Abstract
Atrial fibrillation (AF) and heart failure (HF) are both highly prevalent diseases and are accompanied by a significant disease burden and increased mortality. Although the conditions may exist independently, they often go hand in hand as each is able to provoke, sustain, and aggravate the other. In addition, the diseases share a risk profile with several coinciding cardiovascular risk factors, promoting the odds of developing both AF and HF separately from each other. When the diseases coexist, this provides additional challenges but also opportunities for the optimal treatment. The recommended management of the comorbidities has been much debated in the past decades. In this review, we describe the pathophysiological coherence of AF and HF, illustrate the current knowledge on the management of them as comorbidities of each other and look forward to future developments in this field.
Keywords: Atrial fibrillation, Heart failure, Tachycardiomyopathy, Pathophysiology, HFrEF, HFpEF, Treatment, Catheter ablation, Pulmonary vein isolation
Introduction
Atrial fibrillation (AF) and heart failure (HF) are both highly prevalent diseases, with an estimated number of 33 million individuals that are affected by AF and 26 million by HF worldwide.1,2 The prevalence of both diseases is expected to rise even further in the years to come as a result of increased life expectancy and the increasing prevalence of cardiovascular risk factors and underlying diseases; an alarming trend given that both AF and HF are accompanied by significant morbidity and mortality. Although the conditions may exist independently, they often coexist as each is able to provoke, sustain, and aggravate the other. They strongly affect each other’s outcome, with higher hospitalization rates and a two to three times increase in mortality risk when compared with the separate diseases.3
Numerous studies have been conducted that aimed to elucidate the complex pathophysiological mechanisms between AF and HF, both with reduced (heart failure with reduced ejection fraction, HFrEF) and preserved ejection fraction (heart failure with preserved ejection fraction, HFpEF), and to discover the optimal treatment strategy for the combination of both diseases. In this review, we describe the pathophysiological coherence of AF and HF, illustrate the current knowledge on the management of them as comorbidities and look to future developments in this field.
Pathophysiology
The increased risk of patients with AF to develop HF and vice versa, is attributable to two factors. First, the diseases are inter-related pathophysiologically and as such can provoke and sustain each other. Secondly, both diseases share a risk profile with several coinciding cardiovascular risk factors, increasing the odds of developing both conditions separately from each other.
Atrial fibrillation-induced heart failure
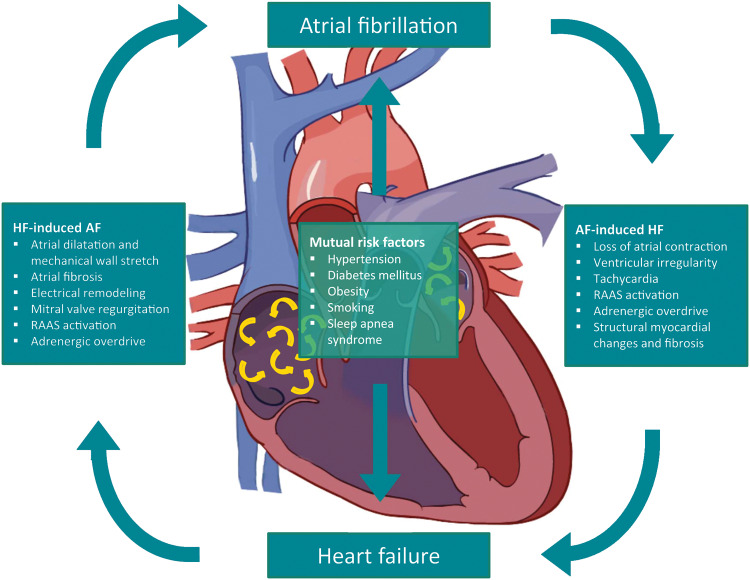
AF is able to provoke the development of HF via different mechanisms. The arrhythmia causes several immediate haemodynamic changes, which may contribute to decreased cardiac output and acute HF. In addition, continuous AF or frequent AF paroxysms may lead to persistent or irreversible structural changes causing impaired systolic and diastolic function not only of the atria but also the ventricles. Mechanisms responsible for the acute and chronic development of HF in AF patients include loss of atrial contraction, irregular heart rate, (persistent) tachycardia, neurohumoral activation, and structural myocardial changes (Figure 1).
