpc.114.125419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.G. , Stork, W. & Mudgett, M.B. (2013) Xanthomonas type III effector XopD desumoylates tomato transcription factor SlERF4 to suppress ethylene responses and promote pathogen growth. Cell Host & Microbe, 13, 143–154. 10.1016/j.chom.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Tian, Y. , Xu, J. , Fu, X. , Gao, J. , Wang, B.O. et al. (2018) A tomato ERF transcription factor, SlERF84, confers enhanced tolerance to drought and salt stress but negatively regulates immunity against Pseudomonas syringae pv. tomato DC3000. Plant Physiology and Biochemistry, 132, 683–695. 10.1016/j.plaphy.2018.08.022 [DOI] [PubMed] [Google Scholar]
- Licausi, F. , Ohme‐Takagi, M. & Perata, P. (2013) APETALA 2/Ethylene Responsive Factor (AP2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytologist, 199, 639–649. 10.1111/nph.12291 [DOI] [PubMed] [Google Scholar]
- Lu, W. , Deng, F. , Jia, J. , Chen, X. , Li, J. , Wen, Q. et al. (2020) The Arabidopsis thaliana gene AtERF019 negatively regulates plant resistance to Phytophthora parasitica by suppressing PAMP‐triggered immunity. Molecular Plant Pathology, 21, 1179–1193. 10.1111/mpp.12971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, K.C. , Dombrecht, B. , Manners, J.M. , Schenk, P.M. , Edgar, C.I. , Maclean, D.J. et al. (2005) Repressor‐and activator‐type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome‐wide screen of Arabidopsis transcription factor gene expression. Plant Physiology, 139, 949–959. 10.1104/pp.105.068544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokryakov, M.V. , Abdeev, I.A. , Piruzyan, E.S. , Schaad, N.W. & Ignatov, A.N. (2010) Diversity of effector genes in plant pathogenic bacteria of genus Xanthomonas . Microbiology, 79, 58–65. 10.1134/S002626171001008X [DOI] [Google Scholar]
- Mücke, S. , Reschke, M. , Erkes, A. , Schwietzer, C.‐A. , Becker, S. , Streubel, J. et al. (2019) Transcriptional reprogramming of rice cells by Xanthomonas oryzae TALEs. Frontiers in Plant Science, 10, 162. 10.3389/fpls.2019.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva, R. , Ji, C. , Atienza‐Grande, G. , Huguet‐Tapia, J.C. , Perez‐Quintero, A. , Li, T. et al. (2019) Broad‐spectrum resistance to bacterial blight in rice using genome editing. Nature Biotechnology, 37, 1344–1350. 10.1038/s41587-019-0267-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Z. , Hu, Y. , Zhang, J. , Huguet‐Tapia, J.C. , Block, A.K. , Park, S. et al. (2019) Xanthomonas translucens commandeers the host rate‐limiting step in ABA biosynthesis for disease susceptibility. Proceedings of the National Academy of Sciences of the United States of America, 116, 20938–20946. 10.1073/pnas.1911660116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, A.L.A. , Carazzolle, M.F. , Abe, V.Y. , de Oliveira, M.L.P. , Domingues, M.N. , Silva, J.C. et al. (2014) Identification of putative TAL effector targets of the citrus canker pathogens shows functional convergence underlying disease development and defense response. BMC Genomics, 15, 157. 10.1186/1471-2164-15-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Quintero, A.L. , Lamy, L. , Gordon, J.L. , Escalon, A. , Cunnac, S. , Szurek, B. et al. (2015) QueTAL: A suite of tools to classify and compare TAL effectors functionally and phylogenetically. Frontiers in Plant Science, 6, 545. 10.3389/fpls.2015.00545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Quintero, A.L. , Rodriguez‐R, L.M. , Dereeper, A. , López, C. , Koebnik, R. , Szurek, B. et al. (2013) An improved method for TAL effectors DNA‐binding sites prediction reveals functional convergence in TAL repertoires of Xanthomonas oryzae strains. PLoS One, 8, e68464. 10.1371/journal.pone.0068464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecka, A. , Jedrzejczak‐Rey, N. & Bednarek, P. (2015) Secondary metabolites in plant innate immunity: Conserved function of divergent chemicals. New Phytologist, 206, 948–964. 10.1111/nph.13325 [DOI] [PubMed] [Google Scholar]