Figure 1.
Pathophysiological relationship between atrial fibrillation and heart failure. AF, atrial fibrillation; HF, heart failure; RAAS, renin–angiotensin–aldosterone system.
Loss of atrial contraction
In normal sinus rhythm, the atrial contraction contributes ∼20–25% of the total left ventricular (LV) stroke volume, with maximum effect at heart rates between 50 and 80 beats per minute. When diastolic dysfunction is present, the contribution of the atrial contraction becomes more important due to the decreased passive filling.4 As such, the (sudden) loss of atrial contraction during AF episodes accompanied by the corresponding decrease in stroke volume can contribute to the development of HF, especially in patients with diastolic dysfunction.
Irregularity
The irregularity of ventricular contractions during AF may negatively impact systolic and diastolic function, even when ventricular rates are sufficiently treated with rate controlling drugs. This is partially caused by the beat-to-beat variability in duration of the diastolic interval, resulting in variable LV filling and end-diastolic volume. In addition, shorter cycle lengths affect the filling and release of calcium from the sarcoplasmic reticulum in greater extents than longer cycle lengths. Hereby, the myocardial contractibility and total cardiac output during irregular rhythms are decreased compared with regular rhythms with the same average frequency.5
Tachycardia
In the absence of rhythm or rate modulating drugs, AF is often accompanied by high ventricular rates. Continuous high heart rates may, independent of the cause of the tachycardia, lead to abnormal calcium signalling between the cardiomyocyte surface membrane and the sarcoplasmic reticulum, as well as decreased calcium levels in the sarcoplasmic reticulum. The resulting altered excitation–contraction coupling of the cardiomyocyte causes decreased myocardial contractility, smaller stroke volume, and LV dilatation, also referred to as tachycardiomyopathy. Several animal studies demonstrated a correlation between a higher rate and longer duration of rapid ventricular pacing and the severity of LV systolic dysfunction.6 Both relatively short episodes of tachycardia with high frequency and longer episodes with moderate frequencies may thus cause tachycardiomyopathy.
Neurohumoral activation
The reduced cardiac output resulting from the loss of atrial contraction, irregularity, and tachycardia accompanying AF may cause activation of several neurohumoral pathways, including the renin–angiotensin–aldosterone system (RAAS) and adrenergic system. Increased levels of angiotensin and aldosterone cause vasoconstriction, fluid retention, and increased blood pressure. When elevated during longer periods of time, however, RAAS hormones also lead to structural changes including cardiomyocyte hypertrophy, apoptosis, and adverse structural remodelling in the atrial and ventricular wall, promoting the development of systolic and diastolic LV dysfunction.7 Additionally, the increased sympathetic stimulation during AF results in increased contractility and heart rate in an attempt to maintain sufficient cardiac output. Although this may be beneficial in the short term, it may cause development and deterioration of HF in the long term.
Structural myocardial changes
The combined effects of haemodynamic alterations and overactivated regulatory mechanisms may cause permanent effects on the structural integrity of the atrial and ventricular myocardium. Although the systolic function of tachycardiomyopathy patients usually recovers when the arrhythmia is discontinued, prolonged AF may cause permanent damage. The extracellular matrix is particularly susceptible to long-term changes such as interstitial fibrosis, i.e. increased fibroblast activity and deposition of collagen and elastin fibres. Notably, these interstitial adjustments predominantly develop in the recovery phases between episodes of tachycardia, not during the higher ventricular rates itself.8 Even in patients with normal LV systolic ventricular function, cardiac magnetic resonance (CMR) imaging in AF patients reveals increased levels of diffuse interstitial ventricular fibrosis, associated with the AF burden but independent of other risk factors such as ischaemic heart disease and systolic dysfunction.9 These remnants of the arrhythmia episodes may cause increased LV stiffness and diastolic dysfunction.
Heart failure-induced atrial fibrillation
The increased risk of HF patients developing AF can primarily be explained by structural atrial remodelling, mitral valve regurgitation, and altered neurohumoral balances (Figure 1).
Structural remodelling of atria in heart failure
Both HFrEF and HFpEF are often associated with increased atrial filling pressures, although the mechanisms responsible may be different. HFrEF is characterized by reduced LV ejection fraction and increased end-diastolic LV volume. In HFpEF, the end-diastolic volume is usually not increased, but LV relaxation is disturbed. The elevated LV pressure in both types of HF causes increased atrial filling pressure, which in turn lead to a cascade of structural changes in the atrial wall that are strongly associated with AF.
The first step in this cascade is atrial dilatation and mechanical atrial wall stretch due to the elevated atrial filling pressures. Wall stretch may be present in strongly varying extents through different parts of the atria, with peaks around the pulmonary vein ostia, LA appendage ridge, the high posterior wall, anterior wall regions, and the septal regions.10 Atrial stretch provokes atrial scarring and fibrosis, predominantly in the areas where it is most severe. It is likely that atrial dilatation and atrial fibrosis are important factors for the occurrence and maintenance of AF. In dilated atria, multiple circuits coexist. Fibrosis leads to inhomogeneities in conduction and refractoriness and the arrhythmia itself causes persistent shortening of refractoriness. All of these changes favour re-entry.11
Mitral regurgitation
Mitral regurgitation is common in HF, with different underlying etiologies for HFrEF and HFpEF. In HFrEF, structural ventricular remodelling and LV dilatation may lead to secondary mitral regurgitation, whereas HFpEF may induce atrial functional mitral regurgitation predominantly due to annular dilatation and anterior leaflet flattening.12 Moderate or severe mitral regurgitation causes left atrial volume and pressure overload, resulting in increased local atrial wall stress, thus promoting the development of AF. The severity of regurgitation is correlated with the development of AF. Notably, AF itself may cause atrial functional mitral regurgitation similar to the manner in which HFpEF does, thereby indirectly sustaining itself.
Neurohumoral changes in heart failure
The decreased cardiac output during acute and chronic HF, similarly to that during AF, often causes RAAS and sympathetic activation. Besides their impact on HF development, these neurohumoral changes promote atrial remodelling and increase susceptibility for AF as well. The structural myocardial changes in the atria following increased RAAS hormone levels lead to increased development and sustenance of AF. Furthermore, sympathetic stimulation causes increased early and delayed afterdepolarizations, increased focal firing and favourable conditions for re-entry, thus increasing the susceptibility for AF.13 Importantly, as these neurohumoral changes are both a cause of and a result from AF as well as HF, a continuous process is created in which the presence of (one of) the diseases may provoke or deteriorate both itself and the other.
Mutual risk factors
Additionally, AF and HF share a common risk profile, increasing the possibility of developing both conditions separately from each other. Both HF and AF are more commonly seen in older patients with cardiovascular risk factors such as hypertension, diabetes mellitus, obesity, smoking, and sleep apnoea syndrome.