- Pierce, E.J. & Rey, M.C. (2013) Assessing global transcriptome changes in response to South African cassava mosaic virus [ZA‐99] infection in susceptible Arabidopsis thaliana . PLoS One, 8, e67534. 10.1371/journal.pone.0067534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postnikova, O.A. , Minakova, N.Y. , Boutanaev, A.M. & Nemchinov, L.G. (2011) Clustering of pathogen‐response genes in the genome of Arabidopsis thaliana . Journal of Integrative Plant Biology, 53, 824–834. 10.1111/j.1744-7909.2011.01071.x [DOI] [PubMed] [Google Scholar]
- Poveda, J. , Eugui, D. & Velasco, P. (2020) Natural control of plant pathogens through glucosinolates: An effective strategy against fungi and oomycetes. Phytochemistry Reviews, 19, 1045–1059. 10.1007/s11101-020-09699-0 [DOI] [Google Scholar]
- Shen, X. , Liu, H. , Yuan, B.I.N. , Li, X. , Xu, C. & Wang, S. (2011) OsEDR1 negatively regulates rice bacterial resistance via activation of ethylene biosynthesis. Plant, Cell & Environment, 34, 179–191. 10.1111/j.1365-3040.2010.02219.x [DOI] [PubMed] [Google Scholar]
- Sowden, R.G. , Watson, S.J. & Jarvis, P. (2018) The role of chloroplasts in plant pathology. Essays in Biochemistry, 62, 21–39. 10.1042/EBC20170020 [DOI] [PubMed] [Google Scholar]
- Streubel, J. , Baum, H. , Grau, J. , Stuttman, J. & Boch, J. (2017) Dissection of TALE‐dependent gene activation reveals that they induce transcription cooperatively and in both orientations. PLoS One, 12, e0173580. 10.1371/journal.pone.0173580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streubel, J. , Pesce, C. , Hutin, M. , Koebnik, R. , Boch, J. & Szurek, B. (2013) Five phylogenetically close rice SWEET genes confer TAL effector‐mediated susceptibility to Xanthomonas oryzae pv. oryzae . New Phytologist, 200, 808–819. 10.1111/nph.12411 [DOI] [PubMed] [Google Scholar]
- Tang, X. , Xiao, Y. & Zhou, J.M. (2006) Regulation of the type III secretion system in phytopathogenic bacteria. Molecular Plant‐Microbe Interactions, 19, 1159–1166. 10.1094/MPMI-19-1159 [DOI] [PubMed] [Google Scholar]
- Tariq, R. , Ji, Z. , Wang, C. , Tang, Y. , Zou, L. , Sun, H. et al. (2019) RNA‐Seq analysis of gene expression changes triggered by Xanthomonas oryzae pv. oryzae in a susceptible rice genotype. Rice, 12, 44. 10.1186/s12284-019-0301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, T.T. , Pérez‐Quintero, A.L. , Wonni, I. , Carpenter, S.C. , Yu, Y. , Wang, L. et al. (2018) Functional analysis of African Xanthomonas oryzae pv. oryzae TALomes reveals a new susceptibility gene in bacterial leaf blight of rice. PLoS Pathogens, 14, e1007092. 10.1371/journal.ppat.1007092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui, T. , Kato, W. , Asada, Y. , Sako, K. , Sato, T. , Sonoda, Y. et al. (2009) DEAR1, a transcriptional repressor of DREB protein that mediates plant defense and freezing stress responses in Arabidopsis . Journal of Plant Research, 122, 633. 10.1007/s10265-009-0252-6 [DOI] [PubMed] [Google Scholar]
- Van Loon, L.C. , Geraats, B.P. & Linthorst, H.J. (2006) Ethylene as a modulator of disease resistance in plants. Trends in Plant Science, 11, 184–191. 10.1016/j.tplants.2006.02.005 [DOI] [PubMed] [Google Scholar]