Hypertension and sleep apnoea may cause structural myocardial changes such as LV hypertrophy and interstitial fibrosis, leading to increased filling pressures and provoking the development of HF and AF. Obesity, diabetes mellitus, and smoking cause a pro-inflammatory state, creating an environment in which a patient is more susceptible to both diseases.14 In addition, besides their direct effects these risk factors contribute to the development of ischaemic heart disease, which is one of the most prevalent causes of HF and is associated with an increased risk of developing AF.15
Treatment considerations
Heart failure management
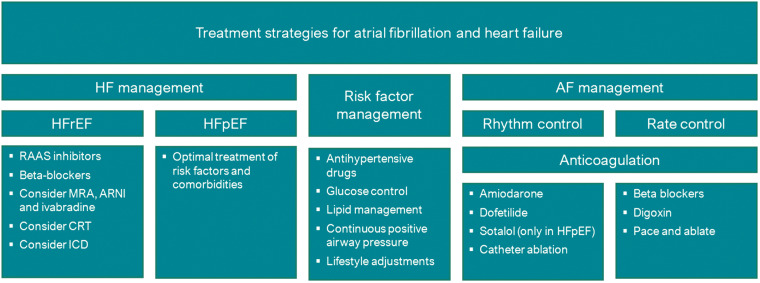
Standard treatment for HFrEF patients, independently of the presence or absence of AF, involves at least treatment with RAAS inhibitors and beta-blockers (Figure 2).16 Given the close involvement of the RAAS and the sympathetic nervous system in developing and maintaining AF, treatment with inhibitors of these pathways are thought to not just inhibit HF progression, but also to reduce structural atrial remodelling and prevent AF in at-risk patients. Indeed, these pharmacological interventions seem to have the potential to reduce the rate of new-onset AF in this population. However, a meta-analysis based on individual patient data comparing beta-blockers with placebo in 1677 patients with concomitant HF and AF did not demonstrate a beneficial effect, in contrast to HF patients in sinus rhythm.17 This may be caused by the questionable positive effect of strict rate control in AF patients and the increased risk of longer pauses in excessive rate control.18,19 However, these findings did not lead to changed treatment recommendations for AF patients in the most recent guidelines.
Figure 2.
Treatment strategies for atrial fibrillation and heart failure. AF, atrial fibrillation; CRT, cardiac resynchronization therapy; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter-defibrillator; RAAS, renin–angiotensin–aldosterone system.
For HFpEF, the optimal treatment strategy remains unclear. As no single drug has yet demonstrated a survival benefit in this complex and heterogeneous population, the cornerstone of the treatment of these patients remains treatment of underlying comorbidities.16 Notably, up to 80% of HFpEF patients are still prescribed RAAS inhibitors or beta-blockers, presumably mainly for the treatment of common cardiovascular comorbidities such as hypertension and coronary artery disease.
Risk factor management
There has been extensive research studying the effect of strict risk factor management on AF burden. Positive effects from weight loss, blood pressure management, lipid management, and treatment of obstructive sleep apnoea syndrome have been demonstrated in patients with (lone) AF.14 In a population with both AF and HF, strict risk factor management reduced AF burden as well.20 Less is known about the effect of risk factor management on HF in this population. In addition, the optimal target weight for patients with concomitant AF and HF remains unclear. Although associated with a higher arrhythmia burden in AF patients, obesity actually improves prognosis in the HF population.21 This phenomenon has become known as the obesity paradox, and its effect on prognosis of combined HF and AF remains to be determined.
Atrial fibrillation management
AF can be treated with either rhythm control, i.e. attempting to maintain sinus rhythm, or rate control, i.e. allowing AF to persist but controlling the frequency of ventricular contractions (Figure 2).22 In light of the negative effect AF can have on HF, adopting a rhythm control strategy would be expected to be beneficial in terms of survival and disease progression. This theory is supported by the recently published EAST trial, which confirmed the positive effects of rhythm control in patients with early AF.23 However, most antiarrhythmic drugs are contraindicated in HF patients, providing a challenge to the pursuit of rhythm control in this patient category. The only available options are amiodarone and dofetilide in HFrEF patients and amiodarone, dronedarone, and dofetilide in HFpEF patients, while dofetilide is not widely available in Europe.14 Amiodarone, although a potent antiarrhythmic drug, is known for its extracardiac side-effects and high discontinuation rate, limiting its low-threshold prescription.
Studies comparing rhythm and rate control in patients with AF and HF did not demonstrate benefit of medication-based rhythm control over rate control in terms of major clinical endpoints.24 A recent meta-analysis comparing rhythm and rate control in a total of 2486 patients demonstrated comparable rates of mortality, stroke, and thromboembolic events between the two groups.25 The hospitalization rate was higher in the rhythm control arm, mainly driven by the need for repeated cardioversion, adjustment of antiarrhythmic therapy and adverse drug reactions. However, the lack of improvement may be the limited efficacy of drugs in maintaining sinus rhythm, in addition to the harmful side-effects of currently available antiarrhythmic therapies.
Catheter ablation in heart failure patients with paroxysmal or persistent atrial fibrillation
In light of these limitations of medical therapy, more potent options to maintain sinus rhythm, such as invasive treatment with catheter ablation, might be effective to improve outcome. Considerable advancements in this technique have been made in the past years and it has proven to be an effective treatment to reduce AF burden and complaints. Several observational studies investigated if these results can be extrapolated to the HF population. Indeed, positive effects of catheter ablation were demonstrated in HFrEF patients on important surrogate outcomes such as LV ejection fraction, quality of life, and exercise capability. Similar to in HFrEF, observational data of HFpEF patients suggests that catheter ablation is associated with decreased HF symptoms, as well as with regression of echocardiographic diastolic dysfunction parameters.26 However, randomized trials confirming these promising results are not yet available in the HFpEF population.
In the HFrEF population, the first randomized trial comparing catheter ablation with pharmacological treatment was published in 2011.27 This small study in 38 patients did not demonstrate an improvement on the primary endpoint of LV ejection fraction following catheter ablation compared with pharmacological rate control. However, this lack of effect might be attributable to the modest success percentage of maintaining sinus rhythm of only 50%. In subsequent randomized studies, which all achieved higher success rates from catheter ablation, positive results on LV ejection fraction, improved functional capacity, and quality of life were demonstrated.28–30
In 2016, the AATAC trial demonstrated a trend towards lower mortality and hospitalizations following catheter ablation when compared with amiodarone, although the study was not powered to demonstrate significant effects.31 After these promising results, the outcomes of the CASTLE-AF were eagerly awaited, as this was the first sufficiently powered study to demonstrate possible effects on clinical endpoints. Indeed, the CASTLE-AF described an important reduction in the composite endpoint of death and HF hospitalizations, from 44.6% in the standard medical therapy group to 28.5% in the AF ablation group [hazard ratio 0.62 (0.43–0.87)].32 In contrast, the most recent study comparing catheter ablation with pharmacological treatment, the CABANA trial, did not demonstrate a significant difference in the primary composite endpoint of mortality, disabling stroke, serious bleeding, or cardiac arrest between the two groups.33
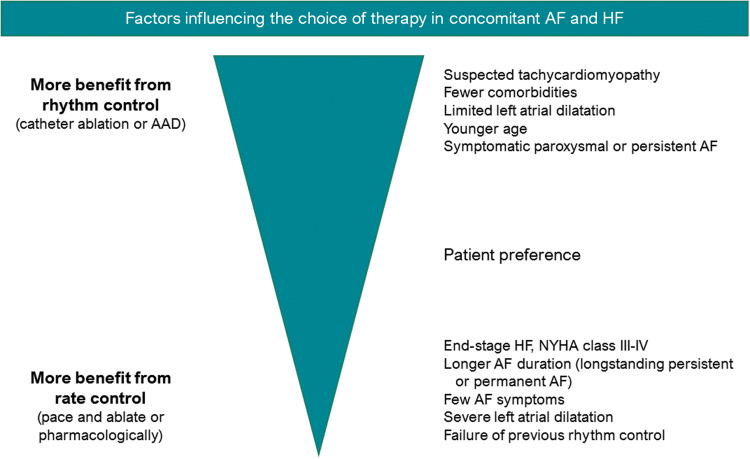
A meta-analysis combining efficacy data of all seven aforementioned randomized trials found that catheter ablation was associated with significantly lower mortality (relative risk reduction of 49%), hospitalization (relative risk reduction of 56%), improved LV ejection fraction, and improved quality of life.25 Still, it is important to note that the positive reported effects of catheter ablation are strongly dependent on several factors, such as patient characteristics, HF aetiology, follow-up duration, and ablation strategy (Figure 3). Hence, although catheter ablation has demonstrated favourable effects on important clinical endpoints, as well as functional status and quality of life, careful patient selection, and selection of ablation technique remains a point of attention.