- Vicente, J.G. & Holub, E.B. (2013) Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Molecular Plant Pathology, 14, 2–18. 10.1111/j.1364-3703.2012.00833.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L.I. , Rinaldi, F.C. , Singh, P. , Doyle, E.L. , Dubrow, Z.E. , Tran, T.T. et al. (2017) TAL effectors drive transcription bidirectionally in plants. Molecular Plant, 10, 285–296. 10.1016/j.molp.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Wang, W. , Yang, J. , Zhang, J. , Liu, Y.‐X. , Tian, C. , Qu, B. et al. (2020) An Arabidopsis secondary metabolite directly targets expression of the bacterial type III secretion system to inhibit bacterial virulence. Cell Host & Microbe, 27, 601–613. 10.1016/j.chom.2020.03.004 [DOI] [PubMed] [Google Scholar]
- Wu, D. , von Roepenack‐Lahaye, E. , Buntru, M. , de Lange, O. , Schandry, N. , Pérez‐Quintero, A.L. et al. (2019) A plant pathogen type III effector protein subverts translational regulation to boost host polyamine levels. Cell Host & Microbe, 26, 638–649. 10.1016/j.chom.2019.09.014 [DOI] [PubMed] [Google Scholar]
- Yuan, X. , Yu, M. & Yang, C.H. (2020) Innovation and application of the type III secretion system inhibitors in plant pathogenic bacteria. Microorganisms, 8, 1956. 10.3390/microorganisms8121956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaka, A. , Grande, G. , Coronejo, T. , Quibod, I.L. , Chen, C.‐W. , Chang, S.‐J. et al. (2018) Natural variations in the promoter of OsSWEET13 and OsSWEET14 expand the range of resistance against Xanthomonas oryzae pv. oryzae . PLoS One, 13, e0203711. 10.1371/journal.pone.0203711 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 (a) Positions of primer annealing sites for the amplification of short fragments of the TALE gene. (b) Amplification of TALE gene fragments in Xanthomonas campestris pv. campestris strains from different races
FIGURE S2 Alignment of coding regions of the TALE genes from strains XY1‐1, XY1‐2, XY2‐1, and XY2‐2 (this work) and CN12, CN17, and CN18
FIGURE S3 Black rot symptoms developed at 12–14 days after inoculation of Brassica oleracea TO‐1000DH3 plants with TALE‐bearing Xanthomonas campestris pv. campestris strains (XY1‐1, XY1‐2, XY2‐1, XY2‐2)
FIGURE S4 Relative expression of ERF121 in the leaf tissues of Brassica oleracea TO1000DH3 plants grown in a growth room (top diagram) or greenhouse (bottom diagram) on syringe infiltration with Xanthomonas campestris pv. campestris strains or mock at 6, 12, 24, and 48 hr postinoculation
FIGURE S5 Relative expression of ERF121 in the leaf tissues of Brassica oleracea TO1000DH3 plants grown in a greenhouse or growth room after mock inoculation with 10 mM MgCl2 at 6, 12, 24, and 48 hr postinoculation
TABLE S1 Similarity between amino acid sequences of the different TALE‐activated ERFs
TABLE S2 Expression of ERF120, ERF121, and ERF122 genes from Arabidopsis thaliana changes under stress conditions and treatment with defence‐associated plant hormones
FILE S1 Supplementary materials and methods. Bacterial strains, growth conditions, and Xanthomonas campestris pv. campestris genomic DNA extraction; TALE gene amplification and sequencing; phylogenetic analyses, EBE prediction; plant material, growth conditions and leaf inoculation; TALE target validation assays by quantitative reverse transcription PCR
Data Availability Statement
Sequences of TALE genes from this study were deposited in GenBank at https://www.ncbi.nlm.nih.gov/genbank/ under accession numbers MT828881, MT828882, MT828883, and MT828884.