Figure 3.
Therapy choice for atrial fibrillation and concomitant heart failure. AAD, antiarrhythmic drugs; AF, atrial fibrillation; HF, heart failure, NYHA, New York Heart Association.
Pace and ablate (rate control) in heart failure patients with permanent atrial fibrillation
For patients with permanent AF, a pace and ablate strategy [atrioventricular junction ablation combined with cardiac resynchronization therapy (CRT)] may be considered. In this strategy the persistence of AF and the associated loss of atrial contractions is accepted, while biventricular pacing assures strict rate control and regular ventricular contractions. Several studies have demonstrated markedly improved HF outcomes of a pace and ablate strategy compared with pharmacological rate control in patients with and without a previous CRT indication.34 However, when pace and ablate is compared with catheter ablation aimed to achieve rhythm control, rhythm control demonstrated superior results. Therefore, it seems reasonable to reserve a pace and ablate strategy for those patients in whom catheter ablation is expected to be ineffective (e.g. due to comorbidities, severely dilated atria or permanent AF) or in whom previous catheter ablation has failed (Figure 3).
Conclusion
The optimal therapy for coexisting AF an HF remains a topic of debate. In light of the harmful effect AF can have on HF, adopting a rhythm control strategy could be expected to be beneficial in terms of survival and disease progression. However, pharmacological rhythm control does not lead to significant health gain when compared with rate control in this patient population. Catheter ablation is an effective option to achieve rhythm control without the unfavourable effects of antiarrhythmic drugs, and seems to improve the HF prognosis as well. However, several unaddressed questions remain in spite of the current evidence, in particular with regards to the optimal patient selection for this invasive therapy. Further studies with long-term follow-up may clarify the remaining uncertainties.
Conflict of interest: none declared.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ. et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M. et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–33. [DOI] [PubMed] [Google Scholar]
- 3. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA. et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–5. [DOI] [PubMed] [Google Scholar]
- 4. Zakeri R, Moulay G, Chai Q, Ogut O, Hussain S, Takahama H. et al. Left atrial remodeling and atrioventricular coupling in a canine model of early heart failure with preserved ejection fraction. Circ Heart Fail 2016;9:1–11. [Google Scholar]
- 5. Clark DM, Plumb VJ, Epstein AE, Kay GN.. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol 1997;30:1039–45. [DOI] [PubMed] [Google Scholar]
- 6. Hendrick DA, Smith AC, Kratz JM, Crawford FA, Spinale FG.. The pig as a model of tachycardia and dilated cardiomyopathy. Lab Anim Sci 1990;40:495–501. [PubMed] [Google Scholar]
- 7. Hartupee J, Mann DL.. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol 2017;14:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spinale FG, Zellner JL, Johnson WS, Eble DM, Munyer PD.. Cellular and extracellular remodeling with the development and recovery from tachycardia-induced cardiomyopathy: changes in fibrillar collagen, myocyte adhesion capacity and proteoglycans. J Mol Cell Cardiol 1996;28:1591–608. [DOI] [PubMed] [Google Scholar]
- 9. Ling LH, Kistler PM, Ellims AH, Iles LM, Lee G, Hughes GL. et al. Diffuse ventricular fibrosis in atrial fibrillation: noninvasive evaluation and relationships with aging and systolic dysfunction. J Am Coll Cardiol 2012;60:2402–8. [DOI] [PubMed] [Google Scholar]
- 10. Hunter RJ, Liu Y, Lu Y, Wang W, Schilling RJ.. Left atrial wall stress distribution and its relationship to electrophysiologic remodeling in persistent atrial fibrillation. Circ Arrhythm Electrophysiol 2012;5:351–60. [DOI] [PubMed] [Google Scholar]
- 11. Stiles MK, John B, Wong CX, Kuklik P, Brooks AG, Lau DH. et al. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the “second factor”. J Am Coll Cardiol 2009;53:1182–91. [DOI] [PubMed] [Google Scholar]
- 12. Deferm S, Bertrand PB, Verbrugge FH, Verhaert D, Rega F, Thomas JD. et al. Atrial functional mitral regurgitation: JACC review topic of the week. J Am Coll Cardiol 2019;73:2465–76. [DOI] [PubMed] [Google Scholar]
- 13. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S.. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 2014;114:1500–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C. et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020; 1–126, Doi:10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 15. van Veldhuisen DJ, van den Heuvel AF, Blanksma PK, Crijns HJ.. Ischemia and left ventricular dysfunction: a reciprocal relation? J Cardiovasc Pharmacol 1998;32:S46–51. [DOI] [PubMed] [Google Scholar]
- 16. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ. et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 17. Rienstra M, Damman K, Mulder BA, Van Gelder IC, McMurray JJ, Van Veldhuisen DJ.. Beta-blockers and outcome in heart failure and atrial fibrillation: a meta-analysis. JACC Heart Fail 2013;1:21–8. [DOI] [PubMed] [Google Scholar]
- 18. Mulder BA, Van Veldhuisen DJ, Crijns HJ, Tijssen JG, Hillege HL, Alings M. et al. Lenient vs. strict rate control in patients with atrial fibrillation and heart failure: a post-hoc analysis of the RACE II study. Eur J Heart Fail 2013;15:1311–8. [DOI] [PubMed] [Google Scholar]
- 19. Van Gelder IC, Rienstra M, Crijns HJ, Olshansky B.. Rate control in atrial fibrillation. Lancet 2016;388:818–28. [DOI] [PubMed] [Google Scholar]
- 20. Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brugemann J. et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J 2018;39:2987–96. [DOI] [PubMed] [Google Scholar]
- 21. Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO.. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 2013;1:93–102. [DOI] [PubMed] [Google Scholar]
- 22. Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T. et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347:1834–40. [DOI] [PubMed] [Google Scholar]
- 23. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A. et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 24. Hagens VE, Crijns HJ, Van Veldhuisen DJ, Van Den Berg MP, Rienstra M, Ranchor AV. et al. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 2005;149:1106–11. [DOI] [PubMed] [Google Scholar]
- 25. Chen S, Purerfellner H, Meyer C, Acou WJ, Schratter A, Ling Z. et al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: a stratified pooled analysis of randomized data. Eur Heart J 2020;41:2863–73. [DOI] [PubMed] [Google Scholar]
- 26. Rattka M, Pott A, Kuhberger A, Weinmann K, Scharnbeck D, Stephan T. et al. Restoration of sinus rhythm by pulmonary vein isolation improves heart failure with preserved ejection fraction in atrial fibrillation patients. Europace 2020;22:1328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacDonald MR, Connelly DT, Hawkins NM, Steedman T, Payne J, Shaw M. et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart 2011;97:740–7. [DOI] [PubMed] [Google Scholar]
- 28. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V. et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol 2014;7:31–8. [DOI] [PubMed] [Google Scholar]
- 29. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A. et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI Study. J Am Coll Cardiol 2017;70:1949–61. [DOI] [PubMed] [Google Scholar]
- 30. Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL. et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol 2013;61:1894–903. [DOI] [PubMed] [Google Scholar]
- 31. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D. et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC Multicenter Randomized Trial. Circulation 2016;133:1637–44. [DOI] [PubMed] [Google Scholar]
- 32. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L. et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 33. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE. et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA Randomized Clinical Trial. JAMA 2019;321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brignole M, Pokushalov E, Pentimalli F, Palmisano P, Chieffo E, Occhetta E. et al. A randomized controlled trial of atrioventricular junction ablation and cardiac resynchronization therapy in patients with permanent atrial fibrillation and narrow QRS. Eur Heart J 2018;39:3999–4008. [DOI] [PubMed] [Google Scholar]